Abstract
Objective(s):
This study was conducted to evaluate the cerebroprotective effect of methanolic leaf extract of Punica granatum (MePG) in Wistar rats.
Materials and Methods:
The MePG was initially assessed for in vitro antioxidant activity, and later evaluated on LPS-induced RAW 264.7 cell line assay. Finally, the MePG was evaluated against ischemia-reperfusion (I/R) induced brain injury in Wistar rats.
Results:
In DPPH, FRAP and ORAC assays, the MePG has exhibited potent antioxidant activity. Further, the MePG has significantly inhibited the generation of nitrite, ROS and TNF-α in LPS-induced RAW 264.7 cell lines. Besides, global ischemia followed by reperfusion caused significant changes in the neurological and behavioral functions in I/R control animals compared to sham control. Additionally, in the I/R control group there was a substantial decrease in the catalase and superoxide dismutase activities; Likewise, reduced glutathione levels reduced and lipid peroxidation levels enhanced significantly. Also, pro-inflammatory cytokines such as TNF-α, IL-6, and ICAM-I were increased and the levels of IL-10 was decreased significantly. Furthermore, the I/R insult caused increase in brain volume and cerebral infarct formation. Similarly, histopathology of the brain tissue revealed hallmarks like necrosis, leukocyte infiltration, cerebral edema and vascular congestion in I/R control. Notably, MePG (200 and 400 mg/kg) pretreatment for 7 days, has attenuated all the I/R-persuaded pathological changes compared to I/R control. In addition, the LC-MS/MS analysis showed presence of acteoside, apigenin, gallic acid, gossypin, pentagalloyl glucose, quercetin, and rutin as major ingredients in the MePG.
Conclusion:
These findings suggest that the MePG possesses significant cerebroprotective activity.
Key Words: Antioxidant, Cerebral ischemia, Neuroprotection, Punica granatum, Stroke
Introduction
Stroke is considered as one among the most common and major cerebrovascular pathological conditions (1). According to the recent survey, stroke is the second most cause of death (around 65 lakh deaths per year), and primary cause of severe and prolonged debility (2). Ischemic stroke accounts for more than 80% of the overall incidences of stroke, and it is a consequence of decreased blood supply to a small portion (focal) of the brain or complete brain (global) (3).
Many studies have reported that oxidative stress plays a crucial role in ischemia-reperfusion (I/R) brain injury (4). Though oxidative metabolism is highly essential for the survival of neurons, the exaggeration of the same leads to generation numerous oxidative free radicals (5). Under normal physiology, the reactive oxygen species are controlled by endogenous antioxidant defense mechanisms associated with the living system such as catalase, superoxide dismutase, glutathione system and so on, and thus maintains homeostasis (6). During I/R injury, the excess generation of reactive oxygen species leads to depletion or failure of endogenous antioxidant defense systems, and lead to accumulation of enormous free radicals (7). These toxic chemical entities initiate multifaceted cascades of various inflammatory events and apoptosis, that consequences in neuronal cell death. The injury to the neuronal tissue is displayed as functional (neurological, cognitive) and motor deficits, along with morphological (cerebral edema, infarction) and histopathological alterations of the brain tissue (8).
Based on the clear understanding of the disease, it is evident that the compounds belong to calcium channels blockers, excitatory neurotransmitter receptor blockers/antagonists, anti-inflammatory, antioxidants, and anti-apoptotic class may be beneficial in the treatment of cerebral stroke (9, 10).
In this context, various parts of Punica granatum L. (Punicaceae) have been extensively used in the traditional system of Indian medicine to treat varieties of illnesses (11, 12), and various parts of the plant have been scientifically proved for diverse biological activities such as antioxidant (13), anticonvulsant (14, 15), anti-Parkinson (16), anti- neuroinflammatory (17), anti-Alzheimer’s (18, 19), memory enhancing (20), and protection against neonatal hypoxic-ischemic brain injury (21, 22). Considering the strong literature reports on antioxidant and neuroprotective actions of P. granatum, this study was designed to evaluate the effect of methanolic leaf extract of P. granatum on global I/R induced brain injury in Wistar rats.
Materials and Methods
Drugs and Chemicals
Quercetin, 2-thiobarbituric acid (TBA), 5-5-dithiobis (2-nitrobenzoic acid) (DTNB), trichloroacetic acid (TCA), and 2,3,5-triphenyltetrazolium chloride stain (TTC) were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, Maharashtra, India). ELISA kits were procured from Krishgen (Krishgen Biosystems, Mumbai, India). LC-MS grade solvents were procured from Merck Ltd (Mumbai, Maharashtra, India).
Plant material collection, handling, and extraction process
The leaves of P granatum L. were harvested from Doddathekahalli, Sidlaghatta (T), Chikkaballapura (D), Karnataka, India during May-June. The plant material was recognized and authentified by Dr Madhavachetty, Associate professor, Botany Department, SV University, Tirupati, Andhra Pradesh, India. A sample of the plant part is conserved with voucher number 1213. The plant material was shade dried, powdered, and extracted at 60 °C for 48 hr, with 70 % methanol using Soxhlet apparatus (14).
In vitro antioxidant assays
The MePG was evaluated using DPPH, FRAP and ORAC assays in in vitro.
DPPH assay
This assay was performed as per standard procedure mentioned in the literature. Shortly, 100 µl of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) (in ethanol) was added to 100 µl of varied concentrations of the test drug solution prepared in water, and the mixture was incubated at room temperature for 30 min. Later, the absorbance was measured at 517 nm (23).
The DPPH radical scavenging capacity was calculated using the following equation:
DPPH Scavenged (%) =)
The assay was carried out in triplicate for each sample and the results were expressed as IC50 (µg/ml) values.
FRAP assay
This experiment was conducted as per well-established method given in the literature. The basic principle involves reduction of ferric ion to ferrous ion at low pH, which results in the formation of a colored complex called ferrous–tripyridyltriazin. The intensity of color formed is directly proportional to quantity of the product formed and it is estimated by recording the absorbance at 593 nm. The results are expressed as FRAP value (24).
ORAC assay
This experiment was carried out as per previously reported procedure by Rafiq et al. The Peroxyl radical formed from 2,2’-azobis (2-amidinopropane) dihydrochloride reacts with fluorescein (substrate) to give bright purple colored fluorescence. The capability of the test drug to decline the intensity of fluorescence is used as a measure of antioxidant activity. Results are expressed as Trolox equivalent/g of fresh sample, also called as ORAC value (25).
In vitro RAW 264.7 cell line assay
RAW 264.7 cells were procurement from the National Centre for Cell Science (NCCS), Pune, India and all the cell line experiments were carried out as per Viswanatha et al., and Mathew et al. (26, 27).
Experimental animals
Inbred male Wistar rats (200–220 g, 8-10 weeks old) were preserved in polypropylene cages under standard housing conditions. The experimental animals were provided with standard rat diet and purified water ad libitum. All the experimental procedures were approved by the IAEC and the experiments were performed as per CPCSEA guidelines (CPCSEA, India).
Study plan
Group classification
Fifty male Wistar rats were allocated into five groups (n=10, G-1 to G-5), G-1 and G-2 named as Sham control, and I/R control respectively, these two groups received vehicle (0.5% CMC); the G-3 animals received quercetin (20 mg/kg, PO) which is a reference standard, whereas G-4 and G-5 groups received oral doses of MePG at 200 mg/kg, and 400 mg/kg, respectively. All the treatments were given for a span of seven days.
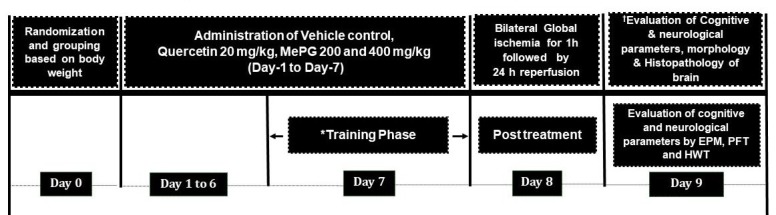
On day-8, all the experimental animals, excluding G-1 were exposed to 60 min of ischemia followed by 24 hrs reperfusion as per previously mentioned procedure (26). Subsequently, all the animals were evaluated for neurological, cognitive, and motor functions. Later, all the animals were sacrificed using isoflurane. The brain tissue was quickly collected, weighed, and the brain volume was assessed by displacement method, and subjected for the measurement of cerebral infarct size, biochemical estimations, and histopathology (Figure 1).
Figure 1.
Illustration of detailed experimental protocol
Note: EPM, elevated plus maze test; PFT, pole fall test; HWT, hanging wire test. *Training Phase: All the animals were trained in elevated plus maze test to move from open arm to closed arm (Transfer latency) on day 5, 6 and 7. In pole fall test (PFT) and hanging wire test (HWT) all the animals were trained only on Day 7. †Evaluation on Day 9: All the animals were evaluated in elevated plus maze test for transfer latency (memory); While the fall off time in seconds were evaluated for all the animals in pole fall test and hanging wire test (motor coordination).
Formulation and administration of MePG
The MePG was suspended in 0.5 % carboxy methyl cellulose for oral administration. The concentrations used for administration are 20 and 40 mg/ml. The sham control and I/R control animals received 0.5% CMC, at 10 ml/kg dose volume.
Evaluation parameters
Neurological score
There are four categories (0 to 3) of neurological findings were noted as follows: 0=no observed neurological deficits; 1=contralateral forelimb flexion with wrist flexion and shoulder adduction; 2=reduced resistance to lateral push; and 3=circling movements toward the ipsilateral side (28).
Elevated plus maze test
On Day-7 of the experiment, all the animals were trained on the elevated plus maze as per the previously published method (29). The transfer latency noted during training period is named as acquisition, whereas the transfer latency recorded after I/R insult is considered as index of retrieval and named as retention latency (29).
Evaluation of motor functions
Hanging wire test (HWT) and pole fall test (PFT)
These tests were performed as per previously explained procedure by Viswanatha et al. (29).
Brain morphology
Brain volume was estimated by displacement method, and used as an index of edema, while the TTC staining was performed as per published literature, which is used to determine cerebral infarction (28).
Biochemical estimations
Using the brain homogenate the biochemical parameters were determined as per standard methods published in the literature, namely the total protein content was estimated as per the Lowry’s method (30); the catalase activity as per Luck (31), lipid peroxidation was determined as per Wills (32), reduced glutathione was estimated as per Sedlak and Lindsay (33). While, SOD activity was measured using commercially available superoxide dismutase estimation kit (Sigma-Aldrich, USA), as per manufacturer instructions (34).
Assessment of inflammatory markers
The TNF-α, IL-6, ICAM-I and IL-10 levels in the brain homogenate was assessed using commercially available ELISA kits (Krishgen Biosystems, Mumbai, India).
Histopathological evaluation
In short, the brain tissue was fixed in 10% v/v neutral buffered formalin, and embedded in paraffin wax and made into 5 µm thick longitudinal section using microtome. Subsequently, the sections were stained with hematoxylin, and eosin for histopathological evaluation.
Characterization of methanolic leaf extract of P. granatum
Total Phenolic content
Total phenolic content in the extract was determined by Folin-Ciocalteu test (35).
LC-MS/MS investigation of MePG
The LC-MS/MS analysis was carried out using API 3000 Triple Quadrupole LC/MS/MS Mass Spectrometer (Perkin-Elmer Sciex Instruments, USA) and electron spray ionization source with an electron multiplier detector and a chromatographic system.
The amount of identified compounds present in the extracts were quantified using the equation given below (28).
Statistical analysis
The results of in vivo experiments were expressed as mean±SEM, and compared by One-way ANOVA followed by Tukey’s multiple comparison test using Graph pad version 5.01 (Graph Pad Software, San Diego California USA). The P<0.05 was considered to be statistically significant.
Results
In vitro antioxidant activity
Initially, MePG was assessed in DPPH, FRAP, and ORAC assays for its antioxidant activity. In these assays, the MePG showed effective antioxidant property with a IC50 of 69.50±4.0 in DPPH assay, with a FRAP and ORAC value of 989.74±68.9 and 1229.8±117.3 respectively, in FRAP and ORAC assays. The results are depicted in Table 1.
Table 1.
Evaluation of in vitro antioxidant activity of methanolic leaf extract of P. granatum
| Sample |
Antioxidant activity
*
|
||
|---|---|---|---|
|
DPPH Assay
(IC 50- µg/ml) |
FRAP assay
(mg/g FeSO 4 equivalents |
ORAC assay
(TE/g) |
|
| MePG | 69.50 ± 4.0 | 989.74 ± 68.9 | 1229.8 ± 117.3 |
| Vitamin C | 25.30 ± 1.8 | 1416.81 ± 121.3 | 3418.4 ± 221.2 |
Note: DDPH, 2, 2, Diphenyl-1-picrylhydrazyl; FRAP, Ferric reducing antioxidant power; ORAC, oxygen radical absorbance capacity; TE, Trolox equivalents; MePG, Methanolic leaf extract of P. granatum.
The values are expressed as mean ± SD for three trials carried out for each assay.
In vitro RAW 264.7 cell line assay
In continuation with antioxidant assays, the MePG was assessed for its effect on generation of nitrite, ROS and TNF-α in LPS-activated RAW 264.7 cell lines. The cytotoxicity assessed by MTT assay revealed that the MePG exhibits very negligible cytotoxicity with IC50 (µg/ml of 1826.7±157.7. Thus, for all remaining experiments doses were selected between 12.5 to 200 µg/ml.
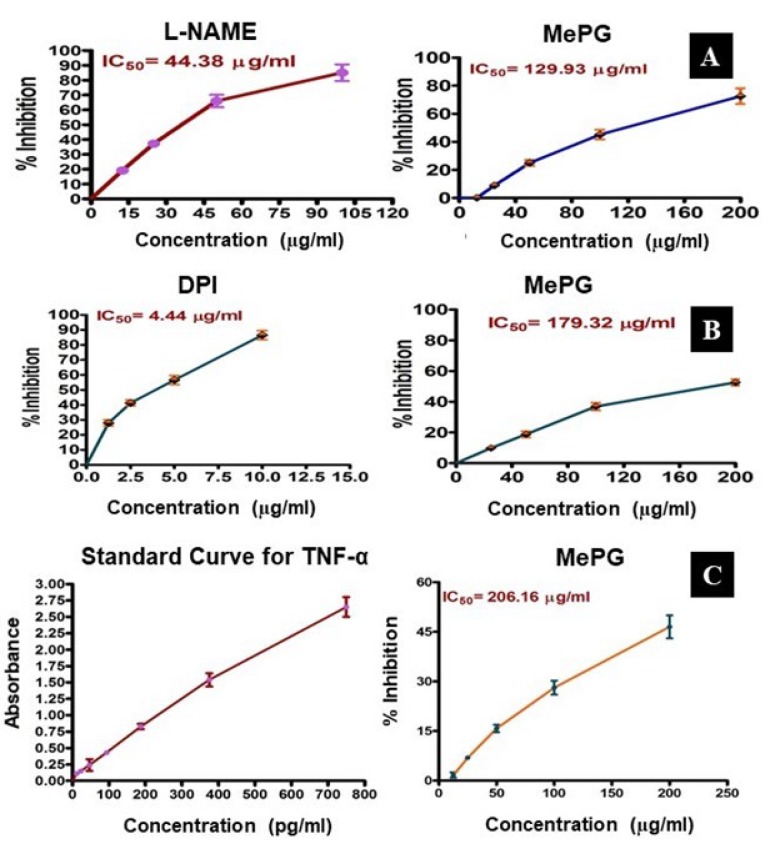
Notably, the MePG has significantly inhibited the generation of nitrite and ROS with IC50 values of 179.32
µg/ml and 129.93 µg/ml respectively. Likewise, the standard compounds DPI and L-NAME have displayed significant inhibition with an IC50 of 4.44 and 44.38 µg/ml respectively. Also, the MePG has shown significant inhibition of TNF-α generation with an IC50 of 206.16 µg/ml. These outcomes further witnessed the strong antioxidant property of MePG and also gave a clue about the strong anti-inflammatory property of MePG (Figure 2A, 2B, 2C).
Figure 2.
Effect of MePG on LPS-stimulated RAW 264.7 cell lines in vitro Note: MePG–methanolic leaf extract of Punica granatum, L-NAME: NG-Nitro-L- arginine methyl ester, TNF-α: tumor necrosis factor alpha. A–LPS -induced ROS generation, B–LPS -induced nitrite elevation, C–LPS- induced TNF-α elevation. Figure Legend: All the values (IC50 values) are expressed as mean±SEM (N=3). The MePG (12.5 to 200 µg/ml) was evaluated against LPS-stimulated ROS, nitrite and TNF-α generation in vitro. The MePG has significantly inhibited the LPS-stimulated ROS, nitrite and TNF-α generation in RAW 264.7 cell lines
Effect of MePG on I/R-induced brain injury
In our earlier study, we have evaluated the anticonvulsant activity of MePG in experimental animals and we observed that the MePG exhibits potent anti-epileptic activity through GABA mediated mechanisms. In this study, the MePG showed significant antioxidant and anti-inflammatory activities. Considering these outcomes, we have further assessed the significance of MePG (200 and 400 mg/kg) treatment against I/R-induced brain injury in Wistar rats.
Effect of MePG on I/R-induced neurological, motor, and cognitive deficits
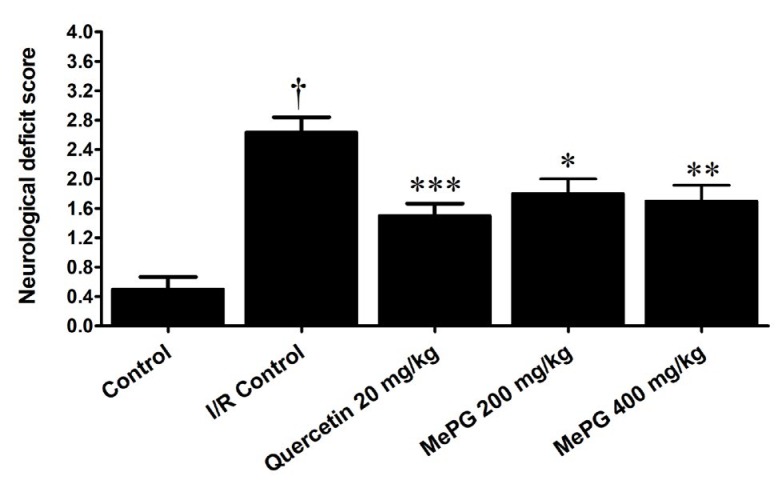
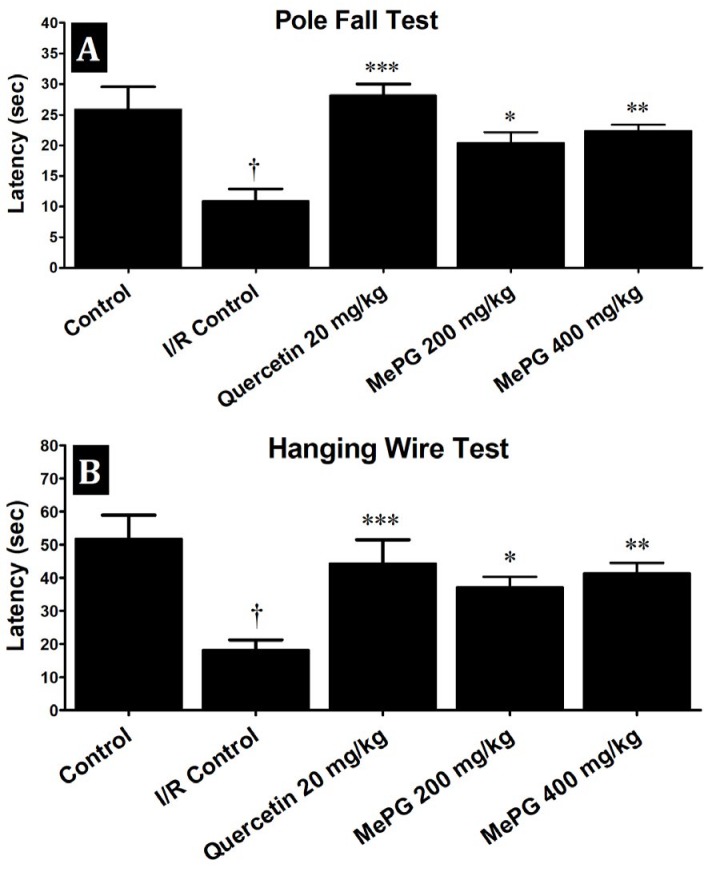
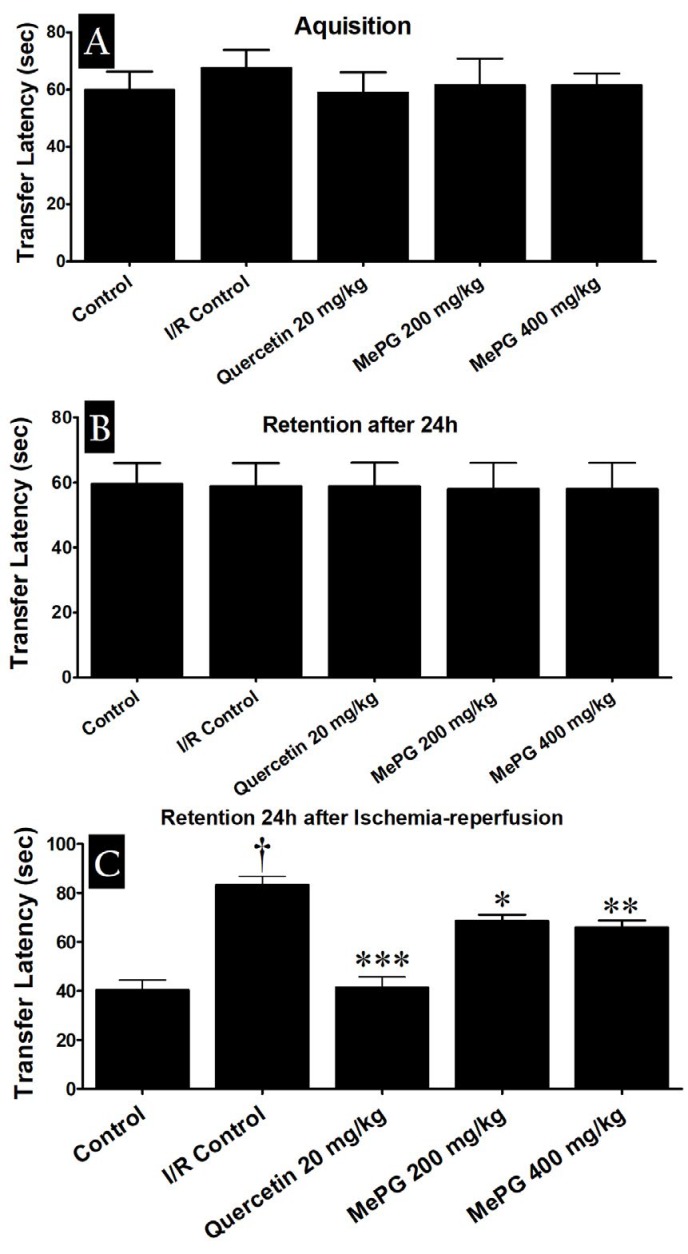
In present study, severe neurological deficits were observed in the I/R control animals (F 4, 46 =16.27, P< 0.01) compared to sham control (Figure 3). Moreover, in motor functional tests, a substantial decline in the falloff latencies were noted, in both PFT (F 4, 46 =9.18, P<0.01) and HWT (F 4, 43 =7.99, P<0.01) in I/R control group compared to sham control (Figure 4A and 4B). Besides, the EPM test performed before I/R induction revealed that there was no significant difference in TL among the different groups (during training phase). In contrary, after subjecting to I/R insult, there was a significant increase in TL was noted in I/R control group compared to sham control (F 4, 42 =28.06, P<0.01) (Figure 5A, 5B and 5C). Remarkably, quercetin (20 mg/kg, PO) and MePG (200 and 400 mg/kg, PO) pretreatment for 7 days, have offered substantial protection against I/R-induced neurological (F 4, 46 =16.27, P<0.001; P<0.01; P<0.001, respectively), motor (F 4, 46 =9.18; F 4, 43 =7.99, P<0.001; P<0.01; P<0.001, respectively), and cognitive deficits (F 4, 42 =28.06, P<0.001; P<0.05; P<0.001, respectively) compared to I/R control group (Figure 3; Figure 4A and 4B; Figure 5A, 5B and 5C).
Figure 3.
Effect of MePG on I/R-induced neurological deficits in Wistar rats
Note: I/R, Ischemia reperfusion; MePG–Methanolic leaf extract of Punica granatum. Figure Legend: All the values are expressed as mean± SEM (N=10); means of various groups were statistically compared by analysis of variance followed by Tukey’s multiple comparison test using Graph Pad version 5.01. †P<0.01 compared with control. *P<0.05, **P<0.01, *** P<0.001 compared with I/R control. Control: Normal neurological functions, I/R Control: Showing significant deterioration of neurological functions, Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing significant protection against I/R-induced neurological deficits.
Figure 4.
Effects of MePG on I/R-induced motor deficits in Wistar rats
Note: I/R, Ischemia reperfusion; MePG–Methanolic leaf extract of Punica granatum. Figure Legend: All the values are expressed as mean± SEM (N=10); means of various groups were statistically compared by analysis of variance followed by Tukey’s multiple comparison test using Graph Pad version 5.01. †P<0.01 compared with control. **P< 0.01, *** P<0.001 compared with I/R control. Control: Normal motor functions, I/R Control: Showing significant deterioration of motor functions, Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing significant protection against I/R-induced motor deficits.
Figure 5.
Effect of MePG on I/R-induced cognitive impairment in Wistar rats (elevated plus maze test)
Note: I/R, Ischemia reperfusion; MePG–Methanolic leaf extract of Punica granatum. Figure Legend: All the values are expressed as mean±SEM (N=10); means of various groups were statistically compared by analysis of variance followed by Tukey’s multiple comparison test using Graph Pad version 5.01. †P<0.01 compared with control. *P<0.05, **P<0.01, ***P<0.001 compared with I/R control. Control: Normal cognitive functions, I/R Control: Showing significant deterioration of cognitive functions, Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing significant protection against I/R-induced cognitive deficits
Effect of MePG on I/R-induced cerebral edema and infarction
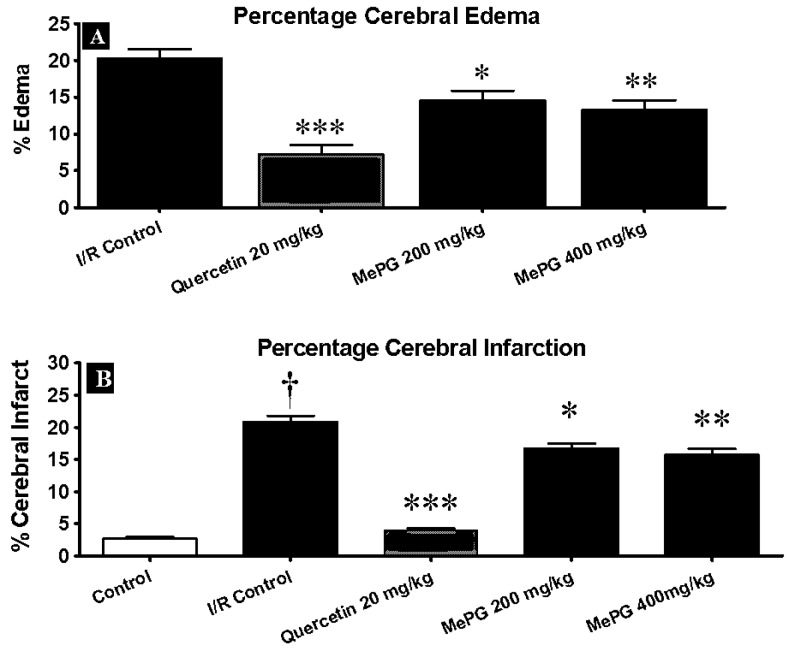
Followed by I/R insult, the I/R control group has exhibited obvious pathological changes, as observed in TTC staining (Figure 6 and 7). Additionally, the I/R control animals (F 3, 32 =16.96, P <0.01) showed increase in brain volume by 20.34±1.31 % compared to sham control group (Figure 6). Further, the TTC staining revealed that there was approximately 20.96±0.87 % (F 4, 25 =131.1, P<0.01) of the infarct area in the I/R control group (Figure 6). These observations (cerebral edema and infarction) were consistent with the previously published reports.
Figure 6.
Effect of MePG on I/R-induced cerebral edema and Infarction in Wistar rats
Note: I/R, Ischemia reperfusion; MePG–Methanolic leaf extract of Punica granatum. Figure Legend: All the values are expressed as mean ± SEM (N=3); means of various groups were statistically compared by analysis of variance followed by Tukey’s multiple comparison test using Graph Pad version 5.01. †P<0.01 compared with control. *P<0.05, **P<0.01, ***P<0.001 compared with I/R control. I/R Control: Showing significant cerebral edema and cerebral infarction, Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing significant protection against I/R-induced cerebral edema and cerebral infarction
Figure 7.
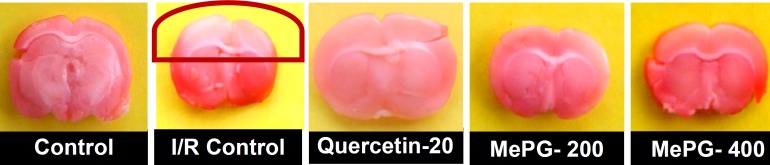
Brain morphology of various groups after I/R injury (TTC staining)
Note: I/R, ischemia-reperfusion; TTC, 2, 3, 5-triphenyltetrazolium chloride; MePG, Methanolic extract of Punica granatum leafs. Figure Legend: Control: normal morphology of brain tissue; I/R control: showing the pale whitish crown indicating the non-viable brain tissue due to I/R injury; Quercetin: close to normal morphology of brain observed in control; MePG 200 mg/kg: showing very minimal brain injury as indicated by very slight pale colored crown; MePG 400 mg.kg: showing normal morphology of brain.
However, the sham control animals displayed 1.22±0.1% infarct area, out of total area of the brain. Interestingly, seven days pretreatment with quercetin (20 mg/kg; P<0.01) and MePG (200 and 400 mg/kg ; P <0.01 and P<0.001, respectively) have offered significant protection against I/R-induced cerebral edema and infarction in Wistar rats (Figure 6 and 7).
Estimation of biochemical parameters
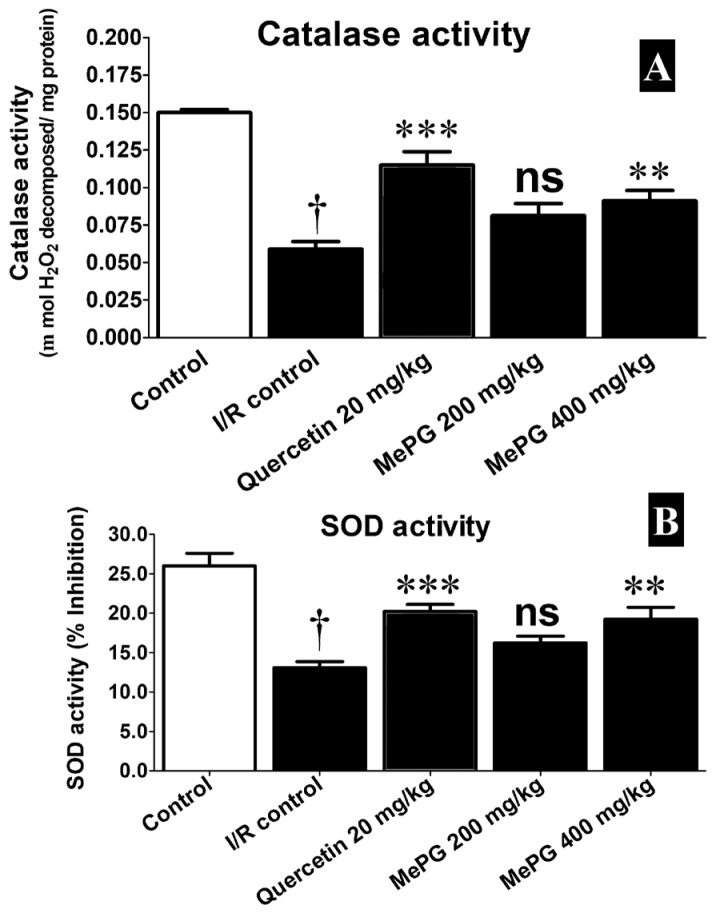
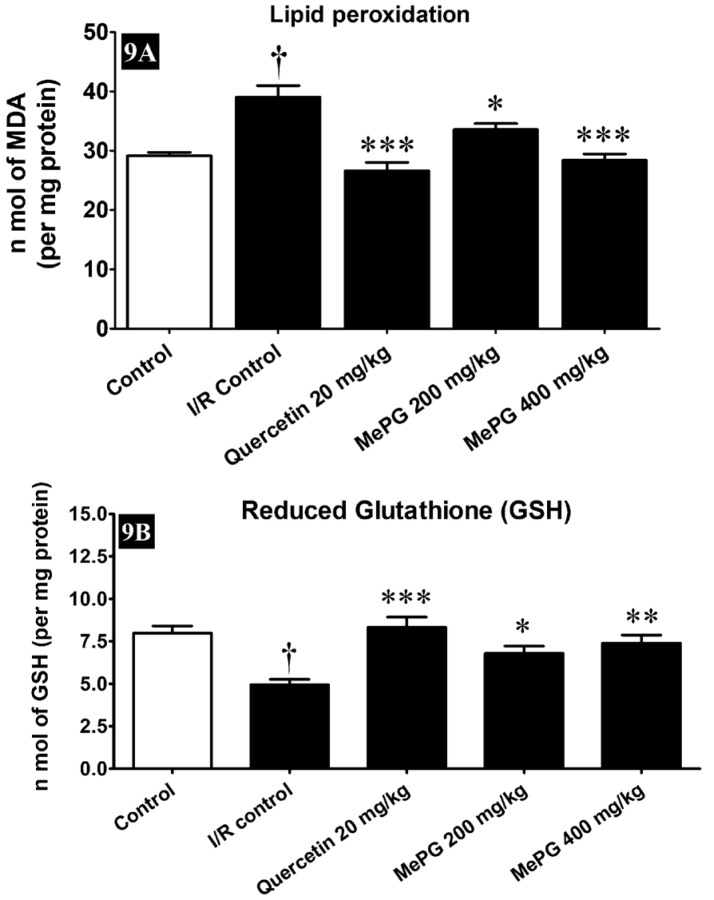
The cerebral ischemia and reperfusion is well-known to triggers the formation of enormous amount of free radicals and results in the brain injury. In the present study, the ischemia and reperfusion has deteriorated the defensive antioxidant systems and increased the generation of toxic free radicals, it was clearly evident in I/R control group compared to sham control. Predominantly, the enzyme activities of catalase (F 4, 35 =27.08, P<0.01), and SOD (F 4, 35 =16.90, P<0.01) were significantly diminished, associated with substantial decline in the GSH levels (F 4, 35 =8.45, P<0.01), along with increase in MDA (F 4, 35 =15.07, P<0.01) levels in the I/R control group compared to sham control. Remarkably, pretreatment with quercetin (20 mg/kg, PO), and MePG (200 and 400 mg/kg, PO) for seven days, have shown significant protection against I/R-induced altered enzymatic (increased catalase and SOD activities) and non-enzymatic antioxidant systems (increased GSH, reduced MDA), the results are given in Figure 8A, 8B, 9A, and 9B.
Figure 8.
Effect of MePG on I/R-induced altered catalase and SOD activities in Wistar
Note: I/R, ischemia reperfusion; MePG, methanolic leaf extract of Punica granatum; ns, not significant. Figure Legend: All the values are expressed as mean±SEM (N=3); means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph pad version 5.01. †P<0.01 compared to control; **P<0.01, ***P<0.001 compared to I/R control. Control: Normal SOD and catalase activities; I/R Control: showing deteriorated enzyme activities of SOD and catalase; Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing better SOD and catalase activities compared to I/R control group
Figure 9.
Effect of MePG on I/R induced altered GSH and lipid peroxidation in Wistar rats
Note: I/R, ischemia reperfusion; MePG, methanolic leaf extract of Punica granatum. Figure Legend: All the values are expressed as mean±SEM (N=3); means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph pad version 5.01. †P<0.01 compared to control; *P<0.05, **P<0.01, ***P<0.001 compared to I/R control. Control: normal levels of GSH and LPO; I/R Control: showing reduced GSH levels with increased LPO; Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing restored GSH and LPO levels compared to I/R control group
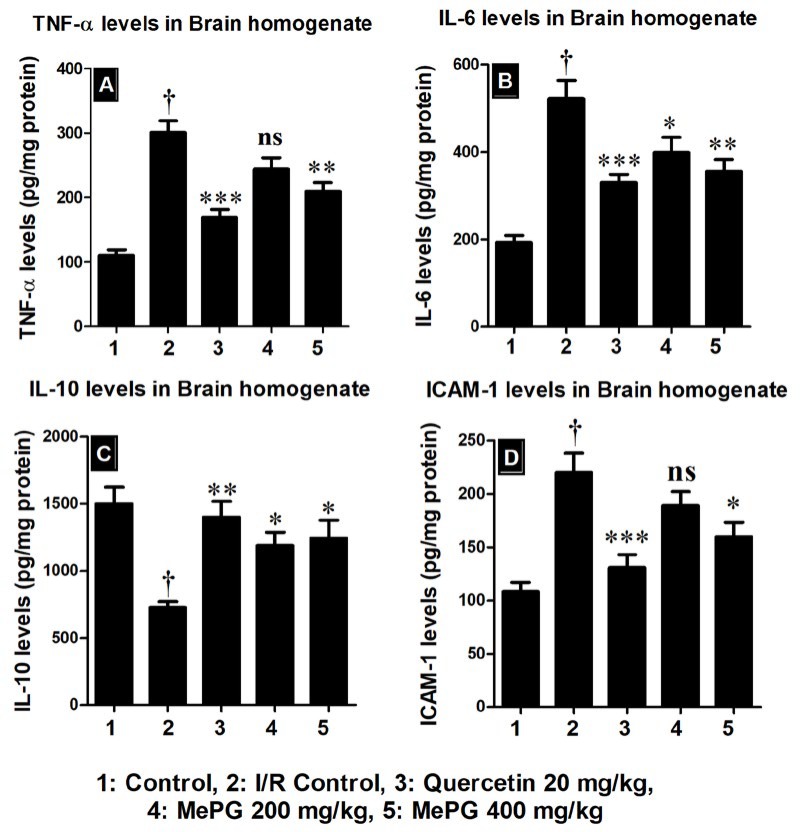
Additionally, the pro-inflammatory markers namely, TNF-α (F 4, 25 =26.15, P<0.01), IL-6 (F 4, 25 =16.93, P<0.01) and ICAM-I (F 4, 25 =11.24, P<0.01) were significantly increased, along with profound decrease in the levels of IL-10 (F 4, 25 = 7.61, P<0.01) in the I/R control group compared to sham control. Interestingly, the quercetin and MePG pretreatments have enhanced the levels of anti-inflammatory cytokine (IL-10) and decreased the levels of pro-inflammatory cytokines (TNF-α, IL-6, and ICAM-I) compared to I/R control. The results are given in Figure 10A, 10B, 10C, and 10D.
Figure 10.
Effect of MePG on I/R-induced altered TNF-α, IL-6, IL-10 and ICAM-I levels
Note: I/R, ischemia reperfusion; MePG, methanolic leaf extract of Punica granatum; ns, not significant. Figure Legend: All the values are expressed as mean±SEM (N=6); means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph pad version 5.01. †P<0.01 compared to control; *P<0.05, **P<0.01, ***P<0.001 compared to I/R control. Control: normal levels of cytokines; I/R Control: showing increased pro-inflammatory cytokines and reduced anti-inflammatory cytokine levels; Quercetin, MePG (200 mg/kg) and MePG (400 mg/kg): showing reduced pro-inflammatory cytokines and increased anti-inflammatory cytokine levels compared to I/R control group
Histopathology of brain
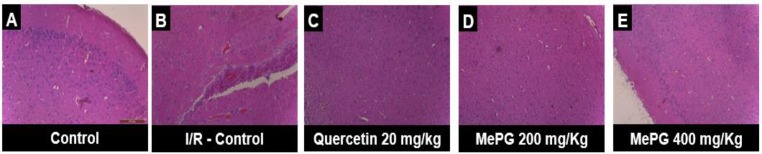
In the present study, histopathology of sham control group showed normal anatomical structure of the brain tissue. However, the I/R-control animals have exhibited clinical signs of I/R-induced brain injury, such as vascular congestion, cerebral edema, and leukocyte infiltration, accompanying with the necrosis of the brain tissue. Interestingly, MePG (400 mg/kg, PO) and quercetin (20 mg/kg, PO) treatments have ameliorated the I/R induced histopathological changes significantly, and showed very mild cerebral edema and vascular congestion. However, MePG (200 mg/kg, P.O.) treated group displayed minimal vascular congestion, inflammatory changes and cerebral edema as a sign of mild protection against I/R injury. Prominently, the MePG (400 mg/kg, PO) and quercetin (20 mg/kg, PO) have completely abolished the I/R-induced necrosis of brain tissue. These findings suggest that, the MePG and quercetin have substantially alleviated I/R-induced histological changes in the brain tissue. The histopathological images are depicted in Figure 11, and the severity scoring is given in Supplementary data file 1.
Figure 11.
Effect of MePG on I/R-induced histological changes of brain tissue Note: The pictures represent the histopathology of the brain tissue of various treatment groups along with control and I/R control groups.
Figure Legend: (A) Normal structure of the brain, (B) I/R control group showing cerebral edema, vascular congestion, leukocyte infiltration, and necrosis of brain tissue, (C) mild vascular congestion with edema of brain parenchyma, absence of necrosis, (D) very negligible characters of I/R-induced neuroinflammation and absence of necrosis, as a sign of protection against I/R-induced brain injury, (E) very minimal characters of I/R-induced damage and absence of necrosis (Hematoxylin & Eosin staining, 100X magnification).
Chemical characterization of MePG
Total phenolic content (TPC)
The MePG exhibited a TPC of 95.67 mg gallic acid equivalents per gram of the extract (9.56%).
LC-MS/MS analysis of MePG
The LC-MS/MS analysis of MePG showed the occurrence of acteoside, apigenin, gallic acid, gossypin, pentagalloyl glucose, quercetin, and rutin. Additionally, LC-MS/MS quantification showed that, the MePG comprised of 11653.09 ng/mg of pentagalloyl glucose, 685.26 ng/mg of gallic acid, 462.41 ng/mg of apigenin, 294.23 ng/mg of rutin, 38.03 ng/mg of quercetin, 21.13 ng/mg of gossypin, 7.96 ng/mg of acteoside as major phytoconstituents. The results of LC-MS/MS investigations are given in Supplementary data file 2 and 3.
Discussion
In our earlier study, we have assessed the anti-epileptic activity of P. granatum leaf extracts and we have observed that the MePG exhibits noteworthy anti-epileptic property in animal models of epilepsy (14).
Ischemia-reperfusion and other forms of oxidative stress generate extremely reactive and toxic free radicals by numerous biochemical reactions such as electron transport chain (ETC). These free radicals, particularly oxidative and nitrosative radicals further activate the expansion of these toxic chemicals, through reactions like fenton reaction. The RNS and ROS formed will interact with vital biomolecules such as cellular proteins, lipids, and nucleic acids, and cause denaturation. The denaturation consequences in the abnormal functioning of the cell/tissues located at the site of injury. In these lines, many studies have proved that the secondary metabolites of plant origin such as flavonoids, glycosides, saponins and polyphenolic compounds, are capable of scavenging and/or neutralizing the deleterious effects of these toxic free radicals. Hence it is perceived that, these molecules could counter the significances of stroke, either by preventing the formation of free radicals and/or scavenging the generated radicals (6).
In this study, the total phenolic content analysis revealed that the MePG contains 95.67 mg GE/g of phenolic content. Further, LC-MS/MS analysis of MePG showed the presence of pentagalloyl glucose, gallic acid, apigenin, and rutin as major phytoconstituents, thus the MePG is established to contain a high amount of bioactive compounds such as polyphenols. It is widely reported that phenolic compounds such as phenolic acids, tannins, flavonoids, and phenyl propanoids are well-established to possess potent free radical scavenging and antioxidant activities. There exists a linear relationship between the total phenolic content and antioxidant activity, also these molecules are well established to play a crucial role in the prevention of NO and peroxynitrite production (35-37). In line with the above statement, the MePG showed highly significant free radical scavenging and antioxidant activities in the in vitro antioxidant assays.
Based on the chemical profiling and in vitro antioxidant assay outcomes, the MePG was further assessed for its consequence on generation of nitrite, ROS and TNF-α in LPS-activated RAW 264.7 cell lines in vitro.
The murine macrophage cell line RAW 264.7, is a widely used test for assessing the antioxidant and anti-inflammatory activity of the test compounds. The incubation of RAW 264.7 cells with lipopolysaccharides, activate the complex cascade of inflammatory events that produces inflammatory cytokines such as TNF-α, IL-6, IL-1β, IL-8, and augments the expression of nuclear factors such as NF- κB and AP-1. Also generates enormous amount of ROS, and nitrite along with the activating the nuclear enzyme called poly ADP-ribose polymerase (PARP). Importantly, in this assay both anti-inflammatory and antioxidant activity of the test compounds can be assessed concurrently. The ROS, and nitrite estimations could help as markers of antioxidant activity and pro-inflammatory cytokine (TNF-α, IL-6, IL-1β, IL-8) estimation served as a marker of anti-inflammatory activity.
In the present study, the MePG has significantly alleviated the generation of nitrite, ROS and TNF-α in RAW 264.7 cell line assay and thus showed potent anti-inflammatory and antioxidant activities. Further, the MePG produced very low level of cytotoxicity in MTT assay, with an IC50 of 1826.7 ± 157.7 µg/ml.
Based on the notable outcomes of in vitro antioxidant and RAW 264.7 cell line assays, the MePG was further evaluated against global ischemia and reperfusion-induced brain injury in Wistar rats.
BCCO followed by reperfusion in rats, is a well-known and most widely used model for screening the cerebroprotective effect of test drugs (38). In literature, the role of ROS in the pathogenesis of I/R-induced brain injury has been well documented (7, 9, 29). It is understood that, during cerebral ischemia the ROS are generated mainly through activation of phospholipase A2 and inhibition of electron transport chain (29). Moreover, the reperfusion phase after the acute cerebral ischemia, will aggravate the generation of toxic free radicals (7). Evidently, the neuronal tissue is highly enriched with polyunsaturated fatty acids and known to have a very weak endogenous antioxidant defense system, and hence the brain tissue is regarded as highly sensitive to oxidative/free radical damage (38). Therefore, the free radical produced throughout the I/R would denaturate the biomolecules of the neurons through complex cascades of events, which lead to neuroinflammation, and apoptotic neuronal cell death. The damage caused to the neuronal tissue will be demonstrated as neurological, cognitive, motor, and behavioral functional deficits along with the morphological and histological changes of the brain tissue (29).
In this study, we have evaluated the neurological, cognitive, and motor functions to explore the effect of MePG on I/R-induced functional deficits. Additionally, the cerebral edema, infarct formation, and histopathological evaluations were performed.
In the observations, I/R control animals exhibited noticeable neurological, cognitive, and motor functional deficits compared to sham control. Nevertheless, pretreatment with MePG and quercetin have offered significant protection against I/R-induced neurological, cognitive, and motor functional deficits.
Moreover, the I/R and other forms of brain injuries are often linked with the cerebral edema. The increase in brain volume intend increase the intracranial pressure, the increased intracranial pressure results in the neuronal injury due to compression, and the end consequences of all these events is diminished blood supply to the site of injury (39). Consistent with the previously published reports, in this study the I/R control animals showed a substantial increase in brain volume compared to sham control. Especially, the MePG and quercetin treatments have offered noteworthy protection against I/R-induced cerebral edema.
In the present study, the morphology of I/R control animals exhibited significantly high levels of cerebral infarction compared with sham control, which is a cardinal sign of severe brain damage; nevertheless, MePG and quercetin pretreatments have ameliorated the I/R-induced cerebral infarction compared to I/R control group.
Further, during the I/R-injury enormous amount of free radicals are generated due to oxidative stress, initially superoxide radical is formed, and further, a cascade of various biochemical reactions trigger the generation of ROS and RNS. The endogenous antioxidant defense mechanism comprises of SOD, catalase and glutathione systems, are noticed to be highly distressed during the I/R-induced brain injury, which is accompanied by increased lipid peroxidation (quantified by estimating the levels of MDA) in the brain homogenate (29).
To investigate the above mentioned hypothesis, we have assessed SOD and catalase enzyme activities along with the quantification of MDA and GSH levels in the brain homogenate. In the observations, the I/R control animals exhibited a decline in SOD and catalase enzyme activities, along with the reduction in GSH, and increase in the MDA levels compared to sham control. In contrary, pretreatment with quercetin and MePG showed significant protection against I/R-induced altered antioxidant enzyme (SOD and catalase) activities, and sustained the levels of MDA and GSH near to sham control. These outcomes are in mutual agreement with the antioxidant activity of MePG observed in the in vitro assays.
In the literature it is well reported that, throughout I/R-injury the activated microglia generate an enormous amount of pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, and ICAM-I; associated with substantial decline in the levels of anti-inflammatory cytokines like IL-10 (40).
To explore the possible anti-neuroinflammatory property of MePG, we have assessed TNF-α, IL-6, ICAM-I and IL-10 levels in the brain homogenate by ELISA. Consistent with literature reports, significant elevation of TNF-α, IL-6, and ICAM-I with a substantial decline in IL-10 levels was observed in the I/R control. However, pretreatment with quercetin and MePG have attenuated the I/R- induced elevation of pro-inflammatory cytokines (TNF-α, IL-6, ICAM-I), and increased the levels of anti-inflammatory cytokine (IL-10) compared to I/R control group.
Moreover, histopathological evaluation of brain tissue further confirmed the outcomes recorded in the functional, morphological and biochemical estimations. Interestingly, sham control animals showed normal histology of brain without any abnormalities, while the I/R control group exhibited indications of brain injury such as congestion of blood vessels, cerebral edema, leukocyte infiltration, and necrosis of brain tissue. Nevertheless, quercetin and MePG pretreatments have alleviated the I/R-induced histological changes and displayed substantial protection against I/R-induced brain injury.
Further, mentioning on the phytoconstituents accountable for the cerebroprotective effect, the MePG was found to be rich in phenolic compounds, and LC-MS/MS analysis of MePG showed the existence of acteoside, apigenin, gallic acid, gossypin, pentagalloyl glucose, quercetin, and rutin as major phytoconstituents.
Remarkably, several studies have shown that the phytoconstituents of MePG, such as apigenin (41), gallic acid (42), gossypin (43), pentagalloyl glucose (29), quercetin (44), and rutin (45, 46) are scientifically well established for their protective effects against I/R-induced brain injury. Hence, the cerebroprotective activity of MePG witnessed in the present study is believed to be due to the synergistic effect of these compounds.
Conclusion
Based on these findings, we conclude that methanolic leaf extract of P. granatum exhibits substantial cerebroprotective action against I/R-induced brain injury in Wistar rats.
Acknowledgment
The authors greatly acknowledge Ms Radiant Research Services Pvt Ltd, Peenya Industrial Area, Bangalore; Ms Vittarthaa Life Sciences Pvt Ltd, Bommasandra industrial Area, Bangalore and Dr Nandakumar Krishnadas, Professor of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal for providing all the facilities and valuable technical support to carry out the research work.
Conflicts of Interest
The authors declare that there is no conflict of interests.
References
- 1.Ringelstein EB, Nabavi DG. Cerebral small vessel diseases: cerebral microangiopathies. Curr Opin Neurol. 2005;18:179–188. doi: 10.1097/01.wco.0000162861.26971.03. [DOI] [PubMed] [Google Scholar]
- 2.Guzik A, Bushnell C. Stroke Epidemiology and Risk Factor Management. Continuum. 2017;23:15–39. doi: 10.1212/CON.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 3.Mahajan SK, Kashyap R, Sood BR, Jaret P, Mokta J, Kaushik NK, Prashar BS. Stroke at moderate altitude. JAPI. 2004;52:699–702. [PubMed] [Google Scholar]
- 4.Allen C, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 5.Albarracin SL, Stab B, Casas Z. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012;15:1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 6.Tsai PJ, Tsai TH, Yu CH, Ho SC. Evaluation of the NO-suppressing activity of several Mediterranean culinary spices. Food Chem Toxicol. 2007;45:440–447. doi: 10.1016/j.fct.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues FTS, de Sousa CNS, Ximenes NC, Almeida AB, Cabral LM, Patrocinio CFV, et al. Effects of standard ethanolic extract from Erythrina velutina in acute cerebral ischemia in mice. Biomed Pharmacother. 2017;96:1230–1239. doi: 10.1016/j.biopha.2017.11.093. [DOI] [PubMed] [Google Scholar]
- 8.Rao A, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- 9.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42:S24–27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 11.Kritikar K, Basu B. Indian Medicinal Plants. Bombay: International Book Publishers; 2007. [Google Scholar]
- 12.Nadkarni K. Indian Material Medica. Bombay: Bombay Popular Prakashan; 1976. [Google Scholar]
- 13.Basiri S. Evaluation of antioxidant and antiradical properties of Pomegranate (Punica granatum L) seed and defatted seed extracts. J Food Sci Technol. 2015;52:1117–23. doi: 10.1007/s13197-013-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viswanatha GL, Venkataranganna MV, Prasad NBL. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J Intercult Ethnopharmacol. 2016;5:415–421. doi: 10.5455/jice.20160904102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braidy N, Selvaraju S, Essa MM, Vaishnav R, Al-Adawi S, Al-Asmi A, et al. Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid Med Cell Longev . 2013;2013:685909. doi: 10.1155/2013/685909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapias V, Cannon JR, Greenamyre JT. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol Aging . 2014;35:1162–76. doi: 10.1016/j.neurobiolaging.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DaSilva NA, Nahar PP, Ma H, Eid A, Wei Z, Meschwitz S, et al. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr Neurosci. 2017;7:1–11. doi: 10.1080/1028415X.2017.1360558. [DOI] [PubMed] [Google Scholar]
- 18.MM Essa, S Subash, M Akbar, S Al-Adawi, GJ Guillemin. Long term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of Alzheimer’s disease. PLoS One . 2015;10:e0120964. doi: 10.1371/journal.pone.0120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morzelle MC, Salgado JM, Telles M, Mourelle D, Bachiega P, Buck HS, et al. Neuroprotective effects of Pomegranate Peel Extract after Chronic Infusion with Amyloid–β Peptide in Mice. PLoS One . 2016;11:e0166123. doi: 10.1371/journal.pone.0166123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adiga S, Trivedi P, Ravichandra V, Deb D, Mehta F. Effect of Punica granatum peel extract on learning and memory in rats. Asian Pac J Trop Med. 2010;3:687–90. [Google Scholar]
- 21.West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Dev Neurosci. 2007;29:363–72. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loren DJ, Seeram NP, Schulman RN, Holtzman DM. Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury. Pediatr Res . 2005 ;57:858–64. doi: 10.1203/01.PDR.0000157722.07810.15. [DOI] [PubMed] [Google Scholar]
- 23.Anturlikar S, Rafiq M, Azeemuddin M, Viswanatha G, Jagadeesh M, Rao KS, et al. Free radical scavenging and hepatoprotective activity of HD-03/ES in experimental models. J Exp Integr Med. 2012;2:161–166. [Google Scholar]
- 24.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 25.Rafiq M, Azeemuddin M, Anturlikar SD, Viswanatha GL, Patki PS. Application of oxygen radical absorbance capacity (ORAC) assay in the estimation of antioxidant value of botanicals. Oxid Antioxid Med Sci. 2012;1:87–90. [Google Scholar]
- 26.Viswanatha GL, Venkataranganna MV, Prasad NBL, Hanumanthappa S. Chemical characterization and cerebroprotective effect of methanolic root extract of Colebrookea oppositifolia in rats. J Ethnopharmacol. 2018;223:63–75. doi: 10.1016/j.jep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Mathew G, Jacob A, Durgashivaprasad E, Reddy ND, Unnikrishnan MK. 6b,11b-Dihydroxy-6b,11b-dihydro-7H-indeno[1,2-b]naphtho[2,1-d]furan-7-one (DHFO), a small molecule targeting NF-kappaB, demonstrates therapeutic potential in immunopathogenic chronic inflammatory conditions. Int Immunopharmacol. 2013;15:182–189. doi: 10.1016/j.intimp.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Viswanatha GL, Kumar LM, Rafiq M, Kavya KJ, Thippeswamy AH, Yuvaraj HC, et al. LC-MS/MS profiling and neuroprotective effects of Mentat(R) against transient global ischemia and reperfusion-induced brain injury in rats. Nutrition. 2015;31:1008–1017. doi: 10.1016/j.nut.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Viswanatha GL, Shylaja H, Mohan CG. Alleviation of transient global ischemia/reperfusion-induced brain injury in rats with 1, 2, 3, 4, 6-penta-O-galloyl-beta-d-glucopyranose isolated from Mangifera indica. Eur J Pharmacol. 2013;720:286–293. doi: 10.1016/j.ejphar.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Luck H Catalase, H.O., B., editors. Methods of Enzymatic analysis. New York: Academic Press; 1971. pp. 885–893. [Google Scholar]
- 32.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 34.Moukdar F, Robidoux J, Lyght O, Pi J. Daniel KW. Collins S.Reduced antioxidant capacity and diet-induced atherosclerosis in uncoupling protein-2-deficient mice. J Lipid Res. 2009;50:59–70. doi: 10.1194/jlr.M800273-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 36.Choi SY, Ko HC, Ko SY, Hwang JH, Park JG, Kang SH, et al. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol Pharm Bull. 2007;30:772–778. doi: 10.1248/bpb.30.772. [DOI] [PubMed] [Google Scholar]
- 37.Conforti F. Menichini F. Phenolic compounds from plants as nitric oxide production inhibitors. Curr Med Chem. 2011;18:1137–1145. doi: 10.2174/092986711795029690. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya M, Sako K, Yura S, Yonemasu Y. Local cerebral glucose utilisation following acute and chronic bilateral carotid artery ligation in Wistar rats: relation to changes in local cerebral blood flow. Exp Brain Res. 1993;95:1–7. doi: 10.1007/BF00229648. [DOI] [PubMed] [Google Scholar]
- 39.Hillard CJ. Role of cannabinoids and endocannabinoids in cerebral ischemia. Curr Pharm Des. 2008;14:2347–2361. doi: 10.2174/138161208785740054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soliman ML, Puig KL, Combs CK, Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem. 2012;123:555–567. doi: 10.1111/j.1471-4159.2012.07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C, Tu FX, Chen X. Neuroprotective effects of apigenin on acute transient focal cerebral ischemia-reperfusion injury in rats. Zhong Yao Cai. 2008;31:870–873. [PubMed] [Google Scholar]
- 42.Sun J, Li YZ, Ding YH, Wang J, Geng J, Yang H, et al. Neuroprotective effects of gallic acid against hypoxia/ reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014;1589:126–139. doi: 10.1016/j.brainres.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 43.Chandrashekhar VM, Ganapathy S, Ramkishan A, Narsu ML. Neuroprotective activity of gossypin from Hibiscus vitifolius against global cerebral ischemia model in rats. Ind J Pharmacol. 2013;45:575–580. doi: 10.4103/0253-7613.121367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivera F, Costa G, Abin A, Urbanavicius J, Arruti C, Casanova G, et al. Reduction of ischemic brain damage and increase of glutathione by a liposomal preparation of quercetin in permanent focal ischemia in rats. Neurotox Res. 2008;13:105–114. doi: 10.1007/BF03033562. [DOI] [PubMed] [Google Scholar]
- 45.Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastava P, Khan MB, et al. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009;1292:123–135. doi: 10.1016/j.brainres.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 46.Jang JW, Lee JK, Hur H, Kim TW, Joo SP, Piao MS. Rutin improves functional outcome via reducing the elevated matrix metalloproteinase-9 level in a photothrombotic focal ischemic model of rats. J Neurol Sci. 2014;339:75–80. doi: 10.1016/j.jns.2014.01.024. [DOI] [PubMed] [Google Scholar]