Abstract
BACKGROUND
Continuous albuterol administration (CAA) is commonly used in hospitalized patients for treatment of asthma exacerbations. Due to higher dose requirements, CAA requires large volumes of albuterol obtained from multidose vials containing benzalkonium chloride (BAC). BAC is a common pharmaceutical preservative and potent bronchoconstrictor, which may antagonize the bronchodilation effects of albuterol. Some institutions are using preservative-free (PF) albuterol for their CAA. However, no published data currently exist to support the extended sterility or stability of this formulation.
OBJECTIVE
To evaluate the sterility and stability of PF-albuterol.
METHODS
Sterility testing was conducted for PF- and BAC-albuterol when stored at room temperature. Samples were incubated for 10 days in aerobic and anaerobic blood culture media to assess for bacterial growth. Stability of both albuterol formulations at high (0.67 mg/mL) and low (0.17 mg/mL) concentrations was determined at room temperature and under refrigeration. High performance liquid chromatography was used to evaluate samples up to 168 hours after preparation.
RESULTS
No bacterial growth was witnessed from either albuterol formulation at day 10 of observation. Both high and low concentrations of PF-albuterol and BAC-albuterol were stable at room temperature for up to 168 hours. There were no differences in stability between storage conditions for any formulation.
CONCLUSIONS
Under the current study conditions, there was no difference in sterility or stability for PF-albuterol when compared with BAC-albuterol. Thus, based on the findings of this study, PF-albuterol is sterile and stable up to 168 hours when stored at room temperature or under refrigerated conditions. The findings of this study do not confirm the therapeutic efficacy of PF-albuterol compared with BAC-albuterol for the treatment of asthma exacerbations. Further studies are warranted to determine the efficacy of PF-albuterol verses BAC-albuterol when used for CAA.
Keywords: albuterol, asthma, benzalkonium compounds, drug stability, pharmaceutical preservatives
Introduction
Asthma is a chronic inflammatory disease of the airways affecting more than 6 million children and leading to nearly 1.6 million emergency department visits per year.1,2 Acute management of asthma exacerbations involves a multitude of agents including oxygen, intermittent albuterol, systemic corticosteroids, ipratropium, and magnesium sulfate. In some cases of severe acute asthma exacerbation, the use of continuous albuterol administration (CAA) may be indicated.2 Due to higher dosing requirements over an extended duration of time, CAA requires a larger volume of albuterol solution, which is often obtained from a multidose vial containing benzalkonium chloride (BAC).
BAC is a common preservative used in pharmaceuticals; however, it is also considered a potent broncho-constrictor. BAC doses as small as 46 mcg have been shown to trigger bronchoconstriction, and this effect is cumulative, prolonged, and correlates directly with basal airway responsiveness.3,4 The multidose albuterol vials currently used for CAA at our institution contain 50 mcg of BAC per 2.5 mg of albuterol.5 A patient receiving CAA at 20 mg/hr will therefore receive approximately 400 mcg of BAC per hour. In the 1987 study conducted by Beasley et al,3 the mean concentration of BAC required to provoke a 20% drop in forced expiratory volume in 1 second was 300 mcg (range 46 to 708 mcg). In addition, Zhang et al4 saw a 20% drop in forced expiratory volume in 1 second with doses ranging from 124 to 1947 mcg. More recently, the package insert for BAC-albuterol was updated to include information stating, “albuterol sulfate inhalation solution contains the preservative benzalkonium chloride. Benzalkonium chloride has been associated with bronchospasm in a dose-dependent manner. In patients who receive larger doses (e.g., continuous nebulization) of albuterol sulfate inhalation solution and bronchospasm does not resolve, consider a trial of short-acting bronchodilator that does not contain the preservative benzalkonium chloride.”5
In response, some institutions are using preservative-free (PF) albuterol formulations for their CAA. Unfortunately, no data currently exist to support the sterility or stability of PF-albuterol when used in this manner. The primary objective of this study is to evaluate the sterility and stability of a PF-albuterol formulation compared with the BAC formulation.
Materials and Methods
CAA at Norton Children's Hospital is administered using the Misty Finity Continuous Nebulizer (CareFusion, Model number: 002503, Yorba Linda, CA) at a rate of 30 mL/hr. Albuterol dilutions to be administered are prepared in 8-hour aliquots to a total volume of 240 mL. The albuterol dose added to normal saline diluent is determined based on the patient's weight (Table 1). The study was designed as a usability stability study to mimic clinical conditions for the typical use of the product.
Table 1.
Continuous Albuterol Administration Dosing at Norton Children's Hospital
| Patient Weight, kg | ||||
|---|---|---|---|---|
| < 11 | 11–20 | 21–40 | > 40 | |
| Dose, mg/hr* | 5 | 10 | 15 | 20 |
| Concentration, mg/mL | 0.17 | 0.33 | 0.5 | 0.67 |
| BAC amount, mcg/hr | 100 | 200 | 300 | 400 |
* Eight-hour batches are prepared with normal saline to a total volume of 240 mL, and all doses are nebulized at 30 mL/hr.
The products tested for sterility and stability include an albuterol sulfate multidose vial (active albuterol 5 mg/mL, preservative-containing, Hi-Tech Pharmaceuticals, Amityville, NY) and single-use albuterol sulfate mini nebs (active albuterol 2.5 mg/0.5 mL, PF, Nephron Pharmaceuticals, West Columbia, SC). Monobasic potassium phosphate and high-performance liquid chromatography (HPLC) grade methanol were from Fisher Scientific (Pittsburgh, PA). Trifluoroacetic acid (TFA) and albuterol sulfate reference standard were from Sigma Aldrich (St. Louis, MO).
Sample Preparation. Samples of PF-albuterol were prepared using either 16 unit-of-use vials (0.67 mg/mL concentration) or 4 unit-of-use vials (0.17 mg/mL concentration), and PF, sterile normal saline for irrigation to a total volume of 60 mL. BAC-albuterol was prepared at a total volume of 60 mL using the included dropper from the multiuse vial with 8 or 2 mL for the high and low concentrations, respectively.
All albuterol solutions for stability were prepared in the bench research laboratory at Sullivan University College of Pharmacy at room temperature and open to air. Solutions were stored in sealed plastic bottles and protected from light in room temperature stability chambers or refrigerated conditions. At assigned time points, samples were withdrawn from stability chambers or refrigeration.
Sterility. We examined PF-albuterol and BAC-albuterol at the high and low concentrations when stored at room temperature and exposed to light, up to 168 hours after preparation. All samples were prepared, handled, and stored non-sterilely in the pharmacy at Norton Children's Hospital to mimic clinical practice. Two samples from each albuterol preparation were tested. For the first sample from each preparation, 10 mL of the dilution was aseptically inoculated into an aerobic blood culture bottle (BD BACTEC Plus Aerobic/F medium). To detect the presence of obligate anaerobes, the second sample of 10 mL from each preparation was inoculated into an anaerobic blood culture bottle (BD BACTEC Lytic/10 Anaerobic/F medium). All samples were placed on a BACTEC FX continuously monitoring blood culture instrument and incubated at 35°C for 10 days. The method used would be expected to detect a large majority of clinically significant bacteria and yeasts in the first 72 hours.6 However, to improve detection of slowly growing organisms and very low levels of contamination that could delay detection, samples were incubated for a total of 10 days.
HPLC Analysis. HPLC analysis was performed on a Dionex Ultimate 3000 system using a modified USP assay method for albuterol sulfate.7 A YMC ODS-A HPLC column (150 × 4.6 mm I.D., 5 μm) was used, and the mobile phase was 25 mmoL monobasic potassium phosphate (pH 3 and methanol [90:10, v/v) and 1% TFA). The flow rate was set at 1 mL/min, the injection volume was 25 mcL, and the ultraviolet/visible spectroscopic detector was set at 276 nanometers. Samples were diluted for HPLC and analyzed at a 20-minute run time with albuterol sulfate eluting at a retention time of 11.6 minutes. Albuterol sulfate was chosen as the standard to correlate with commercially available product. Data analysis was performed using Dionex Chromeleon (Dionex, Sunnyvale, CA) software. The method was validated for linearity, specificity, precision, accuracy, and limits of quantitation over the concentration range used and deemed suitable for albuterol analysis.
Stability Testing. Formulations were considered stable if concentrations were within 10% of the reference concentration over time.8 Both the PF and BAC-containing albuterol formulations were prepared in normal saline at the high and low concentrations used at our institution. They were then divided into 4 equal aliquots, stored at room temperature (20–25°C) and under refrigeration (2–8°C). Each sample was evaluated hourly via HPLC for 8 hours, then again at 24, 48, 72, and 168 hours. Stability was assessed by evaluating the percent of drug remaining when compared with the theoretical reference range. Mean values and standard deviations were calculated.
Statistical Analysis. Pairs of groups were compared by paired t-test. Differences between groups were considered significant at p < 0.05. Values for all measurements are expressed as mean ± SD.
Results
Sterility. No bacterial growth was detected by the BACTEC FX instrument in all aerobic and anaerobic bottles, for both the high and low concentrations of PF-albuterol, throughout 10 days of incubation.
Stability. After 8 hours, both concentrations of PF-albuterol and BAC-albuterol maintained stability, irrespective of storage condition (Figures 1 and 2). To determine if albuterol solutions could be prepared in advance, samples were monitored daily for 72 hours and again at hour 168 (Table 2). Statistical analysis using a paired t-test was conducted among concentrations to be compared with prior time points (i.e., each formulation at hour 0 versus hour 1 and so on) and p-values were calculated at 95% confidence level (Table 2). In general, there was not enough evidence to claim that the population mean was different among the points tested at the 0.05 significance level. Thus, the percent remaining average concentration of drug between time points was statistically insignificant for the duration of the test. PF-albuterol and BAC-albuterol were stable at 72 hours when stored under refrigeration and at room temperature, and both remained stable under these conditions through hour 168.
Figure 1.
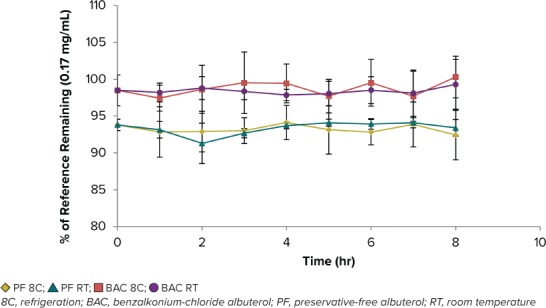
PF-albuterol and BAC-albuterol stability (n = 4) at the low concentration (0.17 mg/mL) over 8 hr at room temperature and refrigeration.
Figure 2.
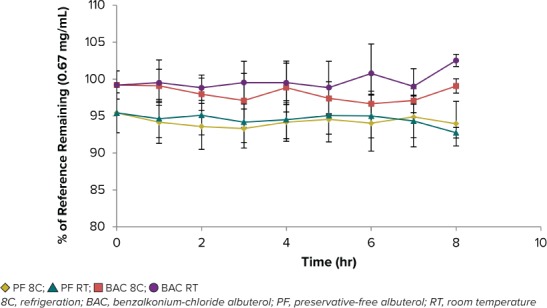
PF-albuterol and BAC-albuterol stability (n = 4) at the high concentration (0.67 mg/mL) over 8 hr at room temperature and refrigeration.
Table 2.
Mean Percent ± Standard Deviation Remaining for BAC-albuterol and PF-albuterol Over Time
| Concentration, mg/mL | Time (hr) | ||||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 168 | |
| 0.17 | |||||
| PF 8C | 93.8 ± 0.76 | 90.81 ± 1.35 | 92.59 ± 5.16 | 93.86 ± 1.19 | 93.19 ± 0.36 |
| p value | 0.027 | 0.546 | 0.696 | 0.281 | |
| PF RT | 93.8 ± 0.76 | 94.33 ± 5.43 | 92.91 ± 1.84 | 92.63 ± 1.01 | 96.72 ± 5.83 |
| p value | 0.862 | 0.671 | 0.854 | 0.193 | |
| BAC 8C | 98.49 ± 2.1 | 102.88 ± 6.9 | 100.56 ± 4.7 | 98.09 ± 2.4 | 100.2 ± 1.99 |
| p value | 0.389 | 0.662 | 0.31 | 0.227 | |
| BAC RT | 98.49 ± 2.1 | 98.8 ± 1.41 | 98.09 ± 1.81 | 98.02 ± 1.02 | 98.70 ± 0.98 |
| p value | 0.675 | 0.517 | 0.886 | 0.022 | |
| 0.67 | |||||
| PF 8C | 95.43 ± 2.71 | 91.43 ± 1.13 | 90.47 ± 2.13 | 93.09 ± 3.01 | 94.03 ± 2.86 |
| p value | 0.047 | 0.159 | 0.064 | 0.239 | |
| PF RT | 95.43 ± 2.71 | 91.34 ± 1.49 | 93.97 ± 0.4 | 93.5 ± 1.68 | 93.47 ± 2.43 |
| p value | 0.125 | 0.021 | 0.672 | 0.968 | |
| BAC 8C | 99.21 ± 1.9 | 98.29 ± 2.17 | 98.56 ± 2.16 | 98.12 ± 2.56 | 98.49 ± 1.74 |
| p value | 0.126 | 0.652 | 0.651 | 0.548 | |
| BAC RT | 99.21 ± 1.9 | 99.85 ± 4.04 | 99.99 ± 4.19 | 99.66 ± 3.67 | 99.67 ± 2.81 |
| p value | 0.601 | 0.859 | 0.613 | 0.983 | |
8C, refrigeration; BAC, benzalkonium-chloride albuterol; PF, preservative-free albuterol; RT, room temperature
At high and low concentrations, PF-albuterol compared with BAC-albuterol had a lower concentration in relation to theoretical values. This lower concentration was maintained through the entirety of the study regardless of time, albuterol concentration, or storage condition.
Discussion
Due to concern surrounding the bronchoconstricting effects of BAC in preservative-containing albuterol solutions, there is a need to determine if a PF-albuterol product may be safely used in patients receiving CAA. To our knowledge, this is the first study to evaluate the sterility and stability of PF-albuterol for use in CAA. Based on the findings of this study, we can conclude that a PF-albuterol dilution is sterile and stable for up to 168 hours when stored at either room temperature or under refrigeration.
Additional limitations to the study include the incapability to extrapolate results during administration via nebulization, the small number of samples examined, the inability to test multiple albuterol manufacturers, the lack of assessment for mold contamination, and the baseline difference in concentration between PF-albuterol and BAC-albuterol. It is important to note the results of this study are only applicable to the storage of PF-albuterol, as the sterility and stability during albuterol nebulization was not tested. Additionally, each solution in theory should have the same concentration after preparation; but as seen in our study, the concentration of PF-albuterol was consistently lower than BAC-albuterol. One explanation for this observation would be our method of preparation. When preparing PF-albuterol, the solution was expelled from unit-ofuse vials, each containing 0.5 mL of albuterol. The high concentration required 64 unit-of-use vials to be emptied into 208 mL of normal saline to prepare an 8-hour inhalation. During this process, an exact volume was not measured, which may have resulted in some drug being left in the original packaging. This process differed from BAC-albuterol preparation. BAC-albuterol was prepared using the provided 0.5-mL dropper, which likely resulted in a more accurate amount of albuterol added to the final product. Additionally, PF-albuterol nebules have been found to contain lower drug concentrations than conventional preservative-containing preparations, which could also contribute to the lower amount of drug we observed at time zero.9
Conclusion
PF-albuterol evaluated in this study is sterile and stable for up to 168 hours when stored at room temperature or under refrigeration. A difference in baseline concentration between the 2 preparations was observed; however, this may be due to the preparation process and could be overcome by more accurately measuring the volume of PF-albuterol added to the diluent. This study provides sterility and stability data for CAA using PF unit-of-use albuterol vials until a larger volume vial of PF-albuterol can be manufactured.
Acknowledgment
This abstract was presented on May 4, 2017, at the Pediatric Pharmacy Advocacy Group 2017 Annual Meeting in Charlotte, North Carolina and on July 15, 2017, the American Association of Colleges of Pharmacy 2017 Annual Meeting in Nashville, Tennessee.
ABBREVIATIONS
- BAC
benzalkonium chloride
- CAA
continuous albuterol administration
- HPLC
High-performance liquid chromatography
- PF
preservative-free
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The corresponding author had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.United States Department of Health and Human Services. National Center for Health Statistics Asthma, United States, 2015. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/fastats/asthma.htm Accessed September 9, 2018.
- 2.National Asthma Education and Prevention Program Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma – summary report. J Allergy Clin Immunol. 2007;120(5):94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Beasley CRW, Rafferty P, Holgate ST. Bronchoconstrictor properties of preservatives in ipratropium bromide (Atrovent) nebulizer solution. Br Med J. 1987;294(6581):1197–1198. doi: 10.1136/bmj.294.6581.1197-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YG, Wright WJ, Tam WK et al. Effect of inhaled preservatives on asthmatic subjects. II. Benzalkonium chloride. Am Rev Respir Dis. 1990;141(6):1405–1408. doi: 10.1164/ajrccm/141.6.1405. [DOI] [PubMed] [Google Scholar]
- 5.Albuterol Sulfate Inhalation Solution 0.5% [package insert] Amityville, NY: Hi-Tech Pharmaceutical Co., Inc; 2018. [Google Scholar]
- 6.BACTEC Plus Aerobic/F Culture Vials and BACTEC Plus Anaerobic/F Culture Vials [package inserts] Sparks, Maryland: Becton, Dickinson and Company; 2015. [Google Scholar]
- 7.National Formulary Monograph for Albuterol Tablets United States Pharmacopeia 38th rev., and the national formulary. 33rd ed. Rockville, MD: United States Pharmacopeia; 2015. pp. 2072–2073. [Google Scholar]
- 8.O'Donnell PB, Bokser AD. Remington: the Science and Practice of Pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. p. 986. [Google Scholar]
- 9.MacNeish CF, Coates AL, Meisner D et al. A comparison of pulmonary availability between ventolin (albuterol) nebules and ventolin (albuterol) respirator solution. Chest. 1997;111(1):204–208. doi: 10.1378/chest.111.1.204. [DOI] [PubMed] [Google Scholar]


