Abstract
Introduction:
Organophosphates are widely used pesticides that have been show to affect child neurodevelopment. Previous studies that explored their potential effects on Autism Spectrum Disorder (ASD) either relied on proxies of external exposure or on questionnaires completed by the parents to identify autism-like behaviors but do not provide a clinical diagnosis of ASD.
Aims:
We studied the associations between prenatal biologic markers for exposure to organophosphate pesticides and the risk of having a child with ASD or non-typical development (NTD).
Method:
We analyzed 203 mother-child pairs of the ongoing MARBLES (Markers of Autism Risk in Babies – Learning Early Signs) mother-child cohort, which enrolls mothers who are either pregnant or planning a pregnancy and having an elevated risk for the expected child to develop ASD. Seven metabolites of organophosphate pesticides were assessed in repeated urine samples collected during pregnancy. At 36 months, children were assessed with measures of cognitive function and adaptive behaviors, and with two gold-standard diagnostic instruments for ASD: the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview-Revised. Children were classified in one of the following groups: ASD (n = 46), non-typical development (NTD, n = 55) and typically developing (TD, n = 102).
Results:
After adjustment for potential confounders, organophosphate metabolite concentrations were not associated with an increased risk of ASD or NTD when boys and girls were studied together. After stratification by sex, dimethylthiophosphate (DMTP) pregnancy concentration tended to be associated with an increased ASD risk among girls (OR for a doubling in the DMTP concentration: 1.64 (95%CI, 0.95; 2.82)) but not among boys (OR: 0.84, 95%CI: 0.63; 1.11).
Discussion:
This is the first study of clinically confirmed diagnoses of ASD that utilized repeated measurements of organophosphate metabolites during pregnancy to explore the associations between these pesticides and ASD risk in children. The association we observed among girls, as well as the lack of association in boys, need to be replicated in further studies with similar design and larger sample size. In light of the higher baseline risk for ASD in this cohort, generalizability to children lacking a first degree relative affected by ASD is unknown.
Keywords: Autism spectrum disorder, Biomarkers, Developmental concerns, Prenatal exposure, Prospective cohort, Organophosphate pesticides
Introduction
Organophosphate (OP) pesticides are a class of insecticides widely used throughout the world. While they were phased out for most residential uses by the U.S. Environmental Protection Agency in the early 2000s (Clune et al. 2012), they are still applied in agriculture for insect control on food crops (Shelton et al. 2012), on golf courses and some other uses. In the general population, exposure to OP mainly occurs through inhalation from agricultural spray drift and ingestion of residues on food products. An interventional study in 23 children showed that replacing their conventional diet with organic food items for 5 consecutive days led to significant reduction in urinary OP pesticide metabolite concentrations (Lu et al. 2006). OP pesticides were initially developed as nerve gases for chemical warfare and then adapted for insect control at lower doses. They affect mammalian and insect nervous systems by irreversibly inhibiting the enzyme acetyl cholinesterase (AChE) that breaks down the neurotransmitter acetylcholine. Inhibition of AChE leads to accumulation of acetylcholine in the synapses, that can result in acute neurologic symptoms. In addition to high-dose effects on AChE inhibition, toxicological studies have suggested that OP pesticides can down-regulate serotonin receptors, induce oxidative stress and alter calcium and potassium homeostasis (reviewed by Shelton et al, 2012 (Shelton et al. 2012)). In addition, a magnetic resonance imaging study in 40 children aged 5.9–11.2 years found brain morphological changes in the group with high cord blood concentrations of chlorpyrifos (Rauh et al. 2012).
Regarding the effects of OP pesticides on child neurodevelopment, epidemiological studies that measured the parent compounds in blood or their metabolites in urine reported associations with abnormal reflexes in neonates (Engel et al. 2007; Young et al. 2005; Zhang et al. 2014), poorer mental development in toddlers assessed with the Bayley scales of early learning (Eskenazi et al. 2007)), and poorer working memory and intellectual quotient in children (Bouchard et al. 2011; Horton et al. 2012; Rauh et al. 2011). Only a few studies have looked at Autism Spectrum Disorder (ASD), a neurodevelopmental disorder characterized by impairments in social interactions and communication and a pattern of stereotyped behaviors or sensory sensitivities, for which increased prevalence has been observed in the past decades in the United States (Centers for Disease Control and Prevention 2012). One study did not report an association (Millenson et al. 2017) while the five others reported increased risk of an ASD diagnosis or ASD like symptoms in association with OP pesticide exposure during pregnancy (Eskenazi et al. 2007; Furlong et al. 2014; Rauh et al. 2006; Roberts et al. 2007; Shelton et al. 2014). These studies on ASD were limited by the fact that they relied on 1) mandated reports of commercial pesticide use near the houses of the participants as a surrogate of exposure (Roberts et al. 2007; Shelton et al. 2014) which does not take into account exposures occurring through other sources like food; or 2) by the use of questionnaires completed by the parents to identify autism-like behaviors but do not provide a clinical diagnosis of ASD (Eskenazi et al. 2007; Furlong et al. 2014; Millenson et al. 2017; Rauh et al. 2006).
Our aim was to study prenatal exposure to OP pesticides in relation to children’s diagnosis of ASD and non-typical development (NTD). We relied on the MARBLES prospective cohort, which obtained repeated urine samples during pregnancy to assess OP exposure and confirmed all diagnoses at 3 years using diagnostic gold-standard assessments for ASD.
Methods
Population
We analyzed the ongoing MARBLES cohort that started in 2006 and enrolls women at high risk for having a child with ASD. Selection criteria are 1) having a biological child diagnosed with ASD and so being at elevated risk to have another child with this disorder (close to 20% (Ozonoff et al. 2011) compared to about 1.5% in the general population); 2) being pregnant or planning a pregnancy and being biologically able to become pregnant; 3) living within an approximate 2 hour drive to the UC Davis MIND (Medical Investigation of Neurodevelopmental Disorders) Institute clinic; 4) being at least 18 years old. A few women (n = 2) did not match the first criteria of inclusion but were enrolled since they were at high risk of having a child with ASD (e.g., mother had an identical twin with an ASD child). Eligible families are identified using the list of families receiving state-funded services for a child with ASD, provided to us by the California Department of Developmental Services. Some families are also referred by other research studies at the UC Davis MIND Institute or by health and service providers, or learn about the study at outreach events. To confirm the ASD diagnosis of the older sibling, the study psychologist requests the record of an ASD diagnosis, i.e., an evaluation made by a psychologist using the ADOS (Autism Diagnostic Observation Schedule, (Lord et al. 2000)). If neither the parent nor the clinician provides such a record, then the study psychologist administers the ADOS to the child and the Social Communication Questionnaire (SCQ) to the mother (Rutter et al. 2003). Based on these assessments, the older sibling is either deemed to meet criteria for ASD, in which case the mother is enrolled, or not, in which case the mother is ineligible.
For the current study, we included all active MARBLES participants who were born between 2006 and March 2014, for whom pregnancy urine samples were available and who did not drop out before the examination of the child at 3 years of age. A comparison of mother-child pairs included in this analysis versus those not included is provided in the Supplemental Material, Table S3. Those not included lacked urine samples, dropped out before the 3-year visit or had an incomplete exam at 3 years. The MARBLES study has been approved by the institutional review boards for the State of California and the University of California Davis. The parents of all participants signed an informed consent before being enrolled in the study.
Collection of urine samples
Beginning at enrollment and throughout pregnancy, mothers of the MARBLES study were asked to collect for each trimester up to three first morning void urine samples (referred as spot samples in the manuscript) and one 24-hour urine sample collected on a different day than the spot samples. Mothers collected spot samples, each one week apart, and then stored these spot urine samples in their home freezer until collection by study staff during a home visit. The 24-hour urine samples included all urine voids for a 24-hour period starting at 8 am the day before the study staff made a home visit. Participants used collection hats that were emptied into the 24-hour container after each void. Twenty-four hour samples were stored in the mother’s home refrigerator. The MARBLES Study personnel pick up urine samples during home visits and transported them to UC Davis for storage at −80°C. Urine samples were then thawed and aliquoted as follows: for a given trimester if the woman collected only one or two urine samples (spot or 24 hour sample) both samples were analyzed individually (not pooled). If three or more samples (spots and 24 hour samples) were available, generally the first sample of that trimester was analyzed as an individual sample and the remaining samples were pooled together (pooled within-trimester, within-subject). Pooling enabled us to take advantage of the repeated measurements per subject to reduce exposure measurement error while keeping the total cost of OP pesticide metabolite measurements reasonable..
Assessments of OP metabolites
In the present study, urine samples (pools, spots and 24-hour) from the 2nd and 3rd trimesters of pregnancy were shipped overnight on dry ice to Emory University’s Rollins School of Public Health (RSPH) for OP metabolite quantification. Specific gravity was measured on all samples (spot, pools, 24-hour) prior to shipment. Dimethylphosphate (DMP), diethylphosphate (DEP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP), diethylthiophosphate (DETP) and diethyldithiophosphate (DEDTP) along with 3,5,6-trichloro-2-pyridinol (TCPy), a specific metabolite of chlorpyrifos and chlorpyrifos-methyl, were measured using either gas-chromotography or high-performance liquid chromatography coupled with tandem mass spectrometry with both quantification and confirmation ions monitored. Full details on the methods have been previously published (Olsson et al. 2004; Prapamontol et al. 2014). DEDTP was not detected in any of the urine samples and was not included in our analysis.
Assessment of child neurodevelopment and diagnostic outcome
Children were assessed at 3 years of age using the ADOS, a semi-structured interview during which the clinician observes the child’s social interaction, communication, play and imaginative use of materials (Lord et al. 2000), and the Mullen Scales of Early Learning (MSEL), using the four subscales that measure fine motor, visual reception, expressive and receptive language (Mullen 1995). In addition, the parents completed the SCQ to screen for symptoms of autism spectrum disorder (Berument et al. 1999). Parents of children demonstrating signs of ASD on either the ADOS or SCQ were additionally interviewed with the Autism Diagnostic Interview-Revised (ADI-R), a standardized instrument that probes for ASD symptoms throughout the child’s life. Using their ADOS and MSEL scores, we classified the children in one of the following groups: ASD (n = 46), non-typical development (NTD, n = 55) and typically developing (TD, n = 102). Criteria of inclusion in each group are detailed in Supplemental Material, Table S2.
Statistical analysis
We performed multiple imputation using the mi impute chained command in Stata 14 (White et al. 2011) to impute OP concentrations that were below the limit of detection. Imputation models included the OP concentrations along with the following variables: home owner vs. renter status, season and date of birth, maternal height and weight, maternal age and specific gravity. We restricted the imputed values to be in the following range: ≥ 0 and < LOD. OP pesticide metabolite concentrations were then standardized for specific gravity (sg) using the following formulae: CSG = C × [(SGmean – 1)/(SG – 1)], where CSG is the specific gravity corrected biomarker concentration, SGmean is the arithmetic mean of the specific gravity in our study population, and C is the measured OP concentration. We computed the following sum of the molar concentrations: ∑DEP (molar sum of the diethylphosphate metabolites: DEP and DETP), ∑DMP (molar sum of the dimethylphosphate metabolites: DMP, DMTP, DMDTP) and ∑DAP (molar sum of DEP, DETP, DMP, DMTP and DMDTP). Some organophosphorus insecticides such as Malathion can be metabolized into the three DAP included in ∑DMP or ∑DEP. However, degradation of others such as Naled and Diazinon only lead to one or two DAPs (Supplementary Material, Table S6). For these compounds, it might be relevant to study individual metabolite in addition to the molar sums that include some of their metabolites but also metabolites that are not related to them. For this reason, in this manuscript we also presented results for individual OP metabolites that were detected in more than 60% of the samples (namely DEP, DMTP).
We fit adjusted multinomial logistic regression models predicting the odds of ASD and NTD, each relative to TD. We used the mean of the log2-transformed sg-standardized biomarker concentrations as a proxy of the pregnancy exposure. In addition to studying the mean of the OP concentrations over the 2nd and 3rd trimester we also computed the average OP concentration in mid (corresponded to the 2nd trimester) and late pregnancy (3rd trimester).
Compliance to the urine sampling protocol was variable and on average, women collected 5 (Standard Déviation = 2.6) urine samples (spot and / or 24-hour) over the second and third trimesters (Supplemental Material, Table S1). To take into account the unbalanced number of urine samples per participant and the correlations among samples that were averaged together from the same woman to form that woman’s (possibly trimester-specific) average exposure, we used analytical weights. Weights reflected the “effective number” (Ching-Ping 2011) of samples used to quantify a given woman’s average exposure for a given measurement: weightij = ni / (1 + (ni - 1) ICCj), where ni is the number of individual urine samples that were pooled together for woman i (from a given trimester, if applicable) and ICCj is the within-woman intraclass correlation coefficient for compound j measurements from individual urine samples. Intraclass correlation coefficients for each compound were estimated using compound-specific between-mother and within-mother variance component estimates (for hypothetical individual urine sample measurements) from maximum-likelihood analysis of pooled-sample and spot-sample observations, using a user-written program in SAS PROC NLMIXED that accounted for the variable number of samples in each of these observations. The estimated ICCs were 0.09 for TCPy; 0.12 for DMTP; 0.19 for DEP and ∑DMP; and 0.18 for ∑DMP and ∑DAP.
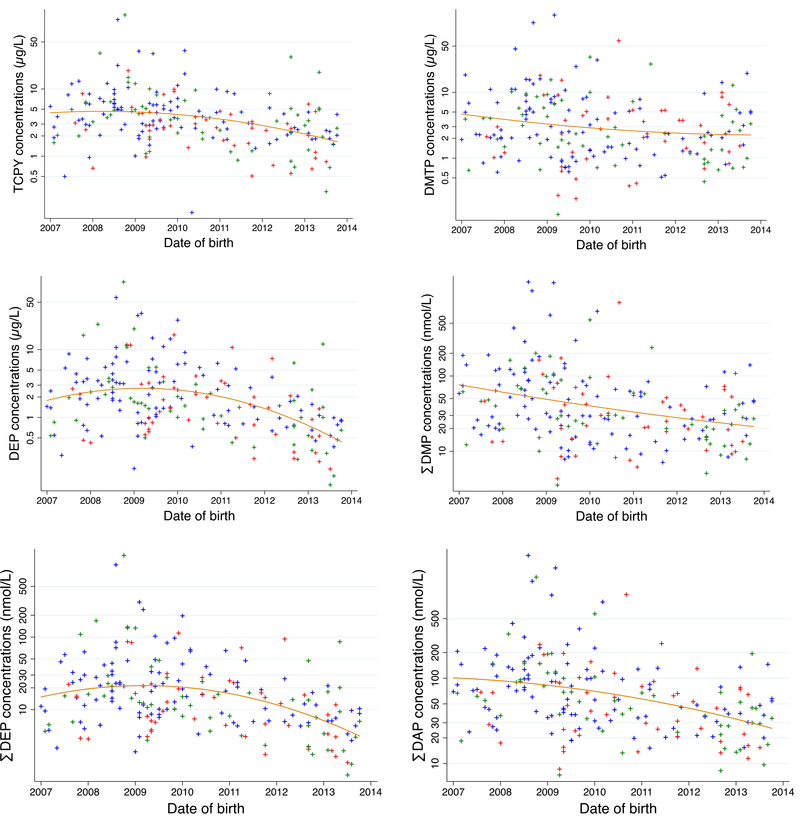
Adjustment factors were chosen a priori based on a directed acyclic graph constructed using the published literature on risk factors for ASD (Supplemental Material, Figure S1). We adjusted for the minimal sufficient set given by the Dagitty software (Textor et al. 2011). The set included socio-economic status, maternal body mass index (BMI) before pregnancy (continuous), season (categorical) and date of birth (continuous). Adjustment for date of birth was necessary because of the opposing time trends observed for ASD diagnosis and OP concentrations in our study population: 67% of the ASD children were indeed born after 2010 compared to 45% of the TD children (Table 1) while OP concentrations tended to decrease with time (Figure 1). As a measure of socio-economic status we used home ownership status (yes / no) rather than parental education since the number of parents of ASD children in the lowest education group “high school, some college (no degree)” was small (N = 6). In sensitivity analysis, we ran additional models adjusted for 1) the use of prenatal vitamins during the first month of pregnancy, 2) pyrethroid exposure estimated using the average concentrations of 3-phenoxybenzoic acid, a general metabolite of pyrethroid pesticides, also measured in the repeated urine samples collected during pregnancy, 3) gestational age (a potential mediator of the effects of OP on ASD and NTD), 4) maternal age, 5) paternal age. To investigate the impact of extreme values we also performed an analysis in which we removed those mothers in the top 2.5 percent of the OP pesticide urinary concentration. We explored potential sex specific effects by adding an interaction term between OP concentrations and sex in the regression models and by performing stratified analysis. Finally, we excluded 6 twins (3 pairs) and performed an analysis restricted to singleton births.
Table 1:
Characteristics of the study population, n = 203 mother-child pairs of the MARBLES cohort with OP assessments during pregnancy and diagnosis at 3 years
| TD | ASD | NTD | ASD versus TDa | NTD versus TDa | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Child sex | 0.03 | 0.20 | ||||||
| Female | 48 | 47 | 13 | 28 | 20 | 36 | ||
| Male | 54 | 53 | 33 | 72 | 35 | 64 | ||
| Year of birth | 0.01 | 0.19 | ||||||
| 2007–2008 | 33 | 32 | 4 | 9 | 11 | 20 | ||
| 2009 | 24 | 24 | 11 | 24 | 13 | 24 | ||
| 2010–2011 | 24 | 24 | 14 | 30 | 12 | 22 | ||
| 2012–2014 | 21 | 21 | 17 | 37 | 19 | 35 | ||
| Birth status | 0.44 | 0.10 | ||||||
| Singleton | 97 | 95 | 45 | 98 | 55 | 100 | ||
| Has a twin | 5 | 5 | 1 | 2 | 0 | 0 | ||
| Child race/ethnicity | 0.10 | 0.14 | ||||||
| White non Hispanic | 53 | 52 | 17 | 37 | 19 | 35 | ||
| Hispanic | 27 | 26 | 17 | 37 | 17 | 31 | ||
| Otherb | 22 | 22 | 12 | 27 | 19 | 35 | ||
| Birth season | 0.30 | 0.26 | ||||||
| Warm months (May - Oct) | 56 | 55 | 21 | 46 | 25 | 45 | ||
| Cold months (Nov - Apr) | 46 | 45 | 25 | 54 | 30 | 55 | ||
| Maternal age (years) | 0.39 | 0.52 | ||||||
| < 30 | 26 | 25 | 8 | 17 | 10 | 18 | ||
| 30 to 35 | 32 | 31 | 13 | 28 | 21 | 38 | ||
| > 35 | 44 | 43 | 25 | 54 | 24 | 44 | ||
| Home owner | 0.15 | 0.34 | ||||||
| Yes | 67 | 66 | 23 | 50 | 31 | 56 | ||
| No | 34 | 33 | 20 | 43 | 22 | 40 | ||
| Missing values | 1 | 1 | 3 | 7 | 2 | 4 | ||
| Parental education | 0.66 | 0.96 | ||||||
| High school, some college (no degree) | 17 | 17 | 6 | 13 | 9 | 16 | ||
| Bachelor degree | 59 | 58 | 29 | 63 | 33 | 60 | ||
| Graduate or professional degree | 26 | 25 | 9 | 20 | 13 | 24 | ||
| Missing values | 0 | 0 | 2 | 4 | 0 | 0 | ||
| Prepregnancy BMI (kg/m2) | 0.32 | 0.48 | ||||||
| < 25 | 59 | 58 | 20 | 43 | 27 | 49 | ||
| 25 to 30 | 23 | 23 | 13 | 28 | 13 | 24 | ||
| > 30 | 20 | 20 | 12 | 26 | 15 | 27 | ||
| Missing values | 0 | 0 | 1 | 2 | 0 | 0 | ||
p-values for Pearson’s chi-squared test
include Black, Multiracial and Asian
Figure 1: Average OP metabolite concentrations according to the child date of birth (n = 203 mother child pairs from the MARBLES study).
Blue crosses represent TD children, red crosses ASD children and green crosses NTD children.
Abbreviations: DEP: Diethylphosphate, DMTP: Dimethylthiophosphate, TCPy: 3,5,6-trichloro-2-pyridinol, ∑DAP: molar sum of DEP, DETP, DMP, DMTP and DMDTP, ∑DEP: molar sum of DEP and DETP, ∑DMP: molar sum of DMP, DMTP, and DMDTP.
All analyses were performed using STATA/SE (College station, TX 77845, version 14) and SAS (Institute Inc. 2016. Sas 9.4. Carny, NC).
Results
Study Population Demographics
Among the 203 women included, 74 were recruited during their first trimester of pregnancy, 71 during the second and 58 during their third trimester. The male to female ratio was 1.1, 2.5 and 1.8 among TD, ASD and NTD, respectively. TD children tended to be born in the earlier years of the study than ASD and NTD children (Table 1), and included a higher proportion of white non-Hispanics (52%) compared to ASD and NTD (about 35%, Table 1). In the TD groups, 66% of the parents owned their home compared to 50% in the ASD group (p-value for Pearson’s chi-squared test = 0.15). MARBLES mothers with missing outcomes or exposure tended to have a lower socio-economic status (a higher percentage of their parents did not own their house) and to have daughters, as compared with those included in our analyses (Supplemental Material, Table S3).
OP concentrations in maternal urine
TCPy, DMTP and DEP were detected in most of the urine samples with frequency of detection ranging from 89% for DEP to 95% for TCPy. The highest median was observed for TCPy (2.6 μg/L) followed by DMTP (1.8 μg/L) and DEP (0.9 μg/L) (Table 2).
Table 2:
Maternal urinary concentrations of OP metabolites among 203 mother-child pairs of the MARBLES cohort (mean of the concentrations measured in repeated urine samples collected during pregnancy)
| Whole study population |
TD |
ASD |
NTD |
ASD versus TDa | NTD versus TDa | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | %> LOD | N | Percentiles |
N | Percentiles |
N | Percentiles |
N | Percentiles |
|||||||||||
| 5 | 50 | 95 | 5 | 50 | 95 | 5 | 50 | 95 | 5 | 50 | 95 | |||||||||
| TCPy (ng/mL) | 0.1 | 95 | 203 | <LOD | 2.61 | 15.74 | 102 | <LOD | 2.65 | 18.3 | 46 | 0.34 | 2.36 | 9.03 | 55 | 0.41 | 2.69 | 18.12 | 0.11 | 0.77 |
| DMP (ng/mL) | 0.35 | 51 | 203 | <LOD | 0.44 | 10.07 | 102 | <LOD | <LOD | 12.0 | 46 | <LOD | 0.50 | 5.23 | 55 | <LOD | 0.46 | 9.37 | 0.62 | 0.55 |
| DMTP (ng/mL) | 0.25 | 94 | 203 | < LOD | 1.80 | 18.4 | 102 | <LOD | 1.90 | 23.1 | 46 | 0.35 | 2.05 | 18.4 | 55 | <LOD | 1.60 | 15.94 | 0.65 | 0.13 |
| DMDTP (ng/mL) | 0.25 | 34 | 203 | <LOD | <LOD | 5.88 | 102 | <LOD | <LOD | 9.83 | 46 | <LOD | <LOD | 3.64 | 55 | <LOD | <LOD | 4.27 | 0.03 | 0.08 |
| ∑DMP (μmol/mL) | 203 | 6.37 | 26.0 | 225 | 102 | 6.73 | 23.51 | 296 | 46 | 6.57 | 27.91 | 164 | 55 | 5.89 | 26.0 | 193 | 0.78 | 0.10 | ||
| DEP (ng/mL) | 0.25 | 89 | 203 | < LOD | 0.91 | 13.4 | 102 | <LOD | 1.13 | 13.4 | 46 | <LOD | 0.79 | 10.2 | 55 | <LOD | 0.83 | 17.0 | 0.06 | 0.06 |
| DETP (ng/mL) | 0.25 | 34 | 203 | < LOD | < LOD | 1.70 | 102 | <LOD | <LOD | 1.93 | 46 | <LOD | <LOD | 1.55 | 55 | <LOD | <LOD | 1.87 | 0.01 | 0.09 |
| ∑DEP (μmol/mL) | 203 | 2.23 | 8.39 | 115 | 102 | 2.23 | 10.12 | 117 | 46 | 2.23 | 6.58 | 67.8 | 55 | 2.23 | 7.56 | 122 | 0.01 | 0.01 | ||
| ∑DAP(μmol/mL) | 203 | 11.71 | 40.8 | 304 | 102 | 12.09 | 43.24 | 345 | 46 | 12.09 | 40.03 | 210 | 55 | 10.33 | 35.1 | 312 | 0.30 | 0.04 | ||
p-value for Wilcoxon rank test
Abbreviations: DEP: Diethylphosphate, DETP: Diethylthiophosphate, DMDTP: Dimethyldithiophosphate, DMP: Dimethylphosphate, DMTP: Dimethylthiophosphate, TCPy: 3,5,6-trichloro-2-pyridinol, ∑DAP: molar sum of DEP, DETP, DMP, DMTP and DMDTP, ∑DEP: molar sum of DEP and DETP, ∑DMP: molar sum of DMP, DMTP, and DMDTP
Associations between the average pregnancy exposure and child diagnosis
After adjustment for home owner status, maternal BMI before pregnancy, season and date of birth, the multinomial ORs for the associations between OP pesticide metabolites and the risk for the child developing ASD or NTD were below one and ranged between 0.80 (95%CI: 0.57; 1.12) for the association between TCPy and ASD and 0.99 (95%CI: 0.79; 1.25) for the association between DMTP and ASD. None of these associations was significant (all p-values ≥ 0.15, Table 3). Additional adjustment for the use of prenatal vitamins during the first month of pregnancy, pregnancy pyrethroid exposure, gestational age at birth, maternal or paternal age did not change these null results (Supplemental Material, Table S4). Excluding the 6 twins of our study population or the mothers in the top 2.5 percent of the OP pesticide concentrations led to similar results (Supplemental Material, Table S4).
Table 3:
Adjusteda Multinomial Odds Ratios for Autism Spectrum Disorder and Non Typically Developing children in relation to OP pesticide metabolite urinary concentrationsb, MARBLES study
| ASD versus TD |
NTD versus TD |
|||
|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |
| A) Whole pregnancy exposure (N = 101 TD, 42 ASD, 53 NTDc) | ||||
| TCPy | 0.80 | [0.57; 1.12] | 0.99 | [0.74; 1.32] |
| DMTP | 0.99 | [0.79; 1.25] | 0.86 | [0.69; 1.06] |
| DEP | 0.94 | [0.74; 1.20] | 0.92 | [0.74; 1.15] |
| ∑DMP | 0.92 | [0.73; 1.15] | 0.86 | [0.69; 1.06] |
| ∑DEP | 0.91 | [0.72; 1.16] | 0.91 | [0.73; 1.13] |
| ∑DAP | 0.89 | [0.68; 1.17] | 0.85 | [0.66; 1.08] |
| B) Analysis restricted to samples collected in the 2nd trimester of pregnancy (n = 66 TD, 28 ASD, 40 NTD c) | ||||
| TCPy | 0.77 | [0.53; 1.13] | 0.90 | [0.65; 1.25] |
| DMTP | 1.11 | [0.83; 1.48] | 0.96 | [0.74; 1.25] |
| DEP | 1.03 | [0.76; 1.38] | 0.89 | [0.68; 1.18] |
| ∑DMP | 1.01 | [0.76; 1.35] | 0.94 | [0.72; 1.22] |
| ∑DEP | 0.94 | [0.70; 1.28] | 0.85 | [0.64; 1.12] |
| ∑DAP | 1.00 | [0.72; 1.40] | 0.89 | [0.66; 1.21] |
| C) Analysis restricted to samples collected in the 3 rd trimester of pregnancy (n = 98 TD, 39 ASD, 48 NTDc) | ||||
| TCPy | 0.95 | [0.71; 1.27] | 1.06 | [0.82; 1.36] |
| DMTP | 1.01 | [0.82; 1.24] | 0.86 | [0.71; 1.04] |
| DEP | 0.92 | [0.74; 1.16] | 0.97 | [0.80; 1.18] |
| ∑DMP | 0.97 | [0.78; 1.19] | 0.86 | [0.70; 1.04] |
| ∑DEP | 0.91 | [0.73; 1.13] | 0.95 | [0.79; 1.16] |
| ∑DAP | 0.93 | [0.73; 1.19] | 0.88 | [0.71; 1.10] |
Adjustment factors were: home ownership, prepregnancy BMI, season and date of birth
OR represents the change in the odds of the outcomes for a doubling in the OP concentrations standardized for specific gravity
Analysis restricted to the mother-child pairs with OP concentrations, diagnosis at 3 years and non-missing covariates.
Abbreviations: DEP: Diethylphosphate, DMTP: Dimethylthiophosphate, TCPy: 3,5,6-trichloro-2-pyridinol, ∑DAP: molar sum of DEP, DETP, DMP, DMTP and DMDTP, ∑DEP: molar sum of DEP and DETP, ∑DMP: molar sum of DMP, DMTP, and DMDTP.
DMDTP, DETP, DETP and DMP were not studied separately because they were detected in less than 60% of the urine samples analyzed.
Correlation between the second and third trimester-average concentrations ranged between 0.16 for TCPy and 0.41 for ∑DMP (Supplemental Material, Table S5). When we looked at specific time windows during pregnancy (i.e., conducting separate analyses in samples collected during the 2nd trimester or the 3rd trimester) none of the OP metabolites was associated with the risk of having a child with ASD or NTD (all p-value ≥ 0.12, Table 3). After stratification for sex, among boys, OP concentrations tended to be associated with a decreased risk of ASD and all of the observed ORs were below 1 (Table 4). Among girls, DMTP tended to be associated with an increased risk of having a child with ASD (OR for a doubling in the DMTP concentration: 1.64 (95%CI, 0.95; 2.82, p-values for interaction = 0.09, Table 4). No clear association was observed between ∑DMP and ASD risk among girls (OR = 1.38, 95%CI: 0.85; 2.25). These results have to be interpreted cautiously given the small number of females with ASD in our study population (n = 12). After stratification for sex none of the OP metabolite concentrations was strongly associated with NTD risk (Table 4).
Table 4:
Adjusteda Multinomial Odds Ratios for Autism Spectrum Disorder and Non Typically Developing children in relation to OP urinary concentrationsb among boys and girls studied separately
| Girls (N = 48 TD, 12 ASD, 18 NTD)c |
Boys (N = 53 TD, 30 ASD, 35 NTD)c |
P-value for interaction | |||
|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | ||
| A) ASD versus TD | |||||
| TCPy | 1.09 | [0.54; 2.22] | 0.69 | [0.45; 1.06] | 0.56 |
| DMTP | 1.64 | [0.95; 2.82] | 0.84 | [0.63; 1.11] | 0.09 |
| DEP | 1.15 | [0.66; 1.99] | 0.83 | [0.61; 1.13] | 0.65 |
| ∑DMP | 1.38 | [0.85; 2.25] | 0.78 | [0.58; 1.05] | 0.24 |
| ∑DEP | 1.07 | [0.62; 1.84] | 0.80 | [0.59; 1.09] | 0.65 |
| ∑DAP | 1.29 | [0.73; 2.27] | 0.75 | [0.54; 1.06] | 0.44 |
| B) NTD versus TD | |||||
| TCPy | 1.02 | [0.63; 1.67] | 0.91 | [0.62; 1.34] | 0.63 |
| DMTP | 0.89 | [0.60; 1.31] | 0.81 | [0.61; 1.07] | 0.78 |
| DEP | 0.96 | [0.66; 1.42] | 0.83 | [0.62; 1.12] | 0.51 |
| ∑DMP | 0.90 | [0.62; 1.30] | 0.80 | [0.61; 1.06] | 0.73 |
| ∑DEP | 0.92 | [0.63; 1.35] | 0.83 | [0.62; 1.11] | 0.63 |
| ∑DAP | 0.90 | [0.59; 1.38] | 0.78 | [0.56; 1.07] | 0.64 |
adjustment factors were: home ownership, prepregnancy BMI, season and date of birth
OR represents the change in the odds of the outcomes for a doubling in the OP concentrations standardized for specific gravity
Analysis restricted to the mother-child pairs with OP concentrations, diagnosis at 3 years and non-missing covariates.
Abbreviations: DEP: Diethylphosphate, DMTP: Dimethylthiophosphate, TCPy: 3,5,6-trichloro-2-pyridinol, ∑DAP: molar sum of DEP, DETP, DMP, DMTP and DMDTP, ∑DEP: molar sum of DEP and DETP, ∑DMP: molar sum of DMP, DMTP, and DMDTP.
DMDTP, DETP, DETP and DMP were not studied separately because they were detected in less than 60% of the urine samples analyzed.
Discussion
None of the OP metabolites assessed in the current study was significantly associated with increased risk of ASD or NTD when boys and girls were studied together. This null finding might be explained by the small sample size and the lower OP concentrations compared to previous studies looking at the associations with ASD, implying that a larger sample size would be needed to detect similar associations. Windows of exposure might also be an issue since we used urinary OP concentrations measured in urine samples collected at random time points during the 2nd and 3rd trimesters while, for example, studies that relied on the annual Pesticide Use Report were able to re-construct exposure across the entire pregnancy (Shelton et al. 2014). After stratification for sex, our results suggested an association between DMTP urinary concentration and risk of ASD among girls but not among boys. No association was observed between ∑DMP, that included DMTP along with DMP and DMDTP, and ASD among girls. These results should be interpreted cautiously, and requires replication in another study with larger sample size, since only 12 girls with an ASD diagnosis were included in our analysis.
Urinary concentrations of OP
The decrease in OP concentrations with time (Figure 1) observed in our study population was likely a result of the U.S. EPA’s action to restrict the manufacture and sale of OP products for the home use market. Compared to previous studies assessing OP metabolites during pregnancy and ASD, urinary concentrations tended to be lower in our study population. The median ∑DAP concentration was 40.8 nmol/L in our study population compared to 59.9 nmol/L in the HOME study (Millenson et al. 2017), 82 nmol/L in the Mount Sinai study (Engel et al. 2007) and between 107 to 141 nmol/L, depending on the trimester, in the CHAMACOS study (Raanan et al. 2015). Similarly, the median concentration for TCPy in our study population was 2.61 ng/ml compared to 3.5 to 4.6 ng/ml in CHAMACOS depending of the trimester (Eskenazi et al. 2007) and up to 7.6 ng/ml in the Mount Sinai cohort (Berkowitz et al. 2004). Differences in OP concentrations can be explained by the recruitments that occurred earlier in previous studies (1999–2000 in CHAMACOS, 1998–2002 in the Mount Sinai cohort and 2003–2006 in the HOME study) compared to ours (2006–2014). The U.S. Environmental Protection Agency indeed banned OP pesticides for most residential uses in the early 2000s (Clune et al. 2012) and a decrease in OP concentrations have been reported between 1988–1994 and 2003–2004 in NHANES (Clune et al. 2012). The removal of OP pesticides from residential usage may have led to more intermittent exposures in our study population, which would imply greater misclassification relative to the true average concentrations within the time periods studied. In addition, sources of OP exposure may be substantially different compared to previous studies, leading to differences in the distribution of specific compounds.
Associations with ASD and NTD
Among previous studies that investigated the associations between prenatal exposure to OP pesticides and ASD, one study did not report association (Millenson et al. 2017) while five studies reported increased risk of ASD or ASD like symptoms in association with OP exposure (Eskenazi et al. 2007; Furlong et al. 2014; Rauh et al. 2006; Roberts et al. 2007; Shelton et al. 2014). The association observed in the Roberts et al study was no longer significant after correction for multiple testing (Roberts et al. 2007). Differences in study results might come from discrepancies in study design, outcome and exposure assessment. Our study population is indeed the first to enroll children with a high risk for ASD. Some of that elevated risk is due to genetic factors, which were not directly measured. Thus, environmental factors associated with ASD in these multiplex families (> 1 child with autism) might differ from those affecting ASD children in simplex families, due to greater genetic vulnerabilities, on average. Three studies, including ours, relied on clinical diagnosis of ASD (Roberts et al. 2007; Shelton et al. 2014), while the others assessed scale scores on the CBCL and SRS, two instruments completed by the parents that obtain data on symptoms common in ASD or in other types of pervasive developmental delay. Regarding exposure assessment, two studies examined agricultural pesticide uses near the homes (Roberts et al. 2007; Shelton et al. 2014). Although this approach provides valid estimates of agricultural exposures, it does not capture other sources of OP pesticides such as dietary routes of exposure. The other studies measured OP metabolites in urine collected during pregnancy (Eskenazi et al. 2007; Furlong et al. 2014; Millenson et al. 2017; Rauh et al. 2006) or the parent compounds in umbilical cord blood collected at delivery (Rauh et al. 2006). However, they relied on a small number of biological samples (usually no more than 2), which, considering the high variability reported for OP metabolite concentration in urine (intraclass correlation coefficients ranging from 0.14 to 0.31 for ∑DAP (Spaan et al. 2015) and of 0.41 for TCPy (Fortenberry et al. 2014)), is likely to lead to exposure measurement error. If this error is of classical type (i.e, the average of many replicate measurements is expected to approximate the true individual level) attenuation of the effect estimates would be expected (Perrier et al. 2016). Despite the fact that we collected more urine samples (on average 5 samples per mother) than previous studies, we did not demonstrate an association between prenatal urinary concentration of OP pesticide metabolite concentrations and ASD or NTD risk in our overall sample; however, our results were suggestive of an association between DMTP and ASD among girls. Among studies on organophosphate pesticides and child neurodevelopment, a few performed sex-stratified analysis (reviewed by (Gonzalez-Alzaga et al. 2014)). Among them, Bouchard et al. reported a negative association with child IQ that was not modified by child sex (p-value for interaction > 0.3 (Bouchard et al. 2011)) while the others reported deleterious effects among boys but not among girls in ADHD (Marks et al. 2010), working memory (Horton et al. 2012) and ASD like symptoms (Furlong et al. 2014).
Limitations
Urinary OP metabolite concentrations reflect direct exposure to OP parent compounds that were metabolized endogenously by the enrolled women, but also direct exposure to OP metabolites that are naturally present in the environment. These OP metabolites result from the photolysis of the parent compounds in food or indoor dust (Clune et al. 2012), are non toxic and are mostly excreted unchanged in urine. The part of the urine concentrations from each source (parent compounds metabolized by the women versus OP metabolites naturally present in the environement) is unlikely to vary across diagnostic group and so this should not introduce differential measurement error with regard to the child’s outcome in our study. Except for TCPy, a specific metabolite of chlorpyrifos, OP metabolites are not specific and reflect exposure to multiple OP parent compounds. While we adjusted for several confounders we cannot rule out residual bias due to unmeasured confounders or confounders measured with error. PON1 enzyme has been associated with neurodevelopmental outcomes (Eskenazi et al. 2010) and is also involved in the detoxification pathway of several chemicals including some OP pesticides. Individuals with certain PON1 polymorphisms might be more susceptible to the effects of these chemicals (Engel et al. 2007). We do not have information regarding PON1 genetic polymorphism and enzyme activity, however, even with such information our small sample size may have limited our ability to examine the interaction between the polymorphism and OP pesticides. An additional limitation in our study design is the variable number of urine samples used to estimate average exposures, which we addressed using an ad hoc analytical weighting strategy. In simulation studies (Perrier et al. 2016), we have found that the analytical weighting strategy yields more accurate effect size estimates than a naive strategy that treats all exposure measurements as equally precise, but further methodological research is warranted to fully justify this approach
Conclusion
The MARBLES Study is a high risk longitudinal cohort which gave us the ability to prospectively assess prenatal OP exposure with the use of multiple urine samples collected throughout pregnancy and clinically confirmed classifications of ASD and NTD at 3 years. We did not observe association between either prenatal exposure to OP pesticides and ASD or NTD when boys and girls were studied together. After stratification for sex, DMTP tended to be associated with an increased risk of having a child with ASD among girls. Given the relatively small sample size, both the association we observed among girls, as well as the lack of association in boys, needs to be replicated in studies with larger sample size.
Supplementary Material
Acknowledgement:
We acknowledge the MARBLES Staff and the study participants.
Funding: This research was supported by the following grants: R01ES020392, R01ES014901, P42ES04699 and P01 ES011269 and by the U.S. Environmental Protection Agency (Grant 8354320), the UC Davis MIND Institute, and an unrestricted gift grant from the JB Johnson Foundation. Claire Philippat is funded by a grant from Fondation de France (grant 2015-00059545). The funding sources were not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- ADI-R
Autism Diagnostic Interview-Revised
- ADOS
Autism Diagnostic Observation Schedule
- BMI
Body Mass Index
- DEP
diethylphosphate
- DETP
diethylthiophosphate
- DEDTP
diethyldithiophosphate
- DMP
dimethylphosphate
- DMTP
dimethylthiophosphate
- DMDTP
dimethyldithiophosphate
- OP
Organophosphate pesticides
- SCQ
Social Communication Questionnaire
- ∑DAP
molar sum of DEP, DETP, DMP, DMTP and DMDTP
- ∑DEP
molar sum of DEP and DETP
- ∑DMP
molar sum of DMP, DMTP, and DMDTP
Footnotes
Competing financial interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, et al. 2004. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environmental health perspectives 112(3): 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. 1999. Autism screening questionnaire: diagnostic validity. The British journal of psychiatry : the journal of mental science 175: 444–451. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environmental health perspectives 119(8): 1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2012. Prevalence of autism spectrum disorders-Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR Surveill Summ 61(3): 1–19. [PubMed] [Google Scholar]
- Ching-Ping C 2011. The design effects of cluster sampling on the estimation of mean lengths and total mortality of reef fish. Fisheries Research(109): 295–302. [Google Scholar]
- Clune AL, Ryan PB, Barr DB. 2012. Have regulatory efforts to reduce organophosphorus insecticide exposures been effective? Environmental health perspectives 120(4): 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. 2007. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. American journal of epidemiology 165(12): 1397–1404. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, et al. 2010. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environmental health perspectives 118(12): 1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental health perspectives 115(5): 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenberry GZ, Meeker JD, Sanchez BN, Barr DB, Panuwet P, Bellinger D, et al. 2014. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: distribution, temporal variability, and relationship with child attention and hyperactivity. International journal of hygiene and environmental health 217(2–3): 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong MA, Engel SM, Barr DB, Wolff MS. 2014. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environment international 70: 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alzaga B, Lacasana M, Aguilar-Garduno C, Rodriguez-Barranco M, Ballester F, Rebagliato M, et al. 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicology letters 230(2): 104–121. [DOI] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. 2012. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicology and teratology 34(5): 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, et al. 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30(3): 205–223. [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. 2006. Organic diets significantly lower children’s dietary exposure to organophosphorus pesticides. Environmental health perspectives 114(2): 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environmental health perspectives 118(12): 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenson ME, Braun JM, Calafat AM, Barr DB, Huang YT, Chen A, et al. 2017. Urinary organophosphate insecticide metabolite concentrations during pregnancy and children’s interpersonal, communication, repetitive, and stereotypic behaviors at 8 years of age: The home study. Environmental research 157: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. 1995. Mullen Scales of Early Learning. Circle Pines, MN. [Google Scholar]
- Olsson AO, Baker SE, Nguyen JV, Romanoff LC, Udunka SO, Walker RD, et al. 2004. A liquid chromatography--tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Anal Chem 76(9): 2453–2461. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, et al. 2011. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 128(3): e488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology 27(3): 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapamontol T, Sutan K, Laoyang S, Hongsibsong S, Lee G, Yano Y, et al. 2014. Cross validation of gas chromatography-flame photometric detection and gas chromatographymass spectrometry methods for measuring dialkylphosphate metabolites of organophosphate pesticides in human urine. International journal of hygiene and environmental health 217(4–5): 554–566. [DOI] [PubMed] [Google Scholar]
- Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B. 2015. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environmental health perspectives 123(2): 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental health perspectives 119(8): 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118(6): e1845–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences of the United States of America 109(20): 7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. 2007. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environmental health perspectives 115(10): 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Berument S, Lord C, Pickles A. 2003. Social Communication Questionnaire (SCQ): Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Shelton JF, Hertz-Picciotto I, Pessah IN. 2012. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environmental health perspectives 120(7): 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environmental health perspectives 122(10): 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan S, Pronk A, Koch HM, Jusko TA, Jaddoe VW, Shaw PA, et al. 2015. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. Journal of exposure science & environmental epidemiology 25(3): 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, Hardt J, Knuppel S. 2011. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology (Cambridge, Mass) 22(5): 745. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in medicine 30(4): 377–399. [DOI] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, et al. 2005. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology 26(2): 199–209. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han S, Liang D, Shi X, Wang F, Liu W, et al. 2014. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: a birth cohort study in Shenyang, China. PloS one 9(2): e88491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.