Abstract
Background
γ-Aminobutyric acidergic (GABAergic) dysfunction and immune activation have been implicated in the pathophysiology of schizophrenia. Preclinical evidence suggests that inflammation-related abnormalities may contribute to GABAergic alterations in the brain, but this has never been investigated in vivo in humans. In this multimodal imaging study, we quantified cerebral GABA plus macromolecule (GABA+) levels in antipsychotic-naive people at clinical high risk for psychosis and in healthy volunteers. We investigated for the first time the association between GABA+ levels and expression of translocator protein 18 kDa (TSPO; a marker of microglial activation) using positron emission tomography (PET).
Methods
Thirty-five people at clinical high risk for psychosis and 18 healthy volunteers underwent 3 T proton magnetic resonance spectroscopy to obtain GABA+ levels in the medial prefrontal cortex (mPFC). A subset (29 people at clinical high risk for psychosis and 15 healthy volunteers) also underwent a high-resolution [18F]FEPPA PET scan to quantify TSPO expression. Each participant was genotyped for the TSPO rs6971 polymorphism.
Results
We found that GABA+ levels were significantly associated with TSPO expression in the mPFC (F1,40 = 10.45, p = 0.002). We found no significant differences in GABA+ levels in the mPFC (F1,51 = 0.00, p > 0.99) between people at clinical high risk for psychosis and healthy volunteers. We found no significant correlations between GABA+ levels or residuals of the association with TSPO expression and the severity of prodromal symptoms or cognition.
Limitations
Given the cross-sectional nature of this study, we could determine no cause-and-effect relationships for GABA alterations and TSPO expression.
Conclusion
Our findings suggest that TSPO expression is negatively associated with GABA+ levels in the prefrontal cortex, independent of disease status.
Introduction
γ-Aminobutyric acidergic (GABAergic) dysregulation and immune activation have been separately implicated in the pathophysiology of schizophrenia, but the in vivo relationship between these 2 systems has never been investigated in parallel in the human brain.
Postmortem and neuroimaging studies support a role for dysregulation of the primary inhibitory neurotransmitter system, γ-aminobutyric acid (GABA), in the pathophysiology of schizophrenia.1,2 Postmortem studies have consistently reported deficits in the expression of fast-spiking, parvalbuminpositive GABAergic interneurons in the prefrontal cortex of patients with schizophrenia and reductions in mRNA and protein levels of GAD67, a major GABA-synthesizing enzyme. 3–6 In addition, a recent study reported reduced cerebrospinal fluid concentrations of GABA in patients following a first episode of psychosis compared with healthy controls.7 Recent advances in magnetic resonance spectroscopy (1H-MRS) allow for the in vivo quantification of cerebral GABA levels. Several GABA 1H-MRS studies have been published in schizophrenia, particularly in the medial prefrontal cortex (mPFC).8 While some studies report reductions in GABA levels in patients with schizophrenia,9–12 others have reported increased13–15 or unaltered levels9,11,13,16–18 in the mPFC. However, these discrepancies appear to reflect differences in voxel placement and/or whether the patients had previous exposure to antipsychotics. This idea is supported by a study that reported elevated GABA levels in the mPFC of unmedicated patients with schizophrenia, but in not in medicated patients. 13 Further, a recent study reported elevated GABA levels in antipsychotic-naive patients following a first episode of psychosis that normalized after 4 weeks of antipsychotic treatment.19 Differences in methodology, duration, phase of illness or demographics (e.g., age, sex or smoking status) may also explain these inconsistencies. In this context, studying people at clinical high risk (CHR) for psychosis provides an unparalleled opportunity to investigate the neurobiological changes underlying schizophrenia, without the confounding influence of antipsychotic medication. Only 3 studies have investigated GABA levels in people at CHR for psychosis. Wang and colleagues20 reported unaltered GABA levels in the mPFC of antipsychotic-naive people at CHR (21 people at CHR and 23 healthy volunteers). Similarly, a recent 1H-MRS study also failed to detect differences in GABA levels in the mPFC of antipsychotic-naive people at CHR (21 people at CHR and 20 healthy volunteers),21 whereas another study reported increased GABA levels in the mPFC (23 people at CHR and 24 healthy volunteers).22
Converging evidence from preclinical, genetic and peripheral studies have implicated immune-related abnormalities in the pathophysiology of schizophrenia.23 Microglia play a critical role in both healthy and diseased states of the central nervous system.24 In the developing and mature central nervous system, microglia are involved in synaptic pruning and maturation, and in maintaining synaptic plasticity.24 On the other hand, microglia also function as inflammatory cellular mediators in response to tissue damage or brain insult.25 Neuroinflammation is characterized (at least in part) by microglial activation.26 Microglial activation is associated with elevated expression of a mitochondrial protein, translocator protein 18 kDa (TSPO), making TSPO a suitable marker for quantifying microglial activation in vivo.27 Currently, we can quantify TSPO expression in vivo using positron emission tomography (PET). The most replicated finding across TSPO PET studies in schizophrenia suggests no differences in TSPO expression using second-generation radioligands, 28–31 although a recent study reported a significant reduction in patients with schizophrenia compared with healthy volunteers,32 also supported by a recent meta-analysis. 33 Three other studies reported elevated TSPO expression in people with schizophrenia and first-episode psychosis,34–36 but these studies used the first-generation radioligand [11C]PK11195, which has several known limitations. 37 Only 2 studies have investigated TSPO expression in people at CHR for psychosis, and both reported a lack of group differences between people at CHR and healthy volunteers using total distribution volume (VT), a validated outcome measure for second-generation TSPO radioligands.38,39
Accumulating evidence suggests that GABAergic signalling can modulate inflammatory processes, and that immune processes can induce reciprocal changes in the GABAergic system, providing a potential link between immune activation and GABAergic alterations in schizophrenia.40 Preclinical studies have consistently shown alterations in gene expression across GABAergic pathways and reductions in GABAergic parvalbumin-positive interneurons following prenatal exposure to inflammatory stimuli.41–43 Activation of GABA receptor activity attenuates immune activation, while inhibition of receptor activity increases inflammatory responses. 44–46 In fact, studies have shown that microglial activation is negatively regulated by GABAergic neurotransmission. 45,47 As well, GABA receptors are present on immune cells, including astrocytes and microglia, suggesting a potential role for GABA in neuroimmune responses.48,49 Moreover, GABAergic deficits can lead to glutamatergic hyperactivity, resulting in toxicity-mediated neuronal death, excessive glial activation and neuroinflammation.50,51 Although there is a close relationship between GABAergic and immune dysfunction in schizophrenia, the in vivo relationship between these 2 systems has yet to be investigated in humans. In the present study, we investigated GABA plus macromolecule (GABA+) in the mPFC of antipsychotic-naive people at CHR for psychosis, making this the largest GABA 1H-MRS study in this population (35 people at CHR and 18 healthy volunteers). We also investigated, to our knowledge for the first time, the in vivo association between GABA+ levels and TSPO expression using 3 T 1H-MRS and high-resolution PET, respectively. Based on previous evidence, we hypothesized that there would be a negative association between GABA+ levels and TSPO expression in the brain.
Methods
Participants
We initially enrolled 36 people at CHR for psychosis and 18 healthy volunteers. Most of the people in the CHR group were antipsychotic-naive (n = 30).
People in the CHR group were included if they met diagnostic criteria for prodromal risk syndrome as assessed by the Criteria of Prodromal Syndromes.52 They were excluded if they had a current axis I disorder as determined by the Structured Clinical Interview53 for DSM-IV Axis I disorders (SCID-I). Healthy volunteers were included if they did not have a history of past psychoactive drug use and/or first-degree relatives with a major mental disorder. All participants were excluded if they had a clinically significant medical illness, had a current diagnosis of alcohol or substance use/dependence, were pregnant or breastfeeding, or had metal implants that precluded an MRI scan. In the CHR group, we assessed clinical status and the severity of prodromal symptoms using the Structured Interview for Psychosis-Risk Syndromes, the Scale of Psychosis-Risk Symptoms, the Calgary Depression Scale for Schizophrenia for depression symptoms and the Apathy Evaluation Scale for apathy. We assessed neurocognitive performance using the Repeatable Battery for the Assessment of Neuropsychological Status.54
This study was approved by the research ethics board at the Centre for Addiction and Mental Health in Toronto, Canada. All participants provided written informed consent after procedures had been explained thoroughly.
Data acquisition and analysis: 1H-MRS
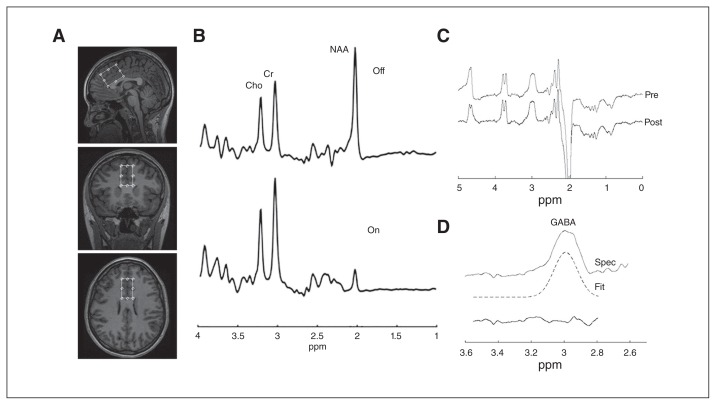
All participants underwent a 1H-MRS scan on a 3 T MR-750 scanner (General Electric Medical Systems) equipped with an 8-channel head coil. Head position was fixed at the centre of the head coil with tape strapped across the forehead and soft padding to minimize head motion. A 24 mL (20 × 40 × 30 mm3) MRS voxel was positioned as shown in Figure 1A. The voxel called mPFC was composed of 65% cingulate gyrus and 28% superior frontal gyrus (Appendix 1, Fig. 2S, available at jpn.ca/170201-a1). We performed shimming using the manufacturer-supplied automated shimming routine (AUTOSHIM) to adjust magnet homogeneity. We measured GABA levels using a modification of standard single-voxel, double-spin echo data acquisition by inserting a pair of frequency-selective radiofrequency (RF) pulses before and after the second volume-selective, 180° RF pulse (MEGA-PRESS). 13,55–59 This J-editing approach used frequency-selective RF pulses to alter the temporal evolution of the strongly coupled spins in the GABA C2, C3 and C4 multiplets. The frequencies of these editing RF pulses were centred to suppress the C3 resonance of GABA at 1.9 ppm in the “on” condition and at 7.5 ppm in the “off” condition (Fig. 1B). This editing RF pulse pair inhibited and allowed J-modulation of the coupled GABA spin system, such that subtraction of the 2 subspectra yielded the J-edited GABA resonance free from the overlapping creatine and aspartate resonances. However, our data acquisition did not exclude the underlying macromolecule, so the GABA observed in this study was GABA+. We averaged 8 excitations for each “on” and “off” frequency cycle, resulting in 66 data frames. We combined these multichannel raw MRS data sets in the time domain based on coil sensitivity derived from unsuppressed water signal, weighted by the sum of the squares of the signal intensities from each channel (Fig. 1B).60 Subtraction of the “on” from the “off” condition yielded only the J-edited GABA C4 resonance (Fig. 1C). Acquisition parameters for the measurements were as follows: echo time 68 ms, repetition time 1500 ms, bandwidth = 5 kHz, number of excitations = 528 (512 water-suppressed, 16 water-unsuppressed), data points = 4096. The data acquisition implementation used in this study has been validated in a large-scale, multivendor, multisite study, with the aim of understanding the factors that impact GABA measurement outcome with a coefficient of variation of the entire healthy volunteer cohort of 12% for GABA.61
Fig. 1.
(A) Axial, sagittal and coronal views of the voxel placement in the medial prefrontal cortex. (B) Typical fitting results from a patient: magnetic resonance spectroscopy acquired for the “off” and “on” conditions (see text). (C) Difference between the “on” and “off” spectra before (pre) and after (post) signal alignment using Gannet software. (D) Gaussian lineshape fitting of edited GABA resonance showing GABA resonance at 3.0 ppm, fitted signal and residual. Cho = choline; Cr = creatine; fit = model of best fit; GABA = γ-aminobutyric acid; NAA = N-acetylaspartate; ppm = parts per million; pre = before spectral alignment; post = after spectral alignment; spec = GABA-edited spectrum.
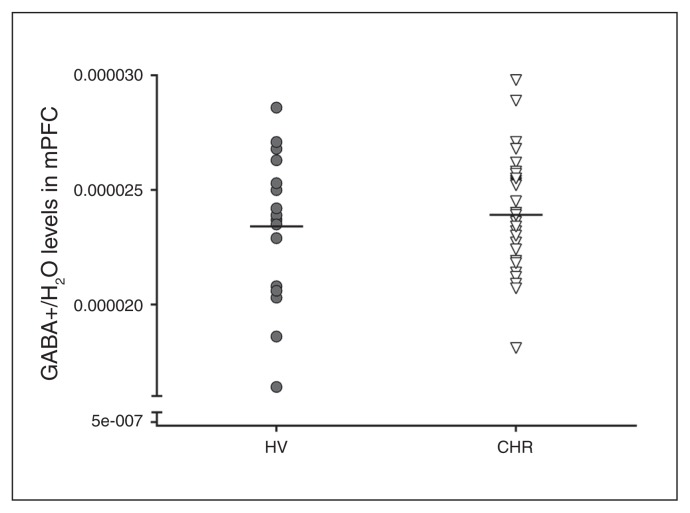
Fig. 2.
GABA+/H2O levels in the medial prefrontal cortex (mPFC) of healthy volunteers (HV; n = 18) and people at clinical high risk (CHR) for psychosis (n = 35). GABA+ = γ-aminobutyric acid plus macromolecule.
All postprocessing and analysis were performed with Gannet62,63 using Gaussian line shape fitting, with modifications to allow the output of GABA+ area (Fig. 1D). The data were zero-padded to 32 000 points, and we used line-broadening of 3 Hz. The ratio of GABA+ to unsuppressed water peak areas has been reported.64 Spectra that exceeded a full width at half maximum of unsuppressed water resonance greater than 10 Hz were excluded from further analysis.
To estimate the fractions of grey matter, white matter and cerebrospinal fluid in the voxel, we created volume images and a segmentation mask for the mPFC from the MRS raw data file and 3D T1-weighted images using an in-house MATLAB-based code. We then performed segmentation using statistical parametric mapping software (SPM8; Wellcome Centre for Human Neuroimaging) to determine fractional tissue composition in the voxel.
Data acquisition and analysis: PET and structural MRI
Data from PET and MRI were available for a subset of our sample (29 people at CHR and 15 healthy volunteers). As well, 26 people at CHR and 13 healthy volunteers were included in our previous cohorts,39 including 1 manuscript in submission. The PET and MRI scans were obtained on average 16.09 days apart. The PET data acquisitions have been described in detail elsewhere.29,30 Briefly, we obtained proton-density-weighted and T1-weighted brain magnetic resonance images for each participant using a 3 T MR-750 scanner. We performed all [18F]FEPPA scans using a high-resolution research tomograph (CPS/Siemens). Each participant was given an intravenous bolus injection of 186.53 ± 11.01 MBq of [18F]FEPPA for 125 min. Arterial and manual blood samples were taken to measure radioactivity in blood and the relative proportion of radiolabelled metabolites. We collected arterial blood for the first 22.5 min at a rate of 2.5 mL/min after radioligand injection, using an automatic blood sampling system (Model PBS-101, Veenstra Instruments). We took manual blood samples at −5, 2.5, 7, 12, 15, 20, 30, 45, 60, 90 and 120 min relative to the time of injection. Dispersion and metabolite-corrected plasma input function were generated as previously described.30 We extracted time–activity curves for the mPFC using a validated in-house imaging pipeline regions of mental interest.65 The region of interest was delineated using proton-density MRIs. We derived VT in the mPFC from the time–activity curve and plasma input function using a 2-tissue compartment model, which has been validated for [18F]FEPPA quantification.66
Genotyping: rs6971 polymorphism
A polymorphism in the TSPO gene (rs6971) affects the binding affinity of second-generation radioligands, including [18F]FEPPA.67,68 We genotyped all participants on the basis of this polymorphism as high-affinity (C/C), mixed-affinity (C/T) or low-affinity (T/T) binders, as described elsewhere.68,69
Statistical analysis
We tested differences in participant characteristics between the CHR group and healthy volunteers using χ2 tests for categorical variables (e.g., sex) and analysis of variance for continuous variables (e.g., age). To test for differences in GABA+ levels in mPFC between groups, we used univariate analysis of variance, with GABA+ levels as the dependent variable and group (CHR v. healthy volunteers) as a fixed factor. To test our hypothesis regarding the association between [18F]FEPPA VT and GABA+ levels in mPFC, we used a general linear model with [18F]FEPPA VT in mPFC as the dependent variable; group and TSPO genotype (categorical variable, high-affinity binders or mixed-affinity binders) as fixed factors; and GABA+ levels as a covariate. We tested the main effects of group, TSPO genotype and GABA+ levels, and the interaction between GABA+ levels and group. We removed the GABA+ × group interaction from the final model because it was not significant. Owing to the large-scale difference between GABA+ levels (10−5 range) and [18F]FEPPA VT (101 range), we standardized GABA+ levels before the analysis by subtracting the mean and dividing the difference by the standard deviation. To test for potential confounding effects of sex and age on GABA+ levels and association with TSPO expression, we added both factors as covariates to the model. We also explored the associations between the residuals of the general linear model and clinical and neuropsychological measures in people at CHR for psychosis using bivariate correlations. We performed statistical analyses using SPSS version 23.0 (IBM Inc.). We set the significance level at p < 0.05.
Results
The demographic and clinical characteristics of the participants are shown in Table 1. One member of the CHR group was excluded because of motion during the 1H-MRS scan that could not be corrected. We found no significant differences in sex and age between the CHR group and the healthy volunteers (Table 1), or in TSPO genotype or PET parameters (Appendix 1, Table S1). Five members of the CHR group were on low-dose antipsychotic treatment with risperidone (1 taking 0.5 mg and 2 taking 1 mg), quetiapine (75 mg) or aripiprazole (5 mg) at the time of the PET scan. All participants had had a negative urine drug screen, except for 1 member of the CHR group, who had a positive urine drug screen for benzodiazepines (1-time use) and 4 members of the CHR group who had a positive urine drug screen for cannabis, but no other drugs. The averaged unsuppressed water line width for GABA+ data acquisition, full width at half maximum, was 7.39 ± 0.85 Hz for healthy volunteers and 7.57 ± 1.14 Hz for the CHR group. We observed no significant differences in full width at half maximum between groups (Appendix 1, Table S2).
Table 1.
Demographic and clinical characteristics of study participants
| Measure | Healthy volunteers (n = 18) | People at clinical high risk for psychosis (n = 35) | Statistical test | p value |
|---|---|---|---|---|
| Age, yr, mean ± SD | 21.28 ± 1.99 | 20.57 ± 1.63 | F = 1.91 | p = 0.17 |
| Sex, male: female | 6:12 | 19:16 | χ2 = 2.09 | p = 0.15 |
| Current drug use, n* | ||||
| Nicotine | 0 | 8 | — | — |
| Cannabis | 0 | 4 | — | — |
| Lifetime history of recreational drug use (use > 10 times), n | ||||
| Cannabis | 0 | 17 | — | — |
| MDMA | 0 | 1 | — | — |
| Cocaine | 0 | 1 | — | — |
| LSD | 0 | 1 | — | — |
| Barbiturate | 0 | 1 | — | — |
| Antipsychotic use, n† | 0 | 5 | — | — |
| SOPS, mean ± SD | ||||
| Total | — | 36.03 ± 11.81 | — | — |
| Positive | — | 11.20 ± 3.76 | — | — |
| Negative | — | 11.37 ± 5.94 | — | — |
| Disorganization | — | 3.74 ± 2.65 | — | — |
| General | — | 8.86 ± 4.31 | — | — |
| AES, mean ± SD‡ | — | 39.06 ± 9.19 | — | — |
| CDSS, mean ± SD | — | 5.51 ± 4.03 | — | — |
| RBANS, total, mean ± SD | — | 90.94 ± 13.95 | — | — |
AES = Apathy Evaluation Scale; CDSS = Calgary Depression Scale for Schizophrenia; CHR = clinical high risk for psychosis; LSD = lysergic acid diethylamide; MDMA = 3,4-methylenedioxymethamphetamine (ecstasy); SOPS = Scale of Psychosis-Risk Symptoms; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; SD = standard deviation.
All participants underwent a urine drug screen at baseline for cannabis, ethanol, methadone and cocaine. Baseline results were negative, except for 1 member of the CHR group with a positive result for benzodiazepine, and 4 members of the CHR group with a positive result for cannabis.
Participants at clinical high risk for psychosis who were currently on antipsychotic treatment: 0.5 mg risperidone (n = 1), 1.0 mg risperidone (n = 2), 75 mg quetiapine (n = 1), and 5 mg aripiprazole (n = 1).
Score missing for 2 participants.
GABA+ levels in the mPFC
We found no significant differences in GABA+ levels in mPFC between the CHR group and healthy volunteers (35 CHR and 18 healthy volunteers; F1,51 = 0.00, p . 0.99; 2.14% higher in the CHR group v. the healthy volunteers; Fig. 2). We obtained similar results after controlling for sex and age (F1,49 = 0.00, p > 0.99). Results remained the same when excluding members of the CHR group who were tobacco users, or were positive for cannabis, benzodiazepine or antipsychotic use. We found no significant differences in grey matter, white matter or cerebrospinal fluid fractions in the mPFC voxel between the CHR group and the healthy volunteers (Appendix 1, Table S2). We also found no significant correlations between GABA+ levels and symptom severity or clinical and neuropsychological measures (Appendix 1, Table S3).
GABA+ and [18F]FEPPA VT in the mPFC
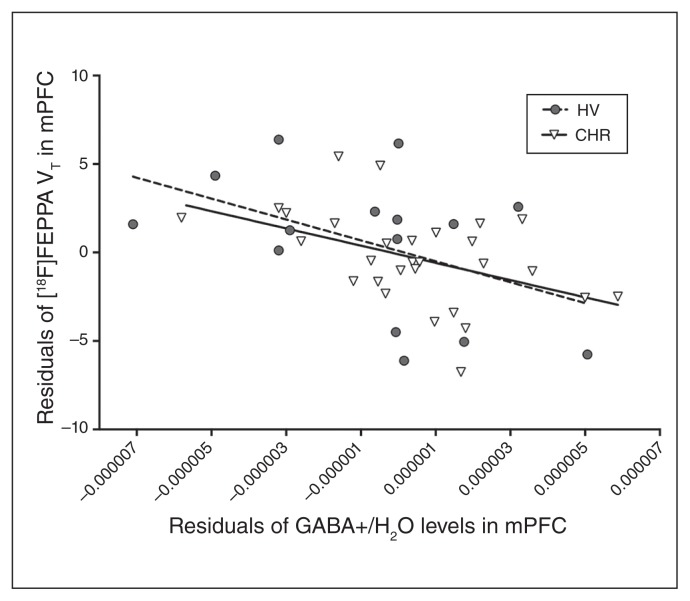
We observed a significant negative association between GABA+ levels and [18F]FEPPA VT in the mPFC (β = −1.47, SE = 0.45; F1,40 = 10.45, p = 0.002; Fig. 3). We obtained similar results after controlling for sex and age (β = −1.43, SE = 0.46; F1,38 = 9.42, p = 0.004). Results remained the same when excluding members of the CHR group who were tobacco users, or were positive for cannabis, benzodiazepine or antipsychotic use. We found no significant correlations between residuals of the model and symptom severity or clinical and neuropsychological measures. The lack of group differences in [18F]FEPPA VT between CHR and healthy volunteers has been reported elsewhere (Appendix 1, Table S1).39
Fig. 3.
Associations between residuals of regressing [18F]FEPPA VT on TSPO genotype and residuals of regressing GABA+/H2O levels in medial prefrontal cortex (mPFC) on TSPO genotype in people at clinical high risk (CHR) for psychosis (r = −0.49, p = 0.008) and healthy volunteers (HV; r = −0.53, p = 0.053). GABA+ = γ-aminobutyric acid plus macromolecule; TSPO = translocator protein 18 kDa; VT = total distribution volume.
Discussion
This is, to our knowledge, the first in vivo study to investigate GABA+ levels in the mPFC of people at CHR for psychosis and their association with TSPO expression. As previously reported, we observed no significant differences in GABA+ levels or [18F]FEPPA VT in the mPFC between members of the CHR group and healthy volunteers. We reported a significant negative association between GABA+ levels and TSPO expression in the mPFC. We found no correlations between GABA+ levels in the mPFC or residuals of the model and severity of prodromal symptoms or neuropsychological scores.
GABA+ levels in the mPFC
We found no significant differences in GABA+ levels in the mPFC between the CHR group and the healthy volunteers (35 people at CHR and 18 healthy volunteers). We also found no associations between GABA+ levels and the severity of prodromal symptoms or clinical and neuropsychological measures. Of the 3 1H-MRS GABA studies in people at CHR for psychosis, 2 studies reported unaltered GABA levels in the mPFC, and the other reported elevated GABA levels in people at CHR compared with healthy volunteers. Such discrepancies in the findings are most likely due to differences in the positioning of the 1H-MRS voxel in the mPFC. A recent study using a smaller sample and a voxel that overlapped with ours (i.e., it was more dorsally placed in the mPFC) reported unaltered GABA levels in antipsychotic-naive people at CHR compared with healthy controls.21 Of the 2 studies with the 1H-MRS voxel more ventrally placed in the mPFC, 1 reported unaltered GABA levels,20 but the other reported increased GABA levels22 in people at CHR compared with healthy volunteers. This discrepancy could be attributable to methodological rigour, given that the MEGA-PRESS spectrum shown in the former study was of poorer quality (a very broad GABA resonance) 20 than the spectrum shown in the study that reported mPFC GABA elevations.15 Future studies should consider standardizing voxel placement and size, naming voxels consistently and (if possible) quantifying GABA levels in both dorsal and ventral brain regions to clarify whether regional differences exist in the mPFC in people at CHR for psychosis. However, consistent with our findings, a recent meta-analysis of 1H-MRS GABA in schizophrenia failed to reveal group differences in the mPFC.8 Longitudinal clinical follow-up revealed that 7 of the 35 people at CHR in this study (20%) converted to psychosis. Although this was a cross-sectional study and conclusions were limited by small sample size, we found no significant differences in GABA+ levels in the mPFC between converters at CHR and nonconverters (F1,35 = 1.53, p = 0.23; Appendix 1, Figure S1).
GABA+ and [18F]FEPPA VT in the mPFC
We found a significant negative association between GABA+ levels and [18F]FEPPA VT, suggesting that regardless of clinical diagnosis, higher TSPO expression is associated with lower GABA levels. Although this is the first in vivo study to investigate the link between immune activation and the GABAergic system in the brain, accumulating evidence from preclinical studies suggests a potential link between these 2 systems. Preclinical studies have consistently shown that immune activation alters components of the GABAergic system in the brain, including reductions in GABA receptor expression and function, 70,71 neuronal density72,73 and the expression of GAD65 and GAD67, 2 major GABA-synthesizing enzymes.71,74 Similarly, reduced GABA levels in the prefrontal cortex of mice have been reported following prenatal exposure to inflammatory stimuli. 75 Further, supporting our results, overexpression of interleukin-6, specifically in glial cells, decreased GABA-positive and parvalbumin-positive neurons in adult mouse hippocampus. 76 In addition, GABAA and GABAB receptor agonists can reduce microglial activation45 and attenuate the release of proinflammatory cytokines,44 while inhibition of GABA receptor activity increases inflammatory responses.46 Our results provide, to our knowledge, the first in vivo evidence linking GABA levels and TSPO expression, but future studies should investigate the mechanisms underlying this association.
Limitations
Interpretation of the results of this study should consider the following limitations. First, the proton MRS voxels are relatively large because of the low concentration of GABA in the human brain. Our mPFC voxel contained approximately 65% cingulate gyrus and 28% superior frontal gyrus (Appendix 1, Figure S2). In addition, the GABA quantification reported in this study was a combination of GABA molecule and mobile macromolecule (up to 50% of total GABA signal),77 termed GABA+. The contribution of this macromolecule to the total GABA signal in healthy participants has been documented using the same J-editing approach.78 However, it is not known whether this mobile macromolecule might differ in people at CHR for psychosis.
Second, there have been inconsistencies in the placement of 1H-MRS voxels across GABA studies in schizophrenia, particularly in the mPFC.8 We acknowledge that the failure of this study to detect group differences in GABA+ levels between people at CHR for psychosis and healthy volunteers may be due to the more dorsal placement of our 1H-MRS voxel in the mPFC — consistent with Modinos and colleagues21 — compared with a more ventral placement.22
Third, in neurochemical brain-imaging studies, relatively small sample sizes represent a potential limitation; however, to our knowledge, this is the largest GABA 1H-MRS study in people at CHR for psychosis. In addition, given the novelty of our findings in linking TSPO expression and GABA levels, this study can serve as a framework for future studies.
Fourth, although we acquired scans on 2 separate days, both [18F]FEPPA PET and GABA 1H-MRS have satisfactory test–retest reliability, and no changes in PET and MRI machine stability and CHR pathophysiology are expected in our time interval (about 16 days).
Fifth, the voxel used for 1H-MRS in mPFC was larger than the region of interest used with PET and also included the dorsal part of the anterior cingulate cortex, but this was unlikely to affect the results presented.
Sixth, although an increase in [18F]FEPPA binding was mostly attributed to microglial activation, studies show that astrocytes and vascular endothelial cells also express TSPO.79 However, this does not affect the overall conclusion of our study, given that astrocytes are also involved in the immune response. Further studies are needed to evaluate the contribution of these components to the TSPO signal. In addition, the link between TSPO expression and microglial activation is not yet well understood. Several studies have shown marked elevations in TSPO expression in activated microglia, 80–82 including a study by Sandiego and colleagues83 that reported robust increases in [11C]PBR28 binding (a second-generation TSPO radioligand) following a lipopolysaccharide challenge, confirming that TSPO is upregulated during immune activation; however, other studies have shown a decrease in TSPO in proinflammatory states.84
Seventh, the [18F]FEPPA outcome measure, VT, is a composite measure that includes both specific and nonspecific-plus-free binding, and to date, it is unknown whether the nonspecific-plus-free binding is different between groups. Further, in this study we did not correct [18F]FEPPA VT for the plasma-free fraction of the radioligand (fp), because it has been shown to substantially increase the variability.85
Finally, we acknowledge that GABA does not provide a full picture of excitatory and inhibitory balance in the brain. However, exploratory analysis in our data set revealed no significant group differences between mPFC glutamine + glutamate or inhibitory index,86 or associations with TSPO expression in the mPFC (Appendix 1, Table S4, Table S5, Figure S3, Figure S4). Similarly, we found no group differences in mPFC N-acetylaspartate/creatine or choline/creatine ratios between people at CHR for psychosis and healthy volunteers, or associations with TSPO expression in the mPFC (Appendix 1, Table S4, Table S5).
Conclusion
The results of this study suggest a link between GABA+ levels and TSPO expression in the mPFC, independent of disease status.
Acknowledgements
The authors thank the excellent staff of the Research Imaging Centre at the Centre for Addiction and Mental Health — in particular Peter Truong for his Gannet GABA processing and for generating the brain region composition of the magnetic resonance spectroscopy voxel, and the Focus on Youth Psychosis Prevention clinic. The authors are grateful to Felix Raschke of National Centre for Radiation Research in Oncology, Dresden, Germany, for the voxel masking code.
Footnotes
Competing interests: R. Mizrahi has received speaker fees from Otsuka Lundbeck Canada. No other competing interests declared.
Funding: This work was supported by the National Institutes of Health (NIH) R01 grant MH100043 to R. Mizrahi.
Contributors: R. Mizrahi designed the study. All authors acquired and analyzed the data. N. Sailasuta and R. Mizrahi wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Chiapponi C, Piras F, Piras F, et al. GABA system in schizophrenia and mood disorders: a mini review on third-generation imaging studies. Front Psychiatry. 2016;7:61. doi: 10.3389/fpsyt.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–40. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- 3.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–66. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 6.Volk D, Austin M, Pierri J. Decreased glutamic acid decarboxylase messenger RNA expression in a subset of prefrontal cortical G-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–45. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 7.Orhan F, Fatouros-Bergman H, Goiny M, et al. CSF GABA is reduced in first-episode psychosis and associates to symptom severity. Mol Psychiatry. 2018;23:1244–50. doi: 10.1038/mp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egerton A, Modinos G, Ferrera D, et al. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry. 2017;7:e1147. doi: 10.1038/tp.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marenco S, Meyer C, Kuo S, et al. Prefrontal GABA levels measured with magnetic resonance spectroscopy in patients with psychosis and unaffected siblings. Am J Psychiatry. 2016;173:527–34. doi: 10.1176/appi.ajp.2015.15020190. [DOI] [PubMed] [Google Scholar]
- 10.Marsman A, Mandl RC, Klomp DW, et al. GABA and glutamate in schizophrenia: a 7 T 1 H-MRS study. Neuroimage Clin. 2014;6:398–407. doi: 10.1016/j.nicl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–104. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland LM, Krause BW, Wijtenburg SA, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21:198–204. doi: 10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–59. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 14.Öngür D, Prescot AP, McCarthy J, et al. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–70. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhilei Y, Yajing Z, Zhenhua S, Li M, et al. Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls. Shanghai Jingshen Yixue. 2015;27:341. doi: 10.11919/j.issn.1002-0829.215130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandt AS, Unschuld PG, Pradhan S, et al. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: a 1 H MRS Study at 7Tesla. Schizophr Res. 2016;172:101–5. doi: 10.1016/j.schres.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto N, Yoshimura R, Kakeda S, et al. No alterations of brain GABA after 6 months of treatment with atypical antipsychotic drugs in early-stage first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1480–3. doi: 10.1016/j.pnpbp.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Tayoshi S, Nakataki M, Sumitani S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 19.De la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, et al. Prefrontal and striatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol Psychiatry. 2018;83:475–83. doi: 10.1016/j.biopsych.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Tang Y, Zhang T, et al. Reduced γ-aminobutyric acid and glutamate+glutamine levels in drug-naïve patients with first-episode schizophrenia but not in those at ultrahigh risk. Neural Plasticity. 2016;2016 doi: 10.1155/2016/3915703. 3915703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Modinos G, Simsek F, Horder J, et al. Cortical GABA in subjects at ultra-high risk of psychosis: relationship to negative prodromal symptoms. Int J Neuropsychopharmacol. 2018;21:114–19. doi: 10.1093/ijnp/pyx076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Fuente-Sandoval C, Reyes-Madrigal F, Mao X, et al. Corticostriatal GABAergic and glutamatergic dysregulations in subjects at ultra-high risk for psychosis investigated with proton magnetic resonance spectroscopy. Int J Neuropsychopharmacol. 2015;19(3):pyv105. doi: 10.1093/ijnp/pyv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–9. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen M-K, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JA, Das A, Ray SK, et al. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venneti S, Wiley CA, Kofler J. Imaging microglial activation during neuroinflammation and Alzheimer’s disease. J Neuroimmune Pharmacol. 2009;4:227–43. doi: 10.1007/s11481-008-9142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coughlin JM, Wang Y, Ambinder E, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C] DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6:e777. doi: 10.1038/tp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafizi S, Tseng H-H, Rao N, et al. Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F] FEPPA. Am J Psychiatry. 2017;174:118–124. doi: 10.1176/appi.ajp.2016.16020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenk M, Selvanathan T, Rao N, et al. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull. 2015;41:85–93. doi: 10.1093/schbul/sbu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takano A, Arakawa R, Ito H, et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C] DAA1106. Int J Neuropsychopharmacol. 2010;13:943–50. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- 32.Collste K, Plavén-Sigray P, Fatouros-Bergman H, et al. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C]PBR28. Mol Psychiatry. 2017;22:850–6. doi: 10.1038/mp.2016.247. [DOI] [PubMed] [Google Scholar]
- 33.Plavén-Sigray P, Matheson GJ, Collste K, et al. Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: a meta-analysis using individual participant data. Biol Psychiatry. 2018;84:433–42. doi: 10.1016/j.biopsych.2018.02.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doorduin J, De Vries EF, Willemsen AT, et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–7. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 35.Holmes SE, Hinz R, Drake R, et al. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: a [11C](R)-PK11195 positron emission tomography study. Mol Psychiatry. 2016;21:1672–9. doi: 10.1038/mp.2016.180. [DOI] [PubMed] [Google Scholar]
- 36.Van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C] PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–2. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Vivash L, O’Brien TJ. Imaging microglial activation with TSPO PET: lighting up neurologic diseases? J Nucl Med. 2016;57:165–8. doi: 10.2967/jnumed.114.141713. [DOI] [PubMed] [Google Scholar]
- 38.Bloomfield PS, Selvaraj S, Veronese M, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hafizi S, Da Silva T, Gerritsen C, et al. Imaging microglial activation in individuals at clinical high risk for psychosis: an in-vivo PET study with [(18)F] FEPPA. Neuropsychopharmacology. 2017;42:2474–81. doi: 10.1038/npp.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley T, Cryan JF, Downer EJ, et al. Inhibiting neuroinflammation: the role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav Immun. 2016;54:260–77. doi: 10.1016/j.bbi.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Fatemi SH, Reutiman TJ, Folsom TD, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovanoli S, Weber L, Meyer U. Single and combined effects of prenatal immune activation and peripubertal stress on parvalbumin and reelin expression in the hippocampal formation. Brain Behav Immun. 2014;40:48–54. doi: 10.1016/j.bbi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Meyer U, Nyffeler M, Yee BK, et al. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–86. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Lee M, Schwab C, Mcgeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–65. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Huang D, Xu J, et al. Tiagabine protects dopaminergic neurons against neurotoxins by inhibiting microglial activation. Sci Rep. 2015;5:15720. doi: 10.1038/srep15720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song D-K, Suh H-W, Huh S-O, et al. Central GABAA and GABAB receptor modulation of basal and stress-induced plasma interleukin-6 levels in mice. J Pharmacol Exp Ther. 1998;287:144–9. [PubMed] [Google Scholar]
- 47.Fontainhas AM, Wang M, Liang KJ, et al. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charles KJ, Deuchars J, Davies C, et al. GABA B receptor subunit expression in glia. Mol Cell Neurosci. 2003;24:214–23. doi: 10.1016/s1044-7431(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 49.Kuhn SA, van Landeghem FK, Zacharias R, et al. Microglia express GABA B receptors to modulate interleukin release. Mol Cell Neurosci. 2004;25:312–22. doi: 10.1016/j.mcn.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Chaparro-Huerta V, Rivera-Cervantes M, Flores-Soto M, et al. Proinflammatory cytokines and apoptosis following glutamateinduced excitotoxicity mediated by p38 MAPK in the hippocampus of neonatal rats. J Neuroimmunol. 2005;165:53–62. doi: 10.1016/j.jneuroim.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 51.Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 53.First MB, Spitzer RL, Gibbon M, et al. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition: SCIDI/P. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 54.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 55.Chen C-MA, Stanford AD, Mao X, et al. GABA level, gamma oscillation, and working memory performance in schizophrenia. Neuroimage Clin. 2014;4:531–9. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geramita M, van der Veen JW, Barnett AS, et al. Reproducibility of prefrontal γ-aminobutyric acid measurements with J-edited spectroscopy. NMR Biomed. 2011;24:1089–98. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- 57.Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998;11:266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 58.Sailasuta N, LeRoux P, Hurd R, et al. Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3 T. Proc Intl Soc Magn Reson Med. 2001;9:1011. [Google Scholar]
- 59.Shungu DC, Mao X, Gonzales R, et al. Brain γ-aminobutyric acid (GABA) detection in vivo with the J-editing 1H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test–retest reliability. NMR Biomed. 2016;29:932–42. doi: 10.1002/nbm.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright SM, Wald LL. Theory and application of array coils in MR spectroscopy. NMR Biomed. 1997;10:394–410. doi: 10.1002/(sici)1099-1492(199712)10:8<394::aid-nbm494>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 61.Mikkelsen M, Barker PB, Bhattacharyya PK, et al. Big GABA: edited MR spectroscopy at 24 research sites. NeuroImage. 2017;159:32–45. doi: 10.1016/j.neuroimage.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai X, Edden RA, Gao F, et al. Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J Magn Reson Imaging. 2015;41:1326–31. doi: 10.1002/jmri.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edden RA, Puts NA, Harris AD, et al. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid — edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–52. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 66.Rusjan PM, Wilson AA, Bloomfield PM, et al. Quantitation of translocator protein binding in human brain with the novel radio-ligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab. 2011;31:1807–16. doi: 10.1038/jcbfm.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizrahi R, Rusjan PM, Kennedy J, et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [18F]-FEPPA. J Cereb Blood Flow Metab. 2012;32:968–72. doi: 10.1038/jcbfm.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suridjan I, Boileau I, Bagby M, et al. Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. J Psychiatr Res. 2012;46:890–7. doi: 10.1016/j.jpsychires.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Richetto J, Calabrese F, Meyer U, et al. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain Behav Immun. 2013;33:190–200. doi: 10.1016/j.bbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 71.Richetto J, Calabrese F, Riva MA, et al. Prenatal immune activation induces maturation-dependent alterations in the prefrontal GABA-ergic transcriptome. Schizophr Bull. 2014;40:351–61. doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nouel D, Burt M, Zhang Y, et al. Prenatal exposure to bacterial endotoxin reduces the number of GAD67-and reelin-immunoreactive neurons in the hippocampus of rat offspring. Eur Neuropsychopharmacol. 2012;22:300–7. doi: 10.1016/j.euroneuro.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Novais A, Crouzin N, Cavalier M, et al. Tiagabine improves hippocampal long-term depression in rat pups subjected to prenatal inflammation. PLoS One. 2014;9:e106302. doi: 10.1371/journal.pone.0106302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gruol DL, Vo K, Bray JG. Increased astrocyte expression of IL-6 or CCL2 in transgenic mice alters levels of hippocampal and cerebellar proteins. Front Cell Neurosci. 2014;8:234. doi: 10.3389/fncel.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, et al. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–78. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samland H, Huitron-Resendiz S, Masliah E, et al. Profound increase in sensitivity to glutamatergic-but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73:176–87. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- 77.Aufhaus E, Weber-Fahr W, Sack M, et al. Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: a MEGA-PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med. 2013;69:317–20. doi: 10.1002/mrm.24257. [DOI] [PubMed] [Google Scholar]
- 78.Kegeles LS, Mao X, Gonsalez R, et al. Evaluation of anatomic variation in macromolecule contribution to the GABA signal using metabolite nulling and the J-editing technique at 3.0 T. Proc Intl Soc Mag Reson Med. 2007;15:1391. [Google Scholar]
- 79.Rizzo G, Veronese M, Tonietto M, et al. Kinetic modeling without accounting for the vascular component impairs the quantification of [11C]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014;34:1060–9. doi: 10.1038/jcbfm.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hannestad J, Gallezot J-D, Schafbauer T, et al. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012;63:232–9. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hillmer AT, Sandiego C, Hannestad J, et al. In vivo imaging of translocator protein, a marker of activated microglia, in alcohol dependence. Mol Psychiatry. 2017;22:1759. doi: 10.1038/mp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mirzaei N, Tang SP, Ashworth S, et al. In vivo imaging of microglial activation by positron emission tomography with [11C]PBR28 in the 5XFAD model of Alzheimer’s disease. Glia. 2016;64:993–1006. doi: 10.1002/glia.22978. [DOI] [PubMed] [Google Scholar]
- 83.Sandiego CM, Gallezot J-D, Pittman B, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci U S A. 2015;112:12468–73. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Notter T, Coughlin JM, Gschwind T, et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry. 2018;23:323. doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- 85.Hines CS, Fujita M, Zoghbi SS, et al. Propofol decreases in vivo binding of 11C-PBR28 to translocator protein (18 kDa) in the human brain. J Nucl Med. 2013;54:64–9. doi: 10.2967/jnumed.112.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ajram LA, Horder J, Mendez M, et al. Shifting brain inhibitory balance and connectivity of the prefrontal cortex of adults with autism spectrum disorder. Transl Psychiatry. 2017;7:e1137. doi: 10.1038/tp.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]