Abstract
Background
Striatal dysfunction has been proposed as a pathomechanism for negative symptoms in schizophrenia. There is consensus that negative symptoms can be grouped into 2 dimensions: apathy and diminished expression. Recent studies suggest that different neural mechanisms underlie these dimensions, but the relationship between regional resting-state cerebral blood flow (rCBF) and negative symptom dimensions has not been investigated.
Methods
This study included 29 patients with schizophrenia and 20 healthy controls. We measured rCBF in the striatum using arterial spin labelling (ASL) MRI. We assessed negative symptoms using the Brief Negative Symptom Scale.
Results
In the ventral and dorsal striatum, rCBF was not different between patients with schizophrenia and controls. However, we did find a positive association between the severity of apathy and increased rCBF in the ventral and dorsal striatum in patients with schizophrenia. This effect was not present for diminished expression.
Limitations
All patients were taking atypical antipsychotics, so an effect of antipsychotic medication on rCBF could not be excluded, although we did not find a significant association between rCBF and chlorpromazine equivalents.
Conclusion
The main finding of this study was a specific association between increased striatal rCBF and the negative symptom dimension of apathy. Our results further support the separate assessment of apathy and diminished expression when investigating the neural basis of negative symptoms. The ASL technique can provide a direct and quantitative approach to investigating the role of rCBF changes in the pathophysiology of negative symptoms.
Introduction
For decades, it has been hypothesized that striatal dysfunction is a fundamental mechanism underlying symptoms of schizophrenia.1 Robust findings in the literature have shown increased striatal dopamine synthesis in schizophrenia as measured by positron emission tomography (PET) and single-photon emission computed tomography (SPECT).2,3 Studies using functional MRI (fMRI) to assess striatal response to rewards have shown a decreased signal in unmedicated patients with schizophrenia.4–6 Results in medicated patients have been heterogeneous,7–9 indicating a complex relationship between dopamine dysregulation and fMRI findings.
More recently, it has been suggested that an increase in dopamine turnover could be accompanied by an increased perfusion of striatal areas.10,11 Arterial spin labelling (ASL) imaging allows for an absolute measure of regional cerebral blood flow (rCBF). Previous studies have suggested an increase in striatal rCBF in patients with schizophrenia11–14 and at a high risk for psychosis,15 but these findings were not fully consistent;11,16–18 further research is clearly needed.
Schizophrenia is a disorder with heterogeneous symptom expression along its course, and negative symptoms have a strong effect on long-term morbidity and poor functional outcome.19,20 There is now consensus that negative symptoms can be divided into 2 dimensions:21–23 apathy, which consists of anhedonia, avolition and asociality, and diminished expression, which combines the symptoms of blunted affect and alogia. In fMRI studies, an association between ventral striatal hypoactivation and negative symptoms (in particular, apathy) has repeatedly been reported.8,24–26 A recent study also found dorsal striatal hypoactivation in response to reward and apathy.27
These fMRI results do not reflect absolute hypoactivation in the striatum; rather, they represent a decreased signal difference between rewarding and nonrewarding stimuli. Therefore, the absolute measure of rCBF provided by ASL can offer additional information about the neural basis of symptoms. Most studies investigating ASL have not focused specifically on the striatum; they have used whole-brain analysis.12,14,28 Nevertheless, a few have reported associations between striatal rCBF and symptoms. Kindler and colleagues11 showed a positive correlation between striatal rCBF and positive symptoms in patients with treatment-resistant auditory hallucinations. Zhuo and colleagues16 found increased rCBF in striatal and auditory areas in patients with auditory verbal hallucinations.
In addition to these ASL studies on rCBF in the striatum, earlier PET studies have reported an association between negative symptoms and reduced rCBF at rest and during an attentional task in frontal and parietal regions.29–31 However, these studies did not address the distinction between apathy and diminished expression. Liemburg and colleagues32 found apathy to be related to abnormal activation in parietal and thalamic regions during a planning task, but did not specifically investigate striatal rCBF. Overall, negative symptoms seem to be associated with reduced rCBF, particularly in frontal regions, but regional specificity has yet to be determined.
The main goal of this study was to investigate the association between striatal rCBF and negative symptoms. There is evidence that apathy and diminished expression show different associations with behavioural33,34 and neurobiological correlates, suggesting differences in pathophysiology. Therefore, this distinction is of high relevance when investigating striatal rCBF. The paucity of reported associations between striatal rCBF and negative symptoms could result from the fact that until now apathy and diminished expression have not been addressed separately, even though the striatum might play very different roles in their pathophysiology.
Based on the extant (albeit limited) evidence for increased striatal resting-state rCBF in patients with schizophrenia,24,26 our first hypothesis was that these patients would show increased rCBF in the ventral and dorsal striatum compared with controls. Our second and main hypothesis was that apathy would be associated with altered rCBF in the ventral and dorsal striatum. Because no study has previously reported striatal rCBF in relation to specific dimensions of negative symptoms, we could not make predictions about the directionality of the effects.
Methods
Participants
Twenty-nine patients with schizophrenia and 20 healthy controls, matched at a group level for age and sex, were included in the present study. We recruited patients from outpatient and inpatient units of the Psychiatric Hospital of the University of Zurich and affiliated institutions. We recruited healthy controls from the community via advertisement.
We conducted the Mini-International Neuropsychiatric Interview to confirm diagnosis.35 Patients were clinically stable and had been on a stable dose of medication for at least 2 weeks before testing. Inpatients were at the end of their hospitalization, engaging in a multimodal therapy program and activities outside the hospital. The average duration of hospitalization for patients with schizophrenia in Switzerland is longer than in most other countries, so the majority of patients would have been treated as outpatients in other health care systems.
Exclusion criteria for patients were any other DSM-IV axis I disorder; acute psychotic symptoms (i.e., scores higher than 4 on the positive subscale of the Positive and Negative Syndrome Scale [PANSS]36); extrapyramidal adverse effects (i.e., a total score higher than 2 on the Modified Simpson–Angus Scale [MSAS]37); and lorazepam dosage higher than 1 mg/d. If patients met the criteria for cannabis abuse or dependency, they were also excluded from the study. Participants were excluded if they had any alcohol use disorder based on lifetime criteria. Smoking was not an exclusion criterion, but participants did not smoke for 2 hours before the ASL scans. Controls were excluded if any neuropsychiatric diagnosis was present in the structured Mini-International Neuropsychiatric Interview.35 Any participants with a neurologic disorder were excluded.
The Ethics Committee of the Canton of Zurich approved the project, and participants gave written informed consent to participate in the study. The ability of each participant with schizophrenia to provide informed consent was evaluated by the treating psychiatrist.
Clinical and neuropsychological assessment
We assessed negative symptoms using the Brief Negative Symptom Scale.38 We calculated the 2 dimensions of negative symptoms as follows: the apathy dimension consisted of the anhedonia, avolition and asociality items; the diminished expression dimension included the blunted affect and alogia items.39 Other assessment instruments used were the PANSS,36 the Calgary Depression Scale for Schizophrenia,40 the Global Assessment of Functioning scale41 and the Personal and Social Performance scale.42 We assessed cognition using a brief neurocognitive test battery (see our previous studies33,34 for details) to compute a composite cognitive ability score for each participant. The following domains were included in the battery: verbal learning, verbal and visual short-term and working memory, processing speed, planning, and semantic and phonemic fluency.
MRI data acquisition
We acquired MRI data on a Philips Achieva 3.0 T wholebody scanner (Best). We employed resting-state pseudocontinuous ASL (pCASL) perfusion-weighted scans. Owing to superior signal-to-noise ratio, pCASL is considered to be a more reliable method than other ASL sequences.43,44 We based the imaging parameters for pCASL on the sequence developed by Dai and colleagues.45 The plane was positioned parallel to the imaging volume, with a 20 mm labelling gap between the imaging volume and the labelling volume. The ASL parameters for the single-shot, gradient-echo, echo planar imaging sequence were as follows: repetition time 4400 ms, echo time 20 ms, flip angle 90°, field of view 240 × 161 × 240 mm, spacing 3 mm, matrix size 80 × 80, 23 slices with a slice thickness of 7 mm and no gap, SENSE 2.5, postlabelling delay 1525 ms, label duration 1650 ms, number of dynamics 75 (duration 667.9 s). One dynamic consisted of a control and a labelled image. We also acquired high-resolution anatomic images (repetition time 8.1 ms, echo time 3.7 ms, field of view 240 × 240 mm2, voxel size 1 × 1 × 1 mm) using a standard T1-weighted 3D magnetizationprepared rapid gradient echo sequence.
Calculation of cerebral blood flow
We performed image data processing and analysis using the ASLtoolbox46 running in MATLAB (MathWorks, Inc.) and compatible with SPM12 statistical parametric mapping software (Wellcome Trust Centre for Neuroimaging, implemented in MATLAB). For each participant, we conducted image preprocessing, including independent realignment for labelled and unlabelled images, spatial smoothing (6 × 6 × 14 mm kernel), perfusion-weighted image construction and calculation, and normalization to the Montreal Neurological Institute template (for ASL data, rCBF calculations should be performed before spatial normalization46). We recorded equilibrium brain tissue magnetization (M0) images in a separate run for each participant using the same parameters as for the pCASL sequence, apart from repetition time (10 s). Next, we calculated unique cerebral spinal fluid M0 values per participant for each session (corrected for T2* decay using a T2* value of 74.9 ms); we took the relevant H2O partition coefficient from the literature47 and considered it in the calculation of each perfusion-weighted image. We generated perfusion-weighted image series by simple subtraction of the label and control images, and then conversion to absolute mean rCBF image series.
Region-of-interest image analysis
We derived predefined regions of interest (ROIs) for the ventral and dorsal striatum from previous key publications that used fMRI (Fig. 1). Yip and colleagues48 defined ROI coordinates (Montreal Neurological Institute) for the ventral striatum according to a meta-analysis by Knutson and Greer49 (left: x = −12, y = 10, z = −2; right: x = 10, y = 8, z = 0; both 9 mm spheres); we have also used these coordinates in previous studies.25,50 We also adopted coordinates for the dorsal striatum ROI from Yip and colleagues48 (left: x = −9, y = 3, z = 15; right: x = 9, y = 3, z = 15; both 9 mm spheres). We generated the ROIs using the Wake Forest University Toolbox.51 For each ROI (ventral or dorsal striatum) we extracted mean rCBF using the MarsBaR toolbox (http://marsbar.sourceforge.net).
Fig. 1.
Regions of interest of the (A) ventral and (B) dorsal striatum.
To compare the mean cortical (grey matter masked) cerebral blood flow (CBF) between groups, we extracted the mean CBF for each group from 90 cortical brain regions (AAL atlas, http://neuro.imm.dtu.dk/wiki/Automated_Anatomical_Labeling) and applied an unpaired 2-tailed t-test.
Statistical analysis
We conducted statistical analyses using IBM SPSS Statistics version 22. We tested demographic comparisons using a χ2 test, t tests and Mann–Whitney U tests if the criterion of normal distribution was not met. To test our first hypothesis, we calculated t tests for group comparisons of rCBF between patients and controls for each ROI (ventral and dorsal striatum). We confirmed normal distribution with a Shapiro–Wilk test, and tested homogeneity of variance using a Levene test. Both assumptions were met in the current sample. We also calculated analyses of covariance to control for potential sociodemographic differences between the patient and control groups.
To test the main hypothesis, we calculated Spearman correlation coefficients (rs) between negative symptoms (apathy and diminished expression) and rCBF in the ventral and dorsal striatum. We used a Steiger z test to calculate the difference between the 2 negative symptom dimensions as dependent variables and rCBF as the common independent variable.
To address potentially confounding factors in the patient group, we calculated Spearman correlation coefficients between rCBF in the ventral and dorsal striatum and age, positive symptoms, chlorpromazine equivalents, depressive symptoms and cognitive impairments via the composite cognitive ability score. Only age showed a significant association with rCBF, as well as apathy. Therefore, we calculated non-parametric partial correlations to account for the effects of age on the correlation between negative symptoms and rCBF.
All primary analyses described above related to bilateral striatal regions; we had no a priori hypotheses about differences between the left and right striatum.
Results
Demographic and clinical characteristics
Demographic and clinical characteristics are summarized in Table 1. The groups showed no significant differences with respect to age or sex. Controls had a higher educational level and higher cognitive scores.
Table 1.
Demographic, psychopathological and clinical characteristics of study participants
| Characteristic | Patients, mean ± SD* n = 29† |
Healthy controls, mean ± SD* n = 20 |
Statistical test‡ | p value |
|---|---|---|---|---|
| Age, yr | 27.7 ± 7.2 | 30.6 ± 6.6 | t47 = 1.45 | 0.15 |
| Sex, female:male | 7:22 | 6:14 | χ21 = 0.21 | 0.65 |
| Education, yr | 11.4 ± 2.8 | 14.2 ± 2.5 | U = 101.5 | ≤ 0.001 |
| Smoking, pack-years | 5.7 ± 13.7 | 2.4 ± 4.8 | U = 239.5 | 0.25 |
| Duration of illness, yr | 7.2 ± 7.1 | — | — | — |
| Age of onset, yr | 21.0 ± 4.8 | — | — | — |
| Chlorpromazine equivalents, mg/d | 497.7 ± 407.4 | — | — | — |
| BNSS score | ||||
| Apathy (motivation and pleasure) | 15.5 ± 6.9 | — | — | — |
| Diminished expression | 8.6 ± 7.0 | — | — | — |
| SANS score§ | ||||
| Apathy | 11.9 ± 5.6 | — | — | — |
| Diminished expression | 10.4 ± 9.6 | — | — | — |
| PANSS score¶ | ||||
| Positive | 6.4 ± 2.5 | — | — | — |
| Negative | 12.0 ± 5.2 | — | — | — |
| Disorganized | 4.7 ± 2.0 | — | — | — |
| Excited | 4.5 ± 0.7 | — | — | — |
| Depressed | 5.4 ± 2.4 | — | — | — |
| Total | 47.7 ± 10.2 | — | — | — |
| CDSS total score | 2.1 ± 2.5 | — | — | — |
| GAF score | 58.1 ± 9.6 | — | — | — |
| PSP total score | 58.2 ± 9.1 | — | — | — |
| Cognition (composite cognitive ability)** | −0.85 ± 0.80 | 0 ± 0.58 | t47 = 4.07 | ≤ 0.001 |
| MWT IQ | 24.5 ± 6.0 | 28.7 ± 2.8 | t47 = 2.89 | 0.006 |
BNSS = Brief Negative Symptom Scale; CDSS = Calgary Depression Scale for Schizophrenia; GAF = Global Assessment of Functioning; MWT IQ = Multiple Word Test Intelligence Quotient; PANSS = Positive and Negative Syndrome Scale; PSP = Personal and Social Performance scale; SANS = Scale for the Assessment of Negative Symptoms; SD = standard deviation.
Unless otherwise indicated.
All patients were receiving atypical antipsychotics at the time of testing.
We investigated potential group differences using 2-sample t tests for continuous data and χ2 tests for categorical data. For data with non-normal distribution, we applied Mann–Whitney U tests.
Apathy includes avolition/apathy and anhedonia/asociality; diminished expression includes affective flattening or blunting and alogia.
Positive factor = P1, P3, P5, P9; negative factor = N1, N2, N3, N4, N6, G7; disorganized factor = P2, G5, N11; excited factor = P4, P7, G8, G14; depressed factor = G2, G3, G6.
Cognition data have been z-transformed based on the data of the control group for each test separately. The composite cognitive ability score was computed as the mean of the z-transformed test scores at the participant level.
Patients took the following antipsychotic monotherapies: clozapine (2 patients), olanzapine (1 patient), quetiapine (2 patients), amisulpride (2 patients), risperidone (7 patients), paliperidone (2 patients) and aripiprazole (2 patients). Several patients took a combination of antipsychotic medications: clozapine and aripiprazole (1 patient), clozapine and amisulpride (1 patient), olanzapine and aripiprazole (1 patient), olanzapine and quetiapine (1 patient), olanzapine and risperidone (1 patient), amisulpride and quetiapine (1 patient), risperidone and quetiapine (3 patients), risperidone and aripiprazole (1 patient), and aripiprazole and quetiapine (1 patient).
CBF differences between groups
To test our first hypothesis, we compared rCBF in the ventral and dorsal striatum between groups and found that patients and controls did not differ significantly (Table 2). Thus, we could not confirm our hypothesis that patients with schizophrenia would show altered rCBF in the striatum.
Table 2.
Mean rCBF values by region of interest and group
| Region of interest | Patients, mean ± SD | Healthy controls, mean ± SD | Statistical test | p value |
|---|---|---|---|---|
| Ventral striatum | 33.8 ± 7.9 mL/100 g/min | 31.5 ± 9.3 mL/100 g/min | t47 = −0.91 | 0.37 |
| Dorsal striatum | 28.4 ± 9.1 mL/100 g/min | 26.1 ± 7.8 mL/100 g/min | t47 = −0.95 | 0.35 |
rCBF = regional cerebral blood flow; SD = standard deviation.
After we controlled for educational level and cognitive score, patients with schizophrenia showed a trend toward higher rCBF in the ventral striatum (F1,45 = 3.37, p = 0.07), but not in the dorsal striatum (F1,45 = 1.89, p = 0.18).
To control for differences in total grey matter CBF between patients (mean ± SD 41.4 ± 8.8 mL/100 mg/min) and controls (mean ± SD 41.7 ± 8.7 mL/100 mg/min) we used a t test, which showed no significant differences between groups (t47 = 0.11, p = 0.91). We also addressed potential differences in total grey matter volume between patients (mean ± SD 671.8 ± 51.4 mm3) and controls (mean ± SD 677.7 ± 46.23 mm3), and found no significant differences between groups (t47 = 0.41, p = 0.68).
No participants were excluded because of excessive motion. Patients and controls did not differ significantly with respect to motion parameters.
Correlation between CBF and negative symptoms
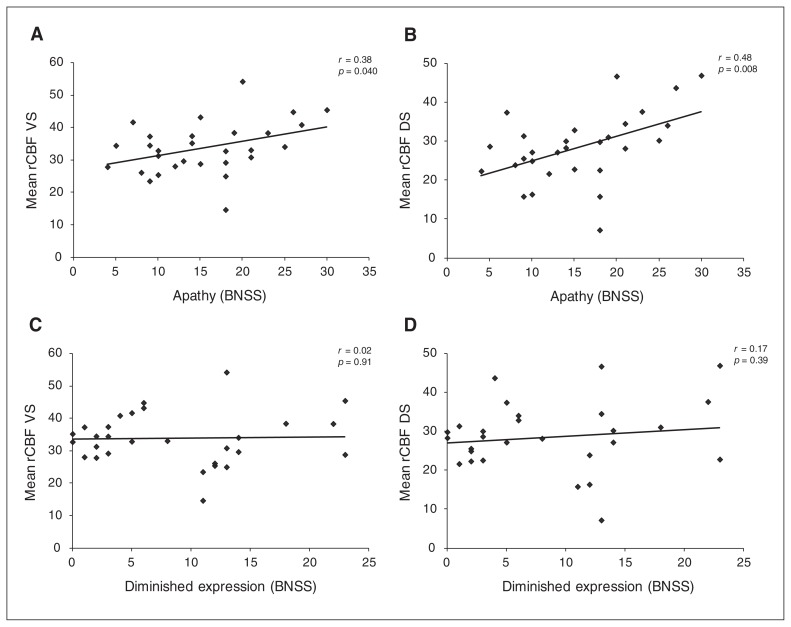
To test our main hypothesis, we calculated Spearman correlations between rCBF in the striatum and the 2 negative symptom dimensions in the patient group (Table 3). We found a significant positive correlation between ventral striatal rCBF and apathy (Fig. 2a and Table 3), and between dorsal striatal rCBF and apathy (Fig. 2b and Table 3). This finding provides evidence that patients with more apathy show higher rCBF in the striatum. We found no significant correlation between ventral striatal rCBF and diminished expression (Fig. 2c and Table 3) or between dorsal striatal rCBF and diminished expression (Fig. 2d and Table 3). The results of the Steiger z test were nearly significant (ventral striatum: z = 1.88, p = 0.06; dorsal striatum: z = 1.73, p = 0.08). In other words, the correlation between rCBF and apathy was stronger than that between rCBF and diminished expression. For the Brief Negative Symptom Scale total score, we observed a trend-level correlation with rCBF in the dorsal striatum (r27 = 0.34, p = 0.07), but not in the ventral striatum (r27 = 0.20, p = 0.29).
Table 3.
Spearman correlations between apathy and diminished expression and the left and right ventral and dorsal striatum in patients with schizophrenia
| Mean rCBF | Apathy | Diminished expression | ||
|---|---|---|---|---|
|
|
|
|||
| r27 | p value | r27 | p value | |
| Left and right ventral striatum | 0.38 | 0.040 | 0.02 | 0.91 |
| Left and right dorsal striatum | 0.48 | 0.008 | 0.17 | 0.39 |
| Left ventral striatum | 0.32 | 0.09 | 0.009 | 0.96 |
| Right ventral striatum | 0.40 | 0.030 | 0.003 | 0.99 |
| Left dorsal striatum | 0.44 | 0.017 | 0.19 | 0.32 |
| Right dorsal striatum | 0.51 | 0.005 | 0.10 | 0.61 |
rCBF = regional cerebral blood flow.
Fig. 2.
Spearman correlation, including significance test, of (A) mean rCBF of the left and right ventral striatum with apathy; (B) mean rCBF of the left and right dorsal striatum with apathy; (C) mean rCBF of the left and right ventral striatum with diminished expression; (D) mean rCBF of the left and right dorsal striatum with diminished expression. BNSS = Brief Negative Symptom Scale; DS = dorsal striatum; rCBF = regional cerebral blood flow; VS = ventral striatum.
We found that rCBF did not correlate significantly with the following potentially confounding variables: PANSS positive factor, Calgary Depression Scale for Schizophrenia for depressive symptoms, chlorpromazine equivalents and composite cognitive ability score (Table 4). However, we did find significant positive correlations between age and rCBF of the ventral and dorsal striatum. Therefore, we calculated non-parametric partial correlations to control for the effects of age on the correlation of rCBF with apathy. The association between apathy and rCBF in the dorsal striatum was at trend level (r26 = 0.34, p = 0.08), and the association with the ventral striatum was no longer significant (r26 = 0.25, p = 0.19).
Table 4.
Spearman correlations between striatal rCBF, confounding variables and PANSS negative factor scores in patients with schizophrenia
| Correlation factor | Mean rCBF ventral striatum | Mean rCBF dorsal striatum | ||
|---|---|---|---|---|
|
|
|
|||
| r27 | p value | r27 | p value | |
| PANSS positive factor | −0.15 | 0.44 | −0.24 | 0.21 |
| PANSS negative factor | 0.17 | 0.39 | 0.30 | 0.12 |
| CDSS total score | 0.23 | 0.24 | 0.35 | 0.06 |
| Chlorpromazine equivalents | −0.05 | 0.80 | −0.17 | 0.39 |
| Composite cognitive ability score | 0.27 | 0.17 | 0.11 | 0.58 |
| Age | −0.39 | 0.04 | −0.50 | 0.006 |
CDSS = Calgary Depression Scale for Schizophrenia; PANSS = Positive and Negative Syndrome Scale; rCBF = regional cerebral blood flow.
In an exploratory analysis, we evaluated the associations with apathy and diminished expression in the left and right striatum, and found the same pattern as in the bilateral analysis (Table 3).
We also performed an exploratory voxel-wise analysis of rCBF in the prefrontal cortex and anterior cingulate cortex. The statistical threshold was set at a peak-level family-wise error rate correction of p = 0.05. No voxels were significantly associated with apathy in the patient group.
Discussion
We observed no significant differences in striatal rCBF between patients with schizophrenia and healthy controls. Importantly, apathy — but not diminished expression — was associated with dorsal striatal rCBF and (to a lesser extent) ventral striatal rCBF.
For this reason, our first hypothesis concerning group differences could not be confirmed. This was at odds with some studies, which reported increased striatal rCBF in patients with schizophrenia,11,12,14,16 but other studies did not observe these effects.17,18 Potential explanations for these differences between studies include variations in image acquisition and data analysis. Most importantly, the patient populations differed in numerous ways. In our study, we specified inclusion and exclusion criteria to assess primarily for negative symptoms, so our patients had low levels of positive symptoms. In contrast, patients in the study by Kindler and colleagues11 had treatment-resistant auditory hallucinations. Another important factor might be the type of antipsychotic medication used for treatment.14,52,53 In our study, all patients were treated with atypical antipsychotic medication, which in fMRI studies has been shown to attenuate group differences in striatal activation.4,8,25
Regarding our main hypothesis, we found a significant association between the severity of apathy and rCBF in both the ventral and dorsal striatum, a relationship that we did not find for diminished expression. This differential effect for the 2 negative symptom dimensions might account at least in part for the lack of consistent previous findings for the rCBF correlates of negative symptoms. In our study, an aggregation of overall negative symptoms would have led to a nonsignificant finding. The only other ASL study that specifically assessed apathy evaluated rCBF during planning-task performance, during which the authors observed reduced parietal and thalamic perfusion.32 However, they did not report striatal perfusion, and comparison with our resting-state approach is difficult. It seems to be important for future ASL studies to assess both dimensions of negative symptoms separately, because different neural mechanisms may underlie these symptoms.21,33,54
While the distinction between apathy and diminished expression (the 2 negative symptom dimensions) has received very limited interest in previous ASL studies, the blood-oxygen-level–dependent (BOLD) fMRI literature has provided evidence for dissociation of their neural correlates. For instance, Kirschner and colleagues25 reported reduced activity in the ventral striatum during reward anticipation that correlated with apathy, but not with diminished expression. For the dorsal striatum, an association between reduced activity and avolition — but not anhedonia — has been shown. Importantly, reduced activity in these fMRI studies reflects attenuated signal differences between rewarding and nonrewarding stimuli. Therefore, an association of apathy with both a reduced task-related fMRI signal and increased resting-state rCBF in the striatum is not contradictory.
At this point, a mechanistic explanation for the association of apathy with striatal rCBF remains speculative. Several studies have found increased rCBF in the striatum to be related to higher dopaminergic activity.55,56 In addition, a PET study reported an association between increased dorsal striatal dopamine release and negative symptoms,57 which might seem at odds with the observation that decreased dopamine availability can lead to apathy in neurologic patients and in animal models.57–59 However, the hypothesis of aberrant salience attribution in schizophrenia proposes that increased dopaminergic activity in the striatum leads to difficulties in distinguishing between relevant and irrelevant stimuli.54,60 This model has also been employed to account for the attenuated striatal reward signal in fMRI studies that has been associated with negative symptoms.61 Thus, this inability to differentiate relevant and (in particular) rewarding stimuli could lead to a decrease in goal-directed behaviour and promote apathy.62
We found no significant correlation between positive symptoms or cognition and striatal rCBF. For positive symptoms, it needs to be kept in mind that the aim of the study was to investigate the neural correlates of negative symptoms, and patients with significant positive symptoms were excluded, considerably reducing variance. Cognitive deficits were not an exclusion criterion, but patients had to be able to take part in this relatively demanding study, and the overall cognitive performance of the patient group was less than 1 standard deviation below the control group.
Surprisingly, we did not find a significant group difference in rCBF of the striatum, despite the relationship between apathy and striatal rCBF. Striatal rCBF was slightly higher in patients than in controls, although this difference was not significant. This type of pattern can best be explained by a difference between patients and controls that is present only for patients with a high level of apathy. In addition, treatment with antipsychotics might have attenuated group differences in striatal rCBF to some extent.63
We observed that greater age was associated with reduced striatal rCBF and lower apathy, although effects of age have not been reported in previous studies of patients with schizophrenia. However, our finding was consistent with previous reports of reduced rCBF with increasing age in healthy individuals. 64,65 Interestingly, Bijanki and colleagues66 found a negative relationship between age and negative symptoms, as well as white-matter integrity measured by diffusion tensor imaging, emphasizing the need to include age as a confounding variable. Our finding that younger patients showed stronger apathy than older participants might seem surprising, but it was consistent with the study by Bijanki and colleagues. 66 Overall, the inclusion of age in partial correlations of apathy and striatal rCBF attenuated the association, but the effect in the dorsal striatum remained at trend level.
Limitations
This study provides evidence for a positive association between increased striatal rCBF and the negative symptom dimension of apathy. However, several limitations need to be taken into account in the interpretation of these findings. First, our sample size was moderate; these findings require replication in a larger sample, which would also allow for further evaluation of the effect of age on the observed associations. Second, our sample was recruited with the aim of investigating the neural correlates of negative symptoms, and is thus not representative of the entire population of patients with schizophrenia. Different associations between symptoms and striatal rCBF could be found in patients with higher levels of positive or depressive symptoms. Third, all patients in our study took second-generation antipsychotic medication. Previous research has suggested an influence of antipsychotic medication on striatal rCBF.52,63,67,68 While we did not observe an association between striatal rCBF and antipsychotic dose, we cannot exclude a potential effect of antipsychotic medication. Thus, future studies should include nonmedicated patients and patients taking first-generation antipsychotics to generalize the relationship between apathy and striatal activity to these populations.
Conclusion
The association between increased striatal rCBF and the negative symptom dimension of apathy, but not diminished expression, provides further evidence for the assumption of different underlying neural bases. These dimensions should be considered separately in future research on negative symptoms. Furthermore, ASL seems to provide a direct and quantitative technique for investigating negative symptoms, circumventing the limitations of task-based measures often employed for BOLD-fMRI and the invasiveness of PET and SPECT. This may qualify ASL as an alternative technique for developing biomarkers that reflect the pathomechanisms of negative symptoms.
Acknowledgements
This study was supported by the Swiss National Science Foundation (grant no. 105314_140351 to S. Kaiser). P.N. Tobler was supported by the Swiss National Science Foundation (PP00P1_128574, PP00P1_150739, CRSII3_141965 and 00014_165884). The authors thank M. Bischof for his support in data acquisition, A. Manoliu for his support with layout of the figures, and all patients and healthy volunteers for their participation.
Footnotes
Competing interests: P. Tobler has received grant support from Pfizer. E. Seifritz has received grant support from H. Lundbeck and has served as a consultant and/or speaker for AstraZeneca, Otsuka, Takeda, Eli Lilly, Janssen, Lundbeck, Novartis, Pfizer, Roche and Servier. S. Kaiser has received speaker honoraria from Roche, Lundbeck, Janssen and Takeda. He receives royalties for cognitive test and training software from Schuhfried. None of these activities is related to the present study. All other authors declare no biomedical financial interest or potential conflicts of interest.
Contributors: M. Kirschner, M.N. Hartmann-Riemer, E. Seifritz, P. Stämpfli, P.N. Tobler and S. Kaiser designed the study. M. Kirschner and M.N. Hartmann-Riemer acquired the data, which K. Schneider, L. Michels, M. Kirschner, A. Burrer and S. Kaiser analyzed. K. Schneider, L. Michels, E. Seifritz and S. Kaiser wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–86. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [18F/11C]-DOPA PET studies. Schizophr Bull. 2013;39:33–42. doi: 10.1093/schbul/sbr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen MØ, Rostrup E, Wulff S, et al. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol Psychiatry. 2012;71:898–905. doi: 10.1016/j.biopsych.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Schlagenhauf F, Huys QJM, Deserno L, et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–80. doi: 10.1016/j.neuroimage.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waltz JA, Schweitzer JB, Ross TJ, et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427–39. doi: 10.1038/npp.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlagenhauf F, Juckel G, Koslowski M, et al. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196:673–84. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- 9.Simon JJ, Biller A, Walther S, et al. Neural correlates of reward processing in schizophrenia — relationship to apathy and depression. Schizophr Res. 2010;118:154–61. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Sander CY, Hooker JM, Catana C, et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci U S A. 2013;110:11169–74. doi: 10.1073/pnas.1220512110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kindler J, Schultze-Lutter F, Hauf M. Increased striatal and reduced prefrontal cerebral blood flow in clinical high hisk for psychosis. Schizophr Bull. 2018;44:182–92. doi: 10.1093/schbul/sbx070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinkham A, Loughead J, Ruparel K, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194:64–72. doi: 10.1016/j.pscychresns.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L, Qin W, Zhuo C, et al. Combination of volume and perfusion parameters reveals different types of grey matter changes in schizophrenia. Sci Rep. 2017;7:435. doi: 10.1038/s41598-017-00352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Zhuo C, Qin W, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res. 2015;63:28–35. doi: 10.1016/j.jpsychires.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Allen P, Chaddock CA, Egerton A, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173:392–9. doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo C, Zhu J, Qin W, et al. Cerebral blood flow alterations specific to auditory verbal hallucinations in schizophrenia. Br J Psychiatry. 2017;210:209–15. doi: 10.1192/bjp.bp.115.174961. [DOI] [PubMed] [Google Scholar]
- 17.Ota M, Ishikawa M, Sato N, et al. Pseudo-continuous arterial spin labeling MRI study of schizophrenic patients. Schizophr Res. 2014;154:113–8. doi: 10.1016/j.schres.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Horn H, Federspiel A, Wirth M, et al. Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry. 2009;194:130–8. doi: 10.1192/bjp.bp.107.045633. [DOI] [PubMed] [Google Scholar]
- 19.Milev P, Ho B, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 20.McGlashan TH, Fenton WS. The positive-negative distinction in schizophrenia. Review of natural history validators. Arch Gen Psychiatry. 1992;49:63–72. doi: 10.1001/archpsyc.1992.01820010063008. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–45. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36:359–69. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messinger JW, Trémeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31:161–8. doi: 10.1016/j.cpr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radua J, Schmidt A, Borgwardt S, et al. Ventral striatal activation during reward processing in psychosis. JAMA Psychiatry. 2015;72:1243–51. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 25.Kirschner M, Hager OM, Bischof M, et al. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. J Psychiatry Neurosci. 2016;41:152–61. doi: 10.1503/jpn.140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187:222–8. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- 27.Mucci A, Dima D, Soricelli A, et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol Med. 2015;45:1765–78. doi: 10.1017/S0033291714002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guimarães TM, Machado-de-Sousa JP, Crippa JAS, et al. Arterial spin labeling in patients with schizophrenia: a systematic review. Arch Clin Psychiatry (São Paulo) 2016;43:151–6. [Google Scholar]
- 29.Lahti AC, Holcomb HH, Medoff DR, et al. Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am J Psychiatry. 2001;158:1797–808. doi: 10.1176/appi.ajp.158.11.1797. [DOI] [PubMed] [Google Scholar]
- 30.Lahti AC, Weiler MA, Holcomb HH, et al. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–30. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 31.Wang CS-M, Yang Y-K, Chen M, et al. Negative symptoms and regional cerebral blood flow in patients with schizophrenia: a single photon emission computed tomography study. Kaohsiung J Med Sci. 2003;19:464–9. doi: 10.1016/S1607-551X(09)70492-9. [DOI] [PubMed] [Google Scholar]
- 32.Liemburg EJ, Dlabac-De Lange JJLAS, Bais L, et al. Neural correlates of planning performance in patients with schizophrenia — relationship with apathy. Schizophr Res. 2015;161:367–75. doi: 10.1016/j.schres.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Hartmann MN, Hager OM, Reimann AV, et al. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr Bull. 2015;41:503–12. doi: 10.1093/schbul/sbu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann-Riemer MN, Hager OM, Kirschner M, et al. The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr Res. 2015;169:427–32. doi: 10.1016/j.schres.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 35.Lecrubier Y, Weiller E, Herugeta T. Mini International Neuropsychiatric Interview, German version 5.0.0. Munich: Psychiatrischen Universitätsklinik München; 1999. [Google Scholar]
- 36.Kay S, Opler L, Lindenmayer J. The positive and negative symptom scale (PANSS): rationale and standardisation. Br J Psychiatry. 1989;7:59–67. [PubMed] [Google Scholar]
- 37.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 38.Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–5. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strauss GP, Hong LE, Gold JM, et al. Factor structure of the brief negative symptom scale. Schizophr Res. 2012;142:96–8. doi: 10.1016/j.schres.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–51. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 41.Frances A, Pincus HA, First MB. The global assessment of functioning scale (GAF) Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- 42.Juckel G, Schaub D, Fuchs N, et al. Validation of the Personal and Social Performance (PSP) scale in a German sample of acutely ill patients with schizophrenia. Schizophr Res. 2008;104:287–93. doi: 10.1016/j.schres.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 43.Tancredi FB, Gauthier CJ, Madjar C, et al. Comparison of pulsed and pseudocontinuous arterial spin-labeling for measuring CO2-induced cerebrovascular reactivity. J Magn Reson Imaging. 2012;36:312–21. doi: 10.1002/jmri.23658. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, Wang DJJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011;33:940–9. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai W, Garcia DM, de Bazelaire C, et al. Continuous flow driven inversion for arterial spin labelling using pulsed radiofrequency and gradient fields. Magn Reson Med. 2008;60:1488–97. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–9. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herscovitch P, Raichle ME. What is the correct value for the brain–blood partition coefficient for water? J Cereb Blood Flow Metab. 1985;5:65–9. doi: 10.1038/jcbfm.1985.9. [DOI] [PubMed] [Google Scholar]
- 48.Yip SW, Worhunsky PD, Rogers RD, et al. Hypoactivation of the ventral and dorsal striatum during reward and loss anticipation in antipsychotic and mood stabilizer–naive bipolar disorder. Neuropsychopharmacology. 2015;40:658–66. doi: 10.1038/npp.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc B Biol Sci. 2008;363:3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kirschner M, Hager OM, Muff L, et al. Ventral striatal dysfunction and symptom expression in individuals with schizotypal personality traits and early psychosis. Schizophr Bull. 2018;44:147–57. doi: 10.1093/schbul/sbw142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 52.Lahti AC, Weiler MA, Holcomb HH, et al. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34:2675–90. doi: 10.1038/npp.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Handley R, Zelaya FO, Reinders AATS, et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272–82. doi: 10.1002/hbm.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strauss GP, Horan WP, Kirkpatrick B, et al. Deconstructing negative symptoms of schizophrenia: avolition — apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–90. doi: 10.1016/j.jpsychires.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohlin KE, Sebastianutto I, Adkins CE, et al. Impact of L-DOPA treatment on regional cerebral blood flow and metabolism in the basal ganglia in a rat model of Parkinson’s disease. Neuroimage. 2012;61:228–39. doi: 10.1016/j.neuroimage.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sander CY, Hooker JM, Catana C, et al. Imaging agonist-induced D2/D3 receptor desensitization and internalization in vivo with PET/fMRI. Neuropsychopharmacology. 2016;41:1427–36. doi: 10.1038/npp.2015.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 58.Salamone JD, Koychev I, Correa M, et al. Neurobiological basis of motivational deficits in psychopathology. Eur Neuropsychopharmacol. 2015;25:1225–38. doi: 10.1016/j.euroneuro.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 59.Pagonabarraga J, Kulisevsky J, Strafella AP, et al. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–31. doi: 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- 60.Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–85. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goozee R, Handley R, Kempton MJ, et al. A systematic review and meta-analysis of the effects of antipsychotic medications on regional cerebral blood flow (rCBF) in schizophrenia: association with response to treatment. Neurosci Biobehav Rev. 2014;43:118–36. doi: 10.1016/j.neubiorev.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 64.Parkes LM, Rashid W, Chard DT, et al. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–43. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Zhu X, Feinberg D, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2012;68:912–22. doi: 10.1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- 66.Bijanki KR, Hodis B, Magnotta VA, et al. Effects of age on white matter integrity and negative symptoms in schizophrenia. Schizophr Res. 2015;161:29–35. doi: 10.1016/j.schres.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lahti AC, Holcomb HH, Weiler MA, et al. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53:601–8. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- 68.Eisenberg DP, Yankowitz L, Ianni AM, et al. Presynaptic dopamine synthesis capacity in schizophrenia and striatal blood flow change during antipsychotic treatment and medication-free conditions. Neuropsychopharmacology. 2017;42:2232–41. doi: 10.1038/npp.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]