Abstract
Oxygen is a vital source of energy necessary to sustain and complete embryonic development. Not only is oxygen the preferred driving force for many cellular functions and metabolism, but it also is involved in regulating stem cell fate, morphogenesis, and organogenesis. Low oxygen levels are the naturally preferred microenvironment for most processes during early development and drive mainly proliferation. Later, more oxygen, as well as nutrients are needed for organogenesis and morphogenesis. Therefore, it is critical to maintain oxygen levels within a narrow range as required during development. Modulating oxygen tensions is performed via oxygen homeostasis mainly through the function of hypoxia-inducible factors. Through the function of these factors, oxygen levels are sensed and regulated in different tissues, starting from their embryonic state to adult development. To be able to mimic this process in a tissue engineering setting, it is important to understand the role and levels of oxygen in each developmental stage, from embryonic stem cell differentiation to organogenesis and morphogenesis. Taking lessons from native tissue microenvironments, researchers have explored approaches to control oxygen tensions, such as hemoglobin-based, perfluorocarbon-based and oxygen generating biomaterials, within synthetic tissue engineering scaffolds and organoids with the aim of overcoming insufficient or non-uniform oxygen levels and nutrient supply.
Keywords: Embryonic development, Hypoxia, Morphogenesis, Organoids, Oxygen bioavailability, Oxygen delivery, Tissue engineering
2. Introduction
The presence of oxygen in Earth’s early atmosphere allowed for the evolution of complex multicellular life, and eventually our human species. Oxygen made abundant ~2.3 billion years ago [Canfield, 2005], provided a readily available energy source for many cellular functions, including metabolism, respiration, and proliferation. Thus, it is of no surprise that oxygen plays a vital role in development, cell and tissue maintenance/basic metabolism as well as cell and tissue repair. Beginning in the 1970s, a substantial body of evidence has been constructed indicating the importance of oxygen availability in early development. During development, the levels of oxygen vary in each stage depending on the specific requirements of the developing embryo [Stamati et al., 2011]. Therefore, precise control of oxygen levels is pivotal to reaching developmental milestones during embryogenesis.
Molecular oxygen is involved in the most cell, and tissue metabolic processes and mitochondrial respiration accounts for the majority of O2 consumption in humans [Boveris et al., 1999] where the aerobic metabolism of glucose generates adenosine-5’-triphosphate (ATP). Additionally, the citric acid cycle and β-oxidation of fatty acids are tightly coupled to the process of oxidative phosphorylation and production of ATP. These ATP molecules then provide the required energy for the majority of cell physiological processes. Thus, oxygen bioavailability is vital to all cell functions and asserts its influence from the beginning of conception and continuing throughout each phase of embryogenesis. Likewise, any attempt in tissue engineering towards the regeneration of a lost or damaged structure seems incomplete without considering oxygen requirements and signaling during organogenesis and development [Lovett et al., 2009; Moore et al., 2013].
Tissue engineering has emerged as an exciting and potential solution for providing functional restoration of many human tissues and often utilizes developmental processes [Parveen et al., 2006; Chen and Liu, 2016]. The strategy of tissue engineering aims for the regeneration of specific tissue through the utilization of combinations of cells, scaffolds, chemical factors, and mechanical forces which usually starts in vitro [O’Brien, 2011]. This will only be possible by thoroughly understanding the development process and applying the developmental cues in tissue engineering biomimetic approaches [Ingber et al., 2006]. Synthetic biomimetic materials can help incorporate the developmental processes in tissue-specific, and organ-specific engineering approaches via providing the essential signals and clues to promote tissue differentiation and morphogenesis [Lutolf and Hubbell, 2005].
Despite improved understanding of developmental processes, it is still extremely challenging to create large tissue engineering scaffolds/organoids often because of insufficient oxygen and nutrient supply throughout the structure [Laschke et al., 2006; Farris et al., 2016]. Thus, understanding the connection between oxygen availability and development during processes such as stem cell fate decisions, angiogenesis, and tissue morphogenesis, could lead to more significant advances in improved development of tissue engineering and therapeutic approaches [Van Tuyl et al., 2005; Radisic et al., 2006; Stoppel and Roberts, 2012]. The fact that low oxygen abundance and lack of vasculature in most cases will lead to the failure of a tissue-engineered implant in vivo has inspired an area of research toward developing vehicles to either facilitate oxygen transport or to use topical, direct delivery to a required site [Stoppel and Roberts, 2012; Farris et al., 2016]. This approach includes the development and study of synthetic oxygen carriers [Eisenbrey et al., 2015], oxygen generating matrices [Oh et al., 2009] and perfluorocarbon-associated technologies (PFCs) [Geyer, 1988].
The primary purpose of this review is to introduce the role of oxygen in different stages of development to attain a better understanding of the process and motivate the translation of this knowledge for tissue engineering and organoid approaches. Herein, we will first review embryonic developmental stages with an emphasis on the impact of oxygen levels on stem cell fate, morphogenesis, oxygen sensing mechanisms and oxygen-mediated embryonic angiogenesis. After understanding the interplay of local oxygen environments and developmental processes, we present available tools for modulating oxygen microenvironments towards improved tissue engineering strategies. Finally, we elucidate the impact of strategies of oxygen modulation in the rapidly growing field of tissue organoids.
3. Oxygen in embryonic development
3.1. Roles of oxygen in early embryonic development
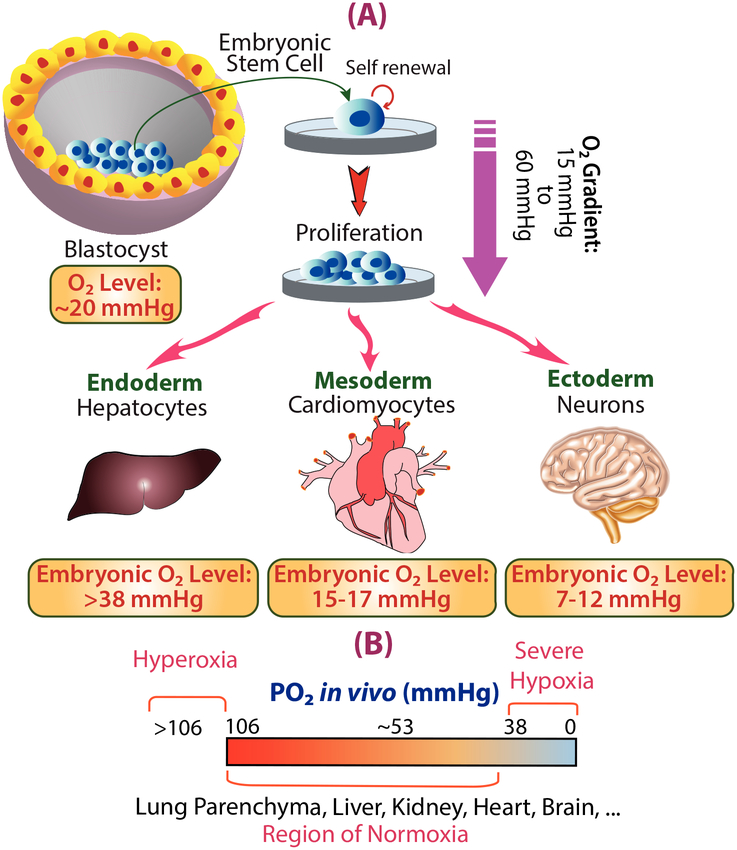
Oxygen regulation is crucial to embryonic development (Fig.1) [Michiels, 2004]. At the very early stages of development, before the formation of the placenta, the embryo experiences a state of physiologic hypoxia. By definition, physiologic hypoxia is the microenvironment with oxygen levels ranging from 1–5% (8–38 mmHg Po2), which is less than adult arterial pressures (10–13.15% oxygen equivalent to 75–100 mmHg Po2). In extreme oxygen limitations, pathologic hypoxia occurs when oxygen levels drop below 1% or 8 mmHg Po2 [Fitzgerald et al., 1999; Ortega et al., 2017]. It is important to note that the exact definition of oxygen hypoxia varies between organs and is different in vitro and in vivo. However, in most tissues venous oxygen concentrations lower than 6% (~46 mmHg Po2) creates mild hypoxic effects, whereas maximum hypoxic responses occur around 0.5–1% of oxygen (~4–8 mmHg Po2) [Shao and Zhao, 2014]. Due to the difficulties of measuring the exact tissue oxygen levels experimentally, the relative changes in oxygen levels at the different stages are not fully known [Hemker et al., 2016].
In terms of the preferred microenvironment for embryonic stem cells (ESC), low oxygen concentration (~2% oxygen, or 15 mmHgPo2) is required for embryonic stem cells to initiate proliferation in the earliest stages of fetal development [Mohyeldin et al., 2010; Abdollahi et al., 2011]. Figure 1 provides a summary of oxygen levels available in each stage of embryonic development. This low oxygen tension is the result of limited connections with maternal circulation during early development and is required for maintaining pluripotency in ESCs [Abdollahi et al., 2011]. In vitro studies also agree with these observations, where ESC cultures maintained at 3–5% oxygen (23–38 mmHg Po2) preserve their embryonic non-differentiated state as compared to cultures exposed to 21% oxygen (160mmHg Po2) [Stamati et al., 2011]. Likewise, Narva et al. have shown that reduced oxygen tension (4% oxygen, 30 mmHg Po2) is crucial for maintaining the pluripotency of human embryonic stem cells (hESCs) to encourage proliferation in an undifferentiated state in vitro [Narva et al., 2013].
Fig. 1.
(A) Oxygen levels in different embryonic development stages. (B) Measures of in vivo O2 levels in different tissues ([Fitzgerald et al., 1999; Simon and Keith, 2008; Mohyeldin et al., 2010; Wang and Zhao, 2010; Abdollahi et al., 2011; Carreau et al., 2011; Ortega et al., 2017]).
The mammalian blastocyst forms during a crucial early stage of development, and consists of an inner cell mass of ESCs inside a fluid-filled membrane (Fig. 1A) [Frankenberg et al., 2016]. The blastocyst serves as the niche and source for ESCs, which maintains the required low oxygen microenvironment for this stage of development. Accordingly, experiments on bovine blastocysts developed in a hypoxic environment (2% oxygen, 15 mmHg Po2) have shown more ESCs in their inner cell mass as compared to normoxic conditions [Harvey et al., 2004]. Further research supports that ESCs in the blastocyst must begin development in hypoxic conditions to successfully generate an embryo [Harvey et al., 2004; Ezashi et al., 2005; Shahbazi et al., 2016]. In parallel, during the very early stages of development, the human placenta forms from a stem cell population termed cytotrophoblasts [Wang and Zhao, 2010], which appear to prefer oxygen tensions of around 18 mmHg Po2 (2.4% oxygen) to form the placenta. Thus, maintaining an undifferentiated state and the eventual fate of placental cytotrophoblasts depends on oxygen availability. Studies indicate the undifferentiated proliferation of cytotrophoblasts occurs in vitro under low oxygen pressures (2% oxygen, 15 mmHg Po2). However, higher oxygen tensions trigger these cells to differentiate into a phenotype required to generate the maternal circulatory system [Genbacev et al., 1997; Simon and Keith, 2008]. In the early stages of pregnancy, the embryonic cytotrophoblasts start growing into the spiral arterioles of the uterus which allows for the formation of the placental circulatory system [Fisher and Burggren, 2007]. These connections established with the maternal vasculature increase the placental oxygen levels from 18 mmHg to 60 mmHg (2.35–7.9% oxygen) [Abdollahi et al., 2011]. In the human embryo, the vascular invasion occurs at embryonic day 9 (E9), and by the end of day 14–16, almost all of the small arterioles are entirely formed, allowing for optimal oxygen and nutrient transfer to the developing fetus [Enders and King, 1991].
3.2. Oxygen as a developmental morphogen
After the formation of the placenta at embryonic day E14.5, morphogenesis initiates to establish the body pattern while guiding tissue architecture towards the adult form [Reddi, 2014]. During the cascade of morphogenesis, physiological hypoxia acts as a signaling cue to guide processes such as cell proliferation and differentiation, which ultimately contributes to embryogenesis [Dunwoodie, 2009]. It was first shown in the 1970s that the development of explanted rat embryos cultured in vitro is highly dependent on oxygen availability when removed from other important maternal supplied factors [New and Coppola, 1970]. New and Coppola considered the number of somites (blocks of the embryonic mesoderm that give rise to vertebrate structures [Cooke, 1975]) as an indicator for embryonic development in rats. They showed that exposing the explanted rat embryos, at the headfold stage, to 60–95% oxygen (456–722 mmHg Po2) in watch glass culture helped the embryos to grow up to 25 somites. Conversely, no growth was observed in watch glass cultures with 20% oxygen (152 mmHg Po2) [New and Coppola, 1970].
Despite these early studies, the role and effects of oxygen availability on mammalian embryo morphogenesis in vivo have not been thoroughly investigated and are poorly understood. This is primarily because mammalian embryo oxygenation depends highly on the maternal supply, and thus the effects of oxygen variation in a mammalian fetus cannot be easily isolated or assessed directly without changing the maternal or placental oxygen levels [Giussani et al., 2007]. The independence of chick embryo development from maternal factors has led many investigators to focus on the influence of oxygen availability on chick embryogenesis. For instance, Metcalfe et al. conducted experiments on chicken eggs to investigate the effects of increased oxygen availability on the growth rate of chick embryos [Metcalfe et al., 1981]. Their experimental design consisted of three oxygenating regimes: 1. eggs covered with a neoprene membrane, to serve as a diffusion barrier limiting oxygen transfer while incubated with 21% oxygen (160 mmHg Po2); 2. uncovered eggs exposed to 21% O2; and 3. eggs incubated with 60% oxygen (456 mmHg Po2). The results of this study indicated a significant, ~10%, delay in embryonic growth for the covered eggs with limited oxygen transfer as compared to the uncovered group. Furthermore, on day 18 the group incubated with 60% oxygen formed significantly heavier eggs (indicative of embryonic growth) in contrast to the control group exposed to 21% oxygen (160 mmHg Po2), confirming that chick embryonic growth is regulated by oxygen availability [Metcalfe et al., 1981]. Similarly, Lourens et al. evaluated the effect of exposure to three different oxygen concentrations (low 17% oxygen (~129 mmHg Po2), normal 21% oxygen (160 mmHg Po2), and high 25% (190 mmHg Po2) on the yolk-free egg masses [Lourens et al., 2007]. Increasing O2 concentration was shown to increase the yolk-free mass and the length of the chick, especially at the three-week time point. More recently, Giussani et al., have studied the effect of oxygen availability on the fetal growth of chick embryos [Giussani et al., 2007]. In an interesting experiment, fertilized eggs laid by sea level hens were incubated in high altitude (~13% oxygen, 100 mmHg Po2) and resulted in increased embryonic mortality as compared to those incubated at the sea level (21% oxygen, 160 mmHg Po2). Decreased growth at the study endpoint (day 20) was also observed in the same conditions, as indicated by head diameter and body length (length of crown-rump).
Another compelling case of oxygen involvement in embryogenesis is in limb morphogenesis and the formation of growth plates. Considering the absence of vasculature in bone growth plates, the cartilaginous structures of new bone are under low oxygen levels. This physiological hypoxia acts as a signal to modulate chondrocytes to differentiate from a proliferative to a terminally differentiated state. It has been shown that within the developing long bone the oxygen tension varies from 21 mmHg in the areas further away from blood supply to 57 mmHg in the hypertrophic regions close to the vasculature [Araldi and Schipani, 2010]. Focusing on each region of developing bone, the cell population present in the pre-hypertrophic areas exist at an oxygen tension of approximately 21 mmHg, are more proliferative which aligns with their more stem cell-like nature. The cell type, population, and oxygen level in the pre-hypertrophic region is contrasted by the hypertrophic region that is comprised of a population of terminally differentiated cells existing at a higher oxygen tension of around 57 mmHg [Araldi and Schipani, 2010; Rankin et al., 2011; Stamati et al., 2011].
3.3. Oxygen homeostasis and sensing mechanisms
After early embryonic morphogenesis and later in development, oxygen sensing mechanisms are required to both establish and maintain oxygen homeostasis. This allows the body to sustain oxygen gradients and maintain levels of oxygen within the range required for development. Oxygen homeostasis is defined as the mechanism through which cellular O2 concentration is maintained within a narrow range with an upper level of 21% oxygen (~160 mmHg Po2) in the upper airway to the minimum of ~1% oxygen (~8 mmHg Po2) at the corticomedullary kidney junctions [Semenza, 2010]. Homeostasis plays a critical role in the survival of all vertebrate species starting from early development continuing to the later stages in adult life. Regulated and optimal oxygen delivery to all cells determines the physiological state of the organism. Sufficient tissue oxygenation depends on the precise development of the embryonic structures to provide a basis for oxygen homeostasis [Michiels, 2004]. At the cell level, defensive mechanisms have been developed to regulate oxygen levels to protect cells from extreme O2 variations [Maltepe and Saugstad, 2009].
Generally, homeostasis pathways work to regulate oxygen levels within the body. Hypoxia-inducible factors (HIFs), prolyl hydroxylases (PHDs), factor-inhibiting HIF-1 (FIH-1), activator protein 1 (AP-1), nuclear factor (NF)-κB, p53 and c-Myc are the most well-understood factors that influence oxygen regulation in vivo [Podar and Anderson, 2010]. Out of these, HIFs are typically considered as the body’s ‘master oxygen sensors’ and belong to a family of transcriptional factors with many downstream actions [Bryant et al., 2018]. The mechanisms related to oxygen sensing and homeostasis through HIFs have been discussed in detail elsewhere and can be found in reviews such as: [Ivan et al., 2001; Maltepe and Saugstad, 2009; Araldi and Schipani, 2010; Semenza, 2010; Zimna and Kurpisz, 2015; Deng et al., 2016; Graham and Presnell, 2017].
3.4. Oxygen-mediated embryonic angiogenesis
Angiogenesis is the developmental process that establishes vasculature and is further regulated by the individual demands of tissues. These demands include a sufficient oxygen supply, removal of excess carbon dioxide and other respiratory gases, and sufficient nutrients [Rouwkema and Khademhosseini, 2016; Yoon and Jones, 2016]. Embryonic angiogenesis initiates with the migration of mostly quiescent endothelial cells that are triggered by hypoxia (<25 mmHg Po2) or oxidative stress [Stamati et al., 2011]. Similarly, the same approximate oxygen tensions have been shown to stimulate the differentiation of varied progenitors derived from the embryonic mesoderm layer into hemangioblasts (hematopoietic or endothelial cells) in vitro [Fraisl et al., 2009]. The required energy for cell growth in the nonvascularized area is usually supplied through anaerobic glycolysis. Interestingly, similar to cancer cells, anaerobic glycolysis makes it possible for endothelial cells to tolerate a wide range of oxygen variations as compared to other cell lineages [Fraisl et al., 2009]. It is noteworthy to mention that although hypoxia is often considered as the driving force for the endothelial cells to initiate the vascularization process[Krock et al., 2011], there is a growing body of literature suggesting that vessel growth is inhibited by both moderate and severe hypoxic conditions via a still unknown mechanism [Faller, 1999; Hutton and Grayson, 2016]. Prior to the formation of the mammalian circulatory system, vascular development starts at a hypoxic condition with oxygen tensions around 23 mmHg. The HIFs largely mediate angiogenesis in hypoxia and among the HIF family, HIF-1 target genes have been shown to play an essential role in angiogenesis. [Schipani et al., 2009]. ARNT (aryl hydrocarbon receptor nuclear translocator: HIF-1β) and HIF-1α are both expressed in ESCs and are required for the regulation of hypoxia-responsive genes and angiogenesis. To test this hypothesis, Maltepe et al. used ARNT-deficient mice (lacking HIF-1β) to investigate the role of ARNT/HIF-1β in the regulation of the genes that are responsive to hypoxia under low oxygen conditions (1.5% oxygen, 12 mmHg Po2) [Maltepe et al., 1997]. The animals did not survive past E10.5, due to the arrested vasculature in the structure of their yolk sac, indicating the importance of HIF expression in embryonic angiogenesis. Furthermore, HIF-deficient mice embryos have been shown to develop improper placental structures as well as impaired vasculature [Dunwoodie, 2009]. Although low oxygen tensions are an activator signal for triggering angiogenesis, prolonged severe hypoxia can prevent the proliferation of blood vessels [Patel et al., 2005; Uno et al., 2007]. Thus, even though hypoxia is the preferred microenvironment to stimulate angiogenesis, low oxygen levels during a long course of time can be harmful to the process.
Oxygen-dependent homeostatic processes also play a role in angiogenesis, where hypoxia acts as a signal to trigger endothelial cells to begin the formation of new vasculature. In this regard, as soon as hypoxia is sensed by the endothelial cells, they activate their master oxygen sensors, the HIFs. HIF-α activates and translocates into the endothelial cell nucleus and attaches to HIF-β. The complex then enters the angiogenic signaling pathway modulating the formation of capillaries and vessels [Pugh and Ratcliffe, 2003; Krock et al., 2011]. Accordingly, hypoxia (<5% oxygen, 38 mmHg Po2) triggers endothelial cells to express angiogenic signaling factors, including vascular endothelial growth factor (VEGF)-A and nitric oxide synthase (eNOS) - resulting in subsequent migration and proliferation of endothelial cells [Cross et al., 2003]. Further, the majority of additional angiogenic factors, such as transforming growth factor-β, platelet-derived growth factor-β, angiopoietin-1 and −2, are direct transcriptional targets of HIFs [Deng et al., 2016]. Another contribution of oxygen in the embryonic angiogenesis process is through modulation of cell-cell attachments to define the degree of vascular bed permeability [Chan et al., 1984; Parks et al., 1984; van Wetering et al., 2002]. In terms of cell-cell adhesion and the permeability of the vascular bed, it has been shown that hypoxia (< 1% O2, 8 mmHg Po2) can loosen endothelial cell junctions to increase the permeability within the arterial walls [Cerutti and Ridley, 2017].
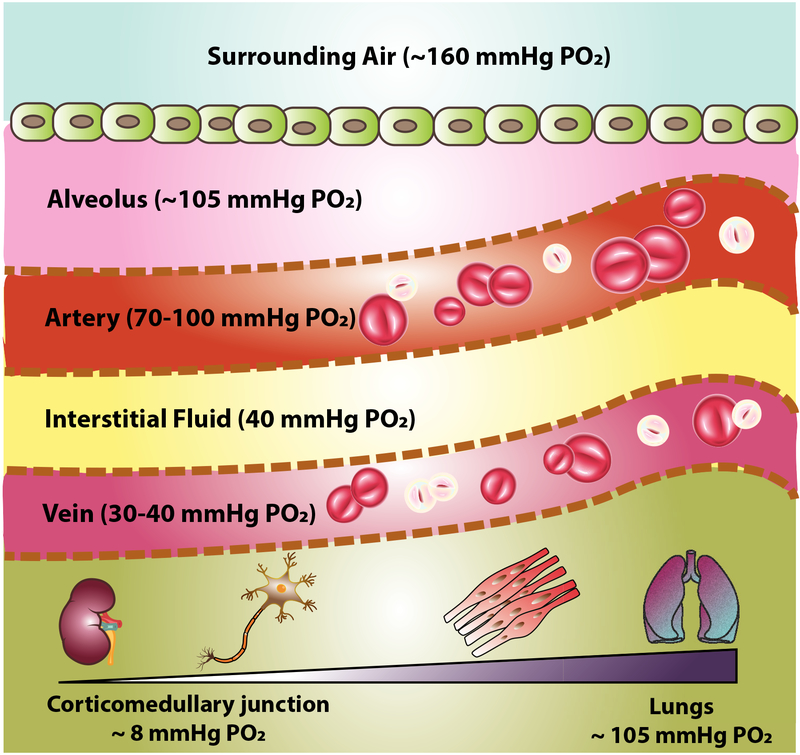
Overall, oxygen gradients, which are detected and maintained via O2 homeostasis mechanisms are the initiating stimuli for the angiogenesis process. Angiogenesis occurs through a sequence of stages starting with the angiogenic stimulus, sprouting of endothelial cells, elongation and branching, the formation of the lumen and finally regression. From a fundamental perspective, angiogenesis in adults is different from the embryonic process in the sense that an embryo needs this process for organ development when oxygen levels are not enough to satisfy developmental requirements. In contrast, in an adult, angiogenesis commonly only occurs as a response to insufficient blood and oxygen supply in instances such as wound healing, reproduction, and exercise. The vascular system of a mature mammalian organism is formed by a large number of vessels providing highways for blood transportation, gas/waste exchange, and oxygen delivery, as well as inflammatory cells and progenitors [Pugsley and Tabrizchi, 2000]. The circulatory system of a mature mammal is mainly comprised of the cardiovascular and lymphatic systems, within which the arteries, capillaries, veins and lymphatic vessels are the main components [Logsdon et al., 2014]. Local oxygen pressures are different within vascular channels based on their function, location and metabolic requirements of the adjacent tissue, the. Figure 2 summarizes oxygen levels throughout the adult organism and the oxygen gradients that are established at the completion of embryonic development, which also should be ultimately achieved in any tissue engineering or organoid strategy. To summarize, low oxygen levels are considered a driving factor for angiogenesis during development in vivo. After formation of the blastocyst and prior to the maturation of the placenta, levels of oxygen and other essential nutrients are insufficient to support the formation of new embryonic structures, which triggers the system to form connections with the maternal vasculature. The inflowing maternal blood provides a route for the exchange of oxygen and other nutrients to the fetal circulatory system [Wang and Zhao, 2010]. Therefore, without supplying the required oxygen and nutrients to the developing organism, the process cannot normally proceed and form a fully developed embryo.
Fig. 2.
Oxygen levels in the adult mammal.
4. Tissue oxygen monitoring and measurement techniques
In order to satisfy the oxygen requirements of a tissue, tissue-engineered construct or an organoid, it is equally important to be able to both monitor and measure the oxygen levels in situ. Currently, challenges, such as the thickness and structure of the scaffold as well as the tissue, limit the effectiveness of oxygen monitoring and measuring techniques within 3D living structures [Weyand et al., 2015]. As mentioned before, oxygen levels directly correlate with cell growth, ECM synthesis and the success of a tissue engineering approach during growth are essential to monitor. Despite the importance, currently available techniques to directly measure the oxygen levels both in vitro and in vivo are often limited to the surface layer of the developing tissue or the surrounding aqueous environment [Weyand et al., 2015]. Some of the most common techniques to directly measure the oxygen levels include delayed fluorescence of endogenously overproduced protoporphyrin IX (PPIX), intravascular (cell-impermeable) phosphorescent probes and cell-penetrating small molecule phosphorescent nanosensors, and solid-state phosphorescent sensors - all of which can be used for imaging and to quantitatively measure cell and tissue oxygenation. Detailed discussion on each of these methods as well as their advantages and limitations can be found in the review paper recently published by Papkovsky [Papkovsky and Dmitriev, 2018].
Direct optical sensing of oxygen is a common method also known as endogenous delayed fluorescence, which uses a metal-free endogenously produced precursor of heme called PPIX as an oxygen-sensing reporter. However, the optical fluorescence signals of the endogenous PPIX reporter is weak and needs to be enhanced in animal or human tissues to enable oxygen measurement. This enhancement is usually done by treating tissues with 5-aminolevulinic acid (ALA) to increase mitochondrial PPIX levels and the specific optical signal. Despite the diverse applications of this method, it presents moderate selectivity and resolution especially for quantitative oxygen measurement.
Another common type of oxygen measurement is performed indirectly via oximetry integrating methods such as pH measurement, lactate concentration or glucose consumption. One common indirect approach is to use the localization and quantification of an drug that undergoes oxidative metabolism, such as EF5/2-nitroimidazole, (2-(2-nitro-1H-imidazole-1-yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide), as have been employed to study hypoxic tumor environments [Evans et al., 2000]. Reductive metabolism activates EF5 which leads to covalent bonding with macromolecules. Increased oxygen availability in the environment causes more inhibition in the reductive mechanism and activation [Koch, 2002]. The intracellular adducts of EF5 can subsequently be detected using antibodies, quantified using immunohistochemistry and correlated with oxygen availability [Evans et al., 2004]. Similar to EF5, pimonidazole has also been used clinically as a hypoxia probe to indirectly measure levels of tumor hypoxia [Nordsmark et al., 2003]. Pimidazole can bind to thiol-containing proteins present in hypoxic cells, which can then be quantitatively detected and converted indirectly to oxygen concentration using point counting and image analysis software methods [Varia et al., 1998]. Among all the indirect methods, polarographic (Clark) electrodes are considered the oximetry gold standard presenting advantages such as good reproducibility and accuracy as well as the low deviation between each sensor and high detection resolution [Park et al., 2007]. The polarographic method uses an electrode made of platinum cathodes and silver anodes linked by an electrolyte bridge. While oxygen is reduced at the cathode surface, the amount of O2 diffusing through the oxygen-permeable membrane increases, which closes the circuit and sends out a current proportional to the amount of oxygen at the measurement site [De Santis and Singer, 2015]. These electrochemical sensors, however, are often faced by challenges such as invasiveness, oxygen consumption by the electrode, and inability to reflect the heterogeneity of tissue oxygen levels [Liu et al., 2011]. Fiber-optic probes are another alternative to measure the tissue oxygen levels which are limited by invasiveness as well as probe fragility [Weyand et al., 2015]. These sensors use a fluorescent dye in a sol-gel coating on the sensor tip to measure the partial pressure of oxygen. The collected signal is then sent to a meter via an optical fiber to be detected and then converted to the equivalent Po2 [Davenport et al., 2016].
A commonly used clinical method for detecting blood oxygen levels uses near-infrared (NIR) spectroscopy of hemoglobin (Hb) saturation to measure mean oxygen levels in large tissue volumes, as well as blood (pulse oximetry). This method is based on the fact that different biological molecules, including Hb, have distinct optical properties when bonded to oxygen [Scheeren et al., 2012]. Based on the Beer-Lambert law, photons can easily pass through the tissue regions with the lowest absorption, such as in small vessels where the light can make multiple passages throughout the tissue. Therefore, the changes in oxygen concentrations can be sensed using a NIR sensor and detected from the light absorbed [Boushel et al., 2001].
A precise method for measuring oxygen values within tissues is electron paramagnetic resonance (EPR), which is invasive and difficult in that it requires the implantation or intravenous injection of a paramagnetic material as well as imaging via positron emission tomography (PET) scanning. The problem with this technique is it’s a limited range of detection to hypoxic conditions and high costs making it less common for clinical use [Schrey et al., 2010]. EPR can detect Po2 within the range of 10–100 mmHg, but becomes increasingly inaccurate beyond this range [Presley et al., 2006]. Similarly, magnetic resonance imaging (MRI) techniques using fluorine contrast agents have also been used to quantitate tissue oxygen levels [De Santis and Singer, 2015; Papkovsky and Dmitriev, 2018]. This method is based on the fact that dissolved oxygen molecules have paramagnetic properties that can affect the relaxation rate of fluorine nuclei, which is linearly proportional to oxygen concentration. MRI can then use the 19F relaxation rate to either monitor the Po2 levels or to create an organ oxygen distribution map. Various perfluorinated compounds can be used as contrast agents, the distribution of which reveals the tissue oxygenation levels. For instance, MRI has been used to visualize tumor oxygenation in live animals after injection of hexafluorobenzene in the target tumor tissue [Ruiz-Cabello et al., 2011]. High costs of using this technique as well as the requirement for patient cooperation can be considered as main drawbacks that limit it’s practical application [Aydogdu et al., 2012].
As described before, oxygen bioavailability plays a vital role, starting from development and later in tissue maintence and regeneration. In order to be able to mimic any natural tissue regeneration process in vitro, it is of great importance to investigate the oxygen levels in the native microenvironment, which will not be possible without accurate and efficient tissue oximetry techniques. This, in turn, helps to maximize the efficiency of any oxygen modulation strategy, it would be ideal to draw the required clues from the natural developmental processes, such as oxygen gradients [Jaenisch et al., 2018]. However, the field is still lacking effective, accurate and straightforward tools to provide us with the quantifiable measures of oxygen, especially within a developing tissue to then translate to a tissue-engineering setting [Barinaga, 1997; Lovett et al., 2009]. Therefore, more research and advances are required in the field of direct tissue oximetry to increase the outcomes of tissue engineering and regenerative medicine.
5. Oxygen-delivery approaches for tissue engineering
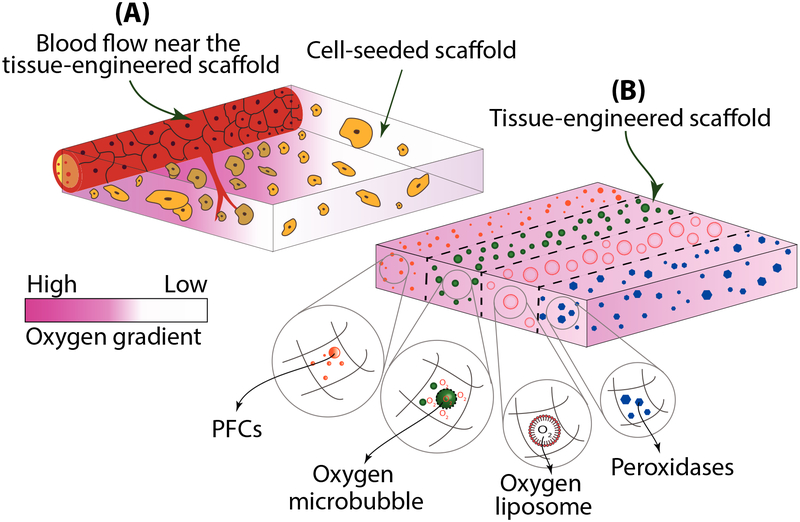
From studying oxygen’s role in the process of embryonic development, it is clear that the formation of tissues is tied closely to oxygen availability within the microenvironment. Thus, not only does this principle apply to embryogenesis, but the same conditions should also be considered in any tissue engineering setting. However, as opposed to embryonic development, during the formation of an engineered tissue, there is no maternal circulation or vasculature to provide enhanced oxygen and nutrient supply for developing engineered tissues [Stamati et al., 2011] Unless adequately addressed, the lack of sustained and prolonged oxygen supply via oxygenating strategies can cause primary limitations in tissue engineered constructs to create large, self-sustaining structures. One strategy to overcome this issue is via the application of engineered oxygen carriers. These carriers have shown the potential to provide a sufficient and prolonged supply of oxygen for tissue engineered approaches both in vitro and in vivo. More specifically, oxygen-controlled release strategies use two primary mechanisms, either via diffusion or by oxygen generating reactions [Gholipourmalekabadi et al., 2016]. In an implantable strategy, a sustained and controlled level of oxygen allows enough time for early neovascularization to occur in vivo to support the engineered tissue allowing maintenance of cellular metabolic activity and signaling [Lovett et al., 2009]. Further, engineered oxygen carriers can provide long-term oxygen sources within the tissue engineered construct as would be required in in vitro approaches where vasculature is not present. The next sections deal with introducing novel oxygen-releasing technologies as well as their potential applications in the emerging field of organoid formation and is summarized in Figure 3.
Fig. 3.
(A) Oxygen levels depend on the distance from the primary blood flow and are location-specific in a vascularized tissue engineering scaffold. (B) Different oxygen carrier strategies shown encapsulated in tissue engineering scaffolds creates a more uniform oxygen gradient (Figure modified from [papStoppel and Roberts, 2012]).
Even if a large enough construct can be created in vitro, an implanted construct often fails to integrate with the host and survive in vivo due to insufficient blood vessel and capillary networks [Griffith and Naughton, 2002]. Oxygen regulates angiogenesis using biological oxygen sensors such as HIF signaling, resulting in the formation of vascular networks and capillaries [Lovett et al., 2009]. In earlier parts of this review, we discussed the role of oxygen in HIF signaling and hypoxia regulation. As discussed above, oxygen is a key regulator of many signaling pathways, which affect essential cellular processes such as differentiation, proliferation, and apoptosis. Thus, every tissue engineering strategy should consider the role of oxygen in both formation/maturation of constructs as well as for successful integration and restoration of function after final in vivo implantation. Important knowledge can be gained from studying how oxygen gradients and levels change during different stages of development, as we have discussed above. With this knowledge, oxygen delivery strategies can be specially formulated to modulate oxygen microenvironments at prescribed levels and to promote specific differentiation/developmental processes in tissue engineering. Currently available platforms for oxygen delivery are broadly classified as hemoglobin-based, perfluorocarbon-based, oxygen generating materials, as well as a few other recently introduced classes (Table 1). A general theme is observed, which is to encapsulate or directly apply the formulations of these oxygenation strategies in tissue-engineered scaffolds to supply tissue-specific oxygen-microenvironment requirements as will be discussed in more detail below.
Table 1:
Oxygen delivery approaches in tissue engineering
| Class | Applications | Oxygen Carrier Strategy | Ref. |
|---|---|---|---|
| Hemoglobin | Artificial red blood cells | Hb encapsulated in liposomes | [Sakai, 2017] |
| Bone, islet cell (pancreas) | HEMOXCell® | [Le Pape et al., 2018; Rodriguez-Brotons et al., 2016] | |
| Hepatocytes (liver) | Hemoglobin-Albumin microspheres | [Lai et al., 2015] | |
| Cardiomyocyte proliferation (heart muscle cell) | Hemoglobin/gelatin/fibrinogen scaffolds | [Ravichandran et al., 2013] | |
| Oxygen generating biomaterials | Skin tissue preserve skeletal muscle homeostasis | Sodium percarbonate | [Harrison et al., 2007; Ward et al., 2013] |
| MSCs | calcium peroxide | [Steg et al., 2017] | |
| Oxygen delivery to tumors, urinary tract | calcium oxide | [Huang et al., 2016; Lv et al., 2016] | |
| Pancreatic islets | Oxysite® | [Coronel et al., 2017] | |
| Perfluorocarbon oxygen carriers | Pancreatic beta cells | Perfluorotributylamine (FC-43) emulsion | [Goh et al., 2010] |
| Liquid ventilation | Perfluorodecalin (Fluosol) emulsion | [Waxman, 1986] | |
| Bone | perfluoro-octane emulsion | [Lee et al., 2015] | |
| Oxygen delivery in cell culture | Bis(perfluorobutyl)ethane emulsion | [Lowe et al., 1998] | |
| Central nervous system tissue engineering | Covalently immobilized Perfluorooctanoyl chloride (MACF) | [Li et al., 2014] | |
| Skin repair/wound healing | MACF | [Wijekoon et al., 2013; Patil et al., 2016] | |
| Recent technologies | Skin tissue/wound healing | photosynthetic engineered microalgae | [Lode et al., 2015; Schenck et al., 2015; Chavez et al., 2016] |
| Cartilage | myoglobin-polymer-surfactant complex | [Armstrong et al., 2015] | |
| Oxygen delivery for tissue engineering | Microtanks | [Cook et al., 2015] | |
| Oxygen delivery for tissue engineering | Endoperoxides | [Benz et al., 2013] |
5.1. Hemoglobin-based oxygen carriers
One strategy to improve in vitro oxygenation is to isolate native hemoglobin from red blood cells and then modify it to improve oxygen-carrying function. Hemoglobin is an iron-containing metalloprotein existing in tetramer form in red blood cells [Jensen et al., 1998]. A hemoglobin tetramer can be conjugated with hemoglobin-based oxygen carriers by various crosslinking and encapsulation techniques as discussed later in this section [Pin et al., 1982; Bianconi et al., 1985]. With such an approach, hemoglobin-based oxygen carriers (HbOCs) can be used to carry and maintain physiological oxygen levels in tissues, similar to their native function.
The earliest HbOC strategies mainly focused on hemoglobin harvested from blood without any modification for use as a blood substitute. Unfortunately, this unmodified cell-free hemoglobin, or methemoglobin, blocks renal tubules leading to kidney failure, while also acting as a pro-inflammatory molecule that induces oxidative toxicity in kidneys, the liver, the central nervous system and in cardiac tissue [Harrison et al., 1947; Kumar and Bandyopadhyay, 2005]. These adverse outcomes are largely a result of their high affinity for oxygen [Buehler et al., 2010], short half-life and their dissociation into dimers from tetramers [Bunn et al., 1969]. To improve half-life and stability of hemoglobin, strategies such as cross-linking [Hathazi et al., 2014; Romagnoli et al., 2015], polymerizing [Espes et al., 2015], coating with polydopamine [Wang et al., 2017], conjugating with dextran [Wang et al., 2017] and complexation with superoxide dismutase [Bian and Chang, 2015] have been studied. The underlying theme behind these strategies is to increase the size and reduce reactivity to diminish the toxicity and renal filtration. The most significant improvement in the application of hemoglobin as an oxygen carrier is the use of bacterially synthesized recombinant hemoglobin. Mutagenesis of Hb can: (i) adjust the dioxygen affinity of Hb over a 100-fold range, (ii) reduce the nitric oxide (NO) scavenging over 30-fold without compromising the dioxygen binding, (iii) slow down the rate of auto-oxidation, (iv) impede subunit dissociation, and (v) diminish irreversible subunit denaturation [Varnado et al., 2013]. Additionally, recombinant Hb helps to eliminate the chance of cross-contamination from mammalian donors [Pishchany et al., 2010]. These improvements in HbOCs have been somewhat successful in overcoming limitations presented during previous HbOC blood substitute applications.
Despite the considerable time and effort spent on the development of Hb-based oxygen carriers, it is important to highlight that cell-free Hb-based artificial blood oxygen carriers to date have not been proved safe in humans, which is concerning because their application poses the risk of myocardial infarction [Natanson et al., 2008]. These HbOCs can induce vascular thrombosis of heart and other organs by scavenging nitric oxide rapidly leading to vasoconstriction [De Caterina et al., 1995; Lin et al., 2001; Rother et al., 2005]. Clinical trials and preclinical studies using HbOCs as artificial blood oxygen carriers have not shown significant benefits to their use especially considering the side effects [Terraneo et al., 2017; Kao et al., 2018]. Thus, careful clinical evaluation of newly developed promising strategies is required, or Hb-based carriers may be better suited for purely in vitro endeavors such as the first phases of many tissue engineering applications.
Recent advances in this field have shown some promise as a tool for assisting tissue engineering strategies by improving respiratory gas transport. Specifically, a combination of protein-based scaffolds with hemoglobin have been formulated to create tissue engineering scaffolds and have shown some recent successes. Specifically, hemoglobin blended with gelatin/fibrinogen scaffolds have been shown to improve O2 diffusivity and exhibited the potential to promote cardiomyogenic differentiation of mesenchymal stem cells (MSCs) [Ravichandran et al., 2013]. In a different approach for liver tissue engineering, hemoglobin-albumin microspheres increased hepatocyte cell viability as dependent on the [Lai et al., 2015]. P50 is defined as the Po2 when oxygen saturation reached 50% in the oxygen dissociation curve. These microspheres were found to have a P50 of up to 12 mmHg. HEMOXCell®, a product created using the extracellular hemoglobin M201 with a P50 of 35 mmHg at 37°C and has been explored in islet cell preservation and bone reconstruction. HEMOXCell has shown the ability to reduce cell hypoxia while restoring functions in encapsulated islet cells in a [Rodriguez-Brotons et al., 2016]. In a bone tissue engineering application, HEMOXCell improved oxygenation to the hypoxic regions and promoted the proliferation of human bone marrow MSCs [Le Pape et al., 2018]. As discussed earlier in this review, stem cells are known to preserve their undifferentiated state and proliferate in hypoxia (<5% or Po2 < 38 mmHg) Thus, human bone marrow MSC proliferation likely prefers a Po2 between mild hypoxia while preserving their differentiation potential. Oxygenating biomaterials or carriers such as HEMOXCell can be used to overcome chronic hypoxia (~1% Po2, Po2 8 mmHg) and sustain a mild hypoxic environment. Thus, it is important to study oxygen tensions required to preserve function and differentiation potential during proliferation in all cells types.
5.2. Oxygen-generating biomaterials
As an alternative to chemical or enzymatic means of oxygen generation and delivery, oxygen-generating approaches have been formulated to meet tissue-specific oxygen demands. Oxygen generating chemicals, like sodium percarbonate, calcium peroxide, magnesium peroxide, and hydrogen peroxide, are decomposed in the biological environment to produce oxygen and certain byproducts in some cases [Camci-Unal et al., 2013; Wang et al., 2017]. Biomaterial strategies typically incorporate an oxygen generation mechanism through these chemical species where the ability to generate oxygen is encapsulated within the scaffolds. The advantage of delivering oxygen via these approaches is their ability to generate oxygen in situ instead of via an external source or reservoir [Camci-Unal et al., 2013]. Oxygen-generating biomaterials provide flexibility and can be used in the form of microspheres [Steg et al., 2017], scaffolds [Coronel et al., 2017], films [Harrison et al., 2007], and electrospun nanofiber mats [Wang et al., 2011].
Recent tissue engineering-based reports have revealed the potential benefits of an oxygen generation approach. One such approach is achievable via calcium peroxide hydrolyses in order to form hydrogen peroxide, which, in turn, reacts with water to form oxygen in the biological environment [Huang et al., 2016]. Coronel et al. have introduced Oxysite®, generating oxygen in situ through calcium peroxide hydrolytic decomposition, and showed its ability to mitigate anaerobic glycolysis and preserve and stimulate insulin release in rat pancreatic islet culture (oxygen gradient of 0.8 × 10−4 mM (0.1 mmHg Po2) on the cell side to 0.3 mM (214 mmHg Po2) in Oxysite) [Coronel et al., 2017]. In a similar approach, calcium peroxide particle-embedded silk fibroin scaffolds (Po2 saturation of 10–11 mmHg over 21 days) were shown to enhance the repair in a dog urethra model suggesting its potential in a urinary tract reconstruction application [Lv et al., 2016]. Finally, the beneficial effects of a poly(trimethylene carbonate) matrix with calcium peroxide particles (PTMC/CaO2 0.05–0.1 mg/L; 0.55–1.7 mmHg Po2 above control) showed step release of oxygen for up to 20 days. This biomaterial showed beneficial maintenance of human MSCs under hypoxic conditions confirmed by increased mitochondrial activity and enhanced proliferation [Steg et al., 2017].
Utilizing enzymatic stimulus for oxygen generation is another important strategy towards oxygen generation. A biomaterial strategy with H2O2−releasing microspheres (maximum oxygen concentration of 18.4%, or 137 mmHg Po2) and bovine liver catalase was created to improve stem cell survival and shown to restore differentiation in the cardiosphere-derived cell (CDC) therapy after myocardial injection [Li et al., 2012]. In this study, the oxygen releasing system improved cell survival for 7 days and preserved their differentiation potential even in hypoxic conditions (<8 mmHg oxygen environment). CDCs are stem cell-like phenotypes derived from atrial or ventricular biopsy specimens of patients undergoing heart surgery. Normal arterial and ventricular oxygen tension is about 75–100 mmHg, and thus it can be concluded that CDCs prefer this oxygen microenvironment to maintain their functions. Accordingly, strategies, like H2O2−releasing microspheres and bovine liver catalase, provide a platform to maintain preferred oxygen tensions to preserve the differentiation potential of CDCs after myocardial injury.
As an alternative to enzyme-catalyzed oxygen generation, endoperoxide mediated approaches have been used. Endoperoxides generate oxygen upon contact with water in biological environments through simple reorganization reactions. Methylated pyridone-derived endoperoxides undergo retro-Diels-Alder reactions in an aqueous environment releasing oxygen in high yields and with half-lives of up to 13 h, however, this study used a fluorescence-based assay that only provided relative measures of oxygen abundance [Benz et al., 2013]. These molecules, in combination with vitamin C, as a singlet oxygen quencher, were shown to significantly improve the survival of 3T3 fibroblasts and rat smooth muscle cells when challenged by a hypoxic environment.
Overall, as oxygen generating materials evolve, it is important to realize their implications on tissue microenvironments and to understand and overcome the limitations presented by each approach. The chemical composition of oxygen-generating materials, such as metal oxides or metal peroxides determines their oxygenation characteristics. Several environmental factors such as pH, temperature, buffer conditions, presence or absence of catalysts or inhibitors affect oxygen generation [Oh et al., 2009]. These challenges present an opportunity to develop the next generation of oxygen-generating materials for improved tissue engineering applications. The oxygen generating biomaterial technologies are interesting and still evolving as a tool for producing tissue engineering constructs. Thus, they hold great potential to evolve as tunable oxygenation strategies in tissue engineering.
5.3. Perfluorocarbon-based carriers
Perfluorocarbons (PFCs) can readily dissolve oxygen in aqueous conditions. The mechanism and kinetics of oxygen binding to PFCs and the release is different from that of native hemoglobin [Guzy et al., 2005]. Solutions of native hemoglobin show sigmoidal O2 dissociation behavior [Lowe et al., 1998], whereas, colloidal suspensions of PFCs, such as perfluorotributylamine, perfluorodecalin, perfluoro-octane, show a linear relationship between oxygen content and oxygen partial pressure (Po2) [Lowe et al., 1998; Spahn, 2000; Khattak et al., 2007]. At standard temperature and pressure, the solubility of O2 in water is 2.2 mM. This value can be much higher, up to 44 mM, for PFCs like bis(perfluorobutyl)ethene, representing a 20-fold increase over O2 solubility in water alone [Lowe et al., 1998]. There is considerable interest in using PFCs as O2 carriers in a variety of tissue engineering applications and as blood substitutes [Veen and Hunt, 2015; Santiesteban et al., 2016].
Owing to their ability to carry oxygen, some PFCs in the past have been approved by the FDA as blood substitute products for specific applications. These products include Fluosol emulsions, and perfluorodecalin emulsified primarily with the synthetic poloxamer Pluronic F-68, both of which were approved for use in coronary artery balloon angioplasty procedures and as liquid ventilation procedures [Waxman, 1986]. Despite FDA approvals, published literature demonstrates a limited understanding of biological interactions with PFCs and not all PFCs have been studied for short and long-term toxicity responses. In one such study, a PFC emulsion of perfluorotributylamine (FC-43) has shown to be biologically inert, non-toxic and does not produce toxic metabolic products in rats [Chubb, 1985]. A few reports show that non-immobilized free PFCs which are highly lipophilic tend to exhibit toxic responses to cells and inhibit growth. However, this is contrasted by in vitro cell toxicity responses observed when using more than 1% of the PFC perfluorooctanoylbromide (PFOB) [Khattak et al., 2007], which is more lipophilic than FC-43. PFOB toxicity most likely arose from the fact that their approach utilized a physical binding process that lacked stability over time allowing the individual PFOB molecules to dissociate then diffuse to cells where they could interfere with vital cellular functions. Despite this toxicity response, the 10% PFOB encapsulation still showed marked enhanced cell function as demonstrated by decreases in lactate dehydrogenase and lactates as well as consumption of glucose. In another study, an encapsulated perfluorotributylamine (FC-43) emulsion was shown to have no significant improvement on the proliferation of pancreatic beta cells (BTC-tet) in hypoxic conditions [Goh et al., 2010]. Further, the perfluorotributylamine (FC-43) emulsion exhibited cellular toxicity marked by a reduction in metabolic activity and viability as compared to no PFC controls. These results were in opposition to earlier successful reports by Waxman, highlighting the need for better PFC formulations and approaches. In an improved PFC stabilization methodology, perfluorooctane (PFO) emulsion-loaded polycaprolactone hollow microparticles demonstrated oxygen release of 3.2 mg/L (66.7 mm Hg Po2) above 5% CO2 equilibrated media for 2 days [Lee et al., 2015]. These hollow particles were studied in vitro and in vivo to suggest its benefits in supporting vascularization and osteocyte proliferation. In vitro toxicity response was studied by cell number quantification, which was reduced by the effects of hypoxia, but PFO emulsion loaded MPs were able to sustain more viable cells as compared to controls suggesting limited direct toxicity.
A small subset of researchers, including ourselves, have suggested a strategy of covalently immobilizing PFCs to polymers as side chains to eliminate the problems associated with emulsification, long-term toxicity, or their stability, while still conferring the benefits of PFCs [Gattas-Asfura et al., 2012; Wijekoon et al., 2013; Chen et al., 2014; Palumbo et al., 2014]. Our group has recently shown that pentafluoropropionic anhydride (Ali5, equilibrium 8 ± 1 mmHg Po2 above control), 2,3,4,5,6-pentafluorobenzaldehyde (Ar5, 89 ± 2 mmHg Po2), and pentadecafluoroctanoylchloride (PFOC) (Ali15, 134 ± 3 mmHg Po2) could be attached covalently to a UV-curable methacrylamide chitosan hydrogel (MACF) and used for tunable oxygen delivery to accelerate full thickness acute wound healing in splinted rat model [Wijekoon et al., 2013; Patil et al., 2016]. Recently, MACF was also shown to improve cellular functions important to dermal wound healing such as cell metabolism, total DNA synthesis, and cell migration even under 1% oxygen (~8 mmHg Po2) hypoxia in both fibroblasts and keratinocytes [Akula et al., 2017]. Additionally, adenosine triphosphate (ATP) quantification revealed that MACF treatments improved cellular ATP levels significantly over controls under both normoxia and hypoxia.
The wound tissue oxygen microenvironment has been previously studied by researchers, and non–healing chronic wounds can exhibit oxygen partial pressures (Po2) as low as 5 mmHg, as compared to healthy skin with a Po2 in the range of 10–40 mmHg [Mutluoglu et al., 2013]. Importantly, vital wound healing processes such as collagen synthesis, angiogenesis, and epithelialization require local oxygen bioavailability with a Po2 ranging from 25 mmHg to 100 mmHg [Tuderman et al., 1977; Edwards et al., 1984]. Biomaterial strategies such as MACF can be used to address this oxygen tension gap in chronic and healthy wound tissues to enhance wound healing. However, later stages of wound healing and angiogenesis require oxygen to support cell proliferation and tissue growth. Thus, understanding the relationship between effects of oxygen availability during steps of angiogenesis and modulating them using biomaterials with different oxygenation potentials temporally can provide a next-generation solution to long-standing problem of tissue engineered scaffold vascularization. In a tissue engineering study with the MACF materials described above, Li et al. encapsulated neural stem progenitor cells (NSPCs) in the three types of MACF hydrogels and studied local oxygen concentrations and cellular responses [Li et al., 2014]. This study demonstrated that MACF’s oxygen concentration in a 3D hydrogel could be tuned based on the fluorine moiety, and oxygen tensions were higher (and the gradient less severe) when using the three MACF formulations available. At the center of cell seed gels, the following oxygen tensions were reported: aromatic PFC Ar5, 124 mmHg, aliphatic short chain Ali5, 121 mmHg and aliphatic long-chain Ali15, 130 mmHg. Thus, the highest oxygen levels were seen when using the PFOC modified version, which contained the most fluorines per substitution. Further, the local oxygen concentration in MACF hydrogels leads to enhanced NSPC cell proliferation and neuronal differentiation suggesting that the measured oxygen tensions translated to enhanced cellular functions. The role of oxygen tension in NSPC maintenance and fate decisions is supported by other studies including one that demonstrated that at high O2 conditions (21%, 160 mmHg Po2) NSPCs tend to prefer neuronal differentiation and at low O2 conditions (2%, 15 mmHg Po2) NSPCs differentiate into glia cells [Xie et al., 2014]. Early populations of NSPCs expand their populations by self-renewal and proliferation in low oxygen tensions (1–5%, 8–38 mmHg Po2) [Clarke and van der Kooy, 2009]. Thus, for neural tissue engineering, it is important to understand the stage of stem cell differentiation, lineage commitment, and tissue development and utilize the appropriate oxygenating material strategy.
In similar approaches, other groups have immobilized linear as well as branched PFCs to hyaluronic acid (HA) [Palumbo et al., 2014], chitosan and alginate [Gattas-Asfura et al., 2012], and have shown benefits for in vitro culture of mammalian or human cells. More researchers are now exploring the potential to immobilize PFCs on biomaterials in tissue engineering. In another recent study, 3-pentadecafluoroheptyl,5-perfluorophenyl-1,2,4-oxadiazole (FOX) molecules were immobilized to graphene oxide to form nano-platforms [Maio et al., 2018]. These nano-platforms can act as oxygen reservoirs, and at a dissolved concentration of 0.33 mg/ml, nano-platforms were able to show the transient release of 431.0 ± 0.5 mmHg oxygen over 200 sec at 37°C. Even at low concentrations, the nano-platforms demonstrated high oxygen content at saturation and diffusion rate as compared to the materials currently used as O2−reservoirs in tissue engineering or regenerative medicine. Another interesting approach for delivering oxygen in vitro and in vivo for diagnosis and treatment applications is using nanoscale technologies such as oxygen reservoir nanodroplets. Oxygen reservoirs created by PFC nano-droplets have been used to overcome tumor hypoxia to enhance cancer radiotherapy (RT). Polyethylene glycol (PEG) stabilized PFC nano-droplets were decorated with TaOx nanoparticles (TaOx@PFC-PEG) to form a multifunctional radiotherapy sensitizer to enhance the effectiveness of radiotherapy against tumors [Song et al., 2017]. TaOx@PFC-PEG nano-droplets with 20% (v/v) perfluorohexane increased tissue oxygenation by releasing 443 mmHg Po2 dissolved oxygen. As a result, enhanced in vivo RT treatment efficacy was realized using TaOx@PFC-PEG as a multifunctional radiosensitizer, which not only could concentrate the effective irradiation dose inside the tumor but was also able to overcome hypoxia-associated radio-resistance.
Importantly, the formulation of perfluorocarbons plays an essential role in limiting or eliminating toxicity concerns. As we briefly discussed covalent immobilization, encapsulation in the biorthogonal/biocompatible matrices, coating emulsion droplets with biopolymers/lipids allow for the stabilization of perfluorocarbons and can drastically reduce bioaccumulation and toxicity. Proper toxicological studies are yet to be performed for some of the most promising compounds and strategies; this must occur before any clinical translation can ensue [Serex et al., 2014; Takahashi et al., 2014]. Thus, newer formulation strategies can potentially open doors for the applications of perfluorocarbon-based oxygen delivery systems in tissue engineering.
5.4. Technological advances in oxygen delivery
Recently, novel technologies have made possible new oxygen delivery applications. These techniques have evolved as an independent oxygen delivery class and are of importance considering their potential for application in tissue engineering. As summarized in Table 1, emerging technologies such as photosynthetic algae, myoglobin-polymer-surfactant complexes, microtanks, and endoperoxides show potential for supplemental oxygenating solutions [Farris et al., 2016]. Photosynthesis is plants’ preferred means to generate energy, and a byproduct of this process is oxygen. This concept was recently utilized to create photosynthetic oxygenating biomaterials using photosynthetic algae. Chávez et al. have shown that photosynthetic biomaterials can produce and provide oxygen (saturation Po2 > 400 mmHg; 18.0 mg/L) independently of blood perfusion by generating chimeric animal-plant tissues during dermal regeneration [Chavez et al., 2016]. They demonstrated the safety and efficacy of photosynthetic biomaterials in vivo after engraftment in a fully immunocompetent mouse skin defect model. In addition to oxygen generation, photosynthetic microalgae can be genetically modified to express the angiogenic recombinant protein VEGF (vascular endothelial growth factor) demonstrating its use as a versatile platform for photosynthetic biomaterials for tissue engineering.
The creation of a membrane binding complex of myoglobin and an anionic polymer-surfactant is another such recently introduced and versatile system. Myoglobin is a protein which has a similar structure to hemoglobin and thus binds oxygen to its structure [Endeward et al., 2010]. This novel polymer complex of myoglobin was delivered to the cytoplasmic membrane of human MSCs [Armstrong et al., 2015]. Pretreating MSCs with this complex was shown to improve tissue distribution and biochemical composition of hyaline cartilage. This direct cell oxygenation avoids the size limitations imposed by diffusion limited mass transport and should enable cartilage, bone, and cardiac tissue engineering.
Another recent ‘microtank’ approach utilized polymeric hollow microspheres which can be hyperbarically loaded with oxygen (total O2 1.04 μmol in 8.5 hr) [Cook et al., 2015]. Polycaprolactone scaffold embedded with microtanks was shown to prolong the survival of human adipose-derived stem cells (hASCs) and human umbilical vein endothelial cells (HUVECs) under hypoxic conditions. The results of this study suggest the microtank approach may be a feasible means of maintaining cell viability in tissue engineered scaffolds during the critical period of vascularization in vivo.
Overall these novel tools are versatile and easily adaptable to the constantly evolving field of tissue engineering and associated methodologies. Interestingly, no recent reports of expansion of these technologies in tissue engineering applications were found. They bear great potential and hence must be explored in various tissue engineering applications and perhaps eventually extended to human studies.
5.5. Oxygenating strategies in spheroids and organoids
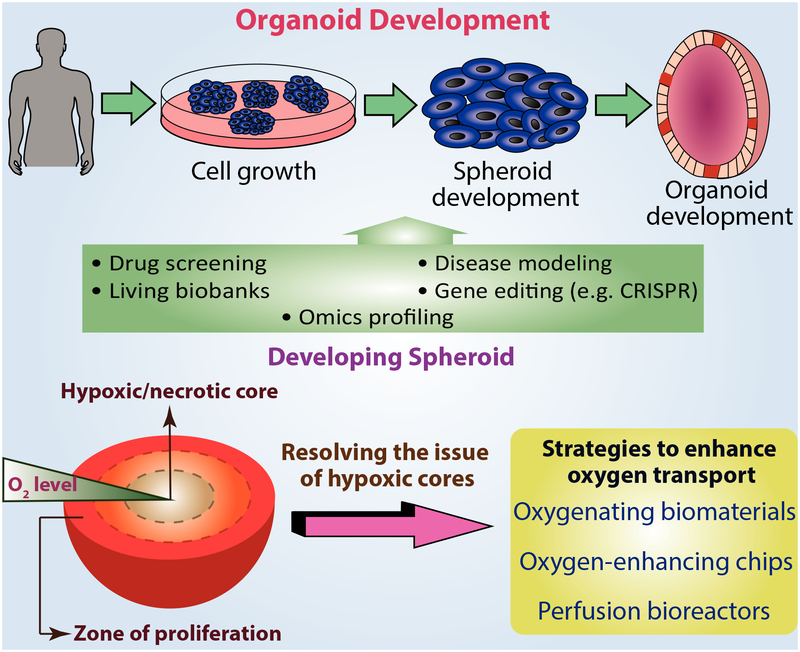
Spheroids and organoids have recently been introduced as a 3D approach in tissue engineering in an attempt to better relate the results of in vitro studies to in vivo conditions. Spheroids and organoids also provide potential tools to help improve the biological relevance of in vitro model platforms to human medicine [Fennema et al., 2013]. By definition, spheroids (or multicellular aggregates) are building blocks of ‘microtissues’ with controllable composition and tailorable biological properties [Mironov et al., 2009]. Spheroids owe their diverse applications in tissue engineering to the 3D microenvironment they provide for cells, and they have been applied to many different cell types, such as stem cells, hepatocytes, and neuronal cells [Fang and Eglen, 2017]. In a 3D environment, cells are subject to a heterogeneous spatial distribution of oxygen diffusion and nutrients. Spheroids are great candidates for mimicking these physiologic in vivo conditions towards forming complex organ-like structures and offer the ability to use human cells to better reproduce and study human responses [Laschke and Menger, 2017]. Despite all the advantages of spheroids for tissue engineering, their application is often challenged by insufficient oxygen diffusion, especially in their cores (Fig. 4). Lack of vasculature in spheroids, which triggers the formation of HIFs, often results in cell apoptosis in the central oxygen and nutrient-deficient regions in prolonged cases of oxygen deficiency [Laschke and Menger, 2017; Lazzari et al., 2017]. To overcome the issue of insufficient oxygen diffusion and the formation of hypoxic cores, especially in cells with high metabolic activity, several in vitro strategies have been developed to prevent necrosis and improve spheroid functionality via direct oxygen delivery [Lou and Leung, 2018]. In line with this approach, Anada et al., have utilized 3D culture chips made of gas-permeable polydimethylsiloxane (PDMS) to enable direct oxygen supply to the cells within their spheroids while maintaining the cell viability and function with an equilibrium oxygen tension of 50–60 mmHg Po2 in the culture media after 5 days [Anada et al., 2012]. Their histochemical analyses showed a significant reduction in hypoxic core formation. In a different approach, Kamoya et al. developed oxygen-permeable spheroid culture chips (Oxy chip) to enable direct oxygen delivery to murine MSCs [Kamoya et al., 2016]. They observed an oxygen partial pressure of 130 mmHg in their culture system after 7 days using their oxygen chips, whereas this number decreased to 70 mmHg in their non-Oxy chip controls. The oxygen delivery was also shown to assist the differentiation MSCs to osteoblasts. Furthermore, spheroids cultured on the Oxy chip showed viable nucleated cores, whereas cell necrosis was observed at the center of the non-Oxy chip controls. Similarly, Pedraza et al. designed a PDMS-encapsulated solid calcium peroxide system (PDMS-CaO2) as an oxygenating biomaterial to successfully overcome the issue of hypoxia-induced spheroid necrosis in islet spheroids [Pedraza et al., 2012]. They showed that a single PDMS-CaO2 disk could improve the oxygen concentration in the culture solution from 0.16 ± 0.017 mM (~114 mmHg Po2) on the first week to an average of 0.073 ± 0.007 mM (~52 mmHg Po2) in 4 weeks of these values over baseline controls of PDMS only disks. The enhanced and prolonged proliferation of β cells was observed when co-cultured with their PDMS-CaO2 disks. In an attempt to assess the oxygen tension profile within a spheroid system made of MSCs, Murphy et al. have utilized oxygen-sensitive microelectrodes to measure the oxygen tension as a function of spheroid diameter and correlated that with the existence of a hypoxic core [Murphy et al., 2017]. In their largest spheroids, containing 60,000 cells, the data showed a 10% decrease in oxygen gradients while moving from the outer spheroid diameter towards the inside core. In spite of low radial oxygen fluctuations, the cell metabolism decreased with increasing spheroid size, possibly due to the adaptive changes in matrix deposition and packing density of the spheroids. These observations indicate that the function of MSC spheroids and their hypoxic cores are non-oxygen-dependent.
Fig. 4.
The overall development and application of organoids emphasizing the issue of inhomogeneous oxygen delivery throughout the developing spheroid.
Moving one step closer towards mimicking the actual properties of tissues, organoids are introduced as in vitro approaches for culturing small fetal or adult organ-like structures for follow-on in vitro study or for in vivo implantation [Lou and Leung, 2018]. An organoid is defined as a group of organ-specific cells that are developed from stem cells or organ progenitors [Fang and Eglen, 2017]. Organoids present promising potential for applications such as organ replacement, modeling of many diseases, drug discovery and safety screening studies [Hu et al., 2018] (Fig. 4). So far, organoids have been formed for intestinal, stomach, liver, kidney, brain and retinal tissue replacements [Xinaris et al., 2015]. However, current organoid technologies face significant challenges, including managing the various cell types in each specific organoid system, while providing the appropriate ECM for each cell type as well as improving the oxygen and nutrient requirements for each particular cell type within the same organoid [Lou and Leung, 2018]. In addition, oxygen diffusion issues lead to the formation of a necrotic core at the center of the organoid, preventing the normal development of the structure [Kelava and Lancaster, 2016]. Considering the fact that size of a cultured organoid depends highly on the maximum diffusion distance of nutrients and in particular oxygen, tackling the oxygen diffusion challenge could result in significant advances in organoid approaches [Akkerman and Defize, 2017].
5.5.1. Bioreactor-based strategies and microfluidics in organoid development
A straightforward strategy to enhance oxygen perfusion within an organoid system is to use higher oxygen levels in a culture setup [Akkerman and Defize, 2017]. However, this approach might cause severe toxicity if not appropriately controlled for oxygen radical formation [Halliwell and Gutteridge, 1984]. Another novel approach to tackle the oxygen diffusion issue is to use spinning bioreactors to provide a driving force for diffusion. In this regard, Qian et al. have developed a spinning bioreactor and successfully cultured forebrain-specific organoids originated from human induced pluripotent stem cells (iPSCs) [Qian et al., 2016]. In a different approach, continuous perfusion bioreactors have been used to maintain the long-term viability of hepatocyte spheroids in a silicon wafer with an array of channels and cell adhesive walls. Bioreactor dimensions, with a channel width of 300 μm, were designed such that achieved perfusion rates of 8.2×10–8 mol/cm3/s met the expected hepatocyte oxygen consumption rates (3.5×10–8 mol/cm3/s). The results indicated long-term maintenance of the spheroids and the corresponding formation of tissue structures [Powers et al., 2002]. Perfusion bioreactors have also been used to develop intestinal [Kim et al., 2007], skeletal muscle [Chromiak et al., 1998], heart [Maidhof et al., 2012], lung [Ott et al., 2010], and liver [Baptista et al., 2011] organoids. Recently DiStefano et al. have developed a rotating-wall vessel (RVW) bioreactor to culture pluripotent stem cells and differentiate them into 3D retinal organoids [DiStefano et al., 2018]. They showed enhanced the proliferation as well as the well-defined differentiation of these cells into neurons and S-cone photoreceptors. Miniaturized spinning bioreactors have also been used to develop region-specific brain organoids derived from pluripotent stem cells [Qian et al., 2018]. Despite the lack of vasculature, these multi-well spinning bioreactors have been able to successfully produce fore-brain, midbrain and hypothalamus organoids over a period of 14 to 84 days, indicating the efficient oxygen and nutrient transport regime.
Despite the promising advantages of spinning bioreactors, developing larger tissue structures is still in need of enhanced oxygen diffusion, especially because organoids cannot grow their own vasculature. To address this issue, microfluidics and bioprinting approaches are currently studying how artificial vessels can be added to tissue engineering scaffolds to reduce oxygen/nutrient deficiencies in organoid cultures, thus allowing for the formation of larger tissue constructs [Zhang et al., 2017]. For example, Zhang et al. have used a composite bio-ink to co-print endothelial cells inside a microfiber hydrogel scaffold [Zhang et al., 2016]. The fluorescent images of their multi-layered scaffold cross-sections showed that incorporated human umbilical vein endothelial cells were able to form tubular patterns similar to that of blood vessel walls. Furthermore, they successfully produced an endothelialized myocardium organoid with controlled anisotropy, which indicates enhanced oxygen transport through the scaffold. Additionally, to make an enhanced platform for cardiac toxicity studies, researchers have used microfluidics technologies to form an endothelialized-myocardium-on-a-chip using a perfusion bioreactor. The design of their bioreactors was such that the perfusion rate of 50 μL/min provided a situation where endothelialized myocardial construct could experience the oxygen concentration of 0.12mM (~85 mmHg Po2) throughout the scaffold.
6. Future directions and concluding remarks
In summary, variations in oxygen bioavailability affect embryonic development starting from the very early stages of blastocyst formation to morphogenesis and organogenesis and continue to be important during angiogenesis and into adulthood. Oxygen contributes to development by signaling proliferation, differentiation, angiogenesis, morphogenesis, and organogenesis processes as well as providing required energy via anaerobic glycolysis. Different levels of oxygen are required at each stage of embryonic development and thus understanding the specific in vivo microenvironment, as well as the levels of oxygen present at each stage during normal fetal development, helps instruct the environments needed for tissue engineering approaches where fine-tuning of cell proliferation and differentiation are essential. These mostly in vitro tissue engineering approaches are a response to diseases and injuries after birth, where the formed embryonic tissues and organs might require repair or replacement. Tissue engineering applies principles of normal development for the sake of secondary tissue development. Therefore, any success in the field of tissue engineering relies on a thorough understanding of the factors involved in the formation of new tissues during development. As one of these major factors, oxygen availability during development and tissue formation in a growing embryo provides clues of how this process can be mimicked in a tissue engineering setting, and further to aid the development of new technologies, such as organoids, to better mimic native tissue microenvironments.
It is worth stating that the findings discussed in this review highlight that the field is lacking agreed upon accurate and reliable technologies to measure the levels of oxygen both in vitro and in vivo. This is especially important in each stage of embryonic development, which could translate to direct design criteria to satisfy specific requirements of adult tissues to form appropriate tissue engineered structures. This would also provide a direct means to recognize the amount of oxygen needed in vitro to form organ-like structures.
Angiogenesis occurs in response to insufficient levels of oxygen as required by a developing tissue or organ in vivo. However, in a tissue-engineered construct or organoid/spheroid, the lack of a vasculature component makes it difficult to mimic the normal developmental process in vitro entirely, and thus inhibits the growth of any newly formed tissue after a certain point. At such a tipping point, a supplemental oxygenating system might be beneficial to support the oxygen requirements of the system to allow further growth and enhance ultimate functions. In this regard, oxygen releasing biomaterials have recently emerged to help overcome the challenges of inadequate oxygen supply within a tissue-engineered structure or organoid. Thus, expanding research on engineered oxygen carriers, present valuable new tools to overcome the challenges caused by inadequate oxygen supply. These approaches are still in their infancy as oxygen-specific design criteria for tissue engineering and organoid systems are still being formulated while new technologies are constantly emerging and evolving to meet these requirements.
7.3.
Funding Sources
The authors would like to acknowledge the NIH (NIDDK R41 DK105704–01A1) and NSF (1647555) for support.
List of abbreviations
- ALA
5-Aminolevulinic Acid
- AP-1
Activator Protein-1
- ARNT
Aryl Hydrocarbon Receptor Nuclear Translocator
- ATP
Adenosine-5’-Triphosphate
- BTC-tet
Pancreatic Beta Cells
- CDC
Cardiosphere-Derived Cell
- DNA
Deoxyribonucleic Acid
- ECM
Extracellular Matrix
- EF5
2-Nitroimidazole
- eNOS
Nitric Oxide Synthase
- EPR
Electron Paramagnetic Resonance
- ESC
Embryonic Stem Cells
- FC-43
Perfluorotributylamine
- FDA
Food and Drug Administration
- FIH-1
Factor-Inhibiting HIF-1
- hASCs
Human Adipose-Derived Stem Cells
- Hb
Hemoglobin
- HbOCs
Hemoglobin-Based Oxygen Carriers
- hESCs
Human Embryonic Stem Cells
- HIFs
Hypoxia-Inducible Factors
- HUVECs
Human Umbilical Vein Endothelial Cells
- iPSCs
Induced Pluripotent Stem Cells
- MACF
Methacrylamide Chitosan Hydrogel
- mmHg
Millimeter Of Mercury
- MRI
Magnetic Resonance Imaging
- MSCs
Mesenchymal Stem Cells
- NF
Nuclear Factor
- NIR
Near-Infrared
- NSPCs
Neural Stem Progenitor Cells
- PDMS
Polydimethylsiloxane
- PEG
Polyethylene Glycol
- PET
Positron Emission Tomography
- PFCs
Perfluorocarbons
- PFO
Perfluorooctane
- PFOB
Perfluorooctanoylbromide
- PFOC
Pentadecafluoroctanoylchloride
- PHDs
Prolyl Hydroxylases
- Po2
Oxygen Partial Pressure
- PPIX
Protoporphyrin IX
- PTMC
Poly(Trimethylene Carbonate)
- RT
Radiotherapy
- RVW
Rotating-Wall Vessel
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Statements
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
8. References
- Abdollahi H, Harris LJ, Zhang P, McIlhenny S, Srinivas V, Tulenko T, DiMuzio PJ (2011) The role of hypoxia in stem cell differentiation and therapeutics. The Journal of surgical research 165(1): 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkerman N, Defize LH (2017) Dawn of the organoid era: 3D tissue and organ cultures revolutionize the study of development, disease, and regeneration. BioEssays: news and reviews in molecular, cellular and developmental biology 39(4). [DOI] [PubMed] [Google Scholar]
- Akula S, Brosch IK, Leipzig ND (2017) Fluorinated Methacrylamide Chitosan Hydrogels Enhance Cellular Wound Healing Processes. Annals of biomedical engineering 45(11): 2693–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anada T, Fukuda J, Sai Y, Suzuki O (2012) An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 33(33): 8430–8441. [DOI] [PubMed] [Google Scholar]
- Araldi E, Schipani E (2010) Hypoxia, HIFs and bone development. Bone 47(2): 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JPK, Shakur R, Horne JP, Dickinson SC, Armstrong CT, Lau K, Kadiwala J, Lowe R, Seddon A, Mann S, Anderson JLR, Perriman AW, Hollander AP (2015) Artificial membrane-binding proteins stimulate oxygenation of stem cells during engineering of large cartilage tissue. Nature Communications 6: 7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydogdu O, Burgu B, Gocun PU, Ozden E, Yaman O, Soygur T, Dursun A, Aydos K (2012) Near infrared spectroscopy to diagnose experimental testicular torsion: comparison with Doppler ultrasound and immunohistochemical correlation of tissue oxygenation and viability. The Journal of urology 187(2): 744–750. [DOI] [PubMed] [Google Scholar]
- Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S (2011) The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology (Baltimore, Md) 53(2): 604–617. [DOI] [PubMed] [Google Scholar]
- Barinaga M (1997) Neuroscience: What Makes Brain Neurons Run?: Science (New York, NY: ), American Association for the Advancement of Science, p 196. [Google Scholar]
- Benz S, Nötzli S, Siegel JS, Eberli D, Jessen HJ (2013) Controlled Oxygen Release from Pyridone Endoperoxides Promotes Cell Survival under Anoxic Conditions. Journal of medicinal chemistry 56(24): 10171–10182. [DOI] [PubMed] [Google Scholar]
- Bian Y, Chang TMS (2015) A novel nanobiotherapeutic poly-[hemoglobin-superoxide dismutasecatalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a rat model with 90 minutes of sustained severe hemorrhagic shock with loss of 2/3 blood volume. Artificial cells, nanomedicine, and biotechnology 43(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianconi A, Congiu-Castellano A, Dell’Ariccia M, Giovannelli A, Burattini E, Durham PJ (1985) Increase of the Fe effective charge in hemoproteins during oxygenation process. Biochemical and biophysical research communications 131(1): 98–102. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M (2001) Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scandinavian journal of medicine & science in sports 11(4): 213–222. [DOI] [PubMed] [Google Scholar]
- Boveris A, Costa LE, Cadenas E, Poderoso JJ (1999) Regulation of mitochondrial respiration by adenosine diphosphate, oxygen, and nitric oxide. Methods in enzymology 301: 188–198. [DOI] [PubMed] [Google Scholar]
- Bryant JD, Brown MC, Dobrikov MI, Dobrikova EY, Gemberling SL, Zhang Q, Gromeier M (2018) Regulation of HIF-1alpha during Hypoxia by DAP5-Induced Translation of PHD2. Molecular and cellular biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler PW, D’Agnillo F, Schaer DJ (2010) Hemoglobin-based oxygen carriers: from mechanisms of toxicity and clearance to rational drug design. Trends in molecular medicine 16(10): 447–457. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Esham WT, Bull RW (1969) The renal handling of hemoglobin: I. Glomerular filtration. Journal of Experimental Medicine 129(5): 909–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camci-Unal G, Alemdar N, Annabi N, Khademhosseini A (2013) Oxygen-releasing biomaterials for tissue engineering. Polymer International 62(6): 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield DE (2005) The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu Rev Earth Planet Sci 33(1): 1–36. [Google Scholar]
- Carreau A, Hafny-Rahbi BE, Matejuk A, Grillon C, Kieda C (2011) Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. Journal of Cellular and Molecular Medicine 15(6): 1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti C, Ridley AJ (2017) Endothelial cell-cell adhesion and signaling. Experimental Cell Research 358(1): 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH, Schmidley JW, Fishman RA, Longar SM (1984) Brain injury, edema, and vascular permeability changes induced by oxygen-derived free radicals. Neurology 34(3): 315–320. [DOI] [PubMed] [Google Scholar]
- Chavez MN, Schenck TL, Hopfner U, Centeno-Cerdas C, Somlai-Schweiger I, Schwarz C, Machens HG, Heikenwalder M, Bono MR, Allende ML, Nickelsen J, Egana JT (2016) Towards autotrophic tissue engineering: Photosynthetic gene therapy for regeneration. Biomaterials 75: 25–36. [DOI] [PubMed] [Google Scholar]
- Chen F-M, Liu X (2016) Advancing biomaterials of human origin for tissue engineering. Progress in polymer science 53: 86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhao M, Liu K, Wan Y, Li X, Feng G (2014) Novel chitosan hydrogel formed by ethylene glycol chitosan, 1,6-diisocyanatohexan and polyethylene glycol-400 for tissue engineering scaffold: in vitro and in vivo evaluation. J Mater Sci: Mater Med 25(8): 1903–1913. [DOI] [PubMed] [Google Scholar]
- Chromiak JA, Shansky J, Perrone C, Vandenburgh HH (1998) Bioreactor perfusion system for the long-term maintenance of tissue-engineered skeletal muscle organoids. In vitro cellular & developmental biology Animal 34(9): 694–703. [DOI] [PubMed] [Google Scholar]
- Chubb C (1985) Reversal of the endocrine toxicity of commercially produced perfluorochemical emulsion. Biology of reproduction 33(4): 854–858. [DOI] [PubMed] [Google Scholar]
- Clarke L, van der Kooy D (2009) Low Oxygen Enhances Primitive and Definitive Neural Stem Cell Colony Formation by Inhibiting Distinct Cell Death Pathways. Stem cells (Dayton, Ohio) 27(8): 1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CA, Hahn KC, Morrissette-McAlmon JBF, Grayson WL (2015) Oxygen Delivery from Hyperbarically Loaded Microtanks Extends Cell Viability in Anoxic Environments. Biomaterials 52: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J (1975) Control of somite number during morphogenesis of a vertebrate, Xenopus laevis. Nature 254(5497): 196–199. [DOI] [PubMed] [Google Scholar]
- Coronel MM, Geusz R, Stabler CL (2017) Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials 129: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L (2003) VEGF-receptor signal transduction. Trends in biochemical sciences 28(9): 488–494. [DOI] [PubMed] [Google Scholar]
- Davenport JJ, Hickey M, Phillips JP, Kyriacou PA (2016) Fiber-optic fluorescence-quenching oxygen partial pressure sensor using platinum octaethylporphyrin. Applied optics 55(21): 5603–5609. [DOI] [PubMed] [Google Scholar]
- De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr., Shin WS, Liao JK (1995) Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. The Journal of clinical investigation 96(1): 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis V, Singer M (2015) Tissue oxygen tension monitoring of organ perfusion: rationale, methodologies, and literature review. British journal of anaesthesia 115(3): 357–365. [DOI] [PubMed] [Google Scholar]
- Deng W, Feng X, Li X, Wang D, Sun L (2016) Hypoxia-inducible factor 1 in autoimmune diseases. Cellular immunology 303: 7–15. [DOI] [PubMed] [Google Scholar]
- DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, Swaroop A (2018) Accelerated and Improved Differentiation of Retinal Organoids from Pluripotent Stem Cells in Rotating-Wall Vessel Bioreactors. Stem Cell Reports 10(1): 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwoodie SL (2009) The role of hypoxia in development of the Mammalian embryo. Developmental cell 17(6): 755–773. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Hallett MB, Campbell AK (1984) Oxygen-radical production during inflammation may be limited by oxygen concentration. Biochemical Journal 217(3): 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbrey JR, Albala L, Kramer MR, Daroshefski N, Brown D, Liu JB, Stanczak M, O’Kane P, Forsberg F, Wheatley MA (2015) Development of an ultrasound sensitive oxygen carrier for oxygen delivery to hypoxic tissue. International journal of pharmaceutics 478(1): 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC, King BF (1991) Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. The American journal of anatomy 192(4): 329–346. [DOI] [PubMed] [Google Scholar]
- Endeward V, Gros G, Jürgens KD (2010) Significance of myoglobin as an oxygen store and oxygen transporter in the intermittently perfused human heart: a model study. Cardiovascular Research 87(1): 22–29. [DOI] [PubMed] [Google Scholar]
- Espes D, Lau J, Banerjee U, Palmer AF, Carlsson P-O (2015) Cotransplantation of polymerized hemoglobin reduces β-cell hypoxia and improves β-cell function in intramuscular islet grafts. Transplantation 99(10): 2077–2082. [DOI] [PubMed] [Google Scholar]
- Evans SM, Hahn S, Pook DR, Jenkins WT, Chalian AA, Zhang P, Stevens C, Weber R, Weinstein G, Benjamin I, Mirza N, Morgan M, Rubin S, McKenna WG, Lord EM, Koch CJ (2000) Detection of hypoxia in human squamous cell carcinoma by EF5 binding. Cancer research 60(7): 2018–2024. [PubMed] [Google Scholar]
- Evans SM, Judy KD, Dunphy I, Jenkins WT, Nelson PT, Collins R, Wileyto EP, Jenkins K, Hahn SM, Stevens CW, Judkins AR, Phillips P, Geoerger B, Koch CJ (2004) Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer research 64(5): 1886–1892. [DOI] [PubMed] [Google Scholar]
- Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A 102(13): 4783–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller DV (1999) Endothelial cell responses to hypoxic stress. Clinical and experimental pharmacology & physiology 26(1): 74–84. [DOI] [PubMed] [Google Scholar]
- Fang Y, Eglen RM (2017) Three-Dimensional Cell Cultures in Drug Discovery and Development. Slas Discovery 22(5): 456–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris AL, Rindone AN, Grayson WL (2016) Oxygen Delivering Biomaterials for Tissue Engineering. Journal of materials chemistry B 4(20): 3422–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema E, Rivron N, Rouwkema J, van Blitterswijk C, de Boer J (2013) Spheroid culture as a tool for creating 3D complex tissues. Trends in biotechnology 31(2): 108–115. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Burggren WW (2007) Role of hypoxia in the evolution and development of the cardiovascular system. Antioxidants & redox signaling 9(9): 1339–1352. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Wang HY (1999) Acetylcholine release from cat carotid bodies. Brain research 841(1–2): 53–61. [DOI] [PubMed] [Google Scholar]
- Fraisl P, Mazzone M, Schmidt T, Carmeliet P (2009) Regulation of angiogenesis by oxygen and metabolism. Developmental cell 16(2): 167–179. [DOI] [PubMed] [Google Scholar]
- Frankenberg SR, de Barros FR, Rossant J, Renfree MB (2016) The mammalian blastocyst. Wiley interdisciplinary reviews Developmental biology 5(2): 210–232. [DOI] [PubMed] [Google Scholar]
- Gattas-Asfura KM, Fraker CA, Stabler CL (2012) Perfluorinated alginate for cellular encapsulation. Journal of biomedical materials research Part A 100(8): 1963–1971. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ (1997) Regulation of human placental development by oxygen tension. Science (New York, NY) 277(5332): 1669–1672. [DOI] [PubMed] [Google Scholar]
- Geyer RP (1988) Perfluorochemicals as oxygen transport vehicles. Biomaterials, artificial cells, and artificial organs 16(1–3): 31–49. [DOI] [PubMed] [Google Scholar]
- Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M, Seifalian AM (2016) Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends in biotechnology 34(12): 1010–1021. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Salinas CE, Villena M, Blanco CE (2007) The role of oxygen in prenatal growth: studies in the chick embryo. The Journal of physiology 585(Pt 3): 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh F, Gross JD, Simpson NE, Sambanis A (2010) Limited beneficial effects of perfluorocarbon emulsions on encapsulated cells in culture: experimental and modeling studies. Journal of biotechnology 150(2): 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Presnell JS (2017) Hypoxia Inducible Factor (HIF) transcription factor family expansion, diversification, divergence and selection in eukaryotes. PLoS ONE 12(6): e0179545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LG, Naughton G (2002) Tissue engineering-current challenges and expanding opportunities. Science (New York, NY) 295(5557): 1009–1014. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT (2005) Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell metabolism 1(6): 401–408. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. The Biochemical journal 219(1): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ (2007) Oxygen producing biomaterials for tissue regeneration. Biomaterials 28(31): 4628–4634. [DOI] [PubMed] [Google Scholar]
- Harrison HE, Bunting H, Ordway NK, Albrink WS (1947) THE PATHOGENESIS OF THE RENAL INJURY PRODUCED IN THE DOG BY HEMOGLOBIN OR METHEMOGLOBIN. The Journal of Experimental Medicine 86(4): 339–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AJ, Kind KL, Pantaleon M, Armstrong DT, Thompson JG (2004) Oxygen-regulated gene expression in bovine blastocysts. Biology of reproduction 71(4): 1108–1119. [DOI] [PubMed] [Google Scholar]
- Hathazi D, Mot AC, Vaida A, Scurtu F, Lupan I, Fischer-Fodor E, Damian G, Kurtz DM, Silaghi-Dumitrescu R (2014) Oxidative Protection of Hemoglobin and Hemerythrin by Cross-Linking with a Nonheme Iron Peroxidase: Potentially Improved Oxygen Carriers for Use in Blood Substitutes. Biomacromolecules 15(5): 1920–1927. [DOI] [PubMed] [Google Scholar]
- Hemker SL, Sims-Lucas S, Ho J (2016) Role of hypoxia during nephrogenesis. Pediatric nephrology (Berlin, Germany) 31(10): 1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JL, Todhunter ME, LaBarge MA, Gartner ZJ (2018) Opportunities for organoids as new models of aging. The Journal of cell biology 217(1): 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Chia W-T, Chung M-F, Lin K-J, Hsiao C-W, Jin C, Lim W-H, Chen C-C, Sung H-W (2016) An Implantable Depot That Can Generate Oxygen in Situ for Overcoming Hypoxia-Induced Resistance to Anticancer Drugs in Chemotherapy. Journal of the American Chemical Society 138(16): 5222–5225. [DOI] [PubMed] [Google Scholar]
- Hutton DL, Grayson WL (2016) Hypoxia Inhibits De Novo Vascular Assembly of Adipose-Derived Stromal/Stem Cell Populations, but Promotes Growth of Preformed Vessels. Tissue Engineering Part A 22(1–2): 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Mow VC, Butler D, Niklason L, Huard J, Mao J, Yannas I, Kaplan D, Vunjak-Novakovic G (2006) Tissue engineering and developmental biology: going biomimetic. Tissue engineering 12(12): 3265–3283. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science (New York, NY) 292(5516): 464–468. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Dubois N, Rasko JE, Deng H, Alvarado AS, Fuchs E, Vunjak-Novakovic G, Kristin B (2018) Challenging Stem Cells: Cell. Cell, pp 1063–1065. [Google Scholar]
- Jensen FB, Fago A, Weber RE (1998) Hemoglobin structure and function. Fish physiology 17: 1–40. [Google Scholar]
- Kamoya T, Anada T, Shiwaku Y, Takano-Yamamoto T, Suzuki O (2016) An oxygen-permeable spheroid culture chip (Oxy chip) promotes osteoblastic differentiation of mesenchymal stem cells. Sensors and Actuators B: Chemical 232: 75–83. [Google Scholar]
- Kao I, Xiong Y, Steffen A, Smuda K, Zhao L, Georgieva R, Pruss A, Bäumler H (2018) Preclinical In Vitro Safety Investigations of Submicron Sized Hemoglobin Based Oxygen Carrier HbMP-700. Artificial organs. [DOI] [PubMed] [Google Scholar]
- Kelava I, Lancaster MA (2016) Dishing out mini-brains: Current progress and future prospects in brain organoid research. Developmental biology 420(2): 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak SF, Chin KS, Bhatia SR, Roberts SC (2007) Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnology and bioengineering 96(1): 156–166. [DOI] [PubMed] [Google Scholar]
- Kim SS, Penkala R, Abrahimi P (2007) A perfusion bioreactor for intestinal tissue engineering. The Journal of surgical research 142(2): 327–331. [DOI] [PubMed] [Google Scholar]
- Koch CJ (2002) Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods in enzymology 352: 3–31. [DOI] [PubMed] [Google Scholar]
- Krock BL, Skuli N, Simon MC (2011) Hypoxia-Induced Angiogenesis: Good and Evil. Genes & cancer 2(12): 1117–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Bandyopadhyay U (2005) Free heme toxicity and its detoxification systems in human. Toxicology letters 157(3): 175–188. [DOI] [PubMed] [Google Scholar]
- Lai Y-T, Sato M, Ohta S, Akamatsu K, Nakao S.-i., Sakai Y, Ito T (2015) Preparation of uniform-sized hemoglobin-albumin microspheres as oxygen carriers by Shirasu porous glass membrane emulsification technique. Colloids and Surfaces B: Biointerfaces 127: 1–7. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rucker M, Junker D, Haufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD (2006) Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue engineering 12(8): 2093–2104. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Menger MD (2017) Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends in biotechnology 35(2): 133–144. [DOI] [PubMed] [Google Scholar]
- Lazzari G, Couvreur P, Mura S (2017) Multicellular tumor spheroids: a relevant 3D model for the in vitro preclinical investigation of polymer nanomedicines. Polymer Chemistry 8(34): 4947–4969. [Google Scholar]
- Le Pape F, Richard G, Porchet E, Sourice S, Dubrana F, Férec C, Polard V, Pace R, Weiss P, Zal F, Delépine P, Leize E (2018) Adhesion, proliferation and osteogenic differentiation of human MSCs cultured under perfusion with a marine oxygen carrier on an allogenic bone substitute. Artificial Cells, Nanomedicine, and Biotechnology 46(1): 95–107. [DOI] [PubMed] [Google Scholar]
- Lee H-Y, Kim H-W, Lee JH, Oh SH (2015) Controlling oxygen release from hollow microparticles for prolonged cell survival under hypoxic environment. Biomaterials 53: 583–591. [DOI] [PubMed] [Google Scholar]
- Li H, Wijekoon A, Leipzig ND (2014) Encapsulated Neural Stem Cell Neuronal Differentiation in Fluorinated Methacrylamide Chitosan Hydrogels. Annals of biomedical engineering 42(7): 1456–1469. [DOI] [PubMed] [Google Scholar]
- Li Z, Guo X, Guan J (2012) An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials 33(25): 5914–5923. [DOI] [PubMed] [Google Scholar]
- Lin G, Macdonald RL, Marton LS, Kowalczuk A, Solenski NJ, Weir BK (2001) Hemoglobin increases endothelin-1 in endothelial cells by decreasing nitric oxide. Biochemical and biophysical research communications 280(3): 824–830. [DOI] [PubMed] [Google Scholar]
- Liu S, Shah SJ, Wilmes LJ, Feiner J, Kodibagkar VD, Wendland MF, Mason RP, Hylton N, Hopf HW, Rollins MD (2011) Quantitative tissue oxygen measurement in multiple organs using 19F MRI in a rat model. Magnetic resonance in medicine 66(6): 1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode A, Krujatz F, Bruggemeier S, Quade M, Schutz K, Knaack S, Weber J, Bley T, Gelinsky M (2015) Green bioprinting: Fabrication of photosynthetic algae-laden hydrogel scaffolds for biotechnological and medical applications. Engineering in Life Sciences 15(2): 177–183. [Google Scholar]
- Logsdon EA, Finley SD, Popel AS, Gabhann FM (2014) A systems biology view of blood vessel growth and remodelling. Journal of Cellular and Molecular Medicine 18(8): 1491–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YR, Leung AW (2018) Next generation organoids for biomedical research and applications. Biotechnology advances 36(1): 132–149. [DOI] [PubMed] [Google Scholar]
- Lourens A, van den Brand H, Heetkamp MJ, Meijerhof R, Kemp B (2007) Effects of eggshell temperature and oxygen concentration on embryo growth and metabolism during incubation. Poultry science 86(10): 2194–2199. [DOI] [PubMed] [Google Scholar]
- Lovett M, Lee K, Edwards A, Kaplan DL (2009) Vascularization Strategies for Tissue Engineering. Tissue Engineering Part B, Reviews 15(3): 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe KC, Davey MR, Power JB (1998) Perfluorochemicals: their applications and benefits to cell culture. Trends in biotechnology 16(6): 272–277. [DOI] [PubMed] [Google Scholar]
- Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature biotechnology 23(1): 47–55. [DOI] [PubMed] [Google Scholar]
- Lv X, Li Z, Chen S, Xie M, Huang J, Peng X, Yang R, Wang H, Xu Y, Feng C (2016) Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 84: 99–110. [DOI] [PubMed] [Google Scholar]
- Maidhof R, Tandon N, Lee EJ, Luo J, Duan Y, Yeager K, Konofagou E, Vunjak-Novakovic G (2012) Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med 6(10): e12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio A, Scaffaro R, Lentini L, Palumbo Piccionello A, Pibiri I (2018) Perfluorocarbons-graphene oxide nanoplatforms as biocompatible oxygen reservoirs. Chemical Engineering Journal 334: 54–65. [Google Scholar]
- Maltepe E, Saugstad OD (2009) Oxygen in health and disease: regulation of oxygen homeostasis- clinical implications. Pediatric research 65(3): 261–268. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC (1997) Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386(6623): 403–407. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, McCutcheon IE, Francisco DL, Metzenberg AB, Welch JE (1981) Oxygen availability and growth of the chick embryo. Respiration physiology 46(2): 81–88. [DOI] [PubMed] [Google Scholar]
- Michiels C (2004) Physiological and Pathological Responses to Hypoxia. The American Journal of Pathology 164(6): 1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30(12): 2164–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell stem cell 7(2): 150–161. [DOI] [PubMed] [Google Scholar]
- Moore M, Moore R, McFetridge PS (2013) Directed oxygen gradients initiate a robust early remodeling response in engineered vascular grafts. Tissue Eng Part A 19(17–18): 2005–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutluoglu M, Cakkalkurt A, Uzun G, Aktas S (2013) Topical Oxygen for Chronic Wounds: A PRO/CON Debate. The Journal of the American College of Clinical Wound Specialists 5(3): 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narva E, Pursiheimo JP, Laiho A, Rahkonen N, Emani MR, Viitala M, Laurila K, Sahla R, Lund R, Lahdesmaki H, Jaakkola P, Lahesmaa R (2013) Continuous hypoxic culturing of human embryonic stem cells enhances SSEA-3 and MYC levels. PLoS ONE 8(11): e78847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM (2008) Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. Jama 299(19): 2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New DA, Coppola PT (1970) Effects of different oxygen concentrations on the development of rat embryos in culture. Journal of reproduction and fertility 21(1): 109–118. [DOI] [PubMed] [Google Scholar]
- Nordsmark M, Loncaster J, Aquino-Parsons C, Chou S-C, Ladekarl M, Havsteen H, Lindegaard JC, Davidson SE, Varia M, West C, Hunter R, Overgaard J, Raleigh JA (2003) Measurements of hypoxia using pimonidazole and polarographic oxygen-sensitive electrodes in human cervix carcinomas. Radiotherapy and Oncology 67(1): 35–44. [DOI] [PubMed] [Google Scholar]
- O’Brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Materials Today 14(3): 88–95. [Google Scholar]
- Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS (2009) Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30(5): 757–762. [DOI] [PubMed] [Google Scholar]
- Ortega JA, Sirois CL, Memi F, Glidden N, Zecevic N (2017) Oxygen Levels Regulate the Development of Human Cortical Radial Glia Cells. Cerebral cortex (New York, NY: 1991) 27(7): 3736–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP (2010) Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine 16(8): 927–933. [DOI] [PubMed] [Google Scholar]
- Palumbo FS, Di Stefano M, Palumbo Piccionello A, Fiorica C, Pitarresi G, Pibiri I, Buscemi S, Giammona G (2014) Perfluorocarbon functionalized hyaluronic acid derivatives as oxygenating systems for cell culture. RSC Advances 4(44): 22894–22901. [Google Scholar]
- Papkovsky DB, Dmitriev RI (2018) Imaging of oxygen and hypoxia in cell and tissue samples. Cellular and Molecular Life Sciences: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Chang J.h., Choi M, Pak JJ, Lee DY, Pak YK (2007) Microfabirated Clark-type Sensor for Measuing Dissolved Oxygen: 2007 IEEE Sensors, pp 1412–1415. [Google Scholar]
- Parks DA, Shah AK, Granger DN (1984) Oxygen radicals: effects on intestinal vascular permeability. The American journal of physiology 247(2 Pt 1): G167–170. [DOI] [PubMed] [Google Scholar]
- Parveen S, Krishnakumar K, Sahoo SK (2006) New Era in Health Care: Tissue Engineering. Journal of Stem Cells & Regenerative Medicine 1(1): 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Chivukula IV, Roy S, Khanna S, He G, Ojha N, Mehrotra A, Dias LM, Hunt TK, Sen CK (2005) Oxygen: from the benefits of inducing VEGF expression to managing the risk of hyperbaric stress. Antioxidants & redox signaling 7(9–10): 1377–1387. [DOI] [PubMed] [Google Scholar]
- Patil PS, Fountas-Davis N, Huang H, Michelle Evancho-Chapman M, Fulton JA, Shriver LP, Leipzig ND (2016) Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. Acta Biomaterialia 36: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL (2012) Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 109(11): 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin S, Alpert B, Michalowicz A (1982) An investigation by iron K-edge spectroscopy of the oxidation state of iron in hemoglobin and its subunits. FEBS letters 147(1): 106–110. [DOI] [PubMed] [Google Scholar]
- Pishchany G, McCoy AL, Torres VJ, Krause JC, Crowe JE Jr, Fabry ME, Skaar EP (2010) Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell host & microbe 8(6): 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar K, Anderson KC (2010) A therapeutic role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell cycle (Georgetown, Tex) 9(9): 1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers MJ, Domansky K, Kaazempur-Mofrad MR, Kalezi A, Capitano A, Upadhyaya A, Kurzawski P, Wack KE, Stolz DB, Kamm R, Griffith LG (2002) A microfabricated array bioreactor for perfused 3D liver culture. Biotechnology and bioengineering 78(3): 257–269. [DOI] [PubMed] [Google Scholar]
- Presley T, Kuppusamy P, Zweier JL, Ilangovan G (2006) Electron paramagnetic resonance oximetry as a quantitative method to measure cellular respiration: a consideration of oxygen diffusion interference. Biophysical journal 91(12): 4623–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nature medicine 9(6): 677–684. [DOI] [PubMed] [Google Scholar]
- Pugsley MK, Tabrizchi R (2000) The vascular system: An overview of structure and function. Journal of Pharmacological and Toxicological Methods 44(2): 333–340. [DOI] [PubMed] [Google Scholar]
- Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL (2018) Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nature protocols 13(3): 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky G, Jacob F, Zhong C, Yoon K.-j., Jeang W, Lin L, Li Y, Thakor J, Berg D, Zhang C, Kang E, Chickering M, Naeun D, Ho C-Y, Wen Z, Christian KM, Shi P-Y, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming G.-l. (2016) Brain Region-specific Organoids using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165(5): 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G (2006) Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue engineering 12(8): 2077–2091. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ, Schipani E (2011) A Central Role for Hypoxic Signaling in Cartilage, Bone, and Hematopoiesis. Current osteoporosis reports 9(2): 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran R, Seitz V, Venugopal JR, Sridhar R, Sundarrajan S, Mukherjee S, Wintermantel E, Ramakrishna S (2013) Mimicking Native Extracellular Matrix with Phytic Acid-Crosslinked Protein Nanofibers for Cardiac Tissue Engineering. Macromolecular Bioscience 13(3): 366–375. [DOI] [PubMed] [Google Scholar]
- Reddi AH (2014) Chapter 11 - Morphogenesis and Tissue Engineering A2 - Lanza, Robert; in Langer R, Vacanti J (eds): Principles of Tissue Engineering (Fourth Edition), Boston, Academic Press, pp 209–223. [Google Scholar]
- Rodriguez-Brotons A, Bietiger W, Peronet C, Langlois A, Magisson J, Mura C, Sookhareea C, Polard V, Jeandidier N, Zal F, Pinget M, Sigrist S, Maillard E (2016) Comparison of Perfluorodecalin and HEMOXCell as Oxygen Carriers for Islet Oxygenation in an In Vitro Model of Encapsulation. Tissue Eng Part A 22(23–24): 1327–1336. [DOI] [PubMed] [Google Scholar]
- Romagnoli S, Zagli G, Ricci Z (2015) Diaspirin Cross-Linked Hemoglobin and Blood Substitutes: Reducing Mortality in Critically Ill Patients, Springer, pp 83–91. [Google Scholar]
- Rother RP, Bell L, Hillmen P, Gladwin MT (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293(13): 1653–1662. [DOI] [PubMed] [Google Scholar]
- Rouwkema J, Khademhosseini A (2016) Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends in biotechnology 34(9): 733–745. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JWM (2011) Fluorine ((19)F) MRS and MRI in biomedicine. NMR in biomedicine 24(2): 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H (2017) Overview of potential clinical applications of hemoglobin vesicles (HbV) as artificial red cells, evidenced by preclinical studies of the academic research consortium. Journal of functional biomaterials 8(1): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiesteban DY, Kubelick K, Dhada KS, Dumani D, Suggs L, Emelianov S (2016) Monitoring/Imaging and Regenerative Agents for Enhancing Tissue Engineering Characterization and Therapies. Annals of biomedical engineering 44(3): 750–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeren TWL, Schober P, Schwarte LA (2012) Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. Journal of Clinical Monitoring and Computing 26(4): 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck TL, Hopfner U, Chavez MN, Machens HG, Somlai-Schweiger I, Giunta RE, Bohne AV, Nickelsen J, Allende ML, Egana JT (2015) Photosynthetic biomaterials: a pathway towards autotrophic tissue engineering. Acta Biomater 15: 39–47. [DOI] [PubMed] [Google Scholar]
- Schipani E, Maes C, Carmeliet G, Semenza GL (2009) Regulation of Osteogenesis-Angiogenesis Coupling by HIFs and VEGF. Journal of Bone and Mineral Research 24(8): 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrey A, Niemi T, Kinnunen I, Minn H, Vahlberg T, Kalliokoski K, Suominen E, Grenman R, Aitasalo K (2010) The limitations of tissue-oxygen measurement and positron emission tomography as additional methods for postoperative breast reconstruction free-flap monitoring. Journal of plastic, reconstructive & aesthetic surgery: JPRAS 63(2): 314–321. [DOI] [PubMed] [Google Scholar]
- Semenza GL (2010) Oxygen homeostasis. Wiley interdisciplinary reviews Systems biology and medicine 2(3): 336–361. [DOI] [PubMed] [Google Scholar]
- Serex T, Anand S, Munley S, Donner EM, Frame SR, Buck RC, Loveless SE (2014) Toxicological evaluation of 6:2 fluorotelomer alcohol. Toxicology 319: 1–9. [DOI] [PubMed] [Google Scholar]
- Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NNM, Campbell A, Devito L, Ilic D, Khalaf Y, Niakan KK, Fishel S, Zernicka-Goetz M (2016) Self-organisation of the human embryo in the absence of maternal tissues. Nature cell biology 18(6): 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Zhao F-Q (2014) Emerging evidence of the physiological role of hypoxia in mammary development and lactation. Journal of Animal Science and Biotechnology 5(1): 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MC, Keith B (2008) The role of oxygen availability in embryonic development and stem cell function. Nature reviews Molecular cell biology 9(4): 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Ji C, Liang C, Song X, Yi X, Dong Z, Yang K, Liu Z (2017) TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials 112: 257–263. [DOI] [PubMed] [Google Scholar]
- Spahn DR (2000) Current status of artificial oxygen carriers. Adv Drug Deliver Rev 40(3): 143–151. [DOI] [PubMed] [Google Scholar]
- Stamati K, Mudera V, Cheema U (2011) Evolution of oxygen utilization in multicellular organisms and implications for cell signalling in tissue engineering. J Tissue Eng 2(1): 2041731411432365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg H, Buizer AT, Woudstra W, Veldhuizen AG, Bulstra SK, Grijpma DW, Kuijer R (2017) Oxygen-releasing poly(trimethylene carbonate) microspheres for tissue engineering applications. Polymers for Advanced Technologies 28(10): 1252–1257. [Google Scholar]
- Stoppel WL, Roberts SC (2012) Oxygen supply for tissue engineering: Engineering Biomaterials for Regenerative Medicine, Springer, pp 41–86. [Google Scholar]
- Takahashi M, Ishida S, Hirata-Koizumi M, Ono A, Hirose A (2014) Repeated dose and reproductive/developmental toxicity of perfluoroundecanoic acid in rats. The Journal of toxicological sciences 39(1): 97–108. [DOI] [PubMed] [Google Scholar]
- Terraneo L, Bianciardi P, Malavalli A, Mkrtchyan G, Spann SN, Lohman J, Samaja M, Vandegriff KD (2017) Hemoglobin extravasation in the brain of rats exchange-transfused with hemoglobin-based oxygen carriers. Artificial Cells, Nanomedicine, and Biotechnology 45(4): 710–716. [DOI] [PubMed] [Google Scholar]
- Tuderman L, Myllyla R, Kivirikko KI (1977) Mechanism of the prolyl hydroxylase reaction. The FEBS Journal 80(2): 341–348. [DOI] [PubMed] [Google Scholar]
- Uno K, Merges CA, Grebe R, Lutty GA, Prow TW (2007) Hyperoxia Inhibits Several Critical Aspects of Vascular Development. Developmental dynamics: an official publication of the American Association of Anatomists 236(4): 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M (2005) Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. American journal of physiology Lung cellular and molecular physiology 288(1): L167–178. [DOI] [PubMed] [Google Scholar]
- van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL (2002) Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. Journal of cell science 115(Pt 9): 1837–1846. [DOI] [PubMed] [Google Scholar]
- Varia MA, Calkins-Adams DP, Rinker LH, Kennedy AS, Novotny DB, Fowler WC, Raleigh JA (1998) Pimonidazole: A Novel Hypoxia Marker for Complementary Study of Tumor Hypoxia and Cell Proliferation in Cervical Carcinoma. Gynecologic Oncology 71(2): 270–277. [DOI] [PubMed] [Google Scholar]
- Varnado CL, Mollan TL, Birukou I, Smith BJ, Henderson DP, Olson JS (2013) Development of recombinant hemoglobin-based oxygen carriers. Antioxidants & redox signaling 18(17): 2314–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen T, Hunt JA (2015) Tissue engineering red blood cells: a therapeutic. Journal of tissue engineering and regenerative medicine 9(7): 760–770. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu Y, Bawa HK, Ng G, Wu Y, Libera M, van der Mei HC, Busscher HJ, Yu X (2011) Oxygen-Generating Nanofiber Cell Scaffolds with Antimicrobial Properties. ACS Applied Materials & Interfaces 3(1): 67–73. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang R, Lu M, You G, Wang Y, Chen G, Zhao C, Wang Z, Song X, Wu Y (2017) Bioinspired polydopamine-coated hemoglobin as potential oxygen carrier with antioxidant properties. Biomacromolecules 18(4): 1333–1341. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao S (2010) Vascular Biology of the Placenta: Cell Types of the Placenta. Chapter 4, San Rafael CA, Morgan & Claypool Life Sciences. [PubMed] [Google Scholar]
- Ward CL, Corona BT, Yoo JJ, Harrison BS, Christ GJ (2013) Oxygen generating biomaterials preserve skeletal muscle homeostasis under hypoxic and ischemic conditions. PLoS One 8(8): e72485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman K (1986) Perfluorocarbons as blood substitutes. Annals of Emergency Medicine 15(12): 1423–1424. [DOI] [PubMed] [Google Scholar]
- Weyand B, Nohre M, Schmalzlin E, Stolz M, Israelowitz M, Gille C, von Schroeder HP, Reimers K, Vogt PM (2015) Noninvasive Oxygen Monitoring in Three-Dimensional Tissue Cultures Under Static and Dynamic Culture Conditions. BioResearch open access 4(1): 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijekoon A, Fountas-Davis N, Leipzig ND (2013) Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing. Acta Biomater 9(3): 5653–5664. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhang J, Lin Y, Gaeta X, Meng X, Dona R Cinkornpumin Wisidagama, J., Koehler Carla M., Malone Cindy S., Teitell Michael A., Lowry William E. (2014) Defining the Role of Oxygen Tension in Human Neural Progenitor Fate. Stem Cell Reports 3(5): 743–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinaris C, Brizi V, Remuzzi G (2015) Organoid Models and Applications in Biomedical Research. Nephron 130(3): 191–199. [DOI] [PubMed] [Google Scholar]
- Yoon AP, Jones NF (2016) Critical time for neovascularization/angiogenesis to allow free flap survival after delayed postoperative anastomotic compromise without surgical intervention: A review of the literature. Microsurgery 36(7): 604–612. [DOI] [PubMed] [Google Scholar]
- Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell’Erba V, Bishop C, Shupe T, Demarchi D, Moretti M, Rasponi M, Dokmeci MR, Atala A, Khademhosseini A (2016) Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110: 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, Yang J, Jia W, Dell’Erba V, Assawes P, Shin SR, Dokmeci MR, Oklu R, Khademhosseini A (2017) 3D Bioprinting for Tissue and Organ Fabrication. Annals of biomedical engineering 45(1): 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimna A, Kurpisz M (2015) Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Research International 2015: 549412. [DOI] [PMC free article] [PubMed] [Google Scholar]