Abstract
Background and Aims:
The objective of this study was to determine the effect of acute IV alcohol infusion on skin blood flow (SBF) response, measured at fingertip and earlobe, and subjective responses associated with SBF in social drinkers.
Methods:
24 social drinkers underwent a computer-assisted alcohol self-infusion study. SBF was measured continuously using laser Doppler flow meter, with the probe placed on the fingertip or earlobe. Perfusion recordings collected at baseline, and at 0-min (0–5 min), 10-min (10–15 min) and 20-min (20–25 min) time-points during the priming phase of IV alcohol self-administration paradigm at low breath alcohol levels of approximately 30 mg%. Subjective response was measured using the Drug Effects Questionnaire (DEQ), and Biphasic Alcohol Effects Scale (BAES).
Results:
Overall SBF (collective data from both fingertip and earlobe); and SBF by each site showed significant drop at 0-min and then subsequent significant elevation with alcohol self-administration. Males showed higher overall SBF at baseline and 0-min than the females. At finger site, lowering in 0-min SBF compared to baseline, and subsequent significant increase at 10-min and 20-min SBF recordings were observed. DEQ measures of “like” and “want more” alcohol were significantly associated with 10- and 20-min SBF recordings collected at finger site.
Conclusion:
The changes in SBF following acute IV alcohol exposure is consistent with the sympathetic response of alcohol on the cardiovascular system. This acute hemodynamic effect characterizes differences in blood flow that are sensitive to relatively low levels of acute alcohol exposure. The association of subjective perceptions with the SBF response provide evidence of the psychophysiological effects of alcohol at low levels of exposure.
Keywords: Alcohol, IV-Self Administration, Perfusion, Skin Blood Flow, Subjective Responses
Introduction
Perfusion or blood flow is regulated by the cardiovascular reactivity that is derived from the responses in the autonomic nervous system (Blessing, 2016). Change in hemodynamic activity from a resting (baseline) state to a subsequent behavioral state could be caused by a psychological or physical challenge (Obrist, 1981). Acute changes in hemodynamic system are evaluated by the measure of perfusion or skin blood flow (SBF) in response to acute challenge prompted by behavioral and pharmacological stressors (Kamarck, Jennings et al. 1992). Acute alcohol exposure could cause alterations in cardiovascular response and associated autonomic system (Doggett, 2018; Weise, 1986; Koskinen, 1994). Stimulating effects of alcohol on heart rate has been reported previously (Conrod, 2001). Such subtle changes appearing with acute alcohol exposure are difficult to register in humans, particularly at lower levels and variable rates of exposure. None-the-less, these changes are important to understand the changes in cardiovascular response due to alcohol (Kupari, 1998; Howes, 1986; Donahue, 1986), as they may help explain how attributes that determine vulnerability to developing cardiovascular conditions could start or progress with alcohol intake. However, there is a gap in the scientific literature in the understanding of cardiovascular responses specially SBF, occurring due to acute alcohol exposure.
Acute intravenous (IV) alcohol administration is an experimental human paradigm that could be used in studying alcohol pharmacokineticsprecisely (Ramchandani, 2009; Kwo, 1998). Our primary aim was to identify the changes in SBF that occur due to acute alcohol exposure using the IV alcohol self-administration paradigm (Stangl, 2016). To test differences in SBF at different sites, we also conducted a consistency test by repeating the experiment, one at the fingertip and the other at the earlobe site.
Studies have examined the subjective responses and physiological effect of acute oral alcohol administration that appeared as acute cardiac responses previously (Vatsalya, 2014, Hu, 2016). Identifying subjective response measures that may be associated with alterations in cardiovascular response could help better identify psychophysiological indicators of the consequences of alcohol intake (Brunelle, 2007). However, association of these changes observed in the subjective perception and corresponding blood distribution originating as cardiovascular response, namely SBF are inconclusive. Thus, we also evaluated the relationship in the effects of acute alcohol exposure on SBF and subjective responses using intravenous alcohol exposure. There are reports on sex differences in the bio-behavioral response to stress stimuli leading to the changes in cardiovascular responses (Dickerson, 2004; Taylor, 2000), however, SBF has not been evaluated to identify sex differences during acute alcohol administration. In our study, we further explored the role of sex in the development of subjective responses along with SBF to acute alcohol exposure.
Subjects and Methods
Recruitment:
The study was approved by the NIH Addictions Institutional Review Board, and conducted at the NIH Clinical Center in Bethesda, MD. This is one of the study aims of a larger clinical trial protocol indexed at ClinicalTrials.gov Identifier: NCT00713492. Twenty-three healthy 21–45 year old social drinkers underwent two intravenous alcohol self-administration (IV-ASA) sessions, each lasting 150 min, using the computer-assisted infusion system (Zimmermann, 2013). We used IV alcohol administration since it provides precise level of blood alcohol compared to oral, which has variability in absorption thus exposure varies greatly between the individuals receiving oral alcohol (Ramchandani, 1999). Age, sex, weight was corrected for while establishing dosing amount for each subject individually. Each session consisted of two phases (Stangl et al., 2016). In the priming phase (25 min), subjects are prompted to push the button four times over the first 10 min (exposure section) to receive small standardized alcohol infusions every 2.5 min apart. This resulted in subjects achieving a fixed target BrAC level of approximately 30 mg% around 10 min across all participants. Following a 15-min latent period, participants underwent an ad lib phase lasting 125 min in which subjects had free access to standardized IV alcohol infusions (max BrAC=100 mg %), and were instructed to recreate a typical drinking experience based off of how they felt from the IV alcohol infusions. Data from the ad lib phase will be presented in a separate report.
Measures:
SBF data was collected using Periflux system LDPM - PF5010 (Laser Doppler Perfusion Monitor) (Oppermann, 2007) starting at baseline and continuing throughout the session, to compare the responses at baseline and during IV alcohol exposure. Blood perfusion measured by LDPM is a relative value (that is generally collected from digits or other body locations) that represents the product of the relative number of moving blood cells that causes Doppler shift and the relative velocity of these cells in the measured volume; these values are expressed in perfusion units (PU). The SBF probe was placed on the fingertip in the first session and on the top posterior-proximal region of the earlobe in the second session. Pilot testing of the SBF measurement following probe placement on the fingertip, as is typically done in these studies, suggested high variance (noise) in baseline measurements mainly due to movement of the hand during the testing. Therefore, an alternate site, the earlobe, was selected as an alternate site. The earlobe site is less prone to measurement noise related to movement and may provide a more stable alternate site for measurement of SBF. In both sessions, subjects were instructed to sit still and minimize any movements, thus establishing a stable baseline prior to the measurement interval, at each time-point. Blood pressure and heart rate were monitored at baseline and during the experiment as standard of care and they did not vary outside the normal range.
The SBF data was analyzed in 5-min epochs at baseline (−5 min till start of infusion), and 0–5, 10–15 and 20–25 min time intervals during the priming phase, and every 15 min during the ad lib phase. In this study, we present the findings collected during the priming phase of the alcohol IV self-administration.
Breath alcohol concentration (BrAC) readings as well as corresponding subjective scales (Alcohol Urge Questionnaire [AUQ], Drug Effects Questionnaire [DEQ], and Biphasic Alcohol Effects Scale [BAES]) were measured (Drummond, 2002; Morean, 2013; Martin, 1993) at baseline, and at regular intervals (0-, 10-, and 20-min) during the priming phase. Recent drinking measures, assessed using Time-line Follow-back (TLFB) for past 90 days (Sobell, 2003), included Total Drinks in the past 90 days (TD90), Drinking Days in past 90 Days (DD90), Drinks per Drinking Day in past 90 Days (DPD90), Average Drinking Days (AvgDD90), Heavy Drinking Days in past 90 Days (HD90).
Statistical Analysis:
Initially, a two-way repeated measures ANOVA was conducted to examine the effect of site of application and timepoint on the SBF measure. There was a main effect of site of application, and as a result, additional analyses were conducted to examine the effect of timepoint on SBF separately for each site. For the analysis of time-related changes in SBF for each site, there were 3 post-hoc contrasts (each timepoint compared to baseline) for which a Bonferroni corrected p-value of 0.05/3 = 0.017 was applied. The relationship between SBF changes and subjective response measures were examined using linear regression analyses with a p-value threshold of 0.05. Recent drinking history (Total Drinks in the past 90 days) and sex were examined as regressors in these analyses. Data from 2 participants at two timepoints were excluded from analysis due to measurement noise artifacts. Data were processed and analyzed using MS Excel 2013 (Microsoft Corp., Redmond WA) and SPSS 22.0 (IBM, Chicago IL).
Results
Demographic and Baseline SBF assessment
Ten female and 13 male subjects participated in this study. There was no significant difference in the age of the participants, however there was an anticipated sex-difference in weight (Table 1). There was large individual variability observed in the SBF measures from both sites. In general, males registered higher baseline SBF at both the sites, with statistically significantly higher values at the fingersite (p=0.047). There were no major differences in recent drinking measures, other than a trend-level higher drinks per drinking day (DPD90) in the males.
Table 1:
Demographic and baseline skin blood flow variables in the study participants.
| Measures | Females (n = 10) | Males (n = 13) | p-value |
|---|---|---|---|
| Age [years] |
26 ± 4.3 | 25.3 ± 3.5 | NS |
| Height [cm] |
167.7 ± 5.2 | 181.7 ± 6.7 | ≤ 0.001 |
| Weight [Kg] | 64.2 ± 7.4 | 88.1 ± 15.2 | ≤ 0.001 |
| Baseline SBF | |||
| Finger-site | 41.4±39.6 | 186.6±209.8 | 0.045 |
| Ear-site | 37.6±41.8 | 66.0±53.2 | NS |
| Drinking History | |||
| TD90 | 55.7±43.1 | 115.1±133.1 | NS |
| NDD90 | 22.7±11.1 | 30.2±25.1 | NS |
| AvgDPD90 | 2.4±1.2 | 3.8±2.1 | 0.078 |
| HDD90 | 5.4±7.4 | 10.2±21.0 | NS |
Data presented as Mean±SD. Statistical significance was set at p≤0.05.
Effect of Alcohol on Skin Blood Flow
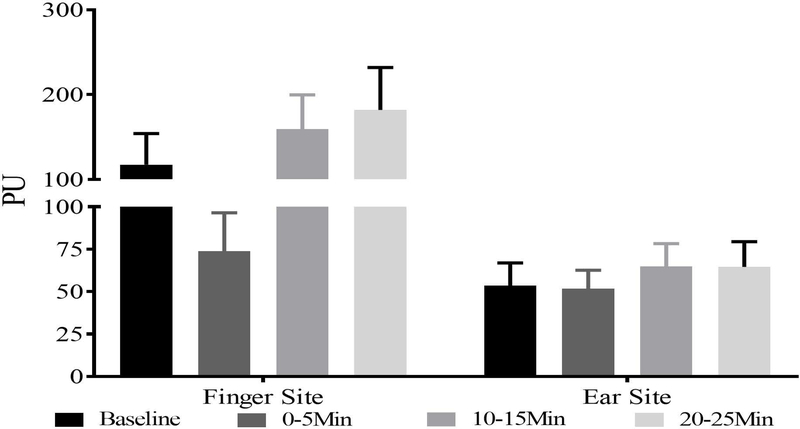
Initial analysis using two-way repeated measures ANOVA showed a significant main effect of site of application, with approximately two-fold higher values for the finger-tip compared to the earlobe. As a result, we examined time-related differences in SBF separately for each site. At the fingertip site, there was a main effect of timepoint (F(3,60)=8.03, p<0.001). SBF decreased by 37% from baseline to the 5-min timepoint (p=0.042), followed by a 116% increase from the 5-min to the 10-min timepoint (p=0.002), and further increase by 14% from 10-min to 20-min (p=0.014). There was no significant effect of recent drinking measures on the SBF measure at the fingertip, however, there was a sex difference observed at baseline, with females showed lower SBF values than males (41.4±39.7 vs. 186.6±209.8; p=0.045). At the earlobe site, there was pattern of increasing SBF with time, however there was no statistically significant effect of timepoint.
Association of SBF with subjective perceptions
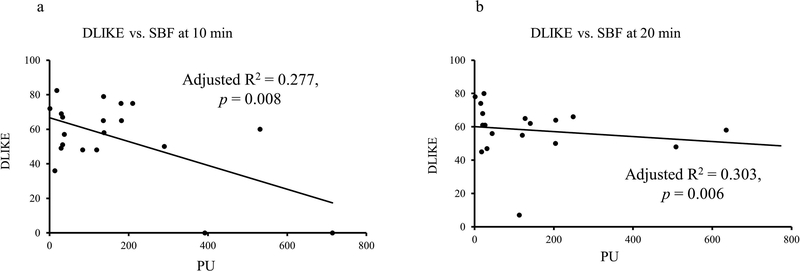
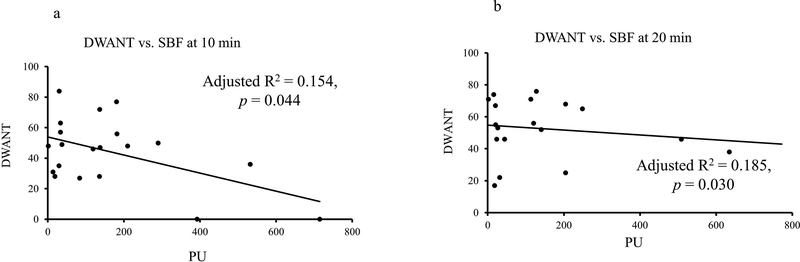
To examine the relationship between SBF changes and subjective perceptions, regression analyses were conducted at each time-epoch. At 10-min, SBF showed a mild, albeit significant, negative association with DEQ Liking (Fig. 2) and DEQ Wanting (Fig. 3). At 10-min timeline, DEQ Liking showed significant association with fingertip site SBF (Fig. 2a); after covarying for TD90 (adjusted R2=0.309, p=0.014). At 20-min timeline, DEQ Liking showed significant association with fingertip site SBF, after covarying for TD90 (adjusted R2=0.315, p=0.013) (Fig. 2b). This relationship persisted between SBF fingertip site and DEQ Wanting response. Unlike DEQ Liking, none of the drinking measures augment the relationship between DEQ Wanting and SBF at fingertip site. There was no association of AUQ and BAES measures and SBF collected at the fingertip site. There was no meaningful association observed between the subjective responses and SBF at the earlobe site.
Figure 2:
Association of subjective responses DEQ “Liking” and SBF recordings collected at the fingertip site. Negative association was found at 10 min. when peak alcohol exposure was achieved. 2a: Association of fingertip site SBF and DLIKE at 10-min time-point. 2b: Association of fingertip site SBF and DLIKE at 20-min time-point.
Figure 3:
Association of subjective responses DEQ “DWANT” and SBF recordings collected at the fingertip site. Negative association was found at 10 min. when peak alcohol exposure was achieved. 3a: Association of fingertip site SBF and DWANT at 10-min time-point. 3b: Association of fingertip site SBF and DWANT at 20-min time-point.
Discussion
The results of this study supported our hypothesis that acute intravenous alcohol administration would show significant changes in skin blood flow (Fig. 1). These alterations started simultaneously with alcohol administration. A previous study showed acute reduction of skin blood flow with cigarette smoking (Waeber, 1984) as an adverse consequence on cardiovascular health. Alcohol also a risk factor for developing cardiovascular complication (Mostofsky, 2016), showed a similar initial drop in SBF. We found a rebound in the skin blood flow levels later during the peak alcohol exposure at 10-min after the initial drop for the fingertip but not the earlobe site. This may be a compensatory response to the initial drop in SBF during the first few min of the infusion. A prior study has shown that compensatory sympathetic response is not just a reflex but is also a reflection of defects in the parasympathetic function (Daly, 1990). At 20-min, skin blood flow at the fingertip site continued to rise, suggesting a continued effect of alcohol.
Figure 1:
Skin blood flow registered during the alcohol infusion session at the fingertip site (panel A) and earlobe (panel B). Significant increases as a function of timepoint were observed for the fingertip site, while no significant effects were observed for the earlobe site.
Overall the SBF values more-or-less represented similar trajectories of recordings as presented at the earlobe site and fingertip site. Some studies have used thermoregulatory effects as external stimulation on vasoconstriction and vasodilation altering SBF responses (Kullmann, 1970). One study showed that subjects with vasoconstriction after alcohol intake performed differently than the subjects who showed vasodilator effects on a motor task (Dengerink, Mead et al. 1978). Vasodilation effects have been reported in preclinical studies targeting neurogenic effects in guinea pigs (Nicoletti, Trevisani et al. 2008). Alcohol consumption might have paradoxical effects on cardiovascular health depending upon the amount and pattern of drinking, main vascular consequence of an acute dose of alcohol has been reported as vasodilation (Hashimoto, Kim et al. 2001, Bau, Bau et al. 2005). Low SBF at 0-min (Fig. 1a, Fig. 1b) recording could have resulted from vasoconstrictive effects of acute alcohol on the cardiovascular-sympathetic activity (Van De Borne, 1997; Vatsalya, 2014). This study has comparatively small sample size and has been conducted as a proof of principal study to observe cardiovascular changes in a highly-controlled human experimental paradigm.
We did not find any significant effect of recent drinking history in the SBF changes following acute alcohol exposure. This lack of effect may be due to the fact that the study participants were social drinkers without heavy drinking patterns and insufficient variability to identify a drinking history influence, and studies have shown that low to moderate drinking could have protective effects on cardiac conditions (Sacco, 1999). There was a baseline sex difference in skin blood flow, with higher values in males. Cardiovascular stress responses to alcohol have been reported as a high risk in males (Stewart, 1992) and both baseline and the initial drop in skin blood flow could suggest the higher sensitivity to these effects in males. Sex differences in SBF at baseline may also be due to anthropomorphic differences in finger volume between males and females. Larger studies are needed to examine these potential sex differences to better understand the cardiovascular pharmacodynamics of alcohol and risk for cardiovascular disease.
Our second aim of this study was supported by the findings from the subjective responses. Subjective responses including alcohol liking and wanting were associated with skin blood flow at the finger-tip site (Fig. 2, Fig. 3). Effects of acute alcohol exposure also showed increasing statistical effect sizes at 20-min recordings compared to 10-min readings for both the liking and wanting responses. These associations at relatively low levels of alcohol exposure (BrACs at or below 30 mg%) suggest that skin blood flow may provide a sensitive physiological correlate of the rewarding and reinforcing effects of acute alcohol. Significant covariate effects of recent drinking measures such as TD90 suggest that drinking patterns may moderate some of the effects of alcohol on skin blood flow and its relationship with subjective perceptions.
This study is not without its limitations. As indicated above, this study was conducted in a relatively small sample of social drinkers and focused on the early priming phase of the session where alcohol levels were consistent across participants. There is no placebo control session in this study, thus it is not possible to distinguish acute pharmacological effects from expectancy effects which could also influence autonomic and cardiovascular reactivity. Having no association of SBF and drinking measure was one of the anticipated limitations of this study in terms of not having additional cohorts of subjects with moderate-to-heavy drinking that could provide information on changes in the perfusion response. Further studies would need to be conducted in larger samples to better understand the effect of factors such as drinking history, sex and level of alcohol exposure. Additionally, we did not statistically adjust for the multiple testing of associations between skin blood flow and the subjective response measures, so these findings should be considered preliminary and would need to be confirmed in future studies. Changes in skin blood flow at higher BrACs levels and correlation with stimulation and sedation effects would provide further evidence of this pharmacodynamic sensitivity. This is particularly relevant as sedative and stimulating effects of alcohol are associated as manifestations of alcohol intoxication contributing to drinking habits (Hendler, 2011; Erblich, 2003).
Data from this study may help improve our understanding of physiological correlates of the subjective response to alcohol, and help establish skin blood flow as a pathophysiological marker of the acute effects of alcohol, both as a correlate of its rewarding effects, and as an indicator of its cardiovascular response, in characterizing the risk of chronic or heavy alcohol drinking.
Acknowledgement:
Authors acknowledge clinical staff of the NIH Clinical Center for the study support.
Grant Support: This investigation was supported by the Division of Intramural Clinical and Biological Research (ZIA-AA000466).
Abbreviations:
- BAES
Biphasic Alcohol Effects Scale
- BrAC
Breath Alcohol Concentration
- CASE
Computer-Assisted Self-Infusion of Ethanol
- DEQ
Drug Effects Questionnaire
- IV
Intravenous
- LDPM
Laser Doppler Perfusion Monitor
- PU
Perfusion Units
- SBF
Skin Blood Flow
Footnotes
Conflict of Interest: All authors declare no conflict of interest and no issues to disclose pertaining to the manuscript publication.
Trial Registration: ClinicalTrials.gov identifier # NCT00713492
Informed Consent: Informed consent was obtained from every participant enrolled in the study protocol.
References
- Bau PFD, Bau CH, Naujorks AA, Rosito GA(2005). “Early and late effects of alcohol ingestion on blood pressure and endothelial function.” Alcohol 37(1): 53–58. [DOI] [PubMed] [Google Scholar]
- Blessing B, & Gibbins I (2016). Autonomic nervous system In Scholarpedia of Touch (pp. 467–477). Atlantis Press. [Google Scholar]
- Brunelle C, Barrett SP, & Pihl RO (2007). Relationship between the cardiac response to acute intoxication and alcohol‐induced subjective effects throughout the blood alcohol concentration curve. Human Psychopharmacology: Clinical and Experimental, 22(7), 437–443. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, & Pihl RO (2001). Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology, 157(1), 20–30. [DOI] [PubMed] [Google Scholar]
- Dengerink HA, Mead JD, Bertilson HS (1978). “Individual differences in response to alcohol. Vasoconstriction and vasodilation.” Journal of studies on alcohol 39(1): 12–18. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological bulletin, 130(3), 355. [DOI] [PubMed] [Google Scholar]
- Doggett TM, Tur JJ, Alves NG, Yuan SY, Tipparaju SM, & Breslin JW (2018). Assessment of Cardiovascular Function and Microvascular Permeability in a Conscious Rat Model of Alcohol Intoxication Combined with Hemorrhagic Shock and Resuscitation In Traumatic and Ischemic Injury (pp. 61–81). Humana Press, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RP, Abbott RD, Reed DM, & Yano K (1986). Alcohol and hemorrhagic stroke: the Honolulu Heart Program. Jama, 255 (17), 2311–2314. [PubMed] [Google Scholar]
- Drummond DC, & Phillips TS (2002). Alcohol urges in alcohol‐dependent drinkers: further validation of the Alcohol Urge Questionnaire in an untreated community clinical population. Addiction, 97(11), 1465–1472. [DOI] [PubMed] [Google Scholar]
- Earleywine M (1994). Anticipated biphasic effects of alcohol vary with risk for alcoholism: a preliminary report. Alcoholism: Clinical and Experimental Research, 18(3), 711–714. [DOI] [PubMed] [Google Scholar]
- Erblich J, & Earleywine M (2003). Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: independent predictors of drinking habits?. Alcoholism: Clinical and Experimental Research, 27(1), 44–50. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Kim S, Eto M, Iijima K, Ako J, Yoshizumi M, Akishita M, Kondo K, Itakura H, Hosoda K, Toba K, Ouchi Y (2001). “Effect of acute intake of red wine on flow-mediated vasodilatation of the brachial artery.” American J Cardiol 88(12): 1457–1460, A1459. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, & Hommer DW (2011). Stimulant and sedative effects of alcohol In Behavioral neurobiology of alcohol addiction (pp. 489–509). Springer Berlin; Heidelberg. [DOI] [PubMed] [Google Scholar]
- Howes LG, & Reid JL (1986). The effects of alcohol on local, neural and humoral cardiovascular regulation. Clinical Science, 71(1), 9–15. [DOI] [PubMed] [Google Scholar]
- Hu TM, Wu MS, Wu WT, Yang FL, & Lee RP (2016). Selective serotonin reuptake inhibitors increase sympathetic activity under heavy alcohol exposure in rat models. Life Sciences. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman‐Weiss E, Johnson PS, Eddy MJ, & Manuck SB (1992). Reliable Measures of Behaviorally‐Evoked Cardiovascular Reactivity from a PC‐Based Test Battery: Results from Student and Community Samples. Psychophysiology, 29(1), 17–28. [DOI] [PubMed] [Google Scholar]
- Koskinen P, Virolainen J, & Kupari M (1994). Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clinical Science, 87(2), 225–230. [DOI] [PubMed] [Google Scholar]
- Kullmann R, Schönung W, & Simon E (1970). Antagonistic changes of blood flow and sympathetic activity in different vascular beds following central thermal stimulation. Pflügers Archiv, 319(2), 146–161. [DOI] [PubMed] [Google Scholar]
- Kupari M, & Koskinen P (1998, November). Alcohol, cardiac arrhythmias and sudden death. In Alcohol and cardiovascular diseases. Novartis Foundation Symposium (Vol. 216, pp. 68–79). [DOI] [PubMed] [Google Scholar]
- Kwo PY, Ramchandani VA, O’Connor S, Amann D, Carr LG, Sandrasegaran K, Kopecky KK, & Li TK (1998). Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology, 115(6), 1552–1557. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, & Swift RM (1993). Development and validation of the biphasic alcohol effects scale. Alcoholism: Clinical and Experimental Research, 17(1), 140–146. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, & O’Malley SS (2013). The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology, 227(1), 177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E, Chahal HS, Mukamal KJ, Rimm EB, & Mittleman MA (2016). Alcohol and Immediate Risk of Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis. Circulation 133(10):979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P, Trevisani M, Manconi M, Gatti R, De Siena G, Zagli G, Benemei S, Capone JA, Geppetti P, Pini LA. (2008). “Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig.” Cephalalgia 28(1): 9–17. [DOI] [PubMed] [Google Scholar]
- Obrist PA (2012). Cardiovascular psychophysiology: A perspective. Springer Science & Business Media. [Google Scholar]
- Oppermann M, Hansen PB, Castrop H, & Schnermann J (2007). Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. American Journal of Physiology-Renal Physiology, 293(1), F279–F287. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Gado MH, Eichling JO, & Ter-Pogossian MM (1976). Correlation between regional cerebral blood flow and oxidative metabolism: in vivo studies in man. Archives of Neurology, 33(8), 523–526. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, & O’Connor S (2009). Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcoholism: Clinical and Experimental Research, 33(5), 938–944. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, & O’Connor S (1999). A Physiologically‐Based Pharmacokinetic (PBPK) Model for Alcohol Facilitates Rapid BrAC Clamping. Alcoholism: Clinical and Experimental Research, 23(4), 617–623. [PubMed] [Google Scholar]
- Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, & Paik MC (1999). The protective effect of moderate alcohol consumption on ischemic stroke. Jama, 281(1), 53–60. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, Lu CS, de Aburto MAK, Chen W, Czernin J, Rapoport SI, Pietrini P, Alexander GE & Schapiro MB (2001). Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. Jama, 286(17), 2120–2127. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Connors GJ, & Agrawal S (2003). Assessing drinking outcomes in alcohol treatment efficacy studies: selecting a yardstick of success. Alcoholism: Clinical and Experimental Research, 27(10), 1661–1666. [DOI] [PubMed] [Google Scholar]
- Stangl BL, Vatsalya V, Zametkin MR, Cooke ME, Plawecki MH, O’Connor S, & Ramchandani VA (2016). Exposure-response relationships during free-access intravenous alcohol self-administration in nondependent drinkers: influence of alcohol expectancies and impulsivity. International journal of neuropsychopharmacology, 20(1), 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Finn PR, & Pihl RO (1992). The effects of alcohol on the cardiovascular stress response in men at high risk for alcoholism: a dose response study. Journal of studies on alcohol, 53(5), 499–506. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, & Updegraff JA (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological review, 107(3), 411. [DOI] [PubMed] [Google Scholar]
- Van De Borne P, Mark AL, Montano N, Mion D, & Somers VK (1997). Effects of alcohol on sympathetic activity, hemodynamics, and chemoreflex sensitivity. Hypertension, 29(6), 1278–1283. [DOI] [PubMed] [Google Scholar]
- Vatsalya V, Momenan R, Hommer DW, & Ramchandani VA (2014). Cardiac reactivity during the ascending phase of acute intravenous alcohol exposure and association with subjective perceptions of intoxication in social drinkers. Alcoholism: Clinical and Experimental Research, 38(5), 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber B, Schaller MD, Nussberger J, Bussien JP, Hofbauer KG, Brunner HR (1984). Skin blood flow reduction induced by cigarette smoking: role of vasopressin. Am J Physiol 247(6), H895–H901. [DOI] [PubMed] [Google Scholar]
- Weise F, Krell D, & Brinkhoff N (1986). Acute alcohol ingestion reduces heart rate variability. Drug and alcohol dependence, 17(1), 89–91. [DOI] [PubMed] [Google Scholar]