Abstract
Introduction:
Olfactory dysfunction is a common symptom of chronic rhinosinusitis (CRS). We previously identified several cytokines potentially linked to smell loss, potentially supporting an inflammatory etiology for CRS-association olfactory dysfunction. In the current study we sought to validate patterns of olfactory dysfunction in CRS using hierarchical cluster analysis, machine learning algorithms, and multivariate regression.
Methods:
CRS patients undergoing functional endoscopic sinus surgery were administered the smell identification test (SIT) preoperatively. Mucus was collected from the middle meatus using an absorbent polyurethane sponge and 17 inflammatory mediators were assessed using a multiplexed flow cytometric bead assay. Hierarchal cluster analysis was performed to characterize inflammatory patterns and their association with SIT scores. The random forest approach was used to identify cytokines predictive of olfactory function.
Results:
110 patients were enrolled in the study. Hierarchical cluster analysis identified 5 distinct CRS clusters with statistically significant differences in SIT scores identified between individual clusters (p<0.001). A majority of anosmic patients were found in a single cluster, which was additionally characterized by nasal polyposis (100%) and a high incidence of allergic fungal rhinosinusitis (50%) and aspirin exacerbated respiratory disease (AERD) (33%). A random forest approach identified a strong association between olfaction and the cytokines IL-5 and IL-13. Multivariate modeling identified AERD, CT score, and IL-2 as the variables most predictive of olfactory function.
Conclusion:
Olfactory dysfunction is associated with specific CRS endotypes that are characterized by severe nasal polyposis, tissue eosinophilia, and AERD. Mucus IL-2 levels, CT score, and AERD were independently associated with smell loss.
Keywords: anosmia, hyposmia, rhinosinusitis, endotype, mucus, cytokine, interleukin, cluster analysis, machine learning
BACKGROUND
Olfactory dysfunction is among the most common symptoms of CRS with a prevalence between 30–80%1,2. Unfortunately, the etiology of CRS-associated olfactory dysfunction remains poorly understood. Olfactory loss in CRS has previously been attributed to an inability of odorants to effectively reach the olfactory cleft, due either to structural abnormalities or presence of nasal polyps3,4 Recent research has suggested that sinonasal inflammation may directly or indirectly affect olfactory neurons and olfactory function5. In animal models, certain cytokines have the ability to adversely affect olfactory neuron function, turnover, and regeneration5–8. An association between olfactory cleft cytokine levels and olfactory function has been partially validated in human tissue by several groups9,10. Recently, our group measured olfactory cleft mucus cytokine levels in CRS patients, and found that objective olfactory function was inversely related to several cytokines, including IL-2, IL-5, IL-6, IL-10, and IL-13. However, this study was limited by a small sample size and was not able to account for potential confounding factors.
In the current study we sought to validate patterns of olfactory dysfunction in CRS using multiple complementary statistical approaches. We previously hypothesized that mucus cytokine levels are reflective of olfactory inflammation and could be predictive of olfactory function11. This study seeks to expand upon this hypothesis by incorporating inflammatory, clinical, and demographic factors, with the ultimate goal of understanding constellations of disease features associated with olfactory dysfunction.
METHODS
Study Design and Population
This study was approved by the Vanderbilt University Institutional Review Board. Patients presented to the Vanderbilt Asthma, Sinus, and Allergy Program (ASAP) and Otolaryngology Clinic at the Vanderbilt Bill Wilkerson Center. CRS was diagnosed according to the European Position Paper on Rhinosinusitis and Nasal Polyps and the International Consensus Statement on Allergy and Rhinology and therefore were initially managed medically12. Patients who chose to undergo endoscopic sinus surgery were prospectively enrolled. Only patients with diffuse, bilateral inflammatory CRS were included, and patients with odontogenic rhinosinusitis, fungus balls, and isolated osteomeatal complex obstruction were excluded. Patients were excluded if they had received systemic steroids within 4 weeks of surgery; had diagnosis of cystic fibrosis, autoimmune, or granulomatous diseases; or were receiving immune-directed monoclonal antibodies. Diagnosis of allergic rhinitis and asthma was recorded. Allergic rhinitis was diagnosed based on positive skin prick testing and/or prior physician diagnosis and clinical history suggestive of seasonal variation of atopic symptoms with improvement following use of topical nasal steroid or oral antihistamines. Asthma was diagnosed based on a positive methacholine challenge or consistent pulmonary function studies, or by prior diagnosis by a pulmonologist. All patients underwent a high resolution CT scan of the paranasal sinuses within 3 months of surgery. Each scan was evaluated by two physicians who were blinded to subject identifiers and diagnosis. A standard Lund Mackay scoring system was used to assess overall extent of CRS. Subjects enrolled in the study also completed the 40-item Smell Identification Test (SIT) immediately prior to surgery, which has been previously validated for olfaction assessment.13. Normative SIT scores were extracted from the Smell Identification Test Administration Manual (Sensonics International; Haddon Heights, NJ). Raw scores were then adjusted for patient age and gender by subtracting the mean normative age- and sex-appropriate SIT score from the total SIT score for each subject10. A negative adjusted SIT score represents reduced sense of smell compared to the mean for that subject’s age and gender.
Mucus Collection and Histopathologic Evaluation of Sinonasal Tissue
At the beginning of surgery, 9 × 24mm polyurethane sponges (Summit Medical; St. Paul, MN) were placed into the middle meatus or ethmoid cavity of each subject under endoscopic guidance as previously reported11. This approach has advantages over other methods for mucus collection, including standardization between subjects and avoidance of specimen dilution. Each sponge was removed after 5 minutes, placed in a sterile microcentrifuge tube and immediately processed. Sponges were placed into a microporous centrifugal filter device (MilliporeSigma; Billerica, MA) and centrifuged at 14,000 x g for 10 minutes to elute mucus. Samples were then gently vortexed and again centrifuged for 5 minutes to remove any cellular debris. Supernatants were removed, placed into a new microcentrifuge tube, and frozen at −80°C for later analysis. Cytokine assays were performed using a multiplex cytokine bead assay (BD Biosciences; Franklin Lakes, NJ) according to the manufacturer’s protocol as previously described14,15.
Sinonasal tissue was collected from the ethmoid bulla or ethmoid sinus in all patients undergoing endoscopic sinus surgery for CRS. Eosinophil and neutrophil counts were obtained from a dedicated, blinded histopathological evaluation of excised tissue by a pathologist and averaged over 5 randomly selected high power fields.
Statistics
Sample size for principal component analysis and subsequent clustering was estimated by establishing a subject to variable ratio of 5 (17 biological variables, 110 subjects) as recommended by Gorsuch and Hatcher16,17. Adequacy of the sample size was verified post hoc by assessing variable communality (heavy loading of variables in retained components). Descriptive statistics and frequency distributions were examined for each biological variable and all were positively skewed. In order to normalize data for subsequent analysis, values were transformed by taking the square root, resulting in elimination or significant reduction of skewing for all variables. A principal component factor analysis with varimax rotation was then performed on the transformed biological variables. Variables with a loading > 0.5 were retained. The appropriate number of factors was selected by analysis of the Scree plot, with a requirement that retained factors explain at least 70% of data variance, and that each factor have an eigenvalue > 1.0. The regression method was then used to calculate a factor score for each subject in each of the five factors. Hierarchical cluster analysis was performed using Ward’s method on squared Euclidian distances using the five factor scores. The hierarchical structure of the data was visualized using a dendogram. The appropriate number of clusters (k) was selected using the Elbow method. Total within sum of squared error (SSE) was calculated for between 2 and 10 clusters and k was chosen at the break point where the SSE started to smooth. Cluster stability was verified using bootstrap analysis, with all clusters having a stability of at least 0.7 (indicating plausible structure and good overall cluster stability)18.
Clusters were then retrospectively compared against the individual components used for analysis, and then against the individual biological variables themselves. Subsequently, clusters were compared against demographic and clinical data. For comparison between groups, normality of data was assessed using the D’Agostino-Pearson omnibus test. Variables with a normal distribution were compared using a student’s t-test or analysis of variance, while nonparametric data was analyzed using the Mann-Whitney test or Kruskal-Wallis test. Comparative data was presented as medians with interquartile range. A p value of < 0.05 was considered statistically significant for all comparisons. Statistical analyses were performed with Prism 6 software (Graphpad; La Jolla, CA), and principal component and hierarchical cluster analysis were performed using R version 3.4 (The R Project for Statistical Computing, Vienna, Austria. http://www.R-project.org/).
The random forest algorithm was used to examine cytokines which were most predictive of SIT score. Analysis was performed in R using the randomForest package19. The training and validation sets each represented half of the samples, chosen at random without replacement. The number of trees generated was 100, 1,000, 10000, and 1,000,000 with 5 variables (i.e., cytokines) chosen at each split. The percent variance explained appeared to level off at ~8% between 100,000 and 1,000,000 trees generated, and therefore the number of trees was not further increased. Variable importance plots were examined for both the training and validation sets for each set of trees generated to verify that the variable importance ordering remained consistent.
Assessment of predictive variables on SIT scores including demographic characteristics and cytokines was assessed with univariate and multivariate regression modeling, performed using STATA (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). In the multivariate model, all clinical factors with P < 0.2 in the univariate modeling were included in models for age- and sex-adjusted olfactory scores. Collinearity diagnostics were performed using the variance inflation factor and when applicable a correlation matrix of the model was utilized to identify variables with collinearity. If a variable was determined to be collinear it was dropped from the model and the model was reanalyzed to determine effect on other predictor coefficients. A value of P < 0.05 was deemed to be statistically significant.
RESULTS:
Study Population and Demographics
Patients included in the study were undergoing functional endoscopic sinus surgery for CRS and completed the validated smell identification test (SIT) immediately prior to their procedure. A total of 110 patients with olfactory testing were enrolled, all of whom are part of an ongoing prospective translational study that has been partially characterized elsewhere11,14,15. A majority of patients had nasal polyps (55%), with comorbid asthma and allergic rhinitis present in 42% and 67% of subjects, respectively (Table 1). Eleven patients had aspirin exacerbated respiratory disease (AERD), while 14 were diagnosed with allergic fungal rhinosinusitis (AFRS). Almost one-half of enrolled subjects had undergone prior endoscopic sinus surgery. Disease burden was significant, with a median CT score of 15.0. The median age- and sex-adjusted SIT score was −7.0 among all CRS patients, with significant differences based on polyp status. CRSwNP (CRS with nasal polyps) patients had a median adjusted SIT score of −20.0 (−5.0 - −26.5) compared to a median score of −3.0 (−1.0 - −7.0) among CRSsNP (CRS without nasal polyps) patients (p <0.0001).
Table 1. Study Population and Demographics.
Values are presented as either the mean ± standard deviation, or median with interquartile range. BOLD, p < 0.05 CRS = chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; yr = year; SNOT- 22 = sino-nasal outcomes test; CT = computed tomography; SIT score = smell identification test score; tissue eos/HPF = tissue eosinophils per high power field; AERD = aspirin exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis
| All CRS | CRSsNP | CRSwNP | P Value | |
|---|---|---|---|---|
| No. (%) | 110 | 49 (45) | 61(55) | * |
| Age, yr | 48.15±13.37 | 49.39±13.86 | 47.15±12.98 | 0.472 |
| Sex, no. male (%) | 59 (54) | 24 (49) | 35 (57) | 0.443 |
| Asthma, no. (%) | 46 (42) | 12 (24) | 34 (56) | 0.007 |
| Allergic rhinitis, no. (%) | 74 (67) | 27 (55) | 47 (77) | 0.024 |
| SNOT-22 score | 44.0 (29.0–58.0) | 48.0 (33.1–57.5) | 43.0 (28.0–61.0) | 0.678 |
| CT score | 15.0 (11.0–20.0) | 12.0 (8.5–14.5) | 18.0 (14.3–22.0) | <0.001 |
| SIT score | −7.0 (−2.0 – −23.1) | −3.0 (−1.0 – −7.0) | −20.0 (−5.0 – −26.5) | <0.001 |
| Prior surgery, no. (%) | 41 (37) | 12 (24) | 29 (48) | 0.017 |
| Tissue eos/HPF | 25.7 (1.7–124) | 2.0 (0.0–25.0) | 80.6 (17.0–226.5) | <0.001 |
| AERD | 11 (10) | 0 (0) | 11 (20) | <0.001 |
| AFRS | 14 (13) | 0 (0) | 14 (25) | <0.001 |
Olfactory Function in Inflammatory CRS Clusters
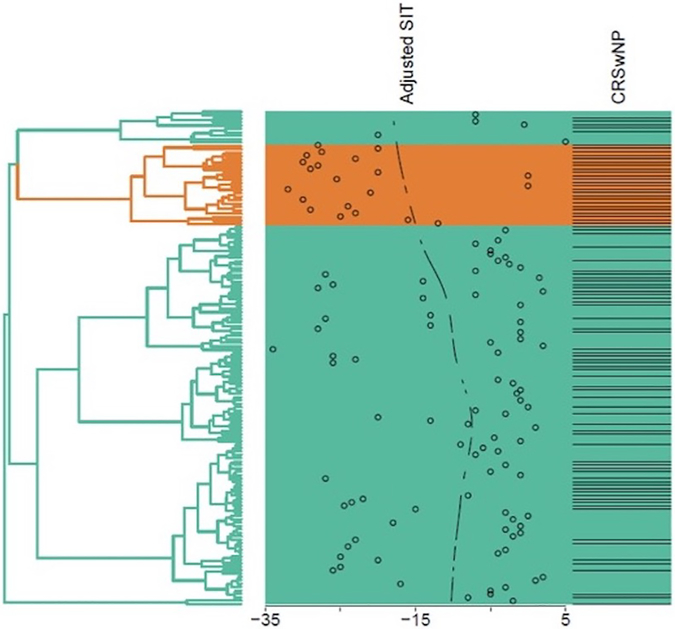
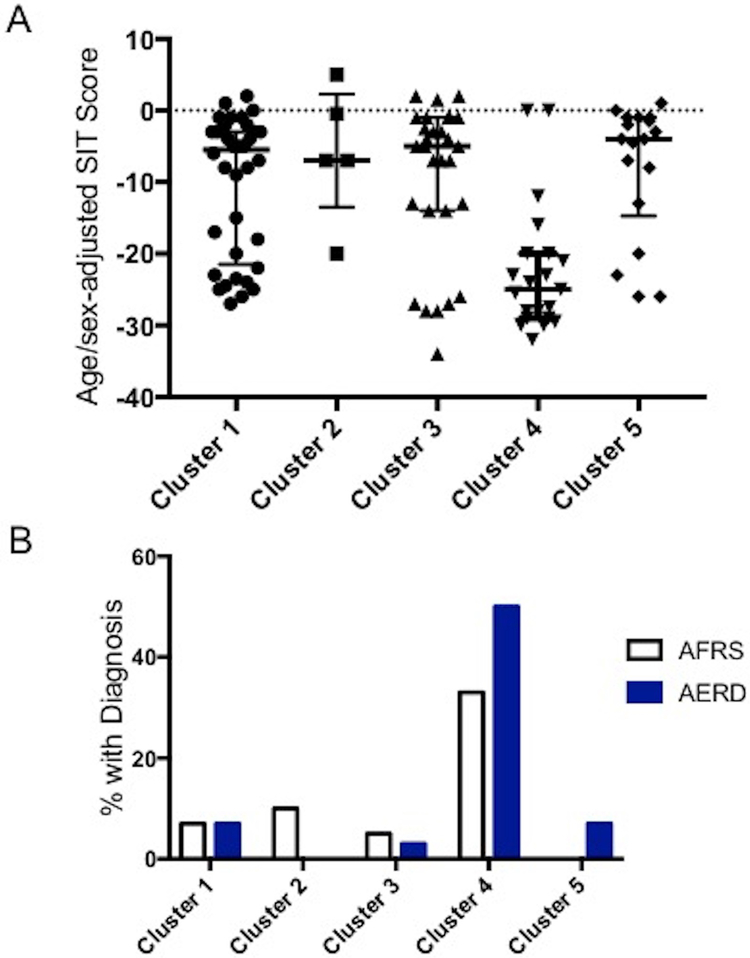
We previously characterized several inflammatory CRS endotypes using hierarchical cluster analysis of mucus cytokines14. In the process of validating these endotypes, we repeated cluster analysis in an updated cohort of 147 patients, 110 of whom had olfactory testing. Hierarchal cluster analysis identified 5 CRS clusters with unique inflammatory signatures (Figure 1). Demographic and clinical characteristics of each cluster are detailed in Table 2. Age- and sex adjusted SIT scores were significantly different between clusters (p<0.001) (Figure 2A). Patients with the worst olfactory function were primarily concentrated within a single cluster (cluster 4) (Figure 1). The median adjusted SIT score in this cluster was −25.0, indicative of total anosmia (Figure 2A)13. More than 80% of patients in this cluster had either AERD or AFRS, both of which varied significantly among all clusters (p<0.001) (Figure 2B). Cluster 4 was associated with a Th2-dominant signature, with elevated levels of IL-5 (p<.001) and IL-13 (p<.001) compared to other clusters (Figure 3).
Figure 1: Dendrogram representing hierarchical cluster analysis of CRS patients and relationship with olfactory function.
Hierarchical cluster analysis was performed using Ward’s method on squared Euclidian distances using 17 cytokines and inflammatory mediators as biological variables. SIT score is recorded in the right panel for individual study subjects and as a continuous mean. Cluster 4, with the lowest SIT scores, is highlighted.
Table 2. Table 2: Characteristics of CRS Clusters.
Values are presented as either the mean ± standard deviation, or median with interquartile range. yr = year; BMI = Body Mass Index; kg = kilogram; m2= meter2; AERD = aspirin exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; SNOT- 22 = sino-nasal outcomes test; CT = computed tomography; SIT score = smell identification test score; tissue eos/HPF = tissue eosinophils per high power field BOLD, p < 0.05
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | p-value | |
|---|---|---|---|---|---|---|
| No. | 46 | 10 | 37 | 24 | 27 | |
| Age (yr) | 47.5 (41.3–57.5) | 66.0 (56.8–70.1) | 46.0 (39.0–58.0) | 46.0 (38.5–53.0) | 53.0 (39.0–60.5) | 0.040 |
| Sex, no. (% female) | 23 (50) | 5 (50) | 22 (59) | 8 (33) | 6 (22) | 0.030 |
| Race, no. (% white) | 39 (85) | 9 (90) | 33 (89) | 19 (79) | 24 (89) | 0.970 |
| Current smoker, no. (%) | 5 (11) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 0.090 |
| BMI (kg/m2) | 28.7 (24.0–34.6) | 28.3 (24.6–31.7) | 30.6 (26.2–33.4) | 29.0 (26.5–32.3) | 29.3 (26.7–34.6) | 0.910 |
| Nasal polyps, no. (%) | 22 (48) | 5 (50) | 20 (54) | 24 (100) | 15 (56) | <0.001 |
| Asthma, no. (%) | 14 (30) | 4 (40) | 16 (43) | 19 (79) | 24 (89) | <0.001 |
| Allergic Rhinitis, no. (%) | 30 (65) | 5 (50) | 21 (57) | 17 (71) | 20 (74) | 0.490 |
| AERD, no. (%) | 3 (7) | 1 (10) | 2 (5) | 8 (33) | 0 (0) | <0.001 |
| AFRS, no. (%) | 3 (7) | 0 (10) | 1 (3) | 12 (50) | 2 (7) | <0.001 |
| SNOT-22 score | 45.6 +/− 16.2 | 45.8 +/− 29.0 | 41.9 +/− 16.4 | 46.1 +/− 22.1 | 50.2 +/− 23.1 | 0.750 |
| CT score | 14.5 (11.1–17.8) | 14.0 (11.0–18.5) | 13.0 (11.0–16.0) | 22.0 (20.0–23.0) | 14.0 (11.0–16.0) | <0.001 |
| SIT score | −5.5 (−20.5–−3.0) | −7.0 (−7.0–−0.5) | −5.0 (−14.0–−1.0) | −25.0 (−29.0–−20.0) | −4.0 (−11.8–−1.1) | <0.001 |
| Prior surgery, no. (%) | 15 (33) | 5 (50) | 14 (38) | 14 (58) | 7 (26) | 0.130 |
| Tissue eos/HPF | 27.5 (2.2–100.0) | 10.0 (5.5–35.5) | 30.0 (3.0–75.0) | 91.5 (50.0–100.0) | 18.0 (0.5–62.5) | 0.001 |
Figure 2: Smell Identification Scores Among Inflammatory CRS Clusters.
(A) SIT scores for individual patients in each cluster are presented as a scatter plot. Bars represent the median and interquartile range. Cluster 4 demonstrates significantly worse SIT scores compared to the other clusters(p<0.001). (B) Cluster 4 was associated with significantly higher prevalence of both AERD and AFRS compared to other clusters (p<0.001).
Figure 3: Cluster 4 is associated with elevated Th2 cytokines and anosmia.
Mucus cytokine levels for individual patients in each cluster are presented as a scatter plot. Bars represent the median and interquartile range. Cluster 4 demonstrates significantly elevated IL-5 (A) (p<.001) and IL-13 (B) (p<.001) compared to the other clusters.
Use of Machine Learning Algorithm to Identify Cytokines Impacting Olfactory Function
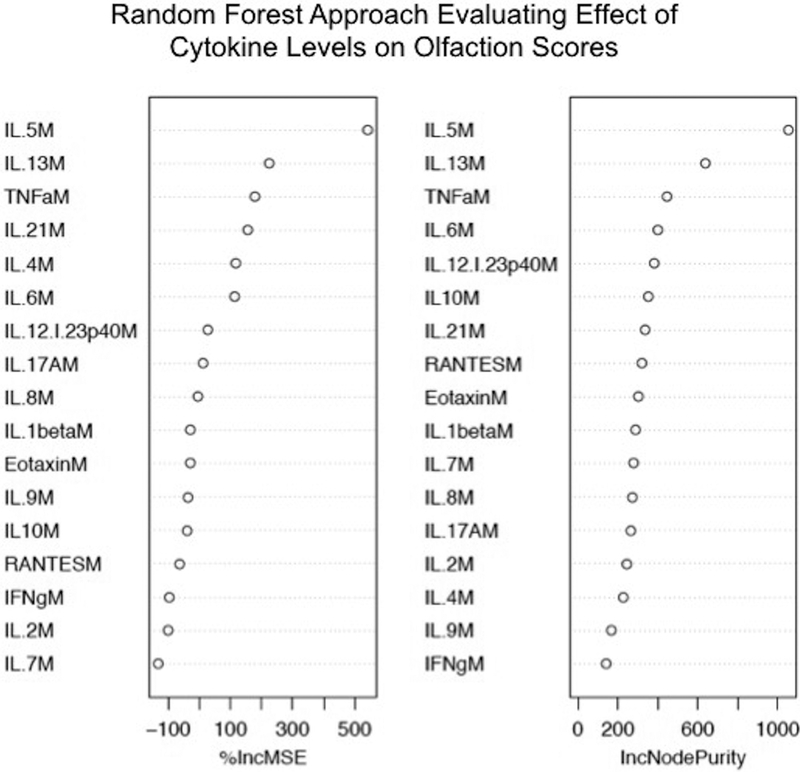
Hierarchical cluster analysis showed a close link between poor olfactory function and a single inflammatory CRS endotype, marked by elevated levels of Th2-associated cytokines. This result is consistent with previous reports that have associated decreased objective olfactory function with Th2 cytokines, including IL-5 and IL-1310,15. We sought to further validate these findings using a random forest model, which is an ensemble machine learning technique that fits decision trees using a random subset of features to predict the outcome of interest. The relative importance of predictors is then computed based on the mean increase in error and decrease in node purity when a variable of interest is excluded from the model. Interestingly, this approach also identified IL-5 and IL-13 as the cytokines most predictive of olfactory function in CRS(Figure 4)19.
Figure 4: Cytokines Predictive of Olfactory Function Using an Ensemble Learning Method.
Each cytokine or inflammatory mediator is ranked based on their relative impact on decision tree construction. Results are presented as the % increase in mean square error (%IncMSE) and the increase in node purity (IncNodePurity), both representative of the impacts of each variable on the overall decision model.
Identification of Variables Affecting Olfactory Function Using Multivariate Regression
Our initial analysis and a small number of preceding studies have identified eosinophilic inflammation and Th2 cytokines as potential mediators of olfactory dysfunction in CRS10,11,15. Small sample sizes have limited the ability of prior studies to account for covariates and other potential confounding factors. We consequently incorporated a large number of demographic, clinical, and inflammatory factors to further analyze CRS-associated olfactory function in our large patient cohort. Univariate regression identified asthma status (p=0.016), polyp status (p <0.001), AERD (p <0.001), CT score (p <0.001), tissue eosinophilia (p=0.002), and prior surgery (p=0.011) as variables predictive of olfactory function. Cytokines associated with olfactory dysfunction included IL-2 (p=0.037), IL-5 (p=0.001), and IL-13 (p= <0.001) (Table 3). After multivariate analysis, only AERD (p=0.015), CT score (p=0.014), and IL-2 (p=0.005) remained as predictive variables (Table 4). IL-5 and IL-13, which were strongly associated with olfactory dysfunction after univariate analysis, demonstrated significant collinearity, and this was verified using a correlation matrix. Removal of either IL-5 or IL-13 from the model did not significantly affect the results or the strength of the model.
Table 3. Olfactory Assessment of All CRS Patients (Age-Sex Adjusted SIT)-Univariate Model.
CRS = chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; CT = computed tomography; SIT score = smell identification test score; tissue eos/HPF = tissue eosinophils per high power field; NCS = nasal corticosteroid medication use; anti-leukotriene = anti-leukotriene nasal medication use; AERD = aspirin exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; IL = interleukin; RANTES = regulated on activation, normal T cell expressed and secreted; TNF = tumor necrosis factor BOLD, P< .05
|
Univariate Regression | |||
|---|---|---|---|
| Variables | Unadjusted β | 95% CI | p value |
| Age | −0.023 | −0.180 to 0.135 | 0.770 |
| Sex | 0.422 | −3.762 to 4.606 | 0.842 |
| Asthma | −5.095 | −9.213 to −0.978 | 0.016 |
| Allergic Rhinitis | 0.909 | −3.535 to 5.353 | 0.686 |
| AERD | −15.162 | −21.488 to −8.835 | <0.001 |
| Current Smoker | 3.943 | −6.048 to 13.933 | 0.436 |
| NCS | 4.504 | −0.555 to 9.564 | 0.080 |
| Anti-Leukotriene | −4.020 | −8.694 to 0.654 | 0.091 |
| Prior Surgery | −5.470 | −9.658 to −1.283 | 0.011 |
| Eos/HPF (mean) | −0.028 | −0.045 to −0.010 | 0.002 |
| Neu/HPF (mean) | 0.033 | −0.094 to 0.160 | 0.609 |
| Culture(+) Purulence | −1.589 | −6.174 to 2.995 | 0.493 |
| CT Score | −1.132 | −1.477 to −0.787 | <0.001 |
| Polyp Status/Phenotype | −10.669 | −14.342 to −6.997 | <0.001 |
| AFRS | −7.761 | −13.660 to −1.863 | 0.010 |
| IL-1β | 0.001 | −0.000 to 0.001 | 0.183 |
| IL-2 | −0.012 | −0.024 to −0.001 | 0.037 |
| IL-4 | −0.433 | −1.212 to 0.345 | 0.272 |
| IL-5 | −0.013 | −0.020 to −0.005 | 0.001 |
| IL-6 | 0.000 | −0.001 to 0.000 | 0.887 |
| IL-7 | −0.003 | −0.095 to 0.089 | 0.950 |
| IL-8 | 0.000 | −5.170e-06 to 0.000 | 0.245 |
| IL-9 | −0.031 | −0.104 to 0.041 | 0.396 |
| IL10 | 0.002 | −0.016 to 0.019 | 0.858 |
| IL-12/I-23p40 | 0.003 | −0.005 to 0.011 | 0.464 |
| IL-13 | −0.036 | −0.051 to −0.020 | <0.001 |
| IL-17α | −0.209 | −0.562 to 0.145 | 0.244 |
| IL-21 | 0.002 | −0.008 to 0.012 | 0.701 |
| TNFα | 0.005 | −0.014 to 0.024 | 0.627 |
| IFNγ | 0.031 | −0.038 to 0.101 | 0.374 |
| Eotaxin | −0.030 | −0.062 to 0.002 | 0.068 |
| RANTES | 0.000 | −0.000 to 0.001 | 0.400 |
Table 4. Objective Olfactory Assessment of All CRS Patients (Age-Sex Adjusted SIT)-Multivariate Model.
CRS = chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; CT = computed tomography; SIT score = smell identification test score; tissue eos/HPF = tissue eosinophils per high power field; NCS = nasal corticosteroid medication use; anti-leukotriene = anti-leukotriene nasal medication use; AERD = aspirin exacerbated respiratory disease; AFRS = allergic fungal rhinosinusitis; IL = interleukin; RANTES = regulated on activation, normal T cell expressed and secreted; TNF = tumor necrosis factor BOLD, P< .05
| Multivariate Regression | Collinearity Statistics | ||||
|---|---|---|---|---|---|
| Variables | Adjusted β | 95% CI | p value | VIF | |
| Age | Not Modeled | * | * | * | |
| Sex | Not Modeled | * | * | * | |
| Asthma | 0.726 | −3.366 to 4.818 | 0.725 | 1.47 | |
| Allergic Rhinitis | Not Modeled | * | * | * | |
| AERD | −9.102 | −16.385 to −1.820 | 0.015 | 1.79 | |
| Current Smoker | Not Modeled | * | * | * | |
| NCS | 1.942 | −2.522 to 6.336 | 0.39 | 1.15 | |
| Anti-Leukotriene | 1.913 | −2.511 to 6.336 | 0.393 | 1.31 | |
| Prior Surgery | −1.623 | −5.464 to 2.218 | 0.404 | 1.24 | |
| Eos/HPF (mean) | −0.007 | −0.024 to 0.010 | 0.418 | 1.44 | |
| Neu/HPF (mean) | Not Modeled | * | * | * | |
| Culture(+) Purulence | Not Modeled | * | * | * | |
| CT Score | −0.569 | −1.019 to −0.118 | 0.014 | 1.93 | |
| Polyp Status/Phenotype | −4.123 | −8.844 to 0.598 | 0.086 | 1.98 | |
| AFRS | −1.959 | −8.319 to 4.402 | 0.542 | 1.58 | |
| IL-1β | Not Modeled | * | * | * | |
| IL-2 | −0.014 | −0.024 to −0.004 | 0.005 | 1.11 | |
| IL-4 | Not Modeled | * | * | * | |
| IL-5 | −0.004 | −0.017 to 0.009 | 0.547 | 4.51 | |
| IL-6 | Not Modeled | * | * | * | |
| IL-7 | Not Modeled | * | * | * | |
| IL-8 | Not Modeled | * | * | * | |
| IL-9 | Not Modeled | * | * | * | |
| IL10 | Not Modeled | * | * | * | |
| IL-12/I-23p40 | Not Modeled | * | * | * | |
| IL-13 | 0.004 | −0.028 to 0.036 | 0.802 | 5.62 | |
| IL-17α | Not Modeled | * | * | * | |
| IL-21 | Not Modeled | * | * | * | |
| TNFα | Not Modeled | * | * | * | |
| IFNγ | Not Modeled | * | * | * | |
| Eotaxin | −0.010 | −0.040 to 0.021 | 0.525 | 1.38 | |
| RANTES | Not Modeled | * | * | * | |
DISCUSSION
This study is the first to utilize hierarchal cluster analysis and machine learning algorithms to assess the relationship between inflammatory cytokines and CRS-associated olfactory dysfunction. It is likewise the first study with an adequate sample size for multivariate analysis of cytokines and other potential contributors to olfactory loss. These findings expand upon our group’s previous work and potentially offer new insight into the potential role of cytokine-associated inflammation in olfactory loss11.
Consistent with prior studies3,20, our data suggests that polyp status alone is not a sufficient predictor of olfactory dysfunction. Most anosmic CRS patients were found in a single CRS disease cluster that was chiefly characterized by nasal polyps (100%), however, the majority of CRSwNP patients did not appear in this cluster. Rather, characteristics of this cluster were suggestive of a more severe form of CRSwNP, with many patients diagnosed with either AERD (33%) or AFRS (50%), and associated with a strong Th2-dominant inflammatory signature. We previously showed that olfactory cleft mucus cytokine levels correlate with olfactory function in CRSwNP patients, and this was particularly true for the Th2-associated cytokines IL-5 and IL-1314. This association was also seen in our random forest model, again suggesting that IL-5 and IL-13 were the strongest predictors of olfactory dysfunction in CRS. Surprisingly, our multivariate regression modelling did not identify either cytokine as independent predictors of olfactory dysfunction. This was largely due to collinearity of both IL-5 and IL-13 with other variables in the model, suggesting that these cytokines may be markers of more severe disease. This hypothesis is partially supported by a recent mouse study, which showed that allergic inflammation associated with elevated olfactory epithelium Th2 cytokines reduces the number of immature olfactory neurons, but does not affect the number of mature olfactory neurons or olfactory function21.
Though our study did not confirm IL-5 and IL-13 as independent effectors of olfactory loss in CRS, we did identify a potential role for IL-2. Our previous study likewise identified this cytokine as being closely correlated with olfactory function11. IL-2 is a non-specific T-cell effector that regulates immunity and tolerance, but its role in chronic rhinosinusitis is poorly defined. A recent study showed that IL-2 may be associated with elevated IgD levels and presence of pathogenic bacteria in CRSsNP patients22. Potential functional relationships between IL-2 and the olfactory epithelium will require further investigation and confirmation.
Our study identified AERD as being independently associated with olfactory dysfunction in CRS. The pathophysiology of smell dysfunction in AERD is unclear, however, recent studies have started to identify factors that differentiate AERD from other CRSwNP patients23. Both the innate and adaptive immune system have roles in AERD pathophysiology and severity24. Both AERD and CRSwNP are associated with eosinophilic tissue inflammation, though studies have generally not shown significant differences in the number of tissue eosinophils in each group. Conversely, the eosinophil degranulation product, eosinophil cationic protein (ECP), is elevated in AERD patients compared to CRSwNP patients. This would suggest that eosinophils may be more highly activated in AERD25,26. Elevation of Th2-associated cytokines has been reported in both CRSwNP and AERD, yet a specific difference in inflammatory signatures has not been clearly defined27. Of note, hierarchical cluster analysis in this and prior studies from our group, do suggest that AERD may be associated with a specific inflammatory CRS endotype14. The relationship between AERD and olfaction is even less clear. Gudziol et al demonstrated that AERD patients have worse olfaction at baseline which subsequently improved after aspirin desensitization, however, no current studies to our knowledge have evaluated inflammatory profiles and olfaction in these patients23. Furthermore, smell testing has not been found to predictive of AERD23. Multiple studies have demonstrated greater disease severity among patients with AERD, based both on endoscopy28 and CT scores29. This would suggest that reduced olfactory identification scores in AERD may be multifactorial, and likely due to collective differences in disease severity, polyp burden, and inflammatory signatures30.
Eosinophilic inflammation in allergic mouse models has previously been shown to have adverse effects on the olfactory epithelium31, and human studies suggest similar findings10,32. While the exact mechanisms of eosinophil-associated olfactory loss remains unclear, it is well established that eosinophilia is correlated with a Th2 inflammatory profile33. Both local neurotoxicity secondary to release of eosinophilic granule proteins34 and eosinophil-associated cytokine effects35 have been postulated as possible mechanisms of eosinophil-associated olfactory loss. Interestingly, while tissue eosinophilia was associated with olfactory loss in our univariate model, multivariate analysis failed to support this link. Rather, our data suggests that eosinophilia may instead simply be indicative of more severe disease, with olfactory dysfunction being one of many indicators of disease severity.
Our study does have some limitations that should be acknowledged. Firstly, this study assessed smell function using the semi-objective smell identification test, rather than using formal and more quantitative assessment tools. While the SIT is a well-established method for assessment of smell function that is highly correlated to threshold testing, it remains possible that some differences in olfactory function could have been overlooked in this study. This possibility is partially supported by a small number of recent studies. For example, Lavin et al. found that Charcot-Leyden crystal protein gene expression in superior turbinate tissue was associated with olfactory thresholds, but not olfactory identification32. Conversely, Schlosser et al. found that elevated olfactory cleft IL-5 levels were associated with worse identification scores, but did not affect thresholds or discrimination2. The relationships identified in the current study will ultimately need to be validated using objective and quantitative olfactory testing. Second, it is possible that mucus cytokine levels may show temporal variations, particularly as relates to CRS and comorbid disease exacerbations. While we have attempted to limit the impact of this potential problem by assessing olfactory function and cytokine levels on the same day, subsequent studies that assess temporal variations in individual cytokines and any potential effects on olfactory function may help to clarify this issue.
To our knowledge, the current study is the largest to date to evaluate potential associations between sinonasal inflammation and olfaction in CRS patients. Strengths of the study include its prospective design, evaluation of a wide array of cytokines and inflammatory mediators, and use of multiple complementary statistical approaches. This study continues to underscore the limitations of phenotypic categorization of CRS, and further suggests that olfactory loss may be more closely associated with endotypic, rather than phenotypic differences.
CONCLUSION
Anosmia in CRS is associated with a Th2-driven inflammatory CRS endotype enriched with AERD and AFRS patients. Previously reported associations between the Th2-associated cytokines IL-5 and IL-13 and olfactory function were not confirmed after multivariate analysis, whereas IL-2 was the only cytokine independently associated with smell dysfunction in CRS. Additionally, disease characteristics that included radiographic severity and presence of AERD were also independently associated with olfactory dysfunction. These results suggest that a combination of inflammatory, clinical, and demographic factors likely contribute to olfactory loss in CRS patients.
Acknowledgments
This project was supported by NIH RO3 DC014809 (J.H.T.), L30 AI113795 (J.H.T.), and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. This work was supported in part by startup funds from Vanderbilt University Medical Center to S.R.D., P30 AI110527, U19AI095227). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Financial disclosures: No relevant disclosures
Conflicts of interest: None
Presented at American Rhinologic Society Fall 2018 Meeting, October 6, 2018, Atlanta, GA
References
- 1.Jiang R-S, Lu F-J, Liang K-L, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol. 2008;22(4):445–448. doi: 10.2500/ajr.2008.22.3195. [DOI] [PubMed] [Google Scholar]
- 2.Kohli P, Naik AN, Harruff EE, Nguyen SA, Schlosser RJ, Soler ZM. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017;127(2):309–320. doi: 10.1002/lary.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope. 2001;111(3):409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishijima H, Kondo K, Yamamoto T, et al. Influence of the location of nasal polyps on olfactory airflow and olfaction. Int Forum Allergy Rhinol. 2018;8(6):695–706. doi: 10.1002/alr.22089. [DOI] [PubMed] [Google Scholar]
- 5.Sousa Garcia D, Chen M, Smith AK, Lazarini PR, Lane AP. Role of the type I tumor necrosis factor receptor in inflammation-associated olfactory dysfunction. Int Forum Allergy Rhinol. 2017;7(2):160–168. doi: 10.1002/alr.21855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner JH, Liang KL, May L, Lane AP. Tumor Necrosis Factor Alpha Inhibits Olfactory Regeneration in a Transgenic Model of Chronic Rhinosinusitis–Associated Olfactory Loss. Am J Rhinol Allergy. 2010;24(5):336–340. doi: 10.2500/ajra.2010.24.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010;30(6):2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. 2010;24(3):192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. Laryngoscope. 2013;123(12). doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 10.Hauser LJ, Chandra RK, Li P, Turner JH. Role of tissue eosinophils in chronic rhinosinusitis–associated olfactory loss. Int Forum Allergy Rhinol. 2017;7(10):957–962. doi: 10.1002/alr.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Chandra RK, Li P, Hull BP, Turner JH. Olfactory and middle meatal cytokine levels correlate with olfactory function in chronic rhinosinusitis. Laryngoscope. 2018. doi: 10.1002/lary.27112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlandi RR, Kingdom TT, Hwang PH. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. Int Forum Allergy Rhinol. 2016;6:S3–S21. doi: 10.1002/alr.21694. [DOI] [PubMed] [Google Scholar]
- 13.Doty RL, Shaman P, Kimmelman CP, Dann M. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2 Pt 1):176–178. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6694486. [DOI] [PubMed] [Google Scholar]
- 14.Turner JH, Chandra RK, Li P, Bonnet K, Schlundt DG. Identification of clinically relevant chronic rhinosinusitis endotypes using cluster analysis of mucus cytokines. J Allergy Clin Immunol. 2018;141(5):1895–1897.e7. doi: 10.1016/j.jaci.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner JH, Li P, Chandra RK. Mucus T helper 2 biomarkers predict chronic rhinosinusitis disease severity and prior surgical intervention. Int Forum Allergy Rhinol. June 2018. doi: 10.1002/alr.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Rourke N, Hatcher L. Factor Analysis and Structural Equation Modeling. A step-by-step approach to using SAS factor Anal Struct Model. 2013:9. doi: 10.1002/9781118411360.wbcla114. [DOI] [Google Scholar]
- 17.Gorsuch RL. Factor Analysis. Work Study. 1983;42(1):10–11. doi: 10.1108/EUM0000000002688. [DOI] [Google Scholar]
- 18.Hennig C Cluster-wise assessment of cluster stability. Comput Stat Data Anal. 2007;52(1):258–271. doi: 10.1016/j.csda.2006.11.025. [DOI] [Google Scholar]
- 19.Breiman L Random Forests. Mach Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 20.Hox V, Callebaut I, Bobic S, Jorissen M, Hellings PW. Nasal obstruction and smell impairment in nasal polyp disease: Correlation between objective and subjective parameters. Rhinology. 2010;48(4):426–432. doi: 10.4193/Rhino10.049. [DOI] [PubMed] [Google Scholar]
- 21.Rouyar A, Classe M, Gorski R, et al. Type 2/Th2-driven inflammation impairs olfactory sensory neurogenesis in mouse chronic rhinosinusitis model. Allergy. July 2018. doi: 10.1111/all.13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min J-Y, Nayak JV, Hulse KE, et al. Evidence for altered levels of IgD in the nasal airway mucosa of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2017;140(6):1562–1571.e5. doi: 10.1016/j.jaci.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudziol V, Michel M, Sonnefeld C, Koschel D, Hummel T. Olfaction and sinonasal symptoms in patients with CRSwNP and AERD and without AERD: a cross-sectional and longitudinal study. Eur Arch Oto-Rhino-Laryngology. 2017;274(3):1487–1493. doi: 10.1007/s00405-016-4366-x. [DOI] [PubMed] [Google Scholar]
- 24.Laidlaw TM, Boyce JA. Aspirin-Exacerbated Respiratory Disease — New Prime Suspects. Longo DL, ed. N Engl J Med. 2016;374(5):484–488. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 25.Stevens WW, Schleimer RP. Aspirin-Exacerbated Respiratory Disease as an Endotype of Chronic Rhinosinusitis. Immunol Allergy Clin North Am. 2016;36(4):669–680. doi: 10.1016/j.iac.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han JK. Subclassification of chronic rhinosinusitis. Laryngoscope. 2013;123(SUPPL. 2). doi: 10.1002/lary.23979. [DOI] [PubMed] [Google Scholar]
- 27.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy Eur J Allergy Clin Immunol. 2006;61(11):1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 28.Jang DW, Comer BT, Lachanas VA, Kountakis SE. Aspirin sensitivity does not compromise quality-of-life outcomes in patients with samter’s triad. In: Laryngoscope. Vol 124; 2014:34–37. doi: 10.1002/lary.24220. [DOI] [PubMed] [Google Scholar]
- 29.Robinson JL, Griest S, James KE, Smith TL. Impact of aspirin intolerance on outcomes of sinus surgery. Laryngoscope. 2007;117(5):825–830. doi: 10.1097/MLG.0b013e3180333121. [DOI] [PubMed] [Google Scholar]
- 30.Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014;133(1). doi: 10.1016/j.jaci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Epstein VA, Bryce PJ, Conley DB, Kern RC, Robinson AM. Intranasal Aspergillus fumigatus exposure induces eosinophilic inflammation and olfactory sensory neuron cell death in mice. Otolaryngol Head Neck Surg. 2008;138(3):334–339. doi: 10.1016/j.otohns.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Lavin J, Min JY, Lidder AK, et al. Superior turbinate eosinophilia correlates with olfactory deficit in chronic rhinosinusitis patients. Laryngoscope. 2017;127(10):2210–2218. doi: 10.1002/lary.26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlaminck S, Vauterin T, Hellings PW, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3-year prospective observational study. Am J Rhinol Allergy. 2014;28(3):260–264. doi: 10.2500/ajra.2014.28.4024. [DOI] [PubMed] [Google Scholar]
- 34.Acharya KR, Ackerman SJ. Eosinophil granule proteins: Form and function. J Biol Chem. 2014;289(25):17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon gamma directly regulates neural precursors in the non-inflammatory brain. J Neurosci. 2010;30(27):9038–9050. doi: 10.1523/JNEUROSCI.5691-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]