Abstract
Iron (Fe) deficiency (FeD) and manganese (Mn) overexposure (MnOE) may result in several neurological alterations in the nervous system. Iron deficiency produces unique neurological deficits due to this elemental role in central nervous system (CNS) development and myelination, which might persist after normalization of Fe in the diet. Conversely, MnOE, is associated with diverse neurocognitive deficits. Despite these well-known neurotoxic effects on the CNS, the influence of FeD and MnOE on the peripheral nervous system (PNS) remains poorly understood. The aim of the present investigation was to examine the effects of developmental FeD and MnOE or their combination on the sciatic nerve of young and adult rats. The parameters measured included divalent metal transporter 1 (DMT1), transferrin receptor (TfR), myelin basic protein (MBP) and peripheral myelin protein 22 (PMP22) expression, as well as Fe levels in the nerve. Our results showed that FeD produced a significant reduction in MBP and PMP22 content at P29, which persisted at P60 after Fe-sufficient diet replenishment regardless of Mn exposure levels. At P60 MnOE significantly increased sciatic nerve Fe content and DMT1 expression. However, the combination of FeD and MnOE produced no marked motor skill impairment. Evidence indicates that FeD appears to hinder developmental peripheral myelination, while MnOE may directly alter Fe homeostasis. Further studies are required to elucidate the interplay between these pathological conditions.
Keywords: iron, manganese, peripheral nervous system, myelination
Introduction
Iron (Fe) and manganese (Mn) are essential dietary elements whose levels are strongly regulated under physiological conditions. Consequently, numerous investigators examined the effects of Fe deficiency (FeD) and Mn overexposure (MnOE) on the central nervous system (CNS) (Park et al., 2007; Seo et al., 2013). Fe deficiency, the most common nutritional deficiency, affects approximately two billion people globally (Beard, 2004; Lee and Okam, 2011). When FeD occurs during the first two years of life, this disorder leads to irreversible damage in the nervous system (Youdim, 2008) even after the reintroduction of an Fe-sufficient (FeS) diet (Beard and Connor, 2003; Badaracco et al., 2008; Ortiz et al., 2004). As shown in developing rats, the peak in Fe uptake in the brain coincides with the point of greatest myelination, with FeD thus leading to alterations in myelin sheath production (Connor, 1994; Morath and Mayer-Pröschel, 2001). For this reason, the majority of neurological disorders associated with FeD have been attributed to hypomyelination (Ortiz et al., 2004; Erikson et al., 2000). Human studies reported a decrease in auditory and visual evoked potentials (Algarin et al., 2003), while investigations in rodents demonstrated a persistent change in resting energy status, neurotransmission and myelination (Rao et al., 2003), as well as altered dopaminergic functions (Nelson et al., 1997) and locomotor activity (Hunt et al., 1994).
Manganese overexposure was reported to be neurotoxic in children (Bouchard et al., 2011; Khan et al., 2012; Lucchini et al., 2012) and associated with cognitive deficits, behavioral disinhibition, decreased IQ and poor school performance (Zoni and Lucchini, 2013; Haynes et al., 2015). Common routes of exposure are contaminated drinking water (Oulhote et al., 2014), contaminated air from smelting factories (Menezes-Filho et al., 2014), tropical fruit acai diet (Santos et al., 2014) and soy-based infant formulas (Tran et al., 2002a). Studies in rodent models of MnOE on CNS developmental effects such as impairment in monoamine levels, learning, memory, motor activity and coordination (Amos-Kroohs et al., 2015; 2016; 2017; Tran et al., 2002b), as well as a reduction in fine motor control (Beudin et al., 2013; 2015) were previously reported.
FeD usually exacerbates tissue Mn absorption due to similarities in uptake mechanisms (Park et al., 2013; Kim and Park, 2014; Meltzer et al., 2010). Both metals possess a classical transferrin (Tf)-bound uptake route through the transferrin receptor (TfR) and an alternative Tf-independent route through divalent metal transporter-1 (DMT1; Tuschl et al., 2013). Both these proteins have high affinity for Mn and Fe (He et al., 2006; Wang et al., 2008) and can be found in various tissues (Chen et al., 2015; Wu et al., 2015). In the CNS, TfR facilitates poliovirus permeation (Mizutani et al., 2016) and enhances macromolecular drugs to permeate the blood-brain barrier (Li et al., 2012), while DMT1 is involved in the pathophysiology of neurodegenerative disorders such as Parkinson’s disease (Lee et al., 2010; Salazar et al., 2008). In summary, neurobehavioral experiments demonstrate that the combination of FeD and MnOE during developmental stages was found to (1) decrease Fe in plasma, (2) increase Mn, TfR, and DMT1 expression at multiple ages in the rat brain (Amos-Kroohs et al., 2015) and (3) produce behavioral deficits in adult rats (Amos-Kroohs et al., 2016; 2017; Tran et al., 2002b).
Despite the well-known neurotoxic effects of FeD on the CNS, the influence of this metal deficiency on peripheral myelination remains poorly understood. Salis et al (2002; 2012) previously demonstrated the pro-differentiating effect of Fe on Schwann cell (SC) maturation and survival. Kabakus et al (2002) noted that FeD is associated with peripheral neuropathies, but whether it is a causative factor or a consequence of the degenerating process remains to be established (Levi and Taveggia, 2014). Some investigators postulated that children with FeD exhibit electrophysiological impairment (Kabakus et al., 2002), while others reported no significant alterations in otherwise healthy children (Akyol et al., 2003). Further, patients with β-thalassemia (Stamboulis et al., 2004) and those submitted to bariatric surgery (Thaisetthawatkul et al., 2004) may develop peripheral neuropathies as a consequence of FeD. In addition, DMT1 is involved in the remyelination process following Wallerian degeneration (Martinez-Vivot et al., 2013; 2015). Regarding the role of Mn, significant amounts were detected in the peripheral nervous system (PNS), although at lower concentrations than in brain (Bourre et al., 1987).
Considering the plethora of effects reported for FeD and MnOE in the CNS, the transport mechanisms shared by Fe and Mn through the nervous system and the role established for Fe in PNS remyelination, it was postulated that FeD and MnOE or their combination may impact PNS myelination similar to that noted in the CNS. The aim of this study was to determine myelin basic protein (MBP), peripheral myelin protein 22 (PMP22) –two markers of peripheral myelin–, Fe levels and transporters DMT1 and TfR in the sciatic nerve in both young and adult rats. In addition, the influence of FeD and MnOE or their combination was examined on motor coordination at both ages using a Rotor-Rod test.
Materials and methods
Animals
Male and nulliparous female Sprague Dawley CD (IGS) rats (Charles River Laboratories, Raleigh, NC; strain #001), approximately 60 days old and weighing approximately 350 and 290 g on arrival, respectively, were acclimatized for no less than one week in the vivarium (AAALAC International accredited) before breeding. Rats were maintained on a 14–10 hr light-dark cycle (lights on 6 am) with controlled temperature (19 ± 1 °C) and humidity (50% ± 10%) throughout the experiment. Rats were housed in a Modular Animal Caging System (Alternative Design, Siloam Spring, AR). HEPA-filtered air was supplied to each cage (Alternative Design, Siloam Spring, AR) with 30 air changes/hr. Reverse osmosis filtered water (SE Lab Group, Napa, CA) and NIH-07 diet (250 ppm Fe and 80 ppm Mn) were provided ad libitum, except during the FeD feeding period. A semicircular stainless steel enclosure was placed in cages for enrichment (Vorhees et al., 2008). Females were separated from males the day a sperm plug was detected (embryonic day 0, E0). Birth was counted as post-natal day 0 (P0). On P1, litters were culled to 10, 5 per gender, using a random number table. On P28, pups were removed from dams into same gender cages (4/cage) until P42, when animals were re-housed (2/cage/gender). All protocols were approved by the Institutional Animal Care and Use Committee and adhered to the NIH Guide on the Care and Use of Laboratory Animals in Research.
Iron deficiency (FeD)
Pregnant females were kept on a standard NIH-07 diet until E15. From E15 to P28, dams were provided one of two purified diets differing only in Fe content (Land O’ Lakes Purina Feed, Evansville, IN). Half the dams were provided an Fe-sufficient diet (350 ppm, FeS, 99 ppm Mn) while the other half received an Fe-deficient diet with a 90% reduction (35 ppm, FeD, 99 ppm Mn). Offspring were breastfed from P0 to P28, placed back on the standard NIH-07 diet at P28, and maintained on this diet throughout the remainder of the experiment (Figure 1). This previously published FeD paradigm (Amos-Kroohs et al., 2015; 2016; 2017) was adapted from Fitsanakis et al. (2009; 2011) and effects on hematological parameters similar to clinical FeD, but not anemia were found (Amos-Kroohs et al., 2015).
Figure 1: Study design.
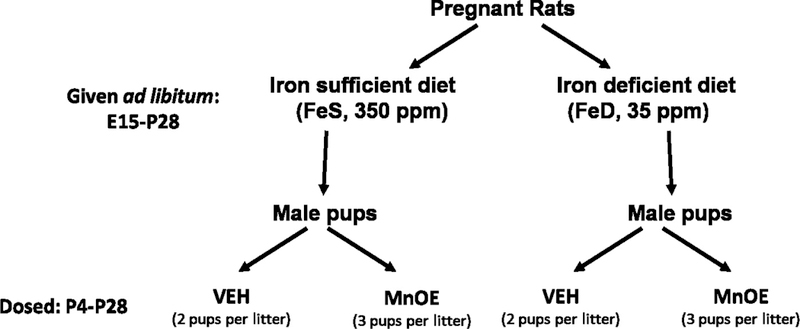
At E15, dams were removed from the NIH-07 diet and placed on a purified FeS or a purified FeD diet, which differed only in Fe content (350 ppm vs 35 ppm). Male offspring were exposed via gavage to either 100 mg/kg MnCl2 or isotonic VEH every other day from P4 to P28. MnOE and FeD diet were continued until P28, when offspring were placed back on an NIH-07 diet for the remainder of the experiment. Two or three male pups per litter were included in each experimental group, with a total of 3 litters included.
Manganese (Mn) overexposure
For these experiments, a split-litter design was used. Within each litter, two males were gavaged 0.01 M anhydrous sodium chloride (VEH, to achieve MnCl2 osmolarity), while three male pups were administered orally 100 mg/kg Mn chloride (MnCl2, MnOE) at a dose of 3 ml/kg (Amos-Kroohs et al., 2016; 2017; Graham et al., 2011) every other day from P4 to P28. The extra male MnOE pup supplemented for anticipated mortality only. Gavage was used to avoid maternal MnOE and its potential influence on maternal-pup interaction. Graham et al (2011) showed that this regime did not increase corticosterone levels above that of untreated littermates. This experimental protocol attempts to replicate oral intake as a common route for exposure during development in humans (Menezes-Filho et al., 2009). Previously investigators reported that these concentrations produce elevated brain and blood Mn values (Amos-Kroohs et al., 2015; Vorhees et al., 2014).
Sciatic nerve collection and preparation
Gender-dependent behavioral and cognitive outcomes were observed in this rat model (Amos-Khroos et al., 2015). However, no gender-dependent differences in myelination or remyelination were detected in the PNS (Aquino et al., 2006; Setton-Avruj et al., 2002; Usach et al., 2011). For these experiments, only sciatic nerves from male offspring were collected to avoid gender-dependent metabolic differences in Mn homeostasis (Carvalho Da Silva et al., 2017) as previously noted in humans. In the light of this evidence, it was decided to experiment only with male sciatic nerves, leaving the analysis in female sciatic nerves for future studies. Male rats in each experimental group (FeS-VEH, FeS-MnOE. FeD-VEH, and FeD-MnOE, Figure 1) were weighed and sacrificed for nerve collection at either P29 or P60 (weights are summarized in Table 1). These sciatic nerves were prepared for immunohistochemistry as previously described (Setton-Avruj et al., 2007; Usach et al., 2011). The number of animals employed in each experimental group is indicated in the corresponding figure legends. Personnel carrying out analyses were blind with respect to group enrollment.
Table 1:
Body weight of male pups at P29 (n=19 per group) and at P60 (n=6 per group) submitted to different diets and Mn exposure during development. Values are expressed as the mean ± SEM. Statistical analysis was performed through two-way ANOVA followed by Bonferroni post-test, where different letters indicate significant differences (p<0.05).
| P29 male rats | P60 male rats | |
|---|---|---|
| FeS-Veh | 98.54 ± 5.03 g A | 359.70 ± 16.67 g A |
| FeS-MnOE | 81.86 ± 4.16 g B | 333.93 ± 20.50 g A |
| FeD-Veh | 83.59 ± 2.67 g AB | 354.00 ± 17.85 g AB |
| FeD-MnOE | 64.28 ± 3.61 g C | 272.20 ± 27.77 g B |
Tissue preparation and immunofluorescence analysis
Briefly, tissue was frozen after fixation and cryopreservation and cut at 16 µm thickness in a cryostat (Zeiss Microm). The sections were mounted on gelatin-precoated glass slides, allowed to dry for at least 1 hr, and rinsed twice in PBS and twice in PBS-Triton X-100 0.1% solution. Sections were incubated in 5% fetal calf serum in PBS for 2 hr at room temperature. Slides were incubated for 18–24 hr in a humid chamber at 4 °C with: anti-rat MBP (1:500, IgG rabbit polyclonal, a kind gift of Dr. Campagnoni, UCLA Neuroscience Research Building Department of Psychiatry and Biobehavioral Sciences. LA, USA), anti-PMP22 (1:200, rabbit polyclional, Santa Cruz Biotech), anti-rat CD71 (1:100, mouse monoclonal IgG (clone Ox-26) (BD) or anti-DMT1 (1:200 goat polyclonal, Santa Cruz Biotech). Goat anti-rabbit Cy3 (1:500) or donkey anti-goat Dylight 488 (1:200) secondary antibodies (Jackson Lab) plus Höechst 32258 (2 µg/ml, Sigma) were used accordingly. Controls were incubated without primary antibodies following Dr. Sapper’s suggestions (Saper, 2005) and validated previously by Usach et al., (2011; 2017). Analysis was performed using an Olympus BX100 epifluorescence microscope.
Iron staining
Sections were processed utilizing a modification of the Perls’ staining method to detect ferric Fe (Bishop and Robinson, 2001; Moos and Mollgard, 1993). Intensification of Perls’ reaction was performed with DAB-Ni sulphate (3´−3´-diaminobenzidine-nickel sulphate; 0.05%:0.01%; Guardia Clausi et al., 2010).
Image analyses and quantification
Microscope images were obtained using a CoolSnap digital camera and Image Pro Plus 5.1 software was used for image analysis. At least 4 images per nerve were analyzed in order to cover all the length of the tissue; in each experimental group and survival time, between 5 and 8 independent nerves (derived from 5–8 different animals) were considered. In each image, the integrated optical density (IOD) was measured in 10 randomly selected fields (33 × 33 µm each). IOD values were expressed in arbitrary units (AU). All data were analyzed and quantified by experimenters who were blind to the experimental design. Figures show representative images from each experimental group and survival time.
Rotor-Rod analysis
From a separate cohort of treated animals, male rats were tested on a San Diego Instruments (SDI, San Diego, CA) Rotor-Rod™ System to determine whether diet, treatment or their combination brought about changes in motor skills. At P29 or P60 time points, rats were first habituated to the non-moving Rotor-Rod for 30 sec and were then conditioned to the moving Rotor-Rod at 12 rpm. Twenty-four hr later, rats were tested once a day for 4 days in a 300 sec program in which the Rotor-Rod continuously accelerated from 12 rpm to 50 rpm at a rate of 0.25 rpm to measure latency to fall. The number of animals used in each experimental group is indicated in the corresponding figure legends. Personnel doing tests were blind with respect to group enrollment.
Quantification and statistical analysis
Sciatic nerve Fe content and DMT1, MBP and PMP22 expression were analyzed by two-way analysis of variance (ANOVA) with diet (FeS or FeD) and treatment (Veh or MnOE) as independent variables. Briefly, and considering the image analysis described in the previous section, an average of all the images obtained from the same sciatic nerve was calculated (between 5–8, depending on the experimental group and the age) and used for the two-way ANOVA. For Rotor-Rod analysis, a two-way ANOVA was employed considering testing days as repeated measures of each rat followed by analyses of covariance for MnOE and body weight. Significant results were then analyzed by Bonferroni post-test. The statistical analysis was re-run using the SAS package and included weight as a covariate. In all cases significance was considered at P<0.05.
Results
Weight and iron content
In agreement with previous findings, both FeD and MnOE in the current study produced a reduction in animal weight at young ages (Table 1; Unger et al., 2007). In accordance with previous observations, FeD-MnOE animals weighed less compared to all other experimental groups, regardless of age (Table 1; Garcia et al., 2006; Amos-Kroohs, 2015). With respect to Fe content in the sciatic nerve, no significant differences were found between groups at younger ages (Figure 2A and 2B). Conversely, at P60, FeD rats exhibited significantly lower Fe levels than FeS rats, which were compensated by MnOE (Figure 2C and 2D). For comparison, an unstained sciatic nerve is also shown (Figure 2E).
Figure 2: Iron content in the sciatic nerve.
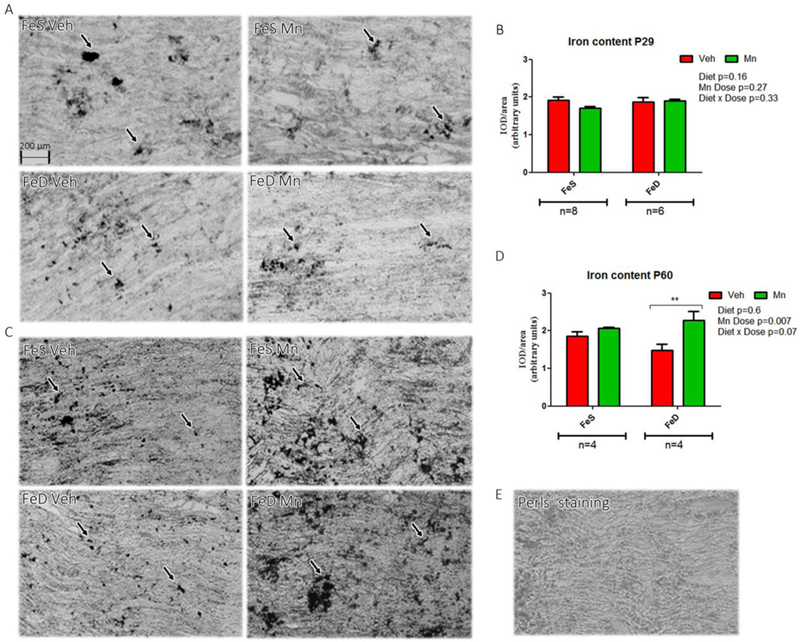
A, Perls’ staining of sciatic nerve from P29 in the FeS (n=8) and FeD (n=6) groups. C, Perls’ staining of sciatic nerve from P60 rats (n=4) in both groups in each experimental condition. B, D: Relative Fe quantification. E: Negative control image of a Perls’ staining. Values are expressed as the mean ± SEM in arbitrary units. Statistical analysis was performed through two-way ANOVA followed by Bonferroni post-test (*p< 0.05).
Myelin protein levels and distribution
MBP expression levels, a major myelin protein and a reliable index of myelination status, were assessed in both young and adult rats. Although the spatial distribution appeared comparable across groups at P29, MBP levels were significantly lower in the FeD group. No overall changes in MBP were induced by MnOE. At P60, MBP levels were also significantly decreased by FeD and further reduced by MnOE, with FeD-MnOE group displaying the lowest MBP content (Figure 3C and D). For reference, a negative control of MBP staining is also illustrated (Figure 3E). In agreement with MBP results, PMP22 values were significantly diminshed at P29 in FeD animals with the FeD-MnOE group exhibiting the lowest levels (Figure 4A and B). An image of negative samples for PMP22 is presented as control (Figure 4E).
Figure 3: MBP levels and distribution in the sciatic nerve.
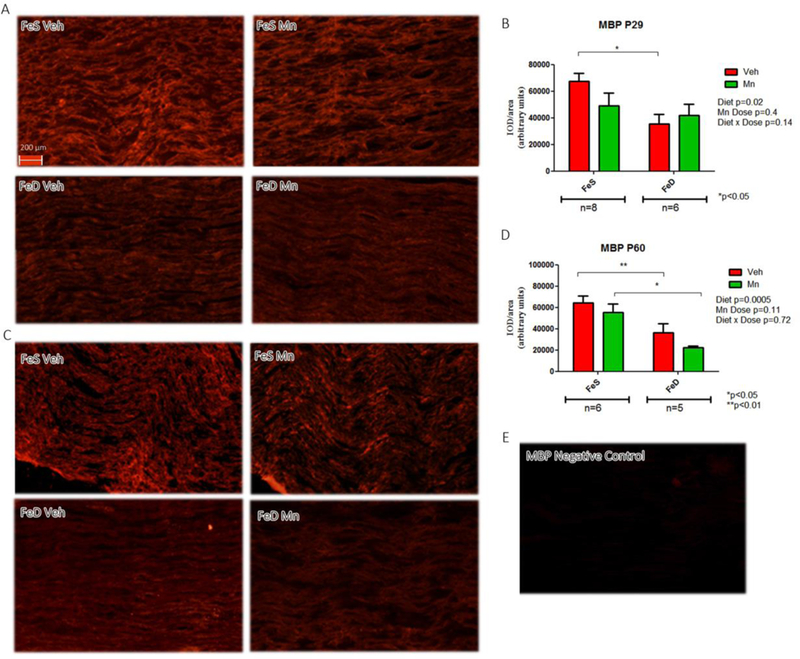
A, MBP immunofluorescence in sciatic nerve slices from P29 in the FeS (n=8) and FeD (n=6) groups. C, MBP immunofluorescence in sciatic nerve slices from P60 rats in the FeS (n=6) and FeD (n=5) groups in each experimental condition. B, D: IOD quantification for MBP. Values are expressed as the mean ± SEM in arbitrary units. Statistical analysis was performed through two-way ANOVA followed by Bonferroni post-test (*p<0.05,). E: Negative control staining for MBP.
Figure 4: PMP22 levels and distribution in the sciatic nerve.
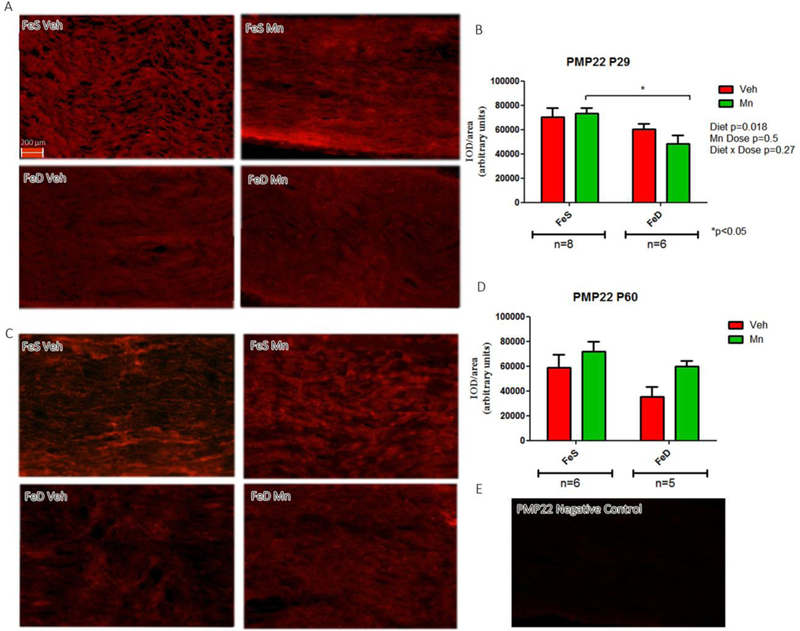
A, PMP22 immunofluorescence in sciatic nerve slices from P29 in the FeS (n=8) and FeD (n=6) groups. C, PMP22 immunofluorescence in sciatic nerve slices from P60 rats in the FeS (n=6) and FeD (n=5) groups in each experimental condition. B, D: IOD quantification for PMP22. Values are expressed as the mean ± SEM in arbitrary units. Statistical analysis was performed through two-way ANOVA followed by Bonferroni post-test (*p<0.05). E: Negative control staining for PMP22.
Fe transporter levels and distribution
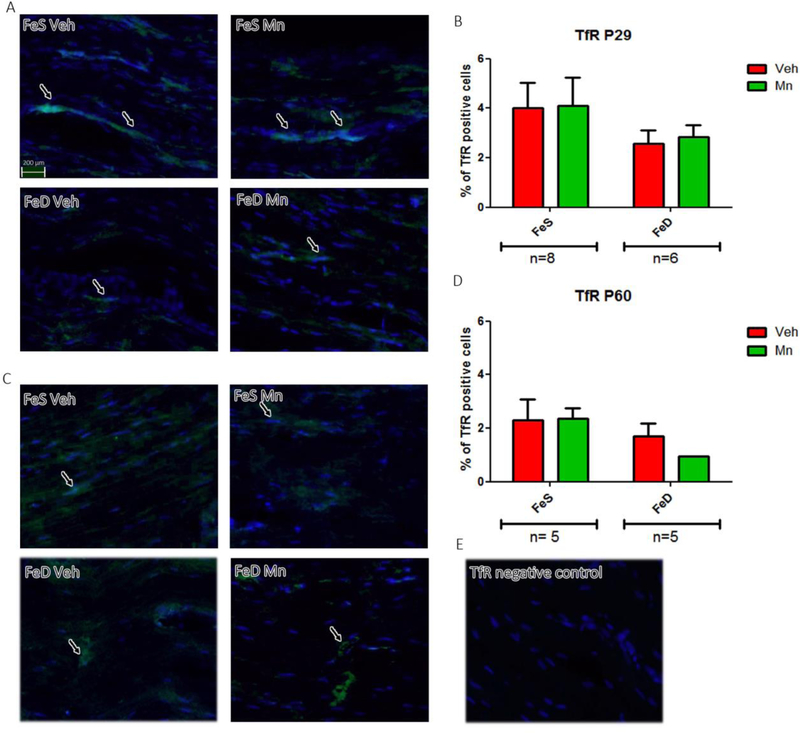
At P29, DMT1 spatial distribution and levels were unaffected by FeD or MnOE (Figure 5A and B). Similarly, no marked interaction between FeD and MnOE was observed in P60 rats, as DMT1 expression in MnOE rats was significantly higher regardless of diet (Figure 5C and D). An image of negative samples for DMT1 is shown as control (Figure. 5E). Finally, no significant differences were detected in TfR values across groups either at P29 (Figure 6A and B) or at P60 (Figure 6C and D). An image of negative samples for TfR is shown as control (Figure 6E).
Figure 5: DMT1 levels and distribution in the sciatic nerve.
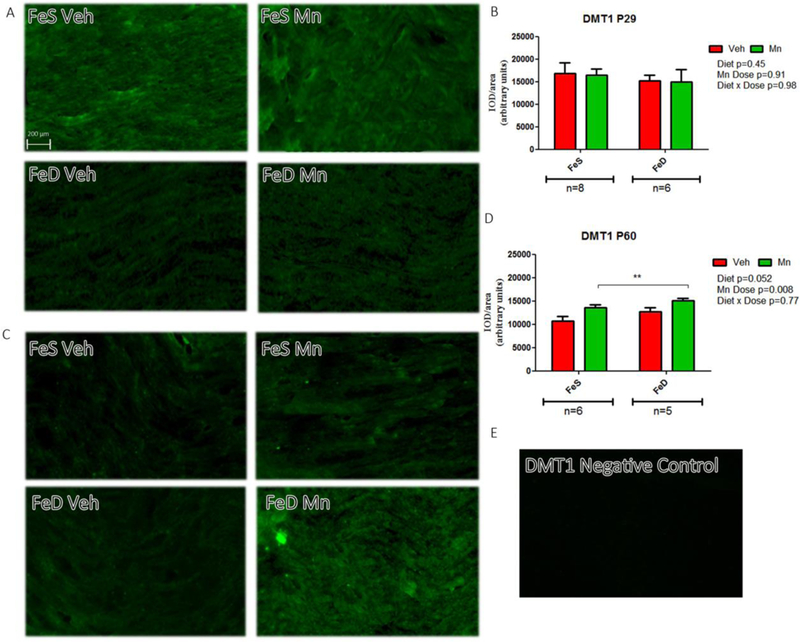
A, DMT1 immunofluorescence in sciatic nerve slices from P29 in the FeS (n=8) and FeD (n=6) groups. C, DMT1 immunofluorescence in sciatic nerve slices from P60 rats in the FeS (n=6) and FeD (n=5) groups in each experimental condition. B, D: IOD quantification for DMT1. Values are expressed as the mean ± SEM in arbitrary units. Statistical analysis was performed through two-way ANOVA followed by Bonferroni post-test (*p < 0.05). E: Negative control staining for DMT1.
Figure 6: TfR levels and distribution in the sciatic nerve.
A, TfR immunofluorescence in sciatic nerve slices from P29 in the FeS (n=8) and FeD (n=6) groups. C, TfR immunofluorescence in sciatic nerve slices from P60 rats (n=5) in both groups in each experimental condition. B, D: IOD quantification for TfR. Values are expressed as the mean ± SEM in arbitrary units. Statistical analysis was performed through two-way ANOVA followed by Bonferroni post-test (*p < 0.05). E: Negative control staining for TfR.
Rotor-Rod performance
In terms of observable motor coordination and performance, P29 animals were not markedly affected by FeD. However, MnOE rats exhibited a significant rise in amount of time spent on the Rotor-Rod (Table 2), particularly skewed by results obtained on day 4. An analysis of covariance demonstrated that weight did not exert a significant influence on performance in the P29 pups. At P60, motor coordination and performance were not markedly affected by FeD, MnOE or their combination.
Table 2:
Rotor-Rod of male pups at P29 (n=9–11 per group) and at P60 (n=6 per group) submitted to different diets and Mn exposure during development. Values are expressed as the mean ± SEM. Statistical analysis was performed through two-way ANOVA; different letters indicate significant differences (p<0.05).
| P29 male rats | P60 male rats | |
|---|---|---|
| FeS-Veh | 113.6 ± 9.3 s A | 75.13 ± 7.13 s A |
| FeS-MnOE | 152.2 ± 24.5 s B | 85.38 ± 7.09 s A |
| FeD-Veh | 100.0 ± 13.1 s AB | 95.47 ± 7.33 s A |
| FeD-MnOE | 114.8 ± 9.9 s AB | 86.09 ± 7.47 s A |
Discussion
Approximately two billion people worldwide suffer from FeD or anemia (de Benoist et al., 2008) and are hence more likely to accumulate Mn in several tissues including the brain (Erikson et al., 2004; Park et al., 2007; Kim et al., 2013). Despite the considerable amount of evidence available regarding FeD-mediated adverse effects on the CNS (Thompson et al., 2007; Ruvin et al, 2012; Kim et al., 2012), few studies are available on the impact on peripheral myelination.
FeD animal models presented with a decrease in blood hematocrit, body weight and locomotor activity (Amos-Kroohs et al., 2016), as well as neurological disorders predominantly attributed to hypomyelination (Rosato-Siri et al., 2018). Regarding MnOE, excessive embryonic Mn intake may be harmful to neural and skeletogenic cell differentiation in vertebrates (Pinsino et al., 2011). Further, high concentrations of Mn in human placenta correlate with enhanced risk of neural tube defects occurrence (Liu et al., 2013). Amos-Kroohs et al (2015) observed increased offspring mortality rate as a consequence of MnOE. For these reasons, the pre-weaning MnOE paradigm was selected, as this results in elevated Mn concentrations in blood and brain (Amos-Kroohs et al., 2015; Vorhees et al., 2014) which is considered to result in learning and memory deficits (Amos-Kroohs et al., 2017). Given that alterations in Fe transport mechanisms may elevate Mn uptake, our experimental model employed a combination of FeD-induced effects and consequent exacerbation of MnOE toxicity (Park et al., 2013; Kim et al., 2014; Meltzer et al., 2010).
Peripheral myelination is a long-spanning process in both rodents and humans. In rodents it is a postnatal event starting 3 days after birth and covering the first 3 weeks of life. In humans, peripheral myelination starts during embryonic life and ends by puberty (Berthold et al., 2005). This information provided the basis for the current study, which was conducted at two distinct time points of PNS development, i.e. P29, a key point in peripheral myelinogenesis, and P60, a steady-state point when myelinogenesis is complete.
FeD promoted a significant reduction in younger age rat MBP levels, which remained low in adulthood despite the re-introduction of an FeS diet. The interaction observed between FeD and MBP levels during development reinforces the notion that nerve damage is associated with high MBP sensitivity and may reflect a blockade of SC progress to a myelinating phenotype and myelin gene expression, indicating a key role for Fe in peripheral myelinogenesis (Martínez-Vivot et al., 2013; 2015). These in vivo findings are in agreement with those reported in vitro showing that Fe plays a role in SC maturation and prevents SC dedifferentiation in culture through an elevation in cyclic adenosine monophosphate (cAMP) and cAMP response element-binding (CREB) phosphorylation, both essential for myelin protein expression (Salis et al., 2012). Dysfunction of human DMT1 is associated with FeD anemia (Mims et al., 2005; Priwitzerova et al., 2005), Fe overload disorders (Hediger et al., 2002; Rolfs et al., 2002), neurodegenerative diseases (Salazar et al., 2008; Zheng et al., 2009), cancer (Brookes et al., 2006; Boult et al., 2008) and inflammation (Martini et al., 2008; Gaudet et al., 2011). Martinez-Vivot et al (2015) demonstrated a positive correlation between DMT1 protein expression and Fe levels and a negative correlation between DMT1 and MBP protein levels in a model of peripheral Wallerian degeneration. FeD constitutes a direct insult on peripheral myelin, while Wallerian degeneration indirectly affects myelin by interrupting SC-axon cross talk. However, both pathological scenarios induce a fall in MBP levels, which promote a rise in DMT1 to enhance Fe uptake for remyelination/regeneration. Interestingly, MnOE effects on MBP, Fe and DMT1 levels were most evident in the FeD rats, which may reflect heightened sensitivity to Mn-induced toxicity triggered by this common developmental nutritional deficiency.
Finally, and despite significant neurocognitive and behavioral impact on the CNS (Amos-Kroohs et al., 2015; 2017; Fitsanakis et al., 2009; 2011) and the influence on peripheral MBP levels, our data showed little impact of FeD on motor coordination and balance. Of note, rotor-rod performance appeared to reflect weight levels in P29 rats with the MnOE groups displaying the longest time spent at the task. In contrast, P60 motor performance appeared to respond to rat age rather than diet, treatment or weight.
These results are by no means inclusive, as more comprehensive behavioral testing would assist in determining whether this decreased sensitivity extends to areas other than gross motor function. Further studies are needed to seek corroboration of these effects in female rats exposed developmentally to this model. In addition, our results suggest vulnerability in myelination during development after FeD and its combination with MnOE.
Conclusions
FeD treatment during development reduced sciatic nerve MBP levels both at young and adult ages, an effect enhanced by MnOE and not reversed by re-introduction of an FeS diet. Further elucidating the interaction between these two pathological conditions and their effects on myelin-dependent neurological processes may render new targets for therapeutic strategies.
Acknowledgements
The authors would like to thank Ms María Marta Rancez for her assistance in the elaboration of this manuscript.
Funding: This work was supported by grants from Universidad de Buenos Aires (UBACYT 20020100101017) and CONICET (PIP 567) to PSA, NIH RO1 ES15689 to MTW, T32 ES007051 to CVV and P30 ES006096 to RMAK.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
References
- Akyol A, Kiylioglu N, Kadikoylu G, Bolaman AZ and Ozgel N 2003. Iron deficiency anemia and restless legs syndrome: Is there an electrophysiological abnormality? Clin Neurol Neurosurg 106: 23–27. [DOI] [PubMed] [Google Scholar]
- Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B 2003. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res 53:217–223 [DOI] [PubMed] [Google Scholar]
- Amos-Kroohs RM, Davenport LL, Gutierrez A A, Hufgard J:R, Vorhees CV, Williams M. and T. 2016. Developmental manganese exposure in combination with developmental stress and iron deficiency: Effects on behavior and monoamines. Neurotoxicol Teratol 56: 55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs RM, Davenport LL, Atanasova N, Abdulla ZI, Skelton MR, Vorhees CV and Williams MT 2017. Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory. Neurotoxicol Teratol 59: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos-Kroohs R:M, Bloor CP, Qureshi MA, Vorhees CV and Williams MT 2015. Effects of developmental exposure to manganese and/or low iron diet: Changes to metal transporters, sucrose preference, elevated zero-maze, open-field, and locomotion in response to fenfluramine, amphetamine, and MK-801. Toxicol Rep 2: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino JB, Musolino PL, Coronel MF, Villar MJ, Setton-Avruj CP 2006. Nerve degeneration is prevented by a single intraneural apotransferrin injection into colchicine-injured sciatic nerves in the rat. Brain Res 1117: 80–91 [DOI] [PubMed] [Google Scholar]
- Badaracco ME, Ortiz EH, Soto EF, Connor J and Pasquini JM 2008. Effect of transferrin on hypomyelination induced by iron deficiency. J Neurosci Res 86: 2663–2673. [DOI] [PubMed] [Google Scholar]
- Beard J. Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, World Health Organization/Centers for Disease Control. 2004.
- Beard JL and Connor JR 2003. Iron status and neural functioning. Annu. Rev. Nutr 23: 41–58. [DOI] [PubMed] [Google Scholar]
- Beaudin SA, Strupp BJ, Lasley SM, Fornal CA, Mandal S and Smith DR 2015. Oral methylphenidate alleviates the fine motor dysfunction caused by chronic postnatal manganese exposure in adult rats. Toxicol. Sci 144: 318–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin SA, Nisam S and Smith DR 2013. Early life versus lifelong oral manganese exposure differently impairs skilled forelimb performance in adult rats. Neurotoxicol. Teratol 38: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold CH, Fraher JP, King RHM and Rydmark M 2005. Microscopic anatomy of the peripheral nervous system. In Peripheral Neuropathy, 4th ed., ed. Dick PJ & Thomas PK. Philadelphia: Elsevier Saunders, pp. 35–91 [Google Scholar]
- Bishop GM and Robinson SR 2001. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res 907: 175–187. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Sauve S, Barbeau B, Legrand M, E Brodeur M, Bouffard T, Limoges E, Bellinger DC, and Mergler D 2011. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119: 138–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boult J, Roberts K, Brookes MJ, Hughes S, Bury JP, Cross SS, Anderson GJ, Spychal R, Iqbal T and Tselepis C. 2008. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin Cancer Res 14: 379–387. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Cloez I, Galliot M, Buisine A, Dumont O, Piciotti M, Prouillet F and Bourdon R 1987. Occurrence of manganese, copper and zinc in myelin. Alterations in the peripheral nervous system of dysmyelinating trembler mutant are at variance with brain mutants (quaking and shivering). Neurochem Int 10: 281–286. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, Berx G, McKie AT, Hotchin N, Anderson GJ, Iqbal T and Tselepis C 2006. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut 55, 1449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Da Silve AL, Ragassi Urbano M., Bertin de Almeida Lopes AC, Carvalho MFH, Buzzo ML, Severo Peixe T., Aschner M, Mesas AE and Bastos Paoliello M. M. 2017. Blood manganese levels and associated factors in a population-based study in Southern Brazil. J Toxicol Environ Health A 80: 1064–1077 [DOI] [PubMed] [Google Scholar]
- Chen AC, Donovan A, Ned-Sykes R and Andrews NC 2015. Noncanonical role of transferrin receptor 1 is essential for intestinal homeostasis. Proc Natl Acad Sci U S A 112: 11714–11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR 1994. Iron acquisition and expression of iron regulatory proteins in the developing brain, manipulation by ethanol exposure, iron deprivation and cellular dysfunction. Dev. Neurosci 16: 233–247. [DOI] [PubMed] [Google Scholar]
- de Benoist B, McLean E, Andersson M and Rogers L 2008. Iodine deficiency in 2007: Global progress since 2003. Food Nutr Bull 29: 195–202. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC and Aschner M 2004. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ 1:334–335:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Jones BC and Beard J 2000. Iron Deficiency alters dopamine transporter functioning in rat striatum. J Nutr 130: 2831–2837 [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Thompson KN, Deery SE, Milatovic D, Shihabi ZK, Erikson KM, W Brown R and Aschner M 2009. A chronic iron-deficient/high-manganese diet in rodents results in increased brain oxidative stress and behavioral deficits in the Morris water maze. Neurotoxicol Res 15: 167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Avison MJ, Erikson KM, Gore JC and Aschner M 2011. Changes in dietary iron exacerbate regional brain manganese accumulation as determined by magnetic resonance imaging. Toxicol Sci 120: 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T and Aschner M 2006. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci 92: 516–525. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG and Ramer MS 2011. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflamm, 8: 110–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Grace CE, Braun AA, Schaefer TL, Skelton MR, Tang PH, Vorhees CV and Williams MT 2011. Effects of developmental stress and lead (Pb) on corticosterone after chronic and acute stress, brain monoamines, and blood Pb levels in rats. Int. J. Dev. Neurosci 29: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia Clausi M., Pasquini LA, Soto EF and Pasquini JM 2010. Apotransferrin-induced recovery after hypoxic/ischaemic injury on myelination. ASN Neuro 2: e00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, Parsons PJ, Aldous KM, Praamsma ML, Beidler C and Dietrich KN 2015. Manganese exposure and neurocognitive outcomes in rural school-age children: The communities actively researching exposure study (Ohio, USA). Environ. Health Perspect 123: 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M and Nebert DW 2006. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol 70: 171–180. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Rolfs A and Goswami T 2002. Iron transport and hemochromatosis. J Invest Med 50: 239S–246S [DOI] [PubMed] [Google Scholar]
- Hunt JR, Zito CA, Erjavec J, Johnson L 1994. Severe or marginal iron deficiency affects spontaneous physical activity in rats. Am J Clin Nutr 59: 413–418 [DOI] [PubMed] [Google Scholar]
- Kabakus N, Ayar A, Yoldas TK, Ulvi H, Dogan Y, Yilmaz B and Kilic N 2002. Reversal of iron deficiency anemia-induced peripheral neuropathy by iron treatment in children with iron deficiency anemia. J Trop Pediatr 48: 204–209. [DOI] [PubMed] [Google Scholar]
- Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, Van GA, Graziano JH and Factor-Litvak P 2012. Manganese exposure from drinking water and children’s academic achievement. Neurotoxicology 33: 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Buckett PD and Wessling-Resnick M 2013. Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS ONE 8: e64944. doi: 10.1371/journal.pone.0064944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Li Y, Buckett PD, Bohlke M, and Thompson KJ 2012. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PLoS One 7: e33533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y and Park S 2014. Iron deficiency increases blood concentrations of neurotoxic metals in children. Korean J Pediat 57:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AI, Okam MM 2011. Anemia in pregnancy. Hematol Oncol Clin North Am 25: 241–259 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kim JS, Yoo JT, Song IU, Kim BS, Jung SL, Yang DW, Kim YI, Jeong DS and Lee KS 2010. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis Assoc Disord 24: 227–33 [DOI] [PubMed] [Google Scholar]
- Levi S and Taveggia C 2014. Iron homeostasis in peripheral nervous system, still a black box? Antioxid Redox Signal 21: 634–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhou L and Shen Y 2012. Recent research progresses of transferrin receptor 1 in central nervous system. Sheng Li Ke Xue Jin Zhan 43: 188–192 [PubMed] [Google Scholar]
- Liu J, Jin L, Zhang L, Li Z, Wang L, Ye R, Zhang Y and Ren A 2013. Placental concentrations of manganese and the risk of fetal neural tube defects. J Trace Elem Med Biol 27: 322–325. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, Salmistraro M, Bontempi E, Zimmerman NJ and Smith DR 2012. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology 33: 687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Vivot R, Goitia B, Usach V and Setton-Avruj PC 2013. DMT1 as a candidate for non-transferrin-bound iron uptake in the peripheral nervous system. Biofactors 39: 476–484 [DOI] [PubMed] [Google Scholar]
- Martinez-Vivot R Copello G, Leal MC, Piñero G, Usach V, Rozenszajn M, Morelli L and Setton-Avruj CP 2015. DMT1 iron uptake in the PNS: bridging the gap between injury and regeneration. Metallomics 7: 1381–1389 [DOI] [PubMed] [Google Scholar]
- Martini R, Fischer S, López-Vales R and David S 2008. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia 56: 1566–1577 [DOI] [PubMed] [Google Scholar]
- Meltzer HM, Brantsaeter AL, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, Stigum H, Ydersbond TA 2010. Low iron stores are related to higher concentration of manganese, cobalt and cadmium in non-smoking Norwegian women in the HUNT 2 study. Envirom Res 110: 487–504 [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, de Carvalho-Vivas C:F, Viana GF, Ferreira JR, Nunes LS, Mergler D and Abreu N 2014. Elevated manganese exposure and school-aged children’s behavior: A gender-stratified analysis. Neurotoxicology 45: 293–300 [DOI] [PubMed] [Google Scholar]
- Menezes-Filho JA, Bouchard M, de P Sarcinelli N and Moreira JC 2009. Manganese exposure and the neuropsychological effect on children and adolescents: A review. Rev Panam Salud Publica 26: 541–548. [DOI] [PubMed] [Google Scholar]
- Mims MP, Guan Y, Pospisilova D, Priwitzerova M, Indrak K, Ponka P, Divoky V and Prchal JT 2005. Identification of a human mutation of DMT1 in a patient with microcytic anemia and iron overload. Blood 105: 1337–1342. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Ishizaka A and Nihei C 2016. Transferrin receptor 1 facilitates poliovirus permeation of mouse brain capillary endothelial cells. J Biol Chem 291: 2829–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T and Møllgård K 1993. A sensitive post-DAB enhancement technique for demonstration of iron in the central nervous system. Histochemistry 99: 471–475. [DOI] [PubMed] [Google Scholar]
- Morath DJ and Mayer-Pröschel M 2001. Iron modulates the differentiation of a distinct population of glial precursor cells into oligodendrocytes. Dev Biol 237: 232–243. [DOI] [PubMed] [Google Scholar]
- Nelson C, Erikson K, Piñero DJ, Beard JL 1997. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J. Nutr 127: 2282–2288 [DOI] [PubMed] [Google Scholar]
- Ortiz E, Pasquini JM, Thompson K, Felt B, Butkus G, Beard J and Connor JR 2004. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J Neurosci Res 77: 681–689. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur ME, Saint-Amour D, Legrand M, Sauve S and Bouchard MF 2014. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ. Health Perspect 122: 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JD, Kim KY, Kim DW, Choi SJ, Choi BS, Chung YH, Han JH, Sung JH, Kwon IH, Mun JH, and. Yu J 2007. Tissue distribution of manganese in iron sufficient or iron-deficient rats after stainless steel welding-fume exposure. Inhal Toxicol 19: 563–572. [DOI] [PubMed] [Google Scholar]
- Park S, Sim CS, Lee H, Kim Y 2013. Blood manganese concentration is elevated in infants with iron deficiency. Biol Trace Elem Res 155: 184–189 [DOI] [PubMed] [Google Scholar]
- Pinsino A, Roccheri MC, Costa C and Matranga V 2011. Manganese interferes with calcium, perturbs ERK signaling, and produces embryos with no skeleton. Toxicol Sci 123: 217–230. [DOI] [PubMed] [Google Scholar]
- Priwitzerova M, Nie G, Sheftel AD, Pospisilova D, Divoky V and Ponka P 2005. Functional consequences of the human DMT1 (SLC11A2) mutation on protein expression and iron uptake. Blood 106: 3985–3987. [DOI] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Gruetterm R Georgieff MK 2003. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J. Nutr 133: 3215–3221 [DOI] [PubMed] [Google Scholar]
- Rolfs A, Bonkovsky HL, Kohlroser JG, McNeal K, Sharma A, Berger UV and Hediger MA 2002. Intestinal expression of genes involved in iron absorption in humans. Am. J. Physiol. Gastrointest. Liver Physiol 282: G598–G607. [DOI] [PubMed] [Google Scholar]
- Rosato-Siri MV, Marziali L, Guitart ME, Badaracco ME, Puntel M, Pitossi F, Correale J, Pasquini JM 2018. Iron availability compromises not only oligodendrocytes but also astrocytes and microglial cells. Mol Neurobiol 55:1068–1081 [DOI] [PubMed] [Google Scholar]
- Ruvin Kumara VM and Wessling-Resnick M 2012. Olfactory ferric and ferrous iron absorption in iron-deficient rats. Am J Physiol Lung Cell Mol Physiol 302: L1280–L1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nuñez MT, Garrick MD, Raisman-Vozari R and Hirsch EC 2008. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci U S A 105: 18578–18583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salis C, Goedelmann CJ, Pasquini JM, Soto EF and Setton-Avruj CP 2002. Holotransferrin but not apotransferrin prevents Schwann cell de-differentiation in culture. Dev Neurosci 24: 214–221. [DOI] [PubMed] [Google Scholar]
- Salis C, Davio C, Usach V, Urtasun N, Goitia N, Martinez-Vivot R, Pasquini JM, and Setton-Avruj CP 2012. Iron and holotransferrin induce cAMP-dependent differentiation of Schwann Cells. Neurochem Int 61: 798–806 [DOI] [PubMed] [Google Scholar]
- da Silva Santos V, de Almeida Teixeira GH and Barbosa F Jr 2014. Açaí (Euterpe oleracea Mart.): A tropical fruit with high levels of essential minerals-especially manganese-and its contribution as a source of natural mineral supplementation. J Toxicol Environ Health A 77: 80–89. [DOI] [PubMed] [Google Scholar]
- Saper CB. 2005. An open letter to our readers on the use of antibodies. J Comp Neurol 493: 477–478. [DOI] [PubMed] [Google Scholar]
- Seo YA, Yuan L and Wessling-Resnick M 2013. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton-Avruj CP, Musolino PL, Salis C, Alló M, Bizzozero O, Villar MJ, Soto EF and Pasquini JM 2007. Presence of alpha-globin mRNA and migration of bone marrow cells after sciatic nerve injury suggests their participation in the degeneration/regeneration process. Exp Neurol 203: 568–578. [DOI] [PubMed] [Google Scholar]
- Setton-Avruj CP Aquino JB, Goedelman CJ, Soto EF, Villar MJ 2002. P0 and myelin basic protein-like immunoreactivities following ligation of sciatic nerve in the rat. Neurochem Res 27: 1293–1303 [DOI] [PubMed] [Google Scholar]
- Stamboulis E, Vlachou N, Drossou-Servou M, Tsaftaridis P, Koutsis G, Katsaros N, Economou-Petersen E and Loutradi-Anagnostou A 2004. Axonal sensorimotor neuropathy in patients with beta-thalassaemia. J Neurol Neurosurg Psychiat 75: 1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaisetthawatkul P, Collazo-Clavell ML, Sarr MG, Norell JE and Dyck PJ 2004. A controlled study of peripheral neuropathy after bariatric surgery. Neurology 63: 1462–1470. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE and Brain JD 2007. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J 21: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Lonnerdal B, Le L, Parker M, Chicz-Demet A and Crinella FM 2002b. Effects of neonatal dietary manganese exposure on brain dopamine levels and neurocognitive functions. Neurotoxicology 23: 645–651 [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Crinella FM, Chicz-Demet A and Lonnerdal B 2002a. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology 23: 635–643. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Mills PB and Clayton PT 2013. Manganese and the brain. Int Rev Neurobiol 110: 277–312 [DOI] [PubMed] [Google Scholar]
- Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC and Beard JL 2007. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr 137: 118–124 [DOI] [PubMed] [Google Scholar]
- Usach V, Goitia B, Lavalle L, Martinez Vivot R. and Setton-Avruj CP. 2011. Bone marrow mononuclear cells migrate to the demyelinated sciatic nerve and transdifferentiate into Schwann cells after nerve injury: Attempt at a peripheral nervous system intrinsic repair mechanism. J Neurosci Res 89: 1203–1217. [DOI] [PubMed] [Google Scholar]
- Usach V, Malet M, López M, Lavalle L, Piñero G, Saccoliti M, Cueto A, Brumovsky P, Brusco A and Setton-Avruj P 2017. Systemic transplantation of bone marrow mononuclear cells promotes axonal regeneration and analgesia in a model of Wallerian degeneration. Transplantation 101: 1573–1586. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Graham DL, Amos-Kroohs RM, Braun AA, Grace CE, Schaefer TL, Skelton MR, Erikson KM, Aschner M and Williams MT 2014. Effects of developmental manganese, stress, and the combination of both on monoamines, growth, and corticosterone. Toxicol Rep 1: 1046–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, L Johnson H and Williams MT 2008. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: Effects of dose and rearing conditions. Int J Dev Neurosci 26: 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Miller S and Zheng W 2008. Intracellular localization and subsequent redistribution of metal transporters in a rat choroid plexus model following exposure to manganese or iron. Toxicol Appl Pharmacol 230: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Song Y, He C, Liu C, Wu R, Fang L, Cong Y, Miao Y and Liu Z 2015. Divalent metal-ion transporter 1 is decreased in intestinal epithelial cells and contributes to the anemia in inflammatory bowel disease. Sci Rep 5: 16344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, 2008. Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotoxicol. Res 14: 45–56. [DOI] [PubMed] [Google Scholar]
- Zheng W, Xin N, Chi ZH, Zhao BL, Zhang J, UY J Li Z and Wang Y 2009. Divalent metal transporter 1 is involved in amyloid precursor protein processing and A beta generation. FASEB J 23: 4207–4217 [DOI] [PubMed] [Google Scholar]
- Zoni S and Lucchini RG 2013. Manganese exposure: Cognitive, motor and behavioral effects on children: a review of recent findings. Curr. Opin. Pediatr 25: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]