Abstract
In this review, Japanese experience of cytoreductive surgery and perioperative chemotherapy is described. The new concept of peritoneal metastasis (PM) type, i.e., trans-mesothelial, trans-lymphatic, and superficial growing metastasis type was proposed in 2012. Surgeons should perform peritonectomy according to the type of PM. Since 1980, Japanese surgical oncologists have been spearheading the use of cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemoperfusion (HIPEC) as treatment for PM from gastric cancer. Two RCTs were conducted to verify the effect of HIPEC for the prophylaxis of peritoneal recurrence after curative resection of advanced gastric cancer. These two studies indicated that HIPEC is effective in preventing peritoneal recurrence of gastric cancer with serosal invasion. In 2002, intraperitoneal chemotherapy using taxans was developed for the treatment of PM from gastric cancer and led to the development of neoadjuvant intraperitoneal/systemic chemotherapy (NIPS), which was reported in 2006. In 2009, extensive intra-operative peritoneal lavage (EIPL) was developed, and contributed to the remarkable improvement in survival of patients with positive lavage cytology as demonstrated by prospective randomized clinical trials. In 2017, the Peritoneal Surface Oncology Group International proposed the value of complete cytoreduction and peritoneal cancer index cut-off as independent prognostic factors after CRS for gastric cancer with PM. Founded in 2016, the Japanese/Asian School of Peritoneal Surface Oncology (JASPSO) trains beginners to perform CRS and HIPEC safely. Sixteen students have already graduated from JASPSO and started to perform the treatment in their home countries.
Keywords: Cytoreductive surgery, Perioperative chemotherapy, Peritoneal metastasis
Introduction
Until the late 1990s, peritoneal metastasis (PM) was considered a terminal stage, and almost all patients with PM died of the disease within a median of 5–7 months after palliative systemic chemotherapy or surgery alone [1, 2].
An innovative treatment for PM (hyperthermic intraperitoneal chemoperfusion (HIPEC)) was developed in 1980 by Spratt, who reported the first case successfully treated with HIPEC [3]. HIPEC has been used since then for the treatment of peritoneal metastasis (PM) from gastrointestinal cancer, and new regimens of systemic chemotherapy have been developed and used to treat PM since the late 1990s. However, the prognosis of patients with PM was poor after HIPEC or systemic chemotherapy alone [4, 5].
In 1995, Sugarbaker developed a new surgical technique of complete removal of PM named “peritonectomy” [6]. In the late 1990s, a paradigm shift occurred in the treatment of PM. Since PM is considered a local disease, confined to the peritoneal cavity, a combination therapy with cytoreductive surgery (CRS) using peritonectomy to remove macroscopic detectable PM and perioperative chemotherapy (POC) to eradicate micrometastases was proposed by the Peritoneal Surface Oncology Group International (PSOGI). This strategy is considered a comprehensive treatment that improves the long-term survival [7–10], and a curative approach in selected patients with limited PM [11]. In this review, the history and the present status of the treatment of PM in Japan will be described.
History of Peritoneal Surface Oncology in Japan
New Concept of Peritoneal Metastasis Formation
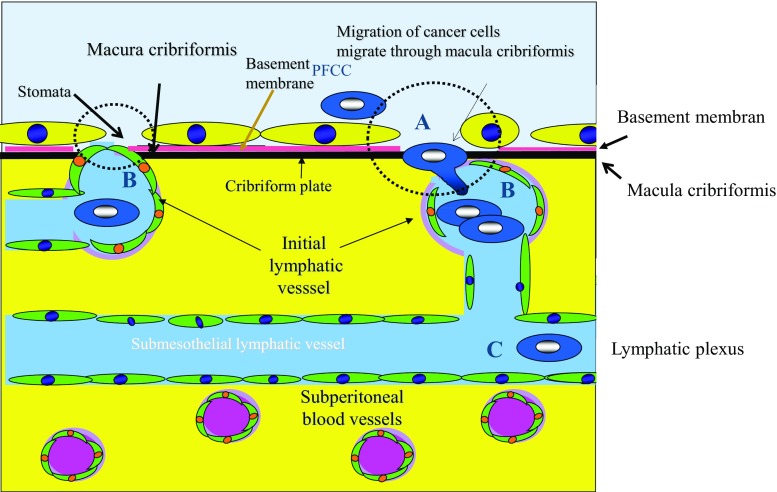
The establishment of PM has long been considered a multi-step process, consisting of (1) detachment of tumor cells from the primary tumor (peritoneal free cancer cells, PFCCs), (2) attachment of PFCCs to distant peritoneal surfaces, (3) invasion of PFCCs into the submesothelial tissue, and (4) proliferation accompanied by angiogenesis and the induction of stromal tissue [12]. The process was named “trans-mesothelial metastasis” by Yonemura [13] and “randomly proximal distribution” by Sugarbaker [14] (Fig. 1). At the completion of the process, cancer cells show step-by-step expression of metastasis-related proteins, such as adhesion molecules, motility factors, matrix digesting enzymes, and angiogenesis factors [13]. In 2012, Yonemura reported new pathways of metastasis, called “trans-lymphatic metastasis” and “superficially growing metastasis.”
Fig. 1.
Three types of peritoneal metastasis
In trans-lymphatic metastasis, PFCCs migrate into the submesothelial lymphatic vessels through mesothelial stomata and holes of macula cribriformis under the mesothelial basement membrane [15]. Macula cribriformis is a collagen plate below the mesothelial basement membrane with many holes, with which blind tips of initial lymphatic vessels connect. Subperitoneal lymphatic vessels, used for trans-lymphatic dissemination, are called initial lymphatics and are found in omental milky spots, parietal peritoneum, and small bowel mesentery. Omental milky spots absorb peritoneal fluid [16], and intraperitoneal inflammatory cells and PFCCs migrate into the initial lymphatic vessels of milky spots [17, 18] (Fig. 2-A). Initial lymphatic vessels have blind loops that extend from the submesothelial lymphatic plexus and blind tips that attach to the holes in the macula cribriformis (Fig. 2-B). PFCCs migrate into the initial lymphatic vessels and proliferate in the lymphatic plexus (Fig. 2-C). The triplet structure of the peritoneum consisting of mesothelial stomata, holes in the macula cribriformis, and initial lymphatic vessels is essential for the formation of trans-lymphatic metastasis (Fig. 2-broken circle) Figure 3. These structures are detected on the parietal peritoneum but not the peritoneum on the anterior upper abdominal wall. The peritoneum of the diaphragm, pelvis, paracolic gutter, Morrison’s pouch, and perihepatic ligaments has a triplet structure but not milky spots. The size of the holes in the macula cribriformis ranges from 5 to 30 μm. In the experimental study, intraperitoneally injected cancer cells induce mesothelial cell contraction and appear in submesothelial lymphatic vessels on day 3 after injection [17]. Clinically, trans-lymphatic metastasis is found in gastric, colorectal, and pancreatic cancer [15].
Fig. 2.
Trans-lymphatic metastasis. Peritoneal free cancer cells (PFCCs) migrate into the superficial lymphatic vessels (initial lymphatics (B)) which attached to the hole in the macula cribriformis (A) (collagen plate just below basement membrane) and mesothelial stomata (gap between mesothelial cells) and proliferate in the lymphatic plexus (C)
Fig. 3.
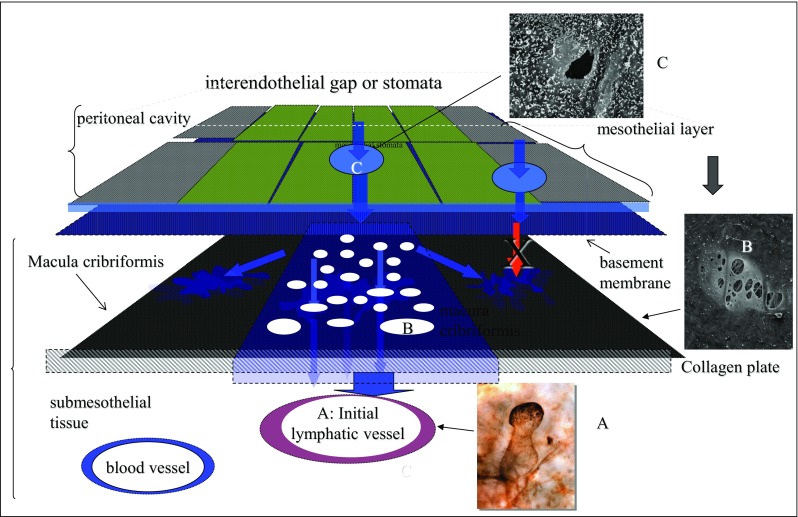
Trans-lymphatic metastasis. Initial lymphatic vessel stained with 5′-Nase enzyme staining (A). Blind loop lymphatic vessel attached to the hole of macula cribriformis (B) and mesothelial stomata (C). The triplet structure consisting of A, B, and C has a big role in the completion of trans-lymphatic metastasis
In contrast, PFCCs from appendiceal mucinous neoplasm (AMN) cannot metastasize through the trans-mesenteric or trans-lymphatic route because PFCCs from AMN are covered with mucinous material and too large (Fig. 4). However, AMN can establish PM in particular peritoneal areas.
Fig. 4.
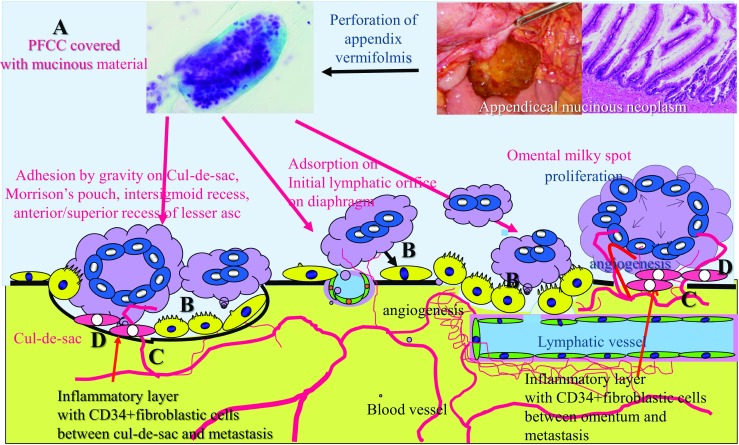
Superficial growing metastasis. Peritoneal free cancer cells (PFCCs) from the primary low-grade mucinous neoplasm are covered with mucinous material (A) and cannot invade the submesothelial tissue through the trans-mesothelial or trans-lymphatic routs because of their large size. PFCCs settle on the pelvic peritoneal surface by gravity, or adsorbed to the omental milky spots or diaphragmatic stomata (B). Then PFCCs proliferate on the peritoneal surface aided by neovascularization with subperitoneal blood capillaries (C) induced by CD34-positive interstitial cells between peritoneum (D, as shown in orange)
PFCCs of AMN settle on the pelvic peritoneal surface by gravity, or adsorbed to the omental milky spots or diaphragmatic stomata (Figs. 3-B and 4) [16]. Then, inflammation by cytokines from PFCCs induces immature stromal tissue between peritoneal surface and tumor layer. These immature stromal cells express CD34. CD34 is a glycoprotein expressed on the surface of interstitial stem cells and immature vascular endothelial cells [19]. CD34 inhibits tissue maturation, and disappears once maturation is complete. These stromal cells in immature interstitial tissue induce neovascularization from subperitoneal blood capillaries (Fig. 4).
This metastasis pattern is named “superficially growing metastasis” by Yonemura (Fig. 5) [11] and “redistribution pattern” by Sugarbaker [14].
Fig. 5.
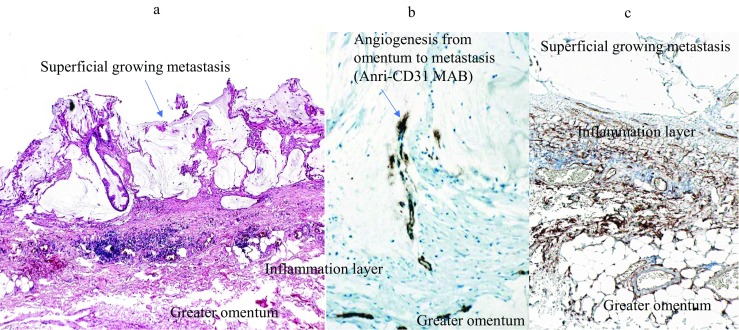
Microscopic findings of superficial growing metastasis. Tumor grows on a layer of the inflammatory cells on the greater omentum (A). Stromal tissue in the inflammatory layer shows positive immunoreaction against anti-CD34 monoclonal antibody (Mab) (C). Newly formed immature blood vessels, stained with anti-CD31 Mab, extended into the superficial growing metastasis from the preexisting blood vessels in the greater omentum (B).
Surgeons should understand the mechanisms of PM formation, and choose to perform peritonectomy options according to the metastatic pattern.
History of HIPEC, Neoadjuvant Intraperitoneal/Systemic Chemotherapy, and Laparoscopic HIPEC in Japan
After the first clinical application of HIPEC in1980 by Spratt, the Japanese surgical oncologist S. Koga began studying HIPEC, and reported the first clinical results of his trial of HIPEC for PM from gastric cancer in 1983 [20]. Then, Yonemura began treating gastric cancer patients with PM using HIPEC in 1986, and reported a case of complete disappearance of PM after HIPEC [21]. Since then, many clinical studies of treatment for PM from gastric and colorectal cancer have been performed [22, 23]. Fujimoto reported a better 3-year survival rate among patients receiving HIPEC + gastrectomy group than gastrectomy alone (46% vs 16%) [23]. Yamaguchi used HIPEC to treat PM from colorectal cancer, and reported significantly longer survival among complete responders [22].
In 1991, Yonemura et al. reported the survival improvement and safety of CRS plus HIPEC for PM from gastric cancer [24]. However, the 5-year (long-term) survival rate remained insufficient.
In 2002, intraperitoneal (IP) administration of taxan compounds was introduced as a gastric cancer treatment [25]. The IP taxan treatment was safe and had a pharmacologic advantage at peritoneal surface [25].
In 2006, Yonemura developed a new bidirectional chemotherapy using IP docetaxel and carboplatin combined with intravenous (IV) methotrexate and 5-fluorouracil [26]. This method was named “neoadjuvant intraperitoneal/systemic chemotherapy (NIPS),” and was believed to increase the treatment area.
In 2010, more powerful NIPS using IP/IV administration of docetaxel and cisplatin in combination with oral administration of S1 was developed [27]. The method effectively reduced peritoneal cancer index (PCI) and eradicated PFCCs [28, 29]. After introduction of NIPS plus CRS for gastric cancer PM, long-term survival was significantly improved, and 5-year survival rates were reported at 18 to 24% [8, 30].
In gastric cancer, complete cytoreduction (CCR-0) is essential for long-term survival [8]. However, CCR-0 can be performed in only 30% of patients with PM at the time of diagnosis [31]. Additionally, PCI cut-off value is an important prognostic factor. All patients with PCI higher than the cut-off level had poor prognosis and died even after complete cytoreduction. In contrast, postoperative survival was significantly more favorable in patients with PCI lower than the cut-off level [8, 9, 32]. According to the meta-analysis by Coccolini F, the PCI cut-off level should be 12 [8]. However, PCI evaluated by preoperative imaging (computed tomography, positron emission tomography) is inaccurate, if the diameter of PM is less than 8 mm [33–35]. Accordingly, PCI should be diagnosed by laparoscopy before CRS. Patients whose PCI is higher than the cut-off level should be excluded from CRS.
Since 2012, HIPEC has been performed on the day of exploratory laparoscopy. Consequently, the method is known as “laparoscopic HIPEC (LHIPEC).” LHIPEC (or closed HIPEC) (relative to open HIPEC as performed under laparotomy) achieves significantly greater drug penetration from the peritoneal surface because intraperitoneal pressure is significantly higher in closed HIPEC [36]. Accordingly, LHIPEC is expected to be more effective than open-type HIPEC.
In 2017, Yonemura first reported the direct effect of HIPEC on PM from gastric cancer in patients treated by LHIPEC [37]. From 2013 to 2016, a total of 53 patients with PM from gastric cancer received LHIPEC two times separated by a 1-month rest interval. Changes in PCI and cytologic status were studied at the time of the first and second laparoscopy. LHIPEC effectively eradicated peritoneal free cancer cells and reduced PCI. Previously, peritoneal cytologic status at CRS had been reported to relate significantly to survival after CRS [38]. In the LHIPEC group, cytology changed from positive to negative in 13 (68%) of 19 patients at the second laparoscopy. Additionally, eight (15%) patients showed complete disappearance of PM and significant reduction of PCI from 14.2 ± 10.7 at the first LHIPEC to 11.8 ± 11.0 at the second laparoscopy.
Additionally, another study was performed after 1 cycle of LHIPEC plus 3 cycles of NIPS. As a result, six (11.5%) patients showed complete disappearance of PM and significantly reduced PCI from 14.8 ± 11.4 at the first LHIPEC to 9.9 ± 11.3 LHIPEC plus 3 cycles of NIPS (P < 0.0001). Adding 3 cycles of NIPS, effectively doubled the mean reduction in PCI levels, significantly increased the number of patients with PCI ≤ 11 from 23 (44.2% of 52 patients) to 35 (67.3%), and changed peritoneal cytology from positive to negative in 22 (71%) of 31patients.
Diffuse involvement of the small bowel and its mesentery is the limiting factor for complete cytoreduction. Yonemura reported that all patients with small bowel PCI ≥ 3 died of disease even after complete cytoreduction [32]. LHIPEC plus 3 cycles of NIPS significantly reduced small bowel PCI. In 2016, PSOGI reported that CCR-0, PCI less than the cut-off value, and small bowel PCI ≤ 2 were the independent prognostic factors [28]. Accordingly, combining LHIPEC and NIPS is a powerful method to reduce small bowel PCI, thereby increasing the complete cytoreduction rate.
No grade 3, 4, or 5 complications of LHIPEC were found, and grade 3 complications of LHIPEC plus NIPS developed in only four patients (7.7%). Accordingly, LHIPEC plus NIPS can be done safely.
Development of a New Method for Detecting Small Peritoneal Metastasis
Even after CCR-0 resection for PM from gastric and colorectal cancer, peritoneal recurrence developed in about 70% of patients [38, 39]. One of the causes of recurrence had to be small PM overlooked by naked eye or palpation during the operation. A new method of detecting PM, named “photodynamic detection (PDD),” using 5-aminolevulinic acid (ALA) was developed. ALA is the natural precursor of protoporphyrin (Pp) IX and heme. In the heme synthesis pathway, ALA is converted to PpIX. After its excess administration, ALA accumulates in cancer cells through the action of the ALA influx transporter on cancer cell membrane. As a result, intracellular PpIX synthesis increases, and PpIX accumulates in cancer cells. PpIX in cancer cells emits a red fluorescence in violet right at 405 nm and renders detectable any PM of diameter of 0.1 mm (Fig. 6) [40–42].
Fig. 6.
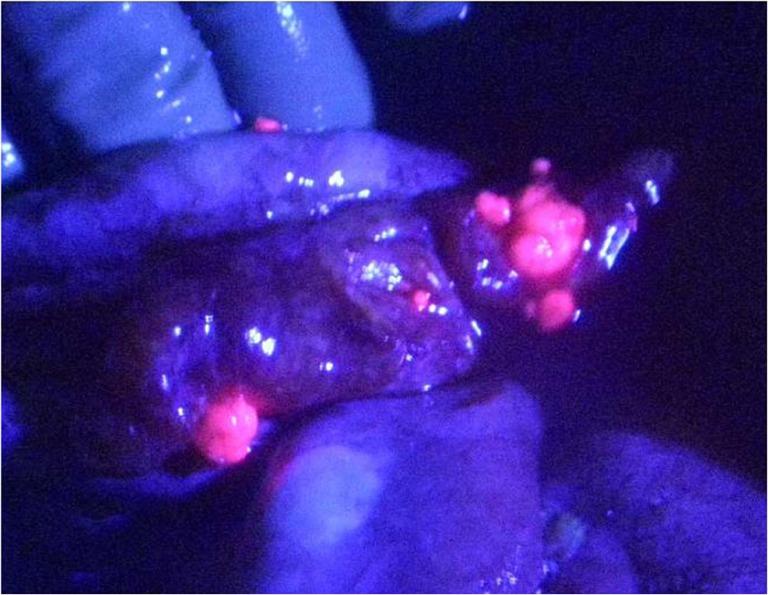
Peritoneal metastasis from ovarian cancer emitted strong red fluorescence when excited by violet light (405 nm) after oral administration of 1 g/patient of 5-aminolevulinic acid, 3 h before laparotomy. Peritoneal metastases were red-fluorescent and small but at least 0.1 mm in diameter can be detected
Initially, PDD for PM had been proposed in an animal model [43]. Normal peritoneum does not photoemit when excited by violet light because of the low PpIX content of normal mesothelial cells [44]. ALA-positive rates were higher in PM from pancreatic cancer, ovarian cancer, colorectal cancer, biliary cancer, small bowel cancer, and mesothelioma (ranging from 50 to 85%), but low in gastric cancer after NIPS and appendiceal mucinous neoplasm. PpIX content was significantly higher in ALA-positive PM than ALA-negative nodules. The PpIX content of PM depends on the expression of the ALA influx transporter, porphyrin efflux transporter [44, 45], and ferrochelatase. There have been no reported permanent side effects of ALA and no transient side effects except transient erythema and nausea [44–48]. ALA PDD is a safe and feasible method of small PM detection, and is indicated for ovarian cancer, mesothelioma, pancreatic cancer, colorectal cancer, biliary cancer, and small bowel cancer [44].
Training Program for CRS and HIPEC in Japan
Morbidity and mortality rates were reported to be significantly higher for those receiving CRS plus HIPEC than those receiving traditional palliative surgery. Between 16 and 68% of patients experienced grade 3 or 4 toxicity according to the common grading criteria and 7–11% of patients needed reoperation [49, 50]. Reported mortality rates were 0–9% [49–51]. Surgeons who want to provide the comprehensive treatment should be very knowledgeable about surgical skills, anatomy, physiology, oncology, chemotherapy, postoperative care, and nutrition, To perform CRS + HIPEC safely within mortality and morbidity limits, beginners have to be trained to carry out the operation techniques, HIPEC methods, and postoperative care under the guidance of experts. The reported learning curve is about 70–130 cases [52, 53]. Peritoneal Surface Oncology Group International (POSGI) started a training program for beginners in 2014 in Europe. The European School of Peritoneal Surface Oncology (ESPSO) is a joint venture of the European Society of Surgical Oncology (ESSO) and PSOGI. The target audience of this 2-year program is chief residents, fellow surgeons, consultant surgeons, gynecologists, and medical oncologists. Topics covered by the training program include (1) peritoneal carcinomatosis (overview and surgical techniques); (2) peritoneal carcinomatosis from colorectal cancer, ovarian cancer, gastric cancer, pseudomyxoma peritonei, and rare peritoneal surface malignancies; (3) palliative care; and (4) experimental and translational research. In addition, students have to attend a special training course held twice a year. Upon successful completion of this program, the candidates obtain European accreditation in Peritoneal Surface Oncology.
In 2016, the Japanese/Asian School of Peritoneal Surface Oncology (JASPSO) was founded as a joint venture with PSOGI. It provides adequate and structured training in the management of PM. Students receive highly specialized knowledge and learn to master the complexity of aggressive CRS combined with NIPS, LHIPEC, HIPEC, early postoperative chemotherapy (EPIC), and systemic chemotherapy. JASPSO’s director is Yutaka Yonemura, and basic and advanced training is provided at Kishiwada Tokushukai Hospital (Osaka) and Kusatsu General Hospital (Shiga). Since 2016, JASPSO has trained 16 students from foreign countries.
The Health Care Structure in Japan and Insurance Coverage for Comprehensive Treatment
At present, the combination of CRS and HIPEC is considered to be standard of care in selected patients with PM from colorectal cancer. However, health insurance coverage for the treatment is different from country to country. Bushati studied the health care structure by web-based survey of 19 expert surgeons from 19 different countries (Australia, Belgium, Brazil, Canada, China, Denmark, France, Germany, Greece, India, Italy, Japan, Netherlands, Singapore, Spain, Sweden, Switzerland, UK, and USA) [54]. CRS plus HIPEC was included in the national guideline in 16 of 19 countries and was approved for reimbursement by health insurance in 17 of 19 countries.
The Japanese guideline for colorectal PM designates CRS plus HIPEC as an experimental treatment and not a recommended treatment. Accordingly, the fee for CRS with HIPEC is not covered by insurance in Japan.
Peritoneal Surface Malignancy Centers in Japan
According to Bushati, more than 430 centers worldwide currently perform CRS + HIPEC, and more than 3800 colorectal cancer patients with PM worldwide annually receive CRS plus HIPEC [54]. There is a huge variation in the number of HIPEC centers per 1 million inhabitants per country, ranging from 0.007 in China to 1.8 in Switzerland.
In Japan, CRS plus HIPEC and HIPEC alone are performed in two and four centers, respectively. The number of CRS plus HIPEC centers in Japan is 0.015 per 1 million inhabitants. In the two big centers of Kishiwada Tokusyukai Hospital and Kusatsu General Hospital, there were 329 CRS + HIPECs and 118 LHIPECs were performed in 2017. During the last 10 years, 2351 patients were treated by CRS with or without HIPEC, and CCR-0 resection was performed in 1431 (61%) patients. Five-year survival rates after CCR-0 resection for patients with PM from pseudomyxoma peritonei, gastric cancer, colorectal cancer, ovarian cancer, mesothelioma, and small bowel cancer were 84%, 14%, 27%, 67%, 40%, and 46%, respectively.
The Contribution of Japan to Peritoneal Surface Oncology—What it Has Given to the World
Since 1980, Japanese surgical oncologists have been spearheading the use of CRS plus HIPEC for treating PM from gastric cancer. Hamazoe [55] and Yonemura [56] have reported the results of RCTs to verify the effect of HIPEC for the prophylaxis of peritoneal recurrence after curative resection of advanced gastric cancer. These two studies indicated that HIPEC is effective in preventing peritoneal recurrence of gastric cancer with serosal invasion.
In 2002, intraperitoneal chemotherapy using taxans was developed by Fushida for the treatment of PM from gastric cancer [25], and led to the development of NIPS reported in 2006 [26].
In 2009, extensive intra-operative peritoneal lavage (EIPL) was developed, and markedly improved the survival of patients with positive lavage cytology in prospective randomized clinical trials [57, 58].
In 2016, the PSOGI group reviewed the latest results of CRS plus HIPEC for gastric cancer with PM [28], and identified completeness of cytoreduction and PCI cut-off as independent prognostic factors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecological malignancy. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Brit J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 3.Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40(2):256–260. [PubMed] [Google Scholar]
- 4.Alexander HR, Fraker DL. Treatment of peritoneal carcinomatosis by continuous hyperthermic peritoneal perfusion with cisplatin. Cancer Treat Res. 1996;81:41–50. doi: 10.1007/978-1-4613-1245-1_5. [DOI] [PubMed] [Google Scholar]
- 5.Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trial N9741 and N9841. Cancer Res. 2012;30(3):263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verwaal VJ, Bruin A, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 8.Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P et al (2015) Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systemic review and meta-analysis. EJSO. 10.1016/j.ejso.2015.03.231 [DOI] [PubMed]
- 9.Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer A muti-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370–2377. doi: 10.1245/s10434-010-1039-7. [DOI] [PubMed] [Google Scholar]
- 10.Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–1581. doi: 10.1245/s10434-011-1631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yonemura Y (2018) Peritoneal cancer index and prognosis. In: Yonemura Y (ed) Comprehensive treatment for peritoneal surface malignancy with an intent of cure. NPO to Support Peritoneal Surface Malignancy, pp 3–55
- 12.Jayne D (2007) Molecular biology of peritoneal carcinomatosis. In: Ceelen WP (ed) Cancer treatment and research. Springer, pp 21–31 [DOI] [PubMed]
- 13.Yonemura Y, Canbay E, Liu Y et al (2013) Trans-lymphatic metastasis in peritoneal dissemination. J Gastroint Dig Syst S12. 10.4172/2161-069X.S12-007
- 14.Sugarbaker PH. Observation concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. In: Sugarbaker PH, editor. Peritoneal carcinomatosis: a multidisciplinary approach. Boston: Kluwar Academic Publisher; 1997. pp. 79–100. [DOI] [PubMed] [Google Scholar]
- 15.Yonemura Y (2012) Trans-lymphatic metastasis. In: Yonemura Y (ed) Atlas and principles of peritonectomy for peritoneal surface malignancy, Published by NPO to Support Peritoneal Surface Malignancy, pp 188–206
- 16.Bettendorf U. Lymph flow mechanism of the subperitoneal diaphragmatic lymphatics. Lymphology. 1978;11(3):111–116. [PubMed] [Google Scholar]
- 17.Tsujimoto H, Takhashi T, Hagiwara A, et al. Site-specific implantation in the milky spots of malignant cells in peritoneal dissemination: immunohistochemical observation in mice inoculated intraperitoneally with bromodeoxyuridine-labeled cells. Br J Cancer. 1995;71:468–472. doi: 10.1038/bjc.1995.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimotsuma M, Takahashi T, Kawata M, Dux K. Cellular subset of the milky spots in the human greater omentum. Cell Tissue Res. 1991;264:599–601. doi: 10.1007/BF00319049. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Flores L, Gutierrez R, Garcia MP, et al. CD34+ stromal cells/fibroblastis/fibrocytes/telocyted as a tissue reserve and principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol Histopathol. 2014;29:831–870. doi: 10.14670/HH-29.831. [DOI] [PubMed] [Google Scholar]
- 20.Koga S, Shimizu N, Maeta M, Hamazoe R, Izumi A. Application of heat combined with antineoplastic agent administration in the treatment if cancer (with special reference to malignancy of the digestive system) Gan to Kagaku Ryoho. 1983;10:358–365. [PubMed] [Google Scholar]
- 21.Fujimura T, Yonemura Y, Fushida S, Urade M, Takegawa S, Kamata T, Sugiyama K, Hasegawa H, Katayama K, Miwa K, Miyazaki I. Continuous hyperthermic intraperitoneal perfusion for the treatment of peritoneal dissemination in gastric cancer and subsequent second-look operation. Cancer. 1990;65(1):65–71. doi: 10.1002/1097-0142(19900101)65:1<65::aid-cncr2820650115>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi A, Tsukioka Y, Fushida S, Kurosaka Y, Kanno M, Yonemura Y, Miwa K, Miyazaki I. Intraperitoneal hyperthermic treatment for peritoneal dissemination of colorectal cancer. Dis Colon Rectum. 1992;35(10):964–968. doi: 10.1007/BF02253499. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto S, Takahashi M, Mutou T, et al. Survival time and prevention of side effects of intraperitoneal perfusion with mitomycin C combined with surgery for patients with advanced gastric cancer. Cancer Treat Res. 1996;81:169–176. doi: 10.1007/978-1-4613-1245-1_14. [DOI] [PubMed] [Google Scholar]
- 24.Yonemura Y, Fujimura T, Fushida S, Takegawa S, Kamata T, Katayama K, Kosaka T, Yamaguchi A, Miwa K, Miyazaki I. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg. 1991;15(4):530–535. doi: 10.1007/BF01675656. [DOI] [PubMed] [Google Scholar]
- 25.Fushida S, Furui N, Kinami S, et al. Pharmacologic study of intraperitoneal docetaxel in gastric cancer patients with peritoneal dissemination. Gan To Kgaku Ryoho. 2002;29(12):2164–2167. [PubMed] [Google Scholar]
- 26.Yonemura Y, Bandou E, Sawa T, Yoshimitsu Y, Endou Y, Sasaki T, Sugarbaker PH. Neoadjuvant treatment of gastric cancer with peritoneal dissemination. EJSO. 2006;32(6):661–665. doi: 10.1016/j.ejso.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Yonemura Y, Endou Y, Sasaki T, et al. Surgical treatment for peritoneal carcinomatosis from gastric cancer. EJSO. 2010;36(12):1121–1138. doi: 10.1016/j.ejso.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Yonemura Y, Canbay E, Li Y et al (2016) A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur J Surg Oncol available in online. 10.1016/j.ejso.2016.03.016 [DOI] [PubMed]
- 29.Yonemura Y, Ayman E, Endou Y, et al. Effects of neoadjuvant intraperitoneal/systemic chemotherapy (bidirectional chemotherapy) for the treatment of patients with peritoneal metastasis from gastric cancer. Int J Surg Oncol. 2012;2012:148420. doi: 10.1155/2012/148420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, Ceresoli M, Montori G, Ansaloni L. Effect of intraperitoneal chemotherapy and peritoneal lavage in positive peritoneal cytology in gastric cancer. Systematic review and meta-analysis. Eur J Surg Oncol. 2016;42(9):1261–1267. doi: 10.1016/j.ejso.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 31.Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal preoperative chemotherapy (HIPEC) in the management of refractory malignant ascites: a multi-institutional retrospective analysis in 52 patients. J Surg Oncol. 2009;100(4):331–334. doi: 10.1002/jso.21321. [DOI] [PubMed] [Google Scholar]
- 32.Yonemura Y, Elnemr A, Endou Y, Ishibashi H, Mizumoto A, Miura M, Li Y. Surgical results of patients with peritoneal carcinomatosis treated with cytoreductive surgery using a new technique named aqua dissection. Gastroenterol Res Pract. 2012;2012:521487. doi: 10.1155/2012/521487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong SH, Shin YR, Roh Y, Jeong EK, Song KY, Park CH, et al. Treatment outcomes of systemic chemotherapy for peritoneal carcinomatosis arising from gastric cancer with no measurable disease: retrospective analysis from a single center. Gastric Cancer. 2013;16:290–300. doi: 10.1007/s10120-012-0182-1. [DOI] [PubMed] [Google Scholar]
- 34.Koh JL, Yan TD, Glenn D, Morris DL. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2009;16:327–333. doi: 10.1245/s10434-008-0234-2. [DOI] [PubMed] [Google Scholar]
- 35.Pôfannenberg C, Knigstainer, Aschoff P, Oksűz MO, Zieker D, Beckers S, et al. (18) F-FDG-PET-CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2009;16:1295–1303. doi: 10.1245/s10434-009-0387-7. [DOI] [PubMed] [Google Scholar]
- 36.Thomas F, Ferron G, Gesson-Paute A, Hristova M, Lochon I, Chatelut E. Increased tissue diffusion of oxaliplatin during laparoscopically assisted versus open heated intraoperative intraperitoneal chemotherapy. Ann Surg Oncol. 2008;15:3623–3624. doi: 10.1245/s10434-008-0115-8. [DOI] [PubMed] [Google Scholar]
- 37.Yonemura Y, Ishibashi H, Hirano M, Mizumoto A, Takeshita K, Noguchi K, Takao N, Ichinose M, Liu Y, Li Y. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol. 2017;24(2):478–485. doi: 10.1245/s10434-016-5487-6. [DOI] [PubMed] [Google Scholar]
- 38.Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric cancer treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol. 2015;21(4):1147–1152. doi: 10.1245/s10434-013-3443-2. [DOI] [PubMed] [Google Scholar]
- 39.Yonemura Y, Canbay E, Shintani H, et al. Treatment failure following complete cytoreductive surgery for peritoneal metastasis from colorectal cancer. Gan To Kagaku Ryoho. 2016;43(12):1435–1439. [PubMed] [Google Scholar]
- 40.Jichlinski P, Forre M, Mizeret J, et al. Clinical evaluation of a method for detecting superficial transitional cell carcinoma of the bladder by light-induced fluorescence of protoporphyrin IX following topical application of 5-aminolevulinic acid: preliminary results. Lasers Surg Med. 1997;20:402–408. doi: 10.1002/(sici)1096-9101(1997)20:4<402::aid-lsm5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko S. Photodynamic applications (PDD, PDT) using aminolevulinic acid in neurosurgery. In: Okura I, Tanaka TR (eds) Aminolevulinic acid. Science, technology and application. SBI ALA Promo Co., Ltd, pp 119–140
- 42.Rodoriguez L, Batle A, Di Verosa G, et al. Study of the mechanisms of uptake of 5-aminolevulinic acid derivatives by PEPT1 and PET2 transporters as a tool to improve photodynamic therapies of tumours. Int J Biochem Cell Biol. 2006;38:1530–1539. doi: 10.1016/j.biocel.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Hino H, Murayama Y, Nakanishi M, Inoue K, Nakajima M, Otsuji E. 5-aminolevulinic acid-mediated photodynamic therapy using light-emitting diodes of different wavelength in a mouse model of peritoneally disseminated gastric cancer. J Surg Res. 2013;185(1):119–126. doi: 10.1016/j.jss.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 44.Yonemura Y, Endo Y, Canbay E, et al. Photodynamic detection of peritoneal metastases using 5-aminolevulinic acid (ALA) Cancers (Basel) 2017;9(3):E23. doi: 10.3390/cancers9030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagiya Y, Furuhara H, Matsumoto K, et al. Expression levels of PEPT1 and ABCG2 play key roles in 5-aminolevulinic acid (ALA)-induced tumor specific protoporphyrin IX (PpIX) accumulation in bladder cancer. Photodiagn Photodyn Ther. 2013;10(3):288–295. doi: 10.1016/j.pdpdt.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Lőning M, Diddens H, Kűpker W, et al. Laparoscopic fluorescent detection of ovarian carcinoma metastasis using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer. 2004;100:1650–1656. doi: 10.1002/cncr.20155. [DOI] [PubMed] [Google Scholar]
- 47.Maruyama Y, Ichikawa D, Koizumi N, et al. Staging fluorescence laparoscopy for gastric cancer by using 5-aminolevulinic acid. Anticancer Res. 2012;32:5421–5427. [PubMed] [Google Scholar]
- 48.Hillermans P, Wimberger P, Reif J, et al. Photodynamic diagnosis with 5-aminolevulinic acid for intraoperative detection of PM of ovarian cancer. A feasibility and dose finding study. Lasers Surg Med. 2016;49(2):169–176. doi: 10.1002/lsm.22613. [DOI] [PubMed] [Google Scholar]
- 49.Yonemura Y, Canbay E, Sako S, Wakama S, Ishibashi H, Hirano M, Mizumoto A, Takao N, Ichinose M, Noguchi K, Motoi S, Liu Y, Li Y, Taniguchi K. Comprehensive treatment using colorectal cancer patients with metachronous peritoneal metastasis. Gan To Kagaku Ryoho. 2017;44(12):1939–1942. [PubMed] [Google Scholar]
- 50.National Institute of Health and Clinical Excellence (NICE). Cytoreduction surgery followed by hyperthermic intraoperative peritoneal chemotherapy for peritoneal carcinomatosis. IPG331. http://www.nice.org.uk/guidance/IPG331 (accessed 12 August 2013)
- 51.Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquival J, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2005;13:635–644. doi: 10.1245/ASO.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 52.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Brit J Surg. 2007;94(11):1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y, Arzahrani NA, Liauw W, et al. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. ANZ J Surg. 2017;87(1–2):49–54. doi: 10.1111/ans.13280. [DOI] [PubMed] [Google Scholar]
- 54.Bushati M, Rovers KP, Sommariva A, Sugarbaker PH, Morris DL, Yonemura Y, Quadros CA, Somashekhar SP, Ceelen W, Dubé P, Li Y, Verwaal VJ, Glehen O, Piso P, Spiliotis J, Teo MCC, González-Moreno S, Cashin PH, Lehmann K, Deraco M, Moran B, de Hingh IHJT. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI) Eur J Surg Oncol. 2018;44(12):1942–1948. doi: 10.1016/j.ejso.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer. 1994;73(8):2048–2052. doi: 10.1002/1097-0142(19940415)73:8<2048::aid-cncr2820730806>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 56.Yonemura Y, de Aletxabala X, Fujimura T, et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepato-Gastroenterology. 2001;48(42):1776–1782. [PubMed] [Google Scholar]
- 57.Masuda T, Kuramoto M, Shimada S, et al. The effects of extensive intraoperative peritoneal lavage therapy (EIPL) in stage IIIB+C and cytology-positive gastric cancer patients. Int J Clin Oncol. 2016;21(2):289–294. doi: 10.1007/s10147-015-0892-6. [DOI] [PubMed] [Google Scholar]
- 58.Yonemura Y, Canbay E, Endou Y, Ishibashi H, Mizumoto A, Miura M, Li Y, Liu Y, Takeshita K, Ichinose M, Takao N, Hirano M, Sako S, Tsukiyama G. Peritoneal cancer treatment. Expert Opin Pharmacother. 2014;15(5):623–636. doi: 10.1517/14656566.2014.879571. [DOI] [PubMed] [Google Scholar]