Abstract
The treatment of peritoneal surface malignancies, either primary or secondary (peritoneal metastasis), has evolved over the past two decades. A nihilistic approach of incurable “carcinomatosis” is changing into treatment of peritoneal metastasis with curative intent. The aim of the present study is to review the current practice, past history, and future of peritoneal surface oncology in Israel. A systematic review of all patients treated in institutions performing cytoreductive surgery (CRS) and hyperthermic intra-peritoneal chemotherapy (HIPEC) for the treatment of peritoneal surface malignancies. Each center provided the following data: start year, number of total cases, number of cases performed in 2017, and the method used (open vs. closed technique). Between 1990 and 2018, there were 1462 patients treated by CRS/HIPEC in Israel by eight different surgical groups in six medical centers. Currently, there are seven surgical groups in six medical centers routinely performing CRS/HIPEC. The annual rate of CRS/HIPEC was 171 cases in 2017 with a range of (4–69 cases/center). This is the first step of establishing an Israeli Peritoneal Surface Oncology Group that will have joined database and perform clinical trials in this challenging field of surgical oncology.
Keywords: Cytoreductive surgery, Hyperthermic intraperitoneal chemotherapy, Peritoneal surface malignancy, Multiple center experiance, Experiance in Israel
Introduction
The treatment of peritoneal surface malignancy (PSM) by cytoreductive surgery (CRS) combined with hyperthermic intra-peritoneal chemotherapy (HIPEC) dramatically changed the outcome of many patients worldwide. The concept of incurable “peritoneal carcinomatosis” treated systemically with little benefit and a short life expectancy has evolved into a new paradigm of combining radical surgery and HIPEC with curative intent and survival benefit [1].
The first documented CRS/HIPEC for low-grade mucinous neoplasm of the appendix (pseudomyxoma peritonei, PMP) was performed in 1979 and published a year later by John Spratt [2]. The ability to perform peritonectomy procedures in a structured manner was first proposed by Sugarbaker in 1995 [3, 4]. Several clinical trials and retrospective analyses of large cohorts of patients positioned CRS/HIPEC as an acceptable option for patients with PMP [5], colorectal peritoneal metastasis [6], diffuse malignant peritoneal mesothelioma (DMPM) [7], and serous carcinoma of the ovary [8].
The concept of CRS/HIPEC for the treatment of PSM was developed in the years 1990–2000 by a few groups of “pioneers.” Joined by “early adaptors” in the years 2000–2010, combined with the development of industrial dedicated HIPEC devices, a major clinical and scientific progress was made. Since 2010, major acceptance by regulatory authorities (Europe) and insurance companies (USA) led many centers worldwide to open PSM programs and perform CRS+HIPEC.
In Israel, the testament of PSM by CRS/HIPEC started in 1990. The first surgical groups, influenced by surgeons from the USA (mainly Dr. Paul H. Sugarbaker) and Japan, started practicing CRS/HIPEC for selected patients. The second generation of “early adopters,” were trained by Israeli “pioneer surgeons” or by surgeons in the USA and Europe were able to develop centers of excellence treating a high number of patients. The third generation of young surgeons with local training combined with training in specialty centers in the USA or Europe is now emerging.
Referral patterns are changing but still many of the patients eligible for CRS/HIPEC are not referred to centers of excellence and managed with systemic therapy only.
The aim of this study is to establish a centralized group of experts in the field of peritoneal surface oncology and to describe the experience gained in Israel in the field of PSM.
Methods
This is a retrospective chart review. All medical centers performing CRS/HIPEC in Israel were contacted. In each center, a principal investigator retrieved the hospital charts of patients who underwent CRS/HIPEC. In the six medical centers, there were nine different groups performing CRS/HIPEC, either in different periods of time or in parallel. In 5/9 groups, data was retrieved from prospectively maintained databases. In 4/9 groups, data was retrieved from hospital records.
For the purpose of the current study, the following parameters were analyzed for each group:
Date of first procedure, total number of procedures performed, number of procedures performed currently (2017, only for active groups), breakdown of performed cases in 2017 according to diagnosis and HIPEC method (open/closed).
For continuous quality control and future clinical research the following parameters are currently collected into a central national web-based database sponsored by the Israeli Society of Surgical Oncology using Red-Cap® platform:
Demographics: Age, gender, date of surgery, co-morbid conditions.
Disease: Diagnosis (cancer type), date of diagnosis, prior surgery, prior chemotherapy, pre-operative peritoneal cancer index (PCI), presence of liver metastasis.
Surgery: Duration of the procedure, estimated blood loss, number of packed red blood cells transfused, number of peritonectomy procedures performed, number of organs resected, operative PCI.
HIPEC: method (open/closed abdomen), duration, cytotoxic agents used, additional intravenous drugs (bi-directional).
Short-term outcomes: hospital stay, ICU stay, surgical complications (by grade), 90-day mortality.
Long-term outcome: Disease status, disease recurrence (site and date), other treatments applied.
In order to evaluate the total number of patients with PSM diagnosed in 2017, The National Cancer Registry (https://www.health.gov.il/UnitsOffice/HD/ICDC/ICR/Pages/default.aspx) data was analyzed for the number of new cases of each cancer type diagnosed in 2017. Currently, there is no data available in the National Cancer Registry on the stage and presence of peritoneal metastasis; therefore, we estimated the number of new cases of PSM for each cancer type according to the data available from other international registries.
Results
All surgeons performing CRS/HIPEC were identified and contacted. There were seven active groups currently performing CRS/HIPEC in six medical centers. Most of the cases are done by three high-volume groups (n = 25/year).
The first case was performed by group #1 in 1990. Until the date of analysis (October 31, 2018), 1462 cases of CRS/HIPEC were performed (Table 1) in Israel.
Table 1.
Distribution of CR/HIPEC cases performed
| Group | Start year | Last case | Number of cases | Number of cases in 2017 | Method | Center |
|---|---|---|---|---|---|---|
| Group #1 (GBM) | 1990 | 2002 | 120 | None | Closed | Center #1 |
| Group #2 (JK) | 1994 | Active | 432 | 38 | Open/closed* | Center #2 |
| Group #3 (SSC) | 1994 | 2014 | 50 | None | Closed | Center #2 |
| Group #4 (MG) | 2003 | Active | 219 | 17 | Open/closed* | Center #1 |
| Group #5 (AN) | 2007 | Active | 443 | 69 | Closed | Center #1 |
| Group #6** (DBS) | 2016 | Active | 62 | 30 | Open | Center #3 |
| Group #7 (RL) | 2009 | Active | 45 | 5 | Closed | Center #4 |
| Group #8 (AH) | 2017*** | Active | 4 | 4 | Closed | Center #5 |
| Group #9 (GS-IA) | 2001 | Active | 87 | 8 | Open | Center #6 |
| Total | – | – | 1462 | 171 | – | – |
*Converted from open to closed-abdomen technique
**PI immigrated to Israel in 2015. Total number of cases performed by the group (total number of cases in Israel)
***PI moved from other center
In the first decade of CRS/HIPEC, cases were done either in the open- or closed-abdomen technique. Since 2007, a gradual shift towards the closed-abdomen technique was observed. Currently all groups, but one, are practicing the closed-abdomen HIPEC technique.
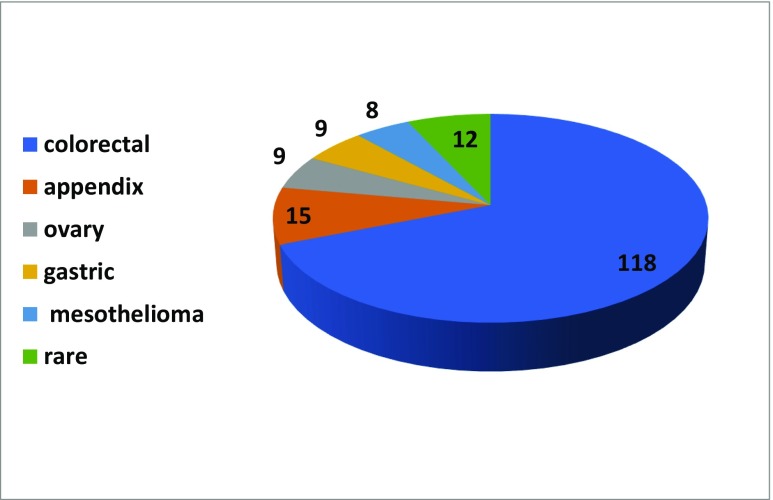
In 2017, a total of 171 of CRS/HIPEC cases (range 4–69/center) were performed. As expected, most of the cases were colorectal PM (n = 118, 69%), followed by appendiceal malignancies (n = 15, 8.8%), ovarian cancer (n = 9, 5.3%), gastric cancer (n = 9, 5.3%), mesothelioma (n = 8, 4.7%), and other rare indications (Fig. 1).
Fig. 1.
Origin of PSM treated in 2017 (n = 171)
According to the Israeli National Cancer Registry, there were 3016 new cases of CRC diagnosed in 2017, 361 cases of ovarian cancer, 701 cases of gastric cancer, and estimated 50 cases of appendiceal cancer. Cases of peritoneal mesothelioma are recorded with pleural mesothelioma; therefore, we assumed a rate of 10–30% of all diagnosed mesotheliomas occur in the peritoneum (Table 2). In PM of CRC and appendiceal origin, it is assumed that the more than 50% of the eligible patients are referred to PSM centers for evaluation and treatment while in PM of gastric or ovarian origin less than 10% of eligible patients are being considered for CRS/HIPEC.
Table 2.
Estimated versus treated cases of PSM in 2017
| Cancer type | New cases | Estimated peritoneal metastasis (%) | Estimated number of cases | Number of cases treated | Cases treated (% of estimated) |
|---|---|---|---|---|---|
| Colorectal | 3016 | 15* | 225 | 118 | 52 |
| Ovary | 361 | 50 | 180 | 9 | 5 |
| Appendix | 30** | 90 | 27 | 15 | 55 |
| Gastric | 701 | 50 | 350 | 9 | 2.6 |
| Mesothelioma | 50 | 10–30*** | 15 | 8 | 53 |
*1361 Patients died of synchronous or meatchronous metastatic CRC in 2017 in Israel assuming about 1500 cases are diagnosed with synchronous or meatchronous metastatic CRC, of which 15% will be PM
**Appendiceal cancers are reported in the National Cancer Registry as “Others”; therefore, it is an estimation based on international ratio of appendiceal/colon cancers
***10–30% are diffuse peritoneal malignant mesothelioma
Discussion
The population of the State of Israel in 2017 was 8,712,000 citizens (www.cbs.gov.il). The National Health Act (1994) provides full medical coverage to all citizens as defined by an annually updated “Health Basket” containing all medical services provided free of charge (https://www.health.gov.il/Subjects/UninsuredRights/HealthInsuranceLawRights/Pages/SalServices.aspx). Medical insurance is provided by four health maintenance organizations (HMO) or “Sick Funds” providing all medical services included in the Health Basket. All services included in the Health Basket are coded with a diagnosis-related group (DRG) code for reimbursement. In 2017, CRS/HIPEC was included in the Health Basket and provided with a DRG code. National guidelines for cancer care are limited; therefore, cancer care is provided according to international guidelines such as NCCN or ASCO guidelines. In the case of PSM, there are no current guidelines.
According to the data available from 2017, 171 cases of CRS/HIPEC were performed in six medical centers. There were three high-volume (more than 25 cases/year) and three low-volume centers.
The majority of patients (n = 118) treated had CRPM. The estimated rate of new cases of CRPM (assuming 15% PM of 50% synchronous and metachronous stage IV patients) nationwide is 225/year. In 2017, there were only 118 cases of CRPM who underwent CRS/HIPEC. Assuming 15% of CC2-3 and additional 20% of patients not eligible for CRS/HIPEC due to co-morbid conditions or high PCI, it is estimated that 70% of eligible with CRPM underwent CRS/HIPEC. The results show awareness of both medical and surgical oncologist to the option of CRS/HIPEC in CRPM compared to the USA or European Union. The high rate of referrals from medical oncologists specializing in gastrointestinal cancers can be explained by the close relationship of surgeons and medical oncologists treating for many years complex gastrointestinal cancers and treating routinely CRC liver metastasis.
The rate of appendiceal neoplasms is not recorded in the National Cancer Registry; therefore, it is estimated that the vast majority of patients eligible for CRS/HIPEC are being referred to PSM centers but there is no evidence to support that. There were only 15 cases of appendiceal neoplasms treated by CRS/HIPEC in 2017 in Israel. It is safe to assume that most of the eligible patients for CRS/HIPEC were evaluated by one of the seven active PSM groups and because co-morbid conditions or high PCI, in invasive cancer cases, were not treated.
There were only nine patients treated in 2017 by CRS/HIPEC for serous carcinoma of the ovary. In 2014, according to the National Cancer Registry, 361 new cases of ovarian cancer were diagnosed, of which 238 (66%) were metastatic at diagnosis. Assuming that the vast majority were peritoneal metastasis (OCPM), with the reduction of 20% of patients not eligible because of co-morbid conditions, it is safe to estimate around 150–180 cases of OCPM were eligible to CRS/HIPEC according to a recently published prospective randomized trial [8]. The peritoneal recurrence rate in patients with ovarian carcinoma FIGO Stage IIIc, undergoing “optimal debulking” combined with systemic therapy, is around 80% in 5 years [9]. Therefore, it can be estimated that an additional 150 cases/year of recurrent disease may be eligible for CRS/HIPEC. It is, therefore, clear that only a minority of patients with OCPM are being offered CRS/HIPEC. This may be explained by the lack of close collaboration between gynecologic-oncologists and surgical oncologists. In order to increase the referral rate of women with ovarian cancer, we will need to incorporate gynecologic-oncologists into our PSM groups and design combined treatment protocols.
There were nine patients treated in 2017 by CRS/HIPEC for gastric cancer. In 2014, according to the National Cancer Registry, 704 new cases of Gastric cancer were diagnosed, of which more than 300 were metastatic at diagnosis. Recurrence rate in 5 years is 60% of which about 50% will present with PM. However, most of the gastric cancer PM cases are not suitable for CRS/HIPEC because of high PCI. Only selected cases are currently being treated by CRS/HIPEC in Israel.
A total of 50 new cases of mesothelioma were diagnosed in Israel in 2017, of which 10–15 were peritoneal mesotheliomas. There were eight cases of peritoneal mesothelioma treated by CRS/HIPEC. The difference between diagnosed cases and treated may stem from patients who are not eligible for CRS/HIPEC because of frailty or sarcomatoid/biphasic mesothelioma. It can be safely estimated that patients with peritoneal mesothelioma eligible for CRS/HIPEC are being evaluated by one of the active PSM groups in Israel.
The evolution of CRS/HIPEC in Israel is almost similar to the global evolution with selected pioneers working in the open abdomen influenced by US surgeons (mainly PH Sugarbaker) and in the closed-abdominal technique, influenced by Japanese surgeons. There is a shift towards the closed-abdomen technique, mainly due to convenience and safety issues.
Most of the cases are concentrated in three high-volume groups performing more than 25 CRS/HIPEC cases/year and another three low-volume groups. The integration of all data from all active centers may lead to better quality assurance, clinical guidelines, and clinical research.
In conclusion, CRS/HIPEC is being practiced for 28 years in Israel. There are high-volume as well as low-volume centers offering high-quality, free of charge care in a timely fashion to multiple patients with PSM. While most of the patients with CRPM and appendiceal cancers are treated, there is a lack of awareness or a lack of referral of patients with advanced or recurrent ovarian cancer.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joseph Klausner and Aviram Nissan contributed equally to this work.
References
- 1.Passot G, Vaudoyer D, Villeneuve L, Kepenekian V, Beaujard AC, Bakrin N, Cotte E, Gilly FN, Glehen O. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113(7):796–803. doi: 10.1002/jso.24248. [DOI] [PubMed] [Google Scholar]
- 2.Spratt JS, Adcock RA, Muskovin M, Sherrill W. McKeown J. clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40(2):256–260. [PubMed] [Google Scholar]
- 3.Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12:703–727. doi: 10.1016/S1055-3207(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 4.Mehta SS, Bhatt A, Glehen O. Cytoreductive surgery and peritonectomy procedures. Indian J Surg Oncol. 2016;7(2):139–151. doi: 10.1007/s13193-016-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 6.Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2462–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 7.Baratti D, Kusamura S, Cabras AD, Bertulli R, Hutanu I, Deraco M. Diffuse malignant peritoneal mesothelioma: long-term survival with complete cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC) Eur J Cancer. 2013;49(15):3140–3148. doi: 10.1016/j.ejca.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 8.van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi: 10.1056/NEJMoa1708618. [DOI] [PubMed] [Google Scholar]
- 9.Zivanovic O, Sima CS, Iasonos A, Bell-McGuinn KM, Sabbatini PJ, Leitao MM, Levine DA, Gardner GJ, Barakat RR, Chi DS. Exploratory analysis of serum CA-125 response to surgery and the risk of relapse in patients with FIGO stage IIIC ovarian cancer. Gynecol Oncol. 2009;115(2):209–214. doi: 10.1016/j.ygyno.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]