Abstract
Incidence of synchronous peritoneal metastases (PM) in colorectal cancer is approximately 5%, with another 5% of the patients develop metachronous PM. Colorectal PM has been hypothesized to be a loco-regional disease rather than a systemic spread, and cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been considered as a viable treatment option. Pelvic exenteration is an established treatment option for locally advanced rectal cancer, but it is associated with significant morbidity. However, there are no studies evaluating the role of such procedure probably because the majority consider it as an exclusion criterion. Here, we present our experience with three cases of locally advanced rectal cancer with PM, treated successfully with pelvic exenteration and CRS-HIPEC.
Keywords: CRS, HIPEC, Pelvic exenteration, Rectal cancer
Introduction
Colorectal cancer is the fourth most prevalent cancer worldwide, and metastatic disease is the predominant cause of death in this disease. The incidence of synchronous peritoneal metastases (PM) in colorectal cancer is approximately 5%, with another 5% of the patients developing clinically relevant metachronous PM during the natural course of the disease [1, 2]. Colorectal PM has been hypothesized to be a loco-regional disease rather than a systemic spread, and cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been considered as a viable treatment option with acceptable morbidity and improved long-term survival [3]. Pelvic exenteration is an established treatment option for locally advanced rectal cancer, but it is associated with significant morbidity and therefore, many investigators do not recommend CRS-HIPEC with pelvic exenteration. However, there are no studies evaluating the role of such procedure probably because the majority consider it as an exclusion criterion. Here, we present our experience with three cases of locally advanced rectal cancer with PM, treated with pelvic exenteration with CRS-HIPEC (Table 1).
Table 1.
Summary of 3 cases in present series
| Variable | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age | 38 | 22 | 23 |
| Gender | Female | Male | Female |
| Location of tumor | Middle 3rd | Lower 3rd | Middle 3rd |
| Tumor differentiation | MDAC | PDAC/Signet | PDAC/Signet |
| Neoadjuvant treatment | SCRT f/b 4# CAPOX | 3# CAPOX f/b NACTRT f/b 4# CAPOX | SCRT f/b 4# CAPOX |
| PCI | 4 | 5 | 6 |
| Extent of surgery | Posterior exenteration + pelvic peritonectomy + omentectomy | Total pelvic exenteration + pelvic peritonectomy + omentectomy | Posterior exenteration + pelvic, paracolic peritonectomy |
| Blood loss (ml) | 500 | 750 | 600 |
| Duration of surgery (h) | 4 | 6 | 4 |
| CC score | 0 | 0 | 0 |
| HIPEC drugs | Adriamycin + Mitomycin | Oxaliplatin | Adriamycin + Mitomycin |
| Postoperative complications | Nil | Anastomotic dehiscence | Paralytic ileus |
| Clavien–Dindo grade (morbidity) | 0 | IIIb | II |
| Hospital stay | 7 | 12 | 10 |
| Pathological stage | pT4N0M1 | pT4N0M1 | pTxN0M1 |
| Last follow-up after surgery (months) | 24 | 12 | 30 |
| Recurrence | No | Yes | Yes |
MDAC moderately differentiated adenocarcinoma, PDAC poorly differentiated adenocarcinoma, SCRT short-course radiotherapy, NACTRT neoadjuvant chemoradiotherapy, CAPOX capecetabine + oxaliplatin-based chemotherapy, HIPEC hyperthermic intraperitoneal chemotherapy
Case 1
A 38-year-old female with symptoms of abdominal pain and constipation was diagnosed with moderately differentiated adenocarcinoma of the middle third rectum infiltrating into the vagina. Diagnostic laparoscopy revealed a peritoneal carcinomatosis index (PCI) of 4. She was treated with short course radiotherapy (SCRT, 25Gy in 5#) followed by 4 cycles of neoadjuvant capecetabine-oxaliplatin chemotherapy (CAPOX). Response assessment MRI revealed excellent response in primary lesion. In view of young age, good performance status, and excellent response to neoadjuvant treatment, she was planned for exenterative surgery. On exploration, PM was confined to pelvic cavity with PCI of 3–4. Posterior exenteration with pelvic peritonectomy and omentectomy was done to achieve complete cytoreduction (CC-0). HIPEC was done using Adriamycin (15 mg/kg/m2) and Mitomycin (15 mg/kg/m2). Intraoperative course and postoperative recovery was smooth and uneventful; she was discharged on postoperative day 8. Her histopathology was suggestive of scanty residual moderately differentiated adenocarcinoma with 7 reactive nodes and metastatic deposits in right ovary (Pt4N0M1). She received 4 cycles of adjuvant CAPOX and doing well with no recurrence at 2 years after surgery.
Case 2
A 22-year-old male was diagnosed with a case of metastatic (PM, PCI-5) signet ring adenocarcinoma of the lower third rectum infiltrating into the urinary bladder trigone. He was treated with 3# CAPOX followed by long-course neoadjuvant chemoradiation (NACTRT) and further 4# CAPOX (in view of positive circumferential resection margin, i.e., CRM, after NACTRT). Post neoadjuvant treatment, disease was stable and he was planned for surgery. He underwent total pelvic exenteration (TPE), pelvic peritonectomy+ omentectomy, and HIPEC (oxaliplatin, 350 mg/kg/m2) to achieve CC-0. Intraoperative course was uneventful. On postoperative day 4, he had ileo-ileal anastomotic leak, which was managed with re-exploration and re-anastomosis. Histopathology revealed residual poorly differentiated adenocarcinoma infiltrating into bladder/ periprostatic tissue with negative regional lymph nodes (Pt4N0M1). He received 4# single agent capecetabine as adjuvant therapy. Follow-up PET-CT at 1 year after surgery showed metastatic left common iliac node, for which chemotherapy was advised but patient refused and is presently under observation.
Case 3
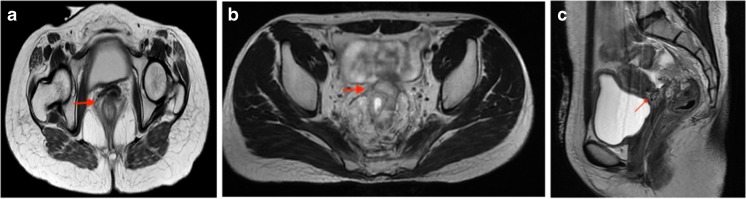
A 23-year-old female was diagnosed with a case of locally advanced lower third rectal cancer infiltrating into the vagina with limited pelvic peritoneal metastasis and PCI of 6. She was treated with SCRT followed by 4# CAPOX. Post neoadjuvant therapy, disease was stable and she underwent posterior pelvic exenteration, pelvic + paracolic peritonectomy with HIPEC to achieve CC-0. Intraoperative course was uneventful with mild paralytic ileus in early postoperative period, managed conservatively. Histopathology revealed complete response in primary lesion with negative regional nodes and metastatic deposits in ovaries, omentum, and peritoneum (pTxN0M1). She received 4# of adjuvant CAPOX. At 30 months of follow-up, she developed recurrence in ileum and underwent ileal resection with retroperitoneal lymphadenectomy followed by chemotherapy. Currently, she is under follow-up and is disease free. Table 1 represents the summary of the three cases and Fig. 1 depicts pre-treatment MRI showing the disease extent.
Fig. 1.
Radiological findings in present case series, (a case 1 case, b 2, and c case 3)
Discussion
PM, commonly referred as peritoneal carcinomatosis, are metastatic deposits on the peritoneal surface throughout the abdominal cavity. Although PM may virtually arise from any organ malignancy, these are most commonly seen with ovarian cancer in females and colorectal cancer in males. PM was traditionally associated with poor life expectancy and treated with palliative care. Median survivals of 15–24 months have been reported with advances in systemic chemotherapy [4, 5] but many patients (60%) fail to complete the complete chemotherapy course due to adverse effects and poor performance status in advanced stages of the disease. Cytoreductive surgery with HIPEC is an effective treatment option for colorectal cancer + PM, with reported median survival of up to 62 months and 5-year survival of around 40% [6].
Role of CRS + HIPEC in colorectal PM has been investigated in two randomized trials. The first study by Verwaal et al. [7] showed a significant survival benefit in patients who underwent CRS + HIPEC followed by adjuvant systemic 5-fluorouracil with leucovorin compared to patients who received systemic 5-fluorouracil with leucovorin alone. However, the true benefit of CRS + HIPEC cannot be predicted precisely in the current period because of the outdated systemic chemotherapy regimen used in this study. Another study by Cashin et al. [8] showed a significant survival benefit in patients treated with CRS + HIPEC compared to patients treated with oxaliplatin-based chemotherapy. A prospectively conducted study by Henderson et al. [9] showed that radical resection with HIPEC for colorectal cancer peritoneal metastases is safe in selected patients and long-term survival is possible.
Recently published phase III randomized trial (PRODIGE 7) has shown lack of survival benefit and increased morbidity with HIPEC in patients of metastatic colorectal cancer [10]. However, HIPEC definitely conferred a survival advantage in patients with higher PCI (11–15 group). Moreover, the chemotherapeutic drug used in this trial was oxaliplatin, and the results of HIPEC may be different with agents other than oxaliplatin.
In non-metastatic setting, colon and rectal cancers are considered separate entities with different treatment approach and prognosis [11, 12]. However, these differences are less evident in patients with PM, who are treated with CRS + HIPEC and colorectal PM are often considered as one disease, regardless of their origin [13]. As a result, a small number of rectal cancer patients are camouflaged by relatively large number of colon cancer patients.
Very few studies are available specifically focusing on rectal cancer patients undergoing CRS + HIPEC for PM, and the results are contradictory. The most recent and largest study included 29 rectal cancer patients and reported similar recurrence and survival rates compared with colon cancer patients, with 5-year survival rates of ~ 30% in both groups [14]. These results are in contrast with two studies with rectal cancer patients who were treated with CRS + HIPEC in which survival was diminished compared to colonic PM patients. However, in large study, investigating prognostic factors for survival after CRS + HIPEC, rectal origin did not seem to influence survival, and outcomes were comparable to patients with colonic PM [15].
Pelvic exenteration has been described as an established treatment option for locally advanced rectal cancer in selected patients. Pelvic exenteration as a part of cytoreductive surgery has been described in ovarian cancer [16]. However, pelvic exenteration as a part of cytoreductive surgery for rectal cancer is still in infancy and considered too morbid a procedure to be combined with HIPEC. Actual data on morbidity and long-term survival following such a procedure are lacking, and therefore subset of patients are probably being denied a chance for effective treatment, and possibly cure. We intended to report our initial experience with three cases of rectal cancer treated with pelvic exenteration and CRS + HIPEC. Operative factors like blood loss, duration of surgery, hospital stay, and perioperative morbidity were acceptable in these selected cases with no postoperative mortality so far. One important task during total pelvic exenteration is monitoring urine output during HIPEC, as urinary conduit is usually made after the HIPEC is over. We prefer to put bilateral ureteric catheters and monitor the urine output during the procedure. All three cases were alive at 12 months after surgery and two had recurrence.
Conclusion
Pelvic exenteration with CRS + HIPEC can be offered in selected cases with acceptable morbidity and probably chances of better survival. Cytoreductive surgery with complete cytoreduction (CC-0) should be aimed and supramajor procedures like pelvic exenteration (either total or partial) can be combined with HIPEC in carefully selected cases. Further studies with larger cohort of patients are required before negating or promoting possibility of such a procedure.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study (retrospective), formal consent is not required.
Authors Statement
Exenterative surgery along with CRS + HIPEC is not performed routinely, due to advances in understanding of disease biology, perioperative care, and more effective systemic therapy; more aggressive surgeries are being offered to improve the outcomes of patients with advanced disease, which were considered non-curable previously. This is the first series describing exenteration with CRS + HIPEC from the Indian subcontinent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rajesh S. Shinde, Email: dr.rajeshinde@gmail.com
Rajgopal Acharya, Email: acharya.mrajgopal@gmail.com.
Naveena AN Kumar, Email: nkoncol@gmail.com.
Sohan Solanki, Email: me_sohans@yahoo.co.in.
Ashwin Desouza, Email: ashwindesouza@gmail.com.
Avanish Saklani, Phone: +917400319886, Email: asaklani@hotmail.com.
References
- 1.Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128(11):2717–2725. doi: 10.1002/ijc.25596. [DOI] [PubMed] [Google Scholar]
- 2.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(5):699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 3.Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19(41):6979–6994. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ., III Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 6.Nadler A, McCart JA, Govindarajan A. Peritoneal Carcinomatosis from colon cancer: a systematic review of the data for cytoreduction and intraperitoneal chemotherapy. Clin Colon Rectal Surg. 2015;28(4):234–246. doi: 10.1055/s-0035-1564431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 8.Cashin PH, Mahteme H, Spång N, Syk I, Frodin JE, Torkzad M, et al. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: a randomised trial. Eur J Cancer. 2016;53:155–162. doi: 10.1016/j.ejca.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Henderson N, Polignano F. PTH-312. 5 year results of radical resection and hipec for colorectal cancer peritoneal metastases. Gut. 2015;64:A546–A547. [Google Scholar]
- 10.Quenet F, Elias D, Roca L Goere D, Ghouti L, Pocard M, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36(18):1. [Google Scholar]
- 11.van Erning FN, van Steenbergen LN, Lemmens VE, Rutten HJ, Martijn H, van Spronsen DJ, et al. Conditional survival for long-term colorectal cancer survivors in the Netherlands: who do best? Eur J Cancer. 2014;50(10):1731–1739. doi: 10.1016/j.ejca.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Van Leersum NJ, Snijders HS, Henneman D, Kolfschoten NE, Gooikar GA, ten Berge MG, et al. The Dutch surgical colorectal audit. Eur J Surg Oncol. 2013;39(10):1063–1070. doi: 10.1016/j.ejso.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Simkens GA, Rovers KP, Nienhuijs SW, de Hingh IH. Patient selection for cytoreductive surgery and HIPEC for the treatment of peritoneal metastases from colorectal cancer. Cancer Manag Res. 2017;9:259–266. doi: 10.2147/CMAR.S119569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simkens GA, van Oudheusden TR, Braam HJ, Wiezer MJ, Nienhuijs SW, Rutten HJ, van Ramshorst B, de Hingh IH. Cytoreductive surgery and HIPEC offers similar outcomes in patients with rectal peritoneal metastases compared to colon cancer patients: a matched case control study. J Surg Oncol. 2016;113(5):548–553. doi: 10.1002/jso.24169. [DOI] [PubMed] [Google Scholar]
- 15.Kwakman R, Schrama AM, van Olmen JP, Otten RH, de Lange-de Klerk ES, de Cuba EM, et al. Clinicopathological parameters in patient selection for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer metastases: a meta-analysis. Ann Surg. 2016;263(6):1102–1111. doi: 10.1097/SLA.0000000000001593. [DOI] [PubMed] [Google Scholar]
- 16.Bacalbasa N, Balescu I. Pelvic exenteration as part of cytoreductive surgery for advanced stage or relapsed ovarian cancer. Eur J Surg Oncol. 2016;42(9):S158. doi: 10.1016/j.ejso.2016.06.252. [DOI] [Google Scholar]