Abstract
To evaluate the clinical outcomes of patients of pseudomyxoma peritonei of appendiceal origin undergoing cytoreductive surgery and HIPEC. Data collected from members, an independent collaborative group of Indian surgeons specializing in the management of peritoneal surface malignancy (INDEPSO), was analyzed retrospectively. Clinicopathological and perioperative outcomes of patients treated for pseudomyxoma peritonei (PMP) of appendicular origin were evaluated. Ninety-one patients were diagnosed with pseudomyxoma peritonei of appendicular origin between March 2013 and December 2017. The median age was 53 years and 60% were females. The median PCI was 27 [range 3–39] and a CC-0/1 resection was achieved in 83.5% patients. The most common histological grade was low-grade PMP, seen in 71.4% cases. The overall rate of grades 3–4 morbidity was 33% (30/91) and the 90-day mortality rate reported was 6.5%. Pulmonary complications and systemic sepsis emerged as the most significant factors affecting morbidity, mortality, and failure to rescue. At a median follow-up of 24 months, the median OS was not reached and the median PFS was 53 months. On univariate and multivariate analysis, high-grade histology, prior chemotherapy, debulking surgery alone without HIPEC, and high PCI > 10 were predictors of poor progression-free survival. The survival and morbidity results of pseudomyxoma peritonei from appendicular origin following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are encouraging. With further awareness and understanding of the disease, and improvement in surgical expertise and learning curve, there is scope for further reduction in morbidity and better improvement in survival.
Keywords: Pseudomyxoma peritonei, Appendiceal tumor, HIPEC
Introduction
Pseudomyxoma peritonei (PMP) is a rare clinical syndrome characterized by the accumulation of mucin within the peritoneal cavity. The clinical entity “PMP syndrome” or “jelly belly” represents a spectrum of disease that ranges from mucinous ascites to frank mucinous adenocarcinoma [1, 2]. The origin of this mucin in most cases is from mucinous tumors arising from the appendix, but is also known to originate from mucinous tumors of the ovary, colon, and seldom, the gall bladder. Around 94% of cases of PMP develop from a mucinous tumor of the appendix [3, 4]. The pathological process of PMP starts with neoplastic transformation of mucin producing goblet cells of the alimentary tract, resulting in the formation of a primary mucinous tumor. As mucin levels increase within the tumor, there is eventual rupture. This is followed by “redistribution” of mucin that follows the pathway of peritoneal fluid and given their lack of cell adhesion molecules, this mucin passively circulates with ease within the peritoneal cavity [5, 6]. Bulky masses comprised of mucinous tumor cells are eventually deposited within the abdominal cavity, giving rise to pseudomyxoma peritonei. This pattern of distribution is unique to PMP and is different from peritoneal metastasis from other cancers, in which metastatic implantation occurs in a random fashion near the site of the tumor rupture/perforation.
The clinical presentation of PMP is insidious and indolent because of which, diagnosis of this clinical entity is made at advanced stages, thus making cure a distant possibility. In the past, the management of patients with PMP essentially comprised of debulking surgeries with the aim to alleviate symptoms arising due to excessive intraabdominal mucinous deposits. Often, multiple debulking procedures would be required to control symptom. This however resulted in dismal 5-year survival rates between 32 and 53% [7, 8]. Attempts to improve survival with the addition of systemic chemotherapy were also unsuccessful. In 2005, Miner et al. reported a 10-year survival of 21% and 12% disease-free survival in 97 patients treated by serial debulking, systemic chemotherapy, and/or delayed intermittent intraperitoneal 5-fluorouracil over a 22-year period in Memorial Sloan Kettering [9]. A paradigm shift in the treatment approach for PMP was witnessed with the introduction of radical cytoreductive surgery which comprised of leaving behind no/very small residual disease and hyperthermic intraperitoneal chemotherapy (HIPEC) by Paul Sugarbaker in the mid-nineties. The rational of HIPEC following an ultra-radical surgery was to target residual microscopic disease or small volume/size macroscopic disease (< 0.25 mm) [10]. An expert consensus panel discussion at the Fifth International Workshop on Peritoneal Surface Malignancy in Milan, Italy (December 4–6, 2006) concluded that there was a survival benefit of the CRS + HIPEC for PMP compared to only debulking surgery [11]. Due to the rarity of this clinical entity, having a robust randomized evidence supporting CRS + HIPEC seems impossible, but sufficient non randomized evidence in literature exists to advocate CRS + HIPEC as the gold-standard treatment for PMP.
The aim of our study was to critically evaluate the clinical outcomes of patients of PMP of appendiceal origin and to add to the continuously growing evidence in literature on the management of PMP.
Materials and Methods
To assess the clinical outcomes, a retrospective analysis of data entered into the registry from March 2013 to Dec 2017 was performed. Data were collected from members of the Indian Network for Development of Peritoneal Surface Oncology (INDEPSO). INDEPSO is an independent collaborative group of Indian surgeons specializing in the management of peritoneal surface malignancy (PM). The demographic data, disease characteristics, details of intraperitoneal chemotherapy, perioperative outcomes, and follow-up details of patients treated for pseudomyxoma peritonei of appendicular origin were captured. The perioperative outcomes were evaluated and complications were graded according to the CTCAE classification version 4.3. Ninety-day morbidity and 90-day mortality were both recorded. Failure to rescue (FTR), defined as death following a complication (30- and 90-day morbidity) was analyzed. We included major (grade 3–4) complications following surgery in analyzing FTR rates.
The goal of CRS was to obtain a complete cytoreduction. The disease extent was quantified using Sugarbaker’s peritoneal cancer index (PCI). Completeness of cytoreduction score (CC-score) was used to describe the residual disease status. The technique of peritonectomy performed was as described by Sugarbaker. The technical details are described elsewhere [10, 12]. Completeness of surgical cytoreduction (CCR) was classified as CCR-0: macroscopically complete; CCR-1: residual disease < 2.5 mm; or CCR-2: residual disease > 2.5 mm. Only after complete cytoreduction, (CCO or CC1), hyperthermic intraperitoneal chemotherapy (HIPEC) was performed at 42.5 °C using the closed abdomen or open abdomen technique. One of the following drugs were used for HIPEC, cisplatin (75 mg/m2) for 60 min, mitomycin C (15 mg/m2) for 90 min, or Oxaliplatin (300 mg/m2) for 30 min. Post-operative complications were graded according to the serious adverse events score (NCI-CTCAE version 4). Postoperative follow-up comprised of physical examination, thoracic and abdominal contrast enhanced tomography, and tumor marker measurements every 4 months during the first 2 years and 6 months thereafter.
Statistical Analysis
Descriptive variables were analyzed using nonparametric tests, χ2 test for categorical variables and Mann-Whitney U test for continuous variables. Clinically relevant variables, as well as variables with a p value < 0.10 on univariate analysis, were evaluated in multivariable logistic regression model. The model was selected using a forward stepwise method. All statistical tests were two-sided, and the significance level was 0.05. Overall Survival (OS) was calculated from the date of surgery to the date of death or last follow-up and progression-free survival (PFS) was determined from the date of surgery to the date of disease progression. Survival curves were calculated using the Kaplan–Meier method and compared using the two-tailed log-rank test. Factors with a p value of < 0.10 on univariate analysis were selected for the multivariate analysis. Multivariate analysis of factors was performed using the Cox proportional hazard model. All statistical analyses were conducted using SPSS version 22.0.0 (IBM Corporation, Armonk, NY, USA).
Results
Ninety-one patients were diagnosed with pseudomyxoma peritonei of appendicular origin between March 2013 and December 2017. The median age was 53 years and 60% were females. More than 50% of patients had undergone prior laparotomy before presenting for definitive treatment. All 91 patients had good performance status with an ECOG of 1.The median PCI was 27 [range 3–39] and a CC-0/1 resection was achieved in 76 (83.5%) patients. Fifteen patients underwent CRS alone or debulking (CC2/CC3 residual disease). Mitomycin C was the commonest drug used for HIPEC Table 1. The median hospital stay was 14 days [range 5–54 days]. Histologically, PMP was graded as low grade, high grade, and high grade with signet cell features. The classification by the American Joint Committee on Cancer (AJCC) and the WHO (2010) was used to grade the tumors. The most common histological grade was low-grade PMP, seen in 71.4% cases. Signet cell histology was seen in 9 (9.9%) cases. No cases of acellular mucin were documented. All patients with high grade and signet cell histology received postoperative adjuvant oxaliplation and 5-FU-based systemic chemotherapy. The demographic and clinical characteristic of these 91 patients are summarized in Table 1. The overall rate of grades 3–4 morbidity was 33% (30/91) and the failure to rescue rate was 23.2%. Pulmonary complications were the most common complications seen Fig. 1. Ninety-day mortality rate reported was 6.5%. Although on univariate analysis, high PCI > 20 was associated with increased risk of mortality (p = 0.018) and failure to rescue (p = 0.03), on univariate and multivariate analysis, pulmonary complications, and systemic sepsis emerged as the most significant factors affecting morbidity, mortality, and failure to rescue Table 2.
Table 1.
Demographic and patient characteristic
| Characteristics | All patients (n = 91) (%) |
|---|---|
| Age (years) | |
| < 50 | 36 (39.6%) |
| > 50 | 55 (60.4%) |
| Median age (years) | 53 |
| Gender | |
| Male | 31 (34.1%) |
| Female | 60 (65.9%) |
| Year of surgery | |
| Before 2014 | 18 (19.8%) |
| After 2014 | 73 (80.2%) |
| Histological subtype of (PMP)a | |
| Low | 65 (71.4%) |
| High | 17 (18.7%) |
| Signet | 9 (9.9%) |
| Prior chemotherapy | |
| Yes | 31 (34.1%) |
| No | 60 (65.9%) |
| Prior surgery | |
| Yes | 47 (51.6%) |
| No | 44 (48.4%) |
| Staging laparoscopy | |
| Yes | 17 (18.7%) |
| No | 74 (81.3%) |
| PCIb | |
| < 20 | 28 (30.8%) |
| > 20 | 63 (69.2%) |
| Median PCI (range) | 27 (3–39) |
| CC-scorec | |
| 0 | 40 |
| 1 | 36 |
| 2 | 8 |
| 3 | 7 |
| CRS + HIPECd | 76 (83.5%) |
| CRS alone | 15 (16.5%) |
| HIPEC regimen | |
| Mitomycin C | 39 (40.7) |
| Cisplatin | 5 (5.5) |
| Oxalipatin | 8 (8.8) |
| Mitomycin + Adriamycin | 24 (26.4%) |
| No of organ resections | |
| < 3 | 37 (40.7%) |
| > 3 | 54 (59.3%) |
| Bowel resected | |
| No | 22 (24.2%) |
| Yes | 69 (75.8%) |
| No of bowel anastomosis | |
| < 1 | 43 (48.4%) |
| > 1 | 26 (28.6%) |
| Duration of surgery | |
| < 8 h | 17 (18.7%) |
| > 8 h | 74 (81.3%) |
| Median duration of surgery (mins) | 540 |
| Median hospital stay (days) | 14 (5–54) |
| Median ICU stay (days) | 4 (0–39) |
| 90 day mortality | 6 (6.5%) |
| 90 day morbidity | 9 (9.9%) |
| Grades 3–4 morbidity | 30 (33%) |
| Failure to rescue rate | 9/39 (23.2%) |
aPseudomyxoma peritonei
bPeritoneal carcinomatosis index
cComplete cytoreduction
dCytoreduction + hyperthermic intraperitoneal chemotherapy
Fig. 1.
Pie chart showing distribution of types of complications
Table 2.
Multivariate analysis of factors affecting morbidity and mortality and failure to rescue
| Variables | Failure to rescue (95% CI) | 90-day morbidity (95% CI) | Grades 3–4 morbidity (95% CI) | 90-day mortality (95% CI) |
|---|---|---|---|---|
| Anastomotic leak | 0.007 (0.002–0.36) | |||
| Cardiovascular | 0.016 (0.023–0.681) | |||
| Systemic sepsis | 0.001 (0.003–0.18) | 0.004 (0.03–0.32) | 0.004(0.003–0.346) | |
| Pulmonary | 0.043 (0.038–0.95) | 0.001 (0.03–0.24) | 0.001 (0.015–0.213) | 0.0069 (0.013–0.48) |
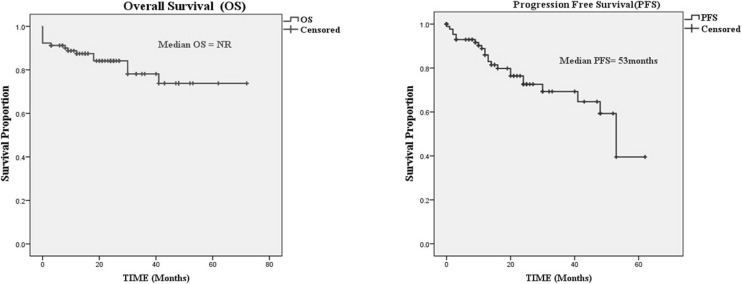
At a median follow-up of 24 months (range 0–72), the median OS was not reached and the median PFS was 53 months (Fig. 2). The 3-year OS and PFS was 78.1% and 69.3% respectively. There were no factors influencing OS. However on univariate and multivariate analysis, high-grade histology, prior chemotherapy, debulking surgery alone without HIPEC, and high PCI > 10 were predictors of poor progression-free survival Table 3.
Fig. 2.
Kaplan-Meir survival curves
Table 3.
Univariate and multivariate analysis affecting progression-free survival (PFS)
| Variable | Median PFS (months) | Univariate log-rank p | Multivariable Cox regression (95% CI) |
|---|---|---|---|
| Prior chemotherapy | 0.040 | 0.008 (1.3–8.1) | |
| Yes | 41 months | ||
| No | 53 months | ||
| CRS + HIPEC | 53 months | 0.05 | 0.016 (1.26–9.8) |
| CRS | 16 months | ||
| PMP grade | 0.001 | 0.03 (1.14–15.67) | |
| Low | Not reached | ||
| High | 12 months | ||
| CC0/CC1 | 53 months | 0.001 | 0.008 (0.1–0.71) |
| CC2/CC3 | 14 months | ||
| PCI | 0.092 | Not significant | |
| < 20 | Not reached | ||
| > 20 | 53 months |
Discussion
At a median follow-up of 36 months, the largest multicentric study of pseudomyxoma peritonei from appendiceal neoplasms reported a median overall survival rate of 16.3 years with a 15-year survival rate of 59% and a the long median progression-free survival rate of 8.2 years. More importantly, this study showed that the combined treatment of cytoreduction and HIPEC was efficacious in prolonging survival [13].
In the largest single institution series of 1000 patients, Moran et al. reported a 5- and 10-year overall survival was 87.4% and 70.3% respectively in the 738 patients who had CC-0/1 compared with 39.2% and 8.1% respectively in patients who had a CC-2/3 resection [14].
Our retrospective multi-institutional registry study of pseudomyxoma peritonei from appendiceal neoplasms showed a median progression-free survival of 53 months (4.5 years). The median overall survival was not reached (NR) in our patients. In particular, in our study, the multivariate analysis of factors affecting progression-free survival demonstrated that debulking alone without HIPEC was associated with poor of progression-free survival. Therefore, our data suggests that optimal cytoreduction seems to be the strongest factor associated with prolonged disease control and the addition of hyperthermic intraperitoneal chemotherapy (HIPEC) improves survival.
Proper selection of patients for treatment is important to ensure that only those patients who actually benefit from the procedure are subjected to it. The most important prognostic indicator is the completeness of cytoreduction (CC) score; a complete cytoreduction indicates that either there is no visible residual disease (CC-0) or the residual tumor deposits measure less than 2.5 mm in size and can be eradicated by the HIPEC (CC-1) [15]. Any residual disease > 2.5 mm (CC-2 residual disease measuring 2.5 mm–5 cm or CC-3 residual disease measuring > 5 cm) is considered debulking. Chua et al. reported a 5-year survival of 85% in patients with CC-0, 80% in patients with a CC-1 resection as opposed to only 24% in patients with gross residual disease (CC-2/3) in the largest multi-institutional study published so far. The difference was statistically significant and was not influenced either by the tumor grade or PCI [13]. Similarly in the largest single institutional study of 1000 patients, the 5 and 10-year overall survival was 87.4% and 70.3% respectively in the 738 patients who had a complete CRS compared with 39.2% and 8.1% respectively in patients with CC-2/3 resections. PCI is an important prognostic factor irrespective of the grade of PMP [14]. In pseudomyxoma peritonei, unlike colorectal and gastric cancers, there is no cut off of the PCI level beyond which a complete cytoreduction should not be attempted. While there is no standard definition of what constitutes extensive disease, Elias et al. have defined it as a PCI of > 28 as “huge PMP” [16]. Some would argue on the futility of performing an ultra-radical surgery on patients with a high PCI; however, even with a high PCI, with complete/near complete cyotreduction, a reasonable progression-free and overall survival can be achieved. For some patients, rather than the actual PCI, it is the anatomical location of the disease which is important. Especially in cases of small bowel involvement where despite a PCI that is not very high, it may be impossible to clear the entire tumor from the bowel surface and its mesentery. The presence of residual unresectable disease at crucial anatomic sites like porta hepatis, small bowel, or its mesentery may nullify the favorable effect of low PCI score on prognosis [17].
The median PCI in our study was 27; in one fourth of our patients, the PMP was high grade but despite this, a CC0/CC1 was achieved in 83% cases and our survival results are comparable to that in literature. In our study although PCI > 20 did not influence OS (p = 0.10) and PFS (p = 0.092), patients enjoyed a fair median OS (NR) and median PFS (53 months) even when cytoreductive surgery was performed with high PCI. Our results therefore reflect a good patient selection and sound surgical expertise in managing this rare disease entity.
Another finding in our study that merits discussion is the impact of prior chemotherapy on poor progression-free survival. In our study, 34% of patients received prior chemotherapy. This may indicate that chemotherapy was administered probably because of increase tumor burden or high-grade histology. This seems logical, as in our study on univariate analysis of factors associated with progression-free survival (PFS), high-grade histology, high PCI > 20, and the use of prior chemotherapy, all were associated with poor PFS, although the significance of a high PCI on PFS was lost on multivariate analysis. This association of prior chemotherapy and a poorer overall and disease-free survival and incidence of higher morbidity has been elucidated in other studies [13, 18, 19].
There is no denying the fact that CRS + HIPEC is an aggressive procedure with its associated morbidity. According to recent reports, the grade, 3 and 4 complication rate range from 22 to 34% and mortality from 0.8–4.1% [20, 21]. A systematic review on cytoreductive surgery and intraperitoneal chemotherapy reported a morbidity and mortality rate of 0–52%. In the PSOGI and the French multicenter registry study, a 24% and 40% major complication rate was observed [13, 22]. The 90-day mortality in our study was 6.5% and the overall grades 3–4 complication rate was 30%. These figures though high are comparable to that reported in literature. The failure to rescue rate of 23.2% in our study is much higher than that reported by other centers (4.4%) [23]. The most common cause of failure to rescue in our patients was sepsis and respiratory complications. Complications like bowel perforations and anastomotic leak led to systemic sepsis and subsequently failure to rescue. Mortality did not occur in most patients who did not develop systemic sepsis.
We do acknowledge the limitations of our study. The study is retrospective in nature and the median follow-up is short and the number of patients is small to draw any robust conclusions. Nevertheless, this study provides a broad overview of the clinical outcomes of treating a rare and difficult disease like PMP at Indian centers.
Conclusion
The clinical outcomes for CRS with HIPEC in PMP are encouraging for patients with low-grade histology amenable to complete cytoreduction. With further awareness and understanding of the disease, and improvement in surgical expertise and learning curve, there is scope for further reduction in morbidity and better improvement in survival.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44–50. doi: 10.4251/wjgo.v2.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12(3):585–603. doi: 10.1016/S1055-3207(03)00026-7. [DOI] [PubMed] [Google Scholar]
- 3.Esquivel J, Sugarbaker PH. Clinical presentation of the Pseudomyxoma peritonei syndrome. Br J Surg. 2000;87(10):1414–1418. doi: 10.1046/j.1365-2168.2000.01553.x. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal AK, Bobiński P, Grzebieniak Z, Rudnicki J, Marek G, Kobielak P, et al. Pseudomyxoma peritonei originating from urachus-case report and review of the literature. Curr Oncol Tor Ont. 2014;21(1):e155–e165. doi: 10.3747/co.21.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219(2):109–111. doi: 10.1097/00000658-199402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmignani CP, Sugarbaker TA, Bromley CM, Sugarbaker PH. Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev. 2003;22(4):465–472. doi: 10.1023/A:1023791229361. [DOI] [PubMed] [Google Scholar]
- 7.Nitecki SS, Wolff BG, Schlinkert R, Sarr MG. The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg. 1994;219(1):51–57. doi: 10.1097/00000658-199401000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gough DB, Donohue JH, Schutt AJ, Gonchoroff N, Goellner JR, Wilson TO et al. Pseudomyxoma peritonei. Long-term patient survival with an aggressive regional approach. Ann Surg. 1994;219(2):112–119. doi: 10.1097/00000658-199402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miner TJ, Shia J, Jaques DP, Klimstra DS, Brennan MF, Coit DG. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg. 2005;241(2):300–308. doi: 10.1097/01.sla.0000152015.76731.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6(8):727–731. doi: 10.1007/s10434-999-0727-7. [DOI] [PubMed] [Google Scholar]
- 11.Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei) J Surg Oncol. 2008;98(4):277–282. doi: 10.1002/jso.21054. [DOI] [PubMed] [Google Scholar]
- 12.Mehta SS, Bhatt A, Glehen O. Cytoreductive surgery and peritonectomy procedures. Indian J Surg Oncol. 2016;7(2):139–151. doi: 10.1007/s13193-016-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IHJT, Ceelen WP, Pelz JO, Piso P, González-Moreno S, van der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(20):2449–2456. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 14.Ansari N, Chandrakumaran K, Dayal S, Mohamed F, Cecil TD, Moran BJ. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2016;42(7):1035–1041. doi: 10.1016/j.ejso.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg. 2004;240(2):278–285. doi: 10.1097/01.sla.0000133183.15705.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benhaim L, Honoré C, Goéré D, Delhorme J-B, Elias D. Huge pseudomyxoma peritonei: surgical strategies and procedures to employ to optimize the rate of complete cytoreductive surgery. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2016;42(4):552–557. doi: 10.1016/j.ejso.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Cytoreductive surgery and perioperative chemotherapy: textbook and video atlas. Woodbury: Cine-Med Publishing; 2013. [Google Scholar]
- 18.Chua TC, Liauw W, Zhao J, Morris DL. Upfront compared to delayed cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei is associated with considerably lower perioperative morbidity and recurrence rate. Ann Surg. 2011;253(4):769–773. doi: 10.1097/SLA.0b013e31820b4dba. [DOI] [PubMed] [Google Scholar]
- 19.Austin F, Mavanur A, Sathaiah M, Steel J, Lenzner D, Ramalingam L, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann Surg Oncol. 2012;19(5):1386–1393. doi: 10.1245/s10434-012-2241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newton AD, Bartlett EK, Karakousis GC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol. 2016;7(1):99–111. doi: 10.3978/j.issn.2078-6891.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuijpers AMJ, Mirck B, Aalbers AGJ, Nienhuijs SW, de Hingh IHJT, Wiezer MJ, van Ramshorst B, van Ginkel RJ, Havenga K, Bremers AJ, de Wilt JHW, te Velde EA, Verwaal VJ. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. 2013;20(13):4224–4230. doi: 10.1245/s10434-013-3145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, Mansvelt B, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2010;36(5):456–462. doi: 10.1016/j.ejso.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Passot G, Vaudoyer D, Villeneuve L, Kepenekian V, Beaujard A-C, Bakrin N, Cotte E, Gilly FN, Glehen O. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113(7):796–803. doi: 10.1002/jso.24248. [DOI] [PubMed] [Google Scholar]