Abstract
The overall aim of this scoping review of the literature is twofold: (1) to provide an overview of all instruments that have been used to assess health-related quality of life (HRQoL) after solid organ transplantation and (2) to provide a list of health items they include to support future studies on the development of a new-generation HRQoL instrument. All studies that administered any form of HRQoL instrument to post-transplant solid organ recipients were identified in a comprehensive search of PubMed (MEDLINE), Embase, and Web of Science, with a cut-off date of May 2018. The search used various combinations of the following keywords: lung, heart, liver, kidney, or pancreas transplantation; quality of life; well-being; patient-reported outcome; instrument; questionnaire; and health survey. In total, 8013 distinct publications were identified and 1218 of these were selected for review. Among the instruments applied, 53 measured generic, 51 organ-specific, 271 domain-specific, and 43 transplant-specific HRQoL. A total of 78 distinct health items grouped into 16 sub-domains were identified and depicted graphically. The majority of publications did not report a logical rationale for the choice of specific HRQoL instrument. The most commonly used types of instruments were generic health instruments, followed by domain-specific instruments. Despite the availability of transplant-specific instruments, few studies applied these types of instruments. Based on the 78 items, further research is planned to develop a patient-centered, transplant-specific HRQoL instrument that is concise, easy to apply (mobile application), and specifically related to the health issues of solid organ recipients.
Electronic supplementary material
The online version of this article (10.1007/s40271-018-0335-3) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| This scoping review provides an overview of all instruments that have been used to assess health-related quality of life (HRQoL) after transplantation. This overview will be useful in the process of instrument selection in future studies. |
| Generic instruments are the most frequently applied HRQoL instruments in solid organ transplantation studies. Despite the availability of transplant-specific instruments, only a few studies have applied these types of instruments to measure HRQoL in transplant patients. |
| There is a need to develop a preference-based, transplant-specific HRQoL instrument. Such an instrument should be easy to apply, and its content should target the health issues of solid organ recipients from the patient perspective. |
Introduction
With respect to the considerable improvements in clinical outcomes in the field of solid organ transplantation (i.e., lung, heart, liver, kidney, and pancreas), there is growing awareness of post-transplant perceived health status or health-related quality of life (HRQoL) [1–4]. Today, the main objectives of organ transplantation include extension of survival, decrease in the level of disability, and improvement of HRQoL [5].
HRQoL is a multi-dimensional concept that refers to the overall impact of health aspects on an individual’s quality of life [6, 7]. More specifically, HRQoL embraces physical symptoms, functional status, psychological states, and social relationships. Together these constitute the domains of the World Health Organization’s (WHO) definition of health [7–10].
Before transplantation, a patient’s HRQoL is significantly reduced due to clinical dysfunction of the failing organ and psychosocial distress. Shortly after transplantation, a significant increase in HRQoL is observed [11, 12]. However, life-long, immunosuppressive regimens are necessary to prevent organ rejection, and chronic exposure to these medications is associated with complications that adversely affect the HRQoL of solid organ transplant recipients [13–15]. Previous studies have emphasized that a considerable proportion of patients are more concerned about HRQoL than about survival [16, 17].
Numerous instruments are available to measure HRQoL of transplant patients. Here, “instrument” refers to any form of self-report questionnaire and rating scale that is used to measure any aspect of an individual’s HRQoL. Most reviews of post-transplant HRQoL studies have focused on frequently used instruments in only one or two organ types, so they may have omitted some less well-known instruments. Recent systematic reviews of the literature on HRQoL in lung, liver, kidney, and pancreas transplant patients have revealed that the most common instruments are the 36-Item Short Form Survey (SF-36) and the EuroQol—5 Dimensions (EQ-5D) [4, 18–22]. These two instruments have proven to be beneficial in measuring the health status and outcomes associated with healthcare interventions [23]. However, these are generic instruments and thus do not contain health items that are specifically relevant to post-transplant patients.
With increased attention being paid to the concept of HRQoL among transplant patients, targeted measurement of HRQoL is becoming more important. An appropriate transplant-specific instrument should cover the full spectrum of HRQoL and assess both general and transplant-specific health issues of patients. Additionally, although many existing HRQoL instruments measure the intensity or frequency of complaints, they lack the ability to measure the impact of these complaints on the health status experienced by patients [24–26]. To measure the latter, specially designed instruments are necessary, derived from methodologies that include the preferences of patients. Special judgmental tasks (e.g., ordering a set of health states or paired comparisons between different health state descriptions) are a central element in such instruments [7, 27]. Embedding patients’ preferences into health-outcome instruments is becoming increasingly important, due to the increasing attention being paid to patient-centered healthcare [28–31].
Some generic preference-based HRQoL instruments, for example, the Health Utilities Index-3 (HUI-3) and EQ-5D, are available. However, their content is not focused on the specific health issues of transplant patients, and the selection of the health items in these instruments is mainly based on expert opinion [24, 31]. Moreover, the determination of the importance of the various health items, which consist of a value-judgment task, is based on a representative community sample [32–35]. Recently, a novel preference-based method has been introduced, which makes it possible for patients themselves to make the value judgements [34, 36]. Therefore, we see a need for a preference-based, patient-centered, transplant-specific HRQoL instrument.
The first step in developing a patient-centered HRQoL instrument for transplant patients is to extract relevant health items from existing instruments [37, 38]. Therefore, we conducted a scoping review of the literature to provide an overview of all instruments that have been used to assess HRQoL after transplantation in major solid organ recipients. Our aim was to find all studies that evaluated any aspects of HRQoL in post-transplant patients and subsequently to identify all instruments and health items used. This study is not directed to the psychometric properties of the instruments or concerned with recommending the best instruments available.
Methods
Study Design and Literature Search Strategy
A scoping literature review was conducted to extract all HRQoL instruments that had been administered to major solid organ transplant recipients. This was not a systematic review, but rather aimed at acquiring adequate information about existing HRQoL instruments to establish a basis for formulating relevant items. To identify relevant studies, the three major electronic databases, namely PubMed (MEDLINE), Embase, and Web of Science, were searched to May 2018. To ensure we included all self-reported instruments that have thus far been applied, different combinations of broad keywords and medical subject heading (MeSH) terms were formulated to cover three domains: (1) solid organ transplantation, i.e., lung, heart, liver, kidney, or pancreas transplantation; (2) quality of life, i.e., quality of life itself, well-being, or patient-reported outcome; and (3) instruments, i.e., questionnaire or health survey. The search strategy was discussed with four experts on epidemiological and transplant studies (PK, EB, KV, and SB) to finalize the list of keywords (Fig. 1).
Fig. 1.
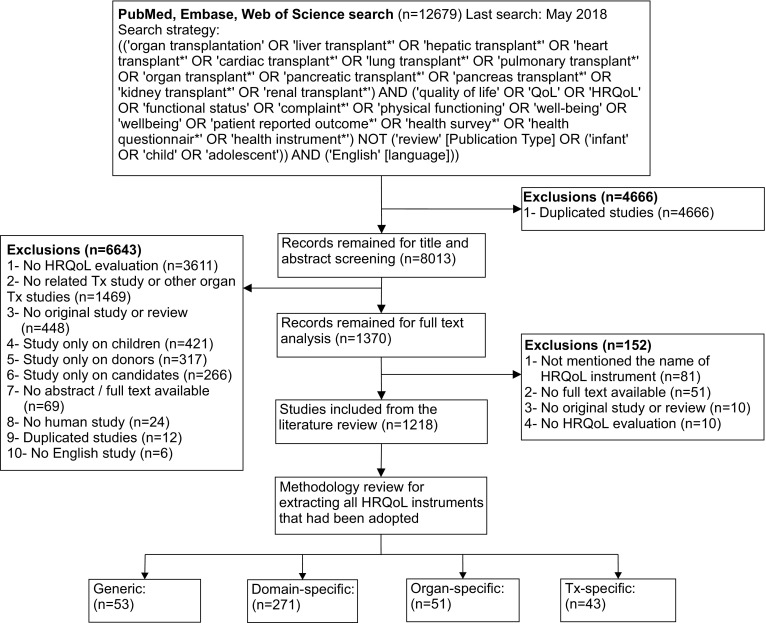
Studies inclusion process. HRQoL health-related quality of life, Tx transplant
Identification of Relevant Literature and Eligibility Criteria
We included all original publications in English if they met the following criteria: (1) human studies in which the participants had been transplanted with at least one of the five major solid organs and were ≥ 18 years old at the time of the study; (2) studies that evaluated symptoms, physical functioning, psychological distress, or social relationships in terms of health outcomes; and (3) studies that used any form of HRQoL instrument.
The finalized list of keywords was then used to select potentially eligible articles for title and abstract review. Because our aim was to include all studies that assessed HRQoL in the post-transplant population, we only excluded publications that clearly did not meet the inclusion criteria, and retained all other references for full text review. If there was any doubt, the full article was retrieved and the methods section was read to check selection criteria. Studies were excluded if they were restricted to donors, organ transplant candidates, pediatric transplant recipients, and family or relatives of the patient. Case studies, editorials, letters to the editor, meta-analyses, systematic reviews, and books were also all excluded from the review. Subsequently, the full text of each eligible paper was reviewed to identify studies that met the inclusion criteria. In addition, if studies included both adult and pediatric participants, the instruments applied were listed in our results (i.e., the number of instruments may be greater than the number of publications, and some pediatric-specific instruments are shown in the results).
Health Items Extraction
We checked the names of the extracted instruments in the different studies to identify the standard unique name for each instrument. In the next step, the names and the items of the extracted instruments were reviewed. Thus, for each instrument, we identified the intended type and dimensions of HRQoL that were assessed (e.g., general health, disease burden, social aspects, etc.). Based on the concepts underpinning the items, we then divided the instruments into four groups: generic (overall) HRQoL; domain-specific HRQoL; disease-/organ-specific HRQoL; and transplant-specific HRQoL.
The transplant-specific HRQoL instruments were reviewed by two authors (AS and KV) and health items were extracted. In this stage, all items were listed regardless of whether they belonged to the concept of HRQoL. If a health item occurred in multiple instruments, the most frequent or shortest phrase was selected for the inventory. In the meetings with all authors, health items were categorized into three broad domains of health: physical, psychological, and social. Items that were clearly irrelevant to the measurement of HRQoL (e.g., religion or income level) were eliminated.
The main aim of this study is to provide an informative pool of items for the development of a new HRQoL instrument. To display the items in a clear and organized way, a technique called HealthFan© was used [24]. All health items included were arranged in a diagram and were classified under three higher order major domains (physical, mental, and social) to create a clear and concise overview. The sub-domains were graphically presented under each major domain, thereby listing the health items in a systematic way.
Results
Based on the search strategy, we identified 4381 articles in PubMed, 5066 in Embase, and 3232 in Web of Science, of which 1218 met the inclusion criteria (Fig. 1). There were 120 related titles (most published before 2000) for which we did not find the full text and therefore were unable to extract data from them. Furthermore, the names of the HRQoL instruments applied were not mentioned in 81 articles. The majority of the publications assessed kidney recipients (525 articles), followed by liver (340 articles), heart (196 articles), lung (131 articles), and pancreas (20 articles) recipients. We also included 138 articles that consisted of two or more different groups of solid organ recipients. We identified 418 distinct instruments that were divided into four groups: generic HRQoL; domain-specific HRQoL; disease-/organ-specific HRQoL; and transplant-specific HRQoL.
Instruments and Outcome Measures
Generic (Overall) HRQoL Instruments
Generic instruments assess global aspects of health status and are thus potentially suitable for a wide range of patient groups. This literature review found that the majority of solid organ transplantation studies applied generic HRQoL instruments. The SF-36 was by far the most frequently used generic instrument in post-transplant HRQoL studies, followed by the Karnofsky Performance Status Scale (KPS), and the Sickness Impact Profile (SIP) (Table 1). The complete list of generic HRQoL instruments is available in Appendix 1 in the electronic supplementary material (ESM).
Table 1.
Characteristics of the top three prominent administered generic and domain-specific health-related quality-of-life instruments
| Instrument name | Instrument type | Items (N) | Domains | Year | Frequency |
|---|---|---|---|---|---|
| 36-Item Short Form Survey (SF-36) [39] | Generic | 36 | Vitality Physical functioning Bodily pain General health perceptions Physical role functioning Emotional role functioning Social role functioning Mental health |
1992 | 460 |
| Karnofsky Performance Status Scale (KPS) [40] | Generic | 11 | Performance status | 1948 | 78 |
| Sickness Impact Profile (SIP) [41] | Generic | 136 | Physical items (ambulation, mobility, and body care/movement) Psychosocial items (social interaction; communication; alertness behavior; emotional behavior; home management; eating; sleep/rest; recreation and pastimes; and work) |
1981 | 75 |
| Hospital Anxiety and Depression Scale (HADS) [43] | Anxiety and depression-targeted | 14 | Mood Interest in activities Anxiety Panic symptoms |
1983 | 107 |
| Pittsburgh Sleep Quality Index (PSQI) [44] | Sleep-targeted | 19 | Subjective sleep quality Sleep latency Sleep duration Habitual sleep efficiency Sleep disturbances Use of sleep medication Daytime dysfunction |
1988 | 26 |
| Social Support Questionnaire (F-SozU) [45] | Social functioning-targeted | 54 | Emotional support Affiliation support Instrumental support Social integration Satisfaction with the received support |
1989 | 13 |
Domain-Specific HRQoL Instruments
Domain-specific instruments measure one particular aspect of health, such as life satisfaction or social functioning. Contrary to generic or organ-specific instruments that cover broad aspects of health, these instruments assess a particular dimension of health in detail. Instruments that assess depressive and/or anxiety symptoms were the most frequently applied domain-specific HRQoL instruments in transplantation studies. Moreover, because insomnia is one of the most frequently reported side effects of immunosuppressive medications [42], sleep quality assessment was frequent in our findings. Social support and life satisfaction instruments were the third most frequently applied domain-specific HRQoL instruments in transplantation studies (Table 1). The complete list of domain-specific HRQoL instruments is available in Appendix 2 in the ESM.
Disease-/Organ-Specific HRQoL Instruments
These instruments are designed to measure the patient’s perceptions of a specific health problem in a particular organ or disease (e.g., respiratory symptoms, gastrointestinal symptoms, heart failure, etc.). The Kidney Disease Quality of Life Short Form (KDQOL-SF) is the most commonly applied organ-specific HRQoL instrument in solid organ transplant patients. Table 2 introduces the characteristics of the most frequently applied kidney-, liver-, lung-, pancreas-, and heart-targeted HRQoL instruments. The complete list of disease-/organ-specific HRQoL instruments is available in Appendix 3 in the ESM.
Table 2.
Characteristics of the top five prominent administered organ- and transplant-specific HRQoL instruments
| Instrument name | Instrument type | Items (N) | Domains | Year | Frequency |
|---|---|---|---|---|---|
| Organ-specific instruments | |||||
| Kidney Disease Quality of Life Short Form (KDQOL-SF) [46] | Kidney-targeted | 79 | 8 SF-36 domains Symptoms/problems Effects of kidney disease Burden of kidney disease Work status Cognitive function Quality of social interaction Sexual function Sleep Social support Staff encouragement Patient satisfaction |
1994 | 38 |
| Liver Disease Quality of Life instrument (LDQOL) [47] | Liver-targeted | 111 | 8 SF-36 domains Symptoms of liver disease Effects of liver disease Concentration Memory Sexual functioning Sexual problems Sleep Loneliness Hopelessness Quality of social interaction Health distress Stigma of liver disease |
2000 | 18 |
| St. George’s Respiratory Questionnaire (SGRQ) [48] | Lung-targeted | 50 | Symptom (illness status such as cough, sputum production, and dyspnea) Activity (activities that cause breathlessness and activities limited by breathlessness) Impact (social functioning and psychological disturbances resulting from airways disease) |
1991 | 17 |
| Diabetes Quality of Life questionnaire (DQOL) [49] | Pancreas-targeted | 62 | Core items (satisfaction, impact, diabetes worry, and social/vocational worry) Auxiliary questions about adolescent patients (schooling experience and family relationships) |
1988 | 14 |
| Minnesota Living with Heart Failure Questionnaire (MLHFQ) [50] | Heart-targeted | 21 | Physical functioning Emotional functioning |
1987 | 5 |
| Transplant-specific instruments | |||||
| End-Stage Renal Disease Symptom Checklist-Transplantation Module (ESRD-SCLTM) [51] | Kidney tx-targeted | 43 | Limited physical capacity Limited cognitive capacity Transplantation-associated psychological distress Cardiac and renal dysfunction Side effects of corticosteroids Increased growth of gum and hair |
1999 | 20 |
| Kidney Transplant Questionnaire (KTQ) [52] | Kidney tx-targeted | 25 | Physical symptoms Uncertainty/fear Fatigue Appearance Emotions |
1993 | 17 |
| Modified Transplant Symptom Occurrence and Symptom Distress Scale (MTSOSD) [53, 54] |
All organs tx-targeted | 59 | Symptom occurrence (cognitive component) exclusively related to the side effects of the immunosuppressant Symptom distress (emotional component) exclusively related to the side effects of the immunosuppressant |
1985 | 17 |
| Transplant Effects Questionnaire (TxEQ) [55, 56] |
All organs tx-targeted | 23 | Worry about the transplant Guilt regarding donor Disclosure Adherence Responsibility |
2002 | 16 |
| Heart Transplant Symptom Checklist [57] | Heart tx-targeted | 92 | Cardiopulmonary symptoms Gastrointestinal symptoms Genitourinary symptoms Dermatological symptoms Neuromuscular symptoms Psychological symptoms |
1992 | 14 |
tx transplant
Transplant-Specific HRQoL Instruments
Transplant-specific instruments were developed to evaluate certain aspects of the health status of patients who receive a graft, such as physical symptoms or medication side effects. Many of these tools are modified modules of disease-specific instruments that contain items pertaining to the transplantation setting and can be used for recipients of a particular kind of organ. A few instruments were designed for transplants of any type and have been used in studies that include various transplanted organs. Table 2 briefly describes the characteristics of the most frequently applied transplant-specific HRQoL instruments. The complete list of transplant-specific HRQoL instruments is available in Appendix 4 in the ESM.
Health Items
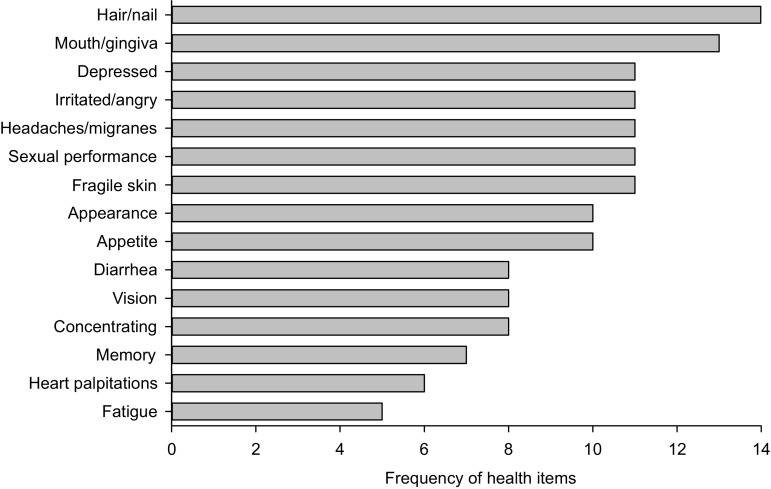
To obtain an overview of the health domains that are currently assessed by transplant-specific instruments, an inventory was made of the health items in these instruments. In total, 576 items were extracted (full list available on request from the author). After elimination of the irrelevant items and merging repetitions and items that assessed similar concepts, 78 distinct health items remained for development by our HealthFan tool. Items that assessed physical symptoms were commonly repeated in different transplant-specific instruments (Fig. 2).
Fig. 2.
Frequency of health items with most repetitions in available transplant-specific health-related quality-of-life instruments
These 78 health items were classified into three broad domains (colored areas): physical, mental, and social (Fig. 3). To provide a visual overview, health items were subdivided into 16 sub-domains (filled-in dots). The class of physical items was subdivided into belly, body heat, chest, eating, energy, pain, physical, respiratory, senses, and skin. The class of mental items was subdivided into cognition, feelings, and worries. The class of social items was subdivided into activities, autonomy, and relationships.
Fig. 3.
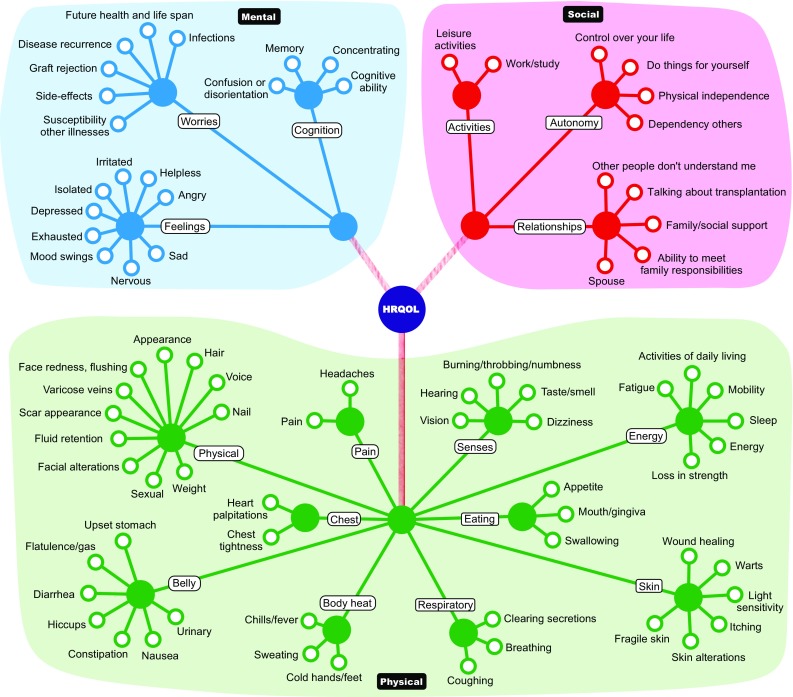
Selected extracted health items organized into physical, mental, and social domains (HealthFan)
Discussion
Ever since the concept of HRQoL was introduced, it has been a challenge to define and measure it [58]. No single instrument can be construed as the gold standard for measuring HRQoL in different populations, particularly when they are heterogeneous. One of the aims of this paper was to identify all HRQoL instruments that have been applied thus far among solid organ transplant patients. On the basis of this review, we compiled a complete list of all HRQoL instruments that have been developed or applied to date in the field of solid organ transplantation as well as a comprehensive list of health items. We discovered that different studies sometimes refer to a particular instrument by different names. We carefully selected the most generally known name corresponding to each instrument. Additionally, we developed a helpful scheme to depict relevant health items.
This review revealed that the majority of publications did not provide a logical rationale for the choice of the HRQoL instrument. Additionally, 81 publications did not mention the name or the reference for the HRQoL instruments that were applied. Moreover, the validity of some of the HRQoL instruments seems questionable, as the studies did not cite a source that described the development and validation procedure. The overview of instruments that we present in this study will be useful in the process of instrument selection in future studies and is conducive to credible findings.
The health assessment instruments most commonly reported in the literature were generic measures, and among these the SF-36 was particularly frequent. These generic instruments have a rich history of assessing psychometric properties and performing validation studies in general populations and many patient groups. Their wide application also enables researchers to compare results from transplant patients with those from the general population. However, these instruments were not developed specifically for transplant patients. Consequently, they do not capture the most salient health domains of organ recipients. Using generic instruments alongside transplant-specific instruments would make the results more comparable. Together they could detect the unanticipated positive or negative effects of transplantation that are not covered by specific instruments. However, relying solely on generic instruments may be insufficient to discover clinically relevant changes in post-transplant patients [59].
Domain-specific instruments, especially those measuring psychological symptoms, were the second most commonly used instruments. The literature has emphasized the importance of assessing psychological issues, due to their high prevalence and their enormous impact on the health status of transplant patients [60–63]. Moreover, as found in this review, the items of some very frequently used transplant-specific HRQoL instruments (e.g., Transplant Effects Questionnaire (TxEQ), Heart Transplant Symptom Checklist, and Heart Transplant Stressor Scale) are restricted to the psychological domain of HRQoL. Although domain-specific instruments provide detailed data on their target domain, they do not give a global sum score that can be interpreted for all domains of HRQoL. We expect that the list of selected health items of domain-specific instruments in the HRQoL studies of post-transplant patients will be very informative.
Disease- or organ-specific HRQoL instruments were the third most frequently applied instruments in studies in transplant patients. These include items that focus on a particular disease or organ. Therefore, in theory, they provide more accurate estimates of HRQoL, with higher consistency and reliability for their target population (i.e., recipients of a specific type of organ). However, the content of these instruments also has certain shortfalls. For example, all of the instruments that we described in the results section were designed to measure HRQoL in patients who had chronic disease of that organ (i.e., before transplantation) and were therefore not, or less, applicable after transplantation. Additionally, it is difficult to interpret the results of organ-specific instruments in heterogeneous patient groups who received different organs. We suggest that the application of organ-specific instruments should be limited to the transplant candidates, since the health issues are usually substantially different after transplantation [3, 15, 64, 65].
Our review revealed that, despite the availability of transplant-specific instruments, only a few studies have applied these types of instruments to measure HRQoL. This low level of application, which has also been observed in some previous reviews [3, 4, 18–20, 66–70], might be explained in several ways. First, transplant-specific instruments are relatively new, meaning that many longitudinal studies started data collection before transplant-specific instruments were available. Second, by applying generic or domain-specific instruments, researchers can compare their results with characteristics of various other populations, whereas transplant-specific instruments restrict the comparability across studies. Third, most transplant-specific instruments (e.g., End-Stage Renal Disease Symptom Checklist-Transplantation Module (ESRD-SCLTM), which includes 43 items, or the Modified Transplant Symptom Occurrence and Symptom Distress Scale (MTSOSD), which includes 59 items) comprise more items than most generic instruments (e.g., EQ-5D, which includes five items, or the SF-36, which includes 36 items), which makes them lengthy and thus less desirable for clinical studies, especially those that require repeated measurements.
We consider that the current transplant-specific instruments have more potential than generic HRQoL instruments for use in post-transplant research. However, given that the content of the available instruments is largely determined by experts rather than patients, it is currently unclear whether the health items included in these instruments are relevant from the perspective of the patients. In addition, the current instruments are not preference-based, meaning the health items are not weighted to generate a single value that expresses the overall quality of the patient’s health condition. This makes the results more complicated to interpret and also not suitable for use in cost-utility studies to support decision makers. Therefore, it might be necessary to develop one, or even a set of, targeted HRQoL measurement instruments for solid organ recipients. The development strategy for such a future instrument must take into account the input of patients at all steps, including (1) item generation based on review of the literature and patient input; (2) item selection; (3) value judgment on the items. Given the increasing use of smartphones and touchscreens, new HRQoL instruments might be devised as mobile applications, which would make them more convenient for patients to use and researchers to apply.
This review had a very broad search strategy, which ensured we included all articles that evaluated HRQoL after solid organ transplantation. We carefully assessed the selection of eligible studies and provided the complete list of HRQoL instruments in our results. The HealthFan listed the health items in a systematic way. In our next study, this graphical arrangement of the health items available will be used to present the items to post-transplant patients to select or add items they consider most important. We believe that, in this way, patients will have a prominent role in the process of developing a generic transplant health-outcome instrument.
Our review has some limitations, which should be mentioned. The inclusion of only English-language publications might limit the results to those instruments that have appeared in an English version. However, we did include non-English instruments if they were published in an English-language article. Another possible limitation is the exclusion of studies that had only assessed pre-transplant or pediatric patients. However, for our purposes, these two groups are not comparable with adult post-transplant patients. Post-transplant issues (e.g., immunosuppressive side effects) do not pertain to pre-transplant patients, while adult issues (e.g., partner relationships and employability) cannot be compared with childhood issues.
Conclusion
We emphasize the need to develop a preference-based, transplant-specific HRQoL instrument that is easy to apply and that targets the health issues of solid organ recipients. The current set of key health items that was collected in this study is a valuable outcome that will be used in the next developmental phase. In the following step, patients’ opinions will be included through focus group meetings, and an online survey will be carried out to derive the content for a new patient-centered, transplant-specific instrument.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
No sources of funding were used to conduct this study or prepare this manuscript.
Author contributions
Ahmad Shahabeddin Parizi conceptualized the project, performed the literature search, and screened abstracts, extracted data, contributed to writing the paper, and approved the final manuscript. Paul F. M. Krabbe conceptualized the project and HealthFan, contributed to writing the paper and approved the final manuscript. Erik Buskens and Stephan J. L. Bakker conceptualized the project, contributed to writing the paper, and approved the final manuscript. Karin M. Vermeulen conceptualized the project, contributed to the search, data extraction, and writing of the paper, and approved the final manuscript.
Conflict of interest
Ahmad Shahabeddin Parizi, Paul F. M. Krabbe, Erik Buskens, Stephan J. L. Bakker, and Karin M. Vermeulen have no conflicts of interest that are directly relevant to this content of this article.
Data availability
The studies used in this review were obtained from the literature. The datasets generated and analyzed during this review are available from the corresponding author on reasonable request.
References
- 1.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transpl. 2011;11:450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 2.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;5:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 3.Tome S, Wells JT, Said A, Lucey MR. Quality of life after liver transplantation. A systematic review. J Hepatol. 2008;48:567–577. doi: 10.1016/j.jhep.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Seiler A, Klaghofer R, Ture M, Komossa K, Martin-Soelch C, Jenewein J. A systematic review of health-related quality of life and psychological outcomes after lung transplantation. J Heart Lung Transpl. 2016;35:195–202. doi: 10.1016/j.healun.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Kugler C, Fischer S, Gottlieb J, Tegtbur U, Welte T, Goerler H, Simon A, Haverich A, Strueber M. Symptom experience after lung transplantation: impact on quality of life and adherence. Clin Transpl. 2007;2:590–596. doi: 10.1111/j.1399-0012.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 6.Karimi M, Brazier J. Health, health-related quality of life, and quality of life: what is the difference? PharmacoEconomics. 2016;34:645–649. doi: 10.1007/s40273-016-0389-9. [DOI] [PubMed] [Google Scholar]
- 7.Krabbe PFM. The measurement of health and health status: concepts, methods and applications from a multidisciplinary perspective. San Diego: Academic Press; 2016. [Google Scholar]
- 8.Murray C. Health systems performance assessment: debates, methods and empiricism. Geneva: World Health Organization; 2003. pp. 301–318. [Google Scholar]
- 9.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273:59–65. doi: 10.1001/jama.1995.03520250075037. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Åberg F, Höckerstedt K, Roine RP, Sintonen H, Isoniemi H. Influence of liver-disease etiology on long-term quality of life and employment after liver transplantation. Clin Transpl. 2012;26:729–735. doi: 10.1111/j.1399-0012.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 12.Shahabeddin Parizi A, Krabbe PFM, Verschuuren EAM, Hoek RAS, Kwakkel-van Erp JM, Erasmus ME, van der Bij W, Vermeulen KM. Patient-reported health outcomes in long-term lung transplantation survivors: a prospective cohort study. Am J Transpl. 2018;18:684–695. doi: 10.1111/ajt.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baranyi A, Krauseneck T, Rothenhäusler HB. Overall mental distress and health-related quality of life after solid-organ transplantation: results from a retrospective follow-up study. Health Qual Life Outcomes. 2013;11:15. doi: 10.1186/1477-7525-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paranjothi S, Yusen RD, Kraus MD, Lynch JP, Patterson GA, Trulock EP. Lymphoproliferative disease after lung transplantation: comparison of presentation and outcome of early and late cases. J Heart Lung Transpl. 2001;20:1054–1063. doi: 10.1016/S1053-2498(01)00314-X. [DOI] [PubMed] [Google Scholar]
- 15.Kugler C, Geyer S, Gottlieb J, Simon A, Haverich A, Dracup K. Symptom experience after solid organ transplantation. J Psychosom Res. 2009;66:101–110. doi: 10.1016/j.jpsychores.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Howell M, Wong G, Rose J, Tong A, Craig JC, Howard K. Eliciting patient preferences, priorities and trade-offs for outcomes following kidney transplantation: a pilot best–worst scaling survey. BMJ Open. 2016;6:e008163. doi: 10.1136/bmjopen-2015-008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transpl. 2001;20:1016–1024. doi: 10.1016/S1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 18.Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51:949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long-term quality of life. Liver Int. 2014;34:1298–1313. doi: 10.1111/liv.12553. [DOI] [PubMed] [Google Scholar]
- 20.Speight J, Reaney MD, Woodcock AJ, Smith RM, Shaw JA. Patient-reported outcomes following islet cell or pancreas transplantation (alone or after kidney) in Type 1 diabetes: a systematic review. Diabet Med. 2010;27:812–822. doi: 10.1111/j.1464-5491.2010.03029.x. [DOI] [PubMed] [Google Scholar]
- 21.Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9:e1001307. doi: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butt Z, Yount SE, Caicedo JC, Abecassis MM, Cella D. Quality of life assessment in renal transplant: review and future directions. Clin Transpl. 2008;22:292–303. doi: 10.1111/j.1399-0012.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 23.Essink-Bot ML, Krabbe PF, Bonsel GJ, Aaronson NK. An empirical comparison of four generic health status measures. The Nottingham Health Profile, the Medical Outcomes Study 36-item Short-Form Health Survey, the COOP/WONCA charts, and the EuroQol instrument. Med Care. 1997;35:522–537. doi: 10.1097/00005650-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Reneman MF, Brandsema KPD, Schrier E, Dijkstra PU, Krabbe PFM. Patients first: towards a patient-centered, instrument to measure impact of chronic pain. Phys Ther. 2018;98:616–625. doi: 10.1093/ptj/pzy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272:619–626. doi: 10.1001/jama.1994.03520080061045. [DOI] [PubMed] [Google Scholar]
- 26.Hamming JF, De Vries J. Measuring quality of life. Br J Surg. 2007;94:923–924. doi: 10.1002/bjs.5948. [DOI] [PubMed] [Google Scholar]
- 27.Krabbe PFM. Thurstone scaling as a measurement method to quantify subjective health outcomes. Med Care. 2008;46:357–365. doi: 10.1097/MLR.0b013e31815ceca9. [DOI] [PubMed] [Google Scholar]
- 28.Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med. 2013;368:201–203. doi: 10.1056/NEJMp1211775. [DOI] [PubMed] [Google Scholar]
- 29.Facey K, Boivin A, Gracia J, Hansen HP, Scalzo AL, Mossman J, Single A. Patients’ perspectives in health technology assessment: a route to robust evidence and fair deliberation. Int J Technol Assess Health Care. 2010;26:334–340. doi: 10.1017/S0266462310000395. [DOI] [PubMed] [Google Scholar]
- 30.Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff. 2010;29:1489–1495. doi: 10.1377/hlthaff.2009.0888. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan M. The new subjective medicine: taking the patient’s point of view on health care and health. Soc Sci Med. 2003;56:1595–1604. doi: 10.1016/S0277-9536(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 32.McKenna SP. Measuring patient-reported outcomes: moving beyond misplaced common sense to hard science. BMC Med. 2011;9:86. doi: 10.1186/1741-7015-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr AJ, Higginson IJ. Measuring quality of life: are quality of life measures patient centered? BMJ. 2001;322:1357–1360. doi: 10.1136/bmj.322.7298.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krabbe PFM. A generalized measurement model to quantity health: the multi-attribute preference response model. PLoS One. 2013;8:e79494. doi: 10.1371/journal.pone.0079494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krabbe PFM, van Asselt ADI, Selivanova A, Jabrayilov R, Vermeulen KM. Patient-centered item selection for a new generic health-status instrument: CS-Base (submitted). [DOI] [PubMed]
- 36.Groothuis-Oudshoorn CGM, van der Heuvel E, Krabbe PFM. An item response theory model to measure health: the multi-attribute preference response model. BMC Med Res Methodol. 2018;18(1):62. doi: 10.1186/s12874-018-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use. 5. Oxford: Oxford University Press; 2015. [Google Scholar]
- 38.DeVellis RF. Scale development: theory and applications. 4. Newbury Park: Sage; 2016. [Google Scholar]
- 39.Brazier J-H, Harper R, Jones N, O’cathain A, Thomas K, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187–193. doi: 10.1200/JCO.1984.2.3.187. [DOI] [PubMed] [Google Scholar]
- 41.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981;19:787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Reilly-Spong M, Park T, Gross CR. Poor sleep in organ transplant recipients: self-reports and actigraphy. Clin Transpl. 2013;27:901–913. doi: 10.1111/ctr.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 44.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 45.Fydrich T, Geyer M, Hessel A, Sommer G, Brähler E. Fragebogen zur sozialen Unterstützung (F-SozU): Normierung an einer repräsentativen Stichprobe. Diagnostica. 1999;45:212–216. doi: 10.1026//0012-1924.45.4.212. [DOI] [Google Scholar]
- 46.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the Kidney Disease Quality of Life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 47.Gralnek IM, Hays RD, Kilbourne A, Rosen HR, Keeffe EB, Artinian L, Kim S, Lazarovici D, Jensen DM, Busuttil RW, Martin P. Development and evaluation of the Liver Disease Quality of Life instrument in persons with advanced, chronic liver disease-the LDQOL 1.0. Am J Gastroenterol. 2000;95:3552–3565. doi: 10.1111/j.1572-0241.2000.03375.x. [DOI] [PubMed] [Google Scholar]
- 48.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 49.Jacobson A, Barofsky I, Cleary P, Rand L. Reliability and validity of a diabetes quality-of-life measure of the Diabetes Control and Complications Trial (DCCT) Diabetes Care. 1988;11:725–732. doi: 10.2337/diacare.11.9.725. [DOI] [PubMed] [Google Scholar]
- 50.Bilbao A, Escobar A, García-Perez L, Navarro G, Quirós R. The Minnesota living with heart failure questionnaire: comparison of different factor structures. Health Qual Life Outcomes. 2016;14:23. doi: 10.1186/s12955-016-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franke GH, Reimer J, Kohnle M, Luetkes P, Maehner N, Heemann U. Quality of life in end-stage renal disease patients after successful kidney transplantation: development of the ESRD symptom checklist—transplantation module. Nephron. 1999;83:31–39. doi: 10.1159/000045470. [DOI] [PubMed] [Google Scholar]
- 52.Laupacis A, Pus N, Muirhead N, Wong C, Ferguson B, Keown P. Disease-specific questionnaire for patients with a renal transplant. Nephron. 1993;64:226–231. doi: 10.1159/000187318. [DOI] [PubMed] [Google Scholar]
- 53.Moons P, De Geest S, Versteven K, Abraham I, Vlaminck H, Moens G, Waer M. Psychometric properties of the “modified transplant symptom occurrence and symptom distress scale”. J Nurs Meas. 2001;9:115–134. doi: 10.1891/1061-3749.9.2.115. [DOI] [PubMed] [Google Scholar]
- 54.Dobbels F, Moons P, Abraham I, Larsen CL, Dupont L, De Geest S. Measuring symptom experience of side-effects of immunosuppressive drugs: the Modified Transplant Symptom Occurrence and Distress Scale. Transpl Int. 2008;21:764–773. doi: 10.1111/j.1432-2277.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- 55.Ziegelmann JP, Griva K, Hankins M, Harrison M, Davenport A, Thompson D, Newman SP. The Transplant Effects Questionnaire (TxEQ): the development of a questionnaire for assessing the multidimensional outcome of organ transplantation - Example of end stage renal disease (ESRD) Brit J Health Psychol. 2002;7:393–408. doi: 10.1348/135910702320645381. [DOI] [PubMed] [Google Scholar]
- 56.Annema C, Roodbol PF, Stewart RE, Ranchor AV. Validation of the Dutch version of the transplant effects questionnaire in liver transplant recipients. Res Nurs Health. 2013;36:203–215. doi: 10.1002/nur.21530. [DOI] [PubMed] [Google Scholar]
- 57.Grady KL, Jalowiec A, Grusk BB, White-Williams C, Robinson JA. Symptom distress in cardiac transplant candidates. Heart Lung. 1992;21:434–439. [PubMed] [Google Scholar]
- 58.Barofsky I. Can quality or quality-of-life be defined? Qual Life Res. 2012;21:625–631. doi: 10.1007/s11136-011-9961-0. [DOI] [PubMed] [Google Scholar]
- 59.von der Lippe N, Waldum B, Brekke FB, Amro AA, Reisæter AV, Os I. From dialysis to transplantation: a 5-year longitudinal study on self-reported quality of life. BMC Nephrol. 2014;15:191. doi: 10.1186/1471-2369-15-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widows MR, Rodrigue JR. Clinical practice issues in solid organ transplantation. In: Llewelyn S, Kennedy P, editors. Handbook of clinical health psychology. Oxford: Wiley; 2003. pp. 280–288. [Google Scholar]
- 61.De Vito Dabbs A, Dew MA, Stilley CS, Manzetti J, Zullo T, McCurry KR, Kormos RL, Iacono A. Psychosocial vulnerability, physical symptoms and physical impairment after lung and heart-lung transplantation. J Heart Lung Transpl. 2003;22:1268–1275. doi: 10.1016/S1053-2498(02)01227-5. [DOI] [PubMed] [Google Scholar]
- 62.Dew MA, Rosenberger EM, Myaskovsky L, Dimartini AF, Devito Dabbs AJ, Posluszny DM, Steel J, Switzer GE, Shellmer DA, Greenhouse JB. Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: a systematic review and meta-analysis. Transplantation. 2015;100:988–1003. doi: 10.1097/TP.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corbett C, Armstrong MJ, Parker R, Webb K, Neuberger JM. Mental health disorders and solid-organ transplant recipients. Transplantation. 2013;96:593–600. doi: 10.1097/TP.0b013e31829584e0. [DOI] [PubMed] [Google Scholar]
- 64.Shetty AA, Wertheim JA, Butt Z. Health-related quality of life outcomes after kidney transplantation. In: Orlando G, Remuzzi G, Williams DF, editors. Kidney transplantation, bioengineering and regeneration: kidney transplantation in the regenerative medicine era. London: Academic Press; 2017. pp. 699–708. [Google Scholar]
- 65.Smeritschnig B, Jaksch P, Kocher A, Seebacher G, Aigner C, Mazhar S, Klepetko W. Quality of life after lung transplantation: a cross-sectional study. J Heart Lung Transpl. 2005;24:474–480. doi: 10.1016/j.healun.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Landreneau K, Lee K, Landreneau MD. Quality of life in patients undergoing hemodialysis and renal transplantation: a meta-analytic review. Nephrol Nurs J. 2010;37:37–44. [PubMed] [Google Scholar]
- 67.Studer SM, Levy RD, McNeil K, Orens JB. Lung transplant outcomes: a review of survival, graft function, physiology, health-related quality of life and cost-effectiveness. Eur Respir J. 2004;24:674–685. doi: 10.1183/09031936.04.00065004. [DOI] [PubMed] [Google Scholar]
- 68.Edgell ET, Coons SJ, Carter WB, Kallich JD, Mapes D, Damush TM, Hays RD. A review of health-related quality-of-life measures used in end-stage renal disease. Clin Ther. 1996;18:887–938. doi: 10.1016/S0149-2918(96)80049-X. [DOI] [PubMed] [Google Scholar]
- 69.Cleemput I, Dobbels F. Measuring patient-reported outcomes in solid organ transplant recipients. PharmacoEconomics. 2007;25:269–286. doi: 10.2165/00019053-200725040-00002. [DOI] [PubMed] [Google Scholar]
- 70.Ceulemans LJ, Lomme C, Pirenne J, De Geest S. Systematic literature review on self-reported quality of life in adult intestinal transplantation. Transpl Rev. 2016;30:109–118. doi: 10.1016/j.trre.2016.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The studies used in this review were obtained from the literature. The datasets generated and analyzed during this review are available from the corresponding author on reasonable request.