Abstract
p-Cresyl sulfate (pCS), a uremic toxin, can cause renal damage and dysfunction. Studies suggest that renal dysfunction increases the prevalence of renal cancer. However, the effect of pCS on the proliferation and migration of renal cancer is unclear. Clear cell renal cell carcinoma (ccRCC) expresses mutant von Hippel-Lindau gene and is difficult to treat. Hypoxia-inducible factor-1α and 2-α (HIF-1α and HIF-2α) as well as microRNA-21 (miR-21) can regulate the proliferation and migration of ccRCC cells. However, the association between HIF-α and miR-21 in ccRCC remains unclear. Therefore, the effects of pCS on ccRCC cells were investigated for HIF-α and miR-21 signals. Our results showed that pCS induced overexpression of HIF-1α and promoted the proliferation and regulated epithelial-mesenchymal transition-related proteins, including E-cadherin, fibronectin, twist and vimentin in ccRCC cells. pCS treatment increased miR-21 expression. Specifically, inhibition of miR-21 blocked pCS-induced proliferation and migration. Taken together, the present results demonstrate that pCS directly induced the proliferation and migration of ccRCC cells through mechanisms involving miR-21/HIF-1α signaling pathways.
Introduction
Clear cell renal cell carcinoma (ccRCC) is the most common subtype of renal cancers1,2. Clinically, approximately 70–80% of ccRCC is found in renal cancers3. Von Hippel-Lindau gene (VHL), a tumor suppressor gene, is found in various cancers4. VHL protein, a member of the E3-ubiquitin ligase complex, can bind to hypoxia-inducible factor-1α and 2-α (HIF-1α and HIF-2α) to cause HIF-α degradation5. A previous study showed that VHL inhibits cell proliferation by interacting with HIF6. However, in most ccRCC cases, VHL is mutated and deficient7,8. Currently, nephrectomy is the major treatment for ccRCC9,10; as ccRCC is not sensitive to various drugs, chemotherapy is not an effective treatment11,12. Therefore, it is important to understand the mechanisms of the proliferation and migration of ccRCC to develop new therapies.
Indoxyl sulfate (IS) and p-cresyl sulfate (pCS) are 2 types of uremic toxins metabolized from tryptophan and tyrosine, respectively, in the intestine and can cause renal dysfunction. Tyrosine is converted into p-cresol through a series of reactions by microbiota in the distal colon, such as deamination, transamination, and decarboxylation. p-Cresol is further detoxified into pCS in the mucosa of the colon and in the liver. In circulation, pCS mostly binds to albumin and is excreted in the kidney. The free fraction of pCS is filtered at the glomerulus and the protein-bound fraction is secreted at the tubular epithelial cells13. The gut microbiota is also altered in patients with chronic kidney disease (CKD) with changes in the composition of microbiota and overgrowth of bacteria in the small intestine and colon, resulting in higher levels of p-cresol14. In CKD patients, excretion is impaired; in patients with hemodialysis, protein-bound pCS is difficult remove by dialysis. pCS accumulates as renal function deteriorates, with 100–200 μM as the mean serum total levels of pCS observed in uremic patients15. Accumulation of pCS causes renal dysfunction and disease progression by inducing oxidative damage and endothelial dysfunction16,17. Previous studies found that renal dysfunction is related to the risk of renal cell carcinoma, particularly ccRCC18,19. pCS can induce proliferation and migration of rat aortic vascular smooth muscle cells20 and epithelial-mesenchymal transition (EMT) in kidney fibrosis21. Based on these studies, we predicted that pCS influences proliferation, EMT, and migration in ccRCC. Therefore, the aim of this study was to determine the effect of pCS on the proliferation and migration of ccRCC cells and the related mechanisms.
HIF-1α and HIF-2α are transcription factors22,23. Most patients with ccRCC have high expression of HIF-1α24, and studies have shown that HIF-1α plays an important role in the proliferation of ccRCC10,24,25 and regulates EMT in ccRCC and tubular epithelial cells26,27. Fibronectin, twist, vimentin, and E-cadherin are associated with EMT and are required for cell migration28,29. Additionally, inhibition of HIF-1α can reduce the migration of ccRCC cells30. These results suggest that HIF-1α plays an important role in the proliferation, EMT, and cell migration of ccRCC. HIF-2α also regulates cell proliferation and migration in ccRCC7,31. Therefore, the expression of HIF-α, EMT-related proteins, and migration were investigated in pCS-treated ccRCC cells in this study.
MicroRNAs, which are 19–25 nucleotides in length, can influence gene expression32. MicroRNAs can interact with the 3′-untranslated regions of mRNAs to cause mRNA degradation and inhibit translation33. MicroRNA-21 (miR-21) is highly expressed in various cancers34. Studies have indicated that miR-21 can induce cell proliferation and EMT in ccRCC35,36. MiR-21 can activate HIF-1α expression in various cells including prostate cancer, retinal pigment epithelia, cervical cancer, and human stem cells37–39, but its role in ccRCC remains unclear. Therefore, the miR-21/HIF-1α axis signals were studied in pCS-treated ccRCC cells to examine cell proliferation and migration.
Results
Effect of pCS on cell proliferation in dose- and time-dependent manner
Cells from two cell lines, ccRCC 786-O and A498, were treated with 20, 50, 100, 200, and 500 μM of pCS for 48 h. Cell proliferation was measured by the WST-1 assay and the optical density of the cell culture was determined. As shown in Fig. 1, cell proliferation by pCS treatment of both cells was increased significantly at 12 h for 786-O cells and 24 h for A498 cells. pCS-induced cell proliferation was time-dependent in both 786-O and A498 cells (Fig. 1a,b). Cells treated with control, 100 or 200 μM pCS for 48 h are shown in Fig. 1c.
Figure 1.
Effect of p-cresyl sulfate (pCS) on proliferation of clear cell renal cell carcinoma (ccRCC) was time-dependent. Various concentrations of pCS (20–500 µM) were tested on 786-O (a) and A498 (b) cells. Cells were assayed for 48 h (c) after treatment with 100 and 200 μM pCS or none (P100, P200, or control). Cell proliferation was measured by WST1 assay as cell optical density at 48 h. Values are represented as the mean ± standard deviation from three independent experiments.
Effect of pCS on HIF-1α, HIF-2α and VHL levels
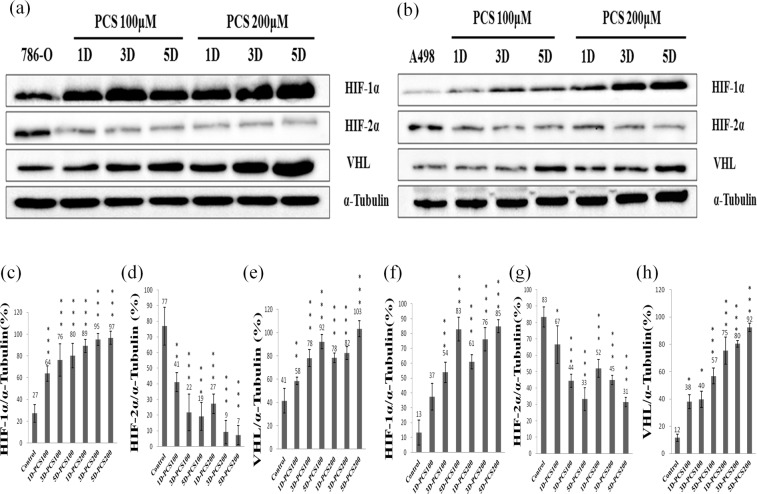
HIF-1α and HIF-2α transcription factors can regulate the cell proliferation of renal cancer5. We investigated whether pCS-induced proliferation was related to HIF-1α and HIF-2α. 786-O and A498 cells were treated with 100 or 200 μM pCS for 5 days. HIF-1α, HIF-2α, and VHL levels and the ratios of their expressions are shown in Fig. 2. pCS-induced cell proliferation of 786-O cells was related to HIF-1α signals and decreased levels of HIF-2α (Fig. 2a–d). Similarly, HIF-1α was increased and HIF-2α was decreased in pCS-treated A498 cells (Fig. 2b,f,g). Interestingly, VHL levels were also increased in both pCS-treated 786-O (Fig. 2a,e) and A498 cells (Fig. 2b,h). This result suggests that HIF-1α signals play an important role in pCS-induced proliferation of ccRCC cells.
Figure 2.
Effect of pCS on HIF-1α, HIF-2α, and VHL levels of 786-O and A498 cells. Cells treated with 100 or 200 μM pCS for 1 day (1D), 3 days (3D), or 5 days (5D) and indicated as 1D-PCS100 or 5D-PCS200. Protein expression in 786-O (a) and A498 (b) cells was assayed by Western blotting. The ratios of HIF-1α, HIF-2α, and VHL to α-tubulin were compared in 786-O cells (c–e) and A498 cells (f–h), respectively. HIF-1α and VHL levels were increased and HIF-2α level decreased in both pCS-treated cells as compared to the control. (*P < 0.05, **P < 0.01 and ***P < 0.001, respectively).
Effect of pCS on EMT and cell migration
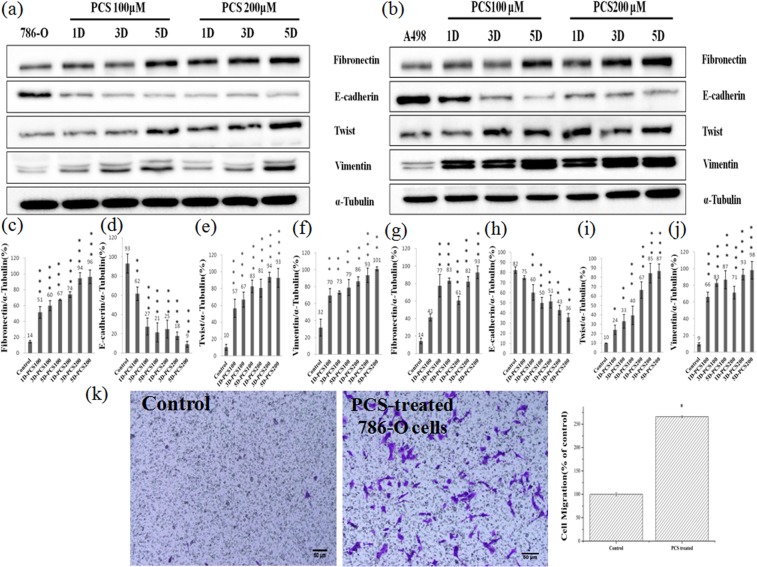
Because HIF-1α regulates EMT-related proteins29, we studied the effect of pCS on EMT in ccRCC cells. EMT-related proteins (including fibronectin, twist, vimentin, and E-cadherin) were determined by Western blotting in pCS-treated 786-O and A498 cells (Fig. 3a,b). The results showed that fibronectin, twist, and vimentin expression was increased, while E-cadherin was decreased in pCS-treated 786-O and A498 cells (Fig. 3) and indicated that pCS induced EMT in ccRCC cells. The effect of pCS on cell migration was further investigated in ccRCC cells. As shown in Fig. 3k, pCS promoted the migration of 786-O cells as compared to the control. The relative density of migration is shown in Fig. 3k. Similarly, pCS promoted the migration of A498 cells (data not shown). These results indicate that pCS induces EMT and migration of ccRCC cells.
Figure 3.
Effect of pCS on expression of EMT-related proteins in 786-O (a) and A498 (b) cells. Western blotting was used and ratios of fibronectin, E-cadherin, twist, and vimentin to α-tubulin were compared in 786-O cells (c–f) and A498 cells (g–j), respectively. The migration and percentage (k) were determined for 786-O cells using a Transwell system with and without 100 μM pCS treatment. Fibronectin, twist, and vimentin expression was increased, while E-cadherin was decreased in pCS-treated 786-O and A498 cells, indicating that pCS induced EMT in ccRCC cells. *P < 0.05, **P < 0.01 and ***P < 0.001, as compared to the control.
Effect of HIF-1α knockdown on pCS-induced proliferation, EMT and migration
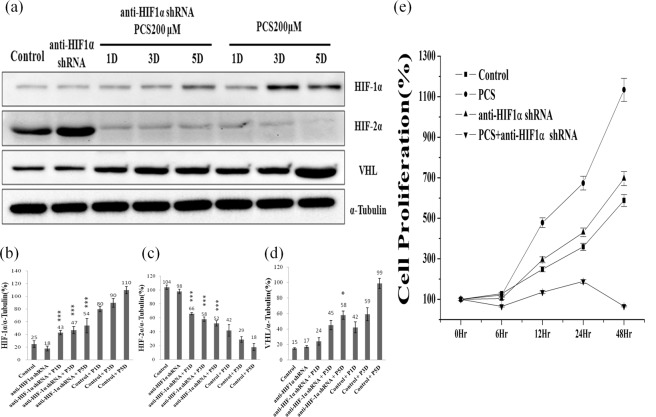
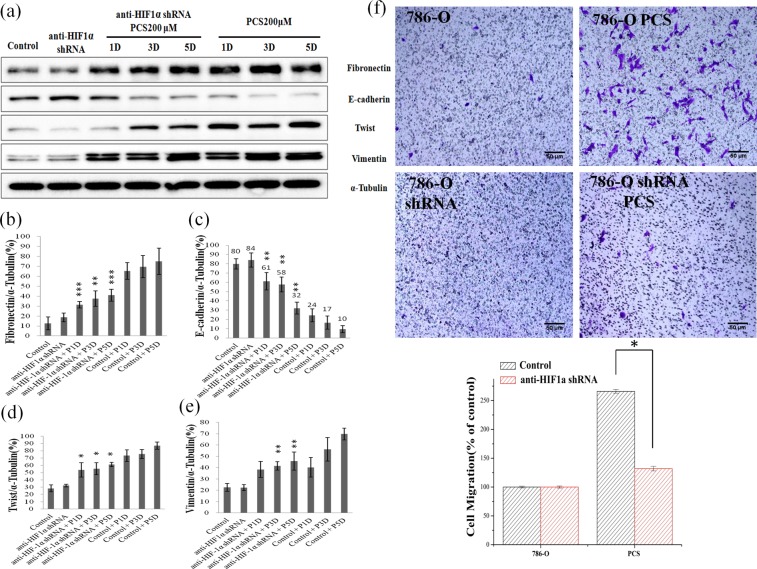
To determine whether pCS induces EMT, migration, and proliferation via HIF-1α, HIF-1α was knocked down with an anti-HIF-1α short hairpin RNA (shRNA). Knockdown of HIF-1α promoted the expression of HIF-2α at days 1, 3, and 5 (Fig. 4a–c). However, knockdown of HIF-1α significantly inhibited VHL expression in pCS-treated 786-O cells only at day 5 (Fig. 4a,d). Additionally, knockdown of HIF-1α inhibited the expression of fibronectin, twist, and vimentin and promoted the expression of E-cadherin (Fig. 5a). pCS-induced cell migration (Figs 3k and 5f) was inhibited by knockdown of HIF-1α (Fig. 5f). Knockdown of HIF-1α also inhibited pCS-induced proliferation of 786-O cells (Fig. 4e). Similar results were observed for pCS-treated A498 cells (data not shown). Overall, these results suggest pCS induced EMT, migration, and proliferation via HIF-1α signals.
Figure 4.
Effect of HIF-1α knockdown on HIF-1α, HIF-2α, and VHL expression in 786-O cells. HIF-1α, HIF-2α, VHL, and α-tubulin levels were assayed by Western blotting (a) with or without shRNA HIF1α in 200 μM pCS-treated cells at day(s) 1–5. Ratios of HIF-1α (b), HIF-2α (c), and VHL (d) to α-tubulin were compared. Knockdown of HIF-1α promoted the expression of HIF-2α at day(s) 1–5 and inhibited VHL expression only at day 5. HIF-1α knockdown inhibits pCS-induced proliferation (e). Values were represented as the mean ± standard deviation from three independent experiments. *P < 0.05 and ***P < 0.001, as compared to the control group. pCS-treated day(s) 1, 3, and 5 are indicated as P1D, P3D, and P5D, respectively. Anti-HIF-1α shRNA indicated as HIF-1α knockdown with shRNA.
Figure 5.
Effect of HIF-1α knockdown on expression of EMT-related proteins in 786-O cells. Protein expression was assayed by Western blotting (a) with or without shHIF1α in 200 μM pCS-treated cells at day(s) 1–5. Ratios of fibronectin (b), E-cadherin (c), twist (d), and vimentin (e) to α-tubulin were compared. HIF-1α knockdown inhibited pCS-induced cell migration (f). pCS-induced cell migration was inhibited by knockdown of HIF-1α. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to the control. Anti-HIF-1α shRNA indicated as HIF-1α knockdown with shRNA. pCS-treated day(s) 1, 3, and 5 are indicated as P1D, P3D, and P5D, respectively. 786-O shRNA cells: HIF-1α knockdown 786-O cells and 786-O shRNA PCS: 786-O shRNA cells treated with pCS.
miR-21 mediates pCS-induced EMT and proliferation of ccRCC
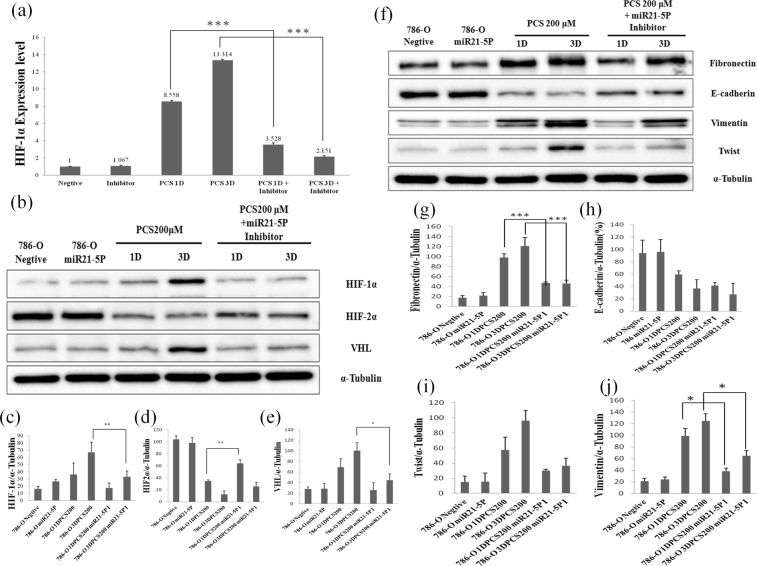
Previous studies demonstrated that miR-21 promotes the proliferation and EMT of ccRCC cells38,39. We examined the role of miR-21 in pCS-induced proliferation and EMT-mediated protein expression. The results showed that the miR21 inhibitor reduced the expression of HIF-1α mRNA at days 1 and 3 (Fig. 6a). Additionally, overexpression of HIF-1α and VHL proteins was inhibited and expression of HIF-2α was promoted in pCS-treated cells with miR-21 inhibitor(Fig. 6b). The inhibitor of miR-21 also significantly inhibited the expression of fibronectin and vimentin in pCS-treated cells (Fig. 6g,j). Overall, our results suggest that pCS increases miR-21 to cause cell proliferation and EMT in ccRCC cells.
Figure 6.
Effect of miR-21 inhibitor on pCS-induced HIF-1α mRNA expression by PCR (a) and HIF-1α, HIF2α, and VHL expression by western blotting (b). HIF-1α levels in 786-O cells were compared with miR-21 inhibitor-treated group (miR21-5P), pCS-treated-1 and 3 day groups (1D and 3DPCS200), as well as pCS plus miR-21 inhibitor-treated-1 and 3 day groups (1DPCS200 miR21-5PI and 3DPCS200 miR21-5PI). Ratios of HIF-1α (c), HIF-2α (d), and VHL (e) to α-tubulin were compared. miR-21 inhibition regulated pCS-induced expression of EMT-related proteins (f). Ratios of fibronectin (g), E-cadherin (h), twist (i), and vimentin (j) to α-tubulin were also compared. The inhibitor of miR-21 significantly inhibited the expression of HIF-1α, fibronectin and vimentin in pCS-treated cells, suggesting that pCS increases miR-21 to cause overexpression of HIF-1α and EMT in 786-O cells. Values are expressed as the mean ± standard deviation from three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001, compared to the pCS-treated group.
Discussion
Previous reports showed that patients with end-stage renal disease (ESRD) generally have high levels of uremic toxins containing IS and pCS40,41. Clinical cases showed that patients with ccRCC are closely associated with ESRD42,43. However, direct evidence linking pCS and ccRCC progression is lacking. Our results demonstrated that pCS induced the proliferation and migration of ccRCC cells and suggested that the progression of ccRCC is related to pCS in the kidneys of patients. Therefore, removing pCS may prevent ccRCC progression in ESRD patients. AST-120, an approved clinical drug, decreases pCS levels44,45 and may be useful for preventing ccRCC progression, but further studies are needed to confirm this aspect. Other methods are also available for decreasing pCS such as renal replacement therapy of hemodiafiltration, dietary intervention, laxative, and pro-, pre-, and syn-biotics13,14.
Accumulation of pCS increases oxidative stress in endothelial cells, reactive oxygen species in cardiomyocytes, and expression of DNA methyltransferase in HK2 cells13. A study showed that pCS activated the renin angiotensin aldosterone system and induced EMT21. Some studies showed that accumulation of reactive oxygen species leads to stabilization of HIF-1α46,47. Another study showed that the angiotensin system increased HIF-1α signals48.
HIF-1α and HIF-2α have similar structures containing transcription and oxygen-dependent degradation domains, but have different functions49. Additionally, both HIF-1α and HIF-2α can regulate cell proliferation and migration7,24,25,28,31. Some studies indicated that HIF1α acts as a renal tumor suppressor and HIF2α as a renal tumor inducer50,51. However, another study showed that HIF-1α inhibits and HIF-2α induces apoptosis52. Nevertheless, both HIF-1α and HIF-2α induce the proliferation of ccRCC cells7,10,25,53. Our results showed that pCS induced HIF-1α overexpression and promoted the proliferation of ccRCC cells. This suggests that HIF-1α plays an important role in promoting the proliferation of ccRCC cells. Our results on pCS-induced HIF-1α and reduced HIF-2α expression agreed with those of previous studies54,55.
VHL can interact with HIF-α to promote the degradation of HIF-α via the ubiquitin system, leading to blockage of HIF-α signals56,57. However, mutant VHL genes are found in most ccRCC cells, including in 786-O and A498 cells58. A study indicated that mutant VHL does not regulate HIF-α signals52. Our results showed that pCS induced HIF-1α and VHL and suppressed HIF-2α expression. We found that overexpression of mutant VHL in pCS-treated 786-O and A498 cells did not inhibit HIF-1α-induced proliferation. This may be explained by the data showing that overexpression of mutant VHL in ccRCC prevented UV-induced apoptosis59. Although the mechanism of pCS-induced overexpression of VHL remains unclear, pCS-induced overexpression of VHL may decrease apoptosis and lead to the progression of ccRCC.
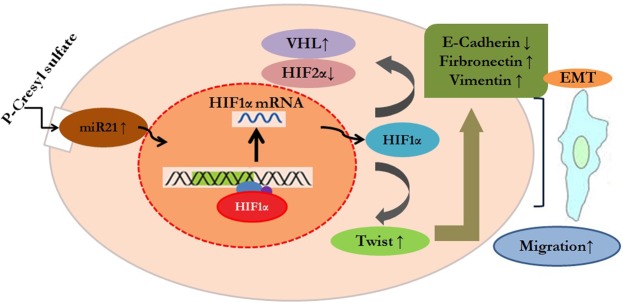
miR-21 in various cancers can activate HIF-1α signals to promote cell proliferation37,38. Both miR-21 and HIF-1α induced proliferation and EMT of ccRCC cells25,28,35,36, but it was unclear whether miR-21 upregulates HIF-1α in ccRCC. Our results showed that inhibition of miR-21 decreased HIF-1α expression in pCS-treated ccRCC cells. Therefore, miR-21 functions upstream of HIF-1α to regulate the proliferation and EMT of ccRCC cells. A hypothetical mechanism is shown in Fig. 7 and summarizes our study results. Briefly, pCS induced overexpression of miR-21, resulting in increased expression of HIF-1α and VHL and decreased HIF-2α. HIF-1α further promote the proliferation and migration of ccRCC cells. VHL is commonly mutated in ccRCC, and there are no reports regarding the relationship between miR-21 and VHL in ccRCC. However, a previous study indicated miR-21 can target VHL to regulate cell growth and that miR-21 inhibition induces VHL expression and inhibits the proliferation of glioblastomas60. In contrast, our results showed that inhibition of miR-21 decreased the expression of VHL and inhibited pCS-induced proliferation in ccRCC cells. Because wild-type VHL in glioblastoma inhibits cell growth while mutant VHL in ccRCC inhibits apoptosis, miR-21 has distinct effects on VHL expression and regulates cell growth in different cells.
Figure 7.
Hypothetical mechanism of pCS-induced proliferation and EMT of ccRCC. pCS treatment increased miR-21 expression in ccRCC and further induced overexpression of HIF-1α. HIF-1α promoted proliferation and regulated epithelial-mesenchymal transition (EMT)-related proteins including E-cadherin, fibronectin, twist, and vimentin to promote migration of ccRCC cells.
In summary, we demonstrated that pCS-induced proliferation and EMT of ccRCC was mainly mediated by miR-21/HIF-1α signals.
Materials and Methods
Materials
p-Cresyl sulfate (pCS) was obtained from Sigma (Sigma-Aldrich, St. Louis, MO, USA). The Millicell cell culture chamber was obtained from Millipore (Millipore, Billerica, MA, USA). Rabbit polyclonal HIF1α antibody (1:1000) was obtained from Genetex (Alton Parkway, CA, USA). Rabbit monoclonal HIF2α antibody (1:1000), rabbit polyclonal VHL antibody (1:1000), rabbit monoclonal E-cadherin antibody (1:1000), rabbit monoclonal vimentin antibody (1:1000), and rabbit polyclonal α-tubulin antibody (1:5000) were from Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal fibronectin antibody (1:2000) and mouse monoclonal twist antibody (1:500) were from Abcam (Cambridge, UK). Secondary antibodies (anti-mouse IgG horseradish peroxidase-linked and anti-rabbit IgG horseradish peroxidase-linked) were form Cell Signaling Technology. Primers were synthesize by Integrated DNA Technologies (Coralville, IA, USA).
Cell culture
A498 and 786-O cells of ccRCC were kindly provided by Dr. Ren-Jun Hsu (Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan). Both cells were cultured with RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Invirogen, Carlsbad, CA, USA), 2 mM l-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, and 1.0 mM sodium pyruvate. The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C.
Cell proliferation assay
Cell proliferation was measured using a WST-1 assay kit (BioVision, Milpitas, CA, USA). The cells were cultured in 96-well culture plates (8 × 103 cells/well). WST-1 reagent was added to the wells of the experimental and control groups at 6, 12, 24, and 48 h. After incubation for 3 h (37 °C, 5% CO2), an aliquot of culture supernatant was measured at 450 nm with a Multiskan™ FC Microplate Photometer (Molecular Devices, Sunnyvale, CA, USA). The absorbance of WST-1 reagent without cells was used as the blank.
SDS-PAGE and western blotting
The cells were collected and washed twice with PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4), and then lysed with NETN buffer (20 mM Tris at pH 8.0, 150 mM NaCl, 1 mM EDTA at pH 8.0, 0.5% Nonidet P-40) plus protease and phosphatase inhibitors (25 mM NaF, 2 mM Na3VO4, 0.1 mM PMSF, 20 μg/mL aprotinin) and sonicated with a sonicator (ChromTech UP-800). After centrifugation at 15,000 × g for 15 min at 4 °C, the soluble extraction containing proteins was collected from the supernatant. The protein concentration was measured with a protein assay kit (Thermo Fischer Scientific, Waltham, MA, USA). Equal quantities (approximately 40 μg) of samples were loaded onto 6%, 10%, and 15% SDS-PAGE gel and separated at a voltage of 100V. Proteins in the SDS-PAGE gel were then transferred to a polyvinylidene fluoride membrane (Millipore). The membrane was treated with 5% skim milk in TBST buffer (TBS containing 0.1% Tween-20) for 1 h at 26.5 °C and then hybridized with primary antibody at 4 °C with gentle agitation overnight. After washing with TBST three times, the membrane was incubated with secondary antibody for 1 h at 26.5 °C. The protein was detected by using the enhanced chemiluminescence detection reagent (GE Healthcare, Little Chalfont, UK) and observed with a Luminescence Image Analysis system (LAS-4000, GE Healthcare). The protein levels were quantified by using Image J software (NIH, Bethesda, MD, USA) and protein percentage was indicated as target protein level/tubulin protein level × 100%.
Cell migration assay
Cell migration was determined by using Millicell cell culture chambers (24-well, 8-μm chambers, Millipore) according to the manufacturer’s instructions. Briefly, the Matrigel was re-hydrated with RPMI 1640 media (1:4) immediately for 1 h before the migration assay. Cells (5 × 104) were suspended in 200 μL serum-free medium then added to the upper chamber of Matrigel-coated filter inserts. After treatment with surfactin, 700 μL RPMI 1640 (containing 10% fetal bovine serum) was added to the bottom well as a chemoattractant. Next, the chambers were incubated for 24 h. Migrated cells attached to the lower surface of the filter. The cells were fixed and stained with 2% ethanol containing 0.2% crystal violet. Migrated cells were counted under a light microscope (40x) (OLYMPUS, IX-71, Tokyo, Japan) and absorbance was measured at 470 nm. The migration percentage was indicated as A470 experimental group/A470 control group × 100%.
Knockdown of HIF-1α
HIF-1α knockdown was performed with specific short hairpin RNAs (shRNAs) delivered by a lentivirus system from the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan) according to the protocol. Control shRNA were produced by using 2.5 μg pLKO.1-Luc, 0.25 μg pMDG, and 2.25 μg pCMV-ΔR8.91 plasmids cotransfected into 293 T cells with Lipofectamine agent (Invitrogen, Carlsbad, CA, USA). Anti-HIF-1α shRNA was produced by using 2.5 μg pLKO.1-HIF-1α, 25 μg pMDG, and 2.25 μg pCMV-ΔR8.91 plasmids cotransfected into 293 T cells with Lipofectamine agent. After 6 h, the medium was replaced with RPMI 1640 containing 1% bovine serum albumin for 24 h. The lentiviral particles with control shRNA or anti-HIF-1α shRNA were collected using a 0.22-μM filter and then stored at −80 °C. For gene knockdown, cells were transduced with the lentiviral particles with 8 μg/mL polybrene. After 24 h, 3 μg/mL puromycin was added to the culture medium and selected for 3 days.
Inhibition of miR-21
Cells were cultured to 50–60% confluence and transfected with a miR-21-5P inhibitor and negative control miRNA inhibitor (Integrated DNA Technologies) by using siLenFectTM lipid reagent (Bio-Rad, Hercules, CA, USA) in serum-free Opti-MEM medium according to the manufacturer’s instructions. The final concentration of the oligomers was 25 nM. After transfection for 24 h, the medium was replaced with fresh RPMI medium containing 10% fetal bovine serum. The levels of miR-21 were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR).
Determination of RNA expression levels
The RNA expression levels of miR-21, HIF1α and were determined by qRT-PCR. The optimized PCR assay of 20 μL PCR volume contained 10 µL of iTaq Universal Probes Supermix, 2 μL of TaqMan Gene Expression Assay, and water to a volume of 20 μL. All reagents were mixed and distributed into a 96-well PCR plate before adding 2 µL of cDNA (1–100 ng). The PCR program was as follows: 95 °C for 30 s, followed by 40 cycles at 95 °C for 1 s and 60 °C for 60 s, during which fluorescence data were collected. Total RNA was extracted using the Purezol kit (Bio-Rad) according to the manufacturer’s protocol. Next, 1 μg of total RNA was used to synthesize cDNA with a cDNA Synthesis kit (Bio-Rad). The expression levels of B2M and HIF1α were quantified by qRT-PCR using the iTaq Universal probe Supermix kit (Bio-Rad) and StepOne plus Real-time PCR system (Applied Biosystems, Foster City, CA, USA). Primers used in this experiment were as follows: HIF1α: 5′-CAACCCAGACA- TATCCACCTC-3′ (forward (F)), 5′-CTCTGATCATCTGACCAAAACTCTA-3′ (reverse (R)). The relative expression level of each gene was calculated by using the 2−ΔΔCt method). All data were obtained from three independent experiments.
Statistical analysis
Data are presented as the mean ± SE from at least three independent experiments. One-way analysis of variance was used to compare the experimental data. Two-way analysis of variance was used to compare data obtained from different treatment concentrations and incubation times. The data were analyzed with SPSS Statistics v18.0 (SPSS, Inc., Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
The present study was supported by grants from the Ministry of Science and Technology, Taiwan (Grant Nos MOST106-2320-B-039-051-MY3 and MOST106-2320-B-039-048) and Ministry of Health and Welfare (MOHW106-TDU-B-212-144-003) and the work was financially supported by the “Drug Development Center, China Medical University” from The Featured Areas Re-search Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Author Contributions
T.S.-W. designed, performed the experiments, analyzed data and wrote the paper. C.W.-W. analyzed the data. C.Y.-W. and R.J.-H. designed the experiments and analyzed the data. Y.R.-P. performed the experiments, analyzed the data. Y.L.-Y. designed the experiments, analyzed the data, and wrote the paper. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39646-9.
References
- 1.Wang D, et al. MicroRNA-30e-3p inhibits cell invasion and migration in clear cell renal cell carcinoma by targeting Snail1. Oncol Lett. 2017;13:2053–2058. doi: 10.3892/ol.2017.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu, N. et al. Percutaneous radiofrequency ablation for renal cell carcinoma vs. partial nephrectomy: Comparison of long-term oncologic outcomes in both clear cell and non-clear cell of the most common subtype. Urol Oncol, 10.1016/j.urolonc.2017.03.014 (2017). [DOI] [PubMed]
- 3.Jin P, Wang J, Liu Y. Downregulation of a novel long non-coding RNA, LOC389332, is associated with poor prognosis and tumor progression in clear cell renal cell carcinoma. Exp Ther Med. 2017;13:1137–1142. doi: 10.3892/etm.2017.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, et al. The Clinicopathological Significance of Epigenetic Silencing of VHL Promoter and Renal Cell Carcinoma: A Meta-Analysis. Cell Physiol Biochem. 2016;40:1465–1472. doi: 10.1159/000453198. [DOI] [PubMed] [Google Scholar]
- 5.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frost J, et al. Potent and selective chemical probe of hypoxic signalling downstream of HIF-alpha hydroxylation via VHL inhibition. Nat Commun. 2016;7:13312. doi: 10.1038/ncomms13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Saez O, Gajate Borau P, Alonso-Gordoa T, Molina-Cerrillo J, Grande E. Targeting HIF-2 alpha in clear cell renal cell carcinoma: A promising therapeutic strategy. Crit Rev Oncol Hematol. 2017;111:117–123. doi: 10.1016/j.critrevonc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, et al. Synergy between von Hippel-Lindau and P53 contributes to chemosensitivity of clear cell renal cell carcinoma. Mol Med Rep. 2016;14:2785–2790. doi: 10.3892/mmr.2016.5561. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, et al. Tubulin cofactor A functions as a novel positive regulator of ccRCC progression, invasion and metastasis. Int J Cancer. 2013;133:2801–2811. doi: 10.1002/ijc.28306. [DOI] [PubMed] [Google Scholar]
- 10.Ji SQ, et al. Down-regulation of CD74 inhibits growth and invasion in clear cell renal cell carcinoma through HIF-1alpha pathway. Urol Oncol. 2014;32:153–161. doi: 10.1016/j.urolonc.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, et al. MEIS1 inhibits clear cell renal cell carcinoma cells proliferation and in vitro invasion or migration. BMC Cancer. 2017;17:176. doi: 10.1186/s12885-017-3155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gryp, T., Vanholder, R., Vaneechoutte, M. & Glorieux, G. p-Cresyl Sulfate. Toxins 9, 10.3390/toxins9020052 (2017). [DOI] [PMC free article] [PubMed]
- 14.Beaumont M, et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. The American journal of clinical nutrition. 2017;106:1005–1019. doi: 10.3945/ajcn.117.158816. [DOI] [PubMed] [Google Scholar]
- 15.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25:1897–1907. doi: 10.1681/asn.2013101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients–protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–337. doi: 10.1111/j.1525-139x.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H. Molecular mechanisms for uremic toxin-induced oxidative tissue damage via a cardiovascular-renal connection. Yakugaku Zasshi. 2013;133:889–895. doi: 10.1248/yakushi.13-00170. [DOI] [PubMed] [Google Scholar]
- 18.Woldu SL, et al. Renal insufficiency is associated with an increased risk of papillary renal cell carcinoma histology. Int Urol Nephrol. 2014;46:2127–2132. doi: 10.1007/s11255-014-0780-4. [DOI] [PubMed] [Google Scholar]
- 19.Satasivam P, et al. Patients with medical risk factors for chronic kidney disease are at increased risk of renal impairment despite the use of nephron-sparing surgery. BJU Int. 2015;116:590–595. doi: 10.1111/bju.13075. [DOI] [PubMed] [Google Scholar]
- 20.Han H, et al. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front Med. 2016;10:320–329. doi: 10.1007/s11684-016-0463-x. [DOI] [PubMed] [Google Scholar]
- 21.Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PloS one. 2012;7:e34026. doi: 10.1371/journal.pone.0034026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang, S. et al. Hypoxia-inducible factor-1alpha activates insig-2 transcription for degradation of HMG CoA reductase in the liver. J Biol Chem, 10.1074/jbc.M117.788562 (2017). [DOI] [PMC free article] [PubMed]
- 23.Polke M, et al. Hypoxia and the hypoxia-regulated transcription factor HIF-1alpha suppress the host defence of airway epithelial cells. Innate Immun. 2017;23:373–380. doi: 10.1177/1753425917698032. [DOI] [PubMed] [Google Scholar]
- 24.Stoyanoff TR, et al. Tumor biology of non-metastatic stages of clear cell renal cell carcinoma; overexpression of stearoyl desaturase-1, EPO/EPO-R system and hypoxia-related proteins. Tumour Biol. 2016;37:13581–13593. doi: 10.1007/s13277-016-5279-4. [DOI] [PubMed] [Google Scholar]
- 25.Xi H, et al. Hypoxia inducible factor-1alpha suppresses Peroxiredoxin 3 expression to promote proliferation of CCRCC cells. FEBS Lett. 2014;588:3390–3394. doi: 10.1016/j.febslet.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, et al. AHNAK2 is a Novel Prognostic Marker and Oncogenic Protein for Clear Cell Renal Cell Carcinoma. Theranostics. 2017;7:1100–1113. doi: 10.7150/thno.18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75:1278–1287. doi: 10.1038/ki.2009.62. [DOI] [PubMed] [Google Scholar]
- 28.Lee, H. M., Hwang, K. A. & Choi, K. C. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol Cell Endocrinol, 10.1016/j.mce.2016.12.026 (2016). [DOI] [PubMed]
- 29.Zhang P, et al. Epithelial-mesenchymal transition is necessary for acquired resistance to cisplatin and increases the metastatic potential of nasopharyngeal carcinoma cells. Int J Mol Med. 2014;33:151–159. doi: 10.3892/ijmm.2013.1538. [DOI] [PubMed] [Google Scholar]
- 30.Song T, et al. MiR-138 suppresses expression of hypoxia-inducible factor 1alpha (HIF-1alpha) in clear cell renal cell carcinoma 786-O cells. Asian Pac J Cancer Prev. 2011;12:1307–1311. [PubMed] [Google Scholar]
- 31.Chen YC, Chien LH, Huang BM, Chia YC, Chiu HF. Aqueous Extracts of Toona sinensis Leaves Inhibit Renal Carcinoma Cell Growth and Migration Through JAK2/stat3, Akt, MEK/ERK, and mTOR/HIF-2alpha Pathways. Nutr Cancer. 2016;68:654–666. doi: 10.1080/01635581.2016.1158292. [DOI] [PubMed] [Google Scholar]
- 32.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 33.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina PP, Slack FJ. MicroRNAs and cancer: an overview. Cell Cycle. 2008;7:2485–2492. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 35.Li X, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor PDCD4 and promotes cell transformation, proliferation, and metastasis in renal cell carcinoma. Cell Physiol Biochem. 2014;33:1631–1642. doi: 10.1159/000362946. [DOI] [PubMed] [Google Scholar]
- 36.Cao J, et al. MicroRNA-21 stimulates epithelial-to-mesenchymal transition and tumorigenesis in clear cell renal cells. Mol Med Rep. 2016;13:75–82. doi: 10.3892/mmr.2015.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu LZ, et al. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PloS one. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song L, et al. MiR-21 modulates radiosensitivity of cervical cancer through inhibiting autophagy via the PTEN/Akt/HIF-1alpha feedback loop and the Akt-mTOR signaling pathway. Tumour Biol. 2016;37:12161–12168. doi: 10.1007/s13277-016-5073-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, et al. Human Stem Cells Overexpressing miR-21 Promote Angiogenesis in Critical Limb Ischemia by Targeting CHIP to Enhance HIF-1alpha Activity. Stem Cells. 2016;34:924–934. doi: 10.1002/stem.2321. [DOI] [PubMed] [Google Scholar]
- 40.Rossi M, et al. Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: an observational study. Nutr Metab Cardiovasc Dis. 2014;24:1035–1042. doi: 10.1016/j.numecd.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Lin CJ, et al. P-cresyl sulfate is a valuable predictor of clinical outcomes in pre-ESRD patients. Biomed Res Int. 2014;2014:526932. doi: 10.1155/2014/526932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue T, et al. Genomic profiling of renal cell carcinoma in patients with end-stage renal disease. Cancer Sci. 2012;103:569–576. doi: 10.1111/j.1349-7006.2011.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi SS, et al. Clear cell papillary renal cell carcinoma: a clinicopathological study emphasizing ultrastructural features and cytogenetic heterogeneity. Int J Clin Exp Pathol. 2013;6:2936–2942. [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto S, et al. Continuous Reduction of Protein-Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci Rep. 2015;5:14381. doi: 10.1038/srep14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CT, et al. Effects of AST-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014;37:76–83. doi: 10.1159/000357641. [DOI] [PubMed] [Google Scholar]
- 46.Dong A, Shen J, Zeng M, Campochiaro PA. Vascular cell-adhesion molecule-1 plays a central role in the proangiogenic effects of oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14614–14619. doi: 10.1073/pnas.1012859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamberti MJ, et al. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy. PloS one. 2017;12:e0177801. doi: 10.1371/journal.pone.0177801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krick S, et al. Hypoxia-driven proliferation of human pulmonary artery fibroblasts: cross-talk between HIF-1alpha and an autocrine angiotensin system. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:857–859. doi: 10.1096/fj.04-2890fje. [DOI] [PubMed] [Google Scholar]
- 49.Covello KL, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schonenberger D, et al. Formation of Renal Cysts and Tumors in Vhl/Trp53-Deficient Mice Requires HIF1alpha and HIF2alpha. Cancer Res. 2016;76:2025–2036. doi: 10.1158/0008-5472.can-15-1859. [DOI] [PubMed] [Google Scholar]
- 51.Biswas S, et al. Effects of HIF-1alpha and HIF2alpha on Growth and Metabolism of Clear-Cell Renal Cell Carcinoma 786-0 Xenografts. J Oncol. 2010;2010:757908. doi: 10.1155/2010/757908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doonachar A, et al. Differential effects of HIF-alpha isoforms on apoptosis in renal carcinoma cell lines. Cancer Cell Int. 2015;15:23. doi: 10.1186/s12935-015-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan Y, et al. Dicer suppresses the malignant phenotype in VHL-deficient clear cell renal cell carcinoma by inhibiting HIF-2alpha. Oncotarget. 2016;7:18280–18294. doi: 10.18632/oncotarget.7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, et al. Epigenetic regulation of HIF-1alpha in renal cancer cells involves HIF-1alpha/2alpha binding to a reverse hypoxia-response element. Oncogene. 2012;31:1065–1072. doi: 10.1038/onc.2011.305. [DOI] [PubMed] [Google Scholar]
- 55.Raval RR, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/mcb.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hicks KC, Patel TB. Sprouty2 Protein Regulates Hypoxia-inducible Factor-alpha (HIFalpha) Protein Levels and Transcription of HIFalpha-responsive Genes. J Biol Chem. 2016;291:16787–16801. doi: 10.1074/jbc.M116.714139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding XF, Zhou J, Hu QY, Liu SC, Chen G. The tumor suppressor pVHL down-regulates never-in-mitosis A-related kinase 8 via hypoxia-inducible factors to maintain cilia in human renal cancer cells. J Biol Chem. 2015;290:1389–1394. doi: 10.1074/jbc.M114.589226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, et al. Physapubescin selectively induces apoptosis in VHL-null renal cell carcinoma cells through down-regulation of HIF-2alpha and inhibits tumor growth. Sci Rep. 2016;6:32582. doi: 10.1038/srep32582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoenfeld AR, et al. The von Hippel-Lindau tumor suppressor gene protects cells from UV-mediated apoptosis. Oncogene. 2000;19:5851–5857. doi: 10.1038/sj.onc.1203985. [DOI] [PubMed] [Google Scholar]
- 60.Zhang KL, et al. Blockage of a miR-21/EGFR regulatory feedback loop augments anti-EGFR therapy in glioblastomas. Cancer Lett. 2014;342:139–149. doi: 10.1016/j.canlet.2013.08.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.