Abstract
Wall teichoic acid (WTA) are major constituents of Staphylococcus aureus (S. aureus) cell envelopes with important roles in the bacteria’s physiology, resistance to antimicrobial molecules, host interaction, virulence and biofilm formation. They consist of ribitol phosphate repeat units in which the ribitol residue is substituted with D-alanine (D-Ala) and N-acetyl-D-glucosamine (GlcNAc). The complete S. aureus WTA biosynthesis pathways was recently revealed with the identification of the two glycosyltransferases, TarM and TarS, respectively responsible for the α- and β-GlcNAc anomeric substitutions. We performed structural analyses to characterize WTAs from a panel of 24 S. aureus strains responsible for invasive infections. A majority of the S. aureus strains produced the β-GlcNAc WTA form in accordance with the presence of the tarS gene in all strains assessed. The β-GlcNAc anomer was preferentially expressed at the expense of the α-GlcNAc anomer when grown on stress-inducing culture medium containing high NaCl concentration. Furthermore, WTA glycosylation of the prototype S. aureus Newman strain was characterized in vivo in two different animal models, namely peritonitis and deep wound infection. While the inoculum used to infect animals produced almost exclusively α-GlcNAc WTA, a complete switch to β-glycosylation was observed in infected kidneys, livers and muscles. Overall, our data demonstrate that S. aureus WTA glycosylation is strongly influenced by environmental conditions and suggest that β-GlcNAc WTA may bring competitive advantage in vivo.
Introduction
Staphylococcus aureus (S. aureus) is frequently a commensal symbiont in the human host, and one of the most successful opportunistic pathogens causing severe infections worldwide. The bacteria has acquired the ability to manipulate and evade host immune surveillance and responses1,2. Infection with S. aureus can cause a range of symptoms, from relatively minor skin abscesses/boils to fully disseminated disease including, endocarditis, sepsis and toxic shock syndrome. Invasive disease is associated withc ≥20% mortality rate3.
S. aureus is surrounded by cell surface polysaccharides, including capsular polysaccharides (CPs) and teichoic acids (TAs)4. There are two types of TAs: lipo-TAs (LTA), which are anchored in the cytoplasmic membrane, and cell wall TAs (WTAs), which are covalently linked to peptidoglycan in the bacterial cell wall.
WTAs contribute to staphylococcal adhesion and colonization5,6, and play a role in cell division and biofilm formation7, and their overexpression increases S. aureus virulence8. In addition, D-alanine (D-Ala) residues on TAs contribute to resistance to cationic antimicrobial peptides such as defensins or cathelicidins, and to glycopeptide antibiotics such as vancomycin or teicoplanin9–11.
There is substantial variation in the chemical structure of WTAs among Gram-positive bacteria12. S. aureus WTAs consist of poly(ribitol-phosphate) substituted at the O-2 and O-4 positions of the ribitol residue with D-Ala and α- or β-N-acetylglucosamine (GlcNAc), respectively13–15. S. aureus WTAs have been considered as putative vaccine candidate4,16. The pioneer work in that field was conducted by Nabi Biopharmaceuticals with Antigen 336 (Ag336), also named Polysaccharide 336 (PS336). Ag336 was purified from a strain deposited at ATCC under number ATCC 55804 and used to serotype S. aureus isolates that do not express capsule17. Ag336 was reported as a cell surface polysaccharide consisting of ribitol, GlcNAc and phosphate, and hence reveals to be WTA. However, in contrast to conventional WTA the ribitol residue of Ag336 is substituted at the O-3 and not O-4 position with β-GlcNAc18.
Although S. aureus WTA glycosylation with α- and β-GlcNAc was reported in the 1960s, the glycosyltransferases responsible, TarM and TarS, were only identified recently19,20, While TarM is α-glycosyltransferase, TarS is a β-glycosyltransferase that glycosylates the O-4 of ribitol respectively. The tarS gene is present in near all sequenced S. aureus genomes, with very few exceptions, whereas tarM is absent in a number of strains such as strains of CC5 and the emerging clonal complex CC39821. In general, the presence or absence of the tarS, and tarM genes in S. aureus strains has been used to infer their WTA glycosylation pattern19,21–24. While the presence of the tarM or tarS genes can be easily detected by polymerase chain reaction (PCR), high-throughput structural analyses of WTA, and more specifically WTA glycosylation, remain challenging. Purified WTA samples and nuclear magnetic resonance (NMR) spectroscopy are required, rendering the identification of glycosylation pattern both laborious and time-consuming.
In addition, the potential of individual S. aureus strains to regulate the expression of both α- and β-GlcNAc anomers under different environmental conditions has not been assessed.
Herein, we report structural analyses by a High-Performance Anion-Exchange Chromatography (HPAEC)-based method that has allowed us to characterize WTAs from a panel of 24 S. aureus strains responsible for invasive infections, grown in vitro under normal and stress-inducing culture conditions. We show that a majority of the S. aureus strains produced the β-GlcNAc WTA form, which is consistent with the presence of tarS gene in most of the strains, and we present evidence that S. aureus is able to modify the phenotype of its WTA glycosylation pattern by exposure to environmental stress-inducing culture conditions. Furthermore, we report for the first time the characterization of WTA directly on infected mouse tissues without any purification or bacteria culture step, and we show a preferential switch to β-glycosylation of WTAs in vivo using two mouse infection models, with the Newman strain as prototype strain.
Results
The panel of strains selected is representative for Staphyloccus aureus clinical diversity
In order to study the extent of variation of WTA composition among S.aureus strains, a panel of 24 strains isolated from 1905 to 2005 was selected (Table 1). The genetic diversity and representativeness of the panel was firstly illustrated by the presence of the four accessory gene regulator agr groups. The agr locus controls the expression of genes for virulence factors that play a major role in the virulence of S. aureus. Expression of these genes is controlled by the accessory gene regulator (agr) locus25,26. This locus is recognized as a quorum-sensing system in S. aureus27. Based on polymorphisms in agrB, agrC and agrD genes, four major allelic agr groups (I, II, III and IV)27,28 have been characterized, each associated with specific staphylococcal disease25. Secondly, as the adaptation of S. aureus to the environment has been marked by the acquisition of methicillin-resistance, the collection included both MRSA and MSSA strains as predicted by the detection of the mecA gene29. Thirdly, the chosen panel included the two major capsular genotypes (5 and 8) among S. aureus isolates causing human infections30,31. Fourthly, the strains are representative of the various types of infection as they were isolated from patients with eight different diseases. Finally, we characterized the strains for the presence of WTA glycosyltransferase tarS and tarM genes. All the strains displayed the tarS gene. The tarM gene was present in 11 out of 24 strains representing 45.8% of the panel. Moreover the sequence of tarS and tarM were determined for strains HH0528 1156, HT2005 0756, HT2005 0843 and was found to be identical to the reference sequences.
Table 1.
Staphylococcus aureus strains used in the study.
| Strain name | geographic | isolation | type | genetic characterization* | Source | ||||
|---|---|---|---|---|---|---|---|---|---|
| origin | date | of infection | cap | agr type | mecA | tarS | tarM | ||
| HT2005 0667 | France | 2005 | septic shock | 5 | 2 | S | + | − | HEH, Lyon |
| HT2005 0769 | France | 2005 | pneumonia | 5 | 2 | S | + | − | HEH, Lyon |
| HT2005 0699 | France | 2005 | osteomyelitis | 5 | 2 | R | + | − | HEH, Lyon |
| HT2005 0749 | France | 2005 | toxic shock | 5 | 2 | R | + | − | HEH, Lyon |
| HT2005 0847 | France | 2005 | skin | 5 | 2 | S | + | − | HEH, Lyon |
| HT2005 0659 | France | 2005 | diarrhea | 5 | 2 | S | + | − | HEH, Lyon |
| HT2005 0742 | France | 2005 | septic shock | 5 | 1 | S | + | − | HEH, Lyon |
| HT2005 0499 | France | 2005 | respiratory | 5 | 1 | S | + | − | HEH, Lyon |
| HT2005 0662 | France | 2005 | foodborne | 5 | nd | S | + | − | HEH, Lyon |
| HT2005 0704 | France | 2005 | skin | 8 | 4 | R | + | + | HEH, Lyon |
| HT2005 0828 | France | 2005 | skin | nd | 3 | S | + | + | HEH, Lyon |
| HT2005 0702 | France | 2005 | skin | 5 | 4 | S | + | + | HEH, Lyon |
| HT2005 0689 | France | 2005 | osteomyelitis | 5 | 1 | R | + | + | HEH, Lyon |
| HT2005 0756 | France | 2005 | skin | 5 | 1 | R | + | + | HEH, Lyon |
| HT2005 0837 | France | 2005 | septic shock | 5 | 1 | S | + | + | HEH, Lyon |
| HT2005 0726 | France | 2005 | osteomyelitis | 8 | 2 | S | + | + | HEH, Lyon |
| HT2005 0843 | France | 2005 | skin | 5 | 1 | R | + | + | HEH, Lyon |
| HH0528 1156 | France | 2005 | septic shock | 5 | nd | R | + | + | HEH, Lyon |
| ATCC 55804 | Unknown | Unknown | urinary | 5 | nd | S | + | − | ATCC |
| Newman | UK | 1952 | osteomyelitis | 5 | nd | S | + | + | ATCC |
| Reynolds | USA | 1982 | septic shock | 5 | nd | S | + | − | CIP |
| SA113 | UK | 1943 | unknown | 5 | nd | S | + | + | ATCC |
| Wood46 | Australia | 1905 | skin | 5 | nd | S | + | − | ATCC |
| Wright | USA | 1979 | bacteremia | 8 | nd | S | + | − | CIP |
A chromatographic method was developed to rapidly determine WTA structures
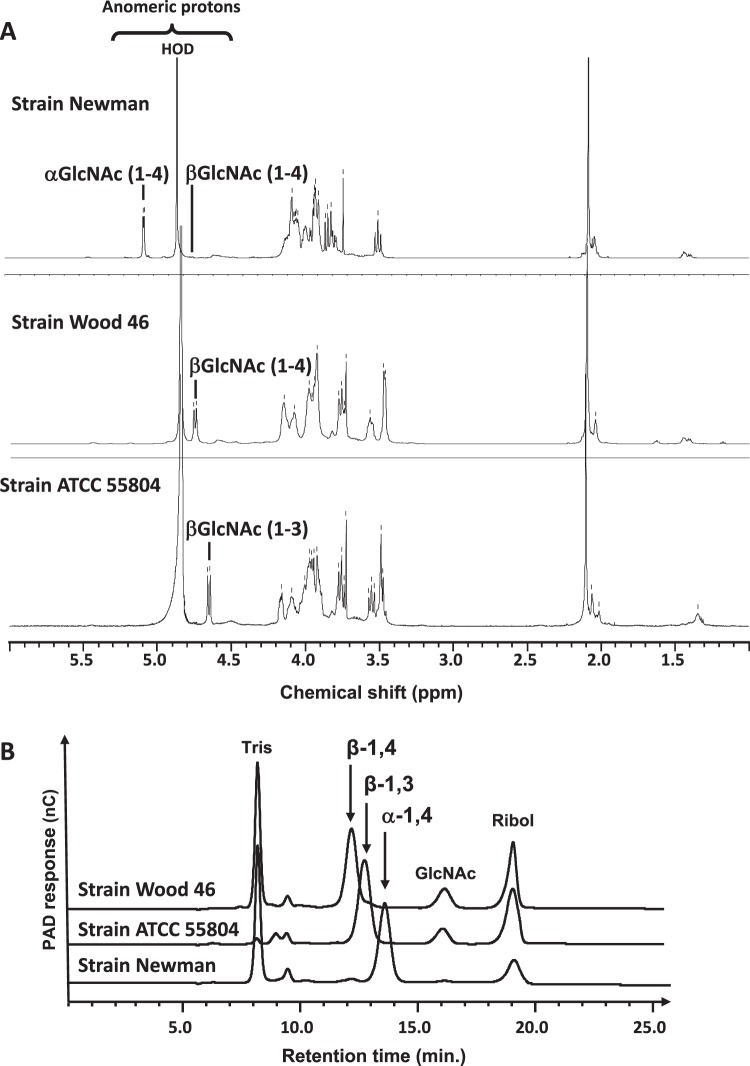
WTAs were extracted and purified from the Newman, Wood 46 and ATCC55804 strains grown in a complex commercially available medium (tryptic soy broth, TSB). Analysis of the monosaccharide composition of the WTAs by High-Performance Anion-Exchange Chromatography with Pulsed Amperometry Detection (HPAEC-PAD) showed the presence of ribitol and glucosamine. It should be noted that GlcNAc is transformed to Glucosamine (GlcN) under the conditions used for WTA hydrolysis. The GlcN/Ribitol molar ratio was found to be 1.06, 1.09, and 1.08 for Newman, Wood46 and ATCC 55804 WTA analysis, respectively (Supplementary Table S1). Proton nuclear magnetic resonance spectra (Fig. 1A) were consistent with a 1,5-poly(ribitol phosphate) polymer in which the ribitol had been substituted by N-acetyl-D-glucosamine (GlcNAc). In accordance with previous reports19,32, the chemical shifts of the GlcNAc anomeric proton showed that WTAs of the Newman and Wood 46 strains were substituted at the O-4 position of ribitol with α-GlcNAc (5.07 ppm) and β-GlcNAc (4.75 ppm), respectively. As reported by Fattom et al.18, the proton NMR spectra of β-GlcNAc(1–4) WTA of the Wood 46 and ATCC 55804 strains were similar and showed a major difference in the chemical shifts of their respective anomeric proton (4.75 ppm for Wood 46 versus 4.66 ppm for ATCC55804; upfield shift of 0.09 ppm), indicating that the ATCC 55804 WTA (Ag336) was substituted with β-GlcNAc (4.66 ppm) at the O-3 position of the ribitol residue.
Figure 1.
Glycosylation pattern of the Newman, Wood 46 and ATCC55804 WTAs. (A) 500 MHz 1H NMR spectra of purified WTAs in D2O at 293 K; (B) HPAEC-PAD chromatograms of GlcNAc-ribitol disaccharides obtained from S. aureus WTA HF-hydrolysis of the Wood 46 strain (β-D-GlcNAc-(1→4)-ribitol), ATCC55804 strain (β-D-GlcNAc-(1→3)-ribitol) and Newman (α-D-GlcNAc-(1→4)-ribitol) strains. GlcNAc: N-acetyl-glucosamine, Ribol: Ribitol, Tris: Tris buffer in samples.
To further obtain quick and efficient structural information on WTAs of several strains, an HPAEC-PAD method was developed. The three purified WTAs were subjected to aqueous hydrogen fluoride (HF) treatment. This resulted in quantitative hydrolysis of phosphodiester bonds in the polysaccharides and the release of GlcNAc-Ribitol disaccharides, which could then be separated on a Carbopac MA1 column using HPAEC-PAD. Each disaccharide differing in GlcNAc glycosidic linkage eluted at a specific retention time: β-D-GlcNAc-(1→4)-ribitol at 12.1 min, β-D-GlcNAc-(1→3)-ribitol at 12.8 min and α-D-GlcNAc-(1→4)-ribitol at 13.5 min (Fig. 1B), along with some GlcNAc and ribitol residues due to acid-lability of the glycosidic bond in the GlcNAc-ribitol moiety in 48% HF33. The ribitol peak area was higher in the chromatograms of β-D-anomers of WTAs as β-D-anomers hydrolyzed more rapidly than α-D-anomers, as reported by Jennings and Lugowski33.
In addition, the HPAEC-PAD method was used to evaluate the proportion of α-GlcNAc(1–4) and β-GlcNAc(1–4) WTAs produced. The proportion was determined from the peak area of each structure relative to the sum of peak areas of all structures detected in the chromatogram (see formulas in Supplementary Methods). The consistency of the method was assessed from 5 independent experiments with purified WTAs of the Newman strain, which revealed to produce 98% and 2% of α-GlcNAc(1–4) and β-GlcNAc(1–4) WTAs, respectively. The Relative Standard Deviation was found at 0.5% for the α-GlcNAc(1–4) WTA (Supplementary Fig. S1). The accuracy of the method was further confirmed by proton NMR analyses of purified WTAs from 4 strains representative of the various proportions of WTAs found in our strain collection. The percentages of α-GlcNAc(1–4) and β-GlcNAc(1–4) WTAs were determined from the integration values of the anomeric protons and found to be similar to HPAEC-PAD results (Supplementary Figs S2 and S3). Therefore, although ribitol residue could be seen on chromatograms, the amount of released ribitol was negligible and had no impact on the calculation of α- and β-GlcNAc anomers.
The purified WTAs of the Newman, Wood 46 and ATCC55804 strains were further used as reference WTA samples for carbotyping of the other panel S. aureus strains.
A majority of the Staphylococcus aureus strains tested produces β-GlcNAc(1-4) WTA
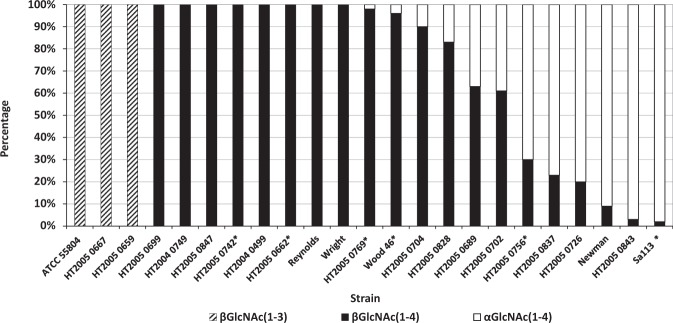
The structure of WTAs from the panel of 24 S. aureus strains was determined by HPAEC-PAD carbotyping using HF-treated bacteria grown on TSB and the proportion of α- and β-GlcNAc WTAs was calculated as described above and in Supplementary Methods. Two independent experiments were performed for 6 out of the 24 strains tested (Supplementary Fig. S4); for the remaining 18 strains only single experiments were performed. Structural variations were observed in WTAs but the majority of strains produced β-GlcNAc WTA (Fig. 2) on TSB. Across the 13 strains possessing only the tarS gene (Table 1), 10 strains exclusively substituted the hydroxyl at position 4 of the ribitol residue with β-GlcNAc while three strains substituted the hydroxyl at position 3 with β-GlcNAc. In the 11 other strains, which possessed both tarS and tarM genes, a mix of both structures was found in nine strains in various proportion, but with higher relative proportions of β-GlcNAc WTAs in four strains. Only two strains produced α-GlcNAc WTAs exclusively.
Figure 2.
Distribution of WTA structures determined by HPAEC-PAD carbotyping of a panel of 24 S. aureus strains grown in TSB. The structures were determined directly from cell growth. The proportion of WTA structures in each strain, calculated as percentage, was determined from a single or two independent experiments for 18 and 6 strains, respectively, as indicated by an asterisk. For the 6 strains, the average percentage values are represented. The percentage was calculated from peak areas using the formulas described in the Supplementary Methods.
α- and β-glycosylation of S. aureus WTA depends on growth media
Eight strains representative of each S. aureus WTA glycosylation pattern and for the presence (five strains) or absence (three strains) of the tarM gene were selected and grown on high-NaCl-containing growth medium (SATA-2)34. The structure of WTAs was determined as previously described and compared to that of bacteria grown under normal conditions (TSB). Modification of the glycosylation pattern was observed for six strains (Fig. 3). Out of the five strains possessing both the tarM and tarS genes, four strains had up to 75–90% increased proportion of β-GlcNAc WTA. Interestingly, the HT2005 0667 and HT2005 0769 strains, which produced exclusively β-GlcNAc-glycosylated WTA at position 3 of the ribitol residue when grown in TSB, switched to a mix of WTAs glycosylated with β-GlcNAc glycosylation either at position 3 or 4 when grown on SATA-2.
Figure 3.
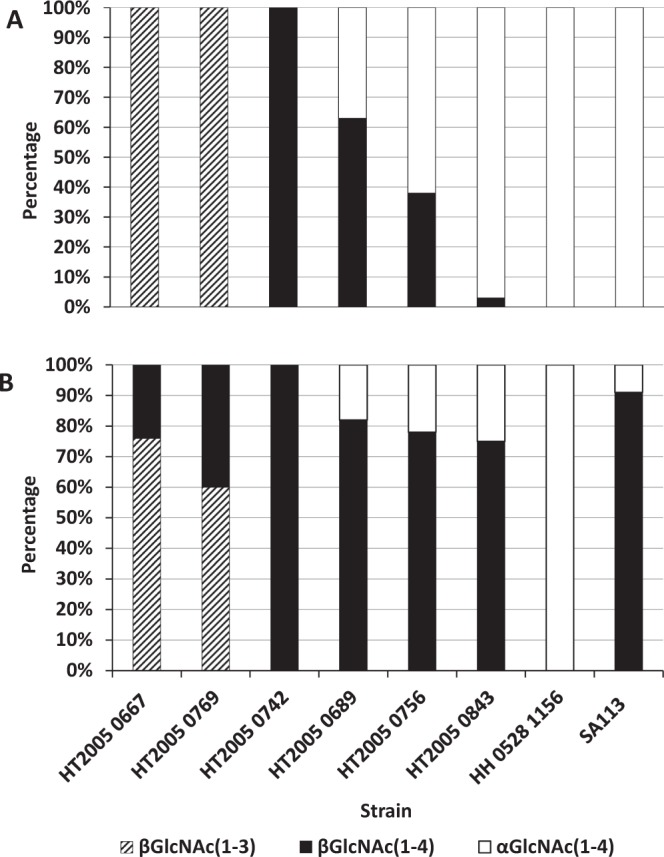
Comparison of WTA structure distribution of 8 S. aureus strains grown in (A) TSB and (B) SATA media. The structures were determined by HPAEC-PAD carbotyping directly from cell growth in the same HPAEC-PAD analysis. The proportion of WTA structures in each strain (calculated as percentage) was determined from single experiments and calculated from peak areas using the formulas described in the Supplementary Methods.
The Newman strain switches from α-GlcNAc WTA glycosylation in vitro to β-glycosylation in vivo
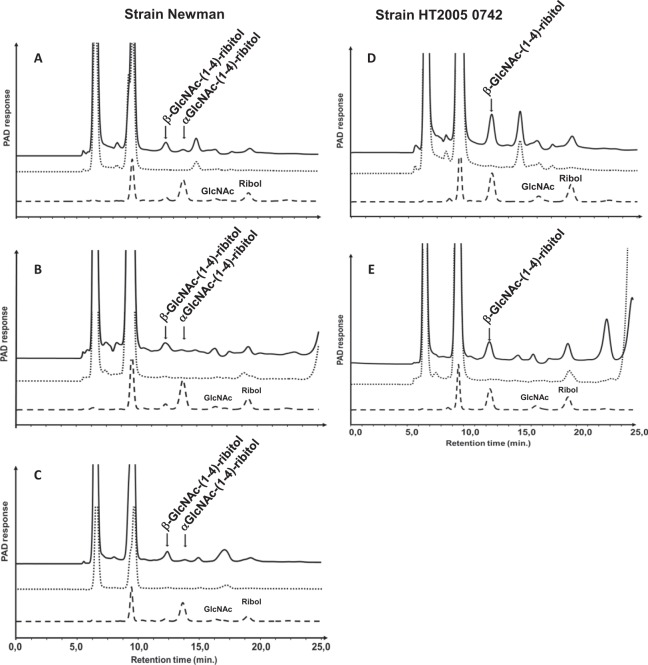
To assess whether structural changes in WTAs also occurred in vivo, two mouse infection models were carried out: the peritonitis model and the deep wound infection model. The Newman strain was used as a prototype strain, as the strain has been shown to produce almost exclusively α-GlcNAc WTA (>90%) under in vitro normal growth conditions despite the presence of both the tarM and tarS genes in the strain (Table 1; Fig. 2). In the peritonitis model, a group of five mice was infected by intraperitoneal route with a non-lethal dose of the Newman strain. Another group of five mice was infected with the HT2005 0742 strain as a control strain producing β-GlcNAc WTA, regardless the in vitro growth conditions (Fig. 3). In the deep wound infection model, one group of five mice was infected with the Newman strain.
Bacterial inocula were obtained from cultures grown on TSB. The structures were analyzed before infection and proven to be exclusively β-GlcNAc WTA in the HT2005 0742 strain, or predominantly α-GlcNAc WTA (>90%) in the Newman strain.
The structure of WTAs was determined by HPAEC-PAD directly on infected tissues without any WTA purification or bacteria culture step from infected organs. Figure 4 shows representative results obtained for each infected organ and each strain. In infected kidneys and livers from the peritonitis model, there was no change in the WTA structure of the HT2005 0742 strain, which retained the β-anomer, in agreement with the presence of the tarS gene only in this strain. In contrast, the WTA structure changed from the α- to the β-GlcNAc anomer in the Newman strain indicating that expression of the later was favored in vivo (Fig. 4). The expression of the β-GlcNAc anomer in the Newman strain was also demonstrated in infected muscles from the deep wound model.
Figure 4.
HPAEC-PAD analyses of WTA from infected mouse organs: kidneys (A,D), livers (B,E), and muscles (C). Chromatograms of GlcNAc-ribitol disaccharides obtained from mouse infected (solid line) with the S. aureus Newman (A–C) and HT2005 742 (D,E) strains compared to chromatograms of disaccharides obtained from the strains grown in TSB and used for mouse infection (dashed line). Samples from kidney of naïve mice were analyzed in parallel as negative reference (dotted line).
Discussion
The WTAs of most S. aureus strains are polysaccharides containing 11–40 ribitol phosphate repeat units with the ribitol residue substituted with D-alanine and GlcNAc at the O-2 and O-4 position, respectively. Earlier studies had revealed strain-specific pattern of α- and β-GlcNAc substitution at position O-4 of the ribitol residue13–15. Interestingly, WTAs isolated from the ATCC 55804 and N315 strains were found to display β-GlcNAc glycosylation at the O-3 position of the ribitol residue18,35 (the present study). An alternative glycosyltransferase, TarP, was recently identified to be responsible for this β-GlcNAc glycosylation35.
The N-acetylglucosaminyl-ribitol linkage of WTA is an immunological determinant in host immune responses; the specificity of elicited antibodies is dependent on the α- or β-GlcNAc anomeric form36 and possibly on the position in the ribitol residue. To design an efficient WTA vaccine antigen, it is crucial to determine the exact WTA glycosylation pattern of a diverse range of staphylococcal strains responsible for human infections, in order to identify the most representative structure so as to ensure the broadest coverage among invasive strains. To date, the distribution of the various WTA structures from a diverse panel of invasive S. aureus strains has never been reported.
In the present study, we selected 24 S. aureus strains representative of clinical isolates. The strains were characterized for the presence of genes encoding the two TarM and TarS glycosyltansferases responsible for modifying WTA with α-GlcNAc and β-GlcNAc respectively19,20. The tarS gene was found in almost all strains, while tarM was found in less than 50% of the strains but always in association with tarS. This is in agreement with previously reported distribution of tarS and tarM on two different panel of strains, where tarS was absent on only one strain and tarM present on 36%24 or 55% of the strains studied21, respectively. In accordance with the presence of the tarS gene in all strains studied, the β-GlcNAc WTA anomeric form was predominant in S. aureus clinical isolates. Brown et al.20 reported that tarS but not tarM expression levels were strongly upregulated by oxacillin treatment, which suggest a role for TarS in β-lactam resistance and highlights the importance of growth environment on gene expression. Our results provide further evidence of environmental influence on α- and β-glycosylation. We have demonstrated on eight strains that in vitro stress-inducing growth conditions (SATA-2 medium containing high NaCl concentration) favor the production of the β-GlcNAc anomer at the expense of the α anomer. Out of the 5 strains displaying both tarM and tarS genes, only one strain (HH0528 1156 strain) did not modify its glycosylation pattern and kept producing exclusively α-GlcNAc WTA. Full genome sequencing was performed on this strain and indicated that both tarS and tarM gene were not mutated and had the potential to express functional enzymes. Our data thus indicate that genetic characterization alone may not accurately reflect the phenotype of WTAs and underline the importance of determining the actual glycotype.
The β-glycosylation of WTAs has been reported at either the O-3 position or the O-4 position 4 of ribitol4,35. We identified three strains (12.5% of the strain tested) that glycosylated exclusively WTA at position 3 when grown in a complex, commercial medium (TSB). Two of these strains (HT2005 0667 and HT2005 0769) were also grown on SATA-2 and interestingly, while the tarP gene has been found dominant over tarS35, their glycosylation pattern was modified to β-GlcNAc glycosylation at both position 3 and 4. Our study demonstrates that S. aureus can modulate the relative amounts of α- and β-glycosylation depending on environmental conditions, as well as the position of β-GlcNAc on ribitol. However, the regulation system that controls the full WTA glycoprofile remains to be determined. By in silico genome scanning, tarM was identified as part of the two-component GraRS regulon and to be positively regulated by this system in vivo37. Although GraRS, was shown to sense and confer resistance to selected cationic antimicrobial peptides38, it was also suggested that this system may respond to other signals like oxidative stress37. While the structure of TarS and TarM have been fully elucidated39,40, their gene regulation remains to our knowledge elusive. Of note, it has been reported that tarS but not tarM expression was strongly upregulated by oxacillin (β-lactam) treatment20.
Recent studies have emphasized the biological significance of the β-GlcNAc WTA anomeric configuration over the α-GlcNAc, and have provided indirect indications of the in vivo preferential selection of the β-form22,41,42. However, none of these studies directly assessed the structure and the diversity of the WTAs expressed. Human sera contain high levels of antibodies directed against S. aureus WTA22,42–44. Anti-β-GlcNAc WTA-IgG level is higher than that of anti-α-GlcNAc WTA-IgG in pooled human IgG fractions and in intact sera from healthy adults and infants22 suggesting that the β-GlcNAc anomer is an immunodominant antigen in staphylococcal infections. More specifically, β-GlcNAc WTA residues are required for induction of anti-WTA IgG-mediated C3 deposition and opsonophagocytosis22,41. Thus, Kurokowa et al.23 hypothesized that β-GlcNAc WTA might be more antigenic than α-GlcNAc WTA or that the β form may be more stable than the α form in vivo.
In the present study, we report for the first time the characterization of WTA directly on infected mouse tissues without any purification or bacteria culture step. Similar to what is observed in humans, S. aureus can induce a diverse spectrum of diseases in mice45 and therefore, two mouse infection models were used. Our study provides direct evidence that in vivo environmental conditions lead preferentially to β-glycosylation of the ribitol residue as stress-inducing culture conditions (high NaCl concentration) does in vitro. The upregulation of β-GlcNAc anomer in the Newman strain was observed in the peritonitis (livers and kidneys) and skin-wound infection (quadriceps muscles) models. While this strain displayed both functional TarS and TarM, only β-glycosylated WTAs were recovered from mice infected organs. Those results are consistent with reports showing that the level of anti-β-GlcNAc WTA antibodies is higher than that of anti-α-GlcNAc WTA antibodies in human sera22.
Dorling et al.46 has proposed that WTA itself would be involved in the evasion of immune recognition, while D-alanylation of WTA would be involved in mediating infection persistence. D-alanylation of TA is decreased when S. aureus are grown in medium containing high NaCl concentration due to the transcriptional repression of the dltABC operon47. Therefore, the decrease of D-alanylation may lead to a decrease of S. aureus resistance to cationic antimicrobial peptides. In that context, S. aureus may upregulates WTA β-glycosylation to overcome the decrease of infection persistence. Indeed, β-glycosylation of WTA is critical for the resistance of S. aureus MRSA strains to β-lactam20 and WTA linkage to peptidoglycan (PG) contributes to the resistance of S. aureus to lysozyme48. These observations support the hypothesis that β-glycosylated WTA could sterically hindered PG, preventing its enzymatic hydrolysis and release of its fragments. Furthermore, heterogeneous Vancomycin-Intermediate S. aureus (hVISA) and MRSA strains are more resistant than Methicilline Sensitive S. aureus (MSSA) strains to opsonophagocytosis and killing by phagocytes in the presence of low concentrations of serum49. Although a role of capsular polysaccharide that has been previously shown to prevent nonspecific killing of S. aureus cannot be excluded50, a molecular epidemiology study conducted from 91 S. aureus isolates from 2004 to 2005 showed that the majority of MRSA isolates, including the most prevalent Community Acquired-MRSA clone, USA300, were unencapsulated51. We suggest that resistance to opsonophagocytosis in MRSA strains may be primarily related to WTA β-glycosylation. In addition, Gautan et al.52 recently reported that WTAs serve as a barrier against opsonin recruitment to the cell wall and contribute to repulsion of peptidoglycan-targeted antibodies. Thus, we propose that expression of WTA β-glycosylation is one of the immune evasion strategies of S. aureus to resist to immune host defense.
In conclusion, the present study provides significant insight into the structural glycosylation diversity of WTAs among S. aureus strains, and demonstrates environmental influence in α- and β-glycosylation of WTAs both in vitro and in vivo. These findings, taken together with previous reports20,22,49,52 suggest that S. aureus with β-GlcNAc WTA may provide a competitive advantage during infection and support β-GlcNAc WTA as an appropriate target for vaccine-based immunotherapy/prophylaxis against invasive S. aureus infections.
Materials and Methods
Bacterial strains, growth conditions and genetic characterization
Overall 24 S. aureus strains were included in the study (Table 1). Most of them (18 strains) were 2005 clinical isolates (HT) from the French National reference Center for Staphylococci (Lyon, France) and were kindly provided by Prof Jérome Etienne (Hôpital Edouard Herriot, Lyon, France) along with their agr allele, capsular and methicillin resistance genotypes characterization34. Seven prototype strains were also included from three different sources: American type Culture Collection (ATCC), Institut Pasteur collection (CIP) and NIAID repository. The presence or absence of S. aureus WTA glycosyltransferase genes tarS and tarM was verified by PCR using gene-specific primers as described previously24. The sequence of tarS and tarM genes were determined for strains HH0528 1156, HT2005 0756, HT2005 0843. DNA was extracted from colonies grown overnight on TSB agar (Difco BD, Pont de Claix, France) by using the GenElute™ Bacterial Genomic DNA Kit Protocol (NA2110-1KT; Sigma-Aldrich, St Quentin Fallavier, France) in presence of Lysostaphin (L7386; Sigma-Aldrich, St Quentin Fallavier, France). The quantity and quality of the genomic DNA were measured with the Nanodrop Spectrophotometer (Thermo Scientific, Waltham, MA), Qubit 3.0 Fluorometer (Life Technologies, Thermo Fischer Scientific) and Dropsense (Trinean, Gentbrugge, Belgium), and by gel electrophoresis. 1 ng of gDNA was used for preparing library with the Nextera XT DNA Library Preparation kit (FC-131-1024-Illumina) containing specific indexes for each library. Briefly, DNA was simultaneously fragmented and tagged with sequencing adapters, followed by an end repair, an A-tailing and the ligation of adaptors containing indexes. After a short amplification step, libraries were purified and then sequenced. The quantity and quality of the libraries were measured with the Qubit 3.0 Fluorometer (Life Technologies) and 2100 bioanalyzer System (Agilent, Santa Clara, CA). Sequencing was performed on MiSeq sequencer (Illumina, San Diego, CA) with the v2 Reagent Kit (MS-102-2002; Illumina) to generate 2 × 150 bp paired-end reads in a batch of 4 samples on the flow-cell. Raw data Quality Control was performed with FastQC (Babraham Bioinformatics, Cambridge, UK). The reads were trimmed and de novo assembled with CLCGenomics Workbench (v8.5; Qiagen, Redwood City CA). Blast was used to retrieve tarS gene sequence in the assembled contigs.
For teichoic acid analysis, bacterial strains were grown either in tryptic soy broth (TSB, Difco), or proprietary medium SATA-2 (93 g/L wheat peptone, 0.25 g/L D-glucose, 41 g/L NaCl and 15 g/L MgCl2) initially developed to increase capsular expression through high concentration of NaCl34.
WTA purification and characterization
WTAs were extracted and purified from the Newman, Wood 46 and ATCC55804 strains grown in 500-mL culture of TSB for 24 h at 37 °C. The cultures were inactivated by treatment with phenol-ethanol (1:1, v/v) to a final concentration of 2%. The cells were collected by centrifugation at 5,000 × g for 1 hour at 4 °C and suspended in 0.05 M Tris, 2 mM MgSO4 pH 7.5 (0.5 g wet weight/mL). The cell suspensions were incubated with lysostaphin (100 µg/mL) at 37 °C for 3 hours with continuous stirring. MgCl2 and benzonase were subsequently added to a final concentration of 1 mM and 50 UI/mL, respectively, and incubated at 37 °C for 4 hours. The final concentration of Tris buffer was adjusted to 50 mM, and CaCl2 and pronase added to a final concentration of 1 mM and 0.5 mg/mL, respectively. Finally, the samples were incubated for 16 hours at 37 °C. The remaining insoluble cell debris were removed by centrifugation at 8,000 × g for 30 min. The supernatants were precipitated with 25% ethanol in presence of 10 mM CaCl2 and stirred for 16 hours at 4 °C. The precipitates were removed by centrifugation at 8,000 × g for 30 min and the supernatants containing WTAs were precipitated with 75% ethanol in presence of 10 mM CaCl2 and stirred for 4 hours at 4 °C, collected by centrifugation at 8,000 × g for 30 minutes and dissolved in water. The samples were dialyzed extensively against water at room temperature. 1 M Tris buffer pH 7.0 was added to a final concentration of 50 mM and loaded onto a Q Sepharose column (GE Healthcare, Uppsala Sweden). WTAs were separated from residuals using a linear gradient 0–0.5 M NaCl in 50 mM Tris buffer pH 7.0. Fractions containing WTA as detected by the modified Elson-Morgan hexosamine assay53 were pooled, extensively dialyzed against water at room temperature and freeze-dried.
Monosaccharide composition of WTAs was determined using High-Performance Anion-Exchange Chromatography with Pulsed Amperometry Detection (HPAEC-PAD) (Thermo Fischer Scientific, Dionex, Sunnyval, CA) as previously described54 and detailed in Supplementary Methods. Proton nuclear magnetic resonance (1H NMR) spectra of WTAs were recorded using a 500-MHz Bruker Avance DRX spectrometer (Bruker Biospin, Wissembourg, France) at 293 K in D2O.
Mouse infection models with S. aureus
Female outbred OF1 mice were obtained from Charles River Laboratories (Saint-Germain-sur-l’Arbresle, France). All mouse procedures were performed under general anesthesia. Animals were housed and handled according to European regulations. The procedures were reviewed and approved by the Sanofi Pasteur animal care committee. Bacterial suspensions were obtained from 50-mL cultures of the Newman or HT2005 0742 strains grown for 20 hours in TSB at 37 °C.
Peritonitis model
Mice were infected by the intraperitoneal route with 1.7 × 106 CFU/500 µL of the Newman strain or 7.0 × 106 CFU/500 µL of the HT2005 0742 strain. Bacterial inocula were prepared extemporaneously by mixing 1:1 sterile 20% hog mucin and 2x concentrated adjusted bacterial suspensions. The mice were euthanized 15 days post-infection. Livers and kidneys were removed.
Deep wound model
The hair from the left thighs was shaved and the area disinfected. An incision measuring 1 cm in length was carried through the skin. The incision was then continued to a depth of 0.5 cm and 0.4 cm in depth into the underlying quadriceps muscles. The muscle incisions were closed with one silk suture and the wounds were inoculated under the suture with 2.5 µL of a Newman S. aureus suspension containing 103 CFU. Finally, the skin incisions were closed with two separated prolene sutures. The mice were treated with 70 µL/20 g Buprecare (Alcyon, Paris, France) injected by intraperitoneal route on a regular basis. Clinical evidence of wound infection, defined as the presence of an abscess and purulent infection within the wound, was observed in all animals two days after the infection. The mice were euthanized three days post-infection. Quadricep muscles were removed.
Infected organs were obtained 15 days and 3 days post-infection in the peritonitis and deep wound models respectively then dissociated and homogenized in sterile phosphate buffered saline (PBS) under aseptic conditions for direct analysis of WTA structure.
WTA carbotyping by HPAEC-PAD from cell growth and infected organs
109 CFUs of S. aureus grown either in TSB or SATA-2 medium until stationary phase were collected by centrifugation at 5,000 × g at 4 °C for 20 min and washed with 0.5 mL of 0.15 M NaCl. Preliminary experiments have showed that the minimal amount of CFU required for the direct detection of WTA in infected organs by HPAEC-PAD is 7 log10 total CFU. Therefore, mouse kidneys, livers and muscles containing more than 7 log10 total CFU were ground and washed twice with 5 mL, 1 mL and 0.5 mL of 0.15 M NaCl, respectively.
The samples containing either the bacterial cells or the ground organs were suspended in 400 µL of aqueous hydrofluoric acid (HF) (48% by mass) and incubated at room temperature overnight. Cell debris were removed by centrifugation and acid removed under a stream of nitrogen at 40 °C. The samples were dissolved in 400 µL of water and passed through a centrifugal filter unit (10 kDa MW cut-off, Ultracel-10, Millipore) to remove proteins and other macromolecules. The disaccharides generated by HF hydrolysis were separated on a Dionex system using a CarboPac MA1 (4 mm × 250 mm) analytical column with a guard column (4 mm × 50 mm) previously equilibrated in 480 mM NaOH at a flow rate of 0.4 ml/min. The disaccharides were separated isocratically using 480 mM NaOH for 40 min. Purified and characterized WTAs from the Newman, Wood46 and ATCC55804 strains were hydrolyzed in the same way and used as references for peak assignement. The proportion of each WTA structure in purified WTAs or strains were calculated as described in Supplementary Methods.
Supplementary information
Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions
Acknowledgements
We thank Prof. Jérome Etienne and Michèle Bes (INSERM, Centre National de Référence des Staphylocoques, Lyon, France) for kindly providing the clinical isolates used in the study, Frédéric Blanc for recording NMR spectra and Christophe Chabanel for performing the mouse challenge model. We are grateful to Virginie Courtois and Nadège Arnaud-Barbe for conducting sequence determination of tarS and tarM genes. We also thank Jean Haensler for critical reading of the manuscript. Editorial assistance was provided by Richard Glover, inScience Communications, Springer Healthcare, UK; funding for this assistance was provided by Sanofi Pasteur.
Author Contributions
N.M., P.T. and B.R. conceived and designed this study. F.T. and V.S. provided the strains. M.B. and F.T. performed the experiments. N.M., M.B., F.T., V.S. and B.R. analyzed and interpreted the data. N.M. and B.R. wrote the manuscript. B.R. has supervised and acted as overall study director. All authors reviewed the manuscript.
Competing Interests
The study was sponsored by Sanofi Pasteur. All authors are employees of Sanofi Pasteur.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39929-1.
References
- 1.Brett M, Bradfute SB. Staphylococcus aureus: current state of prevalence, impact, and vaccine development. Curr. Pharm. Des. 2015;21:2131–2135. doi: 10.2174/1381612821666150310101347. [DOI] [PubMed] [Google Scholar]
- 2.Missiakas D, Schneewind O. Antibodies against a secreted product of Staphylococcus aureus trigger phagocytic killing. J. Exp. Med. 2016;213:1645–1653. doi: 10.1084/jem.20160569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbio.l Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weidenmaier, C., Lee, J. C. Structure and function of surface polysaccharides of Staphylococcus aureus. In: Current Topics in Microbiology and Immunology. (Springer, 2015). [DOI] [PubMed]
- 5.Baur S, et al. A nasal epithelial receptor for Staphylococcus aureus WTA governs adhesion to epithelial cells and modulates nasal colonization. PLoS Pathog. 2014;10:e1004089. doi: 10.1371/journal.ppat.1004247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misawa Y, et al. Staphylococcus aureus colonization of the mouse gastrointestinal tract is modulated by wall teichoic acid, capsule, and surface proteins. PLoS Pathog. 2015;11:e1005061. doi: 10.1371/journal.ppat.1005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atilano ML, et al. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanner S, et al. Wall teichoic acids mediate increased virulence in Staphylococcus aureus. Nat. Microbiol. 2017;2:16257. doi: 10.1038/nmicrobiol.2016.257. [DOI] [PubMed] [Google Scholar]
- 9.Bertsche U, et al. Correlation of daptomycin resistance in a clinical Staphylococcus aureus strain with increased cell wall teichoic acid production and D-Alanylation. Antimicrob. Agents Chemother. 2011;55:3922–3928. doi: 10.1128/AAC.01226-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertsche U, et al. Increased cell wall teichoic acid production and D-alanylation are common phenotypes among daptomycin-resistant methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates. PLoS One. 2013;8:e67398. doi: 10.1371/journal.pone.0067398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koprivnjak T, Weidenmaier C, Peschel A, Weiss JP. Wall teichoic acid deficiency in Staphylococcus aureus confers selective resistance to mammalian group IIA phospholipase A2 and human β-defensin 3. Infect. Immun. 2008;76:2169–2176. doi: 10.1128/IAI.01705-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajagopal M, Walker S. Envelope structures of gram-positive bacteria. Curr Top Microbiol Immunol. 2017;404:1–44. doi: 10.1007/82_2015_5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddiley J, Buchanan JG, Rajbhandary UL, Sanderson AR. Teichoic acid from the walls of Staphylococcus aureus H. Structure of the N-acetylglucosaminylribitol residues. Biochem. J. 1962;82:439–448. doi: 10.1042/bj0820439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddiley J, Buchanan JG, Martin RO, Rajbhandary UL. Teichoic acid from the walls of Staphylococcus aureus H. 2. Location of phosphate and alanine residues. Biochem. J. 1962;85:49–56. doi: 10.1042/bj0850049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanderson AR, Strominger JL, Nathenson SG. Chemical structure of teichoic acid of Staphylococcus aureus, strain Copenhagen. J. Biol. Chem. 1962;237:3603–3613. [PubMed] [Google Scholar]
- 16.Takahashi K, et al. Intradermal immunization with wall teichoic acid (WTA) elicits and augments an anti-WTA IgG response that protects mice from methicillin-resistant Staphylococcus aureus infection independent of mannose-binding lectin status. PLoS One. 2013;8:e69739. doi: 10.1371/journal.pone.0069739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdier I, et al. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J. Clin. Microbiol. 2007;45:725–729. doi: 10.1128/JCM.01572-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fattom, A. I. Methods of protecting against staphylococcal infection. Patent WO2007/053176 (2007).
- 19.Xia G, et al. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J. Biol. Chem. 2010;285:13405–13415. doi: 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown, S. et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. USA 109, 18909–18914 (2012). [DOI] [PMC free article] [PubMed]
- 21.Winstel V, Xia G, Peschel A. Pathways and roles of wall teichoic acid glycosylation in. Staphylococcus aureus. Int. J. Med. Microbiol. 2014;304:215–221. doi: 10.1016/j.ijmm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa K, et al. Glycoepitopes of staphylococcal wall teichoic acid govern complement-mediated opsonophagocytosis via human serum antibody and mannose-binding lectin. J. Biol. Chem. 2013;288:30956–30968. doi: 10.1074/jbc.M113.509893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurokawa K, Takahashi K, Lee BL. The staphylococcal surface-glycopolymer wall teichoic acid (WTA) is crucial for complement activation and immunological defense against Staphylococcus aureus infection. Immunobiology. 2016;221:1091–1101. doi: 10.1016/j.imbio.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Winstel V, et al. Wall teichoic acid glycosylation governs Staphylococcus aureus nasal colonization. MBio. 2015;6:e00632. doi: 10.1128/mBio.00632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarraud S, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoulas G, et al. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J. Infect. Dis. 2003;187:929–938. doi: 10.1086/368128. [DOI] [PubMed] [Google Scholar]
- 28.Dufour P, et al. High genetic variability of the agr locus in Staphylococcus species. J. Bacteriol. 2002;184:1180–1186. doi: 10.1128/jb.184.4.1180-1186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stegger M, et al. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251) Clin. Microbiol. Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 30.Goerke C, Esser S, Kummel M, Wolz C. Staphylococcus aureus strain designation by agr and cap polymorphism typing and delineation of agr diversification by sequence analysis. Int. J. Med. Microbiol. 2005;295:67–75. doi: 10.1016/j.ijmm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Cocchiaro JL, et al. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol. Microbiol. 2006;59:948–960. doi: 10.1111/j.1365-2958.2005.04978.x. [DOI] [PubMed] [Google Scholar]
- 32.Kubler-Kielb J, Coxon B, Schneerson R. Chemical structure, conjugation, and cross-reactivity of Bacillus pumilus Sh18 cell wall polysaccharide. J. Bact. 2004;186:6891–6901. doi: 10.1128/JB.186.20.6891-6901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jennings HJ, Lugowski C. Facile cleavage of some 2-acetamido-2-deoxy-β-D-gluco- and galactopyranosides using aqueous HF. Can. J. Chem. 1980;58:2610–2612. doi: 10.1139/v80-417. [DOI] [Google Scholar]
- 34.Rokbi, B. & Lafont, C. Method for producing strains of overproductive Staphylococcus aureus. Patent WO2006/114500A2 (2006).
- 35.Gerlach D, et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature. 2018;563:705–709. doi: 10.1038/s41586-018-0730-x. [DOI] [PubMed] [Google Scholar]
- 36.Juergens WG, Sanderson AR, Strominger JL. Chemical basis for an immunological specificity of a strain of Staphylococcus aureus. J. Exp. Med. 1963;117:925–935. doi: 10.1084/jem.117.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Falord M, Mäder U, Hiron A, Débarbouillé M, Msadek T. Investigation of the Staphylococcus aureus GraSR Regulon Reveals Novel Links to Virulence, Stress Response and Cell Wall Signal Transduction Pathways. PLoS One. 2011;6:e21323. doi: 10.1371/journal.pone.0021323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang SL, et al. The Staphylococcus aureus two-component regulatory system, GraRS, senses and confers resistance to selected cationic antimicrobial peptides. Infect. Immun. 2012;80:74–81. doi: 10.1128/IAI.05669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobhanifar S, et al. Structure and mechanism of Staphylococcus aureus TarM, the wall teichoic acid α-glycosyltransferase. Proc. Natl. Acad. Sci. USA. 2015;112:E576–E585. doi: 10.1073/pnas.1418084112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobhanifar S, et al. Structure and mechanism of Staphylococcus aureus TarS, the wall teichoic acid β-glycosyltransferase involved in methicillin resistance. Plos One. 2016;12:e1006067. doi: 10.1371/journal.ppat.1006067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JH, et al. Surface glycopolymers are crucial for in vitro anti-wall teichoic acid IgG-mediated complement activation and opsonophagocytosis of Staphylococcus aureus. Infect. Immun. 2015;83:4247–4255. doi: 10.1128/IAI.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung DJ, et al. Specific serum Ig recognizing staphylococcal wall teichoic acid induces complement-mediated opsonophagocytosis against Staphylococcus aureus. J. Immunol. 2012;189:4951–4959. doi: 10.4049/jimmunol.1201294. [DOI] [PubMed] [Google Scholar]
- 43.Lehar SM, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527:323–8. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 44.Hansenova Manaskova S, Bikker FJ, Veerman EC, van Belkum A, Van Wamel WJ. Rapid detection and semi-quantification of IgG-accessible Staphylococcus aureus surface-associated antigens using a multiplex competitive Luminex assay. J. Immunol. Methods. 2013;397:18–27. doi: 10.1016/j.jim.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Kim HK, Missiakas D, Schneewind O. Mouse models for infectious diseases caused by Staphylococcus aureus. J. Immunol. Methods. 2014;410:88–99. doi: 10.1016/j.jim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorling J, Moraes C, Rolff J. Recognition, survival and persistence of Staphylococcus aureus in the model host Tenebrio molitor. Dev. Comp. Immunol. 2015;48:284–290. doi: 10.1016/j.dci.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Koprivnjak T, et al. Cation-induced transcriptional regulation of the dlt Operon of Staphylococcus aureus. J. Bacteriol. 2006;274:3622–3630. doi: 10.1128/JB.188.10.3622-3630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bera A, et al. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J. Bacteriol. 2007;189:280–283. doi: 10.1128/JB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Méhes L, et al. Phagocytosis and intracellular killing of heterogeneous vancomycin-intermediate Staphylococcus aureus strains. J. Med. Microbiol. 2012;61:198–203. doi: 10.1099/jmm.0.029421-0. [DOI] [PubMed] [Google Scholar]
- 50.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 2004;17:218–34. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutter DE, et al. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol. Med. Microbiol. 2011;63:16–24. doi: 10.1111/j.1574-695X.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- 52.Gautam S, Taehan K, Lester E, Deep D, Spiegel DA. Wall teichoic acids prevent antibody binding to epitopes within the cell wall of Staphyococcus aureus. ACS Chem. Biol. 2015;11:25–30. doi: 10.1021/acschembio.5b00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gatt R, Berman ER. A rapid procedure for the estimation of amino sugars on a microscale. Anal. Biochem. 1966;15:161–171. doi: 10.1016/0003-2697(66)90262-4. [DOI] [PubMed] [Google Scholar]
- 54.Talaga P, Bellamy L, Moreau M. Quantitative determination of C-polysaccharide in Streptococcus pneumoniae capsular polysaccharides by use of high-performance anion-exchange chromatography with pulsed amperometric detection. Vaccine. 2001;19:2987–2994. doi: 10.1016/S0264-410X(00)00535-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glycosylation of Staphylococcus aureus cell wall teichoic acid is influenced by environmental conditions