Abstract
The roles of sphingolipids in polycystic ovary syndrome (PCOS) are still unknown. This study aimed to investigate the sphingolipid characteristics for different types of PCOS using liquid chromatography-mass spectrometry (LC-MS). A total of 107 women with PCOS and 37 healthy women as normal controls were studied. PCOS patients were further classified into non-obesity with insulin resistance (IR) (NOIR), obesity with IR (OIR), and non-obesity and non-IR (NIR) subgroups. A total of 87 serum sphingolipids, including 9 sphingosines, 3 sphinganines, 1 sphingosine-1-phosphate (S1P), 19 ceramides (Cers), 1 ceramide-1-phosphate, 44 sphingomyelins (SMs), 4 hexosylceramides, and 6 lactosylceramides (LacCers) were analyzed using an improved sphingolipidomic approach based on LC-MS. Notable elevations in the levels of S1P, Cer, and SM were observed in PCOS patients when compared with healthy women, and SM species with long saturated acyl chains showed potential as novel biomarkers of PCOS. In addition, the level of LacCer was only elevated in NIR, and there was almost no change in NOIR and OIR. This study is the first to report the comprehensive sphingolipidomic profiling of different subgroups of PCOS with or without IR or obesity and suggests that serum sphingolipids might be useful as diagnostic biomarkers for different types of PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine and metabolic disorders in women of reproductive age, and it accounts for 50–70% of anovulatory infertility cases and affects about 5.6% of 19–45-year-old women in China1,2. The clinical manifestations are diverse and include oligomenorrhea/amenorrhea, hirsutism, acne, obesity, polycystic ovaries, and infertility2,3. PCOS is associated with insulin resistance (IR)/hyperinsulinemia, impaired glucose tolerance, and dyslipidemia4–7, and long-term complications include metabolic syndrome, endometrial and breast cancer, and other gynecological tumors8.
The etiology of PCOS remains unclear9. Pathophysiological changes include hypothalamus-pituitary-ovarian axis dysfunction, hyperandrogenism, hyperinsulinemia, insulin resistance (IR), endocrine dysfunction, adrenal dysfunction, etc.10–12. IR is central to the pathogenesis of PCOS, while glucose and lipid disorders are considered to be the general pathology13. Compensatory IR is present in about 40% of women with PCOS14 and mainly affects classical target tissues of insulin action – such as skeletal muscle, adipose, and liver – and induces systemic glucose and lipid metabolism disorders. IR is a physiological condition in which the target organs become resistant to the effects of insulin. That is, the normal response to a given amount of insulin is reduced, thus weakening insulin’s ability to regulate glucose uptake and glucose utilization15.
Several serum/plasma metabolomic studies of PCOS have been carried out using advanced analytical methods, such as liquid or gas chromatography/time-of-flight mass spectrometry (LC or GC/TOF-MS) or nuclear magnetic resonance16–19, and these studies have shown that changes in amino acid metabolism, the tricarboxylic acid cycle, and gut microflora, as well as mild perturbations in lipid and glucose metabolism, are correlated with PCOS20. A lipidomic analysis of serum/plasma samples from PCOS patients was also recently performed, and disturbances in fatty acid, glycerolipid, and glycerophospholipid metabolism were shown to be related to the pathogenesis of IR in PCOS21. In addition, the levels of very long-chain monounsaturated fatty acids were found to be reduced in PCOS patients compared with healthy subjects22. However, there has been a lack of studies focusing on sphingolipid alterations in PCOS.
Sphingolipids are a complex family of compounds that are found in eukaryotes as well as some prokaryotes and viruses, and they comprise a small but vital fraction (2–20%) of the membrane lipids23. They are involved in a variety of biological processes in eukaryotic cells24,25, such as cell proliferation, differentiation, apoptosis, and migration; membrane trafficking; cell-cell interactions; and cell morphology, as well as both intracellular and extracellular signaling26,27. However, previous studies on sphingolipids have found that the changes in the levels of sphingolipids in the body directly affect the intensity of insulin signaling28. Ceramide (Cer), ganglioside monosialo 3 (GM3), and sphingosine-1-phosphate (S1P) have significant regulatory effects on insulin signaling, and studies have shown that Cer and GM3 play negative roles in insulin signaling and promote the development of IR29,30. Conversely, S1P can enhance insulin signaling and inhibit the development of IR31. Abnormal lipid metabolism is closely related to the development of IR, and oxidative damage caused by excessive lipid accumulation can induce IR32, while sphingolipids mediate the development of IR by regulating insulin signaling molecules33. The analysis of whole-serum sphingolipids has been shown to facilitate the understanding of potential mechanisms of some diseases occurrence and development34, but the characteristics of sphingolipids in PCOS are unknown.
In the present study, we explored the potential of sphingolipids as biomarkers for PCOS using an integrated sphingolipid analytical platform that combined the advantages of ultra-high performance liquid chromatography (UHPLC)-quadrupole time-of-flight mass spectrometry and UHPLC coupled with triple quadrupole mass spectrometry (UHPLC-QQQ-MS) to qualitatively and quantitatively analyze the sphingolipids in serum samples from healthy women and from different subgroups of PCOS patients.
Results
Baseline characteristics
A total of 107 PCOS patients and 37 healthy women were enrolled. Thirty-four (31.8%) of the 107 PCOS patients presented with a body mass index (BMI) < 25 kg/m2 and homeostatic model assessment of insulin resistance (HOMA-IR) ≥ 2.14 (the non-obesity with IR (NOIR) group), 41 patients (38.3%) had BMI ≥ 25 kg/m2 and HOMA-IR ≥ 2.14 kg/m2 (the obesity with IR (OIR) group), and 32 patients (29.9%) had BMI < 25 kg/m2 and HOMA-IR < 2.14 kg/m2 (the non-obesity and non-IR (NIR) group). The demographic, endocrine, and glycolipid metabolic features of controls and patients with different types of PCOS are described in Table 1. There were no significant differences in age for any of the groups. PCOS patients in the OIR group had a significantly greater BMI, waist-to-hip ratio (WHR), and HOMA-IR; higher levels of total cholesterol (CHOL), triglycerides (TG), and low-density lipoprotein (LDL); and lower levels of high-density lipoprotein (HDL) compared to controls. Except for BMI and HDL, these characteristics were also significantly increased in the NOIR group compared with the controls. However, PCOS in the NIR group only presented with obviously increased luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio, LH level, and total testosterone (TT) level compared with controls. Overall, these changes in PCOS patients were clinically related to PCOS type and complemented the metabolomics analysis.
Table 1.
Baseline characteristics of the three polycystic ovary syndrome (PCOS) subgroups and control subjects.
| Characteristic | PCOS | Controls | ||
|---|---|---|---|---|
| NOIR | NIR | OIR | ||
| Number | 34 | 32 | 41 | 37 |
| Age (years) | 27.88 (26.30–29.46) | 28.05 (26.96–29.14) | 26.97 (25.56–28.38) | 28.11 (26.33–29.89) |
| BMI (kg/m2) | 21.03 (20.51–21.56) | 20.06 (19.55–20.56) | 28.05 (27.02–29.07)* | 20.98 (20.15–21.81) |
| WHR | 0.83 (0.81–0.85)* | 0.80 (0.78–0.81) | 0.88 (0.86–0.90)* | 0.79 (0.78–0.80) |
| HOMA-IR | 3.25 (3.01–3.48)* | 1.19 (1.10–1.29) | 4.85 (3.99–5.71)* | 1.04 (0.95–1.14) |
| CHOL (mg/dl) | 5.00 (4.74–5.25)* | 4.38 (4.10–4.67) | 5.28 (4.90–5.66)* | 4.38 (4.17–4.58) |
| TG (mg/dl) | 1.30 (1.10–1.49)* | 0.74 (0.68–0.81) | 2.08 (1.39–2.77)* | 0.78 (0.68–0.88) |
| HDL (mg/dl) | 1.33 (1.23–1.42) | 1.51 (1.40–1.61) | 1.19 (1.12–1.27)* | 1.43 (1.34–1.51) |
| LDL (mg/dl) | 3.18 (2.96–3.39)* | 2.61 (2.45–2.77) | 3.34 (3.08–3.60)* | 2.59 (2.44–2.73) |
| LH (mIU/ml) | 9.61 (7.78–11.44)* | 9.83 (7.66–12.00)* | 8.71 (7.42–10.00)* | 4.63 (4.08–5.19) |
| LH/FSH | 1.60 (1.32–1.89)* | 1.41 (1.10–1.71)* | 1.56 (1.34–1.78)* | 0.67 (0.58–0.76) |
| TT (ng/ml) | 0.64 (0.55–0.72)* | 0.60 (0.53–0.67)* | 0.64 (0.57–0.72)* | 0.42 (0.37–0.47) |
Notes: Data are presented as the mean and (95% confidence interval). Differences in continuous variables among groups were analyzed by ANOVA or Kruskal–Wallis test. *p < 0.05.
Abbreviations: NOIR, non-obesity with insulin resistance; OIR, obesity with insulin resistance; NIR, non-obesity and non-insulin resistance; BMI, body mass index; WHR, waist-to-hip ratio; HOMA-IR, homeostasis model assessment of insulin resistance; CHOL, total cholesterol; TG, triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LH, luteinizing hormone; FSH, follicle-stimulating hormone; TT, total testosterone.
Identification of serum sphingolipids
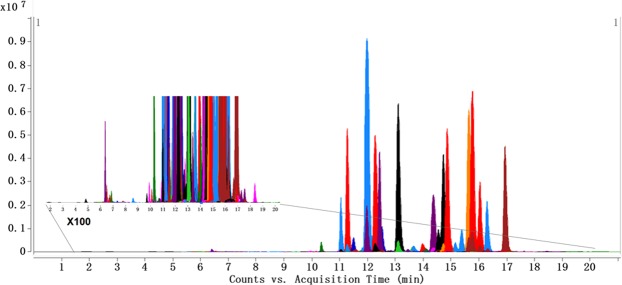
The sphingolipids in human serum were comprehensively profiled using an established sphingolipidomic approach23. A total of 87 sphingolipids were identified and quantified in the serum samples, including 9 sphingosines, 3 sphinganines (Sas), S1P, 19 Cers, 1 ceramide-1-phosphate (Cer1P), 44 sphingomyelins (SMs), 4 hexosylceramides (HexCers), and 6 lactosylceramides (LacCers). The MS and MS/MS data of identified sphingolipids and the multiple reaction monitoring (MRM) transitions used to monitor each sphingolipid are listed in Table 2. The MRM chromatograms of all sphingolipids in human serum are shown in Fig. 1.
Table 2.
Identification and quantification of 87 sphingolipids in human serum using UHPLC-Q-TOF and UHPLC-QQQ MS.
| Q-TOF MS | QQQ MS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subclass | Name | RT (min) | Molecular Formula | Measured m/z | Calculated m/z | Error (ppm) | MS/MS Fragments (m/z) | MRM transition | Fragmentor (V) | CE (V) |
| So | So (d17:1) [I.S.-1] | 6.34 | C17H35NO2 | 286.2748 | 286.2741 | 2.45 | 268.2649 | 286.4 → 268.2 | 80 | 5 |
| So (m18:2) | 8.59 | C18H35NO | 282.2791 | 282.2791 | 0.00 | 264.2691 | 282.3 → 264.3 | 80 | 5 | |
| So (m18:3) | 7.79 | C18H33NO | 280.2639 | 280.2635 | 1.43 | 262.2542 | 280.3 → 262.3 | 80 | 5 | |
| So (d16:1) | 4.85 | C16H33NO2 | 272.2583 | 272.2584 | −0.37 | 254.2833 | 272.3 → 254.3 | 80 | 5 | |
| So (d18:1) | 6.71 | C18H37NO2 | 300.2899 | 300.2897 | 0.67 | 282.2797 | 300.3 → 282.3 | 80 | 5 | |
| So (d18:2) | 6.78 | C18H35NO2 | 298.2743 | 298.2741 | 0.67 | 280.2630 | 298.3 → 280.3 | 80 | 5 | |
| So (d18:3) | 6.34 | C18H33NO2 | 296.2586 | 296.2584 | 0.68 | 278.2473 | 296.3 → 278.2 | 80 | 5 | |
| So (t18:1) | 7.32 | C18H37NO3 | 316.2841 | 316.2846 | −1.58 | 298.2733 | 316.3 → 298.3 | 80 | 5 | |
| So (d22:3) | 7.91 | C22H41NO2 | 352.3205 | 352.3210 | −1.42 | 334.3101 | 352.3 → 334.3 | 80 | 5 | |
| So (d22:3) isomer | 7.62 | C22H41NO2 | 352.3219 | 352.3210 | 2.55 | 334.3100 | 352.3 → 334.3 | 80 | 5 | |
| Sa | Sa (d17:0) [I.S.-2] | 6.57 | C17H37NO2 | 288.2901 | 288.2897 | 1.39 | 270.2795, 60.0453 | 288.4 → 270.2 | 110 | 20 |
| Sa (d16:0) | 5.03 | C16H35NO2 | 274.2743 | 274.2741 | 0.73 | 256.2653 | 274.3 → 256.3 | 110 | 20 | |
| Sa (d18:0) | 6.34 | C18H39NO2 | 302.3056 | 302.3054 | 0.66 | 284.2950 | 302.3 → 284.3 | 110 | 20 | |
| Sa (d20:0) | 6.79 | C20H43NO2 | 330.3376 | 330.3367 | 2.72 | 312.3268 | 330.3 → 312.3 | 110 | 20 | |
| S1P | S1P (d17:1) [I.S.-3] | 6.49 | C17H36NO5P | 366.2406 | 366.2404 | 0.55 | 250.2508 | 366.3 → 250.3 | 105 | 10 |
| S1P (d18:1) | 6.90 | C18H38NO5P | 380.2578 | 380.2560 | 4.73 | 264.2690 | 380.3 → 264.3 | 105 | 10 | |
| Sa1P (d17:0) [I.S.-4] | 6.71 | C17H38NO5P | 368.2571 | 368.2560 | 2.99 | 270.2788 | 368.4 → 270.3 | 120 | 5 | |
| Cer | Cer (d18:1/12:0) [I.S.-5] | 10.92 | C30H59NO3 | 482.4582 | 482.4568 | 2.90 | 464.4463, 282.2790, 264.2691 | 482.6 → 264.3 | 130 | 25 |
| Cer (d18:0/16:0) | 13.75 | C34H69NO3 | 540.5349 | 540.5350 | −0.19 | 266.2837 | 540.5 → 266.3 | 130 | 25 | |
| Cer (d18:0/18:0) | 14.30 | C36H73NO3 | 568.5674 | 568.5663 | 1.93 | 266.2831 | 568.6 → 266.3 | 130 | 25 | |
| Cer (d18:0/22:0) | 17.00 | C40H81NO3 | 624.6278 | 624.6289 | −1.76 | 606.6, 284.2938, 266.2833 | 624.6 → 266.3 | 130 | 25 | |
| Cer (d18:0/23:0) | 18.20 | C41H83NO3 | 638.6446 | 638.6446 | 0.00 | 620.6307, 284.2939, 266.2846 | 638.6 → 266.3 | 130 | 25 | |
| Cer (d18:0/24:0) | 19.14 | C42H85NO3 | 652.6597 | 652.6602 | −0.77 | 634.6402, 284.2925, 266.2838 | 652.7 → 266.3 | 130 | 25 | |
| Cer (d18:1/16:0) | 13.06 | C34H67NO3 | 538.5194 | 538.5194 | 0.00 | 520.5010, 282.2796, 264.2691 | 538.5 → 264.3 | 130 | 25 | |
| Cer (d18:1/18:0) | 14.20 | C36H71NO3 | 566.5503 | 566.5507 | −0.71 | 548.5406, 264.2690 | 566.6 → 264.3 | 130 | 25 | |
| Cer (d18:1/18:1) | 13.06 | C36H69NO3 | 564.5340 | 564.5350 | −1.77 | 264.2685 | 564.5 → 264.3 | 130 | 25 | |
| Cer (d18:1/20:1) | 14.34 | C38H73NO3 | 592.5670 | 592.5663 | 1.18 | 264.2688 | 592.6 → 264.3 | 130 | 25 | |
| Cer (d18:1/22:0) | 17.01 | C40H79NO3 | 622.6133 | 622.6133 | 0.00 | 604.6019, 264.2691 | 622.6 → 264.3 | 130 | 25 | |
| Cer (d18:1/23:0) | 17.72 | C41H81NO3 | 636.6285 | 636.6289 | −0.63 | 618.6185, 264.2689 | 636.6 → 264.3 | 130 | 25 | |
| Cer (d18:1/24:0) | 18.51 | C42H83NO3 | 650.6448 | 650.6446 | 0.31 | 632.6343, 282.2783, 264.2691 | 650.6 → 264.3 | 130 | 25 | |
| Cer (d18:1/24:1) | 17.02 | C42H81NO3 | 648.6280 | 648.6289 | −1.39 | 630.6188, 264.2688 | 648.6 → 264.3 | 130 | 25 | |
| Cer (d18:1/25:0) | 19.05 | C43H85NO3 | 664.6596 | 664.6602 | −0.90 | 646.6434, 282.2795, 264.2695 | 664.7 → 264.3 | 130 | 25 | |
| Cer (d18:1/25:0) isomer | 19.41 | C43H85NO3 | 664.6598 | 664.6602 | −0.60 | 646.6434, 282.2795, 264.2695 | 664.7 → 264.3 | 130 | 25 | |
| Cer (d18:1/26:0) | 20.45 | C44H87NO3 | 678.6741 | 678.6759 | −2.65 | 660.6650, 264.2695 | 678.7 → 264.3 | 130 | 25 | |
| Cer (d18:2/16:0) | 12.41 | C34H65NO3 | 536.5044 | 536.5037 | 1.30 | 262.2549 | 536.5 → 262.3 | 130 | 25 | |
| Cer (d18:2/22:0) | 16.08 | C40H77NO3 | 620.5980 | 620.5976 | 0.64 | 602.5875, 262.2546 | 620.6 → 262.2 | 130 | 25 | |
| Cer (d18:2/23:0) | 16.77 | C41H79NO3 | 634.6115 | 634.6133 | −2.84 | 262.2542 | 634.6 → 262.3 | 130 | 25 | |
| Cer1P (d18:1/12:0) [I.S.-6] | 9.95 | C30H60NO6P | 562.4242 | 562.4231 | 1.96 | 264.2615 | 562.5 → 264.3 | 135 | 25 | |
| Cer1P (d18:1/18:0) | 12.78 | C36H72NO6P | 646.5167 | 646.5170 | −0.46 | 264.2611 | 646.5 → 264.3 | 135 | 25 | |
| SM | SM (d18:1/12:0) [I.S.-7] | 10.30 | C35H71N2O6P | 647.5124 | 647.5123 | 0.15 | 264.2704, 184.0741 | 647.5 → 184.1 | 170 | 20 |
| SM (d18:0/18:0) | 13.65 | C41H85N2O6P | 733.6217 | 733.6218 | −0.14 | 184.0741 | 733.6 → 184.1 | 170 | 20 | |
| SM (d18:1/14:0) | 11.08 | C37H75N2O6P | 675.5420 | 675.5436 | −2.37 | 657.5282, 264.2670, 184.0758 | 675.5 → 184.1 | 170 | 20 | |
| SM (d18:1/16:0) | 12.03 | C39H79N2O6P | 703.5735 | 703.5749 | −1.99 | 685.5649, 264.2703, 184.0734 | 703.6 → 184.1 | 170 | 20 | |
| SM (d18:1/17:1) | 11.75 | C40H79N2O6P | 715.5747 | 715.5749 | −0.28 | 264.2670, 184.0759 | 715.6 → 184.1 | 170 | 20 | |
| SM (d18:1/18:0) | 13.10 | C41H83N2O6P | 731.6056 | 731.6062 | −0.82 | 713.5884, 264.2702, 184.0762 | 731.6 → 184.1 | 170 | 20 | |
| SM (d18:1/18:2) | 11.60 | C41H79N2O6P | 727.5737 | 727.5749 | −1.65 | 264.2671, 184.0749 | 727.6 → 184.1 | 170 | 20 | |
| SM (d18:1/20:0) | 14.37 | C43H87N2O6P | 759.6356 | 759.6375 | −2.50 | 741.6249, 264.2674, 184.0757 | 759.6 → 184.1 | 170 | 20 | |
| SM (d18:1/22:0) | 15.60 | C45H91N2O6P | 787.6680 | 787.6688 | −1.02 | 769.6525, 264.2690, 184.0761 | 787.7 → 184.1 | 170 | 20 | |
| SM (d18:1/23:0) | 16.36 | C46H93N2O6P | 801.6833 | 801.6844 | −1.37 | 264.2675, 184.0744 | 801.7 → 184.1 | 170 | 20 | |
| SM (d18:1/23:1) | 15.18 | C46H91N2O6P | 799.6673 | 799.6688 | −1.88 | 264.2680, 184.0764 | 799.7 → 184.1 | 170 | 20 | |
| SM (d18:1/24:0) | 16.90 | C47H95N2O6P | 815.6989 | 815.7001 | −1.47 | 264.2612, 184.0729 | 815.7 → 184.1 | 170 | 20 | |
| SM (d18:1/24:1) | 16.10 | C47H93N2O6P | 813.6825 | 813.6844 | −2.34 | 795.6724, 264.2606, 184.0759 | 813.7 → 184.1 | 170 | 20 | |
| SM (d18:1/26:1) | 17.02 | C49H97N2O6P | 841.7166 | 841.7157 | 1.07 | 264.2602, 184.0761 | 841.7 → 184.1 | 170 | 20 | |
| SM (d18:2/16:0) | 11.31 | C39H77N2O6P | 701.5585 | 701.5592 | −1.00 | 683.5426, 262.2520, 184.0763 | 701.6 → 184.1 | 170 | 20 | |
| SM (d18:2/16:1) | 10.79 | C39H75N2O6P | 699.5423 | 699.5436 | −1.86 | 262.2523, 184.0765 | 699.5 → 184.1 | 170 | 20 | |
| SM (d18:2/18:0) | 12.31 | C41H81N2O6P | 729.5893 | 729.5905 | −1.64 | 711.5798, 262.2545, 184.0761 | 729.6 → 184.1 | 170 | 20 | |
| SM (d16:1/24:1) | 14.50 | C45H89N2O6P | 785.6548 | 785.6531 | 2.16 | 236.2379, 184.0744 | 785.7 → 184.1 | 170 | 20 | |
| SM (d18:2/22:0) | 14.70 | C45H89N2O6P | 785.6545 | 785.6531 | 1.78 | 262.2533, 184.0742 | 785.7 → 184.1 | 170 | 20 | |
| SM (d18:2/22:1) | 13.60 | C45H87N2O6P | 783.6383 | 783.6375 | 1.02 | 262.2536, 184.0743 | 783.6 → 184.1 | 170 | 20 | |
| SM (d18:2/23:0) | 15.41 | C46H91N2O6P | 799.6710 | 799.6688 | 2.75 | 262.2535, 184.0745 | 799.7 → 184.1 | 170 | 20 | |
| SM (d18:2/24:0) | 15.77 | C47H93N2O6P | 813.6855 | 813.6844 | 1.35 | 262.2527, 184.0743 | 813.7 → 184.1 | 170 | 20 | |
| SM (d18:2/24:1) | 14.80 | C47H91N2O6P | 811.6701 | 811.6688 | 1.60 | 262.2535, 184.0743 | 811.7 → 184.1 | 170 | 20 | |
| SM (d18:2/25:0) | 16.21 | C48H95N2O6P | 827.6995 | 827.7001 | −0.72 | 262.2548, 184.0741 | 827.7 → 184.1 | 170 | 20 | |
| SM (d18:2/25:0) isomer-1 | 16.42 | C48H95N2O6P | 827.6989 | 827.7001 | −1.45 | 262.2548, 184.0741 | 827.7 → 184.1 | 170 | 20 | |
| SM (d18:2/25:0) isomer-2 | 16.72 | C48H95N2O6P | 827.7022 | 827.7001 | 2.54 | 262.2548, 184.0741 | 827.7 → 184.1 | 170 | 20 | |
| SM (d32:0) | 11.43 | C37H77N2O6P | 677.5580 | 677.5592 | −1.77 | 184.0740 | 677.6 → 184.1 | 170 | 20 | |
| SM (d33:0) | 11.90 | C38H79N2O6P | 691.5761 | 691.5749 | 1.74 | 184.0739 | 691.6 → 184.1 | 170 | 20 | |
| SM (d33:1) | 11.51 | C38H77N2O6P | 689.5606 | 689.5592 | 2.03 | 184.0744 | 689.6 → 184.1 | 170 | 20 | |
| SM (d34:0) | 12.43 | C39H81N2O6P | 705.5924 | 705.5905 | 2.69 | 184.0745 | 705.6 → 184.1 | 170 | 20 | |
| SM (d35:0) | 13.20 | C40H83N2O6P | 719.6050 | 719.6062 | −1.67 | 184.0744 | 719.6 → 184.1 | 170 | 20 | |
| SM (d35:1) | 12.54 | C40H81N2O6P | 717.5914 | 717.5905 | 1.25 | 184.0741 | 717.6 → 184.1 | 170 | 20 | |
| SM (d35:1) isomer | 12.37 | C40H81N2O6P | 717.5918 | 717.5905 | 1.81 | 184.0754 | 717.6 → 184.1 | 170 | 20 | |
| SM (d37:1) | 13.76 | C42H85N2O6P | 745.6223 | 745.6218 | 0.67 | 184.0740 | 745.6 → 184.1 | 170 | 20 | |
| SM (d37:2) | 12.85 | C42H83N2O6P | 743.6081 | 743.6062 | 2.56 | 184.0733 | 743.6 → 184.1 | 170 | 20 | |
| SM (d38:0) | 14.90 | C43H89N2O6P | 761.6530 | 761.6531 | −0.13 | 184.0746 | 761.7 → 184.1 | 170 | 20 | |
| SM (d38:2) | 13.46 | C43H85N2O6P | 757.6231 | 757.6218 | 1.72 | 184.0745 | 757.6 → 184.1 | 170 | 20 | |
| SM (d39:2) | 14.09 | C44H87N2O6P | 771.6387 | 771.6375 | 1.56 | 184.0734 | 771.6 → 184.1 | 170 | 20 | |
| SM (d40:0) | 16.10 | C45H93N2O6P | 789.6847 | 789.6844 | 0.38 | 184.0742 | 789.7 → 184.1 | 170 | 20 | |
| SM (d41:0) | 16.85 | C46H95N2O6P | 803.7001 | 803.7001 | 0.00 | 184.0739 | 803.7 → 184.1 | 170 | 20 | |
| SM (d41:3) | 14.31 | C46H89N2O6P | 797.6529 | 797.6531 | −0.25 | 184.0737 | 797.7 → 184.1 | 170 | 20 | |
| SM (d42:4) | 14.00 | C47H89N2O6P | 809.6553 | 809.6531 | 2.72 | 184.0744 | 809.7 → 184.1 | 170 | 20 | |
| SM (d43:1) | 17.62 | C48H97N2O6P | 829.7169 | 829.7157 | 1.45 | 184.0747 | 829.7 → 184.1 | 170 | 20 | |
| SM (d43:1) isomer | 17.35 | C48H97N2O6P | 829.7165 | 829.7157 | 0.96 | 184.0728 | 829.7 → 184.1 | 170 | 20 | |
| SM (t44:4) | 14.82 | C49H93N2O7P | 853.6787 | 853.6793 | −0.70 | 184.0730 | 853.7 → 184.1 | 170 | 20 | |
| HexCer | GalCer (d18:1/12:0) [I.S.-8] | 10.34 | C36H69NO8 | 644.5107 | 644.5096 | 1.71 | 264.2684 | 644.5 → 264.3 | 130 | 30 |
| HexCer (d18:1/20:0) | 14.30 | C44H85NO8 | 756.6327 | 756.6348 | −2.78 | 264.2685 | 756.6 → 264.3 | 130 | 30 | |
| HexCer (d18:1/22:0) | 15.60 | C46H89NO8 | 784.6659 | 784.6661 | −0.25 | 264.2689 | 784.7 → 264.3 | 130 | 30 | |
| HexCer (d18:1/23:0) | 16.30 | C47H91NO8 | 798.6805 | 798.6817 | −1.50 | 264.2676 | 798.7 → 264.3 | 130 | 30 | |
| HexCer (d18:1/24:0) | 16.90 | C48H93NO8 | 812.6973 | 812.6974 | −0.12 | 264.2685 | 812.7 → 264.3 | 130 | 30 | |
| LacCer (d18:1/12:0) [I.S.-9] | 10.13 | C42H79NO13 | 806.5640 | 806.5624 | 1.98 | 788.5498, 482.4589, 264.2683 | 806.7 → 264.3 | 145 | 40 | |
| LacCer (d18:1/16:0) | 10.96 | C46H87NO13 | 862.6259 | 862.6250 | 1.04 | 844.6139, 538.5174, 264.2687 | 862.6 → 264.3 | 145 | 40 | |
| LacCer (d18:1/18:0) | 11.33 | C48H91NO13 | 890.6549 | 890.6563 | −1.57 | 264.2696 | 890.7 → 264.3 | 145 | 40 | |
| LacCer (d18:1/20:0) | 16.95 | C50H95NO13 | 918.6860 | 918.6876 | −1.74 | 264.2688 | 918.7 → 264.3 | 145 | 40 | |
| LacCer (d18:1/22:0) | 15.00 | C52H99NO13 | 946.7192 | 946.7189 | 0.32 | 264.2679 | 946.7 → 264.3 | 145 | 40 | |
| LacCer (d18:1/24:0) | 16.30 | C54H103NO13 | 974.7512 | 974.7502 | 1.03 | 264.2672 | 974.8 → 264.3 | 145 | 40 | |
| LacCer (d18:1/24:1) | 15.10 | C54H101NO13 | 972.7323 | 972.7346 | −2.36 | 264.2650 | 972.7 → 264.3 | 145 | 40 | |
Notes: Abbreviations: MRM, multiple reaction monitoring; RT, retention time; CE, collision energy; So, sphingosine; Sa, sphinganine; S1P, sphingosine-1-phosphate; Cer, ceramide; Cer1P, ceramide-1-phosphate; SM, sphingomyelin; GalCer, galactosylceramide; HexCer, hexosylceramide; LacCer, lactosylceramide. I.S. -1–9 represent the nine internal standards added for MS analysis.
Figure 1.
MRM chromatograms of 87 sphingolipids in human serum.
Differences in serum sphingolipidomes between PCOS patients and healthy women
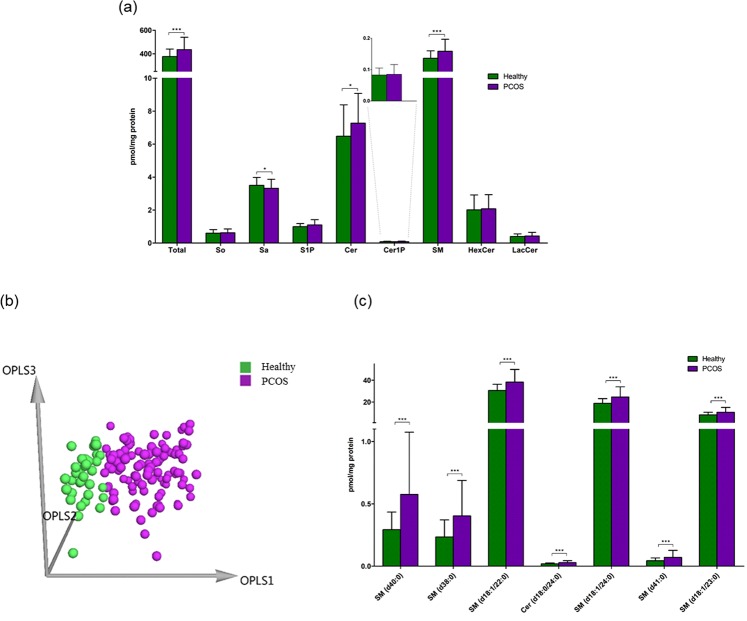
As shown in Fig. 2a, the total sphingolipid content in PCOS patients showed a significant increase (15.9% increase) when compared with healthy women. Among the sphingolipids, three subclasses demonstrated remarkable elevations in PCOS patients, including S1P (10.8% increase), Cer (12.2% increase), and SM (16.3% increase). Sa was the only subclass showing a decrease in PCOS patients. Multivariate analysis was subsequently carried out to discriminate between PCOS patients and healthy women. As shown in Fig. 2b, the three-dimensional (3D) orthogonal partial least squares discriminant analysis (OPLS-DA) score plot demonstrated a clear separation between the PCOS group and the healthy group (R2X = 0.523, R2Y = 0.697, Q2 = 0.533). Sphingolipids with a variable importance plot (VIP) value > 1.5 were regarded as the important variables contributing to the 3D OPLS-DA model. As a result, 7 sphingolipids, including 6 SMs (SM (d40:0), SM (d38:0), SM (d18:1/22:0), SM (d18:1/24:0), SM (d41:0), and SM (d18:1/23:0)) and 1 Cer (Cer (d18:0/24:0)) were selected as potential biomarkers (Fig. 2c).
Figure 2.
(a) The levels of total sphingolipids and of each subclass of sphingolipid in healthy women (n = 37) and PCOS patients (n = 107). Each column represents the mean ± SD (*p < 0.05, ***p < 0.001). So, sphingosine; Sa, sphinganine; S1P, sphingosine-1-phosphate; Cer, ceramide; Cer1P, ceramide-1-phosphate; SM, sphingomyelin; HexCer, hexosylceramide; LacCer, lactosylceramide. (b) The 3D OPLS-DA score plot for the healthy group (n = 37) and the PCOS group (n = 107) (R2X = 0.523, R2Y = 0.697, Q2 = 0.533). (c) The potential biomarkers (SM (d40:0), SM (d38:0), SM (d18:1/22:0), Cer (d18:0/24:0), SM (d18:1/24:0), SM (d41:0), and SM (d18:1/23:0)) for the classification between the healthy group and the PCOS group. Each column represents the mean ± SD (***p < 0.001).
Differences in serum sphingolipidomes between different subgroups of PCOS and healthy women
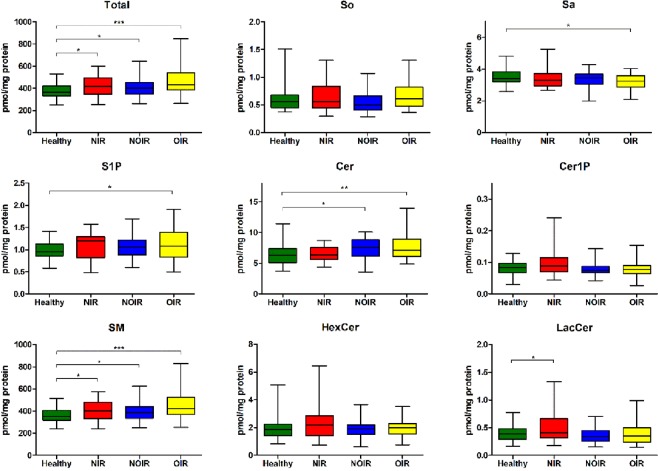
The PCOS group was classified into three subgroups (OIR, NOIR, NIR) as described above for further comparison with the healthy group. As shown in Fig. 3, the levels of total sphingolipids and of each sphingolipid subclass in all three subgroups of PCOS followed similar trends as the overall PCOS group. Notably, OIR showed the most obvious elevation (23.83%) in the total sphingolipid level when compared to the healthy group. All three subgroups of PCOS demonstrated significant increases in the level of SM (OIR > NOIR > NIR), while only NOIR and OIR showed significant increases in the level of Cer (OIR > NOIR), and only OIR showed significant increases in the level of S1P. The level of Sa was decreased in all three subgroups of PCOS, in which only OIR demonstrated a significant change. Unexpectedly, the level of LacCer was only elevated in NIR (28.72%), and there was almost no change in NOIR and OIR.
Figure 3.
Box and whiskers plot showing the levels of total sphingolipids and of each subclass of sphingolipid in healthy women (n = 37), NIR PCOS patients (n = 32), NOIR PCOS patients (n = 34), and OIR PCOS patients (n = 41). *p < 0.05, **p < 0.01, ***p < 0.001. So, sphingosine; Sa, sphinganine; S1P, sphingosine-1-phosphate; Cer, ceramide; Cer1P, ceramide-1-phosphate; SM, sphingomyelin; HexCer, hexosylceramide; LacCer, lactosylceramide.
Differences in serum sphingolipidomes among different subgroups of PCOS patients
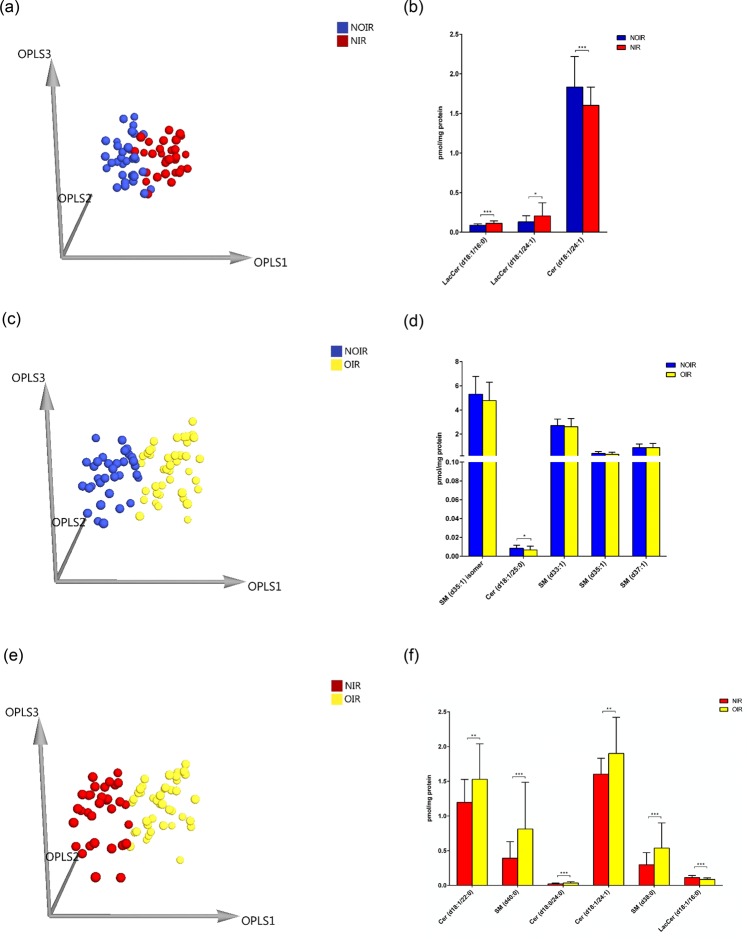
Multivariate statistical analysis was carried out to discriminate between NIR, NOIR, and OIR. Figure 4a shows the 3D OPLS-DA score plot of NOIR and NIR (R2X = 0.499, R2Y = 0.691, Q2 = 0.301), and 3 sphingolipids, including 2 LacCers (LacCer (d18:1/16:0) and LacCer (d18:1/24:1)) and 1 Cer (Cer (d18:1/24:1)), were identified as potential markers (Fig. 4b). Figure 4c shows the 3D OPLS-DA score plot of NOIR and OIR (R2X = 0.481, R2Y = 0.415, Q2 = 0.113), and 1 Cer (Cer (d18:1/25:0)) was identified as a potential marker (Fig. 4d). Figure 4e shows the 3D OPLS-DA score plot of NIR and OIR (R2X = 0.516, R2Y = 0.534, Q2 = 0.416), and 6 sphingolipids, including 3 Cers (Cer (d18:1/22:0), Cer (d18:0/24:0), and Cer (d18:1/24:1)), 2 SMs (SM (d40:0) and SM (d38:0)), and 1 LacCer (LacCer (d18:1/16:0)) were identified as potential markers (Fig. 4f).
Figure 4.
(a) The 3D OPLS-DA score plot for NOIR patients (n = 34) and NIR patients (n = 32) (R2X = 0.499, R2Y = 0.691, Q2 = 0.301). (b) The potential markers (LacCer (d18:1/16:0), LacCer (d18:1/24:1), and Cer (d18:1/24:1)) for the classification between NOIR patients and NIR patients. Each column represents the mean ± SD. *p < 0.05, ***p < 0.001. (c) The 3D OPLS-DA score plot for NOIR patients (n = 34) and OIR patients (n = 41) (R2X = 0.481, R2Y = 0.415, Q2 = 0.113). (d) The potential marker (Cer (d18:1/25:0)) for the classification between NOIR patients and OIR patients. The columns represent the mean ± SD. *p < 0.05. (e) The 3D OPLS-DA score plot for NIR patients (n = 32) and OIR patients (n = 41) (R2X = 0.516, R2Y = 0.534, Q2 = 0.416). (f) The potential markers (Cer (d18:1/22:0), SM (d40:0), Cer (d18:0/24:0), Cer (d18:1/24:1), SM (d38:0), and LacCer (d18:1/16:0)) for the classification between NIR patients and OIR patients. Each column represents the mean ± SD. **p < 0.01, ***p < 0.001.
Discussion
In this study, the serum sphingolipids in healthy controls and PCOS patients were comprehensively investigated using the improved LC-MS–based sphingolipidomic approach23. As a result, 87 sphingolipids were identified and quantified in each sample. Notable elevations in the levels of S1P, Cer, and SM were observed in PCOS patients, and these made the largest contribution to the significant increase in the total sphingolipid content in PCOS patients. Our results are in accordance with a previous study that suggested that PCOS is associated with increased TG and SM and decreased phosphatidylethanolamines and lysophosphatidylcholines in patient plasma35. The multivariate analysis between PCOS patients and healthy women also suggested that SM was the most dominant subclass of sphingolipid involved in the pathogenesis of PCOS because 6 out of 7 potential markers were SMs. Notably, all potential markers, including the one Cer marker, have saturated acyl chains. A previous report showed that the increase in SM species with long saturated acyl chains (18:0, 20:0, 22:0, and 24:0) was closely correlated with the development of metabolic syndrome associated with lipid metabolism, obesity, and IR36. Therefore, we suggest that SM species with long saturated acyl chains might serve as novel biomarkers of PCOS, and further studies should be carried out to confirm this.
In addition to PCOS, other metabolic diseases such as obesity and IR have also been shown to be associated with lipid alterations37. Therefore, we further divided the PCOS patients into three subgroups (NIR, NOIR, OIR) according to their HOMA-IR and BMI values. NIR represented the group without IR or obesity, NOIR represented the group with IR only, and OIR represented the group with both IR and obesity. The levels of total sphingolipids and of each subclass in all three subgroups of PCOS followed similar trends as for all PCOS patients combined, while the subgroups of PCOS with IR or obesity exhibited greater changes than their corresponding subgroups without IR or obesity. As mentioned above, an increase in SM species with long saturated acyl chains was closely associated with lipid metabolism, obesity, and IR. Therefore, the presence of IR or both IR and obesity contributed to the increase in the level of SM in the order of OIR > NOIR > NIR. In addition, plasma Cer was reported to be elevated in obese subjects with type 2 diabetes mellitus, and increased levels of plasma Cer might also be a marker of IR38. However, in our study the occurrence of PCOS seems to have no direct relationship with Cer, and only NOIR and OIR showed significant increases in the level of Cer (OIR > NOIR). Furthermore, S1P was also reported to be elevated in the plasma of obese subjects and to correlate with IR39, and this is the likely explanation for why only OIR demonstrated a significant increase in the level of S1P in our study.
No previous report has discussed the relation between Sa and PCOS. However, because Sa is upstream of Cer in the metabolism pathway of sphingolipids, we speculate that the decrease we observed in Sa might be the result of the upregulation of Cer synthases leading to the accumulation of Cer and further downstream products. However, further studies are needed to confirm this hypothesis.
It is interesting that the level of LacCer was only elevated in NIR, while there was almost no change in NOIR and OIR. This implies that the level of LacCer was significantly increased in PCOS patients who did not suffer from IR and obesity. However, previous studies indicated that there is a negative correlation between gangliosides (complex glycosphingolipids) and insulin responsiveness40, and it was observed that inhibition of glucosylceramide synthase activity reversed IR in several rodent models of obesity41. Therefore, in theory the level of LacCer would be expected to be increased in PCOS patients with IR. However, up to now there have been no studies to investigate the direct interaction of LacCer on insulin responsiveness and the relationship between glucosylceramide synthase activity and IR in humans. Thus the results of the subgroup analysis further suggest that PCOS patients have unique sphingolipid biomarkers that are not caused only by obesity and IR.
In the multivariate statistical analysis of sphingolipid alterations among the three subgroups of PCOS, it was found that the discrimination between the three subgroups of PCOS was unsatisfactory, especially between NOIR and OIR. Only a single sphingolipid showed a significant difference between NOIR and OIR, and this might be explained by the close relationship between IR and obesity, which often cause similar alterations in sphingolipid metabolism. The differentiation between NIR and OIR was comparatively better. These results suggested that alterations in sphingolipid metabolism might play a role in the pathogenesis of PCOS and that sphingolipids might be useful as diagnostic biomarkers for different types of PCOS.
This study represents the first report of a comprehensive sphingolipidomic profiling of PCOS and different subgroups of PCOS with or without IR and/or obesity. The results shed light on the diagnosis and pathogenesis of PCOS. A limitation of the study is that we only detected some of the sphingolipid characteristics for different types of PCOS patients, and the mechanism of alterations of sphingolipid metabolism in PCOS needs further investigation in both human subjects and animal models.
Materials and Methods
Study Participants
PCOS patients aged 18 to 40 years were recruited between January 2014 and May 2015 from the Department of Traditional Chinese Medicine of the First Affiliated Hospital of Guangzhou Medical University. The first woman was recruited on January 2, 2014, and the last one was on May 28, 2015. Age-matched healthy female subjects were selected from community volunteers. PCOS was diagnosed as having two of the following three Rotterdam criteria42: (1) oligomenorrhea/amenorrhea; (2) more than 12 follicles ≤9 mm in diameter or ovarian volume >10 ml on pelvic scanning; and (3) clinical or biochemical hyperandrogenism. Oligomenorrhea is defined as an intermenstrual interval >35 days or <8 menstrual bleedings in the past year. Amenorrhea is defined as an intermenstrual interval >90 days. Biochemical hyperandrogenism is defined as a TT level ≥ 0.6 ng/ml43, and clinical hyperandrogenism is defined as a Ferriman–Gallwey score ≥ 544. Women were excluded if they had other endocrine disorders such as hyperprolactinemia, type I or type II diabetes mellitus, non-classic congenital adrenal hyperplasia, suspected Cushing’s syndrome, androgen-secreting tumors, thyroid diseases, or drug-induced androgen excess. Women who had received any hormonal treatments, Chinese herbal prescriptions, or acupuncture treatments in the past 3 months were also excluded from the study. All controls had regular menstrual cycles and normal hormone levels.
Eligible PCOS patients were further categorized as obese if they had a BMI ≥ 25 (kg/m2) according to the World Health organization (WHO) criteria for Asians45 and were categorized as insulin resistant if they had a HOMA-IR (calculated as [fasting glucose] × [fasting insulin]/22.5)) ≥ 2.1446.
This study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2013–39 and 2016–64). Informed written consent was obtained from all subjects before inclusion.
Body conditions and detection of substances related to endocrine, glucose, and lipid metabolism
The height, weight, waist circumference, hip circumference, and hirsutism scores of all women were recorded, and blood samples were collected in the first 2–5 days of their menstrual cycle at 08.00–10.00 in the morning after fasting for 12 hours. The serum samples were batched and analyzed in the laboratory in the First Affiliated Hospital of Guangzhou Medical University, and serum FSH, LH, estradiol (E2), TT, prolactin, TG, CHOL, LDL, HDL, fasting plasma glucose, and fasting insulin were measured. The intra-assay and inter-assay coefficients of variation were less than 5%. HOMA-IR was calculated to assess changes in insulin sensitivity40. FSH, LH, and TT levels were measured with a Beckman-Coulter Unicel DXi800 automatic chemiluminescence analyzer (Beckman Coulter, Brea, USA), and TG, CHOL, LDL, and HDL levels were measured on a Beckman-Coulter AU5800 automatic biochemical analyzer. Fasting insulin was analyzed using the Modular E170® automatic electrochemiluminescent analyzer (Roche Diagnostics, Mannheim, Germany). Fasting plasma glucose was measured on a Beckman Coulter LX20 automatic biochemical analyzer. All assays were performed based on the instructions of manufacturers and with reagents and materials provide by the manufacturers.
Sample collection and processing
All of the blood samples were collected by staff in the First Affiliated Hospital of Guangzhou Medical University. The blood samples were 5 ml and were centrifuged for 15 minutes at 3,000 rpm within 30 minutes after the blood samples were drawn. The separated serum was stored at −80 °C and was delivered to the State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, within 3 months of being collected.
Sample preparation and sphingolipidomic assays
Extraction of sphingolipids
Serum sphingolipids were extracted using an established method23. Briefly, 20 μl of serum was transferred into a borosilicate glass tube, and 10 μl of internal standards (2.5 μM each) and 0.75 ml of extraction solvent [methanol (MeOH): chloroform, 2:1, v/v] were added. The contents were dispersed in an ultrasonicator at room temperature for 30 seconds, followed by incubation at 48 °C for 12 hours. After cooling, 75 μl of potassium hydroxide in MeOH (1 M) was added and incubated with shaking at 37 °C for 2 hours, after which 3 μl acetic acid was added to neutralize the extract. After centrifugation, the supernatant was stored in a 4 ml bottle, and the residue was re-extracted twice. Finally, the extract was dried by nitrogen and re-dissolved in 150 μl of MeOH for liquid chromatography-mass spectrometry (LC-MS) detection. The quality control (QC) sample was pooled with equal quantities of samples from the different groups for the sphingolipid analysis.
Detection of sphingolipids by LC-MS
Serum sphingolipids were detected with the improved LC-MS–based sphingolipidomic approach23. The chromatographic separation of sphingolipids was performed on an Agilent 1290 Infinity UHPLC system (Santa Clara, CA, USA) with an Agilent Eclipse Plus C18 column (100 mm × 2.1 mm, 1.8 μm) at 40 °C. The mobile phase consisted of (A) MeOH/H2O/formic acid (HCOOH) (60:40:0.2, v/v/v) and (B) MeOH/isopropyl alcohol/HCOOH (60:40:0.2, v/v/v), both containing 10 mM NH4OAc. Qualitative analysis of sphingolipids was performed on an Agilent ultra high definition (UHD) 6550 quadrupole time-of-flight (Q-TOF) MS (Santa Clara, CA, USA). The MS source parameters were as follows: drying gas (nitrogen) temperature 200 °C, drying gas flow 11 L/min, sheath gas (nitrogen) temperature 300 °C, sheath gas flow 12 L/min, capillary voltage 4000 V, nozzle voltage 200 V, nebulizer pressure 40 psi, skimmer voltage 65 V, octopole RF peak 500 V, and fragmentor voltage 175 V. The targeted MS/MS collision energy was set at 20–60 eV. MS spectra and MS-MS spectra were acquired in positive mode with the mass range of m/z 110–1700 and m/z 40–1700, respectively. A reference solution was nebulized for continuous calibration using the reference mass of m/z 922.0098. Quantitative analysis of sphingolipids was carried out on an Agilent 6460 triple quadrupole (QQQ) MS. The MS source parameters were as follows: drying gas (nitrogen) temperature 325 °C, drying gas flow 11 L/min, capillary voltage 4000 V, and nebulizer pressure 30 psi. The optimized parameters for each individual sphingolipid, such as characteristic transition (precursor ion → product ion), fragmentor voltage, and collision energy are shown in Table 2.
Data analysis
An in-house sphingolipid database has been established in our laboratory based on the Agilent MassHunter Personal Compound Database and Library software and information from the LIPID MAPS Lipidomics Gateway. Using this customized sphingolipid database and the “find-by-formula” option in the MassHunter Qualitative Analysis Software (version B.06.00), potential sphingolipids were identified based on a comparison of accurate mass, abundance of the isotopes, and isotope spacing with the calculated theoretical masses and abundances. The structures of potential identified sphingolipids were further determined according to the characterized fragments obtained from high-resolution MS/MS data.
The raw data for quantitative analysis were processed with Agilent MassHunter Quantitative Analysis B.06.00 software. The resulting data were first transferred into a Microsoft Excel-type spreadsheet and then imported into the SIMCA-P+ 14.0 software (Umetrics, Umea, Sweden) for multivariate statistical analysis. Principal component analysis (PCA) was used to visualize general clustering among the different groups. 3D OPLS-DA was carried out to identify differences in sphingolipid expression between the different groups based on their VIP values. Variables that were significantly changed among different samples were selected as potential biomarkers based on VIP > 1.5 and then validated using t-test analysis.
Analysis of variance (ANOVA) or Kruskal–Wallis tests were performed to examine the differences in the clinical characteristics among the different groups using the SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA). Values are shown as the mean and 95% confidence interval, and a value of p < 0.05 was considered statistically significant.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Reference: 2013039 and 2016064) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conclusion
In conclusion, remarkable elevations in the levels of S1P, Cer, and SM were observed in PCOS patients compared to healthy women. Notably, SM (d40:0), SM (d38:0), SM (d18:1/22:0), SM (d18:1/24:0), SM (d41:0) and Cer (d18:0/24:0) showed the most significant alterations, which showed that SM species with long saturated acyl chains were the best candidates to serve as novel biomarkers of PCOS. Our results are in accordance with previous studies suggesting that PCOS is associated with increased SM and that SM species with long saturated acyl chains are closely correlated with the development of metabolic syndrome, obesity, and IR. In all three subgroups of PCOS (NIR, NOIR, OIR), the alteration of sphingolipid levels followed a similar trend as the overall PCOS group except for LacCer, which was only elevated in NIR. Our results suggest that serum sphingolipids might be useful as diagnostic biomarkers for different subgroups of PCOS.
Acknowledgements
We thank Professor Ernest Hung Yu Ng for language correction and critical review of the manuscript. This study was funded by the Natural Science Foundation of Guangdong Province, China (grant number 2015A030310508), the Program for Doctor Startup of Guangzhou Medical University (grant number 2014C29), and the Macao Science and Technology Development Fund (grant number 023/2016/AFJ to WJR).
Author Contributions
J.L., L.X. and J.S. contributed equally to this work. H.M. and J.W. conceived and designed the study. J.L., L.X., J.S. and J.W. drafted and critically revised the manuscript for important intellectual content. J.W., L.X. and J.M. finished the sample preparation and sphingolipidomic assays. C.Z. and W.W. collected and handled the samples. H.M., J.L. and J.W. sought funding and ethics approval. Z.J. and L.Y. provided the technical platform and support. All of the authors contributed to the writing of the manuscript and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jing-Rong Wang, Email: jrwang@must.edu.mo.
Hong-Xia Ma, Email: doctorhongxia@126.com.
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Li R, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum. Repro. 2013;28(9):2562–2569. doi: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- 3.Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecol. Endocrinol. 2010;26(4):281–296. doi: 10.3109/09513590903247873. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin GA, Morales AJ, Yen SS. Serum leptin levels in women with polycystic ovary syndrome: the role of insulin resistance/hyperinsulinemia. J. Clin. Endocrinol. Metab. 1997;82(6):1692–6. doi: 10.1210/jcem.82.6.4028. [DOI] [PubMed] [Google Scholar]
- 5.Pasquali R, Gambineri A. Glucose intolerance states in women with the polycystic ovary syndrome. J. Endocrinol. Invest. 2013;36(8):648–53. doi: 10.1007/BF03346757. [DOI] [PubMed] [Google Scholar]
- 6.Macut D, BjekićMacut J, SavićRadojević A. Dyslipidemian and oxidative stress in PCOS. Front. Horm. Res. 2013;40:51–63. doi: 10.1159/000341683. [DOI] [PubMed] [Google Scholar]
- 7.Li S, et al. Discovery of novel lipid profiles in PCOS: Do insulin and androgen oppositely regulate bioactive lipid production. J. Clin. Endocrinol. Metab. 2017;102(3):810–821. doi: 10.1210/jc.2016-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peigné M, Dewailly D. Long term complications of polycystic ovary syndrome (PCOS) Annales d’Endocrinologie. 2014;75(4):194–199. doi: 10.1016/j.ando.2014.07.111. [DOI] [PubMed] [Google Scholar]
- 9.Vélez LM, Motta AB. Association between polycystic ovary syndrome and metabolic syndrome. Curr. Med. Chem. 2014;21(35):3999–4012. doi: 10.2174/0929867321666140915141030. [DOI] [PubMed] [Google Scholar]
- 10.Petraglia F, Musacchio C, Luisi S, De LV. Hormone-dependent gynaecological disorders: a pathophysiological perspective for appropriate treatment. Best Pract. Res. Clin. Obstet. Gynaecol. 2008;22(2):235–49. doi: 10.1016/j.bpobgyn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Alexander CJ, Tangchitnob EP, Lepor NE. Polycystic ovary syndrome: a major unrecognized cardiovascular risk factor in women. Rev. Cardiovasc. Med. 2009;10(2):83–90. [PubMed] [Google Scholar]
- 12.Alpañés M, et al. Influence of adrenal hyperandrogenism on the clinical and metabolic phenotype of women with polycystic ovary syndrome. Fertil. Steril. 2015;103(3):795–801. doi: 10.1016/j.fertnstert.2014.12.105. [DOI] [PubMed] [Google Scholar]
- 13.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin. Endocrinol (Oxf). 2002;57(3):343–50. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 14.Dong F, et al. Serum metabolomics study of polycystic ovary syndrome based on UPLC-QTOF-MS coupled with a pattern recognition approach. Anal. Bioanal. Chem. 2015;407(16):4683–95. doi: 10.1007/s00216-015-8670-x. [DOI] [PubMed] [Google Scholar]
- 15.Chiu HK, et al. Equivalent insulin resistance in latent autoimmune diabetes in adults (LADA) and type 2 diabetic patients. Diabetes Res. Clin. Pract. 2007;77(2):237–44. doi: 10.1016/j.diabres.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 16.RoyChoudhury S, et al. Polycystic ovary syndrome in Indian women: a mass spectrometry based serum metabolomics approach. Metabolomics. 2017;13:115. doi: 10.1007/s11306-017-1253-4. [DOI] [Google Scholar]
- 17.RoyChoudhury S, et al. Serum metabolomics of Indian women with polycystic ovary syndrome using 1 H NMR coupled with a pattern recognition approach. Molecular BioSystems. 2016;12(11):3407–3416. doi: 10.1039/C6MB00420B. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC medicine. 2012;10:153. doi: 10.1186/1741-7015-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, et al. Comprehensive analysis of serum metabolites in gestational diabetes mellitus by UPLC/Q-TOF-MS. Anal. Bioanal. Chem. 2016;408(4):1125–1135. doi: 10.1007/s00216-015-9211-3. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, et al. Metabonomics Reveals Plasma Metabolic Changes and Inflammatory Marker in Polycystic Ovary Syndrome Patients. J. Proteome. Res. 2012;11(5):2937–2946. doi: 10.1021/pr3000317. [DOI] [PubMed] [Google Scholar]
- 21.Chen YX, et al. UHPLC/Q-TOFMS-based plasma metabolomics of polycystic ovary syndrome patients with and without insulin resistance. J. Pharmaceut. Biomed. Anal. 2016;121:141–150. doi: 10.1016/j.jpba.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XJ, et al. Characterizing plasma phospholipid fatty acid profiles of polycystic ovary syndrome patients with and without insulin resistance using GC-MS and chemometrics approach. J. Pharmaceut. Biomed. Anal. 2014;95:85–92. doi: 10.1016/j.jpba.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Wang JR, et al. Improved sphingolipidomic approach based on ultra-high performance liquid chromatography and multiple mass spectrometries with application to cellular neurotoxicity. Anal. Chem. 2014;86(12):5688–5696. doi: 10.1021/ac5009964. [DOI] [PubMed] [Google Scholar]
- 24.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 25.Mor E, et al. The insulin resistant subphenotype of polycystic ovary syndrome: clinical parameters and pathogenesis. Am. J. Obstet. Gynecol. 2004;190(6):1654–60. doi: 10.1016/j.ajog.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 26.Lahiri S, et al. Kinetic characterization of mammalian ceramide synthases:determination of K(m) values towards sphinganine. FEBS. Lett. 2007;581(27):5289–5294. doi: 10.1016/j.febslet.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Merrill AH, Jr, Wang MD, Park M, Sullards MC. (Glyco) sphingolipidology: an amazing challenge and opportunity for systems biology. Trends Biochem. Sci. 2007;32(10):457–468. doi: 10.1016/j.tibs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Bikman BT. A role for sphingolipids in the pathophysiology of obesity-induced inflammation. Cell Mol. Life Sci. 2012;69(13):2135–2146. doi: 10.1007/s00018-012-0917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inokuchi J. Membrane microdomains and insulin resistance. FEBS Lett. 2010;584(9):1864–1871. doi: 10.1016/j.febslet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54(7):1596–1607. doi: 10.1007/s00125-011-2127-3. [DOI] [PubMed] [Google Scholar]
- 31.Rapizzi. E, et al. Sphingosine 1-phosphate increases glucose uptake through trans-activation of insulin receptor. Cell Mol. Life Sci. 2009;66(19):3207–3218. doi: 10.1007/s00018-009-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollica MP, et al. Fromchronic overfeeding to hepatic injury: role of endoplasmic reticulum stress and inflammation. Nutr. Metab. Cardiovasc. Dis. 2011;21(3):222–230. doi: 10.1016/j.numecd.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Kolter T. A view on sphingolipids and disease. Chem. Phys. Lipids. 2011;164(6):590–606. doi: 10.1016/j.chemphyslip.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Sharma BR, Kim HJ, Rhyu DY. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015;13(1):62. doi: 10.1186/s12967-015-0412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haoula Z, et al. Lipidomic analysis of plasma samples from women with polycystic ovary syndrome. Metabolomics. 2015;11(3):657–666. doi: 10.1007/s11306-014-0726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanamatsu H, et al. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutrition & diabetes. 2014;4(10):e141. doi: 10.1038/nutd.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mika A, Sledzinski T, Stepnowski P. Current progress of lipid analysis in metabolic diseases by mass spectrometry methods. Current medicinal chemistry. 2018;25:1–45. doi: 10.2174/092986732501180122140757. [DOI] [PubMed] [Google Scholar]
- 38.Haus JM, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowalski GM, Carey AL, Selathurai A, Kingwell BA, Bruce CR. Plasma Sphingosine-1-Phosphate Is Elevated in Obesity. Plos One. 2013;8(9):e72449. doi: 10.1371/journal.pone.0072449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nojiri H, Stroud M, Hakomori S. A Specific Type of Ganglioside as a Modulator of Insulin-Dependent Cell Growth and Insulin Receptor Tyrosine Kinase Activity. Possible Association of Ganglioside-Induced Inhibition of Insulin Receptor Function and Monocytic Differentiation Induction in Hl-60 Cells. J. Biol. Chem. 1991;266(7):4531–4537. [PubMed] [Google Scholar]
- 41.van Eijk M, et al. Reducing glycosphingolipid content in adipose tissue of obese mice restores insulin sensitivity, adipogenesis and reduces inflammation. Plos One. 2009;4(3):e4723. doi: 10.1371/journal.pone.0004723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rotterdam ESHRE/ASRM‐Sponsored PCOS consensus workshop group: Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19, 41–47 (2004). [DOI] [PubMed]
- 43.Shi Y, et al. Analysis of clinical characteristics in large-scale Chinese women with polycystic ovary syndrome. Neuro. Endocrinol. Lett. 2007;28(6):807–810. [PubMed] [Google Scholar]
- 44.Zhao X, et al. Defining hirsutism in Chinese women: a cross-sectional study. Fertil. Steril. 2011;96(3):792–796. doi: 10.1016/j.fertnstert.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 45.World WHO Western Pacific Regional Office, IOTF, IASO: The Asia-Pacific perspective: Redefining obesity and its treatment. Sydney; Health Communications Australia (2000).
- 46.Chen X, Yang D, Li L, Feng S, Wang L. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum. Reprod. 2006;21(8):2027–2032. doi: 10.1093/humrep/del142. [DOI] [PubMed] [Google Scholar]