Abstract
Understanding the causes of vaccine failure is important for predicting disease dynamics in vaccinated populations and planning disease interventions. Pathogen exposure dose and heterogeneity in host susceptibility have both been implicated as important factors that may reduce overall vaccine efficacy and cause vaccine failure. Here, we explore the effect of pathogen dose and heterogeneity in host susceptibility in reducing efficacy of vaccines. Using simulation-based methods, we find that increases in pathogen exposure dose decrease vaccine efficacy, but this effect is modified by heterogeneity in host susceptibility. In populations where the mode of vaccine action is highly polarized, vaccine efficacy decreases more slowly with exposure dose than in populations with less variable protection. We compared these theoretical results to empirical estimates from a systematic literature review of vaccines tested over multiple exposure doses. We found that few studies (nine of 5,389) tested vaccine protection against infection over multiple pathogen challenge doses, with seven studies demonstrating a decrease in vaccine efficacy with increasing exposure dose. Our research demonstrates that pathogen dose has potential to be an important determinant of vaccine failure, although the limited empirical data highlight a need for additional studies to test theoretical predictions on the plausibility of reduced host susceptibility and high pathogen dose as mechanisms responsible for reduced vaccine efficacy in high transmission settings.
Introduction
Measurements of vaccine efficacy frequently vary across transmission settings, which limits generalizability of results from preclinical experiments and clinical trials. For example, efficacy of the RTS/S malaria vaccine varies dramatically among location (e.g. 17% efficacy in Burkina Faso to 66% in Kilifi, Kenya)1. Differences in exposure risk among individuals likely contribute to observed differences in vaccine efficacy among clinical trials2. Differences in exposure dose affect disease risk, and high doses of pathogens increase the probability of infection in most host-pathogen systems3. However, the relationship between pathogen dose and vaccine efficacy is less clear, and few studies have tested vaccines across a broad range of pathogen doses4–10, notwithstanding the belief that vaccines may be overcome at high rates of exposure11.
Differences in susceptibility among and within populations also affect risk, thus influencing measurements of vaccine efficacy. Here, we define ‘susceptibility’ as the probability of infection per unit of exposure. Myriad factors may affect susceptibility including age, nutritional status, or infection with other parasites12. Effective vaccines should reduce mean susceptibility thereby decreasing the probability hosts become infected. However, in addition to reducing mean susceptibility, vaccines may also change variance in susceptibility, dependent on the mode of vaccine action, which could further influence population measures of vaccine efficacy.
The mode of vaccine action, or the shape and variance of susceptibility, can influence disease dynamics, and is therefore important to consider in assessing spread and transmission of pathogens through vaccinated populations10,13. Vaccines that have highly polarized modes of action have been termed “all-or-nothing” because hosts are practically either completely protected or unprotected following vaccination. Modes of vaccine action that are less polarized are termed “leaky”, and in the most extreme instance, partially protect each host to an identical degree. In randomized vaccine trials, the distribution of pathogen exposure dose should be balanced by randomization between the vaccinated and control groups. However, the distribution (mean, variance and higher moments) may differ between these groups, while within a group there will be varying levels of pathogen exposure dose10. Here, we examine the impact of variance in susceptibility in vaccinated and unvaccinated individuals on vaccine efficacy across escalating pathogen doses.
Methods
We examined the influence of exposure dose and heterogeneity in susceptibility on vaccine efficacy. We first sampled susceptibility of a host population from a beta distribution with a mean susceptibility of 0.18 for the vaccinated group, and 0.9 for the unvaccinated (control) group, arbitrarily assuming a total number enrolled in the trial of 140,000 (70,000 in each arm). Mean susceptibility values were selected to obtain 80% efficacy at the limit of a low exposure-dose, with a visible decline over the range of doses considered. We then selected a group of 10,000 individuals from both the vaccinated and control groups to challenge at a given dose. From this, we calculated the probability of each individual becoming infected at a given dose using 1 – exp(-dose*susceptibility), where both dose and susceptibility were continuous variables. We performed this simulation for nine different susceptibility shape combinations, with variance ranging from 0.09 (polarized beta distribution) to 9 × 10−7 (homogenous distribution where nearly every individual had a susceptibility equal to the mean). We then took the mean probability of infection for each dose and estimated vaccine efficacy at each using 1 – Risk Ratio (the probability of infection in the vaccine group divided by the probability of infection in control group). Under this model, the probability of becoming infected depends only on susceptibility and the total exposure dose, and therefore exposure could be considered as either dose during a single exposure, or the total number of exposures of fixed or varying dosage.
We compared our results with published estimates of vaccine efficacy obtained from controlled challenge studies based on a systematic literature review using keywords (dose AND vaccine efficacy) OR (dose AND vaccine probability) in PubMed (Fig. S1). Two observers screened the PubMed results, and we restricted our comparisons to studies where vaccinees (vaccinated individuals) and an unvaccinated control group were tested against multiple pathogen doses in the same study, and sample sizes were greater than 2 individuals per group. We also identified several additional studies meeting our criteria based on reviews of reference sections of identified articles. Several studies we identified also varied vaccine dosage, and in cases where vaccine dose did not significantly influence vaccine protection, we combined data among treatment groups. In all other instances, we calculated vaccine efficacy of the most efficacious vaccine type at the most efficacious vaccine dose reported in each study. We analyzed the effect of pathogen dose on vaccine efficacy using a generalized linear mixed model with a binomial distribution and a logit link with disease as a random effect and treatment group interacting with ordinal dose as fixed effects. Statistical models were analyzed using package lme414, and all simulations and analyses were conducted in RStudio v. 1.0.15315.
Results
Simulations showed that vaccine efficacy decreased non-linearly with increasing dose (Fig. 1). As dose increased, vaccine efficacy declined most sharply when the susceptibility distributions in the vaccinated group had lower variance. At moderate doses, highly polarized susceptibility among vaccinees resulted in the highest estimates of vaccine efficacy. At low exposure doses, there was little difference in probability of infection and vaccine efficacy if susceptibility was homogeneous or hump-shaped, despite a three orders of magnitude difference in variance. At the highest doses, vaccine efficacy was close to 0 for groups with homogeneous or hump-shaped modes of vaccine action, whereas vaccine efficacy remained above 40% for polarized distributions. Heterogeneity in susceptibility in the control group had little influence on probability of infection and vaccine efficacy because the mean probability of infection was very high, and no individuals were entirely protected.
Figure 1.
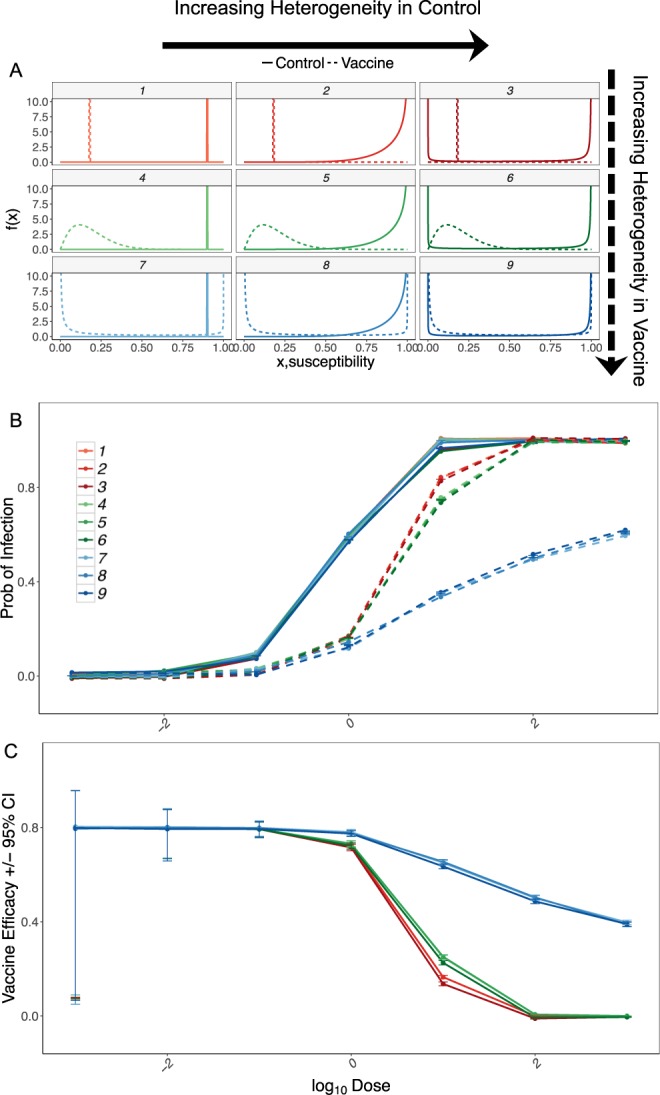
Estimates of vaccine efficacy over a range of pathogen doses allowing for variation in heterogeneity in susceptibility. (A) Susceptibility distributions of vaccinated and control (unvaccinated) groups used in simulation. (B) Estimated probability of infection control (solid lines) and vaccine group (dashed lines) +/− standard error of mean. Colors correspond to susceptibility distributions in panel (A). (C) Measurable vaccine efficacy decreases with increasing exposure dose despite the constancy in intrinsic efficacy given by the susceptibility distributions (intrinsic vaccine efficacy = 0.8). Colors and labels are the vaccine efficacy estimated from the corresponding susceptibility distributions in panel (A). Error bars show 95% confidence intervals.
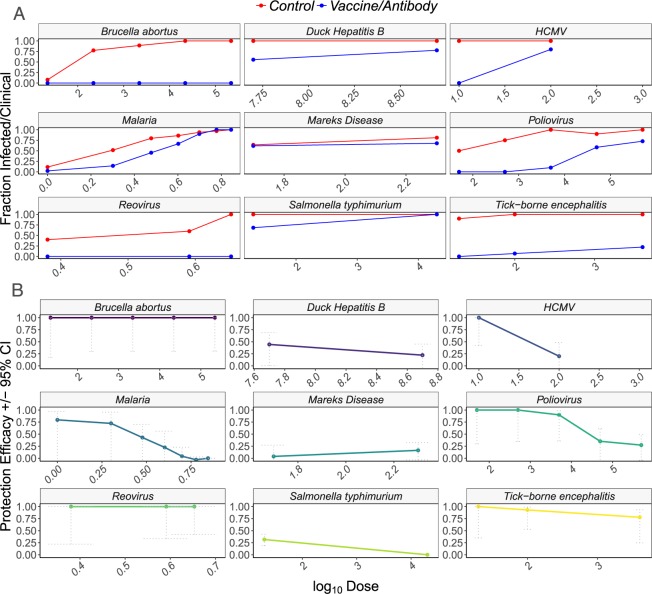
Our systematic review highlighted the paucity of studies that have tested vaccines at multiple pathogen doses. Our broad search criteria identified 5389 articles (as of March 23 2017). Of these, 253 articles were identified for further screening based on information in the abstract, however only 16 studies met the inclusion criteria (Table 1). Nine studies used infection or clinical signs as an outcome variable whereas the others used mortality (Fig. 2). Nine total studies also tested vaccines across more than two different pathogen doses, but only five of these used infection or clinical signs as a response variable.
Table 1.
Studies identified as a result of a systematic review of vaccines tested over multiple pathogen challenge doses. Inclusion criteria included studies where vaccinees (vaccinated individuals) and an unvaccinated control group were tested against multiple pathogen doses in the same study. *Indicates a study in which only two pathogen doses were tested for a seronegative participant group, but three doses were tested across vaccinees.
| Study | No. of Challenge Doses | Study Organism | Pathogen | Response Variable | Citation |
|---|---|---|---|---|---|
| Bosseray 1980 | 5 | Mouse | Brucella abortus | Placental colonization | 25 |
| Bublot 2007 | 6 | Chicken | H5N1 Influenza | Mortality | 32 |
| Chernokhaeva 2016 | 3 | Mouse | Tick-borne encephalitis virus | Infection and Mortality | 31 |
| Churcher 2017 | 4 | Mouse | Malaria | Infection | 28 |
| Cronly-Dillon 1972 | 2 | Mouse | Salmonella sv. typhimurium | Infection | 7 |
| Delagrave 2012 | 4 | Mouse | Herpes simplex virus Type 2 | Mortality | 33 |
| Ghiasi 1997 | 2 | Mouse | Herpes simplex virus Type 1 | Mortality | 34 |
| Henry 1966 | 5 | Human | Poliovirus | Infection | 29 |
| Hatch 1964 | 12 | Mouse | Francisella (Pasteurella) tularensis | Mortality | 35 |
| Islam 2007 | 2 | Chicken | Mareks Disease virus | Infection | 5 |
| Marchart 2003 | 3 | Mouse | Pasteurella multocida | Mortality | 8 |
| Miller 2006 | 2 | Duck | Duck hepatitus B virus | Chronic infection | 26 |
| Plotkin 1989 | 2* | Human | Human cytomegalovirus | Infection | 27 |
| Sebunya 1982 | 3 | Mouse | Haemophilus pleuropneumoniae | Mortality | 36 |
| van Loon 2002 | 3 | Chicken | Reovirus | Isolation from organ | 30 |
| Yamashita 2009 | 2 | Sevenband grouper | Red-spotted grouper nervous necrosis virus | Mortality | 37 |
Figure 2.
The fraction of individuals infected (A) and estimates of vaccine efficacy (B) for Brucella abortus in mice (H.38 B. melitensis killed vaccine25), duck hepatitis b virus in ducks (duck hepatitis b virus surface protein DNA vaccine26), Human cytomegalovirus in humans (Towne cytomegalovirus vaccine27), malaria in mice (anti-circumsporozoite protein, efficacy estimated from total residual sporozoite scores across all bites binned across groups for visualization28), Marek’s disease in broiler chickens (turkey herpesvirus vaccine5), poliovirus in human infants (oral poliovirus vaccine29), reovirus in chickens (attenuated reovirus vaccine30), Salmonella typhimurium in mice (heat-killed S. typhimurium vaccine7), and tick-borne encephalitis in mice (tick-borne encephalitis vaccine31).
Empirical estimates showed that increasing pathogen challenge dose decreased infection vaccine efficacy (treatment and dose interaction: P < 0.0001), although results varied among studies (random effect of disease: variance = 3.43). For some diseases that examined vaccine protection across five or more different pathogen challenge doses (e.g. poliovirus and malaria), vaccine efficacy decreased in accordance with our predictions from the simulation. However, for Brucella abortus and reovirus, which used organ colonization as the response variable, there was no decrease in vaccine efficacy with increasing pathogen dose. While the overall trend aligned with our theoretical predictions, most studies tested a small number of pathogen doses making it difficult to discern whether vaccines were truly not overcome at high pathogen challenge doses or whether the doses studied were just insufficient to overcome vaccines.
Discussion
Our simulation suggests that estimates of vaccine efficacy should be much lower under high pathogen challenge dose regardless of susceptibility heterogeneity. While our review revealed a very small number of studies that have tested vaccines across a broad range of pathogen challenge doses, there is some limited empirical evidence that data aligns with our theoretical predictions. However, two studies appeared to show no consistent trend with dose, and offered hosts complete protection regardless of pathogen dose. Notably, doses of Brucella abortus spanned five orders of magnitude, but no vaccinated hosts were colonized across this large dose range. These findings suggest that these vaccines drastically reduce susceptibility above the range tested in our simulation and highlight a need for additional experiments testing highly efficacious vaccines over multiple pathogen doses.
Additional dose-ranging challenge studies would be useful in that they can be used to both assess vaccine efficacy2,16 and estimate heterogeneity in host susceptibility10,13. These estimates of host heterogeneity can be incorporated into dynamical models to improve predictions of pathogen spread in vaccinated populations10, and also account for the apparent reductions in vaccine efficacy over time in clinical trials2. While human-challenge studies are ethically controversial and only appropriate for some pathogens, animal systems can still provide important insights about the failure of vaccines to protect individuals in high transmission settings, and our review suggests that there is opportunity to rapidly expand theory given the limited data on this topic17. While extrapolation to human systems should be done with care, these studies would help to increase the fundamental understanding of the relationship between host susceptibility, pathogen dose, and vaccine failure.
Across pathogen species and strains, previous studies have noted that pathogens with higher virulence have lower infective doses, suggesting the potential for interactions between low-dose vaccine efficacy and pathogen virulence18,19. While our study examined vaccine efficacy within pathogen species and strains, future work could examine similar relationships across pathogen strains to assess whether vaccine protection is higher at low doses regardless of strain virulence. If vaccines block low dose challenges in both virulent and avirulent strains, this may influence strain competition and pathogen evolution20. However, experiments including clinical trials involving infections that are not always symptomatic (thus in which some infections may go undetected) can produce biased results, and it will be important to design such studies carefully to avoid conflating differences in efficacy with differences in the proportion of infections that are detected21.
Although our simulation showed that increasing pathogen challenge dose decreased vaccine efficacy, variance in susceptibility also affected vaccine protection. When the mode of vaccine action was polarized (e.g. approaching “all-or-nothing”), vaccine protection decayed more slowly with increasing dose. Similar results are expected if exposure accumulates over time and 1 – Rate Ratio is used as the measure of vaccine efficacy16. Interestingly, vaccine protection was similar when distributions of susceptibility were both homogeneous and hump-shaped. The larger change in vaccine protection when susceptibility distributions were highly polarized is likely a consequence of the inclusion of numerous individuals of very low (near 0) susceptibility. Nonetheless, the mode of vaccine action is frequently poorly understood but often assumed to be bimodal, and our results underscore the importance of heterogeneity in susceptibility in influencing vaccine efficacy, particularly in high exposure settings. Understanding the distribution of vaccine protection, rather than just the mean level, can help to improve predictions of vaccine efficacy in heterogeneous populations10.
Studies often speculate about the potential for vaccine failure at high pathogen doses11,22, yet prior to this study, there appears to be little empirical or theoretical basis for this claim. Our results highlight a theoretical basis and some empirical evidence for reductions in vaccine efficacy at high exposure doses, and also reveal the strong potential for heterogeneity in susceptibility to influence vaccine efficacy. Differences in host susceptibility, as have been noted in recent studies on influenza23 and mumps24, can explain age-stratified infection dynamics or disease resurgence that is often attributed to vaccine failure. As both pathogen dose and host susceptibility likely play important roles in disease dynamics in vaccinated populations, disentangling these effects should be an important priority in predicting the public health impact of vaccination programs.
Supplementary information
Acknowledgements
We would like to thank Dr. Karel Brinda for assistance in coding the systematic literature review and Dr. Thomas Churcher for providing an updated data file supplement to his original paper. Funding was provided by the NIH EEID grant R01GM113233. MGMG was funded by Fundação para a Ciência e a Tecnologia (IF/01346/2014) and is a member of the Human Infection Challenge Network for Vaccine Development (HIC-Vac), which is funded by the GCRF Networks in Vaccines Research and Development, which was co-funded by the MRC and BBSRC.
Author Contributions
M.L. and K.E.L. conceived of the study. K.E.L. performed the simulations and analyses. K.E.L., M.D.C, M.K., and S.Y. performed the systematic review. K.E.L. wrote the initial draft, and K.E.L., M.G.M.G., A.R.W., and M.L. revised the manuscript.
Data Availability
All data files are referenced in the manuscript in Table 1 and Fig. 2. Data within those files is available on https://github.com/klangwig/vaccineANDpathogen_dose.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39698-x.
References
- 1.RTS, S., Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. The Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes MGM, Gordon SB, Lalloo DG. Clinical trials: The mathematics of falling vaccine efficacy with rising disease incidence. Vaccine. 2016;34:3007–3009. doi: 10.1016/j.vaccine.2016.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas, C. N., Rose, J. B. & Gerba, C. P. Quantitative microbial risk assessment. First edn, (John Wiley & Sons, Inc., 1999).
- 4.Nygren E, Li BL, Holmgren J, Attridge SR. Establishment of an adult mouse model for direct evaluation of the efficacy of vaccines against Vibrio cholerae. Infect. Immun. 2009;77:3475–3484. doi: 10.1128/IAI.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Islam A, Walkden-Brown SW, Groves PJ, Underwood GJ. Effects of vaccine dose, virus challenge dose and interval from vaccination to challenge on protection of broiler chickens against Marek’s disease virus challenge. Australian Veterinary Journal. 2007;85:348–355. doi: 10.1111/j.1751-0813.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 6.Todd, T. E. et al. Meta-analysis of variables affecting mouse protection efficacy of whole organism Brucella vaccines and vaccine candidates. BMC Bioinformatics14, 10.1186/1471-2105-14-s6-s3 (2013). [DOI] [PMC free article] [PubMed]
- 7.Cronly-Dillon S. Comparative efficacy of whole and disintegrated killed vaccines against Salmonella typhimurium in mice. Journal of medical microbiology. 1972;5:183–189. doi: 10.1099/00222615-5-2-183. [DOI] [PubMed] [Google Scholar]
- 8.Marchart J, et al. Pasteurella multocida- and Pasteurella haemolytica-ghosts: new vaccine candidates. Vaccine. 2003;21:3988–3997. doi: 10.1016/s0264-410x(03)00383-9. [DOI] [PubMed] [Google Scholar]
- 9.Cafaro A, et al. Impact of Viral Dose and Major Histocompatibility Complex Class IB Haplotype on Viral Outcome in Mauritian Cynomolgus Monkeys Vaccinated with Tat upon Challenge with Simian/Human Immunodeficiency Virus SHIV89.6P. Journal of Virology. 2010;84:8953–8958. doi: 10.1128/jvi.00377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langwig, K. E. et al. Vaccine Effects on Heterogeneity in Susceptibility and Implications for Population Health Management. mBio8 (2017). [DOI] [PMC free article] [PubMed]
- 11.Paunio M, et al. Explosive school-based measles outbreak intense exposure may have resulted in high risk, even among revaccinees. Am. J. Epidemiol. 1998;148:1103–1110. doi: 10.1093/oxfordjournals.aje.a009588. [DOI] [PubMed] [Google Scholar]
- 12.Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes MGM, et al. A missing dimension in measures of vaccination impacts. PLoS Pathog. 2014;10:e1003849. doi: 10.1371/journal.ppat.1003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates, D., Maechler, M. & Bolker, B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42, http://cran.r-project.org/package=lme4 (2011).
- 15.RStudio: Integrated Development for R. RStudio, Inc. (Boston, MA, 2015).
- 16.Margheri A, Rebelo C, Gomes MGM. Heterogeneity in disease risk induces falling vaccine protection with rising disease incidence. Dynamical Systems. 2017;32:148–163. doi: 10.1080/14689367.2016.1187115. [DOI] [Google Scholar]
- 17.Cohen, J. Studies that intentionally infect people with disease-causing bugs are on the rise. Science (2016).
- 18.Leggett HC, Cornwallis CK, West SA. Mechanisms of Pathogenesis, Infective Dose and Virulence in Human Parasites. PLOS Pathogens. 2012;8:e1002512. doi: 10.1371/journal.ppat.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid-Hempel P, Frank SA. Pathogenesis, Virulence, and Infective Dose. PLOS Pathogens. 2007;3:e147. doi: 10.1371/journal.ppat.0030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming-Davies AE, et al. Incomplete host immunity favors the evolution of virulence in an emergent pathogen. Science. 2018;359:1030–1033. doi: 10.1126/science.aao2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahn, R., Hitchings, M., Wang, R., Bellan, S. & Lipsitch, M. Analyzing Vaccine Trials in Epidemics With Mild and Asymptomatic Infection. Am J Epidemiol.188, 467–474 (2019). [DOI] [PMC free article] [PubMed]
- 22.Barskey AE, et al. Mumps outbreak in Orthodox Jewish communities in the United States. N. Engl. J. Med. 2012;367:1704–1713. doi: 10.1056/NEJMoa1202865. [DOI] [PubMed] [Google Scholar]
- 23.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science. 2016;354:722–726. doi: 10.1126/science.aag1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. Science translational medicine. 2018;10:eaao5945. doi: 10.1126/scitranslmed.aao5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosseray N. Colonization of mouse placentas by Brucella abortus inoculated during pregnancy. British journal of experimental pathology. 1980;61:361. [PMC free article] [PubMed] [Google Scholar]
- 26.Miller DS, Kotlarski I, Jilbert AR. DNA vaccines expressing the duck hepatitis B virus surface proteins lead to reduced numbers of infected hepatocytes and protect ducks against the development of chronic infection in a virus dose-dependent manner. Virology. 2006;351:159–169. doi: 10.1016/j.virol.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 27.Plotkin SA, Starr SE, Friedman HM, Gönczöl E, Weibel RE. Protective effects of Towne cytomegalovirus vaccine against low-passage cytomegalovirus administered as a challenge. Journal of Infectious Diseases. 1989;159:860–865. doi: 10.1093/infdis/159.5.860. [DOI] [PubMed] [Google Scholar]
- 28.Churcher TS, et al. Probability of Transmission of Malaria from Mosquito to Human Is Regulated by Mosquito Parasite Density in Naïve and Vaccinated Hosts. PLOS Pathogens. 2017;13:e1006108. doi: 10.1371/journal.ppat.1006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henry JL, et al. A study of poliovaccination in infancy: excretion following challenge with live virus by children given killed or living poliovaccine. The Journal of hygiene. 1966;64:105. doi: 10.1017/S0022172400040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Loon A, Suurland B, Van der Marel P. A reovirus challenge model applicable in commercial broilers after live vaccination. Avian Pathology. 2002;31:13–21. doi: 10.1080/03079450120106589. [DOI] [PubMed] [Google Scholar]
- 31.Chernokhaeva LL, et al. Protective immunity spectrum induced by immunization with a vaccine from the TBEV strain Sofjin. Vaccine. 2016;34:2354–2361. doi: 10.1016/j.vaccine.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 32.Bublot M, et al. Efficacy of a fowlpox-vectored avian influenza H5 vaccine against Asian H5N1 highly pathogenic avian influenza virus challenge. Avian diseases. 2007;51:498–500. doi: 10.1637/7624-042706R.1. [DOI] [PubMed] [Google Scholar]
- 33.Delagrave S, et al. Immunogenicity and efficacy of intramuscular replication-defective and subunit vaccines against herpes simplex virus type 2 in the mouse genital model. PLoS One. 2012;7:e46714. doi: 10.1371/journal.pone.0046714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghiasi H, et al. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology. 1997;91:430–435. doi: 10.1046/j.1365-2567.1997.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatch MT, Nicholes PS. Immunogenic substances in culture filtrates and lysates of Pasteurella tularensis. Journal of bacteriology. 1964;88:566–573. doi: 10.1128/jb.88.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebunya T, Saunders J. Studies on immunity to Haemophilus pleuropneumoniae infections in mice. American journal of veterinary research. 1982;43:1793–1798. [PubMed] [Google Scholar]
- 37.Yamashita H, Mori K, Kuroda A, Nakai T. Neutralizing antibody levels for protection against betanodavirus infection in sevenband grouper, Epinephelus septemfasciatus (Thunberg), immunized with an inactivated virus vaccine. Journal of fish diseases. 2009;32:767–775. doi: 10.1111/j.1365-2761.2009.01054.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data files are referenced in the manuscript in Table 1 and Fig. 2. Data within those files is available on https://github.com/klangwig/vaccineANDpathogen_dose.