Abstract
Most of the research effort to understand protective immunity against norovirus (NoV) has focused on humoral immunity, whereas immunity against another major pediatric enteric virus, rotavirus (RV), has been studied more thoroughly. The aim of this study was to investigate development of cell-mediated immunity to NoV in early childhood. Immune responses to NoV GI.3 and GII.4 virus-like particles and RV VP6 were determined in longitudinal blood samples of 10 healthy children from three months to four years of age. Serum IgG antibodies were measured using enzyme-linked immunosorbent assay and production of interferon-gamma by peripheral blood T cells was analyzed by enzyme-linked immunospot assay. NoV-specific T cells were detected in eight of 10 children by the age of four, with some individual variation. T cell responses to NoV GII.4 were higher than those to GI.3, but these responses were generally lower than responses to RV VP6. In contrast to NoV-specific antibodies, T cell responses were transient in nature. No correlation between cell-mediated and antibody responses was observed. NoV exposure induces vigorous T cell responses in children under five years of age, similar to RV. A role of T cells in protection from NoV infection in early childhood warrants further investigation.
Introduction
Noroviruses (NoVs) and rotaviruses (RVs) are leading causes of severe acute gastroenteritis (AGE) in children under five years of age1. RV has been a major cause of AGE requiring hospitalization in children but as a consequence of implementing RV vaccination in >100 countries since 2006, a trend toward NoV predominance is seen2,3. Analysis of NoV-specific antibodies in early childhood indicated ~50% prevalence at the age of 7–12 months that increased to over 90% by the age of five years in Finland4. There is no vaccine available against NoV but two experimental NoV vaccines are in clinical phase5,6 and several in preclinical development7–9.
NoV contains 90 copies of dimeric capsid VP1 proteins that spontaneously form virus-like particles (VLPs) in vitro10. Unlike RV, NoV research lacks efficient cell culture system for virus propagation and thereby most immunological assays, such as surrogate neutralization assay as well as vaccine development, rely on antigenically and morphologically equivalent NoV VLPs11. Recently there has been progress with human intestinal enteroids (HIE) that have been utilized to replicate NoV in vitro, allowing e.g. to measure neutralizing antibodies for NoV with enteroid culture system12,13. Antigenic heterogeneity is a major issue in NoV protection, contributing to the lack of cross-protection between genogroup I (GI) and genogroup II (GII) NoVs and limited immunity between heterogenous strains within these genogroups14–16. Even though there are over 30 genotypes of NoVs infecting humans, for the last two decades GII.4 variants have caused majority of NoV infections17–19.
NoVs and RVs2,20 use polymorphic set of histo-blood group antigen (HBGA) molecules expressed on gut epithelium as cell attachment factors/receptors in strain-specific manner21. Genetic variability of HBGA expression patterns between individuals and subsequent different innate susceptibility to infections complicates interpretation of immune responses and vaccine efficacy studies. For example, individuals with non-secretor status lack expression of certain HBGA molecules essential for infectivity of several NoV strains and thereby are less prone to NoV infections2.
Despite extensive research, immunological mechanisms of protection against NoV and RV infections or vaccination remain unclear22,23. RV humoral and cell-mediated immunity (CMI) has been thoroughly investigated and data supporting the protective role of IgA24,25, neutralizing antibodies26 and T cells27–31 is exhaustive. However, there is still much controversy and debate over the protective effect of these immunological components24,32.
For both NoV and RV, the presence of high preexisting antibody titers in serum may indicate less severe disease and show some correlation with protection33–35. We have previously described the development of NoV-specific IgG responses in the first years of life and found correlation between high strain-specific serum IgG and antibodies blocking HBGA binding to protection from NoV infection4,34,36. However, the results also suggested that antibody response was short-lasting and strain-specific therefore not able to protect from subsequent heterologous NoV infections16,37.
There is evidence from animal models and human studies that RV-specific T cells play a role in viral clearance38, that may be mediated e.g. by secretion of interferon-gamma (IFN-γ). RV infection induces both CD4+ and CD8+ T cell responses, however, the level is much lower compared to other viruses such as cytomegalovirus29,39–41. While human NoV-specific serology is thoroughly investigated, there are very few publications on human NoV-specific CMI responses42–45. Our recent research on NoV-specific T cell responses showed that in addition to antibodies circulating NoV-specific memory T cells are present in healthy adults45. Two human studies have described CD4+ T cells after NoV challenge43,44 and after oral NoV VLP vaccine administration42. In the present study NoV-specific T cell responses in children under five years of age were investigated for the first time.
Results
Serum IgG levels to NoV and RV increase by the age
Serum IgG antibody levels against NoV GI.3 and GII.4 VLPs and RV VP6 at the age 3, 6, 12, 24, 36 and 48 months were analyzed using ELISA. IgG end-point titers stratified according to age are shown in Fig. 1a–c. Antibody levels to all tested antigens increased until the age of 2–3 years and remained at high levels at the age of four years. During the study period all children seroconverted to NoV, either to GI.3 or GII.4 or both, while eight of 10 subjects seroconverted to RV VP6 (Tables 1–3). Lowest levels of NoV (Fig. 1a,b) and RV (Fig. 1c) –specific antibodies were observed at the age of 6 or 12 months following the decrease of maternal antibodies, but the age of observed seroconversion varied from 1 to 4 years of age (Table 3).
Figure 1.
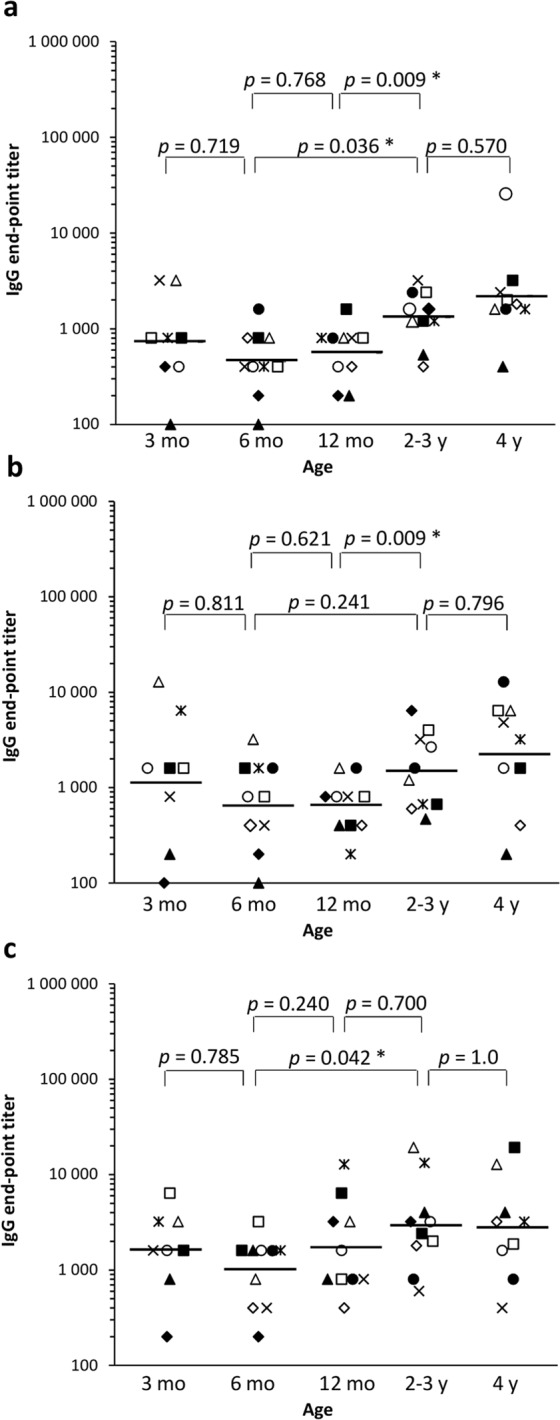
Norovirus (NoV)- and rotavirus (RV)-specific serum immunoglobulin G (IgG) end-point titers. Sera were titrated with 2-fold serial dilutions and IgG antibodies were analyzed against NoV GI.3 (a), NoV GII.4 (b) and RV VP6 (c) in enzyme-linked immunosorbent assay. Shown are end-point titers at the age of 3, 6 and 12 months, mean end-point titers at 2–3 years and 4 years of age. The horizontal line indicates geometric mean titer. Statistical differences were determined using Fisher’s exact test, and a p value of ≤0.05 was considered statistically significant (*). Each symbol denotes an individual child (▲ = Subject 1, ♦ = 2, ○ = 3, □ = 4, × = 5, Δ = 6, • = 7, ◊ = 8, ■ = 9, * = 10).
Table 1.
Seroconversion to norovirus (NoV) and to rotavirus (RV) at different age.
| Donor | 12 mo | 2–3 y | 4 y | |||
|---|---|---|---|---|---|---|
| NoV GI.3/GII.4 | RV | NoV GI.3/GII.4 | RV | NoV GI.3/GII.4 | RV | |
| 1a | −/+ | — | +/− | + | +/− | + |
| 2 | −/+ | + | +/+ | — | −/− | — |
| 3b | −/− | — | +/+ | + | +/− | — |
| 4 | −/− | — | −/+ | + | −/+ | + |
| 5a | −/− | — | +/+ | — | −/− | — |
| 6 | −/− | + | −/− | + | −/+ | — |
| 7 | −/− | — | −/− | — | −/+ | — |
| 8 | −/− | — | −/− | + | +/− | — |
| 9 | −/− | + | −/+ | — | −/− | + |
| 10 | −/− | + | −/+ | — | −/+ | — |
aReceived RV vaccine (3 doses) before 6 months of age.
bReceived RV vaccine (1 dose) before 3 months of age.
−, no seroconversion (<four-fold increase in IgG end-point titer of sequential serum samples). +, seroconversion (≥four-fold increase in IgG end-point titer of sequential serum samples).
Table 3.
Serum norovirus (NoV) and rotavirus (RV)-specific IgG end-point titers and seroconversion.
| Donor | 6 mo IgG titer | Seroconversion IgG titer (age) | ||||
|---|---|---|---|---|---|---|
| GI.3 NoV | GII.4 NoV | RV | GI.3 NoV | GII.4 NoV | RV | |
| 1 | 100 | 50 | 1600 | 800 (2 ya) | 400 (1 y) | 6400 (3 y) |
| 2 | 200 | 200 | 200 | 1600 (2 y) | 800 (1 y) | 3200 (1 y) |
| 3 | 400 | 800 | 1600 | 1600 (2 y) | 3200 (2 y) | 6400 (2 y) |
| 4 | 400 | 800 | 3200 | — | 6400 (3 y) | 3200 (3 y) |
| 5 | 400 | 400 | 400 | 3200 (2 y) | 3200 (2 y) | — |
| 6 | 800 | 3200 | 800 | — | 6400 (4 y) | 25600 (3 y) |
| 7 | 1600 | 1600 | 1600 | — | 12800 (4 y) | — |
| 8 | 800 | 400 | 400 | 1800 (4 y) | — | 3200 (3 y) |
| 9 | 800 | 1600 | 1600 | — | 1600 (3 y) | 6400 (1 y) |
| 10 | 400 | 1600 | 1600 | — | 3200 (3 y) | 12800 (1 y) |
aAge at the seroconversion detected.
−, no seroconversion detected.
NoV-specific maternal IgG antibodies were detected in all subjects at the age of 3 months with geometric mean titers (GMT) 734 for GI.3 (Fig. 1a) and 1234 for GII.4 (Fig. 1b). IgG levels decreased by the age of 6 months (GMT 459 for GI.3 and 696 for GII.4) with no significant change by the 12 months of age (GMTs 566 for GI.3 and 746 for GII.4). A significant increase of NoV GI.3 (Fig. 1a) and GII.4 (Fig. 1b) IgG was observed by the age of 2–3 years (Fig. 1a,b). Both GI.3 (Fig. 1a) and GII.4-specific (Fig. 1b) IgG end-point titers increased by the age of four (GMT 1853 for GI.3 and 2601 for GII.4) although the difference was not statistically significant (p > 0.05). Seven of 10 subjects seroconverted to NoV by the three years of age and the remaining three subjects by the age of four (Table 1).
RV VP6-specific serum IgG levels were high at the 3 months of age (GMT 1600) and decreased by the 6 months of age (GMT 985) when maternal antibodies had vanished (Fig. 1c). No significant change in the GMT from 6 months to 12 months of age (GMT 1970) was detected. A significant increase (p < 0.05) was detected from 6 months to the 2–3 years age (GMT 2907) and the trend was increasing by the age, similarly to NoV IgG (Fig. 1a,b). Seroconversion to RV was detected in eight of 10 subjects during the study period, four at 12 months of age and four subjects at 2–3 year old (Table 1).
NoV and RV induce transient T cell responses in children
T cell responses to NoV GI.3 VLP, NoV GII.4 VLP and RV VP6 developed in a paralleling fashion, as analyzed by ELISPOT IFN-γ (Fig. 2). Five of 10 subject had positive response to NoV and six subjects to RV within the first year of life (Fig. 2 and Table 2). By the age of 4 years only two donors (subject 1 and 2) did not respond to NoV VLPs, and only one subject did not respond to RV VP6 (subject 1). Transient although not statistically significant (p > 0.05) increase in the magnitude of the T cell responses to NoV and RV was observed by the age of 2–3 years, decreasing then by the age of four (Fig. 2). Mean ELISPOT IFN-γ values by the age stratification for NoV GII.4 were 36 at ≤12 months, 90 at 2–3 years and 50 SFC/106 cells at 4 years and congruently with the antibody results, GI.3-specific T cell responses were significantly lower (p = 0.01) (the means: 13, 36 and 31 SFC/106 cells) (Fig. 2). IFN-γ responses to RV VP6 were significantly higher (p = 0.005) than NoV-specific responses, mean values being 88, 183 and 59 SFC/106 cells in the corresponding ages (Fig. 2c). Subject 5 (RV vaccinated) generated the strongest VP6-specific T cell responses, and had also the highest NoV-specific T cell responses (Fig. 2). PBMCs of all donors produced IFN-γ after the PHA stimulation (>1000 SFC/106 PBMCs).
Figure 2.
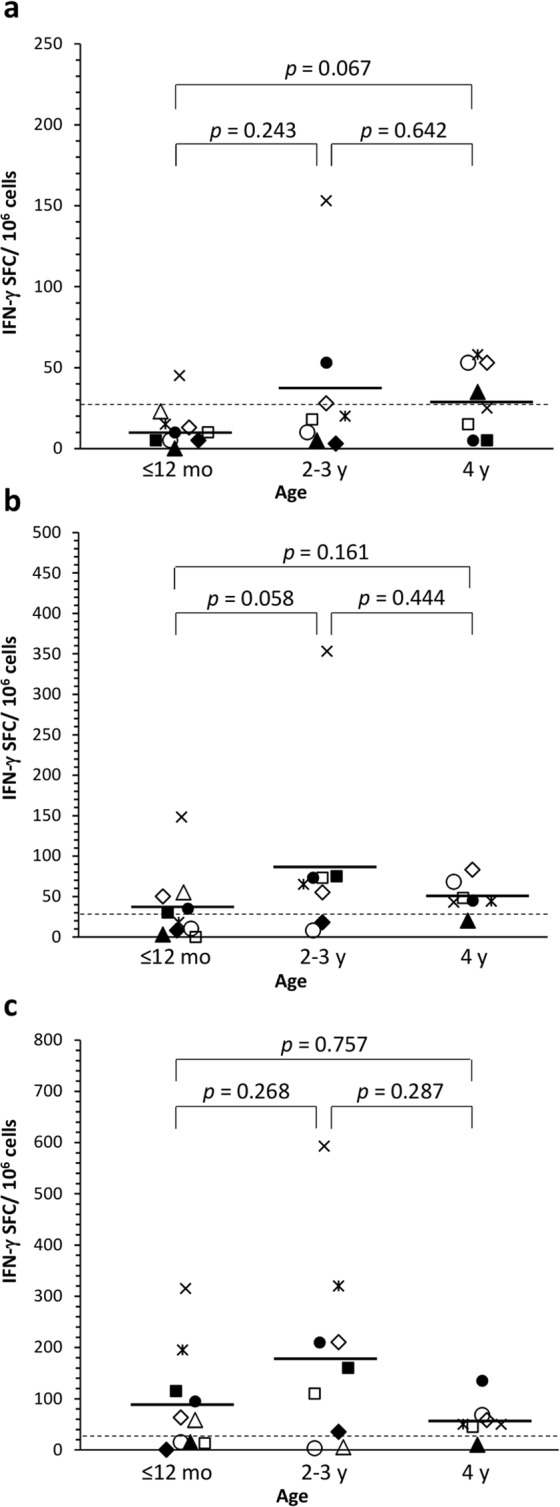
Norovirus (NoV)- and rotavirus (RV)-specific interferon gamma (IFN-γ) production by T cells. Peripheral blood mononuclear cells (PBMC) were stimulated o/n with NoV GI.3 (a) and GII.4 (b) VLPs and RV VP6 (c) (30 µg/ml each) and cytokine production analyzed by enzyme-linked immunospot (ELISPOT) assay. Due to the shortage of cells, PBMCs collected at the age of 6 and 12 months and 2 and 3 years were pooled. Shown are mean IFN-γ spot-forming cells (SFC)/106 PBMCs of two replicate wells of an individual sample. The horizontal line indicates the mean of the age grouped samples. The dashed line represents the positivity cut off (≥26 SFC/106 PBMCs). Statistical differences between the groups were determined by a Mann-Whitney U test, and a p value of ≤0.05 was considered statistically significant.
Table 2.
Positive IFN-γ responses to norovirus (NoV) and to rotavirus (RV) at different age.
| Donor | ≤12 mo | 2–3 y | 4 y | |||
|---|---|---|---|---|---|---|
| NoV GI.3/GII.4 | RV | NoV GI.3/GII.4 | RV | NoV GI.3/GII.4 | RV | |
| 1a | −/− | — | −/− | N/A | +/− | — |
| 2 | −/− | — | −/− | + | N/A | N/A |
| 3b | −/− | — | −/− | — | +/+ | + |
| 4 | −/− | — | −/+ | + | +/− | + |
| 5a | +/+ | + | +/+ | + | +/+ | + |
| 6 | −/+ | + | N/A | N/A | N/A | N/A |
| 7 | −/+ | + | +/+ | + | +/− | + |
| 8 | −/+ | + | +/+ | + | +/+ | + |
| 9 | −/+ | + | N/A/+ | + | N/A/— | N/A |
| 10 | −/− | + | −/+ | + | +/+ | + |
aReceived RV vaccine (3 doses) before 6 months of age.
bReceived RV vaccine (1 dose) before 3 months of age.
−, a negative readout (below the cut-off level) in ELISPOT IFN-γ assay. +, a positive readout (above the cut-off level) in ELISPOT IFN-γ assay. N/A, not available.
Discussion
Antibody responses to NoV and RV have been studied extensively, and while there are publications on T cell-mediated responses to RV in both children and adults29–31,40,41,46, cellular immunity to NoV is investigated in adults42,44,45 but remained uncharacterized in young children. Here we investigated the development of T cell responses together with humoral immunity in early childhood towards both NoV and RV.
Consistent with the predominance of GII.4 NoVs worldwide17 and in Finland19,47, and previous reports showing increased prevalence of NoV-specific antibodies with increasing age in children4,33,34, seroconversion to GII.4 was observed in all subjects analyzed in this study. The high prevalence of GII.4 NoV was reflected similarly to cell-mediated responses, where magnitude of T cell responses to GII.4 was notably higher than to GI.3. GI.3 was chosen as a representative strain in this study, as it was the most prevalent GI NoV circulating in Finland during the study period19. Nevertheless, as the GII.4 strain used in here has not widely circulated during the study years 2002–200747, the responses measured probably reflect cross-reactive responses and may be an underestimate of the total responses. Serum antibody responses to RV in the present study were analyzed using recombinant VP6 protein that induces comparable results to whole RV in ELISA25,48. The majority of RV-specific antibodies are likely directed to highly immunogenic and conserved RV VP649 and seroconversion was observed in 80% of subjects during the study period.
While the cause of reported acute gastroenteritis in the study donors was not determined by the PCR or ELISA, seroconversion was the best marker for presumed infection, as described earlier30,34,35,37. However, seroconversion is not always an adequate measure and some infections may be missed. For instance, the subjects 5 and 7 had NoV- and RV-specific T cell responses already at 12 months of age, indicating early virus exposure, but seroconverted to NoV only at the age of 2 and 4 years, respectively, and no seroconversion to RV was detected at all. Similarly, Mäkelä et al. reported a child with strong proliferative T cell responses to RV without increase in RV antibodies30. Moreover, when measuring virus-specific T cell responses in infants and small children, passively acquired maternal antibodies are not interfering with the results interpretation and in this respect, T cells may be better marker for the infection.
Following RV infection children develop T cells responses that can be detected in peripheral blood40. Due to the low cell numbers, it was necessary to pool PBMC samples of two time points (6 and 12 months, 2 and 3 years), which may have led to underestimation of some immune responses if one of the pooled sample has been negative. However, we assume that the effect is not critical, as pooled sample time points are relatively close to each other. In addition, T cell responses at the age of four were lower, than responses obtained with pooled cells from 2–3 years of age. In the present study the development of both NoV- and RV-specific T cell responses measured by IFN-γ cytokine secretion, showed a similar trend increasing by the age to 2–3 years, followed by a decrease at 4 years. This type of transient T cell immunity to RV in children has been reported earlier30, and our results suggest that NoV-specific T cell responses may follow the same pattern, being similarly short lasting. The acute nature of both NoV and RV infections may induce low frequency of memory T cells, compared to prolonged infections such as herpesvirus50 and furthermore, small children are even less likely to have circulating memory cells due to the limited exposure history and less developed immune system51. In contrast, once reaching high levels, antibody responses to NoV and RV remained high. It could be speculated that protection by NoV-specific antibodies could result in dampening of T cell responses by the age of 4 years. Unfortunately, there are no results on correlation of protective NoV blockade antibody titers and T cell responses published so far. However, large number of subjects and well controlled NoV challenge study would be needed to answer this more reliably. Congruently to our previously published NoV-specific immune responses in adults45, there was no correlation between seroconversion and CMI responses to either NoV or RV (Tables 1, 2, respectively).
The results indicate high individual variation in immune responses to NoV and RV at an early age. This variation can only partly be explained by previous exposure history suggesting that individual variation in immune responsiveness may play a role. The results of the two subjects (Subject 1 and 5) that received three doses of RV vaccine before the age of six months illustrate an example of challenging interpretation of the results in epidemiological and vaccine efficacy studies, interfered by the complexity of human immune system and different innate susceptibility to viruses. One of them (Subject 1) developed high and stable RV IgG response following RV vaccination, however, no CMI response to RV was detected. His NoV-specific IgG responses remained exceptionally low throughout the whole study period that could be due to genetic background of this subject such as non-secretor status and HBGA type (not determined in this study). In contrast, Subject 5 also received RV vaccination at full schedule, but did not seroconvert to RV throughout the study, whereas CMI response to VP6 was high already in the first year of life. NoV-specific immune responses of Subject 5 followed similar pattern to RV-specific responses, with negligible humoral response but exceptionally high CMI response. In addition, three other subjects (6, 7 and 8) also developed T cell responses to NoV and RV without the seroconversion in the first year of life, suggesting that these children primarily respond through CMI rather than humoral response to both AGE viruses.
Even though the number of the subjects is quite low, this study provides novel information on cell-mediated immune responses to NoV in this highly susceptible human population. The results suggest that NoV-specific T cell responses are generated already at an early age, and may have a role in protection similarly to what has been suggested for RV-specific T cells. The transient nature of the CMI responses indicates that serial infections may be needed for the development of stable memory T cell population. Furthermore, it remains to be seen if NoV VLP based vaccine could induce CMI responses capable to contribute to the protection against these infections.
Methods
Study samples
Ten children taking part in the Type I Diabetes Prediction and Prevention (DIPP) study52 were prospectively followed for development of NoV- and RV-specific immune responses for four years. The DIPP study follows children with increased genetic risk for type 1 diabetes from birth and its protocol has been approved by the ethics committee of the Pirkanmaa Hospital District (Permit number: 97193M). A written informed consent to the study has been obtained from the parents of participating families, and all research was performed in accordance with relevant guidelines and regulations. Blood samples were collected at 3, 6, 12, 24, 36 and 48 month of age. Diarrheal history of each subject indicated that all donors, except #2, had at least one episode of diarrhea reported before the age of 3 years, however the cause of acute gastroenteris was not determined. Although the samples were collected in 2002–2007, before introduction of RV vaccination to national immunization program, two of the subjects had received full vaccination series (Subjects 1 and 5) and one subject (Subject 3) received only the first dose. Plasma fractions were stored at −70 °C until analyzed. Peripheral blood mononuclear cells (PBMCs) were separated using BD Vacutainer™ Glass Mononuclear Cell Preparation (CPT) Tubes (Fisher Scientific) according to manufacturer’s instructions. PBMCs were frozen in 10% DMSO in fetal bovine serum (FBS) to liquid nitrogen. Prior to analysis, PBMCs were thawed in the presence of benzonase (50 U/ml), washed and resuspended in culture medium (CM) containing RPMI 1640 with Glutamax® and HEPES (Gibco™ by Thermo Fisher Scientific) supplemented with 10 µg/ml Gentamicin (Gibco™) and 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, USA).
Recombinant protein antigens
NoV GI.3 (GenBank reference strain accession no. AF414403) and GII.4 (AF080551) VLPs and RV VP6 protein (acc. no. GQ477131) used as antigens in analytical methods were produced by a baculovirus expression system (Invitrogen) in Spodoptera frugiperda (Sf9) insect cell cultures and purified as previously described7,53,54. The total protein concentration was quantified with Pierce® BCA Protein Assay (Thermo Scientific, Rockford).
NoV- and RV-specific IgG ELISA
Serum IgG antibody levels against NoV34 and RV55 were analyzed by ELISA as earlier described. Serum specimens were diluted two-fold starting at 1:100 and plated on NoV GI.3, GII.4 VLP or RV VP6 coated (1.0 µg/ml in phosphate-buffered saline, PBS) 96-well half-area microtiter plates (Corning Inc., Corning, NY) blocked with 5% skimmed milk in PBS. Serum dilutions were incubated on plates for 1 h at 37 °C. Bound antibodies were detected with goat anti-human IgG-HRP (Invitrogen, CA, USA) followed by o-phenylenediamine (OPD) substrate (Sigma-Aldrich, MO, USA) and H2O2. Optical density (OD) was measured at λ 490 nm using Victor2 1420 Multilabel Counter (Wallac, PerkinElmer) plate reader. Background signal from the blank wells (wells without serum) was subtracted from all of the OD readings on the plate. Each plate contained known NoV negative and positive control serum sample as an assay control. The cut-off value was determined as the mean OD reading of the negative control serum wells at a dilution 1:200 + 3 × standard error and at least 0.100 OD. End-point titer was expressed as a reciprocal of the final serum dilution giving an OD above the cut-off value. Seroconversion was defined as at least four-fold increase in the titer of successive sera.
NoV- and RV-specific ELISPOT IFN-γ
PBMCs were assayed in an IFN-γ ELISPOT assay according to previously described method45 with slight modifications. Briefly, ninety-six-well nitrocellulose filter plates (Millipore) were coated with anti-human IFN-γ capture antibody (Mabtech) and blocked with 10% FBS in CM. Thawed PBMCs were first rested o/n at +37 °C and 5% CO2 in CM. PBMC collected at 6 and 12 months and 2 and 3 years of age were pooled for adequate cell number for the analysis. Cells were stimulated with 30 µg/ml NoV VLPs (GI.3 or GII.4), 30 µg/ml RV VP6 protein, 50 µg/ml of phytohemagglutinin (PHA, positive control) or left unstimulated (CM only, negative control). PBMCs (0.2 × 106 cells/well) were plated with stimulants and incubated for 20 h at +37 °C and 5% CO2. Biotinylated anti-human IFN-γ antibody (Mabtech) followed by streptavidin-HRP (BD, New Jersey, USA) was used for detection. The spots were developed with Vector Nova Red substrate (Vector Labs, Burlingame, USA) and the plates were analyzed using ImmunoSpot Series II analyzer (CTL Europe, Leinfelden-Echterdingen, Germany). The results are expressed as mean spot forming cells (SFC)/106 PBMCs of the duplicate wells. A positive response (≥26 SFC/106 PBMC) was defined based on the averaged result of PBMCs isolated from the three months blood sample of several donors stimulated with NoV and RV antigens plus 3 × standard deviation (SD) (mean SFC/106 PBMC + 3 × SD).
Statistical analysis
Fisher’s exact test was used to compare the IgG end-point titers between age categories. Mann-Whitney U test of two independent samples was used to analyze ELISPOT IFN-γ results. All hypothesis tests were two-tailed. Statistical analyses were performed using IBM SPSS Statistics (SPSS, Chicago, IL) version 23.0. Statistical significance was defined as a p < 0.05.
Acknowledgements
The personnel of the Vaccine Research Center and Virology Department at the University of Tampere are acknowledged for technical assistance. The DIPP study personnel and participating families are acknowledged for the valuable contribution to the study. DIPP study has been financially supported by grants from several academic research foundations. This funding includes grants from the Juvenile Diabetes Research Foundation (JDRF), Academy of Finland, Sigrid Juselius Foundation and competitive research funding from Pirkanmaa Hospital district.
Author Contributions
Dr. M.M. was responsible for the immunological laboratory analysis, processing the data and writing the manuscript. Professor of Virology H.H., a principal investigator of the DIPP Study, and Dr. T.V., MD, the director of the Vaccine Research Center, critically reviewed the manuscript. Professor M. K., MD, is a principal investigator of the DIPP Study and was responsible for the recruitment of the study subjects. Dr. V.B. is the head of the laboratory of Vaccine Research Center, who designed this study, interpreted the results, and wrote the manuscript with Dr. M. Malm. All authors participated in critical review and revision of the manuscript and approved the final manuscript as submitted.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riera-Montes M, O’Ryan M, Verstraeten T. Norovirus and Rotavirus Disease Severity in Children: Systematic Review and Meta-analysis. Pediatr Infect Dis J. 2018;37:501–505. doi: 10.1097/INF.0000000000001824. [DOI] [PubMed] [Google Scholar]
- 2.Payne DC, Parashar UD, Lopman BA. Developments in understanding acquired immunity and innate susceptibility to norovirus and rotavirus gastroenteritis in children. Curr Opin Pediatr. 2015;27:105–109. doi: 10.1097/MOP.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel M, Glass R, Desai R, Tate JE, Parashar UD. Fulfilling the promise of rotavirus vaccines: how far have we come since licensure? Lancet Infect Dis. 2012;12:561–570. doi: 10.1016/S1473-3099(12)70029-4. [DOI] [PubMed] [Google Scholar]
- 4.Nurminen K, et al. Prevalence of norovirus GII-4 antibodies in Finnish children. J Med Virol. 2011;83:525–531. doi: 10.1002/jmv.21990. [DOI] [PubMed] [Google Scholar]
- 5.Leroux-Roels G, et al. Safety and Immunogenicity of Different Formulations of Norovirus Vaccine Candidate in Healthy Adults: A Randomized, Controlled, Double-Blind Clinical Trial. J Infect Dis. 2018;217:597–607. doi: 10.1093/infdis/jix572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leesun Kim et al. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI insight3 (2018). [DOI] [PMC free article] [PubMed]
- 7.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine. 2011;29:8126–8133. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Tamminen K, Lappalainen S, Huhti L, Vesikari T, Blazevic V. Trivalent combination vaccine induces broad heterologous immune responses to norovirus and rotavirus in mice. PLoS One. 2013;8:e70409. doi: 10.1371/journal.pone.0070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocher J, et al. Intranasal P Particle Vaccine Provided Partial Cross-Variant Protection against Human GII.4 Norovirus Diarrhea in Gnotobiotic Pigs. J Virol. 2014;88:9728–9743. doi: 10.1128/JVI.01249-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad BV, et al. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 12.Ettayebi K, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353:1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koromyslova, A. D., Morozov, V. A., Hefele, L. & Hansman, G. S. Human Norovirus Neutralized by a Monoclonal Antibody Targeting the HBGA Pocket. J Virol, 10.1128/JVI.02174-18 (2018). [DOI] [PMC free article] [PubMed]
- 14.Malm M, et al. Genotype considerations for virus-like particle-based bivalent norovirus vaccine composition. Clin Vaccine Immunol. 2015;22:656–663. doi: 10.1128/CVI.00015-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm M, Tamminen K, Vesikari T, Blazevic V. Type-specific and cross-reactive antibodies and T cell responses in norovirus VLP immunized mice are targeted both to conserved and variable domains of capsid VP1 protein. Mol Immunol. 2016;78:27–37. doi: 10.1016/j.molimm.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Blazevic V, et al. Multiple consecutive norovirus infections in the first 2 years of life. Eur J Pediatr. 2015;174:1679–1683. doi: 10.1007/s00431-015-2591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siebenga JJ, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis. 2009;200:802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 18.Eden JS, et al. The emergence and evolution of the novel epidemic norovirus GII.4 variant Sydney 2012. Virology. 2014;450-451:106–113. doi: 10.1016/j.virol.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puustinen L, et al. Norovirus genotypes in endemic acute gastroenteritis of infants and children in Finland between 1994 and 2007. Epidemiol Infect. 2012;140:268–275. doi: 10.1017/S0950268811000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang X, Liu Y, Tan M. Histo-blood group antigens as receptors for rotavirus, new understanding on rotavirus epidemiology and vaccine strategy. Emerg Microbes Infect. 2017;6:e22. doi: 10.1038/emi.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uusi-Kerttula H, Tamminen K, Malm M, Vesikari T, Blazevic V. Comparison of human saliva and synthetic histo-blood group antigens usage as ligands in norovirus-like particle binding and blocking assays. Microbes Infect. 2014;16:472–480. doi: 10.1016/j.micinf.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Cortes-Penfield NW, Ramani S, Estes MK, Atmar RL. Prospects and Challenges in the Development of a Norovirus Vaccine. Clin Ther. 2017;39:1537–1549. doi: 10.1016/j.clinthera.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucero Y, Vidal R, O’Ryan GM. Norovirus vaccines under development. Vaccine. 2017;36:5435–5441. doi: 10.1016/j.vaccine.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol. 2012;2:419–425. doi: 10.1016/j.coviro.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lappalainen S, Blazevic V, Malm M, Vesikari T. Rotavirus vaccination and infection induce VP6-specific IgA responses. J Med Virol. 2017;89:239–245. doi: 10.1002/jmv.24636. [DOI] [PubMed] [Google Scholar]
- 26.Chiba S, et al. Protective effect of naturally acquired homotypic and heterotypic rotavirus antibodies. The Lancet. 1986;328:417–421. doi: 10.1016/S0140-6736(86)92133-1. [DOI] [PubMed] [Google Scholar]
- 27.McNeal MM, et al. Identification of an immunodominant CD4+ T cell epitope in the VP6 protein of rotavirus following intranasal immunization of BALB/c mice. Virology. 2007;363:410–418. doi: 10.1016/j.virol.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Jaimes MC, Feng N, Greenberg HB. Characterization of homologous and heterologous rotavirus-specific T-cell responses in infant and adult mice. J Virol. 2005;79:4568–4579. doi: 10.1128/JVI.79.8.4568-4579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rojas OL, et al. Human rotavirus specific T cells: quantification by ELISPOT and expression of homing receptors on CD4+ T cells. Virology. 2003;314:671–679. doi: 10.1016/S0042-6822(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 30.Makela M, Marttila J, Simell O, Ilonen J. Rotavirus-specific T-cell responses in young prospectively followed-up children. Clin Exp Immunol. 2004;137:173–178. doi: 10.1111/j.1365-2249.2004.02509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesa MC, Gutiérrez L, Duarte-Rey C, Angel J, Franco MA. A TGF-β mediated regulatory mechanism modulates the T cell immune response to rotavirus in adults but not in children. Virology. 2009;399:77–86. doi: 10.1016/j.virol.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Ryder RW, et al. Evidence of immunity induced by naturally acquired rotavirus and Norwalk virus infection on two remote Panamanian islands. J Infect Dis. 1985;151:99–105. doi: 10.1093/infdis/151.1.99. [DOI] [PubMed] [Google Scholar]
- 34.Malm M, Uusi-Kerttula H, Vesikari T, Blazevic V. High serum levels of norovirus genotype-specific blocking antibodies correlate with protection from infection in children. J Infect Dis. 2014;210:1755–1762. doi: 10.1093/infdis/jiu361. [DOI] [PubMed] [Google Scholar]
- 35.Xu J, et al. Serum Antibody Responses in Children with Rotavirus Diarrhea Can Serve as Proxy for Protection. Clin Diagn Lab Immunol. 2005;12:273–279. doi: 10.1128/CDLI.12.2.273-279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blazevic V, Malm M, Vesikari T. Induction of homologous and cross-reactive GII.4-specific blocking antibodies in children after GII.4 New Orleans norovirus infection. J Med Virol. 2015;87:1656–1661. doi: 10.1002/jmv.24237. [DOI] [PubMed] [Google Scholar]
- 37.Blazevic V, et al. Development and maturation of norovirus antibodies in childhood. Microbes Infect. 2015;18:263–269. doi: 10.1016/j.micinf.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol. 2007;5:529–539. doi: 10.1038/nrmicro1692. [DOI] [PubMed] [Google Scholar]
- 39.Kaufhold RM, et al. Memory T-cell response to rotavirus detected with a gamma interferon enzyme-linked immunospot assay. J Virol. 2005;79:5684–5694. doi: 10.1128/JVI.79.9.5684-5694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Offit PA, Hoffenberg EJ, Santos N, Gouvea V. Rotavirus-Specific Humoral and Cellular Immune Response after Primary, Symptomatic Infection. J Infect Dis. 1993;167:1436–1440. doi: 10.1093/infdis/167.6.1436. [DOI] [PubMed] [Google Scholar]
- 41.Jaimes MC, et al. Frequencies of Virus-Specific CD4+ and CD8+ T Lymphocytes Secreting Gamma Interferon after Acute Natural Rotavirus Infection in Children and Adults. J Virol. 2002;76:4741–4749. doi: 10.1128/JVI.76.10.4741-4749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. doi: 10.1016/S1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 43.Lindesmith L, et al. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol. 2005;79:2900–2909. doi: 10.1128/JVI.79.5.2900-2909.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindesmith LC, et al. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J Virol. 2010;84:1800–1815. doi: 10.1128/JVI.02179-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malm M, Tamminen K, Vesikari T, Blazevic V. Norovirus-Specific Memory T Cell Responses in Adult Human Donors. Front Microbiol. 2016;7:1570. doi: 10.3389/fmicb.2016.01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Offit PA, Hoffenberg EJ, Pia ES, Panackal PA, Hill NL. Rotavirus-Specific Helper T Cell Responses in Newborns, Infants, Children, and Adults. J Infect Dis. 1992;165:1107–1111. doi: 10.1093/infdis/165.6.1107. [DOI] [PubMed] [Google Scholar]
- 47.Huhti L, et al. Genetic analyses of norovirus GII.4 variants in Finnish children from 1998 to 2013. Infect Genet Evol. 2014;26:65–71. doi: 10.1016/j.meegid.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Kavanagh O, et al. A time-resolved immunoassay to measure serum antibodies to the rotavirus VP6 capsid protein. J Virol Methods. 2013;189:228–231. doi: 10.1016/j.jviromet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Svensson L, et al. Serum Antibody Responses to Individual Viral Polypeptides in Human Rotavirus Infections. J Gen Virol. 1987;68:643–651. doi: 10.1099/0022-1317-68-3-643. [DOI] [PubMed] [Google Scholar]
- 50.Torti N, Oxenius AT. Cell Memory in the Context of Persistent Herpes Viral Infections. Viruses. 2012;4:1116–1143. doi: 10.3390/v4071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanto-Salonen K, et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet. 2008;372:1746–1755. doi: 10.1016/S0140-6736(08)61309-4. [DOI] [PubMed] [Google Scholar]
- 53.Huhti L, et al. A comparison of methods for purification and concentration of norovirus GII-4 capsid virus-like particles. Arch. Virol. 2010;155:1855–1858. doi: 10.1007/s00705-010-0768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lappalainen S, Tamminen K, Vesikari T, Blazevic V. Comparative immunogenicity in mice of rotavirus VP6 tubular structures and virus-like particles. Hum. Vaccin Immunother. 2013;9:1991–2001. doi: 10.4161/hv.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinimäki S, Malm M, Vesikari T, Blazevic V. Intradermal and intranasal immunizations with oligomeric middle layer rotavirus VP6 induce Th1, Th2 and Th17 T cell subsets and CD4+ T lymphocytes with cytotoxic potential. Antiviral Res. 2018;157:1–8. doi: 10.1016/j.antiviral.2018.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


