Abstract
Rumex confertus is an alien invasive perennial plant that has increased its range rapidly within central Europe in the last 100 years. This study examined the effects of a commercial fertilizer on the competition between the invasive Rumex confertus and two non-invasive native species R. acetosa or R. conglomeratus in terms of morphological and physiological traits and relative yield. All three Rumex species were grown in the open field with two levels of nutrient availability in field plots. Competition and fertilizer had significant effects on height, relative growth rate (RGR), specific leaf area (SLA) as well as shoot and root biomass of all three species. The fertilized plants had high macronutrient and nitrate contents in leaf tissue. Relative yield of R. confertus was <1, indicating that for this species the effects of interspecific competition were greater than those of intraspecific competition. The results of this experiment indicate that there is interaction between the nutrient status of the soil and the competition between species. Competitive superiority of R. confertus could explain its dominance in grasslands and in disturbed areas, and might explain its great influence on the occurrence of native species because competition intensity was high in fertilized plots.
Introduction
Competition is likely to be an important determinant of plant community structure and is considered one of the most important factors promoting successful invasive potential1,2. Some invasive species are competitive in relation to native species, with the strongest competition occurring between species with similar ecological niches and/or closely related species3,4. With respect to invasion, several researchers5,6 have suggested that the main factors identified to date contributing to plant invasiveness explaining habitat invisibility are evolutionary history, habitat disturbance, propagule pressure, abiotic stress, soil nutrient availability and community structure. The degree of invasion of habitats was correlated with overall availability of nutrients6, and of specific nutrients such as nitrogen (N)7, potassium (K)8, and phosphorus (P)9. The theory of fluctuating resource availability6 proposes that the invasibility of a plant community increases as resource availability increases. Due to the fact that invasive species are usually found in disturbed areas such as agricultural fields, where disturbance reduces competition for nutrients or nutrient enrichment may occur, it can be assumed that they will be the most invasive in nutrient-rich habitats. Agricultural nutrient loading in the form of N, P, and K reaches wetlands via grounder water, surface flow, overland flow and precipitation10–12. Burke & Grime13 manipulated fertilizer in limestone grassland and showed that invasion was strongly related to availability of nutrients. Other studies have showed that hydrological disturbance affected nutrient availability because nitrates were readily leached from oxidized soil during drainage14. Anthropogenic nutrient inputs to the biosphere from fertilizers and atmospheric pollutants now exceed natural sources and enhance plant invasion15. There is also evidence that invasive species may access forms of nutrients that neighbouring (native) species are not using, including amino16.
Nitrogen is limiting nutrient important to fast-growing plants, especially to invasive plants17. Experimental studies showed greater relative performance of invasive species than of native ones in N-enriched treatments, implying reduced success of invasives relative to natives in N-poor sites18–20. Anthropogenic N enrichment due to of agricultural activities or roadside pollutants was shown to increase the number of N-loving invasive species in relation to native ones in N-poor environments, such as coastal, calcareous and sandy grasslands21. Numerous studies on competition demonstrated that excess fertilization and manure production on agricultural lands created surplus N, which was mobile in many soils and often leached to downstream aquatic ecosystems, or volatilized to the atmosphere, could be redeposited elsewhere eventually reaching aquatic ecosystems11. Many successful invaders, for example Australian Acacia spp., Myrica faya, Leucaena leucocephala, in N-limited ecosystems, have symbiotic associations with N-fixing bacteria22.
Potassium is an important macronutrient in plants needed in a number of crucial metabolic processes including photosynthesis and respiration8. A few studies indicated the relationship between invasion success and high K availability in soils8. Other studies, however, showed an opposite pattern. The invasive success of Taraxacum officinalis in the Andes was linked to low soil K availability23.
Eutrophication of most freshwater ecosystems is caused by over enrichment with nutrients, principally phosphorus9. The main sources of excess phosphorus inputs to aquatic ecosystems include domestic treated sewage, industrial discharges, storm drainage, and runoff from agriculture24. Normally, because phosphorus availability (primarily as orthophosphate) is the limiting element for freshwater macrophytes25, reducing phosphorus levels might help in controlling competitiveness of invasive macrophytes24.
Comparison of leaf area, plant height, above- and below-ground dry weight have been used to provide information on aggressiveness of species which might influence their competitive ability26–28. Some studies found that invasive species had higher biomass than non-invasive ones at high nutrient availabilities and that invasive and non-invasive species did not differ at low nutrient availabilities29,30. Competitiveness is often defined by relative yield, i.e. the ratio of yield in mixture to that in monoculture. In mixed swards, the genotype with the higher relative yield is regarded as more competitive31.
While many study focused on the relative competitive strength of alien or exotic vs native species32–34 and the influence of nutrient availability on invasive success35,36, relatively few studies examined experimentally the combined effects of competition and resource availability. Such studies are necessary in order to understand the ability of invasive plants to spread in new areas37–39.
Many invasive species are considered competitively superior to co-existing native species, with the strongest competition expected between species with similar niches. In this study a field experiment with pair-wise competition treatments of three Rumex species widespread in central Europe, i.e. an invasive alien R. confertus Willd. and two native species R. conglomeratus Murr. and R. acetosa L was performed. It is of interest to understand the ecological interactions among the Rumex species because R. confertus is highly invasive in Central Europe, it has spread on meadows and grasslands along river valleys in eastern Europe. Recently, however, R. confertus has colonized roadsides and wastelands40–42. In the majority of published R. confertus studies42,43, the response to nutrient condition was not investigated.
The competitive ability of a species is a combination of two components: competitive effect, the ability of a species to affect other species, and competitive response, the ability of a species to avoid being affected44. In the presented nutrient addition field experiments, a full-factorial randomized complete-block design was used for all combinations of three species as both target and neighbour species in order to address the following questions: (1) Do plant interactions between these three species depend on nutrient addition? (2) Does the relative yield per plant of three species depend on nutrient availability and competitive interactions? and (3) Which plant traits are related to competitive ability of R. confertus?
Specific hypotheses were as follows:
R. confertus would be the strongest competitor in both unfertilized and fertilized plots and would have higher seedling height and shoot biomass than non-invasive species especially at high nutrient availabilities
plants from nutrient fertilized plots would have higher specific leaf area (SLA), leaf macronutrient content than unfertilized ones and
under nutrient limitation all three species would have greater biomass allocation to roots.
Results
Soil solution
Increasing amounts of the fertilizer added to the soil, resulted in enhanced concentrations of N, P and K in the soil solutions (Table 1).
Table 1.
Physicochemical properties (pH, organic matter content, and macronutrient content [N, P, K]) of the soil in the fertilized plots used in the field experiment measured before planting and at end of the experiment.
| Chemical properties | Before planting | End of experiment |
|---|---|---|
| pH-H2O) | 6.7 | 6.3 |
| Organic matter (% dry weight) | 5.5 | 5.6 |
| Total nitrogen (N, %) | 0.4 | 0.6 |
| Phosphorus (P, mg/100 g) | 6.2 | 7.5 |
| Potassium (K, mg/100 g) | 21.4 | 23.4 |
| Texture | ||
| Sand (1.000–0.050 mm) (%) | 38.0 | 38.1 |
| Silt (0.050–0.002 mm) (%) | 54.4 | 54.4 |
| Loam (<0.002 mm) (%) | 7.6 | 7.5 |
Seedling height
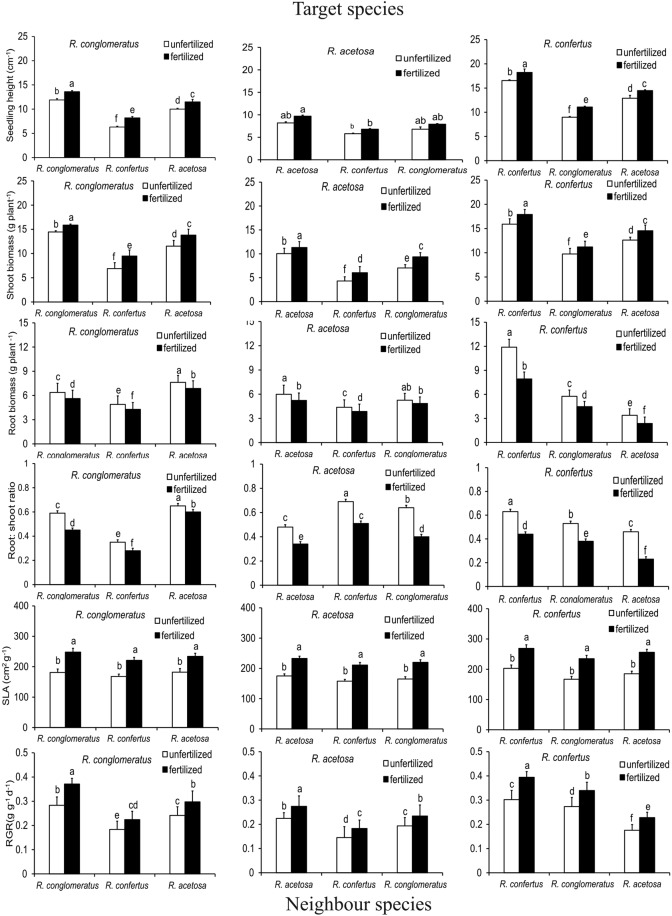
In unfertilized and fertilized plots, the height of both native species was significantly decreased by the invasive species (each P < 0.01). In addition the height of native species in the fertilized monoculture and in the mixture was significantly higher than without the fertilizer (Fig. 1). Analysis of variance showed that the seedling heights of R. confertus, R. conglomeratus and R. acetosa were influenced by competition (for all P < 0.001; Sypplementary Table S1). This analysis also revealed a significant interaction between competition and fertilization (for all P < 0.001; Table 2), i.e. fertilization mediated the effect of competition on the seedling height.
Figure 1.
Effects of competition and nutrient on early growth of Rumex conglomeratus, R. acetosa and R. confertus in each of two nutrient treatments. (n = 5 per species and treatment). The results are means (±SD) for all combinations of target (arranged in columns) and neighbour species (in rows). Different letters denote significant differences between mean values (ANOVA, Tukey’s HSD post hoc test with Bonferroni correction at P < 0.01).
Table 2.
Summary of two-way ANOVA results (F-values and significance levels) of seedlings traits (plant height, shoot and root biomass) or relative growth rate (RGR) in response to competition (presence−absence), fertilization (control vs fertilized) and their interaction in Rumex conglomeratus (a), R. acetosa (b) and R. confertus (c).
| Source of variation | Seedling height | Shoot biomass | Root biomass | SLA | Root:shoot ratio | RGR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | |
| (a) R. conglomeratus | ||||||||||||||||||
| Competition (C) | 1 | 18.6 | <0.001 | 1 | 68.5 | <0.001 | 1 | 9.7 | <0.001 | 1 | 11.2 | <0.01 | 1 | 10.2 | <0.01 | 1 | 46.7 | <0.01 |
| Fertilization (Ft) | 1 | 17.1 | <0.001 | 1 | 57.3 | <0.001 | 1 | 9.2 | <0.001 | 1 | 7.8 | <0.01 | 1 | 8.9 | <0.01 | 1 | 62.3 | <0.01 |
| C × Ft | 1 | 9.6 | <0.001 | 1 | 35.5 | <0.01 | 1 | 4.8 | <0.001 | 1 | 12.5 | <0.01 | 1 | 7.8 | <0.01 | 1 | 36.3 | <0.01 |
| (b) R. acetosa | ||||||||||||||||||
| Competition (C) | 1 | 17.9 | <0.01 | 1 | 57.4 | <0.001 | 1 | 8.8 | <0.01 | 5.8 | <0.01 | 1 | 6.6 | <0.01 | 1 | 41.7 | <0.001 | |
| Fertilization (Ft) | 1 | 16.3 | <0.001 | 1 | 49.2 | <0.001 | 1 | 7.6 | <0.001 | 5.6 | <0.01 | 1 | 7.5 | <0.01 | 1 | 53.5 | <0.001 | |
| C × Ft | 1 | 8.5 | <0.001 | 1 | 45.8 | <0.001 | 1 | 6.2 | <0.001 | 9.2 | <0.01 | 1 | 8.1 | <0.01 | 1 | 43.8 | <0.01 | |
| (c) R. confertus | ||||||||||||||||||
| Competition (C) | 1 | 14.5 | <0.001 | 1 | 18.5 | <0.001 | 1 | 17.8 | <0.001 | 8.5 | <0.01 | 1 | 6.7 | <0.01 | 1 | 47.1 | <0.01 | |
| Fertilization (Ft) | 1 | 15.1 | <0.001 | 1 | 14.9 | <0.001 | 1 | 16.4 | <0.001 | 9.0 | <0.01 | 1 | 8.3 | <0.01 | 1 | 42.7 | <0.001 | |
| C × Ft | 1 | 5.3 | <0.001 | 1 | 4.8 | <0.001 | 1 | 5.7 | <0.001 | 7.6 | <0.01 | 1 | 7.5 | <0.01 | 1 | 26.5 | <0.01 | |
Absence of competition refers to situations where seedlings were grown in monocultures; presence of competition refers to mixtures of seedlings. Data log transformed.
Shoot biomass
Application of the fertilizer significantly increased (for all P < 0.01) the shoot biomass R. conglomeratus and R. acetosa in both monocultures and mixtures. For example, in monocultures the fertilized seedlings of R. confertus, R. conglomeratus and R. acetosa had respectively 18%, 17.6% and 22% higher shoot biomass compared to the unfertilized ones (for each P < 0.01). In both unfertilized or fertilized monocultures the shoot biomass of R. confertus significantly exceeded that of R. conglomeratus (P = 0.0023), which in turn significantly exceeded that of R. acetosa (P = 0.0121). In the mixture, R. confertus caused a marked reduction in the shoot biomass of R. conglomeratus or R. acetosa (Supplementary Table S1). For example, on the unfertilized plots, R. confertus reduced R. acetosa shoot biomass by 25%, a proportion significantly higher than that caused by R. conglomeratus (22%; Fig. 1). A two-way ANOVA showed that shoot biomass production depended on competition and fertilization (C × Ft: F = 4.8, P < 0.001; Table 2).
Root biomass
In the unfertilized monoculture the root biomass of R. confertus significantly exceeded that of R. conglomeratus (P = 0.0013), which in turn significantly exceeded that of R. acetosa (P = 0.0191). In all three species root biomass decreased with increasing nutrient supply (R. confertus: P = 0.0019; R. conglomeratus: P = 0.0078; R. acetosa: P = 0.0035). When R. confertus competed with R. acetosa or R. conglomeratus root biomass of the later two was significantly reduced both with and without the fertilizer. However, the effect was more pronounced for R. acetosa than for R. conglomeratus (each P < 0.001; Fig. 1; Supplementary Table S1). A two-way ANOVA showed that fertilization had significant effect on root biomass of the invasive species (P < 0.001) and two native species (each P < 0.001; Table 2).
Root: shoot ratio
All three Rumex species increased biomass partitioning to roots as nutrient supply decreased (Fig. 1). A two-way ANOVA showed that root: shoot ratio was influenced by competition (Supplementary Table S1), fertilization and the interaction between these factors (for all P < 0.01; Table 2).
Specific leaf area
The increased nutrient supply increased SLA. Within mixtures competition with R. confertus significantly reduced SLA of two native species (Fig. 1). Analysis of variance (ANOVA) showed a significant effect of competition and fertilization on SLA of R. confertus, R. conglomeratus and R. acetosa (for all P < 0.01; Supplementary Table S1) with significant interaction between these factors (for all P < 0.01; Table 2).
Relative growth rate (RGR)
RGR was influenced by competition and fertilization, with interaction between these factors (for all P < 0.01). Addition of the fertilizer increased RGR of all three species in the monoculture and the mixed treatments. However, in the monoculture R. confertus showed the greatest RGR among all species under both nutrient treatments. Compared with the monoculture, R. confertus reduced RGR of R. conglomertaus and R. acetosa under both unfertilized and fertilized conditions (Fig. 1; Supplementary Table S1). To summarize, RGR of all species increased with nutrient availability (ANOVA, for all post hoc P < 0.01; Table 2).
Leaf macronutrient (N, P, K) and nitrate (NO3−-N) contents
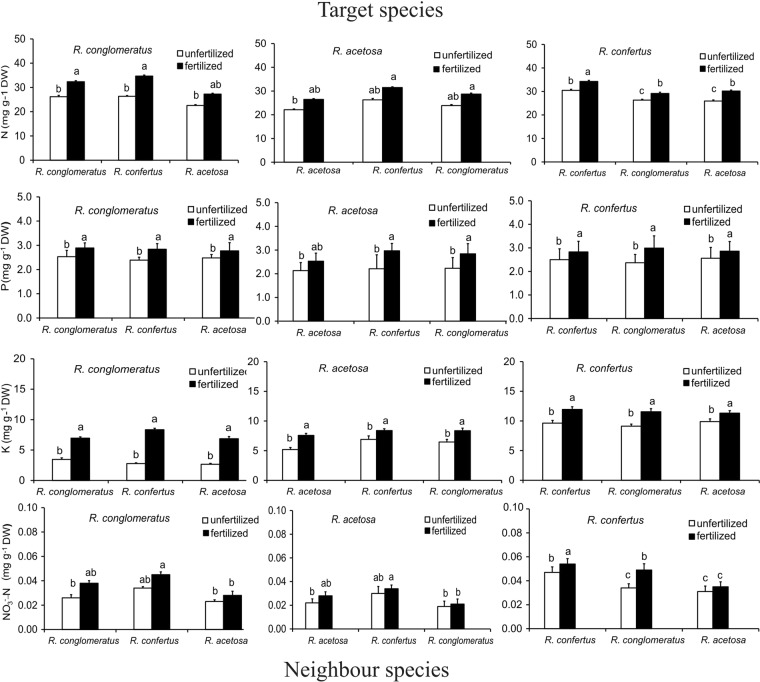
Analysis of variance (ANOVA) of N, P, K or NO3−-N contents indicated significant effects of fertilization. In addition, ANOVA showed that in all mixtures, competition had no effect on leaf nutrient content indicating that N, P, K or NO3−-N content in R. conglomeratus or R. acetosa when grown with R. confertus were not significantly different from those in monocultures (Supplementary Table S2. However, in the fertilized plots the leaf macronutrient or NO3−-N contents of R. confertus plants increased to a greater extent than those of R. acetosa and R. conglomerates plants. For example, in monoculture, leaf NO3−-N content of R. confertus was 46% higher in the fertilized plots as compared to the unfertilized ones, whereas in R. acetosa the increase was only 27% (Fig. 2; Table 3).
Figure 2.
Effects of competition and nutrient fertilizer on N, P, K and NO3−-N contents of leaves of Rumex conglomeratus, R. acetosa and R. confertus seedling in each of two nutrient treatments. (n = 5 per species and treatment). The results are means (±SD) for all combinations of target (arranged in columns) and neighbour species (in rows). Different letters denote significant differences between mean values (ANOVA, Tukey’s HSD post hoc test with Bonferroni correction at P < 0.01). In leaves the nutrient contents per plant estimated as the product of nutrient concentration, expressed as percentage of dry matter obtained from three leaves per plant.
Table 3.
F-values and significance levels from two-way analyses of variance (ANOVA) concerning macronutrient (N, P, K) or nitrate (NO3−-N) concentrations in leaves of Rumex conglomeratus (a), R. acetosa (b) and R. confertus (c).
| Source of variation | N | P | K | NO3−-N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | P | df | F | P | df | F | P | df | F | P | |
| (a) R. conglomeratus | ||||||||||||
| Competition (C) | 1 | 2.3 | >0.05 | 1 | 11.3 | >0.05 | 1 | 8.3 | >0.05 | 1 | 3.6 | >0.05 |
| Fertilization (Ft) | 1 | 11.1 | <0.01 | 1 | 10.5 | <0.01 | 1 | 16.0 | <0.01 | 1 | 11.5 | <0.01 |
| C × Ft | 1 | 5.6 | <0.05 | 1 | 6.0 | <0.05 | 1 | 5.6 | <0.05 | 1 | 10.3 | <0.01 |
| (b) R. acetosa | ||||||||||||
| Competition (C) | 1 | 3.4 | >0.05 | 1 | 16.5 | >0.05 | 1 | 6.6 | >0.05 | 1 | 5.3 | >0.05 |
| Fertilization (Ft) | 1 | 15.4 | <0.01 | 1 | 14.7 | <0.01 | 1 | 12.8 | <0.01 | 1 | 13.6 | <0.01 |
| C × F | 1 | 11.6 | <0.05 | 1 | 8.5 | <0.01 | 1 | 8.8 | <0.05 | 1 | 9.7 | <0.01 |
| (c) R. confertus | ||||||||||||
| Competition (C) | 1 | 4.4 | >0.05 | 1 | 28.5 | >0.05 | 1 | 3.1 | >0.05 | 1 | 2.2 | >0.05 |
| Fertilization (Ft) | 1 | 16.3 | <0.01 | 1 | 17.2 | <0.01 | 1 | 25.4 | <0.01 | 1 | 9.3 | <0.01 |
| C × Ft | 1 | 7.9 | <0.01 | 1 | 10.5 | <0.01 | 1 | 9.8 | <0.05 | 1 | 7.5 | <0.01 |
Main effects are F (fertilization treatment) and C (competition). Interaction C × F between fertilization treatments and competition are also given. Data log transformed.
Relative yields
Based on the observed changes in the total biomass of the harvested plants under both nutrient treatments the relative yields of R. acetosa in relation to R. confertus (RYacetosa+confertus) and of R. conglomeratus to R. confertus (RYconglomeratus+confertus) were significantly (in both P < 0.01) smaller than 1. So R. confertus outperformed both R. acetosa and R. conglomeratus in competition. Moreover, under unfertilized and fertilized conditions the relative yield of R. conglomeratus in relation to R. acetosa (RYconglomeratus+acetosa) was significantly higher (P < 0.01) than 1 which implies that R. conglomeratus outperformed R. acetosa in competition (Table 4).
Table 4.
Relative yields (RYab) of three Rumex species in mixture based on total biomass of single plants in relation to nutrient fertilizer (n = 5).
| Fertility treatment | Unfertilzed | Fertilized |
|---|---|---|
| RYconfertus+acetosa | 0.54 | 0.69 |
| RYconfertus+conglomeratus | 0.58 | 0.75 |
| RYacetosa+confertus | 0.61 | 0.89 |
| RYconglomeratus+confertus | 0.69 | 0.92 |
| RYconglomeratus+acetosa | 1.21 | 1.46 |
| RYacetosa+conglomeratus | 0.58 | 0.83 |
Discussion
The competitive effects of invasive R. confertus on two native species, R. conglomeratus and R. acetosa were examined at different nutrient levels. Competitive ability can be measured in various ways45. In the present study, it was evaluated by calculating the relative yield (RY) per plant for each species. As predicted (hypothesis 1) the invasive R. confertus outperformed the native R. conglomeratus and R. acetosa in competition at both low (control treatment) and high nutrient availability. Plant size (plant height) was shown to be highly correlated with competitive ability46. For example, Gaudet and Keddy47 comparing the short-term effects of 44 wetland species on the biomass of a phytometer (a plant on which the competitive effect of a test plant is measured), showed that the competitive ability of the test plants relative to the phytometer was highly correlated with their above-ground biomass and associated traits, such as plant height. Although R. confertus was not the tallest at the beginning of the experiment, it overtopped both native species rather early. Thus R. confertus is a strong competitor against its shorter native species, especially R. acetosa, which was suppressed by R. confertus in terms of all traits measured (hypothesis 1). In fertile habitats where canopies are well developed, taller plants have an advantage of gaining light in competition with shorter neighbours48.
In all experiments, RY of R. confertus was <1, indicating that for this species the effects of interspecific competition were greater than those of intraspecific competition38. The relative yields of R. acetosa in relation to R. confertus (RYacetosa+confertus) or of R. conglomeratus in relation to R. confertus (RYconglomeratus+confertus) on the unfertilized plots were smaller than on the fertilized ones. So, interspecific competition was lower on fertilized plots because RY was closer to 1. According to Grime2 competition is more intensive at high nutrient levels, which might explain low species diversity in nutrient-rich grassland ecosystems. But, on the other hand Tilman49 stated that the intensity of competition was either independent of or decreases with soil fertility.
In this study nutrient supply increased growth of all three Rumex species but the effect was most pronounced for the invasive R. confertus. A number of other examples were reported in which the competitive ability of a component species was altered by nutrient availability, for example between invasive grass Phragmites australis and a native competitor Spartina pectinata36, between Daucus carota and Chenopodium album50, between invasive Hydrocotyle vulgaris and terrestrial species51 and among nutrient-limited heachland plants38.
The shoot biomass of all three species was strongly influenced by competition especially in the high-nutrient treatment. The shoot biomass of native R. acetosa was reduced in competition with both congeners which shows that R. acetosa is the weakest competitor of the three species. The shoot biomass of R. conglomeratus decreased in two nutrient treatments in competition with R. confertus and increased in competition with R. acetosa. This indicates an intermediate position of native R. conglomeratus in the competitive hierarchy within the members of genus Rumex. The shoot biomass of R. confertus was not significantly affected by the congeners, which also indicates this species competitive superiority.
Three species investigated in this study exhibited significant differences in growth attributes. As predicted (hypothesis 2) SLA of the studied species increased with N, P, K supply. In addition invasive R. confertus had higher SLA than its non-invasive congeners, R. conglomeratus and R. acetosa under the same conditions. These results are consistent with those of other studies that reported that high SLA might be associated with invasive species52–54. Leaves with higher SLA have higher N concentrations55, leading to higher respiration55, carbon assimilation and RGR56. Therefore, high SLA values are characteristic of faster-growing species57. Differences in SLA between species might be caused by differences in leaf thickness or leaf tissue composition. For example leaf tissue density will be greater in the leaves with higher concentration of phenolic compounds (lignin and tannins), or secondary metabolites, resulting in lower SLA57. Experimental studies showed that RGR may be a better indicator of invasiveness than SLA. For example, SLA was more closely correlated with invasiveness than RGR for Acacia and Acer, while RGR was more tightly associated with invasiveness for the Rosaceae53. Invasive species achieved higher RGR than natives primarily by having a higher net rate of dry matter production and/or lower rates of respiration (high NAR, net assimilation ratio) by allocating more biomass to leaves (high LMR, leaf mass ratio), or producing thinner or less dense leaves resulting in more leaf area per unit leaf biomass (high SLA)58. As Grime and Hunt59 showed RGRs of 130 herbaceous species and tree seedlings in the local flora of Sheffield, England, ranged from 0.031 to 0.314 g g−1 d−1 (mean = 0.152). In the present study, mean RGR of R. confertus seedlings in the high-nutrient treatment was 0.35 g g−1 d−1, thus, it was above of the range of RGRs of the 130 species studied by Grime and Hunt59. High seedling RGR under non-limiting resources was found to be the most important trait of six species of dayflowers (Commelinaceae)60, four species of Senecio (Asteraceae)61 and of pine species53. A few other comparisons of RGRs of invasive and non-invasive congeners also generally showed that invasive plants had higher RGR than the native ones29,53,60. In contrast, Bellingham et al.62 did not find any correlation between invasiveness and RGR for seedlings of 33 woody species of gymnosperms and angiosperms. In this study, at both low and high level of fertility I expected R. confertus to be the superior competitor, with R. conglomeratus second and R. acetosa third. The results were as expected in all the species combinations. This suggests that R. confertus has a higher output per unit input and such a species is able to dominate an area quickly. Higher RGR gives R. confertus competitive advantage allowing it to pre-empt resources at an early stage of the growing season.
Phenotypic plasticity can be broadly defined as the ability of plants to alter their morphology and/or physiology in response to varying environmental conditions it may increase the competitive ability of a plant over a range of different resource availabilities63,64. The two common native species, R. acetosa and R. conglomeratus, as well as the invasive species R. confertus allocated relatively more biomass to the roots at low nutrient supply (Fig. 1), possibly increasing their competitive ability for belowground resources. However, both in monocultures or in mixtures the percentage decrease in biomass allocation to the roots in R. confertus exceeded those in both native species (Fig. 1) thus pointing to its higher phenotypic plasticity regarding the partitioning of biomass between shoots and roots.
Similar pattern was found by Aerts et al.38 in evergreen shrubs, Erica tetralix and Calluna vulgaris and perennial grass, Molinia caerulea. High plasticity of R. confertus may have significant effects on seedling establishment and competitive ability, and hence on species distribution65.
Rumex confertus showed significantly higher values of N, P, K or NO3−-N content, than the other Species. Nazaryuk et al.66 showed that the nitrate accumulation in plants depended on three major groups of factors: the amount and kind of a fertilizer applied, treatment with physiologically active substances, and natural and anthropogenic changes in the soil environment. All these factors may be arranged in the following order: fertilizers > physiologically active substances > soil. Nitrate accumulation in plants increased with nitrogen supply, whereas limiting the nitrogen availability reduced nitrate content significantly (see Umar and Iqbar67 for review). Both net absorption and assimilation rates during the growing season are genetically determined, explaining a large variability of plant nitrate content among plant species, and even among cultivars of the same species (see Cárdenas-Navarro et al.68 and references therein). This study showed that the nutrient content levels in the leaves were within the range of the reference values known for R. crispus69 and for R. alpinus70. Changes in the biomass of shoots and roots caused by species competition, did not translate into changes of the amounts of N, P, K or NO3−-N stored in the leaves. This is consistent with Zaller71 results, who tested the competitive ability of R. obtusifolius against grassland species.
Differences between species in root biomass are associated with environmental conditions. Boot and Mensink72 found that species from fertile sites had higher root: shoot ratios at both low and high N supply than did species from infertile sites. Others authors reported that fast-growing species from fertile sites generally had higher capacity to adjust mass allocation (see Hill et al.73 for the list and references). There are reports concerning many higher plant species showing decreased root: shoot ratio correlated with increased growth due to higher nutrient supply74. These results are consistent with the results presented here because the tested species were generally able to modify their root systems in response to nutrient. The hypothesis 3 in the studied species, the relative amount of biomass allocated to the roots was lower in the fertilized variant was confirmed. These findings are similar to those reported by McConnaughay and Coleman74 for the weed species Abutilon theophrasti, Chenopodium album and Polygonum pensylvanicum.
In central Poland, damp meadows on which R. confertus grows are often located below the fields and mineral fertilizers from them flow with water to these neighboring meadows. Thus its success may result from positive response to high nutrient availability. This is consistent with the pattern found in P. australis by Rickey and Anderson36.
Conclusion
The high invasiveness of R. confertus seems to result from its competitive dominance over the other congeners across a very wide range of nutrient availability. The high competitive ability of R. confertus with respect to both R. acetosa and R. conglomeratus was caused by (a) higher SLA, (b) great plant height, and (c) higher potential growth rate. This combination of plant traits reduces light absorption or photosynthate production of R. acetosa and R. conglomeratus thereby limiting their growth.
With strengthened competitive ability of R. confertus, weakened competitive ability in the other species (R. acetosa and R. conglomeratus) and consequent changes in competitive hierarchy, I expect possible changes in species dominance and structure of meadow plant community in central Europe in the future.
Materials and Methods
Study species
The studied Rumex (Polygonaceae) species are biennials with similar life-histories and reproductive characteristics, they coexist in some habitats but with different origin and invasion status in Poland. Rumex acetosa, R. conglomeratus and R. confertus were chosen to test interspecific competition because these species are the most common neighbors in moist to wet meadows and pastures in Poland. All three studied Rumex species reproduce clonally (ramets produced from root and rhizome buds) and sexually from seeds.
According to their ecological indicator values75, R. acetosa (Ellenberg’s N-value = 6) and R. conglomeratus (Ellenberg’s N-value = 8) show similar requirements for soil nutrients. Generally, both species are indicators of high soil nitrogen concentrations75.
Rumex confertus is native to temperate Eastern Europe and Asia where it thrives on meadow-steppes and glades in forest-steppe. Its height varies from 60 to 120 cm. In Poland it occurs predominantly along rivers and invades seminatural vegetation (e.g. meadows, wet ditches, riparian-scrub), but has been recently colonizing disturbed habitats (such as roadsides, railway tracks and embankments), forest clearings and margins76,77. The aggressiveness of R. confertus results from its ability to establish quickly from seed, to flower in its first year and to its fast growth and high seed production some of which can remain viable for very long periods in the soil77.
Rumex conglomeratus grows in wet meadows, stream and river banks, ditches, field margins and gateways, often in places flooded or waterlogged in winter78. This species is characterized by a height similar to that of R. confertus and a broad ecological amplitude. This Eurosiberian Southern-temperate element became widely naturalised so that distribution is now Circumpolar Southern-temperate78.
The third species, R. acetosa occurs in a wide range of habitats, but particularly at waste ground, road sides, disturbed areas, grasslands (mainly pastures), and arable land78. Its height varies from 30 to 80 cm. Rumex acetosa occurs in meadows throughout Europe but rarely in the south as well as in parts of Central Asia. It occurs as an introduced species in parts of North America79,80.
Plant material
Freshly matured seeds of R. acetosa, R. conglomeratus and R. confertus were collected in a wet pasture on 2 September 2016 in the vicinity of Uniejów (51°96′N, 18°79′E), 50 km west of Lodz, central Poland. For each species, I collected a mixed sample of seeds from at least 20 individuals randomly chosen from the whole site. The seeds were stored in paper bags at 4 °C until use.
Experimental design and growth conditions
An experiment was conducted in the experimental garden on a private property in the vicinity of Uniejów, 60 km west of Lodz, where the mean annual temperature was 8.8 °C and mean annual precipitation (rain and snow) was 587.2 mm (meteorological data, Lodz station). The seeds were stratified in a refrigerator at 4 °C for a 16 weeks and then germinated on moist filter paper in a Petri dish in growth cabinet (16 h light, 24 °C, 20 μmol m−2 s−1 PPFD; 8 h dark, 10 °C). After three weeks, uniform seedlings with two leaves were selected and transplanted into plastic pots 25 cm in diameter, 22 cm deep (1962 cm2) filled with a mixture (1: 4, v/v) of sterilized soil and sterilized sand and cultured in a growth room (23 °C; 14 light/10 h dark; 80 μmol m−2 s−1 PPFD, Philips TL 94,) for three months.
Next the same size seedlings of individual species were transferred to field plots, 22 days after germination ([T1], 17 April 2017). Seedling biomass (±SD) at the start of the experiment (T1) was equal for R. confertus (0.41 ± 0.05 g) and R. conglomeratus (0.39 ± 0.04 g) while R. acetosa seedlings had smaller mass (0.28 ± 0.02 g). After planting, the seedlings were grown in the open air during May−August a period of moderate temperature (Table 5).
Table 5.
Mean air temperature at the field site during Rumex seedling growing period for 153 days from 17 April to 17 August 2017.
| April | May | June | July | August | ||
|---|---|---|---|---|---|---|
| Temperature °C | Daily maximum | 13.8 | 18.7 | 22.0 | 24.0 | 23.3 |
| Daily minimum | 3.9 | 7.9 | 11.3 | 13.4 | 12.8 | |
| Daily mean | 8.8 | 13.3 | 16.6 | 18.7 | 18.0 | |
The plants were planted in monocultures and mixtures using a replacement design: each species in monoculture and also in a 1:1 mixture of each of two species. All treatments were carried out with four replicate plots in a randomized block design. A total number of 48 plots (2 nutrient treatments × 6 species-competition combinations × 4 replicates) were used for the experiment.
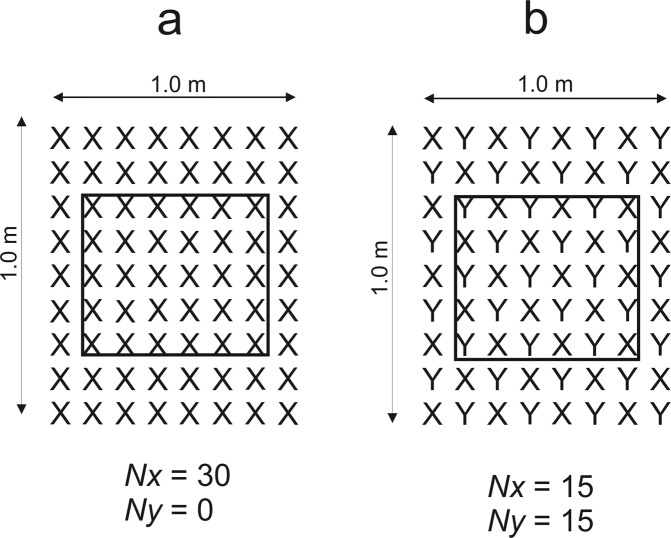
To avoid border effects, only the inner 30 individuals of each plot (6 rows of 5 plants in) in both monocultures or in mixtures were analyzed (Fig. 3).
Figure 3.
Diagram of the arrangement of plants in the plots for the total density of 72 plants m2. Each symbol represents a single plant, separated from its neighbors by 10 cm. (a) species x in monoculture, (b) species x in mixture with species y. Nx = number of plants of species x; Ny = number of plants of species y.
Two nutrient treatments for each species-competition combination were imposed after transplanting the seedlings into plots: unfertilized (control) and fertilized. The commercial (Inco VERITAS S.A., Poland) fertilizer “Azofoska” (40 g m−2 yr−1) containing (%): 5.5 NO3, 8.1 NH4, 6.4 P2O5, 19.1 K2O, 4.5 MgO, 0.27 Fe, 0.045 Mn, 0.18 Cu, 0.045 B, 0.082 Mo, 0.045 Zn was used. The fertilizer was evenly spread by hand and was supplied twice during the growing season, on 25 May and 25 June (20 g each time).
The plots (1 × 1 m) were separated by equally sized (0.5 m wide) buffer zones. There were 8 rows of 9 seedlings in both monocultures and mixture plots. However, plant density of each species in the mixture was half of the density of that species in monoculture. In the plots containing mixtures of seedlings, 36 seedlings of each of the two species were placed alternately in rows at the spacing of 10 cm. Thus each seedling (except at the margin) was surrounded by four seedlings of the other species. To avoid border effects, only the inner 30 individuals of each plot (6 rows of 5 plants: both in monocultures and mixtures) were analyzed (Fig. 3). Watering to field capacity, using tap-water, was carried out every three days.
Soil physical and chemical analysis
In each plot one soil core (3 cm diameter × 5 cm deep) was collected at random and put in a plastic bag. Soil cores were mixed by hand up into a single bulk sample for each plot. Then the soil samples were air-dried (20–25 °C for 3 days) to a constant weight and sieved through a 2-mm mesh screen. The pH was determined in water (ratio 1:1, soil: water). The soil samples (100 g dry soil) were analysed for selected macronutrients: total nitrogen (N), phosphorus (P) and potassium (K). Phosphorus was measured by colorimetric molybdenum blue method in 0.5 M sodium bicarbonate (NaHCO3) extract of soil. Potassium was extracted from soil with calcium lactate (C6H10CaO6) and determined by atomic absorption spectrometer (AAS) method. Total N was determined by the micro-Kjeldahl procedures by the wet oxidation of organic matter using sulfuric acid (H2SO4) and a digestion catalyst. The amount of soil organic matter was estimated as loss on ignition (550 °C, 3 h) and calculated as % dry weight (Table 2). The analyses were performed at the Chemical-Agricultural Station in Lodz.
Harvest and biomass sampling
At the end of the experiment ([T2]; 17 August 2017, 16 weeks following transplantation) height (cm) of the main shoot of each seedling was recorded before they were harvested. In general, ten whole plants of each species-competition combination were randomly collected within both unfertilized and fertilized plots. A total number of 240 seedlings per species (2 nutrient treatments × 3 species combination × 4 replicates × 10 seedlings) were collected for the experiment. To measure shoot biomass, each species shoot (leaves + stem) was cut off at the soil surface. Roots and shoot were carefully washed in the lab. Leaf area was measured on the same day with a CI-202 portable laser leaf area meter (CID Bio-Science, USA). After determination of the area, the leaves were dried for at least 48 h at 70 °C and dry mass was determined. Specific leaf area (SLA; cm2 g−1) was calculated as area per unit mass. Shoots and roots of a single plant were dried to a constant weight for at least 72 h at 70 °C in an oven, and weighed (g). The relative growth rate (RGR) was estimated using the following formula:
| 1 |
where, Wt2 Wt1 are plant dry weight (DW in mg) of seedlings at time T1 and time T2, respectively81.
Relative yield
The effect of competitive response (neighbours) was quantified using relative yield per plant for each species82. The relative yield (RYab) was calculated as:
| 2 |
where, Yab is the dry biomass yield of species a in mixture with species b and Ya is the dry biomass yield of species a in monoculture. If RY = 1, there is absolutely no competition. If RYab > 1 species a outperformed species b in competition, when RYab < 1 the reverse is true38.
Foliar macronutrient and nitrate analyses
Samples of three (youngest fully expanded) leaves were collected from each of 10 different plants separately for each competition treatment. Pooling of three leaves on a plant was necessary to obtain enough tissue for the macronutrient (N, P and K) and nitrate (NO3−-N) analyses. Afterwards, the leaf samples were dried (105 °C for 5 h) and ground with a plant-sample mill and siewed with a 2-mm mesh screen before the sub-samples were weighed with a precision balance. Prior to chemical analyses, the leaf samples of each harvest were combined into four independent sub-samples (100 mg of each). Two thirds being used for macronutrient determination and one third − for nitrate determination. The leaf material was mineralized in a boiling mixture of 10 ml of HNO3 and 5 ml of H2SO4. Phosphorus was determined colorimetrically (see soil properties), potassium by atomic absorption spectrometry (as above), and the total nitrogen content by the micro-Kjeldahl method (as above). Nitrate concentration in plant tissue was determined with a Corning NO3− ion selective electrode (Orion Analyzer Application Bull. No. 7, Determination of nitrate in plant tissue). Leaf macronutrients and tissue NO3−-N contents were as calculated as the total amount of macronutrients and nitrate per unit of dry leaf mass (mg/g dry wt−1).
Data analysis
Normality was verified with the Shapiro-Wilk test. All data were log transformed prior to the analysis if necessary to meet the assumption of normality. Student’s t-test was performed to test for statistical differences in relative yields (RYab) between unfertilized and fertilized plots. For unfertilized and fertilized plots, differences between species in seedling height, shoot biomass, root biomass, SLA and RGR were analysed by one-way ANOVA with post-hoc Tukey’s range tests. The effect of competition (monoculture vs mixture plots), fertilization (control vs fertilized) and their interaction on seedlings traits (plant height, shoot and root biomass) or relative growth rate (RGR) for each species was analyzed using a two-way nested ANOVA. Two-way ANOVA was also performed to test the effect of competition (monoculture vs mixture plots), fertilizatiom (control vs fertilized) and their interaction on concerning macronutrients (N, P, K) or nitrate (NO3−-N) concentrations in leaves of three species. Statistical analyses were performed using the Statistica 13.0 package83.
Supplementary information
Author Contributions
J. Kołodziejek conceived and designed the study, performed the experiments, collected the data, prepared the analysis and figures, and wrote the manuscript.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information File).
Competing Interests
The author declares no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39947-z.
References
- 1.Williamson, M. Biological Invasions. (Chapman and Hall, London, 1996).
- 2.Grime, J. P. Plant Strategies and Vegetation Processes. (Wiley, Chichester, 1979).
- 3.Sala A, Verdaguer D, Vilà M. Sensitivity of the invasive geophyte Oxalis pes-caprae to nutrient availability and competition. Ann. Bot. 2007;99:637–645. doi: 10.1093/aob/mcl289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craine JM, Dybzinsky R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013;27:833–840. doi: 10.1111/1365-2435.12081. [DOI] [Google Scholar]
- 5.Alpert P, Bone E, Holzapfel C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect. Plant Ecol. Syst. 2000;3:52–66. doi: 10.1078/1433-8319-00004. [DOI] [Google Scholar]
- 6.Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol. 2000;88:528–534. doi: 10.1046/j.1365-2745.2000.00473.x. [DOI] [Google Scholar]
- 7.Howard TG, et al. Forest invisibility in communities in southeastern New York. Biol. Invasions. 2004;6:393–410. doi: 10.1023/B:BINV.0000041559.67560.7e. [DOI] [Google Scholar]
- 8.Sardans J, Peñuelas J. Potassium: a neglected nutrient in global change. Global Ecol. Biogeogr. 2015;24:261–275. doi: 10.1111/geb.12259. [DOI] [Google Scholar]
- 9.Schindler DW. Evolution of phosphorus limitation in lakes. Science. 1977;195:260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- 10.Galatowitsch SM, Anderson NO, Ascher PD. Invasiveness in wetland plants in temperate North America. Wetlands. 1999;19:733–755. doi: 10.1007/BF03161781. [DOI] [Google Scholar]
- 11.He WM, Yu GL, Sun ZK. Nitrogen deposition enhances Bromus tectorum invasion: biogeographic differences in growth and competitive ability between China and North America. Ecography. 2011;34:1059–1066. doi: 10.1111/j.1600-0587.2011.06835.x. [DOI] [Google Scholar]
- 12.Grime JP. Competitive exclusion in herbaceous vegetation. Nature. 1973;242:344–347. doi: 10.1038/242344a0. [DOI] [Google Scholar]
- 13.Burke MJW, Grime JP. An experimental study of plant community invasibility. Ecology. 1996;77:776–790. doi: 10.2307/2265501. [DOI] [Google Scholar]
- 14.OldeVenterink H, Davidsson TE, Kiehl K, Leonardson L. Impact of drying and re-wetting on N, P and K dynamics in a wetland soil. Plant Soil. 2002;243:119–130. doi: 10.1023/A:1019993510737. [DOI] [Google Scholar]
- 15.Lovett GH, et al. Effects of Air Pollution on Ecosystems and Biological Diversity in the Eastern United States. Ann. N. Y. Acad. Sci. 2009;1162:99–135. doi: 10.1111/j.1749-6632.2009.04153.x. [DOI] [PubMed] [Google Scholar]
- 16.Lipson D, Näsholm T. The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia. 2001;128:305–316. doi: 10.1007/s004420100693. [DOI] [PubMed] [Google Scholar]
- 17.Lowe PN, Lauenroth WK, Burke IC. Effects of nitrogen availability on competition between Bromus tectorum and Bouteloua gracilis. Plant Ecol. 2003;67:247–254. doi: 10.1023/A:1023934515420. [DOI] [Google Scholar]
- 18.Kolb A, Alpert P, Enters D, Holzapfel C. Patterns of invasion within a grassland community. J. Ecol. 2002;90:871–881. doi: 10.1046/j.1365-2745.2002.00719.x. [DOI] [Google Scholar]
- 19.Liancourt P, Viard-Crétat F, Michalet R. Contrasting community responses to fertilization and the role of the competitive ability of dominant species. J. Veg. Sci. 2009;20:138–147. doi: 10.1111/j.1654-1103.2009.05501.x. [DOI] [Google Scholar]
- 20.James JJ, Drenovsky RE, Monaco TA, Rinella MJ. Managing soil nitrogen to restore annual grass-infested plant communities: effective strategy or incomplete framework? Ecol. Appl. 2011;21:490–502. doi: 10.1890/10-0280.1. [DOI] [PubMed] [Google Scholar]
- 21.Wedin D, Tilman D. Competition among grasses along a nitrogen gradient: initial conditions and mechanisms of competition. Ecol. Monographs. 1993;63:199–229. doi: 10.2307/2937180. [DOI] [Google Scholar]
- 22.Rodríguez-Echeverría S, Crisostomo JA, Nabais C, Freitas H. Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol Invasions. 2009;11:651–661. doi: 10.1007/s10530-008-9280-8. [DOI] [Google Scholar]
- 23.Cavieres LA, Quiroz CL, Molina-Montenegro MA. Facilitation of the non-native Taraxacum officinale by native nurse cushion species in the high Andes of central Chile: are there differences between nurses? Funct. Ecol. 2008;22:148–156. doi: 10.1111/j.1365-2435.2008.01382.x. [DOI] [Google Scholar]
- 24.Carpenter SR. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. PNAS. 2005;102:10002–10005. doi: 10.1073/pnas.0503959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharpley, A. N. et al. Agricultural phosphorus and eutrophication, U.S. (Department of Agriculture, Agricultural Research Service, 2003).
- 26.Lü Y, Wang GQ, Zheng L, Ni HW. Competitiveness of invasive plant Flaveria bidentis with native weed plants. Chinese J. Ecol. 2011;30:577–681. [Google Scholar]
- 27.Manea A, Leishman MR. Competitive interactions between native and invasive exotic plant species are altered under elevated carbon dioxide. Oecologia. 2011;165:735–744. doi: 10.1007/s00442-010-1765-3. [DOI] [PubMed] [Google Scholar]
- 28.Poorter H, Remkes C. Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia. 1990;83:553–559. doi: 10.1007/BF00317209. [DOI] [PubMed] [Google Scholar]
- 29.Maillet J, Lopez-Garcia C. What criteria are relevant for predicting the invasive capacity of a new agricultural weed? The case of invasive American species in France. Weed Res. 2000;40:11–26. doi: 10.1046/j.1365-3180.2000.00171.x. [DOI] [Google Scholar]
- 30.Kolb A, Alpert P. Effects of nitrogen and salinity on growth and competition between a native grass and an invasive congener. Biol. Invasions. 2003;5:229–238. doi: 10.1023/A:1026185503777. [DOI] [Google Scholar]
- 31.Nurjaya, I. G. M. E. & Tow, P. G. Genotype and environmental adaptation as regulators of competitiveness. In Competition and succession in pastures (eds Tow, P. G. & Lazenby, A.), 43−62 (CABI, Sydney, 2000).
- 32.Čuda J, Skálová H, Janovský Z, Pyšek P. Competition among native and invasive Impatiens species: the roles of environmental factors, population density and life stage. AoB Plants. 2015;7:1–39. doi: 10.1093/aobpla/plv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fogarty G, Facelli JM. Growth and competition of Cytisus scoparius, an invasive shrub, and Australian native shrubs. Plant Ecol. 1999;144:27–35. doi: 10.1023/A:1009808116068. [DOI] [Google Scholar]
- 34.Song U. Temperature-dependent performance of competitive native and alien invasive plant species. Acta Oecol. 2017;84:8–14. doi: 10.1016/j.actao.2017.08.001. [DOI] [Google Scholar]
- 35.Cohn EJ, Vanauken OW, Bush JK. Competitive interactions between Cynodon dactylon and Acacia smallii seedlings at different nutrient levels. Amer. Midl. Naturalist. 1989;121:265–272. doi: 10.2307/2426030. [DOI] [Google Scholar]
- 36.Rickey MA, Anderson RC. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. J. Appl. Ecol. 2004;41:888–896. doi: 10.1111/j.0021-8901.2004.00948.x. [DOI] [Google Scholar]
- 37.Sala A, Verdaguer D, Vilà M. Sensitivity of the invasive geophytes Oxalis pes-caprae to nutrient availability and competition. Ann. Bot. 2007;99:637–645. doi: 10.1093/aob/mcl289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aerts R, Berendse F, de Caluwe A, Schmitz M. Competition in heathland along an experimental gradient of nutrient availability. Oikos. 1990;57:310–318. doi: 10.2307/3565959. [DOI] [Google Scholar]
- 39.Suding KN, LeJeune KD, Seastedt TR. Competitive impacts and responses of an invasive weed: dependencies on nitrogen and phosphorus availability. Oecologia. 2004;141:526–535. doi: 10.1007/s00442-004-1678-0. [DOI] [PubMed] [Google Scholar]
- 40.Trzcińska-Tacik H. Studies on the distribution of synanthropic plants. 2. Rumex confertus Willd. in Poland. Fragm. Flor. Geobot. Pol. 1963;9:73–84. [Google Scholar]
- 41.Jehlik V, Sádlo J, Dostálek J, Jarolimova V, Klimeš L. Chorology and ecology of Rumex confertus Willd. In the Czech Republik. Bot. Lithuanica. 2001;7:235–244. [Google Scholar]
- 42.Raycheva T. Rumex confertus (Polygonaceae) in the Bulgarian flora. Bot. Serb. 2011;35:55–59. [Google Scholar]
- 43.Kołodziejek J, Patykowski J. Effect of environmental factors on germination and emergence of invasive Rumex confertus in Central Europe. The Scientific World Journal. 2015;2015:1–10. doi: 10.1155/2015/170176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg DE, Landa K. Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J. Ecol. 1991;79:1013–1030. doi: 10.2307/2261095. [DOI] [Google Scholar]
- 45.Njambuya J, Stiers I, Triest L. Competition between Lemna minuta and Lemna minor at different nutrient concentrations. Aquat. Bot. 2011;94:158–164. doi: 10.1016/j.aquabot.2011.02.001. [DOI] [Google Scholar]
- 46.Walck JL, Baskin JM, Baskin CC. Relative competitive abilities and growth characteristics of a narrowly endemic and a geographically widespread Solidago species (Asteraceae) Am. J. Bot. 1999;86:820–828. doi: 10.2307/2656703. [DOI] [PubMed] [Google Scholar]
- 47.Gaudet C, Keddy PA. Competitive performance and species distribution in shoreline plant communities: a comparative approach. Ecology. 1995;76:280–291. doi: 10.2307/1940649. [DOI] [Google Scholar]
- 48.Weiner J. Asymmetric competition in plant populations. Trends Ecol. Evol. 1990;5:360–364. doi: 10.1016/0169-5347(90)90095-U. [DOI] [PubMed] [Google Scholar]
- 49.Tilman, D. Plant strategies and the dynamics and structure of plant communities. (Princeton University Press, Princeton, NJ, 1988).
- 50.Li B, Watkinson A. R. Competition along a nutrient gradient: a case study with Daucus carota and Chenopodium album. Ecol. Res. 2000;15:293–306. doi: 10.1046/j.1440-1703.2000.00349.x. [DOI] [Google Scholar]
- 51.Liu L, et al. Nutrient enrichment alters impacts of Hydrocotyle vulgaris invasion on native plant communities. Sci Rep. 2016;6:39468. doi: 10.1038/srep39468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Werf A, van Nuenen M, Visser AJ, Lambers H. Contribution of physiological and morphological plant traits to a species’ competitive ability at high and low nitrogen supply: A hypothesis for inherently fast- and slow-growing monocotyledonous species. Oecologia. 1993;94:434–440. doi: 10.1007/BF00317120. [DOI] [PubMed] [Google Scholar]
- 53.Grotkopp E, Rejmánek M, Rost TL. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat. 2002;159:396–419. doi: 10.1086/338995. [DOI] [PubMed] [Google Scholar]
- 54.Knops JMH, Reinchard K. Specific leaf area along a nitrogen fertilization gradient. Amer. Midl. Naturalist. 2000;144:265–272. doi: 10.1674/0003-0031(2000)144[0265:SLAAAN]2.0.CO;2. [DOI] [Google Scholar]
- 55.Reich PB, et al. Scaling of respiration to nitrogen in leaves, stems and roots of higher land plants. Ecol. Lett. 2008;11:793–801. doi: 10.1111/j.1461-0248.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 56.Madani N, et al. Improving ecosystem productivity modeling through spatially explicit estimation of optimal light use efciency. J. Geophys. Res. Biogeosci. 2014;119:1755–1769. doi: 10.1002/2014JG002709. [DOI] [Google Scholar]
- 57.Poorter H, De Jong R. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol. 1999;143:163–176. doi: 10.1046/j.1469-8137.1999.00428.x. [DOI] [Google Scholar]
- 58.James JJ, Drenovsky RE. A basis for relative growth rate differences between native and invasive forb seedlings. Rangeland Ecol. Manage. 2007;60:395–400. doi: 10.2111/1551-5028(2007)60[395:ABFRGR]2.0.CO;2. [DOI] [Google Scholar]
- 59.Grime JP, Hunt R. Relative growth-rate: its range and adaptive significance in a local flora. J. Ecol. 1975;63:393–422. doi: 10.2307/2258728. [DOI] [Google Scholar]
- 60.Burns JH. A comparison of invasive and non-invasive dayflowers (Commelinaceae) across experimental nutrient and water gradients. Divers. Distrib. 2004;10:387–397. doi: 10.1111/j.1366-9516.2004.00105.x. [DOI] [Google Scholar]
- 61.Garcia-Sserrano H, Escarré J, Garnier G, Sans FX. A comparative growth analysis between alien invader and native Senecio species with distinct distribution ranges. Ecoscience. 2005;12:35–43. doi: 10.2980/i1195-6860-12-1-35.1. [DOI] [Google Scholar]
- 62.Bellingham PJ, Duncan RP, Lee WG, Buxton RP. Seedling growth rate and survival do not predict invasiveness in naturalized woody plants in New Zealand. Oikos. 2004;106:308–316. doi: 10.1111/j.0030-1299.2004.13171.x. [DOI] [Google Scholar]
- 63.Reynolds HL, D’Antonio C. The ecological significance of plasticity in root weight ratio in response to nitrogen: Opinion. Plant Soil. 1996;189:75–97. doi: 10.1007/BF02257566. [DOI] [Google Scholar]
- 64.Baker HG. The evolution of weeds. Ann. Rev. Ecol. Syst. 1974;5:1–24. doi: 10.1146/annurev.es.05.110174.000245. [DOI] [Google Scholar]
- 65.Sultan SE. Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology. 2001;82:328–343. doi: 10.2307/2679863. [DOI] [Google Scholar]
- 66.Nazaryuk VM, Klenova MI, Kalimullina FR. Ecoagrochemical approaches to the problem of nitrate pollution in agroecosystems. Russ. J. Ecol. 2002;33:392–397. doi: 10.1023/A:1020995329784. [DOI] [Google Scholar]
- 67.Umar AS, Iqbal M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2006;27:45–57. [Google Scholar]
- 68.Cárdenas-Navarro R, Adamowicz S, Robin P. Nitrate accumulation in plants: a role for water. J. Exp. Bot. 1999;50:613–624. doi: 10.1093/jxb/50.334.613. [DOI] [Google Scholar]
- 69.Gebauer G, Rehder H, Wollenweber B. Nitrate, nitrate reduction and organic nitrogen in plants from different ecological and taxonomic groups of Central Europe. Oecologia. 1988;75:371–385. doi: 10.1007/BF00376940. [DOI] [PubMed] [Google Scholar]
- 70.Rehder H. Nitrogen relations of ruderal communities (Rumicion alpini) in the Northern Calcareous Alps. Oecologia. 1982;55:120–129. doi: 10.1007/BF00386727. [DOI] [PubMed] [Google Scholar]
- 71.Zaller JG. Competitive ability of Rumex obtusifolius against native grassland species: above- and belowground allocation of biomass and nutrients. J. Plant Dis. Protect. 2004;19:345–351. [Google Scholar]
- 72.Boot GA, Mensink M. Size and morphology of root systems of perennial grasses from contrasting habitats as affected by nitrogen supply. Plant Soil. 1990;129:291–299. doi: 10.1007/BF00032425. [DOI] [Google Scholar]
- 73.Hill JO, et al. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil. 2006;286:7–19. doi: 10.1007/s11104-006-0014-3. [DOI] [Google Scholar]
- 74.McConnaughay KDM, Coleman JS. Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology. 1999;80:2581–2593. doi: 10.1890/0012-9658(1999)080[2581:BAIPOO]2.0.CO;2. [DOI] [Google Scholar]
- 75.Ellenberg H, et al. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobot. 1991;18:1–248. [Google Scholar]
- 76.Rechinger, K. H. Rumex L. In Flora Europaea (eds Tutin, T. G. et al.), 82–89 (Cambridge University Press, Cambridge, UK, 1976).
- 77.Stosik T. Generative reproduction efficiency and the population age structure of Rumex confertus Willd. Acta Agrobot. 2006;59:85–93. doi: 10.5586/aa.2006.064. [DOI] [Google Scholar]
- 78.Cavers PB, Harper JL. Rumex obtusifolius L. and R. Crispus L. J. Ecol. 1964;52:737–766. doi: 10.2307/2257859. [DOI] [Google Scholar]
- 79.Meusel, H., Jäger E., & Weinert, E. Vergleichende Chorologie der zentraleuropäischen Flora. (Gustav Fischer Verlag, Jena, 1965).
- 80.Blamey, M., Fitter, R. & Fitter, A. Wild flowers of Britain and Ireland: The Complete Guide to the British and Irish Flora. (A & C Black, London, 2003).
- 81.Hunt, R. Plant growth curves. The Functional Approach to Growth Analysis. (Edward Arnold, London, 1982).
- 82.Harper JL. Approaches to the study of plant competition. Symp. Soc. Exp. Biol. 1961;15:1–39. [Google Scholar]
- 83.Stat-Soft Inc. Statistica for Windows. (Stat-soft, Inc., Tulsa, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information File).