Abstract
The present study is the first report on the application of silver nanoparticles for efficient bacterial transformation. EC50 value of 100 nm silver nanoparticles against E. coli DH5α cells was recorded as 4.49 mg L−1 in toxicity assay. Competency induction in E. coli DH5α cells by treatment with 100 nm silver nanoparticles at a concentration of 1 mg L−1 for 60 min and transformation using three plasmid vectors of different sizes, viz. pUC18, pBR322 and pCAMBIA resulted in tenfold increase in the bacterial transformation efficiency, i.e. 8.3 × 104, 8.0 × 104 and 7.9 × 104 cfu ng−1 of DNA, respectively, even without heat shock compared to the conventional chemical method using 0.1 M calcium chloride (2.3 × 103 cfu ng−1 of DNA).
Keywords: Silver nanoparticles, Competent cells, Bacterial transformation, pUC18 plasmid, pBR322, pCAMBIA, β-Galactosidase
Introduction
Efficient introduction of plasmid DNA into microbial cells by transformation plays a significant role in recombinant DNA technology (Kotnik et al. 2015). Natural genetic transformation is absent in many bacteria mainly due to repulsions caused by the negative charges of both DNA and bacterial membrane and less porosity of the membrane (Asif et al. 2017). Escherichia coli DH5α, the most commonly used bacterial host for cloning has a three-walled membrane. The pore-forming proteins (porins), which are present in high copy number within the outer membrane, behave as a molecular sieve and prevent the passive transport of hydrophilic and high molecular weight molecules such as DNA and proteins into the bacterial cell (Raghava et al. 2011). Therefore, there is necessity for development of controlled artificial transformation methods in bacteria and many approaches have been attempted.
Electroporation and chemical method are being used for artificial microbial transformation (Yoshida and Sato 2009). Cost of the equipment and requirement of very high cell density are the main limitations of electroporation. Chemical method in which calcium chloride is used for competency induction in the cells is simple, cost effective and is widely used. However, the major limitation of this method is the low transformation efficiency (Panja et al. 2006; Liu et al. 2014).
Nanotechnology has evolved as a major interdisciplinary science and has been explored for various biological processes (Pinto-Alphandary et al. 2000). As nanoparticles are smaller (nanoscale 10−9 m) compared to large biological molecules, such as enzymes, receptors and antibodies, they offer unprecedented interactions with biomolecules both on the surface and inside the cell (Cai et al. 2008). Nanoparticle-mediated gene delivery is widely used in the medical field for gene therapy, but its application in genetic transformation in microbes is less exploited (Nagamune 2017). Gold, iron oxide, zinc oxide and silica nanoparticles are the most widely used nanoparticles in molecular biology. However, gold nanoparticles are costly and bacterial exposure to nanoparticles such as zinc oxide increase the transformation efficiency only by two-to-threefold (Wang et al. 2018).
Silver nanoparticles are widely used in medical, pharmacy, food industry, cosmetics, etc., due to its antimicrobial property (Marin et al. 2015). Silver nanoparticles interact with microbial membrane, penetrate inside the cell and increase the porosity of the cell membrane (Dakal et al. 2016). There are no reports on the use of silver nanoparticles for gene transfer in microbes. As silver nanoparticles affect the membrane integrity of microbes they have the potential to be used in gene delivery system with increased transformation efficiency. Hence, in the present study, an attempt was made to evaluate the efficiency of silver nanoparticles in facilitating the uptake of extracellular plasmid DNA of three different sizes, viz. pUC18 (2.6 kb size), pBR322 (4.3 kb) and pCAMBIA (12.3 kb) by E. coli DH5α cells.
Materials and methods
All the chemicals used for the preparation of the culture medium were of analytical grade and procured from Sisco Research laboratories (SRL), India. The antibiotics were purchased from Himedia Laboratories, India. Silver nanoparticles of 100 nm particles size, citrate stabilized at a concentration 20 mg L−1 were purchased from Sigma-Aldrich Chemicals.
Toxicity assay of silver nanoparticles
Different concentrations of silver nanoparticles, viz. 0.01, 0.1, 1, 5, 10, 15 and 20 mg L−1 prepared by diluting with sterile deioninzed water, were used for evaluating the toxicity on E. coli DH5α cells. Toxicity assay was conducted following the protocol of Ivask et al. (2014). Effective concentration 50 (EC50) was calculated using the statistical package SPSS.
Induction of competency in E. coli DH5α
Escherichia coli DH5α cells at the exponential phase (OD600 0.1–0.2) were used for competency induction. The cells were pelleted by centrifuging at 5000 rpm for 5 min in Hermle Z326K microcentrifuge and resuspended in 200 µL of sterile deionized water. Silver nanoparticles at different concentrations below EC50 alone or in combination with 0.1 M calcium chloride and 0.1 M calcium chloride alone were used. Untreated bacterial cells served as control.
In the first set of experiment for induction of competency, 10 µL of different concentrations of silver nanoparticles (0.01, 0.1, 1, 2 and 4 mg L−1) was added to aliquots of 200 µL of bacterial suspension and incubated at different time periods, viz. 30 min, 60 min and 120 min. In the next set, 0.1 M calcium chloride was added along with the different concentrations of silver nanoparticles. Competency induction by 0.1 M calcium chloride was done by following the protocol of Green and Sambrook (2018).
Transformation of competent E. coli DH5α cells using pUC18, pBR322 and pCAMBIA vectors
The competent cells prepared by different treatments were transformed using pUC18 (2.6 kb size) plasmid vector. To the 200 µL of bacterial cells made competent using silver nanoparticles, 1 µL of 1 µg of pUC18 plasmid DNA was added, followed by 1 mL of Luaria Bertani (LB) broth and incubated at 37 °C at 120 rpm for 1 h. The competent cells prepared using combination of calcium chloride and silver nanoparticles and calcium chloride alone were transformed by standard heat shock method (Green and Sambrook 2018).
Efficiency of transformation was checked by culturing the transformed cells by blue white screening in LB medium containing ampicillin (60 mg L−1), X-Gal (30 mg L−1) and IPTG (24 mg L−1) following the standard protocol (Green and Sambrook 2018). Transformation efficiency of the treatments was compared by estimating the number of transformed cells per unit weight of the vector used.
For confirmation of transformation, plasmid was isolated from the white colonies grown in the selection medium, by alkaline lysis method (Birnboim and Doly 1979) and subjected to electrophoresis in 1% agarose gel.
Silver nanoparticle treatment which recorded highest transformation efficiency in first set was used for competency induction in E. coli DH5α cells and transformed with vector pBR322 (4.3 kb) and pCAMBIA (12.3 kb). Transformation efficiency was estimated by counting the number of transformed colonies in appropriate selective antibiotic media for pBR322 (60 mg L−1 ampicillin and 20 mg L−1 tetracycline) and pCAMBIA (50 mg L−1 kanamycin).
Results
Toxicity assay of silver nanoparticles on E. coli DH5α cells
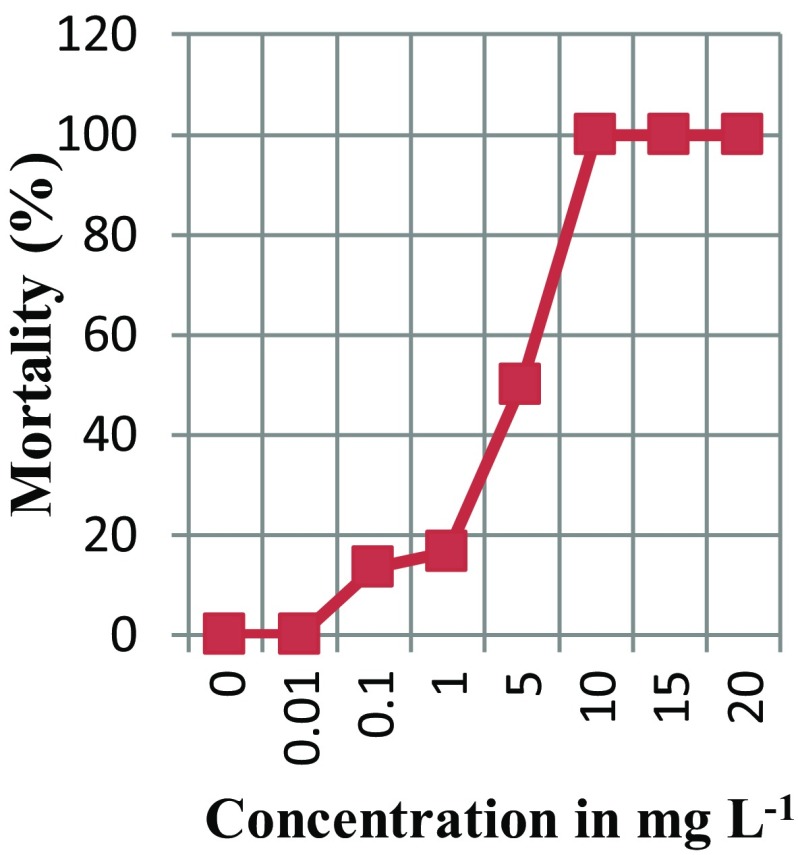
There was a considerable variation in the response of E. coli cells subjected to different concentrations of silver nanoparticles (Table 1). Maximum inhibition of growth (100% mortality) was recorded in E. coli cells treated with 10 mg L−1, 15 mg L−1 and 20 mg L−1 of silver nanoparticles. Minimum inhibition of growth (no mortality) was recorded in E. coli cells treated with 0.01 mg L−1 of silver nanoparticles. On plotting the graph of inhibition vs. concentration of silver nanoparticles (Fig. 1) and carrying out the probability analysis using the statistical package SPSS, EC50 was estimated to be 4.49 mg L−1.
Table 1.
Dose response of E. coli DH5α against silver nanoparticles
| Concentration of AgNPs (mg L−1) | Initial population (cfu mL−1) | Final population (cfu mL−1) | Mortality (%) |
|---|---|---|---|
| 0 | 3 × 107 | 3 × 107 | 0 |
| 0.01 | 3 × 107 | 3 × 107 | 0 |
| 0.1 | 3 × 107 | 2.6 × 107 | 13.4 |
| 1 | 3 × 107 | 2.5 × 107 | 16.6 |
| 5 | 3 × 107 | 1.5 × 107 | 50 |
| 10 | 3 × 107 | 0 | 100 |
| 15 | 3 × 107 | 0 | 100 |
| 20 | 3 × 107 | 0 | 100 |
Fig. 1.
Dose response curve of E. coli DH5α cells
Transformation efficiency of competent cells using pUC18, pBR322 and pCAMBIA vectors and induced by different treatments using silver nanoparticles
Statistical analysis using ANOVA showed significant difference in the transformation efficiency with pUC18 plasmid vector in the bacterial cells subjected to different treatments (Table 2). Silver nanoparticles showed an increase in transformation efficiency ranging from 2.6 × 103 to 8.3 × 104 cfu ng−1 of vector DNA compared to the conventional method using 0.1 M calcium chloride (2.3 × 103 cfu ng−1 of vector DNA). Maximum transformation efficiency (8.3 × 104 cfu ng−1 of vector DNA) was observed when cells were treated with 1 mg L−1 of silver nanoparticles for 60 min. Silver nanoparticles in combination with 0.1 M calcium chloride increased the transformation efficiency compared to 0.1 M calcium chloride alone. However, the transformation efficiency was less compared to silver nanoparticles alone. No colonies were formed in the selection medium from the cells not subjected to any competency induction treatments (control).
Table 2.
Transformation efficiency of competent cells induced by different treatments
| Treatment | Transformation efficiency (cfu ng−1 of DNA) |
|---|---|
| 0.01 mg L−1 silver nanoparticles (30 min) | 1.4 × 103 |
| 0.01 mg L−1 silver nanoparticles (60 min) | 4.5 × 103 |
| 0.01 mg L−1 silver nanoparticles (120 min) | 3.0 × 103 |
| 0.1 mg L−1 silver nanoparticles (30 min) | 1.5 × 104 |
| 0.1 mg L−1 silver nanoparticles (60 min) | 1.7 × 104 |
| 0.1 mg L−1 silver nanoparticles (120 min) | 1.3 × 104 |
| 1 mg L− 1 silver nanoparticles (30 min) | 6.03 × 104 |
| 1 mg L−1 silver nanoparticles (60 min) | 8.3 × 104 |
| 1 mg L−1 silver nanoparticles (120 min) | 4.5 × 104 |
| 2 mg L−1 silver nanoparticles (30 min) | 2 × 104 |
| 2 mg L−1 silver nanoparticles (60 min) | 2.5 × 104 |
| 2 mg L−1 silver nanoparticles (120 min) | 2 × 104 |
| 4 mg L−1 silver nanoparticles (30 min) | 1 × 104 |
| 4 mg L−1 silver nanoparticles (60 min) | 1.5 × 104 |
| 4 mg L−1 silver nanoparticles (120 min) | 1 × 104 |
| 0.01 mg L−1 silver nanoparticles + 0.1 M CaCl2 | 2.6 × 103 |
| 0.1 mg L−1 silver nanoparticles + 0.1 M CaCl2 | 1.65 × 104 |
| 1 mg L−1 silver nanoparticles + 0.1 M CaCl2 | 2.55 × 104 |
| 2 mg L−1 silver nanoparticle + 0.1 M CaCl2 | 8 × 103 |
| 4 mg L−1 silver nanoparticles + 0.1 M CaCl2 | 7.01 × 103 |
| 0.1 M CaCl2 | 2.3 × 103 |
| Control | 0 |
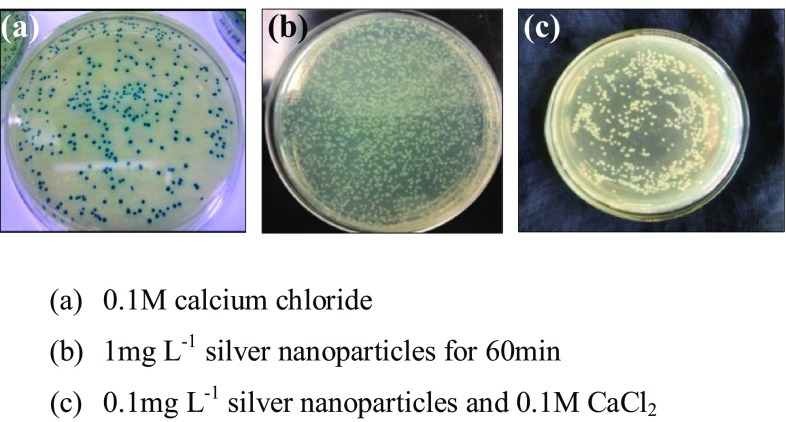
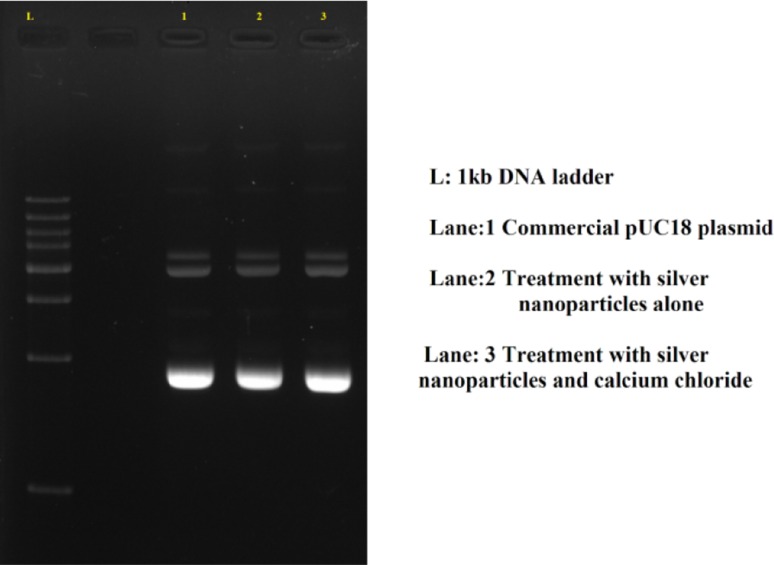
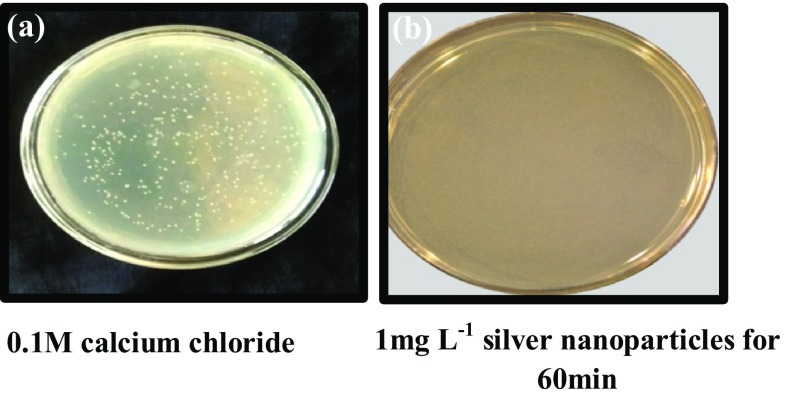
Treatment with silver nanoparticles for competency induction inhibited the lac Z gene expression of pUC18 plasmid vector. All the cells treated with silver nanoparticles showed only white colonies in selection media. The colonies from cells treated with calcium chloride alone, were blue in colour, whereas colonies from cells treated with silver nanoparticles and calcium chloride showed the presence of both white and blue colours (Fig. 2). Agarose gel electrophoresis of the isolated plasmid from white colonies grown in selection medium gave bands of the expected size of commercial pUC18 plasmid confirming transformation (Fig. 3).
Fig. 2.
E. coli DH5α cells treated for competency induction and transformed with pUC18 vector and grown on selection medium
Fig. 3.
Agarose gel profile of plasmid isolated from the white colonies grown in selection medium
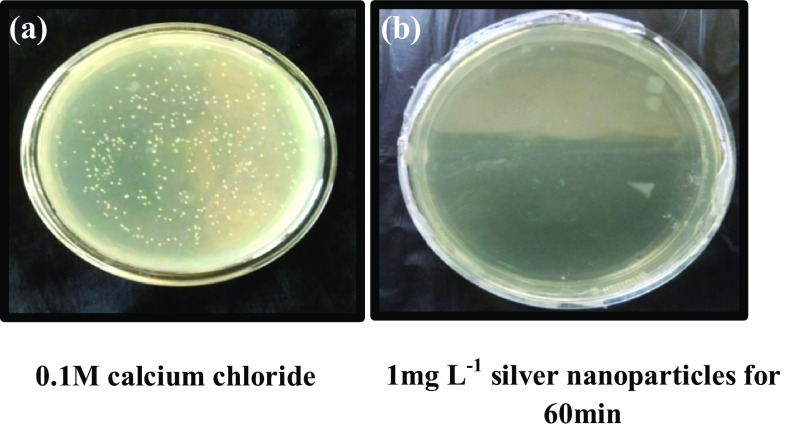
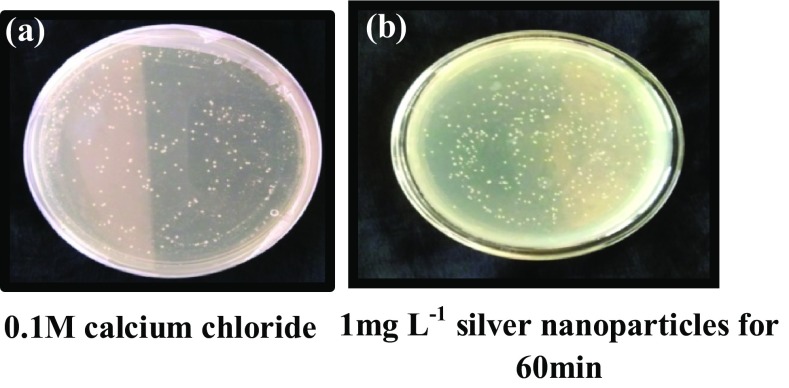
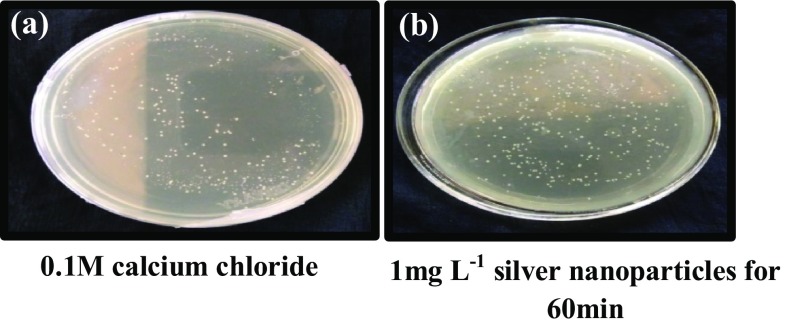
Escherichia coli cells treated with silver nanoparticles (1 mg L−1 for 60 min) and transformed with pBR322 vector failed to grow in selection medium containing both ampicillin and tetracycline (Fig. 4) as well as tetracycline alone (Fig. 5). However, the cells showed tenfold transformation efficiency (8 × 104 cfu ng−1 of DNA), in selection medium containing ampicillin alone compared to control (2.3 × 103 cfu ng−1 of DNA) (Fig. 6).
Fig. 4.
Competent E. coli DH5α cells transformed using pBR322 and selected on LB agar media containing ampicillin and tetracycline
Fig. 5.
Competent E. coli DH5α cells transformed using pBR322 and selected on LB agar media containing tetracycline alone
Fig. 6.
Competent E. coli DH5α cells transformed using pBR322 and selected on LB agar media containing ampicillin
Treatment with 1 mg L−1 silver nanoparticles for 60 min on bacterial cells and transformation pCAMBIA vector also showed increased transformation efficiency (7.9 × 104) in selection medium containing kanamycin compared to calcium chloride and heat shock method (2.3 × 103 cfu ng−1 of DNA) (Fig. 7).
Fig. 7.
Competent E. coli DH5α cells transformed using pCAMBIA vector and grown on LB agar media containing kanamycin
Discussion
Silver nanoparticles at different concentrations, viz. 0.01, 0.1, 1, 5, 10, 15 and 20 mg L−1 were used for testing their toxicity on E. coli DH5α cells in exponential phase. The linear increase in toxicity on E. coli cells on increasing the concentration of silver nanoparticles is in accordance with previous reports (Li et al. 2010; Rudakiya and Pawar 2017). Dziedzic et al. (2016) reported that the toxicity of silver nanoparticles was dose and time dependent.
Treatments including silver nanoparticles for competency induction and transformation using pUC18 vector exhibited increased transformation efficiency even without heat shock. Concentration of silver nanoparticles and time of exposure of cells to silver nanoparticles were found to affect the transformation efficiency. Disruption of the cell membrane by silver nanoparticles might have resulted in increased porosity of the bacterial cells, thereby facilitating increased uptake of extracellular DNA resulting in increased transformation efficiency. Silver nanoparticles at a concentration of 1 mg L−1 exhibited maximum transformation efficiency compared to lower (0.01 mg L−1 and 0.1 mg L−1) and higher concentrations (2 mg L−1 and 4 mg L−1) of silver nanoparticles. At lower concentrations of silver nanoparticles lesser interaction between the silver nanoparticles and the cell membrane might have resulted in reduced induction of porosity and lower transformation efficiency. Whereas low transformation efficiency at higher concentrations of silver nanoparticles might have resulted due to increased interaction between the silver nanoparticles with cell membrane resulting in increased disruption of the cell and increased release of silver ions causing cell damage (Vardanyan et al. 2015). In the present study, the optimum period for the interaction of 1 mg L−1 of silver nanoparticles with cell membrane and the induction of porosity for facilitating extracellular DNA uptake without cell disruption was recorded as 60 min.
Combination of calcium chloride and silver nanoparticles for competency induction and transformation with pUC18 vector exhibited lesser transformation efficiency compared to transformation efficiency exhibited by silver nanoparticles alone. This might have resulted due to greater damage caused in the cell membrane by the interaction of both silver nanoparticles and calcium chloride. Conventional method of competency induction in bacterial cells using calcium chloride is reported to have reduced transformation efficiency (Liu et al. 2014). In the present study also transformation efficiency of the cells treated with calcium chloride alone was found to be lowest among the different treatments tried. According to Ding et al. (2016), higher transformation efficiency was recorded while using nano alumina for transformation of non-competent E. coli (7.4 × 106) and Staphylococcus aureus (2.9 × 105) cells with pBR322. In the present study, tenfold increase in the transformation efficiency was recorded by treating the bacterial cells with 1 mg L−1 of silver nanoparticles for 60 min for competency induction compared to the conventional calcium chloride method.
Blue white selection of transformed cells was not possible when silver nanoparticles were used. The presence of only white colonies from the cells treated with silver nanoparticles and mixture of blue and white colonies in the cells treated with combination of silver nanoparticles and calcium chloride indicates inhibition of β-galactosidase activity in the pUC 18 plasmid vector by the silver nanoparticles. Inhibition of hydrolases, the enzyme class to which β-galactosidase belongs is also reported (Shin et al. 2012). Borase et al. (2015) reported the inhibition of urease (hydrolases) enzyme activity by silver nanoparticles. According to Peyrot et al. (2014), silver nanoparticles are capable of inhibiting soil exoenzymes such as phosphomonoesterase, arylsulfatase, β-D-glucosidase (hydrolases) and leucine-aminopeptidase. Shin et al. (2012) reported the inhibition of enzyme activity of urease, acid phosphatase, arylsulfatase and β-glucosidase in the soil on treatment with silver nanoparticles. Ahmed et al. (2016) have also reported the inhibition of enzyme activity of β-galactosidase by zinc oxide nanoparticles in methicillin-resistant Staphylococcus aureus.
Tetracycline resistance was absent in E. coli cells treated with silver nanoparticles and transformed with pBR322 vector. Tetracycline-resistant gene of pBR322 encodes a 41-kDa inner membrane protein (TetA) which acts as a tetracycline/+ Hantiporter (Allard and Bertrand 1992). The TetA (C) protein, encoded by the tetA (C) gene of plasmid pBR322, is a member of a family of membrane-bound proteins that mediate energy-dependent efflux of tetracycline from the bacterial cell (McNicholas et al. 1992). Stensberg et al. (2011) reported that uptake of silver nanoparticles can stimulate the production of reactive oxygen species (ROS), resulting in oxidative stress and genotoxic effects. Production of ROS results in disruption in the flux of ions and electrons across the mitochondrial membrane. In the present study, the disturbance in the bacterial cell membrane when treated with silver nanoparticles might have disrupted the efflux of ions in the transformed cells. This would have resulted in the failure of growth of the transformed cells in the selection medium containing tetracycline. McShan et al. (2015) reported that silver nanoparticles affect the growth of Salmonella cells in medium containing tetracycline. The tetracycline and silver nanoparticle complex interacts more strongly with the tetracycline-resistant Salmonella cells and causes more release of silver ions, thus creating a temporal high concentration of silver ions near the bacteria cell wall which leads to growth inhibition of the bacteria. Luo et al. (2015) reported highest photocatalysis efficiency for tetracycline degradation by silver nanoparticles due synergistic surface plasma resonance.
In the present study, although the cells treated with silver nanoparticles and transformed using pBR322 failed to grow in selection medium containing tetracycline, they could grow in the medium containing ampicillin. Penicillin and ampicillin belongs to the same group of antibiotics. Mcshan et al. (2015) have reported similar results in case of pencillin. According to them, penicillin did not affect the interaction of silver nanoparticles with the bacteria, and there is no synergistic effect.
Treatment with 1 mg L−1 silver nanoparticles for 60 min on bacterial cells and transformation with pCAMBIA vector also showed increased transformation efficiency (7.9 × 104) in selection medium containing kanamycin (50 mg L−1) compared to calcium chloride and heat shock method (2.3 × 103) and are in similar with results reported by Kumar et al. (2017) while using gold nanoparticles for competency induction and transformation by pCAMBIA 1304 into E. coli DH5α cells.
Conclusion
Escherichia coli DH5α cells treated with silver nanoparticles alone resulted in significant increase in transformation efficiency compared to the calcium chloride while using plasmid vectors of different sizes, viz. pUC18, pBR322 and pCAMBIA. It suggests that silver nanoparticles can aid in developing improved genetic transformation methods in microbes. The actual mechanism of inhibition of β-galactosidase in pUC18 and tetracycline resistance gene activity in pBR322 by silver nanoparticles in the present study needs to be investigated further in future.
Acknowledgements
Research facilities provided by Kerala Agricultural University is gratefully acknowledged.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ahmed KBA, Raman T, Veerappan V. Future prospects of antibacterial metal nanoparticles as enzyme inhibitor. Mater Sci Eng. 2016;68(1):939–947. doi: 10.1016/j.msec.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Allard JD, Bertrand KP. Membrane topology of the pBR322 tetracycline resistance protein. J Biol Chem. 1992;267(25):17809–17819. [PubMed] [Google Scholar]
- Asif A, Mohsin H, Tanvir R, Rehman Y. Revisiting the mechanisms involved in calcium chloride induced bacterial transformation. Front Microbiol. 2017;8(2169):1–5. doi: 10.3389/fmicb.2017.02169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borase HP, Salunkhe RB, Patil CD, Suryawanshi RK, Salunke BK, Wagh ND, Patil SV. Innovative approach for urease inhibition by Ficus carica extract-fabricated silver nanoparticles: an in vitro study. Biotechnol Appl Biochem. 2015;62(6):780–784. doi: 10.1002/bab.1341. [DOI] [PubMed] [Google Scholar]
- Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;7(1831):1–17. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Pan J, Jin M, Yang D, Shen Z, Wang J, Zhang B, Liu W, Fu J, Guo X, Wang D, Chen Z, Yin Z, Qiu Z, Li J. Enhanced uptake of antibiotic resistance genes in the presence of nanoalumina. Nanotoxicology. 2016;10(8):1051–1060. doi: 10.3109/17435390.2016.1161856. [DOI] [PubMed] [Google Scholar]
- Dziedzic A, Kubina R, Bułdak R, Skonieczna M, Cholewa K. Silver nanoparticles exhibit the dose-dependent anti-proliferative effect against human squamous carcinoma cells attenuated in the presence of berberine. Molecules. 2016;21(3):365. doi: 10.3390/molecules21030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Sambrook J. The Hanahan method for preparation and transformation of competent Escherichia coli: high-efficiency transformation. Coldspring Harb Protoc. 2018;18:3. doi: 10.1101/pdb.prot101188. [DOI] [PubMed] [Google Scholar]
- Ivask A, Kurvet I, Kasemets K, Blinova I, Aruoja V, Suppi S, Vija H, Kakinen A, Titma T, Heinlaan M, Visnapuu M, Koller D, Kisand V, Kahru A. Size dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One. 2014;9(7):102–108. doi: 10.1371/journal.pone.0102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotnik T, Frey W, Sack M, Meglic SH, Peterka M, Miklavcic D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015;33(8):480–488. doi: 10.1016/j.tibtech.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Kumar M, Pandey S, Mishra A, Nautiyal CS. Finding a facile way for the bacterial DNA transformation by biosynthesized gold nanoparticles. FEMS. Microbiol Lett. 2017;364(12):1–5. doi: 10.1093/femsle/fnx115. [DOI] [PubMed] [Google Scholar]
- Li WR, Xie XB, Shi QS, Zeng HY, OU-Yang YS, ChenYB Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- Liu XF, Liu L, Wang YG, Wang X, Ma Y, Li Y. The Study on the factors affecting transformation efficiency of E. coli competent cells. Pak J Pharm Sci. 2014;27(3):679–684. [PubMed] [Google Scholar]
- Luo B, Xu D, Li D, Wu G, Wu M, Shi W, Chen M. Fabrication of a Ag/Bi3TaO7 plasmonic photocatalyst with enhanced photocatalytic activity for degradation of tetracycline. ACS Appl Mater Interfaces. 2015;7(31):17061–17069. doi: 10.1021/acsami.5b03535. [DOI] [PubMed] [Google Scholar]
- Marin S, Vlasceanu GM, Tiplea RE, Bucur IR, Lemnaru M, Marin MM, Grumezescu AM. Applications and toxicity of silver nanoparticles: a recent review. Curr Top Med Chem. 2015;15(16):1596–1604. doi: 10.2174/1568026615666150414142209. [DOI] [PubMed] [Google Scholar]
- McNicholas P, Chopra I, Rothstein DM. Genetic analysis of the teta(C) gene on plasmid pBR322. J Bacteriol. 1992;174(24):7926–7933. doi: 10.1128/jb.174.24.7926-7933.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShan D, Zhang Y, Deng H, Ray PC, Yu H. Synergistic antibacterial effect of silver nanoparticles combined with ineffective antibiotics on drug resistant Salmonella typhimurium DT104. J Environ Sci Health C. 2015;33:369–384. doi: 10.1080/10590501.2015.1055165. [DOI] [PubMed] [Google Scholar]
- Nagamune T. Biomolecular engineering for nanobio/bionanotechnology. Nano Converg. 2017;4(9):1–56. doi: 10.1186/s40580-017-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja S, Saha S, Jana B, Basu T. Role of membrane potential on artificial transformation of E. coli with plasmid DNA. J Biotechnol. 2006;127:14–20. doi: 10.1016/j.jbiotec.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Peyrot C, Wilkinson KJ, Desrosiers M, Sauve S. Effects of silver nanoparticles on soil enzyme activities with and without added organic matter. Environ Toxicol Chem. 2014;33(1):115–125. doi: 10.1002/etc.2398. [DOI] [PubMed] [Google Scholar]
- Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents. 2000;13:155–168. doi: 10.1016/S0924-8579(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Raghava S, Giorda KM, Romano FB, Heuck AP, Hebert DN. The SV40 late protein VP4 is a viroporin that forms pores to disrupt membranes for viral release. PLoS Pathog. 2011;7(6):e1002116. doi: 10.1371/journal.ppat.1002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudakiya DM, Pawar K. Bactericidal potential of silver nanoparticles synthesized using cell-free extract of Comamonas acidovaorans: in vitro and in silico approaches. 3 Biotech. 2017;7:92. doi: 10.1007/s13205-017-0728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YJ, Kwak JI, An YJ. Evidence for the inhibitory effects of silver nanoparticles on the activities of soil exoenzymes. Chemosphere. 2012;88:524–529. doi: 10.1016/j.chemosphere.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Stensberg MC, Wei Q, McLamore ES, Porterfield MD, Wei A, Sepulveda MS. Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine. 2011;6(5):879–898. doi: 10.2217/nnm.11.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardanyan Z, Gevorkyan V, Ananyan M, Vardapetyan H, Trchounian A. Effects of various heavy metal nanoparticles on Enterococcus hirae and Escherichia coli growth and proton-coupled membrane transport. J Nanobiotechnol. 2015;13(1):69. doi: 10.1186/s12951-015-0131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang F, Zhao Z, Xu Y, Mao D, Zhua X, Luo Y, Alvarez PJJ. Bacterial exposure to ZnO nanoparticles facilitates horizontal transfer of antibiotic resistance genes. Nano Impact. 2018;10:61–67. [Google Scholar]
- Yoshida N, Sato M. Plasmid uptake by bacteria: a comparison of methods and efficiencies. Appl Microbiol Biotechnol. 2009;83:791–798. doi: 10.1007/s00253-009-2042-4. [DOI] [PubMed] [Google Scholar]