Abstract
For glycemic control in patients with diabetes on peritoneal dialysis (PD), the level of glycated albumin (GA) associated with mortality is unclear. Accordingly, we examined the difference in the association of GA and glycated hemoglobin (HbA1c) with 2-year mortality in a Japanese Society for Dialysis Therapy cohort. We examined 1601 patients with prevalent diabetes who were on PD. Of these, 1282 had HbA1c (HbA1c cohort) and 725 had GA (GA cohort) measured. We followed them for 2 years from 2013 to 2015 and used Cox regression to calculate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for 2-year mortality after adjusting for potential confounders in each cohort. No significant association was found between HbA1c levels and all-cause death HRs before and after adjustment for confounders in the HbA1c cohort. In contrast, the adjusted all-cause death HRs and 95% CIs for GAs < 12.0%, 12.0–13.9%, 16.0–17.9%, 18.0–19.9%, 20.0–21.9%, and ≥22.0%, compared with 14.0–15.9% (reference), were 1.56 (0.32–7.45), 1.24 (0.32–4.83), 1.32 (0.36–4.77), 2.02 (0.54–7.53), 4.36 (1.10–17.0), and 4.10 (1.20–14.0), respectively. In the GA cohort, GA ≥ 20.0% was significantly associated with a higher death HR compared with the reference GA. Thus, GA ≥ 20.0% appears to be associated with a decrease in survival in diabetic patients on PD. There were no associations between HbA1c levels and 2-year mortality in PD patients.
Introduction
Erythrocytes have a shorter life span than normal in dialysis patients, and blood loss and bleeding may also occur during hemodialysis (HD). This can lead to anemia requiring treatment with erythropoiesis-stimulating agents (ESAs), which falsely lowers the glycated hemoglobin (HbA1c) level by raising the proportion of young erythrocytes. As a result, HbA1c levels tend to be low in dialysis patients and glycemic control may be overestimated. In contrast, glycated albumin (GA) level is not significantly associated with erythrocyte life span, hemoglobin level, or ESA dose in patients with diabetes undergoing HD1–4. Thus, GA could be a more robust indicator of glycemic control than HbA1c in patients with diabetes on HD. Even so, the 2012 Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guideline for Diabetes does not recommend GA as a first-line tool for assessing glycemic control5. In patients with no history of cardiovascular events, survival was reported to be significantly longer in those with GA < 20.0% than that in those with GA 20.0–24.5 or >24.5%6. Based on these results, the practice guidelines (2012) published by the Japanese Society for Dialysis Therapy (JSDT) suggests GA levels < 20.0% as a potential target level for glycemic control in patients with diabetes on HD without a history of cardiovascular disease (CVD) and <24.0% for those with such a history7.
The issue of glycemic control may be particularly important in PD patients. However, few studies have examined the association between HbA1c and clinical outcomes in PD patients8,9, and, to our knowledge, no studies have investigated GA levels and mortality in PD patients. Accordingly, to establish the association between glycemic control and mortality—focusing on the ability to predict mortality with GA—we studied a nationwide registry of PD patients in Japan.
Methods
Patients
The data were used in this study were from annual nationwide surveys of patients on dialysis conducted by JSDT and stored in the JSDT Renal Data Registry (JRDR). As described previously, all surveys were conducted by volunteers10,11. The standard analysis file prepared by the JRDR Committee for the present study included data for 314,438 patients who underwent dialysis at 4,268 facilities in the 2013 survey12, 320,448 patients dialyzed at 4,330 facilities in 2014, and 324,986 patients dialyzed at 4,321 centers in 201513. Those diagnosed with diabetes and/or receiving diabetes medications and undergoing PD on 31 December 2013 were followed for 2 years. We excluded patients who had undergone HD, those aged <20 years, those whose records for date of birth, initiation of dialysis, or outcome were incomplete, and those who were considered outliers. In total, 315,631 patients on maintenance dialysis were registered at the end of 2013. After exclusions, 1,601 patients on PD were included in this study (Fig. 1). The HbA1c cohort comprised patients whose HbA1c levels were measured and included 1,282 patients after exclusions. The GA cohort comprised patients in whom glycated albumin (GA) levels were measured and included 725 patients after exclusions. HbA1c and GA levels were both measured in 413 patients, and these patients were included in both cohorts.
Figure 1.
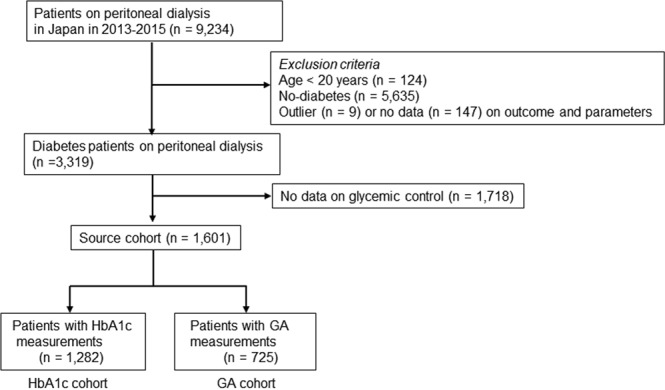
Flowchart of study participants.
Clinical and demographic measures
The following demographic and clinical data were collected: age, sex, duration of dialysis, primary cause of end-stage kidney disease (ESKD), body mass index (BMI), smoking status, use of antihypertensive agents, and history of complications of cardiovascular disease (CVD)—including cerebral infarction, cerebral hemorrhage, myocardial infarction, and limb amputation. Blood samples were collected and assayed at each dialysis facility or hospital, typically within 24 h of collection; and the latest values at the time of the survey were collated including serum albumin, hemoglobin, calcium, phosphate, intact parathyroid hormone (i-PTH), total cholesterol, high-density lipoprotein (HDL) cholesterol, and C-reactive protein (CRP). Using residual renal function (renal Kt/V) and peritoneal dialysis (PD Kt/V), weekly Kt/V urea values were recorded. The sum of renal Kt/V and PD Kt/V was considered as total Kt/V. The dialysate to plasma creatinine ratio (D/P Cr) was obtained using the peritoneal equilibration test. The type of PD fluid used (typically 2.5% glucose or icodextrin dialysate) and whether or not an automated PD system was used were also recorded. Furthermore, the type of treatment was recorded because PD + HD combination therapy, that is, HD treatment 1 day a week and PD on other days, has been approved in Japan since 2010.
We defined three categories of antidiabetic therapy: no medication, insulin therapy, and oral antidiabetic medication only. The no medication group comprised patients managed with diet modification therapy only or those for whom no antidiabetic medication was prescribed. Insulin therapy included insulin injection therapy only or a combination of insulin with an oral antidiabetic agent. Cause of death was classified as cardiovascular death, infection-related death, or other. We defined cardiovascular death as that due to chronic heart failure, pulmonary edema, ischemic heart disease (including acute myocardial infarction, i.e., death within 30 days of onset), arrhythmia, valvular heart disease, endocarditis, other cardiac disease, subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infarction, or other brain disease.
Statistical analysis
The data were expressed as proportions, using the mean ± standard deviation or median (interquartile range) as appropriate. We analyzed categorical variables using the chi-square test and continuous variables using the Student’s t-test. Categorical data were compared between groups with repeated-measures analysis of variance (ANOVA) and Tukey’s honestly significant difference test or Kruskal-Wallis test, as appropriate. Patients who were switched to HD during follow-up were censored from the outcome analysis. We defined reference ranges for the laboratory data such that patients with measured values outside of the following ranges were considered outliers and were excluded from the analysis: height 120–200 cm, body weight 20–150 kg, serum albumin 1.0–5.0 g/dL, CRP < 30 mg/dL, hemoglobin 5.0–20.0 g/dL, i-PTH < 3000 pg/mL, HbA1c 4.0–15.0%, and GA 10.0–50.0%. the conventional method for multivariate regression was used to imputed missing covariate data as appropriate.
We examined the clinical and demographic measures in each cohort by dividing the patients into 7 a priori categories defined by HbA1c values between <5.0% and ≥7.5% at increments of 0.5% (<5.0%, 5.0–5.4%, 5.5–5.9%, 6.0–6.4%, 6.5–6.9%, 7.0–7.4%, and ≥7.5%) and seven a priori categories defined by GA values of <12.0% and ≥22% at increments of 2% (<12.0%, 12.0–13.9%, 14.0–15.9%, 16.0–17.9%, 18.0–19.9%, 20.0–21.9%, and ≥22.0%). We used the 5.5–5.9% HbA1c category as the reference based on previous studies14. We used the 14.0–15.9% GA category as the reference because the standard value of GA was between 12.0 and 16.0%15,16. Survival analyses with Cox proportional hazards regression were used to examine whether basic factors at baseline—including age, sex, PD duration, cardiovascular comorbidity, PD-related factors, and nutritional and inflammatory factors—predicted survival for up to 2 years of follow-up. To examine the relationship between dialysis duration and risk of death, we grouped the patients into six a priori categories by duration of dialysis (<2, 2 to <4, 4 to <6, 6 to <8, 8 to <10, and ≥10 years). Additional analyses were performed with adjustment for PD-related factors, including residual renal function (i.e., anuric or non-anuric status), total Kt/V, use of 2.5% glucose or icodextrin dialysate, and type of treatment (i.e., user or non-user of an automated PD device, and PD alone or combination therapy with HD). Further analyses were performed with adjustments for nutrition-related and inflammation-related factors, including BMI and levels of hemoglobin, albumin, total cholesterol, HDL-cholesterol, calcium, phosphate, i-PTH, and CRP. Age, hemoglobin, and CRP levels were analyzed as continuous variables. We performed unadjusted and adjusted analyses that included all variables for all-cause mortality.
The study was conducted according to the principles of the Declaration of Helsinki, Japanese privacy protection laws, and Ethical Guidelines for Medical and Health Research Involving Human Subjects published by the Ministry of Education, Science and Culture, and the Ministry of Health, Labour and Welfare in 2015. The study protocol was approved by the Medicine Ethics Committee of the Japanese Society for Dialysis Therapy. The study was registered with the University Hospital Medical Information Network (UMIN000018641). The need for informed consent was waived because the study used de-identified information. All analysis was performed using JMP® version 13.0 (SAS Institute, Cary, NC). P-values less than 0.05 were considered statistically significant.
Results
Patients demographic and clinical characteristics
Table 1 shows baseline demographic, clinical, and laboratory characteristics of diabetes patients with and without data on glycemic control (n = 1601 and 1718, respectively). Individuals with missing data were more likely to have diet therapy alone and combination therapy with HD, less likely to have antihypertensive agents, and have lower BMI. The differences in many of the other characteristics were not statistically significant (Table 1). The group of 1601 patients had the following characteristics: mean age 63.9 ± 11.6 years; 71.0% male; dialysis duration 27 (13–50) months; CVD history 24.7%; BMI 24.5 ± 3.9; serum albumin 3.2 ± 0.5g/dL; and hemoglobin 10.8 ± 1.3 g/dL. Baseline renal Kt/V, PD Kt/V, and total Kt/V were 0.4 (0–0.9), 1.3 ± 0.6, and 1.8 ± 0.8, respectively. Combination therapy with PD and HD was undertaken in 20% of the patients. An automated PD system was used in 42.9% of patients. During the 2-year study period, 173 patients (10.8%) died, 20 (1.2%) underwent kidney transplantation, and 499 (31.2%) were switched to HD; 909 (56.8%) patients were alive at the end of the study period.
Table 1.
Demographic, clinical, and laboratory characteristics in patients with diabetes on PD with glycemic indices (n = 1601) and those without glycemic indices (n = 1718).
| Variables | PD patients with HbA1c or GA measurement | PD patients without HbA1c or GA measurement | P value |
|---|---|---|---|
| n (male%) | 1601 (71.0) | 1718 (70.4) | 0.483 |
| Age, years | 63.9 ± 11.6 | 64.0 ± 12.1 | 0.635 |
| PD duration, m | 27 [13–50] | 25 [12–47] | 0.163 |
| Type 1 diabetes, % | 8.9 | 7.7 | 0.172 |
| CVD comorbidity, % | 24.7 | 29.6 | 0.098 |
| Smoking, % | 11.2 | 9.5 | 0.214 |
| Insulin, % | 35.4 | 32.4 | 0.196 |
| Oral antidiabetic agents, % | 42.2 | 38.6 | 0.146 |
| Diet therapy alone, % | 34.5 | 42.2 | 0.001 |
| Antihypertensive agents, % | 83.5 | 79.9 | 0.023 |
| HD combination, % | 20.0 | 17.0 | 0.022 |
| Anuric % | 18.8 | 17.9 | 0.646 |
| Total Kt/V | 1.8 ± 0.8 | 1.8 ± 0.8 | 0.914 |
| 2.5% glucose dialysate use, % | 41.6 | 37.0 | 0.043 |
| Icodextrin user, % | 47.2 | 42.6 | 0.052 |
| D/P Cr | 0.67 ± 0.13 | 0.66 ± 0.12 | 0.204 |
| APD user, % | 42.9 | 43.8 | 0.689 |
| Body mass index, kg/m2 | 24.5 ± 3.9 | 24.1 ± 3.8 | 0.049 |
| Hemoglobin, g/dL | 10.8 ± 1.3 | 10.7 ± 1.4 | 0.251 |
| Serum albumin, g/dL | 3.2 ± 0.5 | 3.2 ± 0.6 | 0.349 |
| Total cholesterol, mg/dL | 172 ± 40 | 176 ± 40 | 0.062 |
| HDL-cholesterol, mg/dL | 46 ± 16 | 46 ± 17 | 0.752 |
| C-reactive protein, mg/dL | 0.16 [0.08–0.46] | 0.20 [0.2–0.55} | 0.106 |
| Calcium, mg/dL | 8.6 ± 0.8 | 8.6 ± 0.8 | 0.997 |
| Phosphate, mg/dL | 5.2 ± 1.3 | 5.2 ± 1.4 | 0.962 |
| Intact-PTH, pg/mL | 152 [76–249] | 142 [80–247] | 0.367 |
APD, automated peritoneal dialysis; CVD, cardiovascular disease; GA, glycated albumin; HD, hemodialysis; HDL, high-density lipoprotein; PD, peritoneal dialysis; PTH, parathyroid hormone.
HbA1c cohort
Table 2 shows hazard ratios (HRs) in the unadjusted ANOVA evaluated as potential predictors of mortality in the HbA1c cohort. There was no significant effect of sex on patient survival (P = 0.185). Older age, longer duration of dialysis, and comorbid CVD were significant predictors of mortality. Lower dialysis dose, assessed by using total Kt/V, was found to be associated with higher risk of mortality. The use of 2.5% glucose dialysate and an anuric state at baseline were significant predictors of mortality. Poor nutritional status and a more severe inflammatory status, indicated by lower hemoglobin, higher CRP, lower serum albumin, and lower BMI, were also associated with higher mortality in patients on PD.
Table 2.
Hazard ratios (with 95% CIs) for variables evaluated as potential predictors of all-cause mortality in the HbA1c cohort.
| Variables | n (%) | HR | 95% CI | P value |
|---|---|---|---|---|
| Male | 919 (71.7) | 1.00 | Reference | Reference |
| Female | 363 (28.3) | 1.27 | 0.89–1.80 | 0.185 |
| Age | ||||
| 1 year increase | 1282 (100) | 1.05 | 1.03–1.06 | <0.0001 |
| Duration of PD (year) | ||||
| <2 | 588 (45.9) | 1.00 | Reference | Reference |
| 2 ≤ < 4 | 365 (28.4) | 1.93 | 1.28–2.91 | 0.002 |
| 4 ≤ < 6 | 196 (15.3) | 2.89 | 1.81–4.61 | <0.0001 |
| 6 ≤ < 8 | 84 (6.6) | 2.64 | 1.32–5.31 | 0.006 |
| 8≤ | 49 (3.8) | 2.19 | 0.94–5.08 | 0.067 |
| Type of diabetes | ||||
| Type 2 | 1184 (92.4) | 1.00 | Reference | Reference |
| Type 1 | 98 (7.6) | 0.78 | 0.40–1.52 | 0.472 |
| CVD comorbidity | ||||
| No | 967 (75.5) | 1.00 | Reference | Reference |
| Yes | 313 (24.5) | 2.65 | 1.86–3.77 | <0.0001 |
| Smoking | ||||
| No | 1023 (89.0) | 1.00 | Reference | Reference |
| Yes | 126 (11.0) | 1.06 | 0.59–1.82 | 0.823 |
| Antihypertensive agent | ||||
| User | 994 (82.9) | 1.00 | Reference | Reference |
| Non-user | 205 (17.1) | 2.27 | 1.51–3.41 | <0.0001 |
| HD combination | ||||
| No | 1028 (80.2) | 1.00 | Reference | Reference |
| Yes | 254 (19.8) | 0.89 | 0.58–1.39 | 0.615 |
| Residual renal function | ||||
| Non-anuric | 762 (81.8) | 1.00 | Reference | Reference |
| Anuric | 169 (18.2) | 1.64 | 1.05–2.50 | 0.030 |
| Type of dialysate | ||||
| 2.5% non-user | 661 (59.7) | 1.00 | Reference | Reference |
| 2.5% user | 446 (40.3) | 1.53 | 1.06–2.28 | 0.025 |
| Type of dialysate | ||||
| Icodextrin non-user | 615 (55.5) | 1.00 | Reference | Reference |
| Icodextrin user | 492 (44.4) | 1.01 | 0.69–1.45 | 0.988 |
| Type of treatment | ||||
| APD non-user | 641 (58.0) | 1.00 | Reference | Reference |
| APD user | 464 (42.0) | 0.71 | 0.49–1.01 | 0.053 |
| Total Kt/V | ||||
| <1.1 | 45 (7.0) | 2.73 | 1.02–7.29 | 0.043 |
| 1.1 ≤ < 1.4 | 78 (12.2) | 1.62 | 0.65–4.06 | 0.297 |
| 1.4 ≤ < 1.7 | 128 (20.0) | 1.00 | Reference | Reference |
| 1.7 ≤ < 2.0 | 170 (26.5) | 0.91 | 0.41–2.05 | 0.825 |
| 2.0≤ | 220 (34.3) | 0.71 | 0.32–1.56 | 0.395 |
| Body mass index (kg/m2) | ||||
| <20.0 | 102 (9.3) | 2.21 | 1.13–4.33 | 0.020 |
| 20 ≤ < 22 | 186 (16.9) | 1.92 | 1.07–3.44 | 0.028 |
| 22 ≤ < 24 | 254 (23.1) | 1.00 | Reference | Reference |
| 24 ≤ < 26 | 202 (18.4) | 1.20 | 0.64–2.23 | 0.567 |
| 26 ≤ < 28 | 164 (14.9) | 1.43 | 0.74–2.76 | 0.278 |
| 28≤ | 191 (17.4) | 1.07 | 0.55–2.08 | 0.837 |
| Serum albumin (g/dL) | ||||
| <2.5 | 87 (7.1) | 5.41 | 2.99–9.78 | <0.0001 |
| 2.5 ≤ < 3.0 | 258 (20.9) | 2.25 | 1.45–3.47 | 0.0003 |
| 3.0 ≤ < 3.5 | 461 (37.4) | 1.00 | Reference | Reference |
| 3.5 ≤ < 4.0 | 336 (27.3) | 0.45 | 0.25–0.76 | 0.004 |
| 4.0≤ | 90 (7.3) | 0.45 | 0.18–1.19 | 0.112 |
| Hemoglobin | ||||
| 1 g/dL increase | 1253 (97.7) | 0.81 | 0.71–0.94 | 0.005 |
| C-reactive protein | ||||
| 1 mg/dL increase | 1126 (87.8) | 1.46 | 1.26–1.70 | <0.0001 |
| Total cholesterol (mg/dL) | ||||
| <130 | 139 (13.4) | 1.01 | 0.55–1.87 | 0.961 |
| 130 ≤ < 150 | 176 (17.0) | 0.75 | 0.41–1.36 | 0.347 |
| 150 ≤ < 170 | 221 (21.3) | 1.00 | Reference | Reference |
| 170 ≤ < 190 | 196 (18.9) | 0.59 | 0.33–1.06 | 0.079 |
| 190 ≤ < 210 | 134 (12.9) | 0.73 | 0.38–1.36 | 0.318 |
| 210≤ | 171 (16.5) | 0.48 | 0.25–0.91 | 0.025 |
| HDL-cholesterol (mg/dL) | ||||
| <30 | 123 (12.1) | 1.44 | 0.80–2.59 | 0.219 |
| 30 ≤ < 40 | 262 (25.8) | 0.87 | 0.52–1.45 | 0.601 |
| 40 ≤ < 50 | 267 (26.3) | 1.00 | Reference | Reference |
| 50 ≤ < 60 | 169 (16.7) | 0.62 | 0.33–1.17 | 0.145 |
| 60 ≤ < 70 | 94 (9.3) | 0.67 | 0.31–1.43 | 0.307 |
| 70≤ | 99 (9.8) | 0.48 | 0.21–1.08 | 0.079 |
| Calcium (mg/dL) | ||||
| <8.0 | 261 (20.7) | 1.47 | 0.92–2.35 | 0.104 |
| 8.0 ≤ < 8.5 | 271 (21.5) | 1.13 | 0.69–1.85 | 0.606 |
| 8.5 ≤ < 9.0 | 332 (26.4) | 1.00 | Reference | Reference |
| 9.0 ≤ < 9.5 | 227 (18.1) | 0.65 | 0.37–1.15 | 0.142 |
| 9.5 ≤ < 10.0 | 118 (9.4) | 0.76 | 0.35–1.67 | 0.502 |
| 10≤ | 49 (3.9) | 1.92 | 0.88–4.19 | 0.100 |
| Phosphate (mg/dL) | ||||
| <4.0 | 198 (15.5) | 1.41 | 0.85–2.31 | 0.178 |
| 4.0 ≤ < 5.0 | 359 (28.1) | 1.02 | 0.65–1.61 | 0.914 |
| 5.0 ≤ < 6.0 | 375 (29.4) | 1.00 | Reference | Reference |
| 6.0 ≤ < 7.0 | 225 (17.6) | 1.02 | 0.61–1.73 | 0.924 |
| 7.0 ≤ < 8.0 | 83 (6.5) | 1.35 | 0.66–2.78 | 0.404 |
| 8.0≤ | 37 (2.9) | 0.22 | 0.02–1.68 | 0.145 |
| Intact-PTH (pg/mL) | ||||
| <60 | 199 (17.7) | 1.40 | 0.82–2.38 | 0.215 |
| 60 ≤ < 140 | 306 (27.2) | 0.87 | 0.51–1.46 | 0.601 |
| 140 ≤ < 220 | 269 (23.9) | 1.00 | Reference | Reference |
| 220 ≤ < 300 | 147 (13.1) | 1.25 | 0.68–2.31 | 0.457 |
| 300 ≤ < 380 | 83 (7.4) | 0.64 | 0.26–1.52 | 0.311 |
| 380≤ | 120 (10.7) | 0.86 | 0.43–1.74 | 0.688 |
CVD, cardiovascular disease; HD, hemodialysis; HDL, high-density lipoprotein; PD, peritoneal dialysis; PTH, parathyroid hormone.
Table 3 shows baseline data for demographics and clinical and laboratory characteristics of PD patients, based on the seven a priori categories according to baseline HbA1c. Higher HbA1c levels were noted to be associated with higher rates of type 1 diabetes and insulin use, 2.5% glucose dialysate, a lower proportion of males, and higher hemoglobin and CRP levels. The rate of transfer from PD to HD was not associated with the a priori HbA1c categories.
Table 3.
Demographic, clinical, and laboratory values in 1282 PD patients, according to categories of HbA1c.
| Variables | All | HbA1c Categories | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <5.0 | 5.0 to 5.4 | 5.5 to 5.9 | 6.0 to 6.4 | 6.5 to 6.9 | 7.0 to 7.4 | ≥7.5 | |||
| n (male%) | 1282 (71.7) | 86 (65.1) | 217 (80.1) | 239 (79.5) | 275 (71.3) | 195 (64.6) | 97 (65.0) | 119 (59.7) | <0.0001 |
| Age (years) | 63.9 ± 11.6 | 61.8 ± 11.7 | 63.3 ± 11.4 | 63.5 ± 11.2 | 64.8 ± 11.7 | 65.2 ± 11.7 | 64.5 ± 11.7 | 63.2 ± 12.1 | 0.198 |
| PD duration (m) | 26 [12–49] | 30 [16–57] | 24 [10–49] | 24 [12–49] | 26 [11–49] | 27 [13–46] | 25 [12–48] | 26 [14–47] | 0.274 |
| Type 1 (%) | 7.6 | 5.8 | 5.9 | 5.9 | 6.6 | 8.2 | 13.4 | 13.5 | 0.037 |
| CVD comorbidity (%) | 24.4 | 23.3 | 20.7 | 22.7 | 27.3 | 24.6 | 23.7 | 30.2 | 0.437 |
| Smoking (%) | 10.9 | 8.9 | 12.7 | 9.2 | 12.7 | 13.5 | 3.2 | 14.4 | 0.259 |
| Insulin (%) | 35.4 | 11.8 | 21.1 | 25.2 | 35.3 | 50.0 | 52.8 | 62.8 | <0.0001 |
| Oral antidiabetic agents (%) | 41.3 | 29.2 | 33.1 | 40.7 | 43.9 | 43.7 | 53.3 | 52.7 | 0.0006 |
| Diet therapy alone (%) | 35.5 | 62.3 | 51.6 | 41.9 | 32.1 | 19.7 | 13.9 | 17.5 | <0.0001 |
| Antihypertensive agents (%) | 82.9 | 81.0 | 87.0 | 86.0 | 84.0 | 80.0 | 81.0 | 71.2 | 0.012 |
| HD combination (%) | 19.8 | 26.7 | 21.0 | 16.3 | 17.5 | 16.4 | 22.6 | 27.7 | 0.070 |
| Anuric (%) | 18.2 | 19.4 | 20.6 | 15.8 | 17.2 | 14.4 | 18.8 | 26.0 | 0.396 |
| Total Kt/V | 1.9 ± 0.9 | 2.1 ± 1.2 | 1.7 ± 0.7 | 1.9 ± 0.9 | 1.8 ± 0.6 | 2.0 ± 1.0 | 1.9 ± 0.6 | 2.0 ± 1.1 | 0.129 |
| 2.5% glucose dialysate use (%) | 40.3 | 30.0 | 36.6 | 39.5 | 38.4 | 42.2 | 49.1 | 51.0 | 0.049 |
| Icodextrin dialysate use (%) | 55.6 | 44.3 | 45.1 | 40.0 | 47.9 | 42.8 | 45.5 | 47.9 | 0.722 |
| D/P Cr | 0.67 ± 0.13 | 0.68 ± 0.13 | 0.67 ± 0.15 | 0.69 ± 0.13 | 0.68 ± 0.12 | 0.66 ± 0.12 | 0.67 ± 0.13 | 0.62 ± 0.12 | 0.043 |
| APD user (%) | 41.9 | 46.4 | 42.7 | 42.8 | 41.0 | 42.2 | 45.2 | 34.7 | 0.776 |
| Body mass index (kg/m2) | 24.5 ± 3.9 | 24.7 ± 3.2 | 24.0 ± 3.5 | 24.2 ± 3.9 | 24.7 ± 3.8 | 25.1 ± 4.3 | 24.6 ± 4.3 | 25.2 ± 4.3 | 0.088 |
| Hemoglobin (g/dL) | 10.8 ± 1.3 | 10.2 ± 1.4 | 10.7 ± 1.3 | 10.7 ± 1.2 | 10.8 ± 1.3 | 10.9 ± 1.3 | 10.9 ± 1.4 | 10.9 ± 1.4 | 0.0009 |
| Serum albumin (g/dL) | 3.2 ± 0.5 | 3.2 ± 0.6 | 3.2 ± 0.5 | 3.3 ± 0.5 | 3.2 ± 0.6 | 3.2 ± 0.5 | 3.2 ± 0.6 | 3.2 ± 0.5 | 0.476 |
| Total cholesterol (mg/dL) | 172 ± 40 | 171 ± 43 | 165 ± 36 | 172 ± 42 | 170 ± 39 | 182 ± 41 | 172 ± 38 | 174 ± 41 | 0.015 |
| HDL-cholesterol (mg/dL) | 47 ± 16 | 50 ± 18 | 48 ± 17 | 49 ± 16 | 45 ± 15 | 44 ± 15 | 46 ± 17 | 42 ± 17 | 0.002 |
| C-reactive protein (mg/dL) | 0.15 [0.08–0.44] | 0.14 [0.10–0.32] | 0.12 [0.07–0.37] | 0.11 [0.05–0.34] | 0.19 [0.09–0.53] | 0.16 [0.09–0.53] | 0.22 [0.10–0.70] | 0.21 [0.10–0.62] | 0.017 |
| Calcium (mg/dL) | 8.6 ± 0.8 | 8.5 ± 0.7 | 8.5 ± 0.8 | 8.5 ± 0.7 | 8.5 ± 0.8 | 8.6 ± 0.8 | 8.7 ± 0.8 | 8.6 ± 0.8 | 0.409 |
| Phosphate (mg/dL) | 5.2 ± 1.3 | 5.3 ± 1.3 | 5.3 ± 1.3 | 5.2 ± 1.1 | 5.1 ± 1.2 | 5.1 ± 1.2 | 5.2 ± 1.3 | 5.4 ± 1.6 | 0.168 |
| Intact-PTH (pg/mL) | 153 [76–248] | 158 [75–200] | 151 [73–241] | 138 [69–242] | 153 [83–241] | 151 [84–287] | 172 [68–273] | 193 [89–290] | 0.246 |
| Switch to HD, n (%) | 396 (30.8) | 24 (27.9) | 68 (31.1) | 97 (33.3) | 85 (30.9) | 58 (29.7) | 31 (31.9) | 33 (27.7) | 0.886 |
| Death (%) | 18.4 | 12.1 | 18.2 | 16.6 | 18.7 | 18.7 | 21.7 | 23.6 | 0.635 |
| Cardiovascular death | 7.4 | 4.9 | 7.9 | 6.7 | 6.9 | 5.8 | 11.3 | 10.5 | 0.719 |
| Infection-related death | 5.1 | 0 | 5.2 | 3.5 | 5.3 | 6.6 | 5.2 | 9.3 | 0.275 |
CVD, cardiovascular disease; HD, hemodialysis; HDL, high-density lipoprotein; PD, peritoneal dialysis; PTH, parathyroid hormone.
Unadjusted all-cause death HRs and 95% CIs for HbA1c < 5.0%, 5.0–5.4%, 6.0–6.4%, 6.5–6.9%, 7.0–7.4%, and ≥ 7.5%, compared with 5.5–5.9% (reference), were 0.69 (0.31–1.53), 1.10 (0.67–1.81), 1.12 (0.68–1.84), 1.12 (0.65–1.92), 1.36 (0.72–2.55), and 1.51 (0.85–2.66), respectively.
Figure 2 shows adjusted death HRs for groups based on HbA1c at baseline. All-cause death HR for each HbA1c category did not differ significantly compared with the reference HbA1c after adjusting separately for basic factors, after adjusting for basic factors plus PD-related factors, and after adjusting for basic factors plus PD- nutrition-, and inflammation-related factors.
Figure 2.
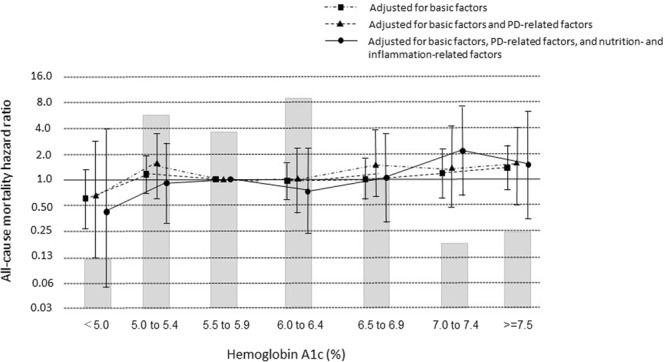
Hazard ratios for all-cause mortality over the entire range of hemoglobin A1c in 1282 patients undergoing peritoneal dialysis (PD), determined by Cox proportional hazards regression analysis. Basic factors include age, sex, dialysis duration, and presence or absence of cardiovascular comorbidity. PD-related factors include anuric or non-anuric state, total Kt/V, use of 2.5% glucose or icodextrin dialysate, and use of an automated PD system. Nutrition-related and inflammation-related factors include BMI and levels of hemoglobin, albumin, total cholesterol, HDL-cholesterol, calcium, phosphate, i-PTH, and CRP. Error bars correspond to 95% confidence intervals.
GA cohort
Table 4 shows hazard ratios (HRs) in the unadjusted ANOVA evaluated as potential predictors of mortality in the GA cohort. There was no significant effect of sex on patient survival. As in the HbA1c cohort, older age, longer duration of dialysis, comorbid CVD, lower total Kt/V, use of 2.5% glucose dialysate, and an anuric state at baseline were significant predictors of mortality. Lower hemoglobin, higher CRP, lower serum albumin, and lower BMI were also associated with higher mortality in patients on PD.
Table 4.
Hazard ratios (with 95% CIs) for variables evaluated as potential predictors of all-cause mortality in the GA cohort.
| Variables | n (%) | HR | 95% CI | P value |
|---|---|---|---|---|
| Male | 514 (70.9) | 1.00 | Reference | Reference |
| Female | 211 (29.1) | 1.13 | 0.71–1.78 | 0.608 |
| Age | ||||
| 1 year increase | 725 (100) | 1.05 | 1.02–1.07 | <0.0001 |
| Duration of PD (year) | ||||
| <2 | 310 (42.8) | 1.00 | Reference | Reference |
| 2 ≤ < 4 | 204 (28.1) | 1.80 | 1.06–3.06 | 0.029 |
| 4 ≤ < 6 | 116 (16.0) | 2.03 | 1.02–4.03 | 0.043 |
| 6 ≤ < 8 | 57 (7.9) | 3.17 | 1.36–7.37 | 0.007 |
| 8≤ | 38 (5.2) | 2.27 | 0.88–5.85 | 0.087 |
| Type of diabetes | ||||
| Type 2 | 637 (87.9) | 1.00 | Reference | Reference |
| Type 1 | 88 (12.1) | 1.09 | 0.57–2.11 | 0.782 |
| CVD comorbidity | ||||
| No | 535 (73.8) | 1.00 | Reference | Reference |
| Yes | 190 (26.2) | 2.69 | 1.71–4.24 | <0.0001 |
| Smoking | ||||
| No | 566 (89.0) | 1.00 | Reference | Reference |
| Yes | 70 (11.0) | 1.22 | 0.58–2.59 | 0.592 |
| Antihypertensive agent | ||||
| User | 551 (83.6) | 1.00 | Reference | Reference |
| Non-user | 108 (16.4) | 1.93 | 1.12–3.33 | 0.017 |
| HD combination | ||||
| No | 553 (76.3) | 1.00 | Reference | Reference |
| Yes | 172 (23.7) | 1.01 | 0.58–1.76 | 0.971 |
| Residual renal function | ||||
| Non-anuric | 429 (79.2) | 1.00 | Reference | Reference |
| Anuric | 113 (20.8) | 1.78 | 1.01–3.22 | 0.041 |
| Type of dialysate | ||||
| 2.5% non-user | 367 (56.4) | 1.00 | Reference | Reference |
| 2.5% user | 284 (43.6) | 2.19 | 1.34–3.59 | 0.002 |
| Type of dialysate | ||||
| Icodextrin non-user | 302 (46.4) | 1.00 | Reference | Reference |
| Icodextrin user | 349 (53.6) | 1.15 | 0.73–1.83 | 0.528 |
| Type of treatment | ||||
| APD non-user | 346 (53.7) | 1.00 | Reference | Reference |
| APD user | 298 (46.3) | 0.74 | 0.48–1.19 | 0.236 |
| Total Kt/V | ||||
| <1.1 | 55 (11.9) | 3.43 | 1.33–8.83 | 0.011 |
| 1.1 ≤ < 1.4 | 64 (13.8) | 2.53 | 0.93–6.93 | 0.068 |
| 1.4 ≤ < 1.7 | 102 (22.0) | 1.00 | Reference | Reference |
| 1.7 ≤ < 2.0 | 107 (23.1) | 0.74 | 0.27–2.05 | 0.573 |
| 2.0≤ | 135 (29.2) | 1.02 | 0.41–2.51 | 0.972 |
| Body mass index (kg/m2) | ||||
| <20.0 | 62 (9.9) | 2.68 | 1.25–5.77 | 0.011 |
| 20 ≤ < 22 | 102 (16.4) | 1.15 | 0.53–2.94 | 0.708 |
| 22 ≤ < 24 | 153 (24.5) | 1.00 | Reference | Reference |
| 24 ≤ < 26 | 123 (19.7) | 0.76 | 0.35–1.64 | 0.488 |
| 26 ≤ < 28 | 84 (13.5) | 0.94 | 0.41–2.19 | 0.895 |
| 28≤ | 100 (16.0) | 0.67 | 0.28–1.58 | 0.364 |
| Serum albumin (g/dL) | ||||
| <2.5 | 47 (6.6) | 12.0 | 5.17–27.9 | <0.0001 |
| 2.5 ≤ < 3.0 | 141 (19.9) | 1.35 | 0.70–2.57 | 0.365 |
| 3.0 ≤ < 3.5 | 283 (40.0) | 1.00 | Reference | Reference |
| 3.5 ≤ < 4.0 | 193 (27.3) | 0.59 | 0.30–1.16 | 0.129 |
| 4.0≤ | 44 (6.2) | 0.17 | 0.02–1.25 | 0.082 |
| Hemoglobin | ||||
| 1 g/dL increase | 711 (98.1) | 0.71 | 0.59–0.84 | <0.0001 |
| C-reactive protein | ||||
| 1 mg/dL increase | 646 (89.1) | 1.39 | 1.18–1.64 | <0.0001 |
| Total cholesterol (mg/dL) | ||||
| <130 | 83 (13.5) | 1.26 | 0.58–2.74 | 0.552 |
| 130 ≤ < 150 | 105 (17.2) | 0.78 | 0.37–1.64 | 0.524 |
| 150 ≤ < 170 | 124 (20.3) | 1.00 | Reference | Reference |
| 170 ≤ < 190 | 108 (17.7) | 0.75 | 0.36–1.53 | 0.432 |
| 190 ≤ < 210 | 89 (14.5) | 0.76 | 0.36–1.63 | 0.492 |
| 210≤ | 103 (16.8) | 0.23 | 0.08–0.60 | 0.003 |
| HDL-cholesterol (mg/dL) | ||||
| <30 | 70 (11.8) | 3.18 | 1.49–6.79 | 0.003 |
| 30 ≤ < 40 | 176 (29.7) | 0.84 | 0.41–1.69 | 0.633 |
| 40 ≤ < 50 | 148 (25.0) | 1.00 | Reference | Reference |
| 50 ≤ < 60 | 89 (15.0) | 1.12 | 0.49–2.51 | 0.791 |
| 60 ≤ < 70 | 59 (10.0) | 0.44 | 0.14–1.39 | 0.165 |
| 70≤ | 50 (8.5) | 0.41 | 0.11–1.50 | 0.179 |
| Calcium (mg/dL) | ||||
| <8.0 | 154 (21.7) | 0.72 | 0.29–1.75 | 0.473 |
| 8.0 ≤ < 8.5 | 159 (22.4) | 0.96 | 0.45–2.03 | 0.918 |
| 8.5 ≤ < 9.0 | 185 (26.1) | 1.00 | Reference | Reference |
| 9.0 ≤ < 9.5 | 116 (16.4) | 0.76 | 0.34–1.68 | 0.508 |
| 9.5 ≤ < 10.0 | 66 (9.3) | 0.78 | 0.29–2.09 | 0.629 |
| 10≤ | 29 (4.1) | 1.83 | 0.64–5.18 | 0.253 |
| Phosphate (mg/dL) | ||||
| <4.0 | 107 (14.8) | 1.47 | 0.75–2.88 | 0.258 |
| 4.0 ≤ < 5.0 | 210 (27.8) | 1.47 | 0.83–2.60 | 0.179 |
| 5.0 ≤ < 6.0 | 221 (30.5) | 1.00 | Reference | Reference |
| 6.0 ≤ < 7.0 | 115 (15.9) | 1.04 | 0.51–2.13 | 0.903 |
| 7.0 ≤ < 8.0 | 58 (8.0) | 1.52 | 0.64–3.58 | 0.335 |
| 8.0≤ | 22 (3.0) | 0.42 | 0.05–3.39 | 0.418 |
| Intact-PTH (pg/mL) | ||||
| <60 | 119 (18.3) | 1.42 | 0.61–3.34 | 0.412 |
| 60 ≤ < 140 | 181 (27.9) | 1.23 | 0.53–2.84 | 0.617 |
| 140 ≤ < 220 | 151 (23.2) | 1.00 | Reference | Reference |
| 220 ≤ < 300 | 88 (13.5) | 1.17 | 0.48–2.85 | 0.718 |
| 300 ≤ < 380 | 44 (6.8) | 0.63 | 0.18–2.12 | 0.461 |
| 380≤ | 67 (10.3) | 1.71 | 0.52–5.57 | 0.368 |
CVD, cardiovascular disease; HD, hemodialysis; HDL, high-density lipoprotein; PD, peritoneal dialysis; PTH, parathyroid hormone.
Table 5 shows baseline data for demographics and clinical and laboratory characteristics of the PD patients based on the seven a priori categories according to baseline GA. Higher GA levels were noted to be associated with older age, type 1 diabetes, insulin use, 2.5% glucose dialysate, and higher CRP levels. The rate of transfer from PD to HD was not associated with a priori GA categories. A higher GA level was associated with higher mortality, especially infection-related death.
Table 5.
Demographic, clinical, and laboratory values in 725 PD patients, according to categories of GA.
| Variables | All | GA Categories | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <12.0 | 12.0 to 13.9 | 14.0 to 15.9 | 16.0 to 17.9 | 18.0 to 19.9 | 20.0 to 21.9 | ≥22 | |||
| n (male%) | 725 (70.1) | 42 (61.9) | 132 (71.2) | 164 (79.3) | 150 (68.0) | 100 (71.0) | 63 (66.7) | 74 (66.2) | 0.153 |
| Age (years) | 63.5 ± 11.7 | 58.5 ± 12.2 | 61.7 ± 11.9 | 64.4 ± 11.9 | 64.4 ± 11.4 | 63.6 ± 11.6 | 63.8 ± 11.0 | 65.0 ± 10.9 | 0.028 |
| PD duration (m) | 29 [14–53] | 27 [15–54] | 24 [13–49] | 24 [13–49] | 33 [14–55] | 37 [19–64] | 29 [12–57] | 27 [13–49] | 0.166 |
| Type 1 (%) | 12.1 | 4.7 | 5.3 | 9.7 | 14.0 | 19.0 | 14.3 | 18.9 | 0.006 |
| CVD comorbidity (%) | 25.8 | 16.7 | 22.7 | 27.4 | 26.7 | 30.0 | 17.5 | 32.4 | 0.241 |
| Smoking (%) | 11.0 | 13.5 | 10.9 | 6.9 | 11.5 | 17.2 | 5.5 | 14.0 | 0.193 |
| Insulin (%) | 37.5 | 30.5 | 20.6 | 27.6 | 39.2 | 47.4 | 50.0 | 65.1 | <0.0001 |
| Oral antidiabetic agents (%) | 43.3 | 37.1 | 36.3 | 46.5 | 41.2 | 49.5 | 45.3 | 46.2 | 0.478 |
| Diet therapy alone (%) | 32.0 | 38.9 | 47.5 | 37.2 | 31.8 | 20.8 | 19.6 | 15.9 | <0.0001 |
| Antihypertensive agents (%) | 83.6 | 84.2 | 87.0 | 84.6 | 84.8 | 76.9 | 89.1 | 76.9 | 0.293 |
| HD combination (%) | 23.7 | 21.4 | 19.7 | 23.1 | 24.6 | 34.0 | 15.8 | 24.3 | 0.163 |
| Anuric (%) | 20.8 | 13.9 | 15.7 | 18.0 | 25.0 | 19.4 | 21.7 | 33.9 | 0.131 |
| Total Kt/V | 1.7 ± 0.7 | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.8 ± 0.9 | 1.6 ± 0.7 | 1.9 ± 0.9 | 1.6 ± 0.6 | 0.224 |
| 2.5% glucose dialysate use (%) | 43.6 | 32.4 | 35.8 | 34.9 | 45.9 | 61.3 | 45.6 | 52.9 | 0.0007 |
| Icodextrin dialysate use (%) | 53.6 | 54.1 | 60.0 | 54.1 | 51.1 | 46.6 | 52.6 | 55.9 | 0.643 |
| D/P Cr | 0.67 ± 0.13 | 0.71 ± 0.14 | 0.69 ± 0.13 | 0.66 ± 0.15 | 0.66 ± 0.12 | 0.67 ± 0.11 | 0.66 ± 0.13 | 0.67 ± 0.11 | 0.273 |
| APD user (%) | 46.2 | 44.7 | 43.3 | 43.4 | 47.7 | 57.3 | 42.1 | 44.8 | 0.439 |
| Body mass index (kg/m2) | 24.3 ± 3.7 | 25.1 ± 3.7 | 24.9 ± 3.9 | 24.1 ± 3.4 | 24.3 ± 3.6 | 23.9 ± 3.7 | 24.7 ± 4.6 | 23.4 ± 3.5 | 0.108 |
| Hemoglobin (g/dL) | 10.8 ± 1.4 | 10.8 ± 1.3 | 10.7 ± 1.3 | 10.9 ± 1.4 | 10.8 ± 1.4 | 10.6 ± 1.4 | 11.0 ± 1.4 | 10.6 ± 1.4 | 0.572 |
| Serum albumin (g/dL) | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.2 ± 0.5 | 3.3 ± 0.5 | 3.2 ± 0.5 | 3.2 ± 0.6 | 3.3 ± 0.4 | 3.2 ± 0.6 | 0.391 |
| Total cholesterol (mg/dL) | 172 ± 40 | 170 ± 43 | 173 ± 34 | 173 ± 41 | 172 ± 41 | 168 ± 41 | 174 ± 43 | 170 ± 41 | 0.962 |
| HDL-cholesterol (mg/dL) | 46 ± 17 | 50 ± 23 | 47 ± 14 | 45 ± 14 | 47 ± 20 | 43 ± 14 | 45 ± 16 | 46 ± 24 | 0.351 |
| C-reactive protein (mg/dL) | 0.17 [0.07–0.50] | 0.12 [0.08–0.42] | 0.17 [0.08–0.38] | 0.15 [0.07–0.44] | 0.17 [0.09–0.48] | 0.17 [0.07–0.62] | 0.11 [0.05–0.46] | 0.35 [0.09–1.01] | 0.003 |
| Calcium (mg/dL) | 8.5 ± 0.8 | 8.5 ± 0.8 | 8.6 ± 0.9 | 8.6 ± 0.8 | 8.6 ± 0.8 | 8.5 ± 0.8 | 8.6 ± 0.8 | 8.4 ± 0.9 | 0.498 |
| Phosphate (mg/dL) | 5.3 ± 1.3 | 5.6 ± 1.6 | 5.4 ± 1.3 | 5.3 ± 1.4 | 5.3 ± 1.2 | 5.1 ± 1.2 | 5.0 ± 1.3 | 5.2 ± 1.3 | 0.285 |
| Intact-PTH (pg/mL) | 152 [74–246] | 151 [79–207] | 148 [68–236] | 155 [75–257] | 128 [67–232] | 138 [68–235] | 194 [85–249] | 198 [81–295] | 0.101 |
| Switch to HD, n (%) | 227 (31.3) | 11 (26.2) | 46 (34.8) | 40 (24.4) | 53 (35.3) | 37 (37.0) | 22 (34.9) | 18 (24.3) | 0.215 |
| Death (%) | 15.9 | 12.9 | 12.8 | 12.9 | 16.5 | 14.3 | 14.6 | 30.3 | 0.002 |
| Cardiovascular death | 7.1 | 10.0 | 7.4 | 6.1 | 5.8 | 5.3 | 7.9 | 11.4 | 0.886 |
| Infection-related death | 7.1 | 0 | 3.9 | 5.3 | 9.0 | 6.9 | 5.4 | 18.8 | 0.025 |
CVD, cardiovascular disease; HD, hemodialysis; HDL, high-density lipoprotein; PD, peritoneal dialysis; PTH, parathyroid hormone.
Unadjusted all-cause death HRs and 95% CIs for GAs < 12.0%, 12.0–13.9%, 16.0–17.9%, 18.0–19.9%, 20.0–21.9%, and ≥22.0%, compared with the reference value, were 1.14 (0.42–3.09), 1.17 (0.58–2.38), 1.50 (0.79–2.86), 1.68 (0.84–3.37), 1.22 (0.51–2.94), and 3.09 (1.66–5.77), respectively. Patients with GA ≥ 22.0% had a significantly higher death HR when compared with the reference GA (P = 0.0003). Figure 3 shows adjusted death HRs for groups based on GA at baseline. The all-cause death HR for GA ≥ 22.0%, compared with the reference GA, was 2.69 (1.43–5.03) after adjusting for basic factors and 4.09 (1.56–10.7) after adjusting for basic factors plus PD-related factors (P = 0.0018 and 0.0025, respectively). However, the all-cause death HR for GA categories 20.0–21.9% and ≥22.0% were 4.36 (1.10–17.0) and 4.10 (1.2–14.0) compared with the reference value after adjusting for basic factors plus PD-, nutrition-, and inflammation-related factors (P = 0.040 and 0.017, respectively).
Figure 3.
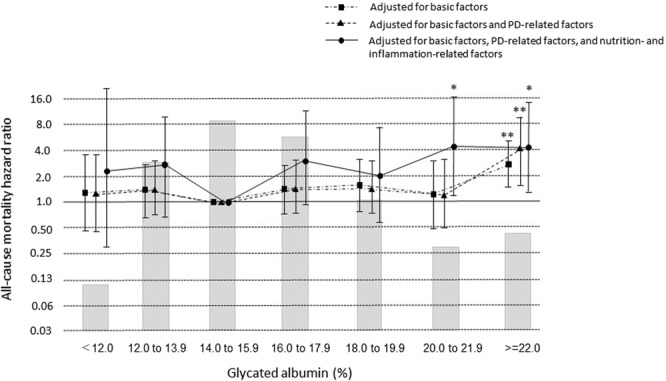
Hazard ratios for all-cause mortality over the entire range of glycated albumin in 725 patients undergoing PD, determined by Cox proportional hazards regression analysis. Basic factors include age, sex, dialysis duration, and presence or absence of cardiovascular comorbidity. PD-related factors include anuric or non-anuric state, total Kt/V, use of 2.5% glucose or icodextrin dialysate, and use of an automated PD system. Nutrition-related and inflammation-related factors include BMI and levels of hemoglobin, albumin, total cholesterol, HDL-cholesterol, calcium, phosphate, i-PTH, and CRP. *P < 0.05, **P < 0.01 vs. GA 14.0 to 15.9% (reference). Error bars correspond to 95% confidence intervals.
Discussion
In this study, we first identified the predictors of 2-year mortality in PD patients. Because survival outcome among dialysis patients might be determined by additional multiple confounding factors—dialysis-related or non-dialysis related—investigations into the control of these factors are difficult to perform. However, we compared mortality rates among glycemic control categories, adjusting for multiple potentially confounding factors. After fully adjusting for these factors, there were no significant differences for death HRs among the HbA1c categories compared with the HbA1c reference value of 5.5–5.9%. However, in this large-scale contemporary cohort of 1,601 PD patients, GA ≥ 20.0% was associated with higher all-cause mortality. This is the first study to suggest that mortality risk for PD patients might differ according to GA level.
Data from the US Renal Data System for 2010 revealed that the overall 1- and 2-year survival rates of patients on PD were 83% and 67.2%, respectively, whereas those of a diabetes subgroup were 80.3% and 61.7%17. A further study from Canada reported 1- and 2-year survival rates of 94% and 89% for patients without diabetes, respectively, and 91% and 76% for patients with diabetes18. Data from single centers in Asia and Europe also showed that survival rates were significantly lower in patients with diabetes on PD than in those without diabetes on PD19. In the present study, the 1- and 2-year survival rates were 88.3% and 84.0%, respectively, which are higher than those reported previously. Older age, longer duration of PD, use of 2.5% glucose dialysate, anuric state, and lower albumin levels and BMI at baseline might have contributed to lower survival rates in this cohort.
Relatively few studies have investigated PD patients with diabetes8,9,20–22. In the largest of the previous studies, which involved 2,798 patients, time-averaged HbA1c ≥ 8% was found to be associated with the highest risk of all-cause mortality9. Another study found that glycemic control was more important in patients who were less than 60 years old20. Infection was a notable cause of death among these patients, particularly those with HbA1c ≥ 8%23. These findings suggest that in PD patients, poor glycemic control adversely impacts survival due to an increased risk of infection. Similar results were obtained in a prospective observational study of diabetes patients on PD; non-cardiovascular mortality primarily due to infection was highest in patients in the highest HbA1c tertile24. The present study revealed no significant differences in the rates of cardiovascular death and infection-related death among the HbA1c categories. However, infection-related death occurred most frequently in patients in the highest GA category.
A meta-analysis reported that high levels of HbA1c (>8.5%) and very low HbA1c levels (<5.4%) were associated with an increased mortality risk25. The Dialysis Outcomes and Practice Patterns Study demonstrated that the relationship between low HbA1c and mortality appeared to be even stronger in patients with indicators of poor nutritional status, including low serum albumin, low BMI, or presence of cachexia26. These data suggest that not only high HbA1c levels but also low HbA1c levels related to malnutrition or anemia are associated with increased mortality in HD patients. Potential contributors to these phenomena include reduced kidney gluconeogenesis, decreased kidney and hepatic insulin clearance, diminished food intake, deficient catecholamine release, protein-energy wasting, and the hypoglycemic effects of HD. Furthermore, patients on dialysis have low HbA1c levels due to renal anemia and use of ESAs. Thus, HbA1c levels could overestimate glycemic control in HD patients and PD patients as well, because of renal anemia and ESA use in PD patients. In contrast, GA is not affected by hemoglobin levels and ESA2–4. Understanding factors associated with mortality remains a priority in clinical care for patients with diabetes on dialysis. Glycemic control is definitely the most important factor for diabetes patients, including those with ESKD. Recent reports have questioned the utility of HbA1c and GA as glycemic markers in HD patients27,28. Although GA may be a more accurate index of glycemic control than HbA1c in HD patients, there is limited evidence of an association between GA and mortality in PD patients. Therefore, concluding that GA is superior to HbA1c as a mortality marker in these patients is premature, although some data suggest that GA is less affected by anemia and serum albumin levels than HbA1c is. Nevertheless, the present study does provide some evidence for the utility of GA in predicting 2-year mortality in a large cohort of PD patients.
According to numerous studies, higher GA levels are associated with higher all-cause or cardiovascular mortality rates in HD patients with diabetes29–31. Remarkably, no significant association was seen between mean HbA1c levels and mortality in these patients. In addition, it was reported that GA, not HbA1c, accurately predicted the risk of death and hospitalization in patients with diabetes on HD (n = 401) or PD (n = 43)32. GA level is a useful index of glycemic control in HD patients, there is little evidence on the relationship between GA levels and the risk of cardiovascular events or prognosis in PD patients.
This study has several limitations. First, as in any annual survey or observational cohort study, sample size and power were limited in the present study. The number of patients who had higher HbA1c or GA categories was small and mortality was low compared with previous reports13,27; an adequate sample size was estimated to be 856 and 1905 patients in the GA cohort and HbA1c cohort, respectively. It must be noted that GA is not a widely available index, and there are limited outcome studies that used GA. Further studies are thus needed to confirm reproducibility. Second, as with any annual survey, our database involves a yearly one-point estimate of glycemic control, rendering analyses with time-averaged GA levels within a year impossible. However, some previous large-database reports have shown similar mortality among initial and time-averaged HbA1c groups26,33. Third, we lacked information about the use of ESA. However, because GA is reported to be unaffected by the life-span of erythrocytes or ESA administration4,34,35, the effect of ESA in the GA-mortality association may be small and hence may not change our results. Finally, blood glucose level data were not available and many patients in this study had either HbA1c or GA measured, not both. Therefore, we were unable to compare GA, HbA1c, and plasma glucose levels. Thus, in the future, prospective studies are warranted to elucidate target GA levels, given that several recommendations for the treatment of diabetes in PD patients are based on longer-term studies of HbA1c levels.
In conclusion, the findings of this study show that a GA > 20.0% was associated with decreased survival, especially infection-related death, in patients with diabetes on PD. No associations were evident between the HbA1c levels and 2-year mortality in PD patients. These results underscore the need for further research on the factors that influence patient outcomes, so as to identify alternative interventions that would improve the outlook for patients undergoing PD.
Acknowledgements
We thank the members of the Committee of the JSDT Renal Data Registry for their contributions, and all the staff of the participating dialysis facilities involved in the survey. The authors declare that they have no other relevant financial interests. Publication of this report was not supported by any grants. No financial support was received for this study.
Author Contributions
Research idea and study design: M.A.; data acquisition: M.A., J.H., A.W., S.N.; data analysis/interpretation: M.A.; statistical analysis: M.A.; supervision: T.H., I.M. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work have been appropriately investigated and resolved. M.A. takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abe M, Kalanter-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat. Rev. Nephrol. 2015;11:302–313. doi: 10.1038/nrneph.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inaba M, et al. Glycated albumin is a better glycemic indicator than glycated hemoglobin values in hemodialysis patients with diabetes: Effect of anemia and erythropoietin injection. J. Am. Soc. Nephrol. 2007;18:896–903. doi: 10.1681/ASN.2006070772. [DOI] [PubMed] [Google Scholar]
- 3.Abe M, Matsumoto K. Glycated hemoglobin or glycated albumin for assessment of glycemic control in dialysis patients with diabetes? Nat. Clin. Pract. Nephrol. 2008;4:482–483. doi: 10.1038/ncpneph0881. [DOI] [PubMed] [Google Scholar]
- 4.Peacock TP, et al. Comparison of glycated albumin and hemoglobin A1c levels in diabetic subjects on hemodialysis. Kidney Int. 2008;73:1062–1068. doi: 10.1038/ki.2008.25. [DOI] [PubMed] [Google Scholar]
- 5. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am. J. Kidney Dis. 60, 850–886 (2012). [DOI] [PubMed]
- 6.Inaba M, et al. Impact of atherosclerosis on the relationship of glycemic control and mortality in diabetic patients on hemodialysis. Clin. Nephrol. 2012;78:273–280. doi: 10.5414/CN106940. [DOI] [PubMed] [Google Scholar]
- 7.Nakao T, et al. Best practice for diabetic patients on hemodialysis 2012. Ther. Apher. Dial. 2015;19(Suppl 1):S40–S66. doi: 10.1111/1744-9987.12299. [DOI] [PubMed] [Google Scholar]
- 8.Wu MS, et al. Pre-dialysis glycemic control is an independent predictor of mortality in type II diabetic patients on continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 1999;19(Suppl 2):S179–183. [PubMed] [Google Scholar]
- 9.Duong U, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Clin. J. Am. Soc. Nephrol. 2011;6:1041–1048. doi: 10.2215/CJN.08921010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino J, et al. Glycated albumin versus hemoglobin A1c and mortality in diabetic hemodialysis patients: a cohort study. Nephrol. Dial. Transplant. 2018;33:1150–1158. doi: 10.1093/ndt/gfy014. [DOI] [PubMed] [Google Scholar]
- 11.Abe M, Hamano T, Wada A, Nakai S, Masakane I. High-Performance Membrane Dialyzers and Mortality in Hemodialysis Patients: A 2-Year Cohort Study from the Annual Survey of the Japanese Renal Data Registry. Am. J. Nephrol. 2017;46:82–92. doi: 10.1159/000478032. [DOI] [PubMed] [Google Scholar]
- 12.Masakane I, et al. An Overview of Regular Dialysis Treatment in Japan (As of 31 December 2013) Ther. Apher. Dial. 2015;19:540–574. doi: 10.1111/1744-9987.12378. [DOI] [PubMed] [Google Scholar]
- 13.Masakane, I. An overview of regular dialysis treatment in Japan (As of 31 December 2014), http://docs.jsdt.or.jp/overview/index.html (2018). [DOI] [PubMed]
- 14.Kalantar-Zadeh K, et al. A1C and survival in maintenance hemodialysis patients. Diabetes Care. 2007;30:1049–1055. doi: 10.2337/dc06-2127. [DOI] [PubMed] [Google Scholar]
- 15.Abe M, et al. Is there a “burnt-out diabetes” phenomenon in patients on hemodialysis? Diabetes Res. Clin. Pract. 2017;130:211–220. doi: 10.1016/j.diabres.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Abe M, et al. Rate of the “burnt-out diabetes” phenomenon in patients on peritoneal dialysis. Diabetes Res. Clin. Pract. 2018;143:254–262. doi: 10.1016/j.diabres.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 17.United states Renal Data System 2010 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2010.
- 18.Fang W, et al. Patient and technique survival of diabetics on peritoneal dialysis: one-center’s experience and review of the literature. Clin. Nephrol. 2008;69:193–200. doi: 10.5414/CNP69193. [DOI] [PubMed] [Google Scholar]
- 19.Chung SH, Heimbürger O, Lindholm B, Lee HB. Peritoneal dialysis patient survival: a comparison between a Swedish and a Korean centre. Nephrol. Dial. Transplant. 2005;20:1207–1213. doi: 10.1093/ndt/gfh772. [DOI] [PubMed] [Google Scholar]
- 20.Adler A, et al. Association between glycemia and mortality in diabetic individuals on renal replacement therapy in the U.K. Diabetes Care. 2014;37:1304–1311. doi: 10.2337/dc13-0553. [DOI] [PubMed] [Google Scholar]
- 21.Yoo DE, et al. Good glycemic control is associated with better survival in diabetic patients on peritoneal dialysis: a prospective observational study. PLos One. 2012;7:e30072. doi: 10.1371/journal.pone.0030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu CC, et al. Predialysis glycemic control is an independent predictor of clinical outcome in type II diabetics on continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 1997;17:262–268. [PubMed] [Google Scholar]
- 23.Park JI, et al. Glycemic control and mortality in diabetic patients undergoing dialysis focusing on the effects of age and dialysis type: A prospective cohort study in Korea. PLoS One. 2015;10:e0136085. doi: 10.1371/journal.pone.0136085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekercioglu N, et al. Glycemic control and survival in peritoneal dialysis patients with diabetes mellitus. Int. Urol. Nephrol. 2012;44:1861–1869. doi: 10.1007/s11255-012-0180-6. [DOI] [PubMed] [Google Scholar]
- 25.Hill CJ, et al. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: A meta-analysis. Am. J. Kidney Dis. 2013;63:84–94. doi: 10.1053/j.ajkd.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez SP, et al. Hemoglobin A(1c) levels and mortality in the diabetic hemodialysis population: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Diabetes Care. 2012;35:2527–2532. doi: 10.2337/dc12-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman BI. A critical evaluation of glycated protein parameters in advanced nephropathy: a matter of life or death: time to dispense with the hemoglobin A1C in end-stage kidney disease. Diabetes Care. 2012;35:1621–1624. doi: 10.2337/dc12-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehrotra R, Kalantar-Zadeh K, Adler S. Assessment of glycemic control in dialysis patients with diabetes: glycosylated hemoglobin or glycated albumin? Clin. J. Am. Soc. Nephrol. 2011;6:1520–1522. doi: 10.2215/CJN.04210511. [DOI] [PubMed] [Google Scholar]
- 29.Fukuoka K, et al. Glycated albumin levels predict long–term survival in diabetic patients undergoing haemodialysis. Nephrology. 2008;13:278–283. doi: 10.1111/j.1440-1797.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 30.Okada T, et al. Association between markers of glycemic control, cardiovascular complications and survival in type 2 diabetic patients with end-stage renal disease. Intern. Med. 2007;46:807–814. doi: 10.2169/internalmedicine.46.6355. [DOI] [PubMed] [Google Scholar]
- 31.Isshiki K, et al. Glycated albumin predicts the risk of mortality in type 2 diabetic patients on hemodialysis: evaluation of a target level for improving survival. Ther. Apher. Dial. 2014;18:434–442. doi: 10.1111/1744-9987.12123. [DOI] [PubMed] [Google Scholar]
- 32.Freedman BI, et al. Glycated albumin and risk of death and hospitalizations in diabetic dialysis patients. Clin. J. Am. Soc. Nephrol. 2011;6:1635–1643. doi: 10.2215/CJN.11491210. [DOI] [PubMed] [Google Scholar]
- 33.Ricks J, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61:708–715. doi: 10.2337/db11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chujo K, et al. Indicators for blood glucose control in diabetics with end-stage chronic renal disease: GHb vs. glycated albumin (GA) J. Med. Invest. 2006;53:223–228. doi: 10.2152/jmi.53.223. [DOI] [PubMed] [Google Scholar]
- 35.Nakao T, et al. Influence of erythropoietin treatment on hemoglobin A1c levels in patients with chronic renal failure on hemodialysis. Intern. Med. 1998;37:826–830. doi: 10.2169/internalmedicine.37.826. [DOI] [PubMed] [Google Scholar]


