Abstract
Objectives
Intra-articular injections of local anaesthetics (LA), glucocorticoids (GC), or hyaluronic acid (HA) are used to treat osteoarthritis (OA). Contrast agents (CA) are needed to prove successful intra-articular injection or aspiration, or to visualize articular structures dynamically during fluoroscopy. Tranexamic acid (TA) is used to control haemostasis and prevent excessive intra-articular bleeding. Despite their common usage, little is known about the cytotoxicity of common drugs injected into joints. Thus, the aim of our study was to investigate the effects of LA, GC, HA, CA, and TA on the viability of primary human chondrocytes and tenocytes in vitro.
Methods
Human chondrocytes and tenocytes were cultured in a medium with three different drug dilutions (1:2; 1:10; 1:100). The following drugs were used to investigate cytotoxicity: lidocaine hydrochloride 1%; bupivacaine 0.5%; triamcinolone acetonide; dexamethasone 21-palmitate; TA; iodine contrast media; HA; and distilled water. Normal saline served as a control. After an incubation period of 24 hours, cell numbers and morphology were assessed.
Results
Using LA or GC, especially triamcinolone acetonide, a dilution of 1:100 resulted in only a moderate reduction of viability, while a dilution of 1:10 showed significantly fewer cell counts. TA and CA reduced viability significantly at a dilution of 1:2. Higher dilutions did not affect viability. Notably, HA showed no effects of cytotoxicity in all drug dilutions.
Conclusion
The toxicity of common intra-articular injectable drugs, assessed by cell viability, is mainly dependent on the dilution of the drug being tested. LA are particularly toxic, whereas HA did not affect cell viability.
Cite this article: P. Busse, C. Vater, M. Stiehler, J. Nowotny, P. Kasten, H. Bretschneider, S. B. Goodman, M. Gelinsky, S. Zwingenberger. Cytotoxicity of drugs injected into joints in orthopaedics. Bone Joint Res 2019;8:41–48. DOI: 10.1302/2046-3758.82.BJR-2018-0099.R1.
Keywords: Chondrocytes, Tenocytes, Intra-articular Drugs, Toxicity
Article focus
This study investigated the effects of intra-articular injection of local anaesthetics (LA), glucocorticoids (GC), hyaluronic acid (HA), contrast agent (CA), and tranexamic acid (TA) on the viability of primary human chondrocytes and tenocytes.
Key messages
Cytotoxicity of intra-articular injected drugs towards chondrocytes and tenocytes depends on the concentration of the drug.
LA (lidocaine, bupivacaine) and triamcinolone acetonide had strong toxic effects on chondrocytes and tenocytes in low dilutions.
Dexamethasone, TA, and CA had only minor effects on chondrocytes and tenocytes whereas HA did not affect cell viability.
Strengths and limitations
Cytotoxicity of clinically relevant intra-articular injectable drugs was compared. To our knowledge, no comparable study has been published.
This study was performed in vitro, and cells were isolated from their extracellular matrix, therefore effects may be reduced in healthy intact cartilage and tendon tissue.
Introduction
The intra-articular injection of medication is common in orthopaedics. Most of these drugs such as local anaesthetics (LA), glucocorticoids (GC), or hyaluronic acid (HA) are used to treat osteoarthritis (OA). It is estimated that 10% of the world’s population who are 60 years or older have significant clinical problems that can be attributed to OA.1 The use of intra-articular injections for the management of OA is increasing.2 An epidemiological analysis of non-operative interventions used in patients with knee OA in the United States in 2009 revealed that, during the previous five years, 43.5% of the patients who underwent total knee arthroplasty (TKA) were preoperatively treated with intra-articular GC injection and 15.4% of patients received viscosupplementation injections such as HA.3
Despite their common usage, little is known about the cytotoxicity of common drugs administered into the joint. The aim of our study was to investigate the effects of LA, GC, HA, contrast agents (CA), and tranexamic acid (TA) on the viability of primary human chondrocytes and tenocytes in vitro.
Materials and Methods
The study design was approved by the Institutional Review Board (protocol number EK 33022017). The following drugs (Table I) were used to investigate their cytotoxity on human chondrocytes and tenocytes: lidocaine hydrochloride 1% (Xylocitin-loc 1%; mibe GmbH, Brehna, Germany); bupivacaine 0.5% (Carbostesin; AstraZeneca GmbH, Wedel, Germany); triamcinolone acetonide (40 mg, TriamHEXAL; HEXAL AG, Holzkirchen, Germany), dexamethasone 21-palmitate (2.5 mg dexamethasone, Lipotalon; Recordati Pharma GmbH, Ulm, Germany); tranexamic acid (500 mg/5 ml, Cyklokapron-Injektionslösung; Pfizer Pharma PFE GmbH, Berlin, Germany); iodine contrast media (300 mg iodine/ml, Solutrast; Bracco Imaging Deutschland GmbH, Konstanz, Germany); hyaluronic acid (1% hyaluronic acid in physiologic saline); and distilled water. Normal saline (NS) served as a control. Chondrocytes and tenocytes were cultured in a medium with three different drug dilutions (1:2, 1:10, and 1:100). The experiments were performed using cells from three different donors.
Table I.
Description of investigated drugs
| Drug | Class of drug | Scope of application/effect/mode of action |
|---|---|---|
| Lidocaine | Local anaesthetic | 1) Temporary amelioration of pain.4
2) Intra-articular use for reduction of anterior shoulder dislocations should be strongly considered as a first-line therapy.5 |
| Bupivacaine | Local anaesthetic | 1) Use in arthroscopic surgery. 2) Continuous intra-articular infusions for analgesia after shoulder surgery.6 |
| Triamcinolone acetonide | Glucocorticoid | 1) A class of steroid hormones that mediate many physiological effects in most vertebrates. 2) One of the most widely prescribed classes of drugs. 3) Involved in many physiological processes including glucose homeostasis, development, metabolism, and programmed cell death. 4) Pharmacological benefits are primarily anti-inflammatory and immunosuppressive.7 |
| Dexamethasone | Glucocorticoid | |
| Tranexamic acid (TA) | Antifibrinolytic haemostatic | 1) Synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules.8
2) Is used to support haemostasis and prevent excessive intra-articular bleeding. 3) Intravenous or intra-articular administration, whereas one single intra-articular dose and two intravenous doses of TA have equivalent efficacy and safety in management of blood loss at total knee arthroplasty.9 |
| Iodine contrast media | Contrast media | 1) Needed to prove successful intra-articular injection or aspiration, or to visualize articular structures dynamically during fluoroscopy. 2) Contrast agents (CAs) that go beyond qualitative visualization and enable quantitative assessments of functional tissue performance represent the next generation of CA. 3) One clinical area where CAs can have a significant impact is in the diagnosis of soft-tissue-based diseases such as osteoarthritis.10 |
| Hyaluronic acid | Cartilage-protective | 1) High-molecular-weight polysaccharide. 2) Important component of synovial fluid and extracellular matrix of articular cartilage. 3) Contributes to the elasticity and viscosity of synovial fluid. 4) Acts as a fluid shock absorber and helps to maintain the structural and functional characteristics of the cartilage matrix.11 |
Isolation of human chondrocytes and tenocytes
Human cartilage from normal areas was harvested from three healthy donors aged 58 and 74 years (both male) and 59 years (female) undergoing TKA after obtaining their informed consent. On the same day, cartilage samples were cut into small pieces, transferred into six-well cell culture plates and treated with collagenase II (0.2% in Dulbecco’s Modified Eagle Medium (DMEM)) for 24 hours at 37°C. After filtration through a 70 µm filter, the solution was centrifuged (five minutes at 1500 rpm), the supernatant was discarded, the remaining cell pellet was resuspended in growth medium (DMEM, 15% FCS, 1% penicillin/streptomycin), and cells were seeded into cell culture flasks and incubated at 37°C and 5% CO2. After seven days, the medium was changed for the first time and cells were cultured until confluency with the subsequent medium changed every two to three days.
A segment of tendon of the proximal long head of biceps was harvested from three healthy donors aged 51 and 59 years (both male) and 61 years (female) undergoing shoulder arthroscopy and biceps tenodesis. The isolation of tenocytes was undertaken as described for the chondrocytes in this study except for the use of collagenase solution; to digest the tendon, collagenase I (0.2% in DMEM) was used.
Investigation of cytotoxicity
Human chondrocytes or tenocytes of the three donors were seeded at 2*104 cells/cm2 and incubated for 24 hours in a growth medium to ensure sufficient cell adhesion. After that, the medium was changed to a growth medium containing the particular drug dilution. Following a 24-hour incubation period, cells were washed with phosphate-buffered saline (PBS), stained for viability analysis, fixed with 4% neutral buffered formaldehyde for histological analysis or frozen at -80°C until biochemical analysis.
Biochemical analysis of cell number
For biochemical determination of cell number, frozen samples were thawed, and cells were lysed in PBS containing 1% Triton X-100 (Sigma-Aldrich Corp., St. Louis, Missouri) for 30 minutes on ice at room temperature. The activity of lactate dehydrogenase (LDH) was quantified using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega Corp., Madison, Wisonsin) according to the manufacturer’s instructions, and correlated with the cell numbers using a calibration line. The experiments were performed in triplicate.
Statistical analysis
Numerical data were statistically analyzed using GraphPad Prism 5.04 software (GraphPad Software Inc., La Jolla, California). All values are expressed as mean and sd. Statistical analysis was performed on quantified data using one-way analysis of variance (ANOVA). For post hoc testing, two tailed non-paired t-tests where NS served as negative control, were performed. Differences were considered significant when p < 0.05.
Histological analysis
For staining with haematoxylin and eosin (H&E), fixed cells were washed with PBS and incubated for three minutes with haematoxylin (Merck KGaA, Darmstadt, Germany). Then the slides were washed again with PBS and incubated for another three minutes with eosin (Merck). Finally, cells were washed with distilled water and examined using a light microscope (Keyence BZ 9000; Keyence Germany GmbH, Neu-Isenburg, Germany).
Live cell staining
Immediately after washing with PBS, live cells were stained with 2.4 µM calcein (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts) in PBS and incubated for 30 minutes at 37°C in the dark. After that, the staining solution was substituted by PBS. Images were taken with the fluorescence microscope Keyence BZ 9000.
Results
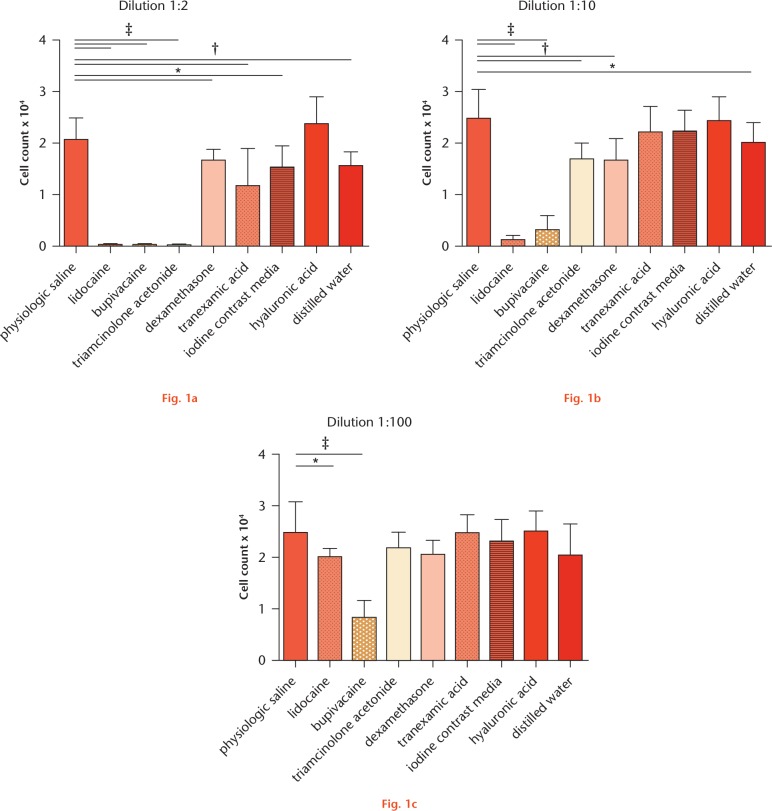
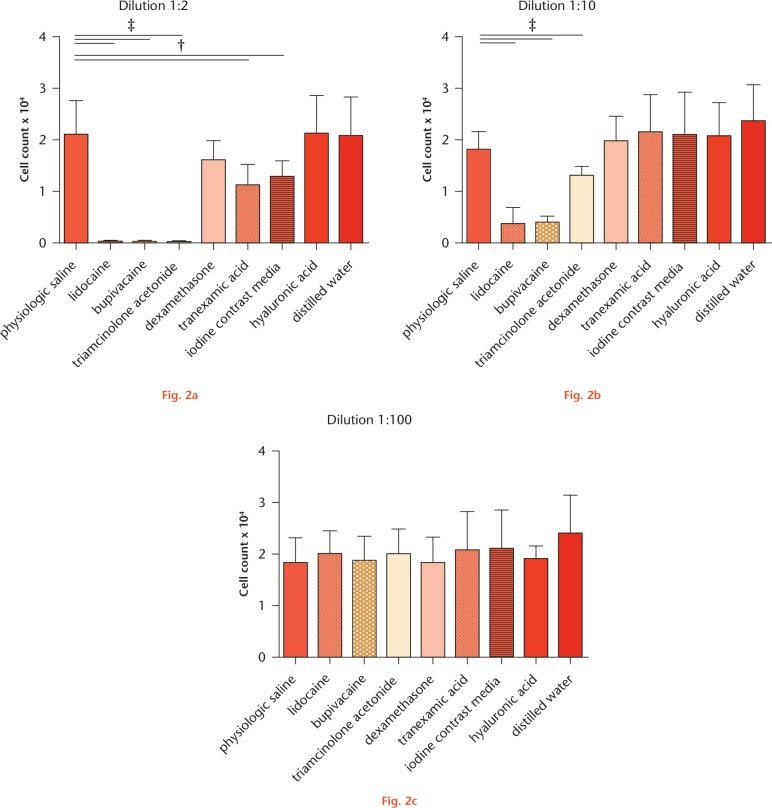
Cell numbers, after culturing chondrocytes and tenocytes at different dilutions of the investigated drugs, are presented in Figures 1 and 2, respectively.
Cell numbers of chondrocytes after 24-hour incubation with different dilutions of the different drugs determined by lactate dehydrogenase (LDH) assay. a) Dilution 1:2, b) Dilution 1:10, c) Dilution 1:100. Results are presented as mean and sd for each drug. For all dilutions, one-way analysis of variance (ANOVA) showed significant differences between the groups (p-value < 0.0001). For post hoc testing, * (p-value < 0.05), † (p-value < 0.01) and ‡ (p-value < 0.001) indicate statistical significance (non-paired t-test) towards normal saline.
Cell numbers of tenocytes after 24-hour incubation with different dilutions of the different drugs determined by LDH assay. a) Dilution 1:2, b) Dilution 1:10, c) Dilution 1:100 Results are presented as mean and sd for each drug. While for dilutions 1:2 and 1:10, one-way ANOVA showed significant differences between the groups (p-value < 0.0001), dilution 1:100 resulted in no significant difference (p-value = 0.4887). For post hoc testing, * (p-value < 0.05), † (p-value < 0.01) and ‡ (p-value < 0.001) indicate statistical significance (non-paired t-test) towards normal saline.
Compared with NS at a dilution of 1:2 LA (lidocaine, bupivacaine), as well as triamcinolone acetonide, TA and iodine contrast media showed strong cytotoxic effects towards chondrocytes and tenocytes as determined by significantly reduced cell numbers. For dexamethasone, a significantly lower number of cells was detected only for chondrocytes in dilution 1:2. HA did not affect cell numbers of both types in each dilution. For chondrocytes, additionally, cell numbers were also decreased when cultured with GC at a dilution of 1:10 and with LA at a dilution of 1:10 and 1:100. Culturing tenocytes with LA and triamcinolone acetonide at a dilution of 1:10 also resulted in significantly reduced cell numbers. At a dilution of 1:100, none of the investigated drugs showed any cytotoxic effects.
Staining with H&E (Figs 3 and 4) showed that cell morphology changed, and cell growth was reduced for both cell types after culturing cells with lidocaine and bupivacaine in dilution 1:2 and 1:10. Morphology of chondrocytes changed from a spindle-like phenotype to narrowed rounded cells while tenocytes had lost their typical spindle-shaped appearance and converted to a polygonal shape. For chondrocytes, toxic effects are also visible for triamcinolone acetonide, TA and iodine contrast media in dilution 1:2, whereas tenocytes showed reactions only for triamcinolone acetonide and TA in dilution 1:2.
Fig. 3.
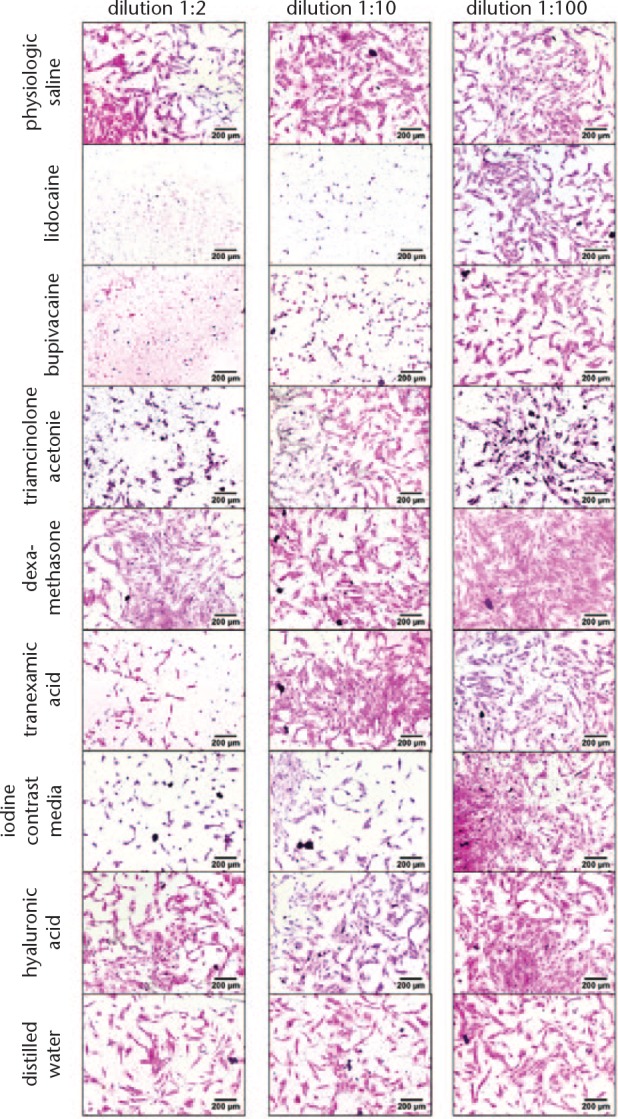
Haematoxylin and eosin (H&E) staining of chondrocytes. Cell nuclei are coloured blue/violet by haematoxylin. Cytoplasm is coloured red/pink by eosin. Normal saline serves as control.
Fig. 4.
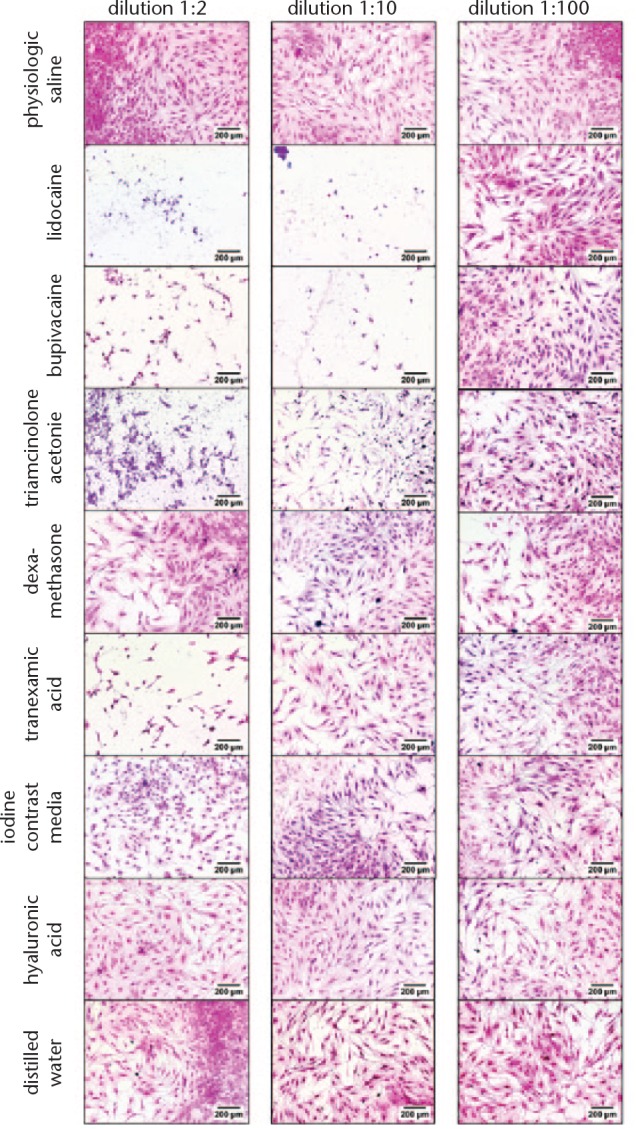
Haematoxylin and eosin (H&E) staining of tenocytes. Cell nuclei are coloured blue/violet by haematoxylin. Cytoplasm is coloured red/pink by eosin. Normal saline serves as control.
Live staining (Figs 5 and 6) of chondrocytes and tenocytes has shown that, for both types of cells, culturing with lidocaine and bupivacaine in dilution 1:2 and 1:10 and culturing with triamcinolone acetonide in dilution 1:2 resulted in a reduction of visible living cells.
Fig. 5.

Live staining of chondrocytes. Live cells are visible as green fluorescent cells and represented here in black. Normal saline serves as control.
Fig. 6.
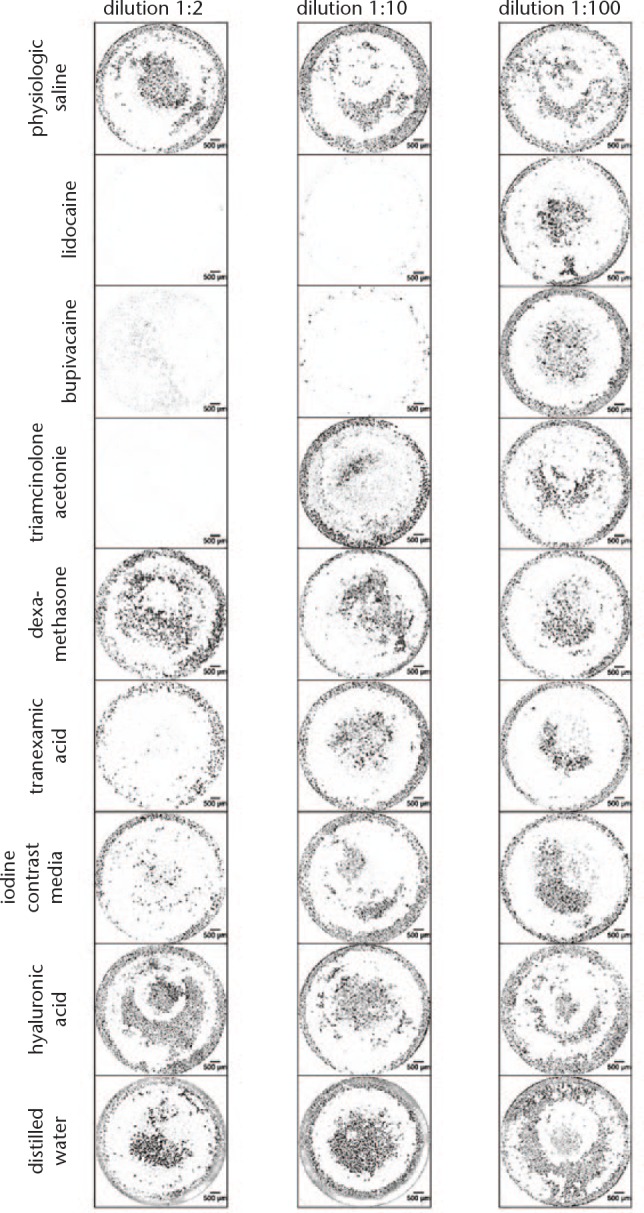
Live staining of tenocytes. Live cells are visible as green fluorescent cells and represented here in black. Normal saline serves as control.
Discussion
We found that toxicity of common intra-articular injectable drugs, assessed by cell viability, is mainly dependent on the dilution of the drug being tested. Drug-incubated chondrocytes and tenocytes showed comparable viability values.
The intra-articular injection of LA, including lidocaine and bupivacaine has been commonly used for the evaluation of atypical pain and is generally accepted as safe.4 Glucocorticoids are known as potent anti-inflammatory agents that act through a variety of mechanisms on different cellular levels.12 However, GC and LA may induce apoptosis in chondrocytes13 and should therefore be used with caution, especially in joints with compromised cartilage.14 Our investigations showed that LA at a dilution of 1:100 resulted in only moderate reduction of cell viability whereas a dilution of 1:10 and 1:2 led to a high cytotoxicity. Our results have demonstrated that the cytotoxic effect of LA is dose-dependent. Karpie and Chu15 also found a dose- and time-dependent cytotoxic effect of lidocaine on bovine articular chondrocytes. Moreover, they had pointed out that an intact articular surface was not protective for lidocaine. In contrast, the intact bovine articular surface has some chondroprotective effects for bupivacaine, which is cytotoxic to bovine articular chondrocytes after an exposure of 15 to 30 minutes.6 We had similar dose-dependent findings when using GC. Triamcinolone acetonide, in particular, reduced cell viability significantly at a dilution of 1:2 and 1:10. In a controlled in vivo study with rats, Sola et al16 found that, after one week, 4 mg/ml triamcinolone resulted in a lower chondrocyte viability as compared with 1 mg/ml triamcinolone. As triamcinolone is a depository drug with prolonged action, and works as an anti-inflammatory agent in controlling symptoms and providing a longer remission effect,17 it is widely used in clinical situations.
Several studies have demonstrated that there are differences between individual drugs of LA and GC. Lidocaine and bupivacaine, in particular, have a dose-dependent effect on chondrocytes18,19 and tenocytes, whereas ropivacaine does not show this effect.20 High concentrations of triamcinolone acetonide may cause irreversible effects in human tenocytes,17 while dexamethasone shows no effect on viability on bovine tenocytes in culture.20 A recent review analyzing the infection rate following total hip arthroplasty found no evidence of an increased infection rate following a previous injection of the joint with GCs, however, the authors stated that the existing level of evidence is low, and high-quality multi-centre randomized trials are needed.21
For TA and CA, reduced cell viability was only observed at a dilution of 1:2. Parker et al22 had shown in their in vitro investigations that there is a dose-dependent effect for TA on human articular chondrocytes. Additionally, they had transferred the results to clinical practice and concluded that a tissue concentration of 10 mg/ml to 20 mg/ml of TA could be expected to be safe.22 In contrast, HA showed no toxicity at all in investigated dilutions. Experimental in vitro investigations have demonstrated that HA treatment may regulate different biochemical pathways involved during cartilage damage.23
The knee joint has about 5 to 9 mls of synovial fluid with a pH of 7 to 8. For intra-articular injection, usually 5 ml to 10 ml of LA or 1 ml to 2 ml of GC are used. According to that, dilution of LA with native synovial fluid is approximately 1:2 and dilution of GC with native synovial fluid is approximately 1:10. Since there is some evidence that high concentrations of LA have toxic effects on chondrocytes and tenocytes, it might be wise to inject a lower concentration of LA or dilute it with saline or HA.
Our study has some limitations. When comparing the in vitro results with clinical intra-articular injection, it should be remembered that, for our experiments, cells were isolated from their native extracellular matrix. As shown by Piper and Kim,24 the cytotoxicity of LA is less when using native cartilage with an intact extracellular matrix. This suggests that the cytotoxicity for chondrocytes and tenocytes demonstrated in our study may be reduced in healthy intact cartilage and tendon. Moreover, the amount of time required by the drug for cellular exposure depends on various factors in humans, such as metabolism of the injected drugs or anatomic nature of the joint injected. To investigate further the effects of intra-articular injected drugs on cartilage and tendons in vivo, a small animal model would be sufficient as a next step; this would allow for histological analysis after a series of injections. Additionally, there are new OA protective therapies, like Torin 1. These will need to be assessed prior to clinical application and will also have to be compared with the clinically established drugs for intra-articular injection.25
In conclusion, we found that the cytotoxicity of intra-articular injected drugs towards chondrocytes and tenocytes depends on the concentration of the drug. LA such as lidocaine and bupivacaine demonstrated the highest toxic effects of the drugs investigated. The indication for intra-articular injection should therefore be defined carefully. Also, GCs had toxic effects on chondrocytes and tenocytes with triamcinolone acetonide more than dexamethasone. TA had only minor effects on chondrocytes and tenocytes. TA is applied intra-articularly, to avoid postoperative bleeding following arthroplasty. We can conclude from our results that an intra-articular injection of TA can also be done in native joints. The effect on chondrocytes and tenocytes is comparable with iodine contrast media, which has been used for decades without clinically reported negative effects. HA does not appear to have any adverse effects in vitro.
Footnotes
Author contributions: P. Busse: Collected and analyzed the data, Wrote the manuscript.
C. Vater: Designed the study, Collected and analysed the data, Reviewed and edited the manuscript.
M. Stiehler: Designed the study, Reviewed and edited the manuscript.
J. Nowotny: Collected the tissue samples, Reviewed and edited the manuscript.
P. Kasten: Designed the study, Reviewed and edited the manuscript.
H. Bretschneider: Collected and analyzed the data, Reviewed and edited the manuscript.
S. B. Goodman: Designed the study, Reviewed and edited the manuscript.
M. Gelinsky: Reviewed and edited the manuscript.
S. Zwingenberger: Designed the study, Analyzed the data, Wrote, reviewed, and edited the manuscript.
ICMJE COI statement: The authors declare that there is no conflict of interest regarding the publication of this article.
Follow us @BoneJointRes
Funding statement
We acknowledge support by the Open Access Publication Funds of the SLUB/TU Dresden.
References
- 1. WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium. The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser 2003;919:i–x, 1-218, back cover. [PubMed] [Google Scholar]
- 2. Xu C, Peng H, Li R, et al. Risk factors and clinical characteristics of deep knee infection in patients with intra-articular injections: A matched retrospective cohort analysis. Semin Arthritis Rheum 2018;47:911-916. [DOI] [PubMed] [Google Scholar]
- 3. Dhawan A, Mather RC, III, Karas V, et al. An epidemiologic analysis of clinical practice guidelines for non-arthroplasty treatment of osteoarthritis of the knee. Arthroscopy 2014;30:65–71. [DOI] [PubMed] [Google Scholar]
- 4. Lee Y-J, Kim SA, Lee S-H. Hyaluronan suppresses lidocaine-induced apoptosis of human chondrocytes in vitro by inhibiting the p53-dependent mitochondrial apoptotic pathway. Acta Pharmacol Sin 2016;37:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fitch RW, Kuhn JE. Intraarticular lidocaine versus intravenous procedural sedation with narcotics and benzodiazepines for reduction of the dislocated shoulder: a systematic review. Acad Emerg Med 2008;15:703–708. [DOI] [PubMed] [Google Scholar]
- 6. Chu CR, Izzo NJ, Papas NE, Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy 2006;22:693–699. [DOI] [PubMed] [Google Scholar]
- 7. Yudt MR, Cidlowski JA. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol Endocrinol 2002;16:1719–1726. [DOI] [PubMed] [Google Scholar]
- 8. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs 1999;57:1005–1032. [DOI] [PubMed] [Google Scholar]
- 9. Subramanyam KN, Khanchandani P, Tulajaprasad PV, Jaipuria J, Mundargi AV. Efficacy and safety of intra-articular versus intravenous tranexamic acid in reducing perioperative blood loss in total knee arthroplasty: a prospective randomized double-blind equivalence trial. Bone Joint J 2018;100-B:152–160. [DOI] [PubMed] [Google Scholar]
- 10. Stewart RC, Patwa AN, Lusic H, et al. Synthesis and Preclinical Characterization of a Cationic Iodinated Imaging Contrast Agent (CA4+) and Its Use for Quantitative Computed Tomography of Ex Vivo Human Hip Cartilage. J Med Chem 2017;60:5543–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun S-F, Chou Y-J, Hsu C-W, Chen W-L. Hyaluronic acid as a treatment for ankle osteoarthritis. Curr Rev Musculoskelet Med 2009;2:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;10:CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farkas B, Kvell K, Czömpöly T, Illés T, Bárdos T. Increased chondrocyte death after steroid and local anesthetic combination. Clin Orthop Relat Res 2010;468:3112–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piper SL, Kramer JD, Kim HT, Feeley BT. Effects of local anesthetics on articular cartilage. Am J Sports Med 2011;39:2245–2253. [DOI] [PubMed] [Google Scholar]
- 15. Karpie JC, Chu CR. Lidocaine exhibits dose- and time-dependent cytotoxic effects on bovine articular chondrocytes in vitro. Am J Sports Med 2007;35:1621–1627. [DOI] [PubMed] [Google Scholar]
- 16. Sola M, Dahners L, Weinhold P, et al. The viability of chondrocytes after an in vivo injection of local anaesthetic and/or corticosteroid: a laboratory study using a rat model. Bone Joint J 2015;97-B:933–938. [DOI] [PubMed] [Google Scholar]
- 17. Harada Y, Kokubu T, Mifune Y, et al. Dose-and time-dependent effects of triamcinolone acetonide on human rotator cuff-derived cells. Bone Joint Res 2014;3:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stueber T, Karsten J, Stoetzer C, Leffler A. Differential cytotoxic properties of drugs used for intra-articular injection on human chondrocytes: an experimental in-vitro study. Eur J Anaesthesiol 2014;31:640–645. [DOI] [PubMed] [Google Scholar]
- 19. Sherman SL, Khazai RS, James CH, et al. In vitro toxicity of local anesthetics and corticosteroids on chondrocyte and synoviocyte viability and metabolism. Cartilage 2015;6:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piper SL, Laron D, Manzano G, et al. A comparison of lidocaine, ropivacaine and dexamethasone toxicity on bovine tenocytes in culture. J Bone Joint Surg [Br] 2012;94-B:856–862. [DOI] [PubMed] [Google Scholar]
- 21. Pereira LC, Kerr J, Jolles BM. Intra-articular steroid injection for osteoarthritis of the hip prior to total hip arthroplasty: is it safe? A systematic review. Bone Joint J 2016;98-B:1027–1035. [DOI] [PubMed] [Google Scholar]
- 22. Parker JD, Lim KS, Kieser DC, Woodfield TBF, Hooper GJ. Is tranexamic acid toxic to articular cartilage when administered topically? Bone Joint J 2018;100-B:404–412. [DOI] [PubMed] [Google Scholar]
- 23. Avenoso A, D’Ascola A, Scuruchi M, et al. Hyaluronan in the experimental injury of the cartilage: biochemical action and protective effects. Inflamm Res 2018;67:5–20. [DOI] [PubMed] [Google Scholar]
- 24. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg [Am] 2008;90-A:986–991. [DOI] [PubMed] [Google Scholar]
- 25. Cheng N-T, Guo A, Cui Y-P. Intra-articular injection of Torin 1 reduces degeneration of articular cartilage in a rabbit osteoarthritis model. Bone Joint Res 2016;5:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]