Abstract
Objectives
The aim of this study was to analyze drain fluid, blood, and urine simultaneously to follow the long-term release of vancomycin from a biphasic ceramic carrier in major hip surgery. Our hypothesis was that there would be high local vancomycin concentrations during the first week with safe low systemic trough levels and a complete antibiotic release during the first month.
Methods
Nine patients (six female, three male; mean age 75.3 years (sd 12.3; 44 to 84)) with trochanteric hip fractures had internal fixations. An injectable ceramic bone substitute, with hydroxyapatite in a calcium sulphate matrix, containing 66 mg of vancomycin per millilitre, was inserted to augment the fixation. The vancomycin elution was followed by simultaneously collecting drain fluid, blood, and urine.
Results
The antibiotic concentration in the drain reached a peak during the first six hours post-surgery (mean 966.1 mg/l), which decreased linearly to a mean value of 88.3 mg/l at 2.5 days. In the urine, the vancomycin concentration reached 99.8 mg/l during the first two days, followed by a logarithmic decrease over the next two weeks to reach 0 mg/l at 20 days. The systemic concentration of vancomycin measured in blood serum was low and decreased linearly from 2.17 mg/l at one hour post-surgery to 0 mg/l at four days postoperatively.
Conclusion
This is the first long-term pharmacokinetic study that reports vancomycin release from a biphasic injectable ceramic bone substitute. The study shows initial high targeted local vancomycin levels, sustained and complete release at three weeks, and systemic concentrations well below toxic levels. The plain ceramic bone substitute has been proven to regenerate bone but should also be useful in preventing bone infection.
Cite this article: M. Stravinskas, M. Nilsson, A. Vitkauskiene, S. Tarasevicius, L. Lidgren. Vancomycin elution from a biphasic ceramic bone substitute. Bone Joint Res 2019;8:49–54. DOI: 10.1302/2046-3758.82.BJR-2018-0174.R2.
Keywords: Bone graft substitute, Antibiotics, Vancomycin, Elution, Bone infection, Prevention
Article focus
The aim of this study was to investigate a new commercially available bone substitute that elutes antibiotics, combining an initial high local release with a sustainable antibiotic level for a sufficiently long time to eradicate or prevent infection effectively.
Key messages
This study simultaneously analyzes drain fluid, blood, and urine to follow the long-term release of vancomycin from a biphasic ceramic carrier in major hip surgery.
High levels of vancomycin were observed during the first weeks at the target area (in drain fluid), with safe and low systemic levels (in blood serum) and complete release from the ceramic bone substitute at one month.
Strengths and limitations
Long-term antibiotic release has been followed in a major hip surgical procedure in sedentary patients with a high risk for prosthetic joint infection.
The antibiotic carrier was inserted in non-infected, well-vascularized bone in patients who had sustained a fragility fracture.
Introduction
Local antibiotic use in the treatment of bone infection is an interesting approach due to the advantage of high local concentrations and low systemic concentrations.1 It has led to a good outcome not only in active infections,2-4 but also when used prophylactically.5
Bone and joint infections constitute a significant and costly societal burden. Whether caused by trauma, tumour surgery, or joint arthroplasty, they may require repeated invasive revision surgery and extensive systemic antimicrobial treatment that can last for years. The measurement of antibiotic distribution at infected and non-infected sites is recommended by the United States Food and Drug Administration (FDA).6 The serious consequences of inadequate drug concentration in target tissues include treatment failure and selection pressure for antibiotic-resistant organisms.
There have been major advances in aseptic and antiseptic routines over the years, but approximately a quarter of high-energy open fractures treated with fixation devices may still become infected.1,7,8
Vancomycin is an effective second-choice systemic antibiotic against Gram-positive pathogens involved in bone infections, and is used as an additive in polymeric bone cements for prosthetic joint infection (PJI) revisions.9 Treatments combining systemic antibiotics with local elution from antibiotic-containing bone cement have been shown to decrease the number of revisions.10 In the last few decades, bone cements containing vancomycin have been recommended, especially in the United States, for PJI prevention in preoperatively screened patients carrying methicillin-resistant Staphylococcus aureus (MRSA).11
More efficient antibiotic carriers, in the form of material that is replaced by normal bone, are desired. This could provide complete antibiotic delivery and bone healing without the need for further surgery to remove the polymeric antibiotic carriers.
In vivo studies have shown a preventive and curative effect of using an injectable vancomycin-containing biphasic ceramic in an osteomyelitis model in rabbits,12 but no clinical long-term pharmacokinetic release study has been reported.
The aim of this study was to investigate the antibiotic elution from a new commercially available bone substitute containing vancomycin (CERAMENT V; BoneSupport AB, Lund, Sweden). The product was developed by adding antibiotics to a clinically well-documented bone-regenerating biphasic ceramic bone graft substitute.13-16 It was predicted that the vancomycin concentration would be elevated locally during the first week after implantation, while the systemic concentration of vancomycin would be maintained at a low level.
Materials and Methods
Nine patients (six female, three male) with trochanteric hip fractures, classified as A1 (n = 1) and A2 (n = 8) according to the AO classification, were included in a single-centre, prospective, observational study. Patients with systemic vancomycin usage before surgery, infection in the hip joint, psychiatric or neurological disorders, renal failure, and/or impaired hearing were excluded.
All nine patients were treated with internal fixation using a dynamic hip screw (DHS). The mean age was 75.3 years (sd 12.3; 44 to 84). An injectable ceramic with 40 wt% hydroxyapatite (HA) embedded in a calcium sulphate matrix, containing 66 mg vancomycin per millilitre (CERAMENT V), was used to augment the bone defects at implantation of the DHS. It was injected into the bone defect in the trochanteric region after placing the DHS but before plate implantation.
A drain was placed under fascia on the posterior side along the DHS plate and inserted by a separate incision three centimetres inferior and in line with the surgical incision. It was a closed suction system that was routinely removed on the second day.
None of the patients had systemic vancomycin during the study period. No patient had any kidney disease or renal insufficiency evaluated by creatinine levels and glomerular filtration rate.
All patients had an extended stay for seven days at the hospital after surgery. Drain fluid, urine, and blood serum samples were collected during this time to analyze the vancomycin release from the ceramic bone graft substitute.
The drain fluid was assessed every six hours for 60 hours (2.5 days) postoperatively. The total volume of the drain fluid was measured for each timepoint. A maximum of 10 ml drain fluid was collected for each sample and centrifuged for ten minutes at 2200 × g or 4000 rpm at room temperature. The supernatant was then separated from the rest and deep frozen at -80°C prior to analysis.
Urine was collected daily during the hospital stay (seven days), and thereafter approximately every three days for a month. During the first four days, it was collected from a urinary catheter, following which morning urine was collected. The samples were homogenized, transferred into two 50 ml tubes, and kept cool in a refrigerator.
Blood serum was assessed every hour for the first six hours post-surgery and every six hours (± one hour) thereafter up to 96 hours post-surgery (total of 21 samples per patient). A minimum of 4 ml of blood was drawn at each timepoint and placed in a 5 ml heparin tube. It was centrifuged for ten minutes at 2200 × g, and the supernatant was transferred to two 5 ml polypropylene tubes and deep frozen at -80°C until analysis.
The vancomycin concentrations in all samples (drain fluid, urine, and blood serum) were analyzed using VANC (vancomycin) reagent in conjunction with UniCel DxC 600/800 Systems and SYNCHRON Systems Vancomycin Calibrator set (both Beckman Coulter, Inc., Brea, California). The detection limit without dilution was 0.1 mg/l.
The study was approved by the local independent ethics committee, and informed consent was obtained from all patients.
Results
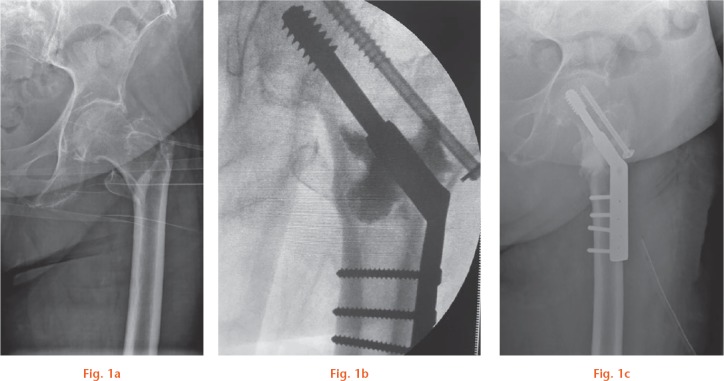
A mean of 9.7 ml (sd 0.7; 8 to 10) of the vancomycin-containing bone substitute was injected into each patient (Figs 1a and 1b).
Fig. 1.
a) Radiograph showing an 85-year-old female patient with a trochanteric fracture (Evan class 4) in her left hip. b) Perioperative fluoroscopy of the same patient taken during injection of the vancomycin containing bone substitute. c) Radiograph of the same patient five weeks postoperatively. There was no change in fracture fixation.
Radiographs were taken immediately postoperatively and at five weeks (Fig. 1). No screw penetration or migration was noted. We did not encounter any wound healing problems. Stitches were removed after two weeks. Eight patients were fully weight-bearing at the last follow-up (between 11 and 13 weeks), and four patients used one crutch.
The elution of vancomycin was followed for up to 60 hours in the drain fluid, for four days in blood, and for 30 days in urine.
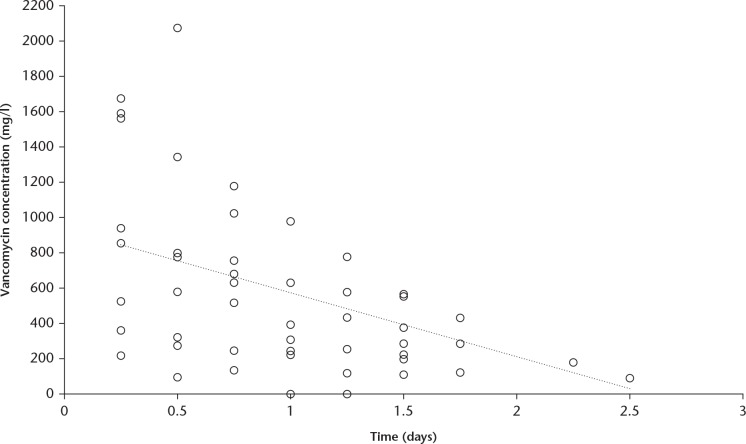
The concentration of vancomycin in the drain fluid reached a peak during the first six hours after surgery, with a mean value of 966.1 mg/l (sd 546.3), which decreased linearly to a mean value of 88.3 mg/l at 2.5 days (Fig. 2). Drain fluid was collected up to 60 hours postoperatively from one patient, up to 42 hours from three patients, up to 36 hours from seven patients, and up to 30 hours from all nine patients.
Fig. 2.
Chart showing the vancomycin concentration measured in the drain fluid after surgery. There was an initial peak followed by a linear decrease. The levels measured in the drain were more than 1000 times higher than those measured in the blood serum and ten times higher than those measured in the urine.
One patient had a myocardial infarction after full weight-bearing on postoperative day 6, and was treated at the intensive care unit and the cardiology unit until she died one month post-surgery. The myocardial infarction was evaluated by the principal investigator (ST) and found to be unrelated to the insertion of the bone graft substitute. This patient was included in the analysis; blood was collected for four days, drain fluid for 1.5 days, and urine for six days.
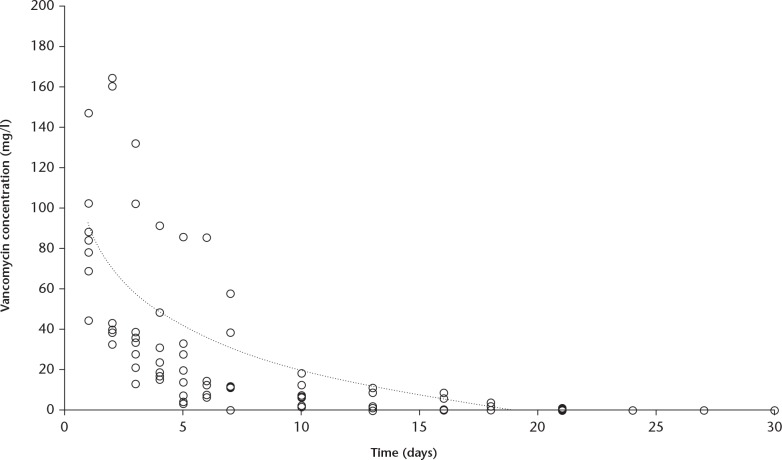
In the urine, the vancomycin concentration reached a mean of 99.8 mg/l (sd 49.8) during the first day, which was maintained during day two (mean 113.2 mg/l (sd 74.8)), followed by a logarithmic decrease over the following two weeks to reach levels below the detection limit (< 0.1 mg/l) at 20 days (Fig. 3). Urine was collected up until day 30 from one patient, day 27 from three patients, and day 24 from eight patients.
Fig. 3.
Chart showing the vancomycin concentration measured in the urine after surgery. There was an initial peak followed by a logarithmic decrease. At approximately 20 days, the vancomycin concentration was below the detection level of 0.1 mg/l.
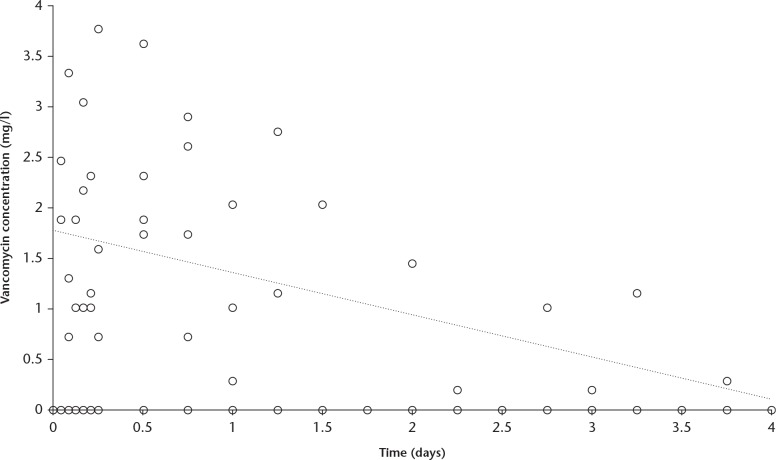
The systemic concentration of vancomycin measured in blood serum was low and decreased linearly from a mean of 2.17 mg/l (sd 0.29) at one hour post-surgery to levels below the detection limit (< 0.1 mg/l) at day 4 post-surgery (Fig. 4). Blood samples were collected until day 4 from all patients.
Fig. 4.
Chart showing the vancomycin concentration measured in the blood serum after surgery. The concentrations remained at a low level and decreased linearly to reach below the detectable concentration (0.1 mg/l) at four days.
Discussion
The vancomycin levels eluted from the ceramic bone substitute were different in the drain fluid, serum, and urine. The highest concentrations were reached in the subcutaneous drain fluid, which was placed close to the implant and therefore regarded as the surrogate local concentration. The local levels measured in the drain fluid were more than 1000 times higher than those in blood during the first week. There was a very high concentration of vancomycin locally, while the systemic concentration of vancomycin was maintained at a safe and low level. The serum levels remained low during the entire study (at least 6.9 times lower than trough levels), indicating that systemic toxicity is unlikely to occur.
Similar observations were made by Wahl et al,17 who concluded that the local application of vancomycin with calcium sulphate as a carrier showed slow release, systemic safety, and a release profile far more interesting than that from poly(methyl methacrylate) (PMMA).
The vancomycin concentration in urine was also initially approximately 100 times higher than in serum, reflecting the fact that levels measured at the implant site were much higher and more sustained than the systemic levels. A detectable vancomycin concentration in urine observed for up to 20 days also indicates prolonged elution of the antibiotic well above minimum inhibitory concentration (MIC) levels. As vancomycin is eliminated in the kidney, the level in urine is a surrogate measure of the local release from the carrier. Vancomycin is approximately 50% protein-bound, with a volume of distribution of 0.4 l/kg to 1.0 l/kg and a β-elimination half-life of three to six hours with normal kidney function. Clearance is linearly related to the glomerular filtration rate.18
It is important that the initial release is high and significantly above the MIC level locally to prevent bacterial adherence leading to an established deep infection.19 The MIC level for most vancomycin-sensitive microorganisms is 2 mg/l.20 In this study, local concentration levels were measured as high as 500 times MIC at six hours, and they were maintained above 200 times MIC for at least 60 hours. This should have allowed for the eradication of any planktonic bacteria that were causing infection and given good preventive support for the bone healing.
Such high local vancomycin concentrations have an effect on biofilms, and it has been shown that even lower local vancomycin concentrations, in which case the vancomycin would be combined with rifampicin, may also have an effect on established Staphylococcus aureus biofilm infection. Zimmerli and Sendi21 reported that the combination of systemic rifampicin (1 mg/l to peak 8 mg/l) and vancomycin (3 mg/l to peak 9 mg/l) was much more effective than monotherapy against biofilm infection in clinical practice.
Recent in vitro studies have not been able to verify the synergistic effect on established biofilm, but the discrepancy may be explained by the systemic peak levels achieved clinically with vancomycin.22,23
Previous studies carried out with the same ceramic bone substitute containing gentamicin showed similar release results.24,25 A high concentration of antibiotics was observed in the drain fluid (i.e. locally), and the concentration observed in the serum never exceeded the corresponding MIC level of 4 mg/l.24 There was also a prolonged antibiotic elution detected in the urine.24 When compared with antibiotic elution from PMMA, the ceramic bone substitute was more efficient, with a higher peak observed during the first couple of days.25 The ceramic bone substitute also presented a higher maintained antibiotic concentration during the first ten days, demonstrating effective prevention of bacterial attachment to the bone bed. The elution from the ceramic bone substitute has been shown not to be dependent only on the surface area, as the elution occurs from the bulk of the entire material as well.24 This is in contrast to PMMA, which is a compact material without minimal microporosity and therefore elutes the antibiotics mainly from the surface.26 Antibiotic particles are embedded in the bulk of PMMA and may give rise to the long-term elution of sub-inhibitory antibiotic concentrations with the risk of inducing antibiotic resistance.27
The ceramic bone substitute consists of HA and calcium sulphate, where the vancomycin does not bind chemically, or electrostatically, to the HA. The elution of vancomycin will therefore be controlled by the microporosity of the material (approximately 30%) and ultimately by the resorption rate of the calcium sulphate phase. In this study, it was not possible to detect vancomycin in the urine more than 20 days post-surgery. However, it is probable that the antibiotic accumulates in the surrounding tissue of the defect and that concentrations above MIC will be locally present for the ensuing weeks, depending on the degree of vascularization of the area.28
In conclusion, this is the first long-term pharmacokinetic report on vancomycin release from a biphasic injectable ceramic bone substitute. The study shows initial high targeted local vancomycin levels (wound drain fluid), sustained and complete release during the first month (verified by the urine concentrations), and systemic concentrations that are well below toxic levels. This material should be useful in preventing bone infection and regenerating new bone tissue.
Footnotes
Author contributions: M. Stravinskas: Performed the surgical part of the study, Edited the manuscript.
M. Nilsson: Collected and analyzed the data, Created all figures, Wrote the manuscript.
A. Vitkauskiene: Performed all chemical analyses in the study, Edited the manuscript.
S. Tarasevicius: Performed and supervised the surgical part of the study, Edited the manuscript.
L. Lidgren: Coordinated and supervised the study, Wrote and edited the manuscript.
ICMJE COI statement: M. Nilsson reports payment from LaliT AB for writing this manuscript, as well as consultancy fees from LaliT AB not related to this study. L. Lidgren reports board membership of, patents with, and stock options with BoneSupport SE not related to this study, as well as board membership of and stock options with Orthocell AU not related to this study.
Ethical review statement: The study was approved by the local ethical committee at the Lithuanian University of Health, Kaunas, Lithuania (No. B-2-44) and written informed consent to participate in the study was obtained from all participants.
Follow us @BoneJointRes
Supplementary Material
Tables showing the long-term release of vancomycin from a biphasic ceramic carrier in blood serum, drain fluid, and urine in major hip surgery.
Funding statement
This work was supported by the Swedish Research Council, grant number VR 2015-06717, VINNOVA innovation agency in Sweden (grant 2017-00269) and Stiftelsen för bistånd åt rörelsehindrade i Skåne, Sweden.
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Cancienne JM, Burrus MT, Weiss DB, Yarboro SR. Applications of local antibiotics in orthopedic trauma. Orthop Clin North Am 2015;46:495-510. [DOI] [PubMed] [Google Scholar]
- 2. Ostermann PA, Henry SL, Seligson D. The role of local antibiotic therapy in the management of compound fractures. Clin Orthop Relat Res 1993;295:102-111. [PubMed] [Google Scholar]
- 3. Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg [Br] 1995;77-B:93-97. [PubMed] [Google Scholar]
- 4. Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local antibiotic therapy in osteomyelitis. Semin Plast Surg 2009;23:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wininger DA, Fass RJ. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob Agents Chemother 1996;40:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. No authors listed. Statement from FDA Commissioner Scott Gottlieb, M.D., on FDA’s efforts to foster discovery and development of new tools to fight antimicrobial-resistant infections. U.S. Food & Drug Administration (FDA), June 2018. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm610503.htm (date last accessed 12 December 2018).
- 7. Jenkinson RJ, Kiss A, Johnson S, Stephen DJG, Kreder HJ. Delayed wound closure increases deep-infection rate associated with lower-grade open fractures: a propensity-matched cohort study. J Bone Joint Surg [Am] 2014;96-A:380-386. [DOI] [PubMed] [Google Scholar]
- 8. Messner J, Papakostidis C, Giannoudis PV, Kanakaris NK. Duration of administration of antibiotic agents for open fractures: meta-analysis of the existing evidence. Surg Infect (Larchmt) 2017;18:854-867. [DOI] [PubMed] [Google Scholar]
- 9. Anagnostakos K. Therapeutic use of antibiotic-loaded bone cement in the treatment of hip and knee joint infections. J Bone Jt Infect 2017;2:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Espehaug B, Engesaeter LB, Vollset SE, Havelin LI, Langeland N. Antibiotic prophylaxis in total hip arthroplasty. Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg [Br] 1997;79-B:590-595. [DOI] [PubMed] [Google Scholar]
- 11. Loc-Carrillo C, Wang C, Canden A, Burr M, Agarwal J. Local intramedullary delivery of vancomycin can prevent the development of long bone staphylococcus aureus infection. PLoS One 2016;11:e0160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lidén E, Sandell V, Kasioptas A, Lindberg F. In vitro characterization of a vancomycin eluting injectable bone graft substitute with examination of concomitant bone remodeling in a rabbit model [abstract]. EBJIS Conference, 2014. https://www.bonesupport.com/assets/PDFs/lindberg-in-vitro-characterisation-of-a-vancomycin-eluting-injectable-bone-graft-substitute.pdf (date last accessed 30 October 2018).
- 13. Abramo A, Geijer M, Kopylov P, Tägil M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J Biomed Mater Res B Appl Biomater 2010;92:281-286. [DOI] [PubMed] [Google Scholar]
- 14. Karr JC. Clinical case presentation: metatarsal delayed union management in a diabetic patient with cerament™ bone void filler. J Diabetic Foot Complications 2010;2;65-68. [Google Scholar]
- 15. Iundusi R, Gasbarra E, D’Arienzo M, Piccioli A, Tarantino U. Augmentation of tibial plateau fractures with an injectable bone substitute: CERAMENT™. Three year follow-up from a prospective study. BMC Musculoskelet Disord 2015;16:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nilsson M, Wang J-S, Wielanek L, Tanner KE, Lidgren L. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg [Br] 2004;86-B:120-125. [PubMed] [Google Scholar]
- 17. Wahl P, Guidi M, Benninger E, et al. The levels of vancomycin in the blood and the wound after the local treatment of bone and soft-tissue infection with antibiotic-loaded calcium sulphate as carrier material. Bone Joint J 2017;99-B:1537-1544. [DOI] [PubMed] [Google Scholar]
- 18. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther 2017;102:459-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howlin RP, Brayford MJ, Webb JS, et al. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 2015;59:111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. No authors listed. Clinical breakpoints and dosing of antibiotics. European Committee on Antimicrobial Susceptibility Testing (EUCAST). EUCAST Clinical Breakpoint Table v. 8.1. 2018. http://www.eucast.org/clinical_breakpoints/ (date last accessed 12 December 2018).
- 21. Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS 2017;125:353-364. [DOI] [PubMed] [Google Scholar]
- 22. Dall GF, Tsang SJ, Gwynne PJ, et al. Unexpected synergistic and antagonistic antibiotic activity against Staphylococcus biofilms. J Antimicrob Chemother 2018;73:1830-1840. [DOI] [PubMed] [Google Scholar]
- 23. Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 2007;5:48-56. [DOI] [PubMed] [Google Scholar]
- 24. Stravinskas M, Horstmann P, Ferguson J, et al. Pharmacokinetics of gentamicin eluted from a regenerating bone graft substitute: in vitro and clinical release studies. Bone Joint Res 2016;5:427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stravinskas M, Nilsson M, Horstmann P, et al. Antibiotic containing bone substitute in major hip surgery: a long term gentamicin elution study. J Bone Jt Infect 2018;3:68-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walenkamp G. Small PMMA beads improve gentamicin release. Acta Orthop Scand 1989;60:668-669. [DOI] [PubMed] [Google Scholar]
- 27. Hendriks JG, van Horn JR, van der Mei HC, Busscher HJ. Backgrounds of antibiotic-loaded bone cement and prosthesis-related infection. Biomaterials 2004;25:545-556. [DOI] [PubMed] [Google Scholar]
- 28. Törholm C, Lidgren L, Lindberg L, Kahlmeter G. Total hip joint arthroplasty with gentamicin-impregnated cement. A clinical study of gentamicin excretion kinetics. Clin Orthop Relat Res 1983;181:99-106. [PubMed] [Google Scholar]