Abstract
Rat pancreatic acinar cells possess only the p21-activated kinase (PAKs), PAK4 of the group II PAK, and it is activated by gastrointestinal hormones/neurotransmitters stimulating PLC and by a number of growth factors. However, little is known generally of cAMP agents causing PAK4 activation, and there are no studies with gastrointestinal hormones/neurotransmitters activating cAMP cascades. In the present study, we examined the ability of VIP and secretin, which stimulate cAMP generation in pancreatic acini, to stimulate PAK4 activation, the signaling cascades involved, and their possible role in activating sodium-potassium adenosine triphosphatase (Na+,K+-ATPase). PAK4 activation was compared with activation of the well-established cAMP target, cyclic AMP response element binding protein (CREB). Secretin-stimulated PAK4 activation was inhibited by KT-5720 and PKA Type II inhibitor (PKI), protein kinase A (PKA) inhibitors, whereas VIP activation was inhibited by ESI-09 and HJC0197, exchange protein directly activated by cAMP (EPAC) inhibitors. In contrast, both VIP/secretin-stimulated phosphorylation of CREB (pCREB) via EPAC activation; however, it was inhibited by the p44/42 inhibitor PD98059 and the p38 inhibitor SB202190. The specific EPAC agonist 8-CPT-2-O-Me-cAMP as well 8-Br-cAMP and forskolin stimulated PAK4 activation. Secretin/VIP activation of Na+,K+-ATPase, was inhibited by PAK4 inhibitors (PF-3758309, LCH-7749944). These results demonstrate PAK4 is activated in pancreatic acini by stimulation of both VIP-/secretin-preferring receptors, as is CREB. However, they differ in their signaling cascades. Furthermore, PAK4 activation is needed for Na+,K+ATPase activation, which mediates pancreatic fluid secretion. These results, coupled with recent studies reporting PAKs are involved in both pancreatitis/pancreatic cancer growth/enzyme secretion, show that PAK4, similar to PAK2, likely plays an important role in both pancreatic physiological/pathological responses.
NEW & NOTEWORTHY Pancreatic acini possess only the group II p21-activated kinase, PAK4, which is activated by PLC-stimulating agents/growth factors and is important in enzyme-secretion/growth/pancreatitis. Little information exists on cAMP-activating agents stimulating group II PAKs. We studied ability/effect of cyclic AMP-stimulating agents [vasoactive intestinal polypeptide (VIP), secretin] on PAK4 activity in rat pancreatic-acini. Both VIP/secretin activated PAK4/CREB, but the cAMP signaling cascades differed for EPAC, MAPK, and PKA pathways. Both hormones require PAK4 activation to stimulate sodium-potassium adenosine triphosphatase activity. This study shows PAK4 plays an important role in VIP-/secretin-stimulated pancreatic fluid secretion and suggests it plays important roles in pancreatic acinar physiological/pathophysiological responses mediated by cAMP-activating agents.
Keywords: p21-activated kinase, PAK4, pancreatic acini, secretin, VIP
INTRODUCTION
The p21-activated kinases (PAKs) are serine/threonine protein kinases that mediate many of the effects of small GTPases: cell control division protein (Cdc42) and Ras-related C3 botulinum toxin substrate (Rac1) (41, 56). PAKs are divided into two groups based on their similarities in structure and homology sequence: group I (PAK1–3) and group II (PAK4–6) (41, 56, 87). The group I PAKs play important roles in signaling cascades involved in regulation of cell survival, cell motility, tumorigenesis, protein synthesis, glucose homeostasis, secretion, and cellular proliferation (41, 56, 79). Group II kinases are reported to play important roles in cytoskeletal dynamics, cell growth, and neoplastic development and its progression (1, 16, 27, 41, 54, 55). However, little is known of the role of group II PAKs in many physiological or pathological processes (55). In recent studies, only one group I PAK (PAK2) and one group II PAK (PAK4) were found in pancreatic acini, and in each case, they were activated by gastrointestinal (GI) hormones/neurotransmitter activating PLC and a number of GI/pancreatic growth factors (48, 55). Both activation of PAK2 and PAK4 in pancreatic acinar cells are reported to be important for enzyme secretion and growth, as well as important for mediating experimental pancreatitis (42, 47, 48, 55).
However, group I PAKs in a number of tissues are activated by cAMP-stimulating agents (2, 4, 37, 44, 71, 78), little information exists in the ability of cAMP-activating agents to activate group II PAKs. No information exists on whether cAMP-activating GI hormones/neurotransmitters can activate group II PAKs, and there are no studies on these stimulant’s effects on PAK4 in pancreatic acinar cells. With a number of GI hormones/neurotransmitters, such as vasoactive intestinal polypeptide (VIP) and secretin, their primary signaling cascade in most tissues is activation of adenylate cyclase (29), including in pancreatic acinar cells, which possess both VIP- and secretin-preferring receptors (5, 24, 33, 35, 57, 59). Therefore, in the present study we examined the ability of the adenylate cyclase activating hormones, VIP and secretin, to activate the group II PAK, PAK4, in pancreatic acinar cells, the signaling cascades involved, and determined whether its activation is needed for stimulation of sodium-potassium adenosine triphosphatase (Na+,K+-ATPase) in these cells, which is essential for mediating stimulation of pancreatic fluid secretion (9, 32, 53).
MATERIALS AND METHODS
Male Sprague-Dawley rats (100–120 g) were obtained from the Small Animals Section, Veterinary Resources Branch, National Institutes of Health (NIH), Bethesda, MD. Stabilized goat anti-rabbit IgG peroxidase conjugated was from Pierce Biotechnology (Rockford, IL). PAK4 (P-21), anti-goat-horseradish peroxidase-conjugated antibodies, 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate sodium salt (8-CPT-2-Me-cAMP), and PAK4 antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit cyclic AMP response element binding protein (CREB) and rabbit anti-phospho-CREB (Ser133), rabbit anti-Na+,K+-ATPase, rabbit anti-phospho Na+,K+-ATPase (Tyr10), rabbit anti-p44/42 rabbit anti-phospho p44/42 (T202/Y204), rabbit anti-p38 rabbit anti-phospho p38 (T180/Y182), and rabbit anti-α/β-tubulin were from Cell Signaling (Beverly, MA). Tris·HCl (pH 8.0 and 7.5) were from Mediatech (Herndon, VA). ESI-09 and protein kinase A (PKA) inhibitor 14–22 Amide PKA Type II inhibitor (PKI) were from Millipore (Billerica, MA). KT-5720 was from Abcam (Cambridge, MA). 2-mercaptoethanol, protein assay solution, sodium lauryl sulfate (SDS), and Tris-glycine-SDS (10×) were from Bio-Rad (Hercules, CA). MgCl2, CaCl2, Tris·HCl 1 M pH 7.5, and Tris-glycine buffer (10×) were from Quality Biological (Gaithersburg, MD). Minimal essential media vitamin solution, amino acids 100×, Dulbecco’s phosphate buffered saline, glutamine (200 mM), 4–20% Tris-glycine gels, and l-glutamine were from Invitrogen (Carlsbad, CA). COOH-terminal octapeptide of cholecystokinin (CCK-8), vasoactive intestinal peptide (VIP), and secretin were from Bachem Bioscience (King of Prussia, PA). Forskolin was from Calbiochem (Burlington, MA). Trypsin inhibitor, HEPES, Tween 20, Triton X-100, GFX (GFX109203X), phenylmethanesulfonylfluoride, ethylenediaminetetraacetic acid, ethylene glycol tetraacetic acid, sucrose, sodium-orthovanadate, sodium azide, SP600125, ouabain, and 8-bromoadenosine 3′,5′-cyclic monophosphate sodium salt (8-Br-cAMP) were from Sigma-Aldrich (St. Louis, MO). Albumin standard, protein G agarose beads, and Super Signal West (Pico, Dura) chemiluminescent substrate were from Pierce (Rockford, IL). Protease inhibitor tablets were from Roche (Basel, Switzerland). Purified collagenase (type CLSPA) was from Worthington Biochemicals (Freehold, NJ). Nitrocellulose membranes were from Schleicher and Schuell Bioscience (Keene, NH). Albumin bovine fraction V was from MP Biomedical (Solon, OH). NaCl, KCl, and NaH2PO4 were from Mallinckrodt (Paris, KY). Nonfat milk Ominlok was purchased from AmericanBio (Natick, MA). PF-3758309 was from APRxBIO (Houston, TX). N2-(3-methoxyphenyl)-N4-[(oxolan-2-yl)methyl]quinazoline-2,4-diamine (LCH-7749944) was from Moltport Gets molecules delivered (Riga, LV). ATPase Colorimetric Assay Kit was from Novus Biologicals (Littleton, CO). PD98059 and SB202190 were from APExBIO (Houston, TX). HJC0197 was from Cayman Chemical (Ann Arbor, MI). ADP-GLO Kinase Assay and PAK4 Kinase Enzyme System were from Promega (Madison, WI). ADP-GLO Kinase Assay + PAK4 Kinase Enzyme System was from Promega.
Pancreatic acini isolation and stimulation.
All animal experiments were approved by the Animal Ethics Committee of the National Institutes of Health and carried out in accordance with the international guiding principles for animal research. Pancreatic acini were obtained by collagenase digestion as described previously (47, 70, 73, 82). After collagenase digestion, dispersed acini were preincubated in standard incubation solution for 2 h at 37°C (47, 70, 73, 82). After preincubation, 1-ml aliquots of dispersed acini were incubated at 37°C with or without stimulants, and then cells were lysed, sonicated, and lysates were centrifuged at 10,000 g for 15 min at 4°C as described previously (47, 55, 70, 82). Protein concentration was measured using the Bio-Rad protein assay reagent.
Inhibition experiments.
Preincubation with ESI-09 and HJC0197, exchange proteins directly activated by cAMP 1 and 2 (EPAC1 and 2) inhibitors; KT-5720 and PKI, PKA inhibitors; PF-3758309 and LCH-7749944, PAK4 inhibitors; adenosine; and three phosphate (ATP)-competitive inhibitors of PAK4 was performed (47, 55) to identify downstream effects of secretin- or VIP-mediated activation of PAK4 and cAMP-response element-binding protein (CREB). Isolated acini preincubated for 1 h with ESI-09, HJC0197, KT-5720, and PKI or 3 h with PF-3758309 or LCH-7749944 and then treated for 15 min with 10 nM secretin or 10 nM VIP. Untreated cells were used as controls. After incubation, cells were processed as below in Western blot analysis.
To select appropriate concentrations of inhibitors, without previous data in the literature for our experimental conditions, we performed preliminary dose-response curves (10, 30, or 100 µM) and time courses (15, 60, or 90 min) of two different EPAC inhibitors, ESI-09 and HJC0197, and two different PKA inhibitors, KT-5720 and PKI (data not shown). These results demonstrated that the maximal inhibitory effect of ESI-09 was at 100 µM, for KT-5720 and PKI at 10 µM, after 60 min of incubation, and for HJC0197 at 10 µM for 90 min. These results agree with those from different studies in different cells (13, 31, 58). For the other inhibitors used, we used conditions and concentrations similar to those previously reported in similar experimental conditions. Specifically, a previous study in pancreatic acini (55) demonstrated that for PD98059, a p44/42 inhibitor, SB202190, a p38 inhibitor, PF-3758309 and LCH-7749944, both PAK4 inhibitors, inhibitory effects were well seen at 10 µM, 10 µM, 0.1 nM, and 30 µM, respectively. For the JNK inhibitor, SP600125, 20 µM was used in in pancreatic β-cells (14) and in rat pancreatic fragments (62).
Western blot analysis.
Western blot analysis was performed as described previously (47, 55). Briefly, cell lysates were separated on 4–20% Tris-glycine gels and transferred to nitrocellulose membranes. After membranes were blocked in buffer containing 50 mM Tris·HCl (pH 8.0), 2 mM CaCl2, 80 mM NaCl, 0.05% Tween 20, and 5% nonfat dry milk, membranes were then incubated with primary antibody overnight at 4°C under constant agitation at antibody dilutions suggested by the supplier. Membranes were washed twice in blocking buffer and then incubated with horseradish peroxidase-conjugated secondary antibody (anti-rabbit, anti-goat) according to the species of the first antibody. Protein bands were measured using GeneTools software from Syngene, which were assessed in the linear detection range.
Coimmunoprecipitation.
Coimmunoprecipitation (Co-IP) studies were performed as previously described (74). Briefly, cell lysates (700 µg/ml) were incubated with 4 µg/l of PAK4 antibody and with 25 µl of protein G-agarose at 4°C overnight. The immunoprecipitates were washed with phosphate-buffered saline and used for the measurement of PAK4 kinase activity.
PAK4 kinase activity assay.
Kinase assays were performed on the immunoprecipitates using the ADP-GLO Kinase Assay from Promega for assessing PAK4 activity according to the manufacturer’s instructions. Briefly, the immunoprecipitates were incubated at 37°C for 15 min in 25 µl of kinase buffer, containing 1 mM dithiothreitol-0.1% BSA and ultrapure ATP (Promega). Reactions were stopped with the addition of 25 μl ADP-GLO reagent (Promega) for 40 min at room temperature. Then, 50 µl of kinase detection reagent were added and were incubated for another 30 min at room temperature. Plates were read using a TECAN infinite M200 reader (TECAN) with an integration time of 1 s per well.
Na+,K+-ATPase activation.
Na+,K+-ATPase activation was assessed by using two approaches. First, Na+,K+-ATPase activity was measured using a colorimetric assay as described previously (86). Second, phosphorylation was assessed by a Western blot assay (M&M 2.2.3.) with a specific antibody, rabbit anti-phospho Na+,K+-ATPase (Tyr10) as previously reported (22, 66). Briefly, isolated pancreatic acini were incubated in reaction buffer, containing 50 mM Tris, 2.5 mM MgCl2, and 0.5 mM ATP. The total ATPase activity was assayed in the reaction mixture. Na+,K+-ATPase-specific activity was calculated by subtracting the total ATPase activity from the ATPase activity assayed in the presence of 1 mM ouabain (Na+,K+ pump inhibitor). The enzyme activity was determined by the amount of inorganic phosphate released from the substrate ATP. The inorganic phosphate concentrations were read using a TECAN infinite M200 reader (TECAN) at a wavelength of 595 nm.
Statistical analysis.
Data from at least three different experiments are presented as means ± SE and were analyzed with the nonparametric Kruskal-Wallis analysis (because the data were not normally distributed) using the GraphPad Prism 6.0 software. P values <0.05 were considered significant.
RESULTS
Ability of VIP and secretin to stimulate PAK4 serine 474 phosphorylation in rat pancreatic acinar cells.
A previous study (55) demonstrated that PAK4, a member of the group II PAKs, was activated by pancreatic secretagogues/neurotransmitters which activated PLC, as well as by endothelin 1. It is unknown if PAK4 can be activated by pancreatic secretagogues/neurotransmitters that activate adenylate cyclase (5, 35, 91). To address this question, rat pancreatic acini were incubated in the absence and presence of VIP and secretin, both pancreatic secretagogues known to activate adenylate cyclase and increase cellular cAMP in pancreatic acini (5, 33, 35, 91). PAK4 activation was assessed by studying the phosphorylation of pS474, which has been shown to be essential for PAK4 protein kinase activity and has been widely used to assess its activation in other studies (7, 21, 54, 55). Both VIP and secretin stimulated an increase in PAK4 serine 474 phosphorylation (149 ± 7 and 138 ± 17% of control, respectively, P < 0.05 vs. control; Fig. 1A). To confirm that the increase in PAK4 phosphorylation stimulated by VIP and secretin was reflecting an increase in kinase activity as reported in a number of other studies (7, 21, 54, 55), we assessed the effect of VIP and secretion directly on PAK4 kinase activity (45). VIP and secretin significantly increased PAK4 activity by 30% (130 ± 3 and 129 ± 2% of control, respectively, P < 0.05 vs. control; Fig. 1B).
Fig. 1.
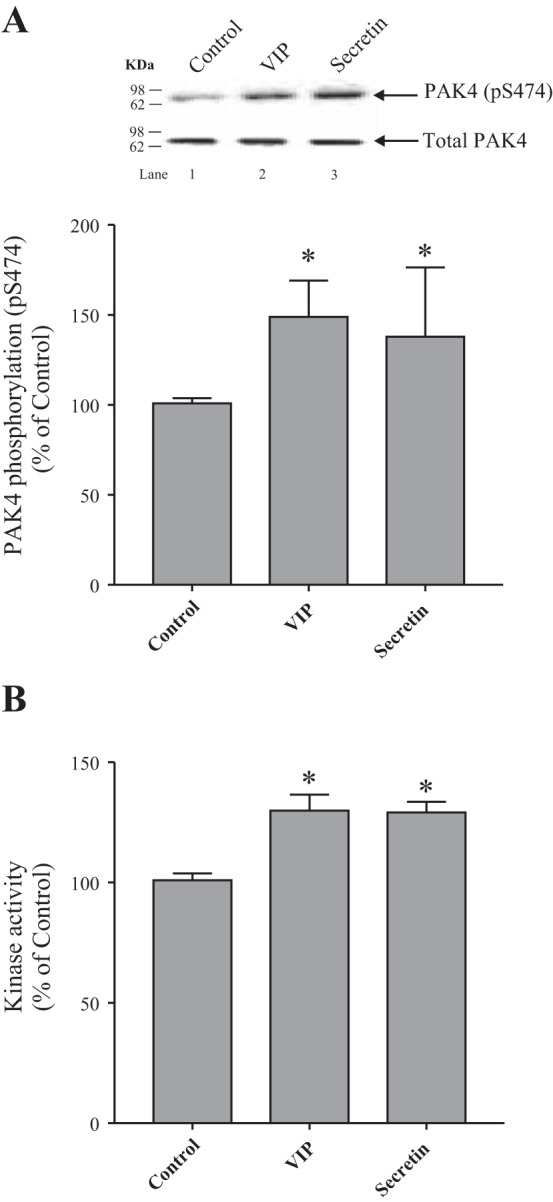
Ability of VIP and secretin to stimulate PAK4 activation assessed by measuring PAK4 phosphorylation (pS474) or PAK4-kinase activity in rat pancreatic acini. A: isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM) or secretin (10 nM) for 15 min and then lysed. Results are expressed as percentages of control phosphorylation. The cell lysates were subjected to Western blot analysis and analyzed using anti-pS474 PAK4 and, as loading control, antitotal PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 6 independent experiments are shown. Bottom: means ± SE of 6 independent experiments. *P < 0.05 compared with the control. B: isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM) or secretin (10 nM) for 15 min, lysed, and then immunoprecipitated. Results are expressed as percent of ADP produced, after 30 min of incubation (Luminescence, RLU: control, 28,864; VIP, 21,314; and secretin, 17,342). Means ± SE of 4 independent experiments. *P < 0.05 compared with the control. PAK, p21-activated kinase; RLU, relative light unit; VIP, vasoactive intestinal polypeptide.
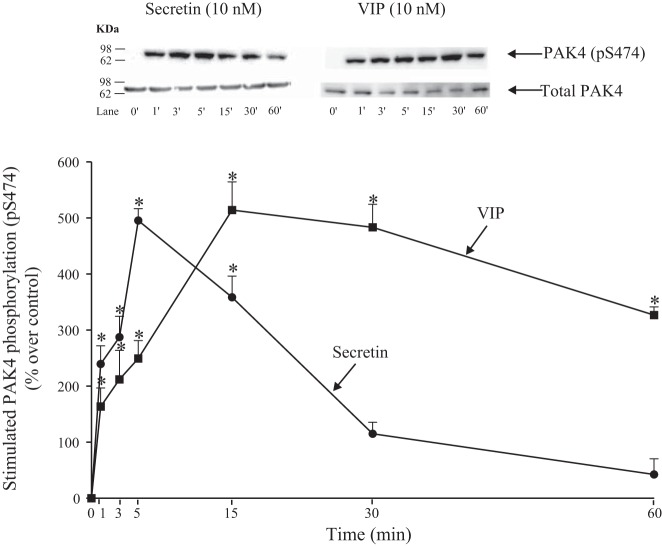
Time course of VIP and secretin stimulation of S474 PAK4 phosphorylation.
Stimulation of S474 PAK4 by VIP and secretin was time-dependent (Fig. 2). VIP produced a rapid increment detected at 0–5 min with a maximum increment after a 15-min incubation time (615 ± 50% of control, P < 0.05 vs. control; Fig. 2). Although the stimulation then slowly decreased over time, it was still present at 60 min (427 ± 15% of control, P < 0.05 vs. control; Fig. 2). Secretin also stimulated a rapid increase by 0.5 min (P < 0.05 vs. control; Fig. 2), but stimulation rapidly decreased and was not maintained for longer than 15 min.
Fig. 2.
Time course of VIP and secretin stimulation of PAK4 S474 phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM) or secretin (10 nM) for the indicated times and then lysed. Western blots were analyzed using anti-pS474 PAK4 and, as loading control, antitotal PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of 3 independent experiments. Results are expressed as percentages of stimulation over the control group. *P < 0.05 compared with the control group (i.e., 0 time). PAK, p21-activated kinase; VIP, vasoactive intestinal polypeptide.
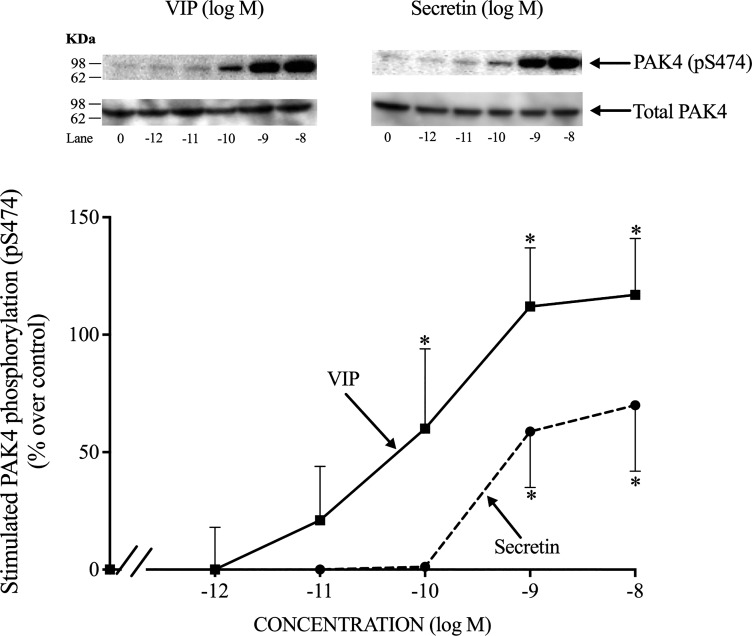
Dose-response effect of VIP and secretin on S474 PAK4 phosphorylation.
Increasing concentrations of VIP and secretin caused increasing stimulation of PAK4 activation, with detectable stimulation at 0.1 nM (Fig. 3) and with maximal stimulation at 10 nM VIP or secretin (217 ± 24 and 170 ± 28% of control, P < 0.05 vs. control, respectively) (Fig. 3). VIPs caused a half-maximal effect at 0.091 ± 0.010 nM (Fig. 3), and the half-maximal effect of secretin was 0.478 ± 0.100 nM (Fig. 3); therefore, secretin was five times less potent than VIP. A 10 nM concentration of both VIP and secretin produced the maximal increase in PAK4 activation, therefore, they had similar efficacies. This maximally effective concentration was used in the next part of the study.
Fig. 3.
Dose-response effect of VIP and secretin to stimulate PAK4 kinase phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of VIP and secretin (at the indicate concentrations) for 15 min and then lysed. Western blots were analyzed using anti-pS474 PAK4 antibody and, as loading control, antitotal PAK4. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of 3 independent experiments. Results are expressed as percentages of stimulation over the control group (VIP 10 nM: 217 ± 24% of control). *P < 0.05 compared with the control group. PAK, p21-activated kinase; VIP, vasoactive intestinal polypeptide.
Effect of pos-receptor stimulants, 8-Br-cAMP or forskolin, on S474 PAK4 kinase phosphorylation.
To investigate further the ability of stimulants to activate the cAMP pathway and stimulate PAK4 activation, both 8-Br-cAMP and forskolin, postreceptor activators of the cAMP signaling cascade, were studied at concentrations known to activate the cAMP signaling cascade in pancreatic acini (24, 35, 77). Both 8-Br-cAMP and forskolin increased PAK4 activation (472 ± 85 and 549 ± 67% of control, respectively, P < 0.05 vs. control; Fig. 4), and this degree of PAK4 activation was comparable to that seen with maximal effective concentrations of VIP or secretin (55). Cholecystokinin-8, which activates the PLC cascade, caused a threefold increase in PAK4 activation (326 ± 29% of control, P < 0.05 vs. control; Fig. 4), and it was twofold less efficacious than the cAMP-activating agents (Fig. 4).
Fig. 4.
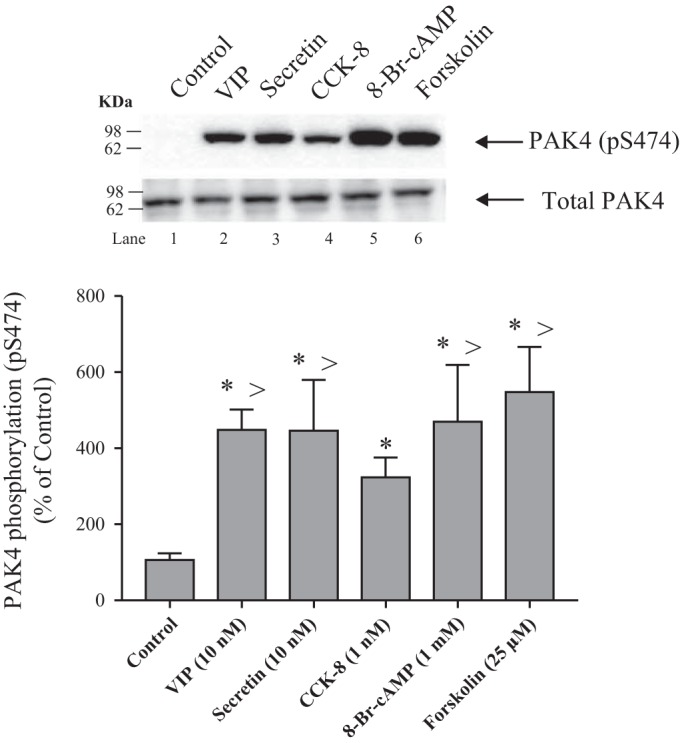
Effect of VIP, secretin, CCK-8, 8-Br-cAMP, and forskolin on PAK4 phosphorylation (pS474) in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM), secretin (10 nM), 8-Br-cAMP (1 mM) or forskolin (25 µM) for 15 min and CCK-8 (1 nM) for 3 min, and then lysed. Results are expressed as percentages of control phosphorylation. The cell lysates were subjected to Western blot analysis and analyzed using anti-pS474 PAK4 and, as loading control, antitotal PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 4 independent experiments are shown. Bottom: means ± SE of 4 independent experiments. *P < 0.05 compared with the control. >P < 0.05 compared with CCK-8 alone. 8-Br-cAMP, 8-Bromoadenosine 3′,5′-cyclic monophosphate sodium salt; CCK-8, cholecystokinin 8; PAK, p21-activated kinase; VIP, vasoactive intestinal polypeptide.
Effects of ESI-09 and HJC0197, EPAC inhibitors, and KT-5720 and PKI, PKA inhibitors, on PAK4, CREB, p44/42, and p38 phosphorylation.
Numerous studies in both pancreatic acini and other tissues demonstrate (12, 51, 58, 85) that cellular increases in cAMP can result in activation of PKA or EPAC. Additionally, numerous older studies demonstrate that in most tissues, activation of PKA is the main mechanism for stimulating CREB phosphorylation (67, 80, 85). Therefore, we compared the effect of secretin and VIP stimulation of PKA or EPAC activation on stimulation of pS474 PAK4 phosphorylation under the same experimental conditions and to their effect on pS133 CREB phosphorylation. To perform these studies, pancreatic acini were preincubated with the selective EPAC inhibitors ESI-09 (100 µM) and HJC0197 (10 µM and 2 h) (13, 31, 58, 92), or the selective PKA inhibitors, KT-5720 (10 µM) and PKI (10 µM) for 1 h (12, 52), and then, treated with secretin (10 nM) or VIP (10 nM) for 15 min (Figs. 5 and 6).
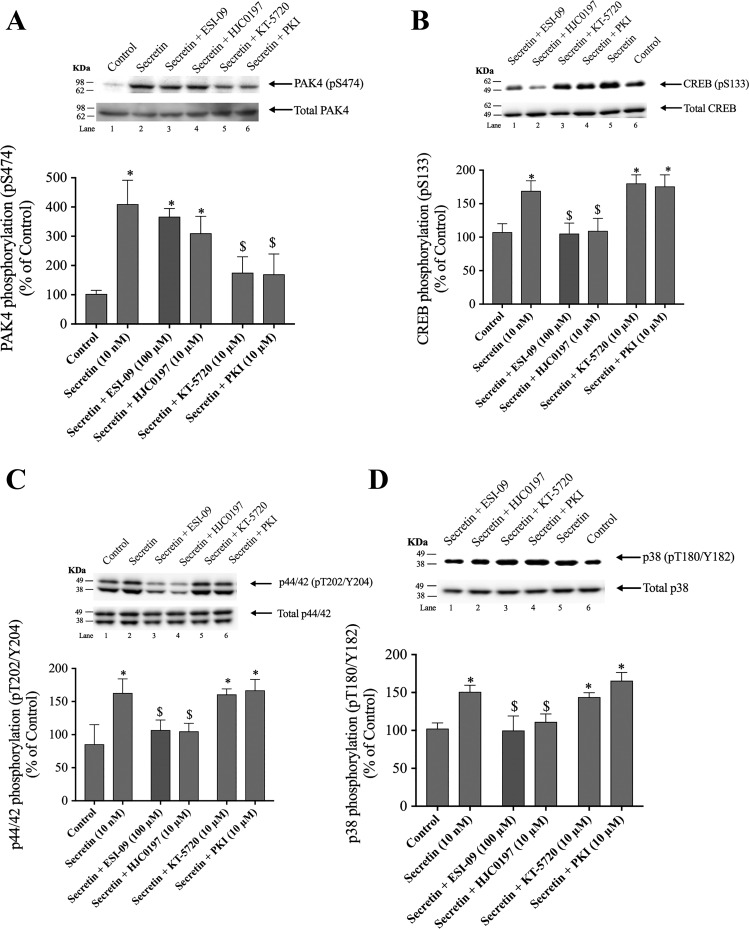
Fig. 5.
Effect of ESI-09 and HJC0197, EPAC inhibitors, and KT-5720 and PKI, PKA inhibitors, on PAK4 activation (A), CREB phosphorylation (B), p44/42 phosphorylation (C), and p38 phosphorylation (D) by secretin in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of ESI-09 (100 µM), HJC0197 (10 µM, 2h), KT-5720 (10 µM), or PKI (10 µM) for 1 h and then incubated with no addition (control) or secretin (10 nM) for 15 min, and then lysed. Western blots were analyzed using anti-pS474 PAK4, pS133 CREB, pT202/Y204 p44/42, or pT180/Y182 p38 and, as loading control, antitotal PAK4, antitotal CREB, antitotal p44/42, and antitotal p38. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of at least 4 independent experiments. Results are expressed as percentages of basal stimulation of the control group. *P < 0.05 compared with the control group; $P < 0.05 compared with secretin alone. PAK, p21-activated kinase; PKI, PKA inhibitor.
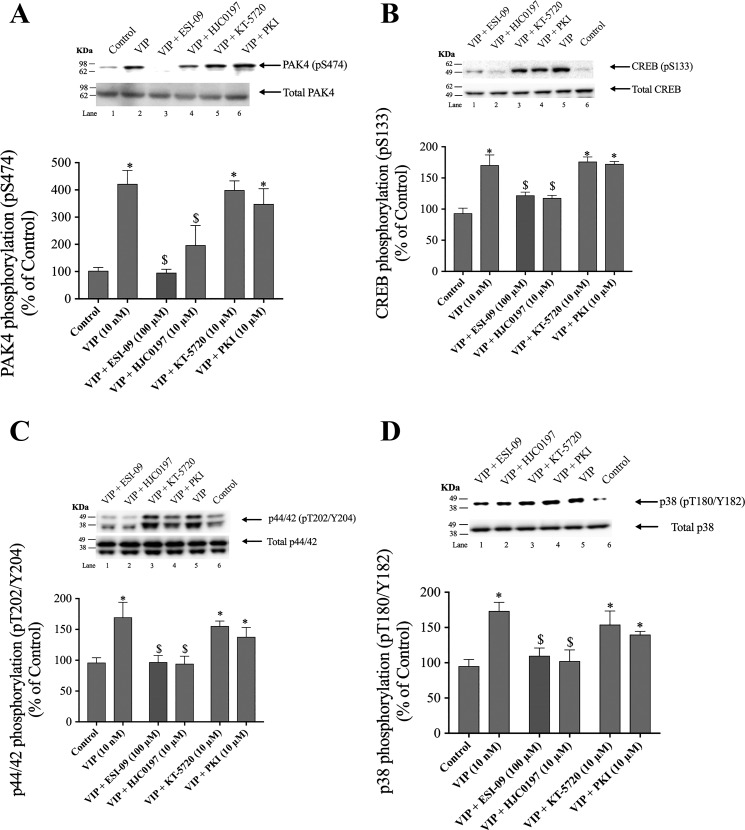
Fig. 6.
Effect of ESI-09 and HJC0197, EPAC inhibitors, and KT-5720 and PKI, a PKA inhibitors, on PAK4 activation (A), CREB phosphorylation (B), p44/42 phosphorylation (C), and p38 phosphorylation (D) by VIP in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of ESI-09 (100 µM), HJC0197 (10 µM, 2 h), KT-5720 (10 µM) or PKI (10 µM) for 1 h and then incubated with no addition (control) or VIP (10 nM) for 15 min, and then lysed. Western blots were analyzed using anti-pS474 PAK4, pS133 CREB, pT202/Y204 p44/42 or pT180/Y182 p38 and, as loading control, antitotal PAK4, antitotal CREB, antitotal p44/42, and antitotal p38. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of at least 4 independent experiments. Results are expressed as percentages of basal stimulation of the control group. *P < 0.05 compared with the control group; $P < 0.05 compared with VIP alone. PAK, p21-activated kinase; PKI, PKA inhibitor; VIP, vasoactive intestinal polypeptide.
Preincubation with both PKA inhibitors, KT-5720 and PKI, reduced the secretin-induced phosphorylation of PAK4 by 75–77% (secretin: 410 ± 37% of control; KT-5720 + secretin: 175 ± 27% of control; PKI + secretin: 170 ± 34% of control, P < 0.05; Fig. 5A, lanes 5 and 6). However, the incubation with ESI-09 and HJC0197 did not alter the increase in PAK4 phosphorylation stimulated by secretin (367 ± 14 and 310 ± 34% of control, P < 0.05 vs. control; Fig. 5A, lanes 3 and 4). In contrast, preincubation with the EPAC inhibitors ESI-09 and HJC0197 but not with KT-5720 or PKI decreased by 85–93% secretin-stimulated CREB phosphorylation (secretin: 169 ± 4%; secretin + ESI-09: 105 ± 6% of control; secretin + HJC0197: 110 ± 6% of control) (Fig. 5B, lanes 1 and 2). Preincubation with the EPAC inhibitors decreased secretin-stimulated p44/42 and p38 phosphorylation by 92–95 and 78–98%, respectively (Fig. 5, C and D, lanes 3 and 4). Neither of the PKA inhibitors had an effect on p44/42 or p38 phosphorylation (Fig. 5, C and D, lanes 5 and 6). In the case of VIP, preincubation with ESI-09 and HJC0197, inhibited completely VIP-induced PAK4 phosphorylation (Fig. 6A, lanes 3 and 4) as well as inhibited VIP-stimulated CREB phosphorylation by 85–93% (122 ± 3 and 123 ± 2% of control, respectively; Fig. 6B, lanes 1 and 2). However, neither VIP-induced PAK4 nor VIP-induced-CREB phosphorylation was affected by KT-5720 or PKI (Fig. 6, A and B). Preincubation with the EPAC inhibitors decreased VIP-stimulated p44/42 and p38 phosphorylation by 89–92 and 86–97%, respectively (Fig. 6, C and D, lanes 1 and 2). Neither of the PKA inhibitors had an effect on p44/42 or p38 phosphorylation (Fig. 6, C and D). These results demonstrate that under the experimental conditions used, PAK4 activation was primarily modulated by a PKA-dependent process with secretin and through an EPAC-dependent process by VIP. In contrast, both secretin- and VIP- induced CREB, p44/42, and p38 phosphorylation were mediated by an EPAC-dependent mechanism and did not require PKA activation.
Time course of 8-Br-cAMP and 8-CPT-2-O-Me-cAMP stimulation of S474 PAK4 phosphorylation and 8-CPT-2-O-Me-cAMP stimulation of S133 CREB activation.
To study in more detail the kinetics of activation of PAK4 by the different cAMP pathways, we next examined the time-dependent activation of PAK4 by 8-Br-cAMP, a nonspecific cAMP analog that has high affinity for both PKA and EPAC (15, 61) and by 8-CPT-2-Me-cAMP, a selective EPAC activator (Figs. 7 and 8) (12, 13, 31, 58, 61, 65). The cAMP analog, 8-Br-cAMP, produced a significant increase after a 15-min incubation time, reaching the maximum after 30 min (880 ± 77% of control, P < 0.05 vs. control; Fig. 7), which was maintained even up to 120 min. Although 8-CPT-2-Me-cAMP also produced a significant increase and a maximal effect after 30 min (558 ± 86% of control, P < 0.05 vs. control; Fig. 7), this increase was twofold lower than that seen with 8-Br-cAMP. Furthermore, the 8-CPT-2-Me-cAMP stimulation of pS474 PAK4 phosphorylation decreased over time more rapidly than seen with 8-Br-cAMP, and it was no longer maintained after 60 min (Fig. 7).
Fig. 7.
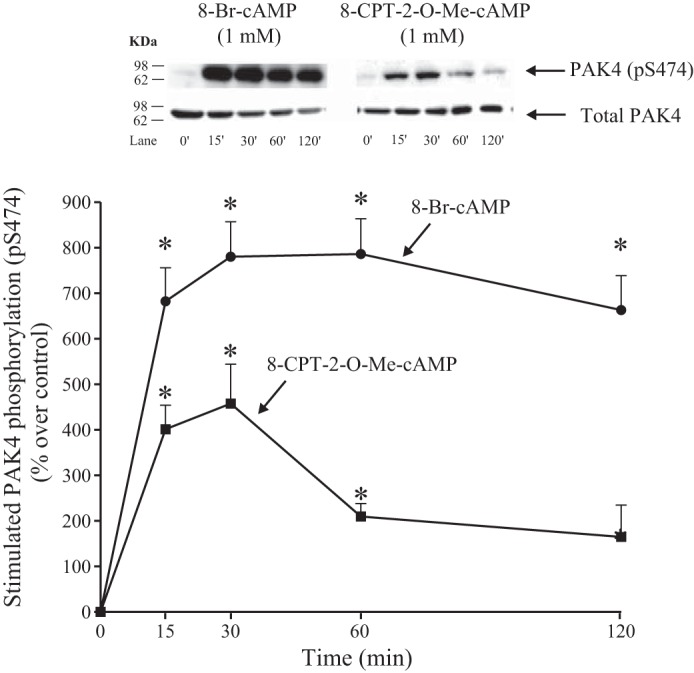
Time course of 8-Br-cAMP and 8-CPT-2-O-Me-cAMP stimulation of PAK4 S474 phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of 8-Br-cAMP (1 mM) or 8-CPT-2-O-Me-cAMP (1 mM) for the indicated times and then lysed. Western blots were analyzed using anti-pS474 PAK4 and, as loading control, antitotal PAK4. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of 3 independent experiments. Results are expressed as percentages of stimulation over the control group. *P < 0.05 compared with the control group (i.e., 0 time). 8-Br-cAMP, 8-Bromoadenosine 3′,5′-cyclic monophosphate sodium salt; 8-CPT-2-O-Me-cAMP, 8-(4-Chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate sodium salt.
Fig. 8.
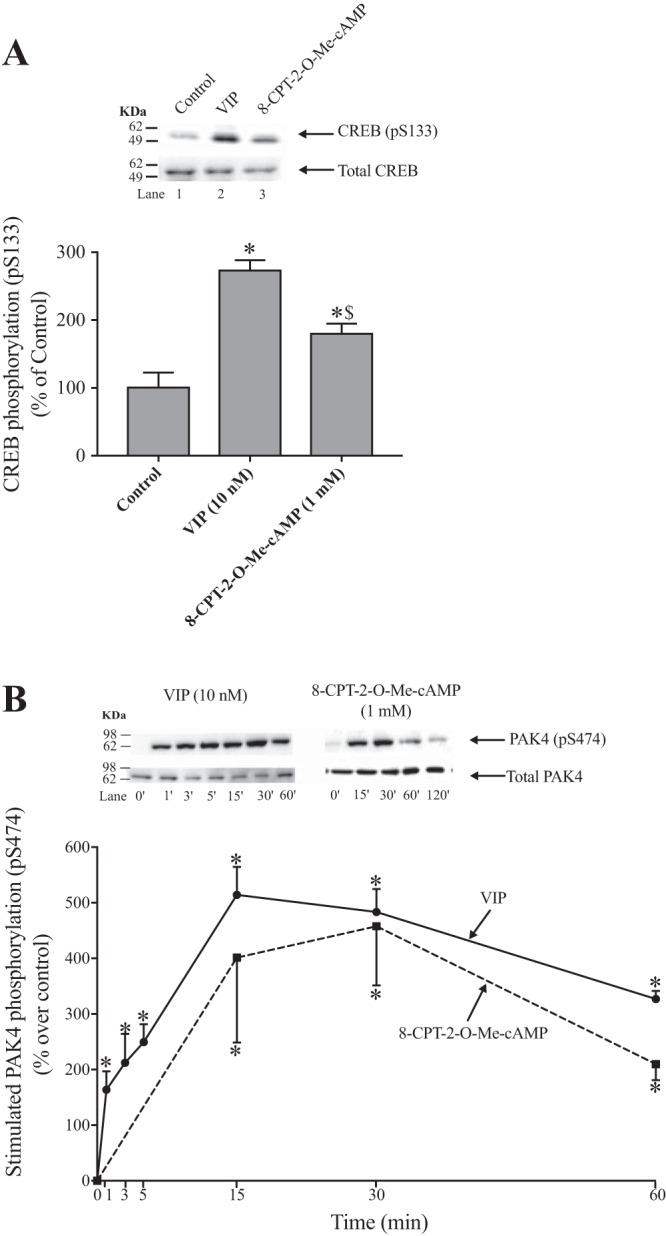
Effect of VIP and 8-CPT-2-O-Me-cAMP on CREB phosphorylation (pS133) (A) and time course of VIP and 8-CPT-2-O-Me-cAMP stimulation of PAK4 S474 phosphorylation (B) in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM) or 8-CPT-2-O-Me-cAMP (1 mM) for 15 min (A) and at the indicated times (B) and then lysed. Western blots were analyzed using anti-pS474 PAK4 or pS133 CREB and, as loading control, antitotal PAK4 or antitotal CREB. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of 3 independent experiments. Results are expressed as percentages of control phosphorylation in (A) and as percentages of stimulation over the control group in (B). *P < 0.05 compared with the control group (i.e., 0 time); $P < 0.05 compared with VIP alone. This experiment was performed at the same time and with the same cells used in the experiments shown in Fig. 7. It was performed under the same conditions and in the same assay as the data in Fig. 7, but the time course was only to 60 min in Fig. 8B and to 120 min in Fig. 7 and the sampling times for VIP were at shorter times in Fig. 8B. In both figures the data of 8-CPT-2-Me-cAMP is the same and the representative blot is the same in both cases up to 60 min. 8-CPT-2-O-Me-cAMP, 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate sodium salt; PAK, p21-activated kinase; VIP, vasoactive intestinal polypeptide.
To investigate further the ability of VIP and 8-CPT-2-Me-cAMP to also stimulate CREB phosphorylation through the cAMP pathway, we examined the ability of these two stimulants to stimulate pS133 CREB phosphorylation (Fig. 8A). VIP and 8-CPT-2-Me-cAMP increased CREB phosphorylation; however, VIP increased CREB activation by a 53% greater amount compared with 8-CPT-2-Me-cAMP (274 ± 15 and 181 ± 14% of control, respectively, P < 0.05 vs. control; Fig. 8A).
To compare in more detail the ability of VIP and 8-CPT-2-Me-cAMP, which both stimulate PAK4 activation through the EPAC pathway, we compared in the same experiment the time courses of their abilities to stimulate PAK4 phosphorylation (Fig. 8B). Both stimulated a maximal increase after a 15-min incubation time; both also stimulated similar increases in PAK4 phosphorylation after 30 min of incubation (VIP: 583 ± 41% and 8-CPT-2-Me-cAMP: 558 ± 106% of control, P < 0.05 vs control; Fig. 8B) and after 60 min of incubation. The results demonstrated both EPAC stimulants had similar kinetics in activating PAK4.
Effects of PD98059 (p44/42 inhibitors), SB202190 (p38 inhibitor), and SP600125 (c-Jun N-terminal kinase inhibitor), in CREB and PAK4 phosphorylation.
In some recent studies in other tissues, various stimulants can activate MAPKs by a cAMP-mediated mechanism resulting in activation of CREB (13, 25, 72). To investigate further the role of p44/42 mitogen-activated protein kinases (p44/42), p38 mitogen-activated protein kinase (p38), or c-Jun NH2-terminal kinase (JNK) activation in VIP- and secretin-stimulated mediating PAK4 and CREB phosphorylation, we first studied the need for secretin’s or VIP’s possible activation of p44/42, p38, or JNK activation in resulting pS474 PAK4 or pS133 CREB phosphorylation. To perform these studies, pancreatic acini were preincubated with PD98059 (10 µM; a p44/42 inhibitor), SB202190 (10 µM; a p38 inhibitor) (6, 63), or SP600125 (20 µM; a JNK inhibitor), for 1 h, and then treated with secretin (10 nM) or VIP (10 nM) for 15 min (Fig. 9).
Fig. 9.
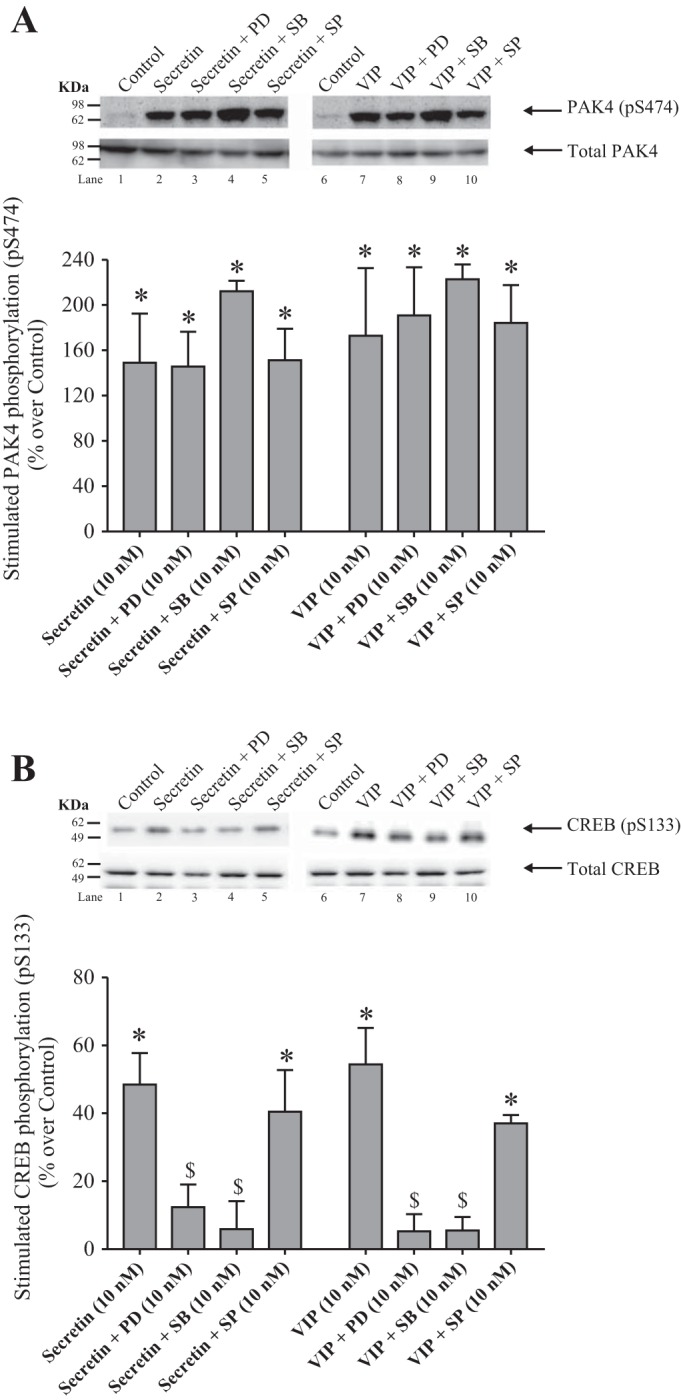
Effect of PD98059 (PD), a p44/42 inhibitor, SB202190 (SB), a p38 inhibitor, and SP600125 (SP), a JNK inhibitor, on PAK4 phosphorylation (A) and on CREB phosphorylation (B) by secretin and VIP in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of PD98059 (10 µM), SB202190 (10 µM) or SP600125 (20 µM) for 1 h and then incubated with no addition (control), secretin (10 nM) of VIP (10 nM) for 15 min and then lysed. Western blots were analyzed using anti-pS474 PAK4 or pS133 CREB and, as loading control, antitotal PAK4 or antitotal CREB. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of at least 4 independent experiments. Results are expressed as percentages of stimulation over the control group. *P < 0.05 compared with the control group; $P < 0.05 compared with secretin or VIP alone. PD, PD98059; SB, SB202190; SP, SP600125. JNK, c-Jun N-terminal kinase; PAK, p21-activated kinase; VIP, vasoactive intestinal polypeptide.
In the case of PAK4 activation, the p44/42, p38 or JNK inhibitors (PD98059, SB202190, and SP600125, respectively) did not affect the secretin- or VIP-induced PAK4 phosphorylation (Fig. 9A). However, preincubation with the p44/42 and p38 inhibitors, PD98059 and SB202190, reduced the secretin-induced phosphorylation of CREB by 80 and 94%, respectively (Fig. 9B, lanes 3 and 4). In the case of VIP, preincubation with PD98059 or SB202190, inhibited completely VIP-induced CREB phosphorylation by 96% (Fig. 9B, lanes 8 and 9). However, the incubation with the JNK inhibitor did not decrease the CREB phosphorylation stimulated by secretin or VIP (Fig. 9B, lanes 5 and 10). These results demonstrate that under the experimental conditions used CREB phosphorylation but not PAK4 activation was primarily modulated by a p44/42- and p38-dependent process by secretin and VIP.
Time course of VIP and secretin stimulation of T202/Y204 p44/42 and T180/Y182 p38 phosphorylation.
Stimulation of T202/Y204 p44/42 and T180/Y182 p38 by VIP and secretin was time-dependent (Fig. 10). In the case of p44/42 activation, secretin and VIP produced a stimulation after 10 min, reaching a maximum after 30 min (164 ± 7 and 168 ± 8% of control, respectively, P < 0.05 vs. control; Fig. 10A), and this was maintained until 60 min. Moreover, secretin and VIP caused a time-dependent stimulation of p38 phosphorylation, with a similar pattern to that observed with p44/42 activation, reaching maximum at 30 min (164 ± 8 and 146 ± 8% of control, P < 0.05 vs. control; Fig. 10B).
Fig. 10.
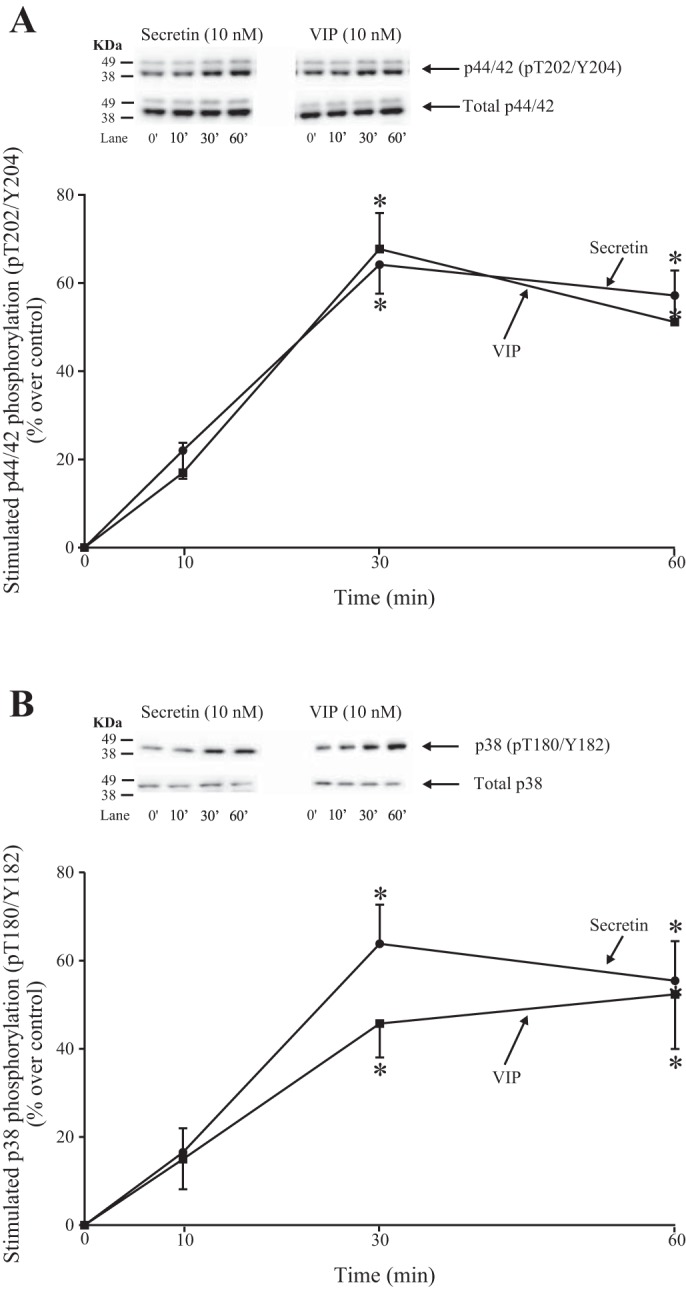
Time course of VIP and secretin stimulation of p44/42 T202/Y204 phosphorylation (A) and p38 T180/Y182 phosphorylation (B) in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM) or secretin (10 nM) for the indicated times, and then lysed. Western blots were analyzed using anti-pT202/Y204 p44/42 or anti-pT180/Y182 p38 and, as loading control, antitotal p44/42 or p38. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of 3 independent experiments. Results are expressed as percentages of stimulation over the control group. *P < 0.05 compared with the control group (i.e., 0 time). VIP, vasoactive intestinal polypeptide.
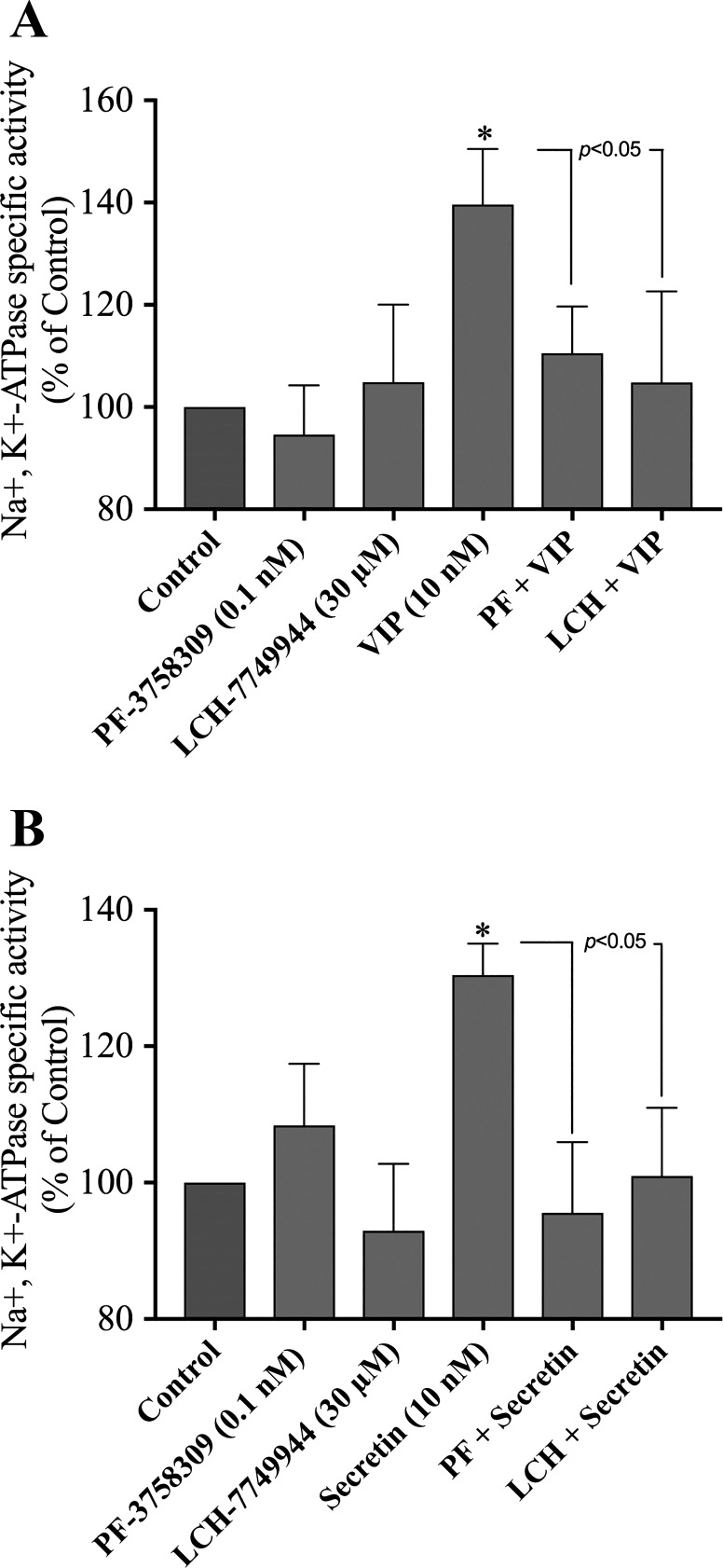
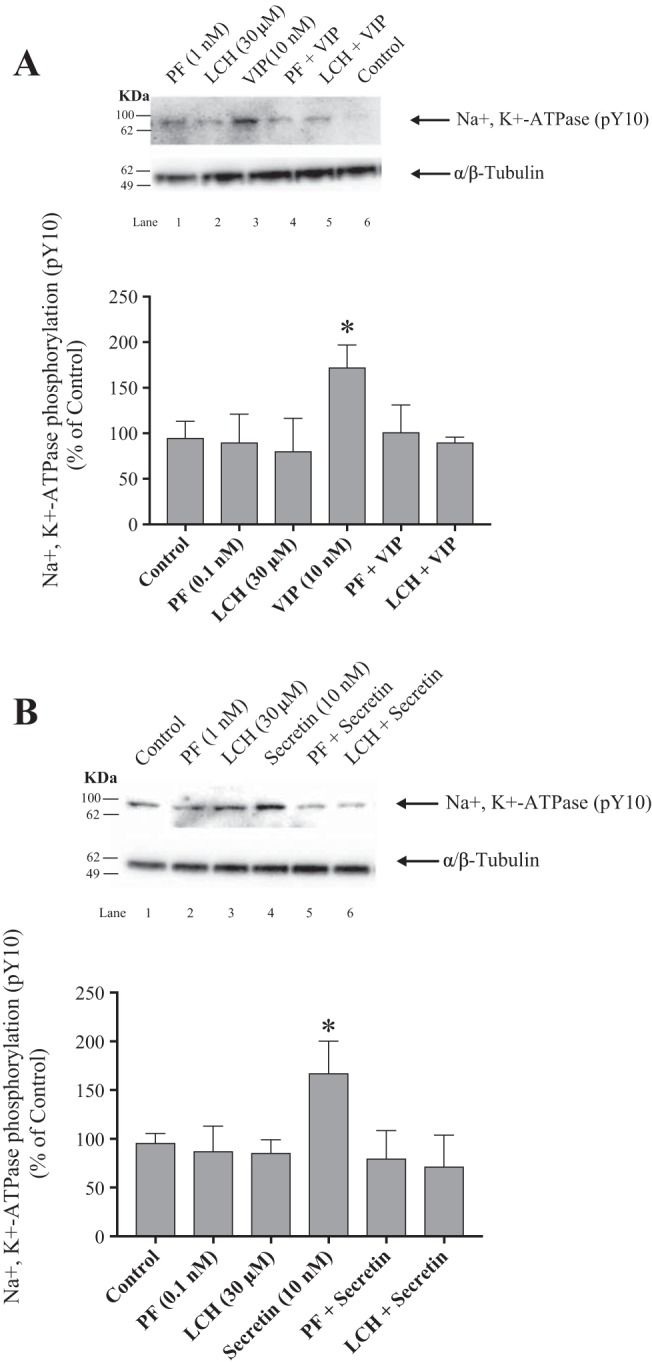
Effects of PF-3758309 and LCH-7749944, PAK4 inhibitors, on Na+,K+-ATPase activity.
One of the main physiological functions of secretin and VIP in the pancreas is to stimulate pancreatic fluid secretion, which is dependent on activation of Na+,K+-ATPase (9, 32, 34, 49, 64). To investigate the possible effect of PAK4 directly on Na+,K+-ATPase activation, we assessed hormonal activation of Na+,K+-ATPase activation by incubating pancreatic acini with the PAK4 inhibitors, PF-3758309 (0.1 nM) and LCH-7749944 (30 µM), and with ouabain (Na+,K+ pump inhibitor as control, 1 mM) for 3 h and then stimulated with VIP (10 nM) or secretin (10 nM) for 15 min.
VIP stimulated Na+,K+-ATPase activity by 39% compared with ouabain alone. Ouabain and PF-3758309 or ouabain and LCH-7749944 completely inhibited the VIP-induced ATPase activity (110 ± 5 and 105 ± 12% of control, respectively (Fig. 11A).
Fig. 11.
Effect of PF-3758309 and LCH-7749944, PAK4 inhibitors, on VIP (A) and secretin (B) stimulation of Na+,K+-ATPase activity in rat pancreatic acini. Isolated pancreatic acini were incubated in the presence of ouabain (used as a control, 1 mM), PF-3758309 (0.1 nM), LCH-7749944 (30 µM) for 3 h, and then incubated with no addition (control), VIP (10 nM) or secretin (10 nM) for 15 min. of Na+,K+-ATPase activity was assayed as described in Materials and Methods with the control being 1 mM ouabain alone and stimulated activity with VIP or secretin with ouabain present. Results are the results of the average of at least 3 independent experiments. Results are expressed as percentages of seen with ouabain alone, i.e., control. *P < 0.05 compared with the control group. LCH, LCH-7749944; PAK, p21-activated kinase; PF, PF-3758309; VIP, vasoactive intestinal polypeptide.
Secretin increased the Na+,K+-ATPase activity by 30% compared with ouabain alone (ouabain + secretin: 130 ± 5% of control; Fig. 11B). Moreover, the presence of ouabain and PF-3758309 or ouabain and LCH-7749944 completely inhibited the secretin-induced ATPase activity (Fig. 11B). Preincubation with ouabain and PF-3758309 or ouabain and LCH-7749944 did not affect the basal level of Na+,K+-ATPase activity (Fig. 11, A and B). These results demonstrate that VIP- and secretin-mediated Na+,K+-ATPase activity is dependent on PAK4 activation.
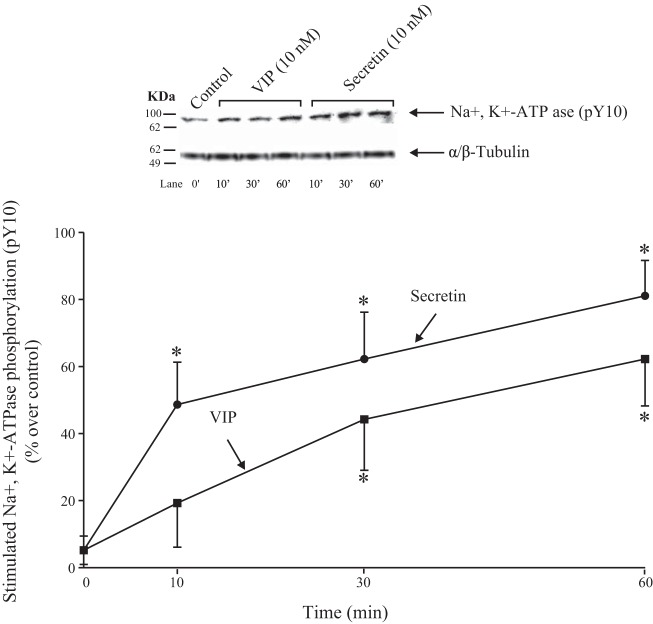
Time course of secretin and VIP stimulation of Y10 Na+,K+-ATPase phosphorylation.
A number of studies report that Na+,K+-ATPase ability can be regulated by phosphorylation (8, 39, 80). Tyr10 phosphorylation of the α subunit of Na+,K+-ATPase is reported to be increased with activation of the Na+,K+-ATPase by a number of stimulants (22, 66). Therefore, we first determined the ability of VIP and secretin to stimulate pY10 Na+,K+-ATPase phosphorylation with its activation. Secretin produced significant stimulation after 10 min, reaching a maximum after 30 min (162 ± 14% of control, P < 0.05 vs. control; Fig. 12), and this was maintained until 60 min. VIP caused a time-dependent stimulation of Na+,K+-ATPase phosphorylation, with similar pattern to that observed with secretin, reaching maximum at 30 min (144 ± 15% of control, P < 0.05 vs. control; Fig. 12). However, VIP was a 11% less efficacious at stimulating Na+,K+-ATPase phosphorylation than secretin at the three different times incubation was studied (10, 30, and 60 min) (Fig. 12).
Fig. 12.
Time course of VIP and secretin stimulation of Na+,K+-ATPase Y10 phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of VIP (10 nM) or secretin (10 nM) for the indicated times, and then lysed. Western blots were analyzed using anti-pY10 Na+,K+-ATPase and, as loading control, anti-α/β-Tubulin. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of 3 independent experiments are shown. Bottom: means ± SE of 4 independent experiments. Results are expressed as percentages of stimulation over the control group. *P < 0.05 compared with the control group (i.e., 0 time). VIP, vasoactive intestinal polypeptide.
Effects of PF-3758309 and LCH-7749944, PAK4 inhibitors, on secretin-stimulated Na+,K+-ATPase activation.
To study further the role of PAK4 in hormonal activation of Na+,K+-ATPase, we determined the effect of PF-3758309 and LCH-7749944, two widely used inhibitors of PAK4 (46, 55, 60, 90) (Fig. 13) on Y10 phosphorylation stimulated by VIP/secretin. Preincubation with PF-3758309 or LCH-7749944, completely inhibited both the VIP- (Fig. 13A top) and secretin-induced (Fig. 13B, bottom) phosphorylation of Na+,K+-ATPase. Neither of the PAK4 inhibitors had an effect on basal Na+,K+-ATPase phosphorylation (Fig. 13, A and B, lanes 1 and 3). These results demonstrate that VIP- and secretin-mediated Na+,K+-ATPase phosphorylation is dependent on PAK4 activation.
Fig. 13.
Effect of PF-3758309 and LCH-7749944, PAK4 inhibitors, on VIP (A) and secretin (B) stimulation of Na+,K+-ATPase Y10 phosphorylation in rat pancreatic acini. Isolated pancreatic acini were incubated in the absence or presence of PF-3758309 (0.1 nM), LCH-7749944 (30 µM) for 3 h and then incubated with no addition (control), VIP (10 nM) or secretin (10 nM) for 15 min, and then lysed. Western blots were analyzed using anti-pY10 Na+,K+-ATPase and, as loading control, anti-α/β-Tubulin. Bands were visualized using chemiluminescence and quantified by densitometry. Top: results of a representative blot of at least 3 independent experiments are shown. Bottom: means ± SE of 4 independent experiments. Results are expressed as percentages of basal stimulation of the control group. *P < 0.05 compared with the control group. LCH, LCH-7749944; PF, PF-3758309; VIP, vasoactive intestinal polypeptide.
DISCUSSION
In general, the activation of PAK4 by tyrosine kinase receptors or by G-protein-coupled receptors mediating changes in PKC or other non-cAMP mediators has been widely described. However, there are no studies on the ability of GI hormone/neurotransmitters, and only a few with other agents, on the ability of cAMP-activating agents to mediate PAK4 activation. Previous studies demonstrate that rat pancreatic acinar cells possess both secretin-preferring and VIP-preferring receptors and that both are coupled to adenylate cyclase (5, 33, 35, 59). Furthermore, studies demonstrate that increases in cAMP in numerous tissues, including pancreatic acini, can activate both PKA, as well as the exchange protein directly activated by cAMP (EPAC) (12, 51, 85). A number of our results support the conclusion that activation of adenylate cyclase resulting in increases in cellular cAMP by either VIP- or secretin-preferring receptors in pancreatic acinar cells can result in PAK4 activation. First, VIP and secretin over concentration ranges known to occupy only VIP- or secretin-preferring receptors, respectively (5, 33, 36), stimulated PAK4 activation. Second, the nonhydrolyzable cAMP analog, 8-Br-cAMP, as well as forskolin, both postreceptor activators of the cAMP pathway, at concentrations known to activate this cascade in pancreatic acini (11, 24, 35, 69); stimulated PAK4 activation and the degree of activation was comparable to that seen with secretin or VIP. Our results have both similarities and differences from the results of the few studies of PAK activation and cyclic AMP in other tissues. Similar to our findings, forskolin can activate PAK4 in papillary thyroid cells (84) and in prostate cancer cells (50), whereas in human mesangial cells, both 8-Br-cAMP and forskolin activated Cdc42, which is the principal upstream activator of PAK4 (10, 27). Although there are no studies on the ability of VIP or secretin to activate PAK4, our results in pancreatic acini on activation of these two receptors are similar to studies reporting activation of some G-protein-coupled receptors, such as those for β-adrenergic agents, prostaglandins, and α-MSH, can stimulate PAK activation via cAMP in a number of different cells (2, 3, 89). Our results with PAK4 activation by secretin and VIP differ from results with the group I PAK, PAK2, in rat pancreatic acini, which is not activated by either VIP or secretin; however, it is activated by PLC-activating GI hormones/neurotransmitters (47, 48).
In pancreatic acini (11, 65), as well as in numerous other tissues, the activation of adenylate cyclase and increasing cellular cAMP levels can stimulate PKA-mediated and EPAC-mediated signaling cascades (58, 83). To distinguish these two possibilities, we used EPAC and PKA inhibitors, as well as selective activators (12, 13, 30, 31, 58, 92). We also compared the results to phosphorylation of the cAMP response-binding protein (CREB), which is needed for CREB-mediating transcriptional activation (67, 80), and is an established signal for PKA activation in many studies, including its activation by secretin and VIP in other tissues (67, 80).
A number of our results support the conclusion that the cAMP signaling cascades in pancreatic acini resulting in PAK4 stimulation differ with stimulation of VIP-preferring and secretin-preferring receptors. In the case of VIP-preferring receptors, PAK4 activation is mediated through cyclic AMP stimulation of EPAC, whereas in the case of the secretin-preferring receptors, it is mediated by PKA. These conclusions are supported by the finding that the PKA inhibitors KT-5720 and PKI (12, 52) but not the EPAC inhibitors ESI-09 and HJC0197 (13, 31, 58, 92) inhibited secretin-stimulated increases in PAK4 activation. Conversely, in the case of VIP-preferring receptor activation the reverse occurred, with inhibition seen with the EPAC inhibitors, ESI-09 and HJC0197 but not the PKA inhibitors KT-5720 and PKI. The ability of selective stimulation of EPAC to cause PAK4 activation was confirmed by using the EPAC-selective agonist, 8-CPT-2-Me-cAMP (12, 13, 31, 58, 65), which stimulated PAK4 activation. Furthermore, the 8-CPT-2-Me-cAMP time course of activation of PAK4 was indistinguishable from that seen with VIP. That PKA activation mediated by cAMP stimulated pathways could stimulate PAK4 activation was also supported by the finding that the stimulation of PAK4 by 8-Br-cAMP or forskolin, both cAMP analogs that can activate PKA and EPAC (15, 61), was greater than that seen with 8-CPT-2-Me-cAMP, which only activates EPAC (12, 31, 58, 65).
A number of our results support the conclusion that cAMP signaling cascade for PAK4 activation, by VIP or secretin, differs from that for their stimulation of pS133 CREB phosphorylation. In contrast to activation of PAK4 by VIP and secretin, which were EPAC- and PKA-mediated, respectively, in the case of CREB S133 phosphorylation, only EPAC activation was required for both receptors. This conclusion was supported by the finding that with both secretin- and VIP-stimulated CREB phosphorylation, the specific EPAC inhibitors ESI-09 and HJC0197 but not the PKA inhibitors KT-5720 and PKI resulted in inhibition. Furthermore, the results of the inhibitor’s study were supported by the finding that the selective EPAC agonist, 8-CPT-2-Me-cAMP, stimulated CREB phosphorylation in pancreatic acini. The differences in this ability of secretin- and VIP-preferring receptor activation to result in stimulation of different cAMP signaling cascades for PAK4 and pCREB has both similarities and differences from other studies in pancreatic acini and in other tissues with VIP, secretin stimulation of other cAMP-activated signaling cascades. Similar to our results, VIP-stimulated Rap1 activation in pancreatic acini is mediated by EPAC (61) and activation of EPAC partially mediates VIP-induced acceleration of calcium waves in pancreatic acini (65). Similarly, VIP stimulation of the cAMP/EPAC pathway increased VEGF expression in prostate cancer cells (23), VIP-mediated antigrowth effects in normal and renal tumor cells (76), and VIP-mediated proinflammatory activity in monocytes (18). Also, similar to our results with the secretin-preferring receptor stimulation of pCREB, secretin stimulates postnatal development of cerebellar cortex neurons (81) and neurite antigrowth in PC-12 cells by a cAMP/PKA signaling pathway (38). In contrast, VIP-stimulated melanogenesis in melanoma cells is mediated by a cAMP/PKA/CREB pathway (88), as well as through a cAMP/PKA pathway for VIP-stimulated expression of calcium channels in colonic smooth muscle cells (68). Also, in contrast to our results, both the cAMP/PKA and cAMP/EPAC pathways mediated secretin and VIP inhibition of electrical activity in suprachiasmatic nucleus cells (40) and the acceleration of calcium waves in pancreatic acinar cells (65). These results demonstrate the marked variation of the cAMP signaling cascade used by VIP and secretin for activation of different signaling cascades in different cells and even in the same cells.
Another important difference, in the ability of VIP and secretin to activate PAK4 and stimulate CREB phosphorylation was the post-EPAC/-PKA pathways involved. In our studies in the case of pCREB (S133) phosphorylation, which was stimulated by EPAC activation with both VIP and secretin, post-EPAC, MAPK kinases activation (both p44/42 and p38) were required. In contrast, MAPK activation was not required for either VIP-mediated EPAC or secretin-mediated PKA activation in pancreatic acini. In most previous studies of the post-cAMP signaling cascades in different cells the activation of CREB, which mediates changes in transcription, was stimulated via PKA (28, 67, 80). This includes studies with VIP and secretin. Specifically, secretin was reported to stimulate, by a PKA mechanism, the PI33S phosphorylation of CREB-mediating chromogranin A transcription (43), stimulate catecholamine biosynthesis and secretion (43), and postnatal developmental changes in cerebellar cortical cells (81). Similarly, VIP stimulates, by a PKA mechanism, the phosphorylation of CREB in microglia cells, which mediate inhibition of NF-κB transcription activity (17), as well as PKA activation of pCREB to modulate proliferative effects on wound healing in bronchial epithelial cells (26).
Similar to our findings with secretin and VIP, a number of recent studies report pCREB phosphorylation can be mediated by other signaling cascades than PKA, particularly by activation of EPAC and MAP kinases (13, 25, 72). Recent studies, both in pancreatic acini and in nonpancreatic acinar cells, suggest that the EPAC stimulation of MAP kinases by EPAC is mediated by Rap1 activation (19, 20, 25, 61). A similar result may be occurring in our study in pancreatic acini, because in another study in pancreatic acini, VIP has been reported to activate Rap1 via an EPAC-dependent mechanism (61). Furthermore, in studies in other tissues with different stimuli activating cAMP-signaling cascades, Rap1 activation subsequently resulting in activation of MAP kinases occurs by a β-Raf-dependent mechanism, raising the possibility of a similar mechanism in pancreatic acinar cells (72).
One of the main physiological roles of secretin and VIP is to stimulate pancreatic fluid secretion (32, 49, 64). Fluid secretion is primarily from pancreatic ductal cells but also involves secretion from centro-acinar cells of the pancreatic acini and it requires activation of Na+,K+-ATPase (9, 32, 53). To explore whether PAK4 has a possible role in pancreatic acinar fluid secretion, we investigate its possible role in secretin- or VIP-stimulated activation of Na+,K+-ATPase. We first studied the effect of PAK4 inhibition on Na+,K+-ATPase activation stimulated by VIP or secretin. Second, we assessed the ability of PAK4 inhibition to affect Na+,K+-ATPase phosphorylation stimulated by secretin or VIP. Our results from both approaches supported an important role for VIP-/secretin-stimulated PAK4 activation for activation of Na+,K+-ATPase by secretin/VIP. The conclusion was directly supported by finding both PAK4 inhibitors, PF-3758309 and LCH-7749944, completely inhibited VIP- or secretin-stimulated ouabain-sensitive increases in Na+,K+-ATPase. Numerous studies show phosphorylation of Na+,K+-ATPase can regulate its activity (49), including its activation by cAMP-stimulating agents (8, 39). Na+,K+-ATPase activation with a number of stimuli is associated with increased Tyr10 phosphorylation of the α subunit (22, 66), and we found that secretin/VIP both stimulated a time-dependent increase in Tyr10 Na+,K+-ATPase phosphorylation. Furthermore, this was completely inhibited by PAK4 inhibitors.
In conclusion, the results of our study are summarized in Fig. 14, comparing the cAMP signaling cascade of VIP/secretin phosphorylation of PAK4 and CREB. Both involve activation of adenylate cyclase and generation of cAMP; however, in the post-cAMP signaling cascades they have similarities and differences. They differ with VIP- and secretin-stimulating PAK4 occurring, by different post-cAMP mediators involving EPAC and PKA, respectively. In contrast, they demonstrate similar post-cAMP signaling cascades for activation of CREB with both stimulating via EPAC. However, activation of CREB differs with both VIP/secretin from their activation of PAK4, because activation of MAP kinases (both p44/42 and p38) is required distal to EPAC activation, whereas for PAK4 activation, distal to EPAC or PKA stimulation, MAP kinase activation is not needed. Lastly, the activation of PAK4 by VIP and secretion is essential for its stimulation of Na+,K+-ATPase, which mediates fluid secretion. These results, coupled with results of a previous study showing PAK4 activation is also essential for pancreatic enzyme secretion by PLC-activating agents (CCK, carbachol) (55), as is PAK2 activation (47), establish a central role for PAK’s (both PAK2 and PAK4) in pancreatic secretory responses. These results, coupled with studies reporting an important role for PAK4 in pancreatic cancer growth/invasiveness (75, 87), and similar to PAK2 (48), in experimental pancreatitis (42), strongly support the conclusion that both group I and group II PAKs both play important roles in pancreatic physiological, as well as pathological, responses.
Fig. 14.
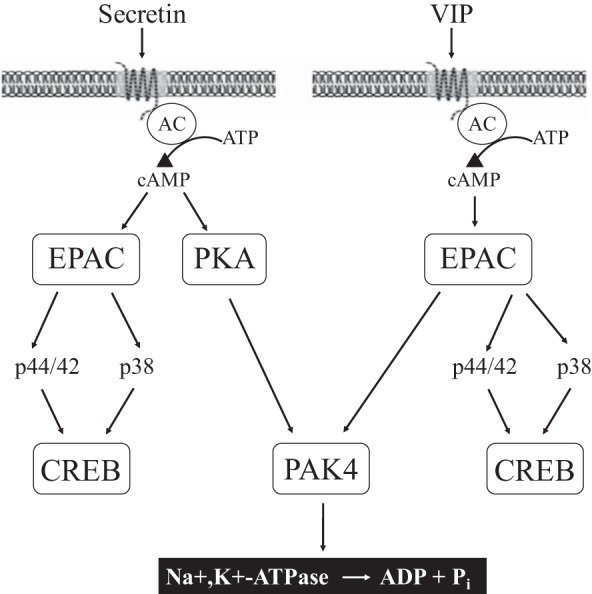
Schematic diagram of signaling cascade for activation of PAK4 in pancreatic acinar cells. In rat pancreatic acinar cells maximal activation of PAK4 by secretin requires activation of PKA and by VIP requires EPAC; however, both secretin and VIP induced CREB phosphorylation through EPAC. 8-Br-cAMP, 8-CPT-2-O-Me-cAMP, and forskolin mediates PAK4 activation. PAK4 activation is important for Na+,K+-ATPase phosphorylation and Na+,K+-ATPase activity. 8-Br-cAMP, 8-Bromoadenosine 3′,5′-cyclic monophosphate sodium salt; 8-CPT-2-O-Me-cAMP, 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate sodium salt; AC, adenylyl cyclase; PAK, p21-activated kinase; Pi, inorganic phosphate; VIP, vasoactive intestinal polypeptide.
GRANTS
This work was partially supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.R.-A. and L.L. performed experiments; I.R.-A., L.L., and R.T.J. analyzed data; I.R.-A., L.L., and R.T.J. interpreted results of experiments; I.R.-A., L.L., and R.T.J. prepared figures; I.R.-A., L.L., and R.T.J. drafted manuscript; I.R.-A., L.L., and R.T.J. edited and revised manuscript; I.R.-A., L.L., and R.T.J. approved final version of manuscript.
REFERENCES
- 1.Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, Belisle B, Minden A. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J 17: 6527–6540, 1998. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann VA, Bister K, Stefan E. Interplay of PKA and Rac: fine-tuning of Rac localization and signaling. Small GTPases 4: 247–251, 2013. doi: 10.4161/sgtp.27281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrows D, He JZ, Parsons R. PREX1 protein function is negatively regulated downstream of receptor tyrosine kinase activation by p21-activated kinases (PAKs). J Biol Chem 291: 20042–20054, 2016. doi: 10.1074/jbc.M116.723882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeser A, Jaffer ZM, Hofmann C, Chernoff J. Role of group A p21-activated kinases in activation of extracellular-regulated kinase by growth factors. J Biol Chem 280: 36609–36615, 2005. doi: 10.1074/jbc.M502306200. [DOI] [PubMed] [Google Scholar]
- 5.Bissonnette BM, Collen MJ, Adachi H, Jensen RT, Gardner JD. Receptors for vasoactive intestinal peptide and secretin on rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 246: G710–G717, 1984. doi: 10.1152/ajpgi.1984.246.6.G710. [DOI] [PubMed] [Google Scholar]
- 6.Blinman TA, Gukovsky I, Mouria M, Zaninovic V, Livingston E, Pandol SJ, Gukovskaya AS. Activation of pancreatic acinar cells on isolation from tissue: cytokine upregulation via p38 MAP kinase. Am J Physiol Cell Physiol 279: C1993–C2003, 2000. doi: 10.1152/ajpcell.2000.279.6.C1993. [DOI] [PubMed] [Google Scholar]
- 7.Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, Jallal B, Smeal T. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem 277: 550–558, 2002. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- 8.Carranza ML, Féraille E, Kiroytcheva M, Rousselot M, Favre H. Stimulation of ouabain-sensitive 86Rb+ uptake and Na+,K+-ATPase α-subunit phosphorylation by a cAMP-dependent signalling pathway in intact cells from rat kidney cortex. FEBS Lett 396: 309–314, 1996. doi: 10.1016/0014-5793(96)01121-0. [DOI] [PubMed] [Google Scholar]
- 9.Case RM, Scratcherd T. The secretion of alkali metal ions by the perfused cat pancreas as influenced by the composition and osmolality of the external environment and by inhibitors of metabolism and Na+, K+-ATPase activity. J Physiol 242: 415–428, 1974. doi: 10.1113/jphysiol.1974.sp010715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahdi A, Miller B, Sorokin A. Endothelin 1 induces β1Pix translocation and Cdc42 activation via protein kinase A-dependent pathway. J Biol Chem 280: 578–584, 2005. doi: 10.1074/jbc.M411130200. [DOI] [PubMed] [Google Scholar]
- 11.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 14: 63–67, 2007. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhuri A, Husain SZ, Kolodecik TR, Grant WM, Gorelick FS. Cyclic AMP-dependent protein kinase and Epac mediate cyclic AMP responses in pancreatic acini. Am J Physiol Gastrointest Liver Physiol 292: G1403–G1410, 2007. doi: 10.1152/ajpgi.00478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Wild C, Zhou X, Ye N, Cheng X, Zhou J. Recent advances in the discovery of small molecules targeting exchange proteins directly activated by cAMP (EPAC). J Med Chem 57: 3651–3665, 2014. doi: 10.1021/jm401425e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YY, Sun LQ, Wang BA, Zou XM, Mu YM, Lu JM. Palmitate induces autophagy in pancreatic β-cells via endoplasmic reticulum stress and its downstream JNK pathway. Int J Mol Med 32: 1401–1406, 2013. doi: 10.3892/ijmm.2013.1530. [DOI] [PubMed] [Google Scholar]
- 15.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Døskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278: 35394–35402, 2003. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 16.Dart AE, Wells CM. P21-activated kinase 4 – not just one of the PAK. Eur J Cell Biol 92: 129–138, 2013. doi: 10.1016/j.ejcb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit CBP-NF-κB interaction in activated microglia. Biochem Biophys Res Commun 297: 1181–1185, 2002. doi: 10.1016/S0006-291X(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 18.El Zein N, Badran B, Sariban E. VIP differentially activates β2 integrins, CR1, and matrix metalloproteinase-9 in human monocytes through cAMP/PKA, EPAC, and PI-3K signaling pathways via VIP receptor type 1 and FPRL1. J Leukoc Biol 83: 972–981, 2008. doi: 10.1189/jlb.0507327. [DOI] [PubMed] [Google Scholar]
- 19.Emery AC, Eiden MV, Eiden LE. Separate cyclic AMP sensors for neuritogenesis, growth arrest, and survival of neuroendocrine cells. J Biol Chem 289: 10126–10139, 2014. doi: 10.1074/jbc.M113.529321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emery AC, Xu W, Eiden MV, Eiden LE. Guanine nucleotide exchange factor Epac2-dependent activation of the GTP-binding protein Rap2A mediates cAMP-dependent growth arrest in neuroendocrine cells. J Biol Chem 292: 12220–12231, 2017. doi: 10.1074/jbc.M117.790329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eswaran J, Lee WH, Debreczeni JE, Filippakopoulos P, Turnbull A, Fedorov O, Deacon SW, Peterson JR, Knapp S. Crystal structures of the p21-activated kinases PAK4, PAK5, and PAK6 reveal catalytic domain plasticity of active group II PAKs. Structure 15: 201–213, 2007. doi: 10.1016/j.str.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Féraille E, Carranza ML, Gonin S, Béguin P, Pedemonte C, Rousselot M, Caverzasio J, Geering K, Martin PY, Favre H. Insulin-induced stimulation of Na+,K+-ATPase activity in kidney proximal tubule cells depends on phosphorylation of the alpha-subunit at Tyr-10. Mol Biol Cell 10: 2847–2859, 1999. doi: 10.1091/mbc.10.9.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Martínez AB, Carmena MJ, Bajo AM, Vacas E, Sánchez-Chapado M, Prieto JC. VIP induces NF-κB1-nuclear localisation through different signalling pathways in human tumour and non-tumour prostate cells. Cell Signal 27: 236–244, 2015. doi: 10.1016/j.cellsig.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Garcia LJ, Pradhan TK, Weber HC, Moody TW, Jensen RT. The gastrin-releasing peptide receptor is differentially coupled to adenylate cyclase and phospholipase C in different tissues. Biochim Biophys Acta 1356: 343–354, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Glas E, Mückter H, Gudermann T, Breit A. Exchange factors directly activated by cAMP mediate melanocortin 4 receptor-induced gene expression. Sci Rep 6: 32776, 2016. doi: 10.1038/srep32776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan CX, Cui YR, Sun GY, Yu F, Tang CY, Li YC, Liu HJ, Fang X. Role of CREB in vasoactive intestinal peptide-mediated wound healing in human bronchial epithelial cells. Regul Pept 153: 64–69, 2009. doi: 10.1016/j.regpep.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ha BH, Morse EM, Turk BE, Boggon TJ. Signaling, regulation, and specificity of the type II p21-activated kinases. J Biol Chem 290: 12975–12983, 2015. doi: 10.1074/jbc.R115.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol 13: 4852–4859, 1993. doi: 10.1128/MCB.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 166: 4–17, 2012. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henquin JC, Nenquin M. Activators of PKA and Epac distinctly influence insulin secretion and cytosolic Ca2+ in female mouse islets stimulated by glucose and tolbutamide. Endocrinology 155: 3274–3287, 2014. doi: 10.1210/en.2014-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20: 10–20, 2008. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hootman SR. Neuroendocrine control of secretion in pancreatic and parotid gland acini and the role of Na+,K+-ATPase activity. Int Rev Cytol 105: 129–181, 1986. doi: 10.1016/S0074-7696(08)61063-3. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Hou W, Katsuno T, Igarashi H, Pradhan TK, Mantey SA, Coy DH, Jensen RT. Rat and guinea pig pancreatic acini possess both VIP1 and VIP2 receptors, which mediate enzyme secretion. Am J Physiol Gastrointest Liver Physiol 278: G64–G74, 2000. doi: 10.1152/ajpgi.2000.278.1.G64. [DOI] [PubMed] [Google Scholar]
- 34.Iwatsuki K, Horiuchi A, Yonekura Y, Chiba S. Inhibitory effect of ouabain and acetazolamide on secretin-stimulated pancreatic exocrine secretion in anaesthetized dog. Clin Exp Pharmacol Physiol 16: 139–145, 1989. doi: 10.1111/j.1440-1681.1989.tb01538.x. [DOI] [PubMed] [Google Scholar]
- 35.Jensen RT. Receptors on pancreatic acinar cells (3rd ed.). In: Physiology of the Gastrointestinal Tract, edited by Johnson LR, Jacobson ED, Christensen J, Alpers DH, Walsh JH. New York: Raven Press, 1994, p. 1377–1446. [Google Scholar]
- 36.Jensen RT, Charlton CG, Adachi H, Jones SW, O’Donohue TL, Gardner JD. Use of 125I-secretin to identify and characterize high-affinity secretin receptors on pancreatic acini. Am J Physiol Gastrointest Liver Physiol 245: G186–G195, 1983. doi: 10.1152/ajpgi.1983.245.2.G186. [DOI] [PubMed] [Google Scholar]
- 37.Ke Y, Lei M, Solaro RJ. Regulation of cardiac excitation and contraction by p21 activated kinase-1. Prog Biophys Mol Biol 98: 238–250, 2008. doi: 10.1016/j.pbiomolbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HS, Yumkham S, Kim SH, Yea K, Shin YC, Ryu SH, Suh PG. Secretin induces neurite outgrowth of PC12 through cAMP-mitogen-activated protein kinase pathway. Exp Mol Med 38: 85–93, 2006. doi: 10.1038/emm.2006.10. [DOI] [PubMed] [Google Scholar]
- 39.Kiroytcheva M, Cheval L, Carranza ML, Martin PY, Favre H, Doucet A, Féraille E. Effect of cAMP on the activity and the phosphorylation of Na+,K+-ATPase in rat thick ascending limb of Henle. Kidney Int 55: 1819–1831, 1999. doi: 10.1046/j.1523-1755.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- 40.Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol 110: 1097–1106, 2013. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar R, Sanawar R, Li X, Li F. Structure, biochemistry, and biology of PAK kinases. Gene 605: 20–31, 2017. doi: 10.1016/j.gene.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma B, Wu L, Lu M, Gao B, Qiao X, Sun B, Xue D, Zhang W. Differentially expressed kinase genes associated with trypsinogen activation in rat pancreatic acinar cells treated with taurolithocholic acid 3-sulfate. Mol Med Rep 7: 1591–1596, 2013. doi: 10.3892/mmr.2013.1355. [DOI] [PubMed] [Google Scholar]
- 43.Mahapatra NR, Mahata M, O’Connor DT, Mahata SK. Secretin activation of chromogranin A gene transcription. Identification of the signaling pathways in cis and in trans. J Biol Chem 278: 19986–19994, 2003. doi: 10.1074/jbc.M207983200. [DOI] [PubMed] [Google Scholar]
- 44.Mascia A, Gentile F, Izzo A, Mollo N, De Luca M, Bucci C, Nitsch L, Calì G. Rab7 regulates CDH1 endocytosis, circular dorsal ruffles genesis, and thyroglobulin internalization in a thyroid cell line. J Cell Physiol 231: 1695–1708, 2016. doi: 10.1002/jcp.25267. [DOI] [PubMed] [Google Scholar]
- 45.Miura K, Nojiri T, Akitake Y, Ando K, Fukuhara S, Zenitani M, Kimura T, Hino J, Miyazato M, Hosoda H, Kangawa K. CCM2 and PAK4 act downstream of atrial natriuretic peptide signaling to promote cell spreading. Biochem J 474: 1897–1918, 2017. doi: 10.1042/BCJ20160841. [DOI] [PubMed] [Google Scholar]
- 46.Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, Kraynov E, Popoff I, Christensen JG, Martinez R, Kephart SE, Marakovits J, Karlicek S, Bergqvist S, Smeal T. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA 107: 9446–9451, 2010. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nuche-Berenguer B, Jensen RT. Gastrointestinal hormones/neurotransmitters and growth factors can activate P21 activated kinase 2 in pancreatic acinar cells by novel mechanisms. Biochim Biophys Acta 1853: 2371–2382, 2015. doi: 10.1016/j.bbamcr.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nuche-Berenguer B, Ramos-Álvarez I, Jensen RT. The p21-activated kinase, PAK2, is important in the activation of numerous pancreatic acinar cell signaling cascades and in the onset of early pancreatitis events. Biochim Biophys Acta 1862: 1122–1136, 2016. doi: 10.1016/j.bbadis.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pallagi P, Hegyi P, Rakonczay Z JR. The physiology and pathophysiology of pancreatic ductal secretion: the background for clinicians. Pancreas 44: 1211–1233, 2015. doi: 10.1097/MPA.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 50.Park MH, Lee HS, Lee CS, You ST, Kim DJ, Park BH, Kang MJ, Heo WD, Shin EY, Schwartz MA, Kim EG. p21-Activated kinase 4 promotes prostate cancer progression through CREB. Oncogene 32: 2475–2482, 2013. doi: 10.1038/onc.2012.255. [DOI] [PubMed] [Google Scholar]
- 51.Parnell E, Palmer TM, Yarwood SJ. The future of EPAC-targeted therapies: agonism versus antagonism. Trends Pharmacol Sci 36: 203–214, 2015. doi: 10.1016/j.tips.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persson E, Voznesensky OS, Huang YF, Lerner UH. Increased expression of interleukin-6 by vasoactive intestinal peptide is associated with regulation of CREB, AP-1 and C/EBP, but not NF-ΚB, in mouse calvarial osteoblasts. Bone 37: 513–529, 2005. doi: 10.1016/j.bone.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 53.Petersen OH, Ueda N. Secretion of fluid and amylase in the perfused rat pancreas. J Physiol 264: 819–835, 1977. doi: 10.1113/jphysiol.1977.sp011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol 21: 3523–3533, 2001. doi: 10.1128/MCB.21.10.3523-3533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos-Alvarez I, Jensen RT. P21-activated kinase 4 in pancreatic acinar cells is activated by numerous gastrointestinal hormones/neurotransmitters and growth factors by novel signaling, and its activation stimulates secretory/growth cascades. Am J Physiol Gastrointest Liver Physiol 315: G302–G317, 2018. doi: 10.1152/ajpgi.00005.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rane CK, Minden A. P21 activated kinases: structure, regulation, and functions. Small GTPases 5: e28003, 2014. doi: 10.4161/sgtp.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raufman JP, Malhotra R, Singh L. PACAP-38, a novel peptide from ovine hypothalamus, is a potent modulator of amylase release from dispersed acini from rat pancreas. Regul Pept 36: 121–129, 1991. doi: 10.1016/0167-0115(91)90200-Z. [DOI] [PubMed] [Google Scholar]
- 58.Robichaux WG III, Cheng X. Intracellular cAMP sensor EPAC: physiology, pathophysiology, and therapeutics development. Physiol Rev 98: 919–1053, 2018. doi: 10.1152/physrev.00025.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodríguez MR, Diez F, Ventimiglia MS, Morales V, Copsel S, Vatta MS, Davio CA, Bianciotti LG. Atrial natriuretic factor stimulates efflux of cAMP in rat exocrine pancreas via multidrug resistance-associated proteins. Gastroenterology 140: 1292–1302, 2011. doi: 10.1053/j.gastro.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 60.Rudolph J, Crawford JJ, Hoeflich KP, Wang W. Inhibitors of p21-activated kinases (PAKs). J Med Chem 58: 111–129, 2015. doi: 10.1021/jm501613q. [DOI] [PubMed] [Google Scholar]
- 61.Sabbatini ME, Chen X, Ernst SA, Williams JA. Rap1 activation plays a regulatory role in pancreatic amylase secretion. J Biol Chem 283: 23884–23894, 2008. doi: 10.1074/jbc.M800754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuel I, Zaheer A, Fisher RA. In vitro evidence for role of ERK, p38, and JNK in exocrine pancreatic cytokine production. J Gastrointest Surg 10: 1376–1383, 2006. doi: 10.1016/j.gassur.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Schäfer C, Ross SE, Bragado MJ, Groblewski GE, Ernst SA, Williams JA. A role for the p38 mitogen-activated protein kinase/Hsp 27 pathway in cholecystokinin-induced changes in the actin cytoskeleton in rat pancreatic acini. J Biol Chem 273: 24173–24180, 1998. doi: 10.1074/jbc.273.37.24173. [DOI] [PubMed] [Google Scholar]
- 64.Schulz I. Bicarbonate transport in the exocrine pancreas. Ann N Y Acad Sci 341: 191–209, 1980. doi: 10.1111/j.1749-6632.1980.tb47172.x. [DOI] [PubMed] [Google Scholar]
- 65.Shah AU, Grant WM, Latif SU, Mannan ZM, Park AJ, Husain SZ. Cyclic AMP accelerates calcium waves in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 294: G1328–G1334, 2008. doi: 10.1152/ajpgi.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahidullah M, Mandal A, Wei G, Delamere NA. Nitric oxide regulation of Na, K-ATPase activity in ocular ciliary epithelium involves Src family kinase. J Cell Physiol 229: 343–352, 2014. doi: 10.1002/jcp.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68: 821–861, 1999. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- 68.Shi XZ, Choudhury BK, Pasricha PJ, Sarna SK. A novel role of VIP in colonic motility function: induction of excitation-transcription coupling in smooth muscle cells. Gastroenterology 132: 1388–1400, 2007. doi: 10.1053/j.gastro.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Slimak GG, Stark HA, Egan JJ, Jensen RT, Gardner JD. Effect of verapamil on the cyclic AMP-mediated pathway for amylase secretion in rat pancreatic acini. Pancreas 8: 212–219, 1993. doi: 10.1097/00006676-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Spanarkel M, Martinez J, Briet C, Jensen RT, Gardner JD. Cholecystokinin-27-32-amide. A member of a new class of cholecystokinin receptor antagonists. J Biol Chem 258: 6746–6749, 1983. [PubMed] [Google Scholar]
- 71.Suh HN, Han HJ. Laminin regulates mouse embryonic stem cell migration: involvement of Epac1/Rap1 and Rac1/cdc42. Am J Physiol Cell Physiol 298: C1159–C1169, 2010. doi: 10.1152/ajpcell.00496.2009. [DOI] [PubMed] [Google Scholar]
- 72.Sun W, Jiao W, Huang Y, Li R, Zhang Z, Wang J, Lei T. Exchange proteins directly activated by cAMP induce the proliferation of rat anterior pituitary GH3 cells via the activation of extracellular signal-regulated kinase. Biochem Biophys Res Commun 485: 355–359, 2017. doi: 10.1016/j.bbrc.2017.02.075. [DOI] [PubMed] [Google Scholar]
- 73.Tapia JA, Ferris HA, Jensen RT, García LJ. Cholecystokinin activates PYK2/CAKβ by a phospholipase C-dependent mechanism and its association with the mitogen-activated protein kinase signaling pathway in pancreatic acinar cells. J Biol Chem 274: 31261–31271, 1999. doi: 10.1074/jbc.274.44.31261. [DOI] [PubMed] [Google Scholar]
- 74.Tapia JA, García-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C-δ kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem 278: 35220–35230, 2003. doi: 10.1074/jbc.M303119200. [DOI] [PubMed] [Google Scholar]
- 75.Thillai K, Sarker D, Wells C. PAK4 pathway as a potential therapeutic target in pancreatic cancer. Future Oncol 14: 579–582, 2018. doi: 10.2217/fon-2017-0458. [DOI] [PubMed] [Google Scholar]
- 76.Vacas E, Fernández-Martínez AB, Bajo AM, Sánchez-Chapado M, Schally AV, Prieto JC, Carmena MJ. Vasoactive intestinal peptide (VIP) inhibits human renal cell carcinoma proliferation. Biochim Biophys Acta 1823: 1676–1685, 2012. doi: 10.1016/j.bbamcr.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 77.van Bogaert P, Soukias Y, Dehaye JP, Lambert M, Poloczek P, Winand J, Mayer R, Christophe J. Adenylate cyclase stimulation by VIP in rat and human parotid membranes. Regul Pept 17: 339–348, 1987. doi: 10.1016/0167-0115(87)90057-7. [DOI] [PubMed] [Google Scholar]
- 78.Veluthakal R, Chepurny OG, Leech CA, Schwede F, Holz GG, Thurmond DC. Restoration of glucose-stimulated Cdc42-Pak1 activation and insulin secretion by a selective epac activator in type 2 diabetic human islets. Diabetes 67: 1999–2011, 2018. doi: 10.2337/db17-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voronina S, Collier D, Chvanov M, Middlehurst B, Beckett AJ, Prior IA, Criddle DN, Begg M, Mikoshiba K, Sutton R, Tepikin AV. The role of Ca2+ influx in endocytic vacuole formation in pancreatic acinar cells. Biochem J 465: 405–412, 2015. doi: 10.1042/BJ20140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Hu XH, Huang ZX, Nie Q, Chen ZG, Xiang JW, Qi RL, Yang TH, Xiao Y, Qing WJ, Gigantelli G, Nguyen QD, Li DW. Regulation of CREB functions by phosphorylation and sumoylation in nervous and visual systems. Curr Mol Med 16: 885–892, 2017. doi: 10.2174/1566524016666161223110106. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Zhang L, Chow BK. Secretin modulates the postnatal development of mouse cerebellar cortex via PKA- and ERK-dependent pathways. Front Cell Neurosci 11: 382, 2017. doi: 10.3389/fncel.2017.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, Frucht H, Haffar BM, Jensen RT. des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem 265: 15695–15703, 1990. [PubMed] [Google Scholar]
- 83.Wang P, Liu Z, Chen H, Ye N, Cheng X, Zhou J. Exchange proteins directly activated by cAMP (EPACs): Emerging therapeutic targets. Bioorg Med Chem Lett 27: 1633–1639, 2017. doi: 10.1016/j.bmcl.2017.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie X, Shi X, Guan H, Guo Q, Fan C, Dong W, Wang G, Li F, Shan Z, Cao L, Teng W. P21-activated kinase 4 involves TSH induced papillary thyroid cancer cell proliferation. Oncotarget 8: 24882–24891, 2017. doi: 10.18632/oncotarget.15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan K, Gao LN, Cui YL, Zhang Y, Zhou X. The cyclic AMP signaling pathway: Exploring targets for successful drug discovery (Review) Mol Med Rep 13: 3715–3723, 2016. doi: 10.3892/mmr.2016.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Y, Dai M, Wilson TM, Omelchenko I, Klimek JE, Wilmarth PA, David LL, Nuttall AL, Gillespie PG, Shi X. Na+/K+-ATPase α1 identified as an abundant protein in the blood-labyrinth barrier that plays an essential role in the barrier integrity. PLoS One 6: e16547, 2011. doi: 10.1371/journal.pone.0016547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeo D, He H, Baldwin GS, Nikfarjam M. The role of p21-activated kinases in pancreatic cancer. Pancreas 44: 363–369, 2015. doi: 10.1097/MPA.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 88.Yuan XH, Yao C, Oh JH, Park CH, Tian YD, Han M, Kim JE, Chung JH, Jin ZH, Lee DH. Vasoactive intestinal peptide stimulates melanogenesis in B16F10 mouse melanoma cells via CREB/MITF/tyrosinase signaling. Biochem Biophys Res Commun 477: 336–342, 2016. doi: 10.1016/j.bbrc.2016.06.105. [DOI] [PubMed] [Google Scholar]
- 89.Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, Kim EG. p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J Invest Dermatol 135: 1385–1394, 2015. doi: 10.1038/jid.2014.548. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, Cheng M, Zhao D, Li F. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett 317: 24–32, 2012. [Erratum in Cancer Lett 349: 159, 2014.] doi: 10.1016/j.canlet.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Zhou ZC, Gardner JD, Jensen RT. Interaction of peptides related to VIP and secretin with guinea pig pancreatic acini. Am J Physiol Gastrointest Liver Physiol 256: G283–G290, 1989. doi: 10.1152/ajpgi.1989.256.2.G283. [DOI] [PubMed] [Google Scholar]
- 92.Zhu Y, Chen H, Boulton S, Mei F, Ye N, Melacini G, Zhou J, Cheng X. Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window”. Sci Rep 5: 9344, 2015. doi: 10.1038/srep09344. [DOI] [PMC free article] [PubMed] [Google Scholar]