Abstract
Cancer-associated thrombosis is a common first presenting sign of malignancy and is currently the second leading cause of death in cancer patients after their malignancy. However, the molecular mechanisms underlying cancer-associated thrombosis remain undefined. In this study, we aimed to develop a better understanding of how cancer cells affect the coagulation cascade and platelet activation to induce a prothrombotic phenotype. Our results show that colon cancer cells trigger platelet activation in a manner dependent on cancer cell tissue factor (TF) expression, thrombin generation, activation of the protease-activated receptor 4 (PAR4) on platelets and consequent release of ADP and thromboxane A2. Platelet-colon cancer cell interactions potentiated the release of platelet-derived extracellular vesicles (EVs) rather than cancer cell-derived EVs. Our data show that single colon cancer cells were capable of recruiting and activating platelets and generating fibrin in plasma under shear flow. Finally, in a retrospective analysis of colon cancer patients, we found that the number of venous thromboembolism events was 4.5 times higher in colon cancer patients than in a control population. In conclusion, our data suggest that platelet-cancer cell interactions and perhaps platelet procoagulant EVs may contribute to the prothrombotic phenotype of colon cancer patients. Our work may provide rationale for targeting platelet-cancer cell interactions with PAR4 antagonists together with aspirin and/or ADP receptor antagonists as a potential intervention to limit cancer-associated thrombosis, balancing safety with efficacy.
Keywords: aspirin, cancer, coagulation, platelets, PAR4, thrombosis
INTRODUCTION
Cancer-associated thrombosis is often the first presenting sign of malignancy and is currently the second leading cause of death in patients with cancer after their malignancy (12, 22, 33). Irrespective of cancer type, measurable activation of platelets and coagulation correlates with the extent of tumor progression and negative clinical outcomes (33, 43). We demonstrated previously that the activation of platelets leads to colon cancer cell proliferation, and that this response may be reversed with aspirin (32, 34).
Increased platelet counts (thrombocytosis) are not uncommon in cancer patients at diagnosis. Thrombocytosis occurs more commonly in advanced malignancies and is a marker for increased risk of cancer-associated thrombosis (44). It is also well established that elevated levels of platelet- and/or tumor-derived extracellular vesicles (EVs) correlate with an increased risk of thrombotic events in patients with cancer (3, 15, 19). However, it is currently unclear whether the majority of procoagulant EVs originate from platelets, cancer cells, or both. In addition, it is unknown whether the release of these procoagulant EVs requires signaling between platelets and cancer cells, or if they are simply a consequence of platelet activation.
Previous in vitro and ex vivo studies have attempted to describe how cancer cells, platelets, and EVs are able to trigger activation of the coagulation cascade and promote thrombus formation (8, 24, 28, 29, 41, 42, 46, 47, 51). Among these studies, only a few have analyzed the simultaneous and potentially synergistic contributions of procoagulant cancer cells and platelets in thrombus formation under physiologically relevant fluid shear flow.
The procoagulant phenotype of cancer cells and EVs is primarily dependent on the expression of a functionally active tissue factor (TF) and exposure of phosphatidylserine (PS) on their outer membrane (10, 45). TF and PS expression allows for the formation of the extrinsic tenase and prothrombinase complexes, respectively, resulting in the generation of the serine protease thrombin. Thrombin plays a central role in cancer-associated thrombosis, either directly, by generating fibrin, or indirectly by eliciting a variety of platelet prothrombotic responses via cleavage of protease-activated receptors (PARs) (9, 50). Human platelets express PAR1 and PAR4, G protein-coupled receptors that are activated by thrombin following proteolytic cleavage of an NH2-terminal site to reveal a tethered ligand that binds the receptor itself and initiates intracellular G protein signaling, culminating in platelet activation, aggregation, and shedding of procoagulant EVs (13, 50). Interestingly, PAR4 has been proposed as the primary receptor mediating the shedding of platelet-derived procoagulant EVs in response to thrombin (13). Yet, despite PAR4 being recently proposed as potential target to prevent thrombotic events, it is unknown whether inhibition of PAR4 can interfere with prothrombotic mechanisms elicited by procoagulant cancer cells (53).
In the present study, we employed a human SW480 colon cancer cell line to investigate platelet-colon cancer cell interactions leading to procoagulant EV production. We determined the cell- and size-specific EV populations arising from colon cancer cell-platelet interactions. By using an in vitro flow assay, we show that SW480 colon cancer cells trigger thrombus formation in a thrombin-dependent manner and we present evidence that PAR4 may be a potential target to limit colon cancer-associated thrombosis. In line with this working hypothesis, we provide clinical evidence from a retrospective cross-sectional analysis that patients with colon cancer have an increased risk for thrombosis independent of covariates (e.g., race, age, sex).
MATERIALS AND METHODS
Reagents.
All the chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) or previously mentioned sources unless specified otherwise (32, 44). Prostacyclin I2 (PGI2) was from Cayman Chemical (Ann Arbor, MI). DAPI solution (H3570) and Alexa Fluor 546-labeled fibrinogen were obtained from Thermo Fisher Scientific (Pittsburgh, PA). Anti-CD41-FITC was from Invitrogen (Carlsbad, CA), anti-CD41-BV421, Annexin V-FITC and Annexin V-binding buffer were from BioLegend (San Diego, CA), PAC-1-FITC, anti-EpCAM-APC, and anti-CD41a- PE-Cy7 were purchased from BD Biosciences (Franklin Lakes, NJ). EpCAM-APC-Cy7 was from Abcore (Ramona, CA). The anti-TF blocking antibody (clone D3H44) was from Genentech (South San Francisco, CA), the anti-TF-PE antibody was from BioLegend, while the anti-TF-FITC was purchased from LSBio (Seattle, WA). All of the anti-TF antibodies used in our study have been previously validated for each experimental application by our lab and others (4, 11, 39). The direct thrombin inhibitor, hirudin, was obtained from CIBA-Geigy Pharmaceuticals (Horsham, UK). Ticagrelor was purchased from Oxchem Corporation (Wood Dale, IL). The anti-factor XI antibody 1A6 and the PAR4 inhibitor VU0652925 were generated as previously described (14, 23).
SW480 cell culture.
The human colon adenocarcinoma cell line, SW480, was purchased from American Type Culture Collection (Manassas, VA). SW480 colon cancer cells were grown as monolayers (37°C in 5% CO2) in DMEM (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% FBS (GIBCO) and 1% penicillin-streptomycin. Cells were detached and prepared as previously described (32).
Isolation of human washed platelets.
Platelets were isolated from human venous blood drawn from healthy volunteers by venipuncture into sodium citrate (1:9; vol/vol), in accordance with an Institutional Review Board-approved protocol at Oregon Health & Science University, as previously described and with written informed consent from the volunteers (44). Briefly, anticoagulated blood was centrifuged (200 g, 20 min) to obtain platelet-rich plasma (PRP). PRP was centrifuged (1,000 g, 10 min) in the presence of PGI2 (0.1 μg/ml) to obtain a platelet pellet. The platelet pellet was resuspended in modified HEPES-Tyrode buffer (129 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCl2; pH 7.3) and washed once via centrifugation at 1,000 g for 10 min in modified HEPES-Tyrode buffer in the presence of prostacyclin (0.1 μg/ml). Purified platelets were resuspended in modified HEPES-Tyrode buffer at the indicated concentrations.
Measurement of SW480-TF activity with a fibrin formation assay.
Human platelet-poor plasma (PPP) was prepared as previously described (40). SW480 colon cancer cells (105 cells/well) were plated, grown for 24 h in 96-well plates, and incubated for 2 h in serum-free medium with 0.3% BSA. A set of control wells without cells was used as the blank. SW480 colon cancer cells were then washed with PBS and treated for 20 min with vehicle or anti-TF antibody (10 µg/ml), before a solution of PPP in the presence of vehicle or 1A6 (20 µg/ml) was added and fibrin formation initiated with 8.3 mM CaCl2. The assay was performed in duplicate in three independent experiments. Fibrin formation was measured as change in turbidity at 405 nm. The time interval required for the solution turbidity to reach the half-maximal value was defined as THalf Max as previously described (39).
Flow cytometric analysis–platelets and colon cancer cell interaction.
Isolated washed platelets and SW480 colon cancer cells were prepared as described above and combined to yield a final concentration of 1 × 106 SW480 cells/ml and 2 × 108 platelets/ml in a total volume of 100 µl. For inhibitory studies, platelets were preincubated with inhibitors [VU652925 (10 µM), aspirin (20 µM), ticagrelor (200 nM)], or vehicle (0.1% DMSO) for 15 min at 37°C. The solution of SW480 cells and platelets was transferred into 5 ml falcon tubes and incubated, at room temperature, for 30 min in the presence of antibodies (anti-CD41-BV421, PAC-1 FITC, and EpCAM-APC) and thrombin (0.1 U/ml). Antibody dilutions were 1:100 for anti-CD41-BV421, 1:100 for PAC-1-FITC and 1:100 for EpCAM-APC. Hirudin (40 µg/ml) was added to stop thrombin activity. Samples were fixed in equal volume of 2% paraformaldehyde (PFA) for 10 min and further diluted to a total volume of 300 μl in PBS containing 0.5% fatty acid-free BSA. Samples were analyzed on the BD FACSymphony A5 Flow Cytometer. A total of 10,000 EpCAM-positive (EpCAM+) events were acquired for each sample. Compensation was performed using OneComp eBeads (Invitrogen).
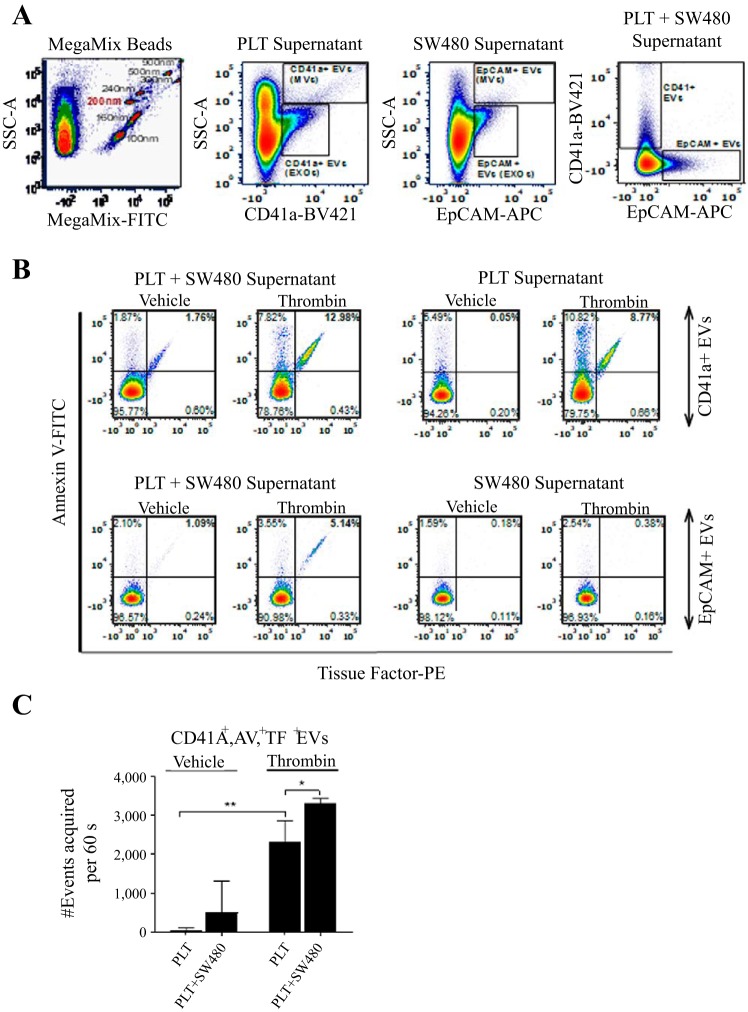
Nanoscale high-resolution flow cytometric analysis of procoagulant cancer and platelet-derived extracellular vesicles.
Isolated washed platelets and SW480 colon cancer cells were combined to yield a final concentration of 1 × 106 SW480 cells/ml and 2 × 108 platelets/ml and incubated in presence of antibodies (anti-CD41-BV421, anti-EpCAM-APC, anti-TF-PE, and Annexin V-FITC) and thrombin (0.1 U/ml) at room temperature for 30 min. Hirudin (40 µg/ml) was added to stop thrombin activity. Platelets, cancer cells and cell debris were removed by centrifugation for 15 min at 1,500 g. The supernatant was collected and diluted to a total volume of 500 µl in 0.1-filtered PBS before extracellular vesicle (EV) analysis by nanoscale high-resolution flow cytometry on a BD Symphony at low instrument pressure (BD Biosciences) as described by our group and others (7, 20, 35, 36, 48). We employed FITC-bright nanobeads (Invitrogen) and Megamix beads (Biocytex) to standardize side light scatter settings (SSC) between experiments and as relative size references as described (7). We defined EVs smaller than 200 nm as exosomes (EXOs) and EVs between 200 nm and 1 µm as microvesicles (MVs). Data were standardized to 1 min collections in triplicate, and the means are reported for each sample for statistical analysis.
Whole mount immunofluorescence and electron microscopy studies.
SW480 colon cancer cells (1 × 106 cells/ml) were pretreated with anti-TF blocking antibody (10 µg/ml) or vehicle for 10 min before incubation with PRP in the presence of 8.3 mM CaCl2 at room temperature for 30 min. PRP and SW480 colon cancer cells in HEPES-Tyrode buffer were used as controls. Hirudin (40 µg/ml) was added to stop thrombin activity in select tubes. Following incubation, cells were pelleted by centrifugation for 15 min at 1,500 g, the supernatant was discarded, and the cell pellets were washed in PBS and fixed in 4% PFA for 10 min. Fixed pellets were then washed in PBS and incubated in blocking buffer [0.1% BSA and 1:100 dilution of Human FC Block (BD Biosciences) in PBS] for 20 min at room temperature. Blocking buffer was removed and pellets were stained with EpCAM-APC-Cy7 at 0.1 mg/ml, anti-TF-FITC antibody (1:100) and PE-Cy7-conjugated anti-CD41 at 0.2 mg/ml for 1 h. Pellets were then washed in PBS, DAPI stained to highlight nuclei, and mounted for imaging using a Spinning Disk Nikon/Yokogawa CSU-W1. Composite images were created using Fiji (ImageJ, National Institutes of Health, Bethesda, MD) software. Three independent experiments were performed. For electron microscopy experiments, samples prepared as above were fixed in 2.5% glutaraldehyde and sections were prepared and imaged using standard methods.
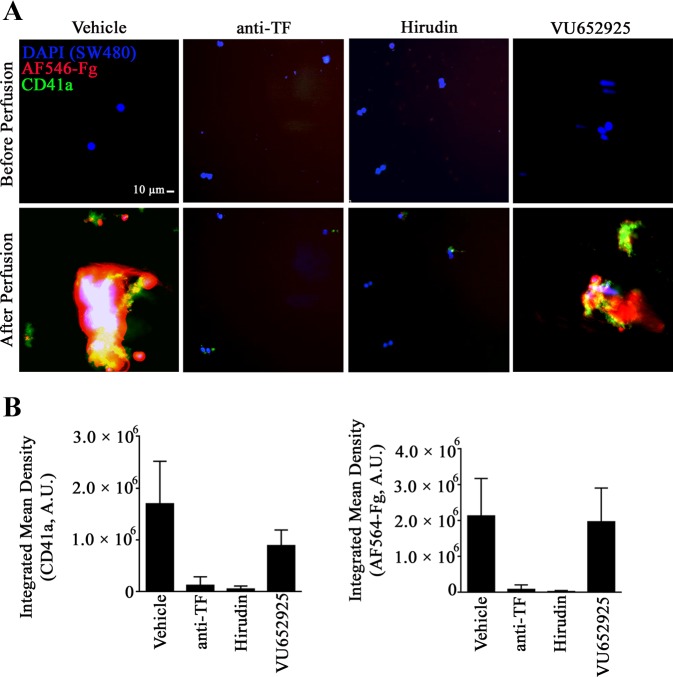
SW480 cancer cell-induced thrombus formation under dynamic flow conditions.
SW480 colon cancer cells (105 cells/well) were plated and grown to line for 24 h in ibiTreat μ-Slide VI 0.1 tissue culture-treated sterile channel slides (Ibidi USA, Fitchburg, WI). After two washes in PBS, SW480 colon cancer cells were incubated for 2 h in serum-free medium with 0.3% BSA and DAPI solution (1:250 dilution). SW480 colon cancer cells were pretreated with anti-TF blocking antibody (10 µg/ml) or vehicle for 10 min before perfusion with PRP stained with anti-CD41a-FITC (1:100 dilution), containing AF546-fibrinogen (40 µg/ml) and recalcified with CaCl2 (10 mM). In selected experiments, PRP was pretreated with hirudin (40 µG/ml) or VU652925 (10 µM) for 10 min before recalcification and perfusion. Perfusion was carried out at a shear rate of 2.5 dyn/cm2 and monitored for 10 min. SW480 cell-induced platelet aggregation and fibrin formation were visualized using a Zeiss ×40 /0.75 NE EC Plan-Neofluar lens on a Zeiss Axiocam MRm camera and Slidebook software (version 5.0; Intelligent Imaging Innovations). Quantitation of fluorescent images were processed by ImageJ. The multicolor images were first split into red, green, and blue channels and fluorescent signal in each channel were selected by intensity thresholding. Properties of selected regions including area and mean intensity were measured and were used to calculate the integrated intensity.
Retrospective cross-sectional clinicopathologic study of thrombosis occurrence in patients with colon cancer.
We performed a retrospective analysis of the Veteran Affairs’ electronic health record in the Corporate Data Warehouse and Cancer Registry. The Stanford University Institutional Review Board deemed the project to be exempt from human subject review. Cancer-associated thrombosis was defined as the occurrence of a pulmonary embolism or venous thromboembolism. ICD-9-CM codes were used to identify these diagnoses (pulmonary embolism included 415.1, 634.6, 635.6, 636.6, 637.6, 638.6, 673.2 and the codes used for deep vein thrombosis were 451.1, 451.2, 451.8, 451.9, 453.2, 453.8, 453.9, 671.3, 671.4, 671.9). Incidence rates (cases per 1,000 person-years) were determined for colon cancer patients and non-cancer patients from January 1, 2000 to January 1, 2010. End points were defined as the first occurrence of thrombosis, death, or loss to follow-up. Age-adjusted relative rates were calculated using a U.S. 2000 Standard Population distribution and 95% confidence intervals were calculated using the normal approximation to the binomial distribution.
Data analysis.
For experimental in vitro studies, data are expressed as means ± SE. Statistical significance of differences between means was determined by Student’s t-test, unless otherwise specified. P < 0.05 was considered statistically significant.
RESULTS
SW480 colon cancer cells induce fibrin formation and platelet adhesion in a TF-dependent manner.
Tissue factor expressed on the surface of cancer cells is considered the principal trigger of coagulation in patients with cancer. Our first experiments were designed to test the ability of SW480 colon cancer cells to generate thrombin and thus induce fibrin formation in a TF-dependent manner. Fibrin formation was observed in plasma after ~200 s (Fig. 1, A and B). To inhibit TF activation, SW480 colon cancer cells were pretreated with blocking anti-TF mAb antibody, whereas 1A6 was used to block the activity of the intrinsic pathway of coagulation in plasma. The presence of blocking anti-TF mAb delayed fibrin formation beyond 600 s. The addition of 1A6 had no effect on the ability of SW480 colon cancer cells to trigger fibrin formation. These results suggest that the ability of SW480 colon cancer cells to generate thrombin is TF-dependent and independent of the intrinsic pathway in vitro.
Fig. 1.
Adhesion and aggregation of human platelets to procoagulant SW480 human colon cancer cells. A and B: SW480 colon cancer cells were grown to confluence in 96-well plates and pretreated with vehicle or a blocking anti-TF antibody (10 µg/ml) for 20 min. Subsequently, PPP pretreated with 20 µg/ml of anti-FXI antibody 1A6 or vehicle was added to SW480 cancer cells and fibrin generation was initiated with the addition of 8.3 mM CaCl2. In A, fibrin formation was measured as change in turbidity at 405 nm. In B, time interval required for the solution turbidity to reach the half-maximal value was defined as THalf Max. For comparing treatments to vehicle, Student’s t-test was used. *P < 0.05, statistically significant. C: immunofluorescent colocalization and ultrastructural verification of platelet adherence to cancer cells. Representative images of three independent experiments revealed cancer cells stained for epithelial cell adhesion molecule (EpCAM-APC-cy7; C,A), highlighting the plasma membrane (arrow) by confocal microscopy. C,B: tissue factor highlighted by FITC-conjugated antibody (arrowhead) showed diffuse staining of cancer cells. CC: composite of cancer cells stained for EpCAM, tissue factor, and platelets stained for CD41a-PE-cy7 (red) showed composite green: red (yellow) punctate pattern (asterisk). C,D and C,E: electron microscopy revealed clusters of platelets (asterisk) adherent to cancer cells (arrow). C,F: in contrast, no adherent platelet aggregates were observed when tissue factor is inhibited. Scale bar, 1 μm. PPP, platelet-poor plasma; TF, tissue factor.
We next explored whether procoagulant cancer cells were able to support binding and induce aggregation of human platelets. To this end, SW480 (106 cells/ml) colon cancer cells were incubated with PRP in the presence of TF-blocking antibody or vehicle, and platelet adhesion to SW480 cancer cells and aggregation was assessed via fluorescent and electron microscopy. For each treatment, 100 platelets and 100 SW480 colon cancer cells were examined. Staining of the cell membrane with EpCAM (purple) and of the nuclei with DAPI (blue) were used to identify SW480 colon cancer cells (Fig. 1C, panel A). As shown in Fig. 1C (panel B), SW480 colon cancer cells showed high TF surface expression (green). Interestingly, platelets (red) adhered predominantly in the regions of high TF expression on SW480 cancer cells, as demonstrated by colocalization (yellow; Fig. 1C, panel C). Moreover, the direct binding of platelets to SW480 colon cancer cells was also confirmed by electron microscopy (Fig. 1C, panels D, E). Importantly, the presence of the TF-blocking antibody, completely prevented the physical interaction of platelets with SW480 (Fig. 1C, panel F).
Role of PAR4 in SW480 colon cancer cell-platelet interactions.
Previous studies have shown that platelets interact with a number of procoagulant cancer cell lines. We aimed to study the role of thrombin in mediating platelet-cancer cell interactions. Thrombin activates platelets via PAR-signaling. While the role of PAR1 in platelet-cancer interactions has been described (6, 38, 49), it is unknown whether PAR4 is involved in platelet-tumor cell cross talk. We investigated whether thrombin promotes platelet-SW480 colon cancer cell interactions and if so, whether PAR4 was required for the cell adhesion and platelet activation. To this end, washed platelets were combined with SW480 colon cancer cells and stimulated with vehicle or thrombin. Platelet adhesion to cancer cells was assessed via flow cytometry as detection of fluorescent platelets (CD41a+) on the surface of SW480 colon cancer cells (EpCAM+) postincubation as shown in Fig. 2A. The state of activation of platelets bound to SW480 was also assessed by quantifying the degree of integrin αIIbβ3 activation (PAC-1 binding; Fig. 2A). As shown in Fig. 2, B and C, the interaction of activated platelets with SW480 colon cancer cells was significantly increased in the presence of thrombin as compared with the baseline level. Importantly, the ability of thrombin to potentiate binding and activation of platelets to SW480 cells was significantly reduced by the PAR4 antagonist. We next investigated whether platelet-SW480 interactions triggered by thrombin could also be inhibited with antiplatelet agents aspirin and ticagrelor, which inhibit the actions of thromboxane A2 (TXA2) and ADP, respectively. As shown in Fig. 2, B and C, thrombin-induced platelet-SW480 cancer cell interactions and activation were impaired by either aspirin or ticagrelor.
Fig. 2.
Flow cytometric analysis of platelet interaction with and activation by SW480 colon cancer cells. Isolated platelets and SW480 colon cancer cells were combined in equal volume to a final concentration of 1 × 106/ml SW480 cancer cells and 2 × 108/ml platelets (A–C). For pharmacological studies, platelets were incubated with inhibitors (aspirin, 20 µM: COX-1 inhibitor; ticagrelor, 200 nM: P2Y12 inhibitor; VU652925, 10 µM: PAR4 inhibitor) or vehicle (0.1% DMSO) for 15 min at room temperature (RT) before incubation with cancer cells. Cell mixture (60 µl) was added to tubes containing 20 µl of antibody and agonist mixtures (thrombin, 0.1 U/ml) and incubated at RT for 30 min. A and B: gating strategy (A) and representative flow cytometric plots (B) of platelet-SW480 cancer cell interactions are shown. Platelets were labeled with BV421-conjugated CD41 antibody (1:100 dilution), and activated platelets were identified by positivity for PAC-1 binding (PAC-1-FITC, 1:100 dilution). Cancer cells were stained with APC-conjugated EpCAM (1:100 dilution). Samples were analyzed on the BD FACSymphony A5 Flow Cytometer. Gates were set to only include SW480 colon cancer cells positive for EpCAM expression. C: bar graphs showing the % of SW480 colon cancer cells bound to total (left) and activated platelets (right). Data are means ± SE (n = 3). For comparing treatments to vehicle, one-way ANOVA-multiple comparison test was used. *P < 0.05, statistically significant. COX-1, cyclooxygenase-1; PAR4, protease-activated receptor 4.
SW480 colon cancer cells incite the release of platelet EVs.
We next aimed to define the composition of EVs and identify whether platelets or SW480 cancer cells were the primary experimental source of EVs. EVs were characterized as described in materials and methods and as shown in Fig. 3. According to the bead tracking analysis, EVs had a mean particle size above 200 nm and were classified as microvesicles (MVs) (Fig. 3A). Staining with Annexin V was used to identify EVs exposing phosphatidylserine. As shown in Fig. 3B, neither SW480 colon cancer cells nor platelets released procoagulant EVs when unstimulated (vehicle). Incubation of platelets with SW480 colon cancer cells led to an increase in procoagulant EVs; importantly the majority of procoagulant EVs were MVs that were shed from platelets (CD41a+/Annexin V/TF positive), while relatively few were released from SW480 colon cancer cells (EpCAM+/Annexin V/TF positive). To assess the ability of thrombin to potentiate release of platelet-derived procoagulant EVs, we incubated the mixture of SW480 colon cancer cells and platelets with 0.1 U/ml of thrombin and stopped the reaction after 30 min with hirudin. As shown in Fig. 3, addition of thrombin to the cellular suspension of platelets and SW480 cancer cells induced a significant increase in platelet-derived procoagulant MVs (CD41a+/Annexin V/TF positive).
Fig. 3.
Nanoscale high-resolution flow cytometry of platelet and colon cancer SW480 cell line extracellular vesicles. A. experiments were standardized using uniform instrument settings based on commercially available fluorescently labeled polystyrene beads (Megamix). At the nanoscale on a BD Symphony machine, side light scatter provides relative size estimates (100 nm-900 nm beads). The beads are fluorescently labeled, which is the advantage of nanoscale flow cytometry because targeted signals emerge from the unlabeled nanoscale “noise”. Platelet or SW480 media stained for CD41 or EpCAM, respectively, revealed both larger microvesicles and smaller exosome populations. B and C: addition of thrombin and cancer cells to platelets led to an increase in tissue factor-positive (TF+), Annexin V-positive (AV+), CD41-positive (CD41a+) platelet EVs (B), which was reproducible in repeated experiments performed in triplicate (C). Data are means ± SE (n = 3). For comparing treatments to each other, Student’s t-test was used. *P < 0.05. EVs, extracellular vesicles; PLT, platelets.
SW480 colon cancer cells induce thrombus formation under flow.
We next aimed to study platelet-colon cancer cell interactions under physiologically relevant levels of fluid shear flow. To this end, recalcified PRP was perfused over immobilized SW480 colon cancer cells. In all experiments, PRP was pretreated with 1A6 to block the activation of the intrinsic pathway of coagulation in plasma. A shown in Fig. 4 and Supplemental Video S1 (Supplemental Material for this article is available online at the Journal website), platelets (green) readily adhered to SW480 colon cancer cells (blue) and were able to form large and stable aggregates and incite fibrin formation (red). To confirm the role of TF and thrombin in the procoagulant activity induced by SW480 colon cancer cells, we used a function-blocking antibody against TF antibody or hirudin, a direct inhibitor of thrombin activity. Both the anti-TF antibody and hirudin fully inhibited SW480-induced thrombus formation (Supplemental Videos S2 and S3), suggesting that SW480 colon cancer cells elicit platelet aggregation and fibrin formation through thrombin generation via selective activation of the extrinsic coagulation cascade. In contrast, pharmacological inhibition of platelet PAR4 did not seem to have an effect on the degree of fibrin formation as measured by fibrinogen binding over time (Supplemental Video S4). Further studies are required to elucidate potential roles for PAR1, PAR4, and receptors including GPIb, P-selectin or CD44 in mediating platelet-tumor cell interactions leading to thrombus formation under flow.
Fig. 4.
Visualization of SW480 colon cancer cells induced platelet aggregation and fibrin formation under flow. SW480 colon cancer cells were grown in Ibidi channel slides and incubated with a blocking anti-tissue factor (TF) antibody (10 µg/ml) or with vehicle for 20 min. Subsequently, recalcified (25 mM CaCl2) platelet-rich plasma (PRP) pretreated with either 40 µg/ml of the thrombin inhibitor hirudin, 10 µM of the PAR4 inhibitor VU652925, or with vehicle and containing 40 µg/ml AF-546 fibrinogen was perfused at 2.5 dyn/cm2 shear stress over SW480 cancer cells for a total time of 10 min. A: representative fluorescent images at ×20 are shown. Platelets were stained with an anti-CD41 antibody (green), fibrin is in red, and cancer cells were stained with the nuclear marker DAPI (blue). Scale bar, 10 µm. B: bar graphs showing data from three fields of view of two experiments (n = 2). AU, arbitrary units. Data are means ± SE.
Thrombosis is associated with colon cancer in patients.
We identified 39,862 colon cancer patients and 10,321,311 control patients (Table 1). The control and colon cancer cohorts varied in age; ~18% of the patients with colon cancer were younger than 50 compared with 48% of the control patients. The colon cancer cohort had slightly more women compared with the control cohort (15% vs. 7%). The distribution of race within the two populations was also similar, and patients were predominantly Caucasian (73%–76%). Overall, the number of venous thromboembolisms (VTEs)/1,000 person-years was found to be higher in colon cancer patients than the control population [10 vs. 2.2, age-adjusted rate ratio: 3.5, 95% confidence interval (CI) 3.4–3.6, P < 0.001]. The median time to first VTE was similar in both populations. Notably, the majority (~68%) of the VTEs in the colon cancer patients occurred following the diagnosis of cancer.
Table 1.
Thrombosis occurrence in patients with colon cancer
| Characteristic | Colon Cancer Patients | Control Patients |
|---|---|---|
| Total, no. (%) | 39,862 (100%) | 10,321,311 (100%) |
| Sex | ||
| Male, no. (%) | 38,760 (85%) | 9,592,001 (93%) |
| Female, no. (%) | 5,845 (15%) | 716,979 (6.9%) |
| Age | ||
| <25, no. (%) | 121 (0.3%) | 1,571,211 (15%) |
| 26–50, no. (%) | 7,081 (18%) | 3,384,087 (33%) |
| 51–75, no. (%) | 27,928 (70%) | 4,690,275 (45%) |
| 76–100, no. (%) | 4,731 (12%) | 675,499 (6.5%) |
| >100, no. (%) | 1 (0.003%) | 424 (0.004%) |
| Follow-up time | ||
| Person-years | 354,603 | 99,782,242 |
| VTE events | ||
| Total | 3,550 | 216,391 |
| After diagnosis, no. (%) | 2,404 (68%) | Not applicable |
| Time to first VTE | ||
| Median (IQR) | 6 (4) | 6 (4) |
| VTE rate (/1,000 person years), 95th CI | 10.0 (9.7–10.3) | 2.2 (2.16–2.18) |
| Relative rate (age adjusted), 95th CI | 3.5 (3.4–3.6) | Reference |
CI, confidence interval; VTE, venous thromboembolism.
In conclusion, the high incidence of thrombotic events in cancer patients has prompted many efforts to elucidate the etiology of cancer-associated thrombosis. The relative contribution of cancer cells, coagulation, or platelets to thrombus formation remains in dispute, however. In this in vitro study, we show that thrombin enhanced the binding of platelets to procoagulant SW480 colon cancer cells and we demonstrate that the enhanced platelet adhesion is dependent on PAR4 activation. In addition, we show that the mechanism of enhanced adhesion of platelets to cancer cells also required secondary soluble mediators of platelet activation, TXA2 and ADP, since this interaction could also be inhibited by aspirin and ticagrelor. By using an in vitro flow assay we show that immobilized SW480 colon cancer cells trigger thrombus formation in a TF- and thrombin-dependent manner and we suggest PAR4 as a potential target to prevent experimental colon cancer cell-associated thrombus formation in vitro. We demonstrate that platelets are the primary experimental source of procoagulant EVs, mainly MVs (defined as larger than 200 nm). We support our in vitro observations with a large retrospective observational study, confirming that patients with colon cancer have an increased risk of developing thrombotic events as compared with patients without known malignancy.
In 1865, Armand Trousseau suggested that thrombotic events were a presenting feature of occult cancer (12, 47a). This finding marked the beginning of a series of studies designed to investigate the molecular mechanisms underlying cancer-associated thrombosis. Subsequent studies have demonstrated that cancer cell-associated TF may be a key player in initiating or promoting thrombin generation leading to fibrin formation, platelet activation, and adhesion to cancer cells, and that this mechanism may even play a role in cancer-associated thrombosis (1, 25, 33). In agreement with this concept, we found a significant reduction in fibrin formation when SW480 colon cancer cells were pretreated with a TF-blocking antibody (Fig. 1A). The observation that cancer cells establish juxtacrine interactions with platelets has already been established by our group and others; however, the majority of these observations were performed in absence of coagulation (2, 26, 27, 31, 34). Herein, we show by immunofluorescent analysis of SW480 colon cancer cell-platelet rich plasma suspensions that a high proportion of the platelets are bound to cancer cell membranes rich in TF; in vitro binding of platelets to cancer cells and cancer-induced thrombus formation under flow were disrupted by targeting TF activity with a specific blocking antibody (Figs. 1B and 4).
Many studies have proposed antiplatelet agents as prophylaxis against cancer-associated thrombosis (30, 32). In this study we demonstrate that aspirin, a cyclooxygenase-1 inhibitor, and ticagrelor, a selective P2Y12 inhibitor, both potent antiplatelet agents, were able to significantly reduce platelet adhesion to and activation by SW480 colon cancer cells in the presence of thrombin in vitro. This result suggests that the cross talk between platelets and cancer cells might be dependent on secondary messengers including TXA2 and ADP. Importantly, while the etiology of this response has yet to be fully determined, our data support the notion that release of TXA2 and ADP caused by thrombin is triggered by activation of PAR signaling. In good agreement with this, pretreatment of platelets with the PAR4 inhibitor seemed to reduce platelet adhesion to SW480 colon cancer cells under flow (Fig. 4). Moreover, we provide evidence that platelet treatment with a PAR4 antagonist prevents thrombin-induced platelet activation and binding to SW480 colon cancer cells (Fig. 2). The potential clinical implication is that antiplatelet agents, such as PAR4 inhibitors, may be effective in preventing thrombotic events in patients with cancer. This would be of crucial importance given the higher risk of VTE in patients with colon cancer observed in our retrospective observational study. Inhibition of PAR4 could dampen the initiation and amplification of platelet activation by procoagulant cancer cells or possibly, inhibit the direct procoagulant effect of platelets or the release of procoagulant EVs by platelets. Moreover, PAR4 has been proposed as the primary PAR family member mediating the shedding of platelet-derived procoagulant EVs (13). Yet, given the genetic and phenotypic differences among cancer cells, inhibition of PAR4 could yield differential responses when different lines of cancer cells are used in vitro or when proposed for use as a therapeutic in select cancers in patients. Future work is warranted to expand this study to a larger number of cancer cell types characterized by diverse genotypic and phenotypic properties.
Activated platelets are known to provide a catalytic surface for the activation of blood coagulation; they enhance the activation of clotting by exposing anionic phospholipids, in particular PS, on their membrane or on EVs shed from their membrane (8). Moreover, there is convincing evidence that circulating EVs likely play a role in coagulation and cancer-associated thrombosis (10, 18, 19). However, the cell source of procoagulant EVs remained undefined. Our in vitro data would suggest that the source is most likely platelets. We show an important enrichment of platelet-derived PS and TF exposing EVs, identified as CD41+/Annexin V+ or CD41+/Annexin V+/TF+ events (Fig. 3). In addition, in accordance with other studies, we found that platelet-derived procoagulant EVs were primarily MVs as compared with EXO-sized vesicles (21). The presence of TF on the membrane of platelet EVs may be necessary to cause localized activate of coagulation factors to recruit more platelets and amplify thrombin generation. However, it remains to be studied whether TF expressed on platelet EVs is endogenous to platelets or is trafficking from SW480 colon cancer cell membranes. The transfer might occur via fusion of cancer cell membranes rich in TF with the platelet membrane. Additional studies will be required to establish whether the expression of TF on platelet EVs contributes to SW480-induced thrombus formation.
Despite decades of important research and clinical studies, there is still an unmet need to develop safer and more effective therapies and prophylactic interventions for cancer-associated thrombosis. Thrombosis in cancer is a dynamic and complex process that may have multiple heterogeneous causes depending on the individual and cancer type. A better understanding of platelet-cancer cell interactions and platelet release of procoagulant EVs may provide rationale for the development of antiplatelet and anticoagulant therapies to prevent cancer-associated thrombosis. Targeting platelet-cancer cell interactions with the use of PAR4 antagonists may be a promising approach for preventing or limiting the risk of VTE in patients with cancer, balancing safety with efficacy. In accordance with this, preclinical and clinical studies have provided evidence that anti-PAR4 therapeutics may be useful in limiting thrombus formation without affecting hemostasis (16, 17, 37, 52).
GRANTS
This work was supported by National Institutes of Health Grants R01HL101972, R01GM116184, R01HL133923, F31HL13623001, and R03HD096173 and the Altarum Institute. This work was also funded by the OHSU/OSU Cancer Prevention and Control Initiative, the Knight Cancer Institute (CEDAR), and the Big Data-Scientist Training Enhancement Program (BD-STEP), which is a collaboration between the VA Employee Education System, the VA Office of Academic Affiliations, the VA Office of Research and Development, and the National Cancer Institute’s Center for Strategic Scientific Initiatives. This material is based on work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development.
DISCLAIMERS
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US Government.
DISCLOSURES
Oregon Health & Science University and A. Gruber have a significant financial interest in Aronora, Inc., a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. None of the other authors have any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
A.M. conceived and designed research; A.M., S.T.T., J.L.S., J.Z.-R., T.S., J.F.H., R.K., R.A., and T.K.M. performed experiments; A.M., S.T.T., Y.Z., X.N., and T.K.M. analyzed data; A.M. interpreted results of experiments; A.M., S.T.T., J.L.S., and T.K.M. prepared figures; A.M. drafted manuscript; A.M., S.T.T., J.J.S., Y.T., T.K.M., and O.J.T.M. edited and revised manuscript; A.M., S.T.T., J.L.S., T.S., J.F.H., R.K., Y.Z., X.N., J.J.S., S.E., M.T.D., H.E.H., A.G., C.D.W., Y.T., T.K.M., and O.J.T.M. approved final version of manuscript.
Supplemental Data
SW480 colon cancer cells induce thrombus formation under flow - .wmv (509 KB)
SW480 colon cancer cell-induced thrombus formation is TF-dependent - .mp4 (150 KB)
Effect of hirudin on SW480 colon cancer cell-induced thrombus formation - .mp4 (192 KB)
Effect of PAR-4 inhibition on SW80 colon cancer cell-induced thrombus formation - .wmv (447 KB)
REFERENCES
- 1.Alves CS, Yakovlev S, Medved L, Konstantopoulos K. Biomolecular characterization of CD44-fibrin(ogen) binding: distinct molecular requirements mediate binding of standard and variant isoforms of CD44 to immobilized fibrin(ogen). J Biol Chem 284: 1177–1189, 2009. doi: 10.1074/jbc.M805144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker-Groberg SM, Itakura A, Gruber A, McCarty OJ. Role of coagulation in the recruitment of colon adenocarcinoma cells to thrombus under shear. Am J Physiol Cell Physiol 305: C951–C959, 2013. doi: 10.1152/ajpcell.00185.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastida E, Ordinas A, Escolar G, Jamieson GA. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood 64: 177–184, 1984. [PubMed] [Google Scholar]
- 4.Berny-Lang MA, Aslan JE, Tormoen GW, Patel IA, Bock PE, Gruber A, McCarty OJ. Promotion of experimental thrombus formation by the procoagulant activity of breast cancer cells. Phys Biol 8: 015014, 2011. doi: 10.1088/1478-3975/8/1/015014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromberg ME, Bailly MA, Konigsberg WH. Role of protease-activated receptor 1 in tumor metastasis promoted by tissue factor. Thromb Haemost 86: 1210–1214, 2001. doi: 10.1055/s-0037-1616053. [DOI] [PubMed] [Google Scholar]
- 7.Cointe S, Judicone C, Robert S, Mooberry MJ, Poncelet P, Wauben M, Nieuwland R, Key NS, Dignat-George F, Lacroix R. Standardization of microparticle enumeration across different flow cytometry platforms: results of a multicenter collaborative workshop. J Thromb Haemost 15: 187–193, 2017. doi: 10.1111/jth.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol 41: 302–310, 2014. doi: 10.1053/j.seminoncol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost 3: 1800–1814, 2005. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 10.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost 6: 1517–1524, 2008. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 11.Davison GM, Nkambule BB, Mkandla Z, Hon GM, Kengne AP, Erasmus RT, Matsha TE. Platelet, monocyte and neutrophil activation and glucose tolerance in South African Mixed Ancestry individuals. Sci Rep 7: 40329, 2017. doi: 10.1038/srep40329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donati MB. Cancer and thrombosis: from Phlegmasia alba dolens to transgenic mice. Thromb Haemost 74: 278–281, 1995. [PubMed] [Google Scholar]
- 13.Duvernay M, Young S, Gailani D, Schoenecker J, Hamm HE. Protease-activated receptor (PAR) 1 and PAR4 differentially regulate factor V expression from human platelets. Mol Pharmacol 83: 781–792, 2013. doi: 10.1124/mol.112.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvernay MT, Temple KJ, Maeng JG, Blobaum AL, Stauffer SR, Lindsley CW, Hamm HE. Contributions of protease-activated receptors PAR1 and PAR4 to thrombin-induced GPIIbIIIa activation in human platelets. Mol Pharmacol 91: 39–47, 2017. doi: 10.1124/mol.116.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvorak HF, Van DeWater L, Bitzer AM, Dvorak AM, Anderson D, Harvey VS, Bach R, Davis GL, DeWolf W, Carvalho AC. Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res 43: 4434–4442, 1983. [PubMed] [Google Scholar]
- 16.French SL, Arthur JF, Lee H, Nesbitt WS, Andrews RK, Gardiner EE, Hamilton JR. Inhibition of protease-activated receptor 4 impairs platelet procoagulant activity during thrombus formation in human blood. J Thromb Haemost 14: 1642–1654, 2016. doi: 10.1111/jth.13293. [DOI] [PubMed] [Google Scholar]
- 17.French SL, Thalmann C, Bray PF, Macdonald LE, Murphy AJ, Sleeman MW, Hamilton JR. A function-blocking PAR4 antibody is markedly antithrombotic in the face of a hyperreactive PAR4 variant. Blood Adv 2: 1283–1293, 2018. doi: 10.1182/bloodadvances.2017015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freyssinet JM. Cellular microparticles: what are they bad or good for? J Thromb Haemost 1: 1655–1662, 2003. doi: 10.1046/j.1538-7836.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 19.Gardiner C, Harrison P, Belting M, Böing A, Campello E, Carter BS, Collier ME, Coumans F, Ettelaie C, van Es N, Hochberg FH, Mackman N, Rennert RC, Thaler J, Rak J, Nieuwland R. Extracellular vesicles, tissue factor, cancer and thrombosis - discussion themes of the ISEV 2014 Educational Day. J Extracell Vesicles 4: 26901, 2015. doi: 10.3402/jev.v4.26901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groot Kormelink T, Arkesteijn GJ, Nauwelaers FA, van den Engh G, Nolte-’t Hoen EN, Wauben MH. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A 89: 135–147, 2016. doi: 10.1002/cyto.a.22644. [DOI] [PubMed] [Google Scholar]
- 21.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94: 3791–3799, 1999. [PubMed] [Google Scholar]
- 22.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 5: 632–634, 2007. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 23.Kravtsov DV, Matafonov A, Tucker EI, Sun MF, Walsh PN, Gruber A, Gailani D. Factor XI contributes to thrombin generation in the absence of factor XII. Blood 114: 452–458, 2009. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima LG, Leal AC, Vargas G, Porto-Carreiro I, Monteiro RQ. Intercellular transfer of tissue factor via the uptake of tumor-derived microvesicles. Thromb Res 132: 450–456, 2013. doi: 10.1016/j.thromres.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 25.McCarty OJ, Jadhav S, Burdick MM, Bell WR, Konstantopoulos K. Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys J 83: 836–848, 2002. doi: 10.1016/S0006-3495(02)75212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarty OJ, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling, and firm adhesion under dynamic flow conditions. Blood 96: 1789–1797, 2000. [PubMed] [Google Scholar]
- 27.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev 33: 231–269, 2014. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer 136: 462–475, 2015. doi: 10.1002/ijc.28997. [DOI] [PubMed] [Google Scholar]
- 29.Mezouar S, Frère C, Darbousset R, Mege D, Crescence L, Dignat-George F, Panicot-Dubois L, Dubois C. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res 139: 65–76, 2016. doi: 10.1016/j.thromres.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Mitrugno A, McCarty OJT. Ticagrelor breaks up the tumor-platelet party. Blood 130: 1177–1178, 2017. doi: 10.1182/blood-2017-07-795898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitrugno A, Sylman JL, Ngo AT, Pang J, Sears RC, Williams CD, McCarty OJ. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: Implications for the oncoprotein c-MYC. Am J Physiol Cell Physiol 312: C176–C189, 2017. doi: 10.1152/ajpcell.00196.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitrugno A, Sylman JL, Rigg RA, Tassi Yunga S, Shatzel JJ, Williams CD, McCarty OJT. Carpe low-dose aspirin: the new anti-cancer face of an old anti-platelet drug. Platelets 29: 773–778, 2018. doi: 10.1080/09537104.2017.1416076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitrugno A, Tormoen GW, Kuhn P, McCarty OJ. The prothrombotic activity of cancer cells in the circulation. Blood Rev 30: 11–19, 2016. doi: 10.1016/j.blre.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitrugno A, Williams D, Kerrigan SW, Moran N. A novel and essential role for FcγRIIa in cancer cell-induced platelet activation. Blood 123: 249–260, 2014. doi: 10.1182/blood-2013-03-492447. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Kastresana A, Telford B, Musich TA, McKinnon K, Clayborne C, Braig Z, Rosner A, Demberg T, Watson DC, Karpova TS, Freeman GJ, DeKruyff RH, Pavlakis GN, Terabe M, Robert-Guroff M, Berzofsky JA, Jones JC. Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep 7: 1878, 2017. doi: 10.1038/s41598-017-01731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan TK. Cell- and size-specific analysis of placental extracellular vesicles in maternal plasma and pre-eclampsia. Transl Res 201: 40–48, 2018. doi: 10.1016/j.trsl.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mumaw MM, de la Fuente M, Noble DN, Nieman MT. Targeting the anionic region of human protease-activated receptor 4 inhibits platelet aggregation and thrombosis without interfering with hemostasis. J Thromb Haemost 12: 1331–1341, 2014. doi: 10.1111/jth.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nierodzik ML, Chen K, Takeshita K, Li JJ, Huang YQ, Feng XS, D’Andrea MR, Andrade-Gordon P, Karpatkin S. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood 92: 3694–3700, 1998. [PubMed] [Google Scholar]
- 39.Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, Gruber A, McCarty OJ. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood 125: 1488–1496, 2015. doi: 10.1182/blood-2014-10-604587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puy C, Tucker EI, Wong ZC, Gailani D, Smith SA, Choi SH, Morrissey JH, Gruber A, McCarty OJ. Factor XII promotes blood coagulation independent of factor XI in the presence of long-chain polyphosphates. J Thromb Haemost 11: 1341–1352, 2013. doi: 10.1111/jth.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramacciotti E, Hawley AE, Farris DM, Ballard NE, Wrobleski SK, Myers DD Jr, Henke PK, Wakefield TW. Leukocyte- and platelet-derived microparticles correlate with thrombus weight and tissue factor activity in an experimental mouse model of venous thrombosis. Thromb Haemost 101: 748–754, 2009. doi: 10.1160/TH08-09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rautou PE, Mackman N. Microvesicles as risk markers for venous thrombosis. Expert Rev Hematol 6: 91–101, 2013. doi: 10.1586/ehm.12.74. [DOI] [PubMed] [Google Scholar]
- 43.Sylman JL, Mitrugno A, Atallah M, Tormoen GW, Shatzel JJ, Tassi Yunga S, Wagner TH, Leppert JT, Mallick P, McCarty OJT. The predictive value of inflammation-related peripheral blood measurements in cancer staging and prognosis. Front Oncol 8: 78, 2018. doi: 10.3389/fonc.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sylman JL, Mitrugno A, Tormoen GW, Wagner TH, Mallick P, McCarty OJT. Platelet count as a predictor of metastasis and venous thromboembolism in patients with cancer. Converg Sci Phys Oncol 3: 023001, 2017. doi: 10.1088/2057-1739/aa6c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost 5: 520–527, 2007. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 46.Thomas GM, Brill A, Mezouar S, Crescence L, Gallant M, Dubois C, Wagner DD. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost 13: 1310–1319, 2015. doi: 10.1111/jth.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med 206: 1913–1927, 2009. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47a.Trousseau A. Phlegmasia alba dolens. Lectures on Clinical Medicine: Delivered at the Hôtel-Dieu, Paris. London: J. E. Adlard, Bartholomew Close, 1865, p 281–332. [Google Scholar]
- 48.van der Vlist EJ, Nolte-’t Hoen EN, Stoorvogel W, Arkesteijn GJ, Wauben MH. Fluorescent labeling of nano-sized vesicles released by cells and subsequent quantitative and qualitative analysis by high-resolution flow cytometry. Nat Protoc 7: 1311–1326, 2012. doi: 10.1038/nprot.2012.065. [DOI] [PubMed] [Google Scholar]
- 49.Villares GJ, Zigler M, Bar-Eli M. The emerging role of the thrombin receptor (PAR-1) in melanoma metastasis–a possible therapeutic target. Oncotarget 2: 8–17, 2011. doi: 10.18632/oncotarget.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vu TK, Wheaton VI, Hung DT, Charo I, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature 353: 674–677, 1991. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- 51.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 119: 5543–5552, 2012. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson SJ, Ismat FA, Wang Z, Cerra M, Narayan H, Raftis J, Gray TJ, Connell S, Garonzik S, Ma X, Yang J, Newby DE. PAR4 (protease-activated receptor 4) antagonism with BMS-986120 inhibits human ex vivo thrombus formation. Arterioscler Thromb Vasc Biol 38: 448–456, 2018. doi: 10.1161/ATVBAHA.117.310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong PC, Seiffert D, Bird JE, Watson CA, Bostwick JS, Giancarli M, Allegretto N, Hua J, Harden D, Guay J, Callejo M, Miller MM, Lawrence RM, Banville J, Guy J, Maxwell BD, Priestley ES, Marinier A, Wexler RR, Bouvier M, Gordon DA, Schumacher WA, Yang J. Blockade of protease-activated receptor-4 (PAR4) provides robust antithrombotic activity with low bleeding. Sci Transl Med 9: eaaf5294, 2017. doi: 10.1126/scitranslmed.aaf5294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SW480 colon cancer cells induce thrombus formation under flow - .wmv (509 KB)
SW480 colon cancer cell-induced thrombus formation is TF-dependent - .mp4 (150 KB)
Effect of hirudin on SW480 colon cancer cell-induced thrombus formation - .mp4 (192 KB)
Effect of PAR-4 inhibition on SW80 colon cancer cell-induced thrombus formation - .wmv (447 KB)