Abstract
Chronic pulmonary diseases, including idiopathic pulmonary fibrosis (IPF), pulmonary hypertension (PH), and chronic obstructive pulmonary disease (COPD), account for staggering morbidity and mortality worldwide but have limited clinical management options available. Although great progress has been made to elucidate the cellular and molecular pathways underlying these diseases, there remains a significant disparity between basic research endeavors and clinical outcomes. This discrepancy is due in part to the failure of many current disease models to recapitulate the dynamic changes that occur during pathogenesis in vivo. As a result, pulmonary medicine has recently experienced a rapid expansion in the application of engineering principles to characterize changes in human tissues in vivo and model the resulting pathogenic alterations in vitro. We envision that engineering strategies using precision biomaterials and advanced biomanufacturing will revolutionize current approaches to disease modeling and accelerate the development and validation of personalized therapies. This review highlights how advances in lung tissue characterization reveal dynamic changes in the structure, mechanics, and composition of the extracellular matrix in chronic pulmonary diseases and how this information paves the way for tissue-informed engineering of more organotypic models of human pathology. Current translational challenges are discussed as well as opportunities to overcome these barriers with precision biomaterial design and advanced biomanufacturing techniques that embody the principles of personalized medicine to facilitate the rapid development of novel therapeutics for this devastating group of chronic diseases.
Keywords: biomanufacturing, biomaterials, chronic obstructive pulmonary disease, in vitro, precision disease modeling, pulmonary engineering, pulmonary fibrosis, pulmonary hypertension, regenerative medicine, tissue-informed engineering
INTRODUCTION
Chronic pulmonary diseases, including idiopathic pulmonary fibrosis (IPF), pulmonary hypertension (PH), and chronic obstructive pulmonary disease (COPD), account for tremendous morbidity and mortality worldwide and impact the lives of hundreds of millions of people daily. In fact, these diseases alone account for 10% of all disability-adjusted life-years globally, a metric that estimates the amount of active and productive life lost due to a condition (160). Unfortunately, many chronic pulmonary diseases are considered irreversible and progressive, resulting in nearly four million premature deaths every year (44). Lung transplantation is currently the only established therapeutic option for all end-stage pulmonary disease, with over 4,000 procedures reported annually (190). Although donor organ shortages have been addressed by innovative approaches to increase the number of available donor lungs, it remains one of the major limitations to treating chronic pulmonary diseases, leading to mortality rates of up to 16% per year for patients currently on waiting lists (190). Preclinical animal models, particularly in rodent species, have been used to study pulmonary disease pathogenesis and to evaluate mechanisms and potential therapeutic interventions. Although certainly informative, it has become increasingly clear that these models alone may not be adequate for providing a complete window into mechanisms of pulmonary disease in humans or for determining drug safety and effectiveness (163).
Current bioengineering approaches propose to address these limitations through the use of mimetic physiological models of human tissue for study of disease progression. In particular, emerging research in lung regeneration is focused on better understanding and characterizing changes in the structure, mechanics, and composition underlying human pulmonary diseases and using this information to inform the development of in vitro models that can be used to identify novel therapeutic strategies (160). Toward achieving this goal, we propose an integrated framework (Fig. 1) that incorporates outputs from advance tissue characterization into engineering tissue-informed disease model platforms using precision biomaterials and advanced biomanufacturing techniques to create more organotypic models of disease. Once verified and validated, the translation of these models into the commercial drug discovery sector could be a gateway to new precision medical treatments. Likewise, the outcomes of these treatments can be evaluated by using the same advanced characterization tools, giving this iterative approach the potential to transform patient care in pulmonary medicine. Here, we review the microenvironmental changes in pulmonary tissue that are key drivers of disease progression, recent developments in engineered models of chronic pulmonary diseases, and opportunities to leverage state-of-the-art precision biomaterials and biomanufacturing techniques to enhance our ability to recapitulate human pathophysiology in vitro.
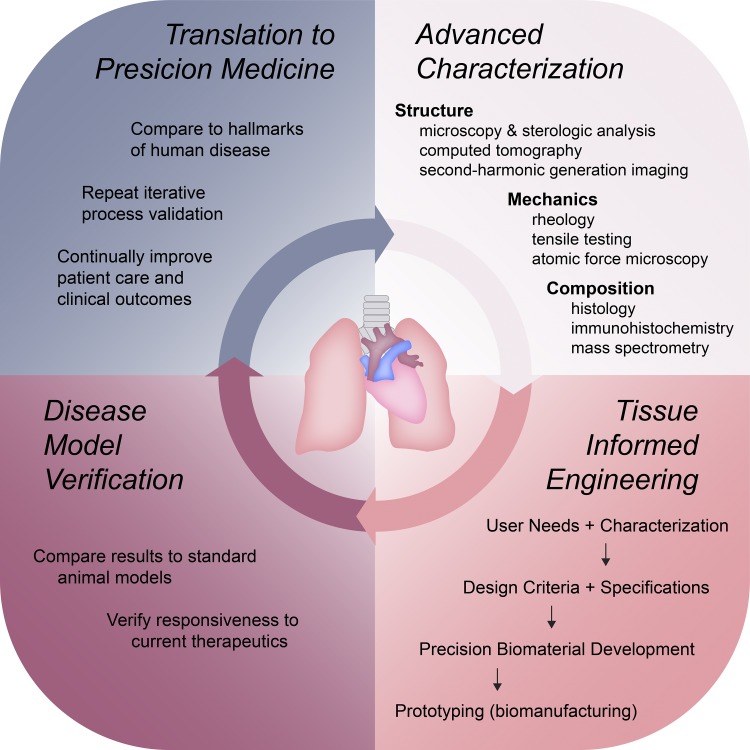
Fig. 1.
Integrated framework for guiding the development and implementation of tissue-informed engineering strategies for modeling human pulmonary disease. 1) Biomaterial design parameters and requirements are elucidated via advanced characterization techniques. 2) These inputs inform and guide the engineering process, resulting in patient- and disease- specific in vitro models. 3) The resulting models can be incorporated into preclinical evaluations to provide greater predictive power for potential therapeutic success in humans. 4) Successful translation of outcomes to innovative patient care builds the foundation for precision medicine. The power of this model lies in its potential for iterative evaluation and continuous improvement.
ADVANCED CHARACTERIZATION OF MICROENVIRONMENTAL CHANGES IN CHRONIC PULMONARY DISEASES
In vivo, cells within the lung are precisely organized into specialized structures that are adapted to the particular function of the tissue through interactions with the extracellular matrix (ECM), a heterogeneous, composite network of soluble and insoluble proteins (73), growth factors (165), and polysaccharides. Structurally, cell-secreted proteins, such as collagens and elastins, provide a mechanical framework to facilitate cell anchorage, cell-cell interactions, and tissue formation. Although, the ECM has historically been viewed as a simple structural support for cells, it is now increasingly recognized as a critical contributor to not only the maintenance of healthy tissue but also to the pathology of disease states (130). It has become clear over a large number of studies that the structure, mechanics, and composition of the microenvironment influence several cellular events, most notably cell adhesion (61, 153), migration (121, 172), proliferation (7, 81), protein expression (87, 185), gene regulation (81), and differentiation (34, 41, 114). While the ECM exerts critical influence over cellular behavior, cells also have the capacity to modify their microenvironment by dynamically synthesizing, degrading, and remodeling the ECM. This elegant cell-ECM connection creates a reciprocal relationship between cells and the biochemical and biomechanical properties exhibited by the surrounding matrix, which when dysregulated is a fundamental contributor to the pathogenesis of many chronic pulmonary diseases (51, 58) (Fig. 2). Advanced characterization of the structure, mechanics and composition of lung and pulmonary vasculature ECM has revealed the complexities of chronic pulmonary disease phenotypes. This knowledge has the potential to revolutionize our understanding of disease etiology and progression and provides a foundation for engineering tissue-informed models of pulmonary disease.
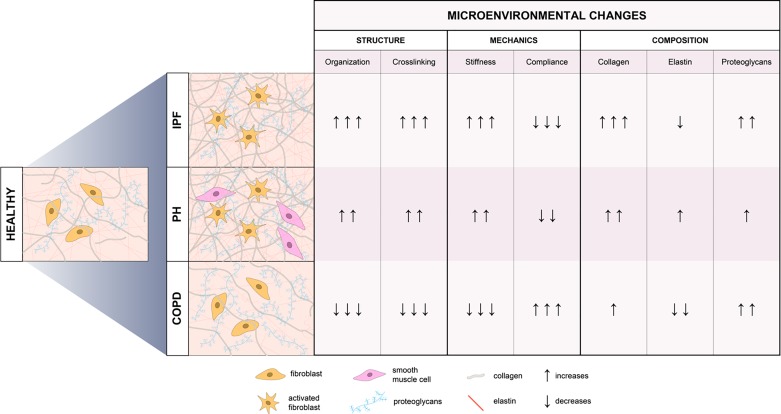
Fig. 2.
Microenvironmental changes observed in chronic pulmonary diseases: idiopathic pulmonary fibrosis (IPF), pulmonary hypertension (PH), and chronic obstructive pulmonary disease (COPD). Highlighted here are distinct structural, mechanical, and compositional signatures revealed by advanced tissue characterization. A comprehensive understanding of pathological extracellular matrix properties is critical to informing the design of engineered in vitro models of pulmonary disease.
Lung Structure Characterization
Lungs are a uniquely complex organ comprising a network of airway branches and vasculature that exhibit hierarchical structure to optimize oxygen exchange in a dynamic environment that expands and contracts during cycles of respiration (123). Macroscale characterization using stereological analysis of microscopic images or computed tomography (CT) imaging has enabled scientists and clinicians to visualize and quantify the complex structures that make up the lung and pulmonary vasculature. Airways decrease in size along this hierarchy and terminate with the smallest, most numerous portion of the respiratory track, alveoli. Stereological analysis of lung tissue sections have revealed that these structures are numerous (274–790 million per lung) and approximately spherical, with an average diameter of 200 μm (123). Similarly, there are 15 orders of arteries between the main pulmonary artery and the capillary beds that surround the alveoli. Clinical CT images and stereological analyses show that these vessels range in size from ~0.02 to 15 mm in diameter (67) within the lung and up to 25 mm in the main pulmonary artery (170). Although CT imaging is a power tool for clinicians to image lung structure in real-time, the lack of resolution at the cellular level limits its practice for investigating detailed mechanisms contributing to pulmonary disease, where the onset and progression of pathogenesis often begins at the cell-ECM interface.
Advances in imaging technology and computing power have enabled 3D tissue reconstructions with micro- and nanoscale resolution to become powerful tools for measuring heterogeneities in pulmonary tissue microarchitecture. For example, fibrotic foci in IPF were traditionally considered to form a large interconnected fibrotic reticulum (35). Recently, however, integrating micro-CT with traditional immunohistochemistry-based histology to create 3D reconstructions of fibrotic lung sections have revealed that fibrotic foci are spread, independent structures lacking interconnection (75). Micro-CT measurements have been further employed to assess narrowing and disappearance of small conducting airways before the onset of emphysematous COPD (113, 178).
Similarly, second-harmonic generation imaging (SHG) is emerging as a complementary method to traditional widefield and confocal microscopy techniques for evaluating macromolecular changes in the ECM without the requirement for antigenic stains (31). This method employs two-photon microscopy to identify nonlinear structural characteristics such as collagen and elastin fiber organization. Images of fibrillar collagen obtained with SHG have confirmed increased ECM deposition in fibrotic regions in human IPF lung tissues (131). Likewise, changes in collagen fibril organization have also been confirmed by SHG in emphysematous COPD lung tissues when compared with healthy donors (1). These advanced imaging techniques will allow clinicians, scientists, and engineers to observe microscale structural changes that evolve during disease progression.
Lung Mechanics Characterization
Lung ECM also provides the mechanical stability and elastic recoil necessary to support stretch and relaxation that must occur during normal respiration. In fact, normal respiratory cycles result in linear distention of 0 to 5% (143) on the tissue level and an astounding 5–25% on the cellular level (143). Thus, the mechanical nature of pulmonary tissues requires both an elastic (energy preserving) and a viscous (energy dissipative) response to mechanical loads (71). One mechanical property that is critical when discussing lung tissue mechanics or materials for disease modeling applications is the stiffness of the material, which is often described as the Young’s or elastic modulus (E). The modulus value for a tissue or biomaterial is calculated by applying stress to a sample and measuring the proportional deformation (strain). Here, we will use the terms modulus and stiffness interchangeably.
Tissue (macroscale) and ECM (microscale) mechanics change with time. Mechanical loads experienced in short time scales (e.g., inflation and deflation of the lung) are generally elastic in nature, meaning the tissue will stretch and recover completely after the mechanical load has been removed. Forces generated over longer time scales (e.g., sustained tissue strain) are met with more viscous behavior, i.e., deformation of the tissue remains after the mechanical load has been removed. Early attempts to measure lung tissue viscoelasticity relied on rheological experiments where small strains were applied in shear to evaluate both the elastic and viscous components of the tissue (48). These same rheological tests are still employed to study the mechanical properties of biomaterials used to recapitulate human tissues in vitro (6). Similarly, to better simulate lung mechanics at higher strain levels, measurements of lung tissue strips under tension have been developed. In these experiments, large tensions have been applied to lung tissues to elucidate the roles of collagen and elastin in the mechanical properties of lung ECM (186). Although these mechanical tests have revealed a wealth of information on lung mechanics, such macroscale measurements fail to describe tissue properties at a scale relevant to cell-ECM interactions.
The introduction of microscale elasticity measurements, such as atomic force microscopy (AFM) (106), has redefined the contributions of tissue mechanics to disease progression at length scales that are relevant to regional changes in ECM and resulting adaptations in cellular function. The average elastic moduli for healthy and fibrotic human lung tissue have been measured to be 2 and 17 kPa, respectively, by AFM (18). These results demonstrate how changes in ECM composition during fibrosis increase average matrix stiffness dramatically. Lui et al. (105) combined AFM with immunohistochemistry to further define the heterogenous lung tissue landscape that develops throughout fibrotic pathogenesis (105). Results derived from healthy and bleomycin-injured rat lungs demonstrated that regions rich in collagen I displayed an overall increase in stiffness from 1.4 to 8.7 kPa, with localized regions as high as 22 kPa.
Similarly, the evaluation of intact lung slices with AFM provides the ability to probe microscale elasticity in distinct regions with complex tissue architecture such as the microvessels commonly indicated in the pathogenesis of PH. This technique has enabled researchers to measure the modulus of pulmonary arteries from healthy and diseased patients with PH. The stiffness of pulmonary arteries was determined to be 2 and 10 kPa, respectively, representing nearly a 10-fold increase in vessel modulus (103). This detailed mapping of microscale elasticity revealed that pulmonary artery stiffening first arises distally in arteries <100 μm, followed by stiffening of more proximal vessels (103). It is clear from both macro- and microscale measurements that pulmonary tissue mechanics change both dynamically and heterogeneously during pathogenesis.
Lung Composition Characterization
The predominant ECM components in the lung are collagens I, III, and IV, elastin, and proteoglycans (25, 188). Collagen I confers tensile strength; collagen III facilitates flexibility; elastin provides recoil properties; and proteoglycans/glycosaminoglycans (GAGs) impart a viscoelastic character to the ECM (167). Insoluble matrix proteins such as collagen and elastin provide the mechanical framework for cell anchorage through integrin binding and support tissue-specific mechanics such as the nonlinear viscoelasticity characteristic of the lung. GAGs reversibly bind and sequester soluble chemical mediators including growth factors and cytokines (12). Traditionally, histological (133) and immunohistochemical (49) methods have identified key biochemical components of lung and vascular ECMs. However, these techniques are limited by availability of antigenic stains as well as optical resolution (76). Emerging mass spectrometry-based proteomic techniques have provided invaluable information on the precise composition of several tissues, greatly increasing the number of uniquely identifiable ECM components (26, 27, 120, 152). Utilizing quantitative mass spectrometry, Gocheva et al. (52) profiled the ECM composition of normal lung, fibrotic lung, primary lung tumors, and lung metastases to the lymph nodes, revealing distinct signatures associated with each tissue and disease (52). In a more recent study, human lung tissues from patients with end-stage COPD or IPF were evaluated using mass spectrometry proteomic analysis. There, researchers discovered unique matrisome signatures for each disease, such as upregulation of ECM-modifying enzymes like matrix metalloproteinases (MMPs) for COPD lungs and impairment in cell communications defined by alterations in cell adhesion laminins for IPF lungs (3).
The lung is a highly organized tissue with unique characteristics apparent at both macroscale and microscale. The ability to study pulmonary disease with both spatial and temporal resolution is critical in understanding disease progression to tailor therapeutic intervention for personalized medicine. Advances in the characterization of tissue structure, mechanics, and composition have allowed researchers to describe pulmonary diseases at a level once unachievable by traditional techniques. It is imperative that this information be incorporated into the design criteria for tissue-informed engineering to create disease models with improved efficacy and predictive strength.
CURRENT TISSUE-INFORMED ENGINEERING STRATEGIES FOR MODELING CHRONIC PULMONARY DISEASES
Until recently, much of our understanding of the mechanisms of pulmonary disease has been acquired by studying cells grown on supraphysiologically stiff tissue culture plates or in animal models where it is difficult to isolate specific cell-matrix interactions (28, 82, 117, 155, 183). Since changes in ECM structure, mechanics, and composition occur dynamically over time, existing in vitro models based on uniform, 2D monolayer culture systems do not adequately reproduce key aspects of pulmonary pathology and the spatiotemporal heterogeneity observed in disease tissue. This disparity between basic research endeavors and clinical observations highlights a critical need to better recapitulate the native ECM in 3D models of study to discover novel mechanistic insights into disease prevention and intervention. Increasingly, researchers have begun to recognize the limitations of traditional preclinical model systems and are now taking advantage of sophisticated engineering strategies to model chronic pulmonary diseases in vitro. Next, we will highlight the synthetic precision biomaterials and biomanufacturing techniques that have improved the physiological relevance of engineered models over the past decade (Table 1).
Table 1.
Summary of biomanufaturing techniques currently used to model chronic pulmonary diseases
| Material | Biomanufacturing Technique | Pathology to Be Modeled | Last Author (Reference) |
|---|---|---|---|
| Human lung tissue | Decellularization | Pulmonary fibrosis | White (18); Bitterman (130); Weiss (177) |
| Human lung tissue | 3D precision-cut lung slices | Pulmonary fibrosis | Wagner (5) |
| Polyacrylamide | Casting | Pulmonary fibrosis | Tschumperlin (104, 106, 110); Zhou (68); Barker (20) |
| Polyacrylamide, cell-derived ECM | Casting | Pulmonary fibrosis | Gonzalez (151) |
| PDMS | Casting | Pulmonary fibrosis | Hinz (10, 101) |
| Collagen | Casting, 3D encapsulation | Pulmonary fibrosis | Sime (42, 125); Mailleux (74); Rosas (173), Erler (36); Monk (144); Hagood (107) |
| Collagen-functionalized alginate | Electrostatic droplet generation | Pulmonary fibrosis | Gomperts (181) |
| PEG (norbornene) | Emulsion polymerization, 3D encapsulation | Pulmonary fibrosis | Anseth (99, 100) |
| Polyacrylamide | Casting | Pulmonary hypertension | Fredenburgh (103) |
| PEG (norbornene) | 3D encapsulation | Pulmonary hypertension | Akins (154) |
| Matrigel | 3D encapsulation | COPD | Davies (22) |
| Human lung tissue | 3D precision-cut lung slices | COPD | Koenigshoff (158) |
| Human lung tissue | Decellularization | COPD | Weiss (180) |
| Gelatin-modified poly (ε-caprolactone) | Casting | COPD | Stam (94) |
| PDMS | Microfluidic device | COPD | Ingber (13) |
ECM, extracellular matrix; PDMS, polydimethylsiloxane; PEG, polyethylene glycol; COPD, chronic obstructive pulmomary disease.
Modeling IPF
IPF is a chronic, progressive interstitial lung disease with a median survival rate of only 2–4 years from diagnosis (140). IPF is a diagnosis of exclusion in patients, who usually present with nonspecific shortness of breath or cough, with restriction on pulmonary function testing and radiographic evidence of usual interstitial pneumonia by CT scan. This disease currently impacts approximately three million patients worldwide with an annual incidence that is predicted to continue rising (111). Given the severity of IPF, surprisingly few therapeutic treatments are currently available to manage symptoms, and none have been shown to improve survival rates (83, 141). The pathophysiology of IPF is known to be based on a dysregulated reparative response characterized by uncontrolled myofibroblast proliferation and activity, including replacement of functional lung tissue with compositionally and mechanically altered ECM with significant spatial and temporal heterogeneity (25, 57, 64, 182, 188).
Emerging evidence indicates that these changes in ECM composition and mechanical properties are not simply a result of fibrotic remodeling but are actually drivers of disease progression, profoundly and continuously altering cell phenotype, function, survival, and fibrotic activity (45, 57, 63, 107, 171). Over the past decade, researchers have become increasingly interested in studying the mechanotransductive mechanisms involved in these cell-matrix interactions as a process that may be amenable for therapeutic intervention. The development of engineered cell and tissue models of pulmonary fibrosis to more closely emulate human lung structure, mechanics, and composition is a promising way to bridge the gap between preclinical animal model results and human clinical trial outcomes. This topic has been reviewed in detail by Sundarakrishnan et al. (164); however, herein we explore the application of precision biomaterials to studying the cellular and molecular mechanisms of IPF.
Researchers began to design experiments to interrogate mechanosensing in pulmonary fibroblasts by using a biomaterial that was both familiar and readily available in cell biology laboratories: polyacrylamide. Polyacrylamide has been widely used in biochemistry and molecular biology to separate large macromolecules, such as proteins, for visualization and quantification since 1959 (138). Coincidentally, this class of materials, known as hydrogels, were also well suited for mimicking the physiological microenvironment in healthy and diseased lung tissue due to their ability to simulate the high water content and mechanical properties of pulmonary tissues. Hydrogels, highly cross-linked polymer networks, absorb large volumes of water while retaining the ability to be created in specific shapes with precise mechanical properties, decorated with cell-adhesive ligands and/or tethered signaling factors, and tuned to degrade on demand. Such platforms can be fabricated from a vast library of natural and synthetic materials, offering a broad array of precision and tunability for pulmonary disease modeling.
To investigate the influence of variations in lung tissue stiffness on fibroblasts, Liu et al. (105) fabricated polyacrylamide hydrogels with a one-dimensional (1D) stiffness gradient with modulus values ranging from healthy tissue levels (E = 1 kPa) to beyond fibrotic tissue (E = 67 kPa) by using photopolymerization to control the level of cross-linking within the hydrogel. Increased light exposure during the polymerization process resulted in higher cross-linking levels and thus higher stiffness values across the material. Liu and colleagues used these techniques to demonstrate that increasing matrix stiffness strongly suppresses expression of cyclooxygenase-2 (COX-2) and production of an autocrine inhibitor of fibrogenesis, prostaglandin E2 (PGE2) in human lung fibroblasts (105). This pioneering work is one of the first studies to suggest a feedback relationship between matrix stiffening and fibroblast activation. Liu and colleagues also used this system to reveal a connection between matrix stiffness and yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), which influences fibroblast proliferation, ECM production, and fibrosis (104). Marinković et al. (109) used the same polyacrylamide hydrogel and a simple casting method to show that matrices with normal physiological lung tissue stiffness (E = 1 kPa) reduced the proliferative and contractile response of IPF fibroblasts to normal levels and that these responses could be modulated through inhibition of the Rho kinase pathway. Using similar techniques, Sava et al. (151) functionalized polyacrylamide hydrogels with deceullarized IPF ECM to decouple cellular responses to matrix composition from matrix mechanics. Human microvascular pericytes grown on these human lung ECM-conjugated hydrogels with tunable mechanical properties exhibited phenotypic transition quantified by expression of α-smooth muscle actin (α-SMA) in response to increases in local substrate stiffness independent of receptor-ligand interactions with IPF ECM. Further, the use of Nintedanib, a tyrosine-kinase inhibitor approved for IPF treatment, reduced the expression of the α-SMA and detection of crosslinked collagen by in pericytes (151). 2D polyacrylamide hydrogels have also been used to indicate that the serum response factor megakaryoblastic leukemia 1 (MKL1) activation plays a role in transducing mechanical stimuli from the ECM to the fibrogenic program that promotes fibroblast activation to the myofibroblast phenotype (68) and that increased epithelial cell contraction on stiff, fibronectin-coated surfaces leads to integrin-mediated transforming growth factor-β1 (TGFβ1) activation (20).
Polydimethylsiloxane (PDMS) elastomers, commonly known as silicones, are hydrophobic, cross-linked biomaterials that have been widely used in medicine for over 60 years. The unique and tunable chemical and physical properties of silicones result in excellent biocompatibility and durability for a variety of applications. Balestrini et al. (10) took advantage of this versatile material to show that fibrotic activity as measured by proliferation, cell contractility, expression of α-SMA, deposition of ECM, and secretion of TGFβ1 was low on 2D castings of soft substrates and high on pathologically stiff substrates, as predicted. Further experiments then revealed that explanting and culturing lung fibroblasts for 3 weeks on stiff substrates resulted in sustained myofibroblast activation even after cells were subsequently cultured exclusively on soft substrates for 2 weeks, suggesting that fibroblasts retain a mechanical memory of substrate mechanical conditions over time (10). A successive study using the same PDMS platform supported the concept that fibroblasts exhibit mechanical memory and identified the microRNA miR-21 as a long-term memory keeper of fibrogenic activity (101).
It is clear from these studies that changes in the physical microenvironment impact gene and protein expression as well as signal transduction during disease progression. Therefore, exploiting precision biomaterials and advanced biomanufacturing techniques to elucidate the mechanisms that control the process of mechanosensing is crucial for translation to precision therapeutic interventions for IPF. Although 2D substrates and conventional polymer casting techniques have been traditionally used as the primary model for studying mechanosensing in IPF, researchers recognize that cellular responses are both dimension dependent and structure dependent (40). In vitro models of pulmonary fibrosis have, as a result, moved toward 3D microenvironments to fully recapitulate cellular responses to heterogeneous changes in the local physical environment. A few studies have used a variety of natural materials, including collagen (36, 42, 74, 125, 144, 173), decellularized tissues (177), and functionalized alginate bead templates (181). Wilkinson et al. (181) fabricated alginate beads approximately the size of a single alveolus (~180 µm) by using a custom electrostatic droplet generator. These templates were then coated with fetal lung fibroblasts or induced pluripotent stem cell-derived mesenchymal stem cells and aggregated within a bioreactor to form 3D engineered models of distal lung architecture. This templating system is an example of how advanced biomanufacturing techniques can be applied to create 3D models of disease; however, it is still limited by the use of natural materials. Although natural materials such as collagen (36, 42, 74, 125, 144, 173), complex mixtures of ECM, and growth factors like Matrigel (32, 66, 98) and alginate (181) have provided key insights into pulmonary disease in vitro, these materials are not conducive to controlled modification, systematic testing, and iterative improvement, tools that are crucial in understanding the mechanisms of dynamic disease onset and progression.
Lewis et al. (99) have used polyethylene glycol (PEG)-based hydrogel systems as a complementary approach to designing precision models of pulmonary fibrosis. Photodegradable PEG-based microsphere templates were used to recreate the 3D architecture of the alveolar epithelium by first seeding the microspheres with either a tumor-derived alveolar epithelial cell line (A549) or primary mouse alveolar epithelial type II (ATII) cells, embedding the cell-coated templates into a synthetic PEG-based biomaterial platform to control local microenvironmental cues and then degrading the templates to create open cyst-like structures. This system has been subsequently used as a 3D coculture platform to demonstrate that fibroblasts in the surrounding matrix respond to changes in epithelial cells by increasing proliferation, migration, and MMP activity when cocultured with cancerous cells (100). Over the past decade, in vitro models of pulmonary fibrosis have evolved from 2D substrates with uniform stiffness to precisely designed 3D models that incorporate dynamic spatiotemporal control over local microenvironmental cues. These precision biomaterials in combination with advanced biomanufacturing techniques exhibit the potential to allow researchers to study cellular responses to pulmonary diseases with higher control and resolution than ever before (188).
Modeling PH
Similar to pulmonary fibrosis, PH, and in particular pulmonary arterial hypertension (PAH), is a deadly disease characterized by profibrotic remodeling of pulmonary vasculature. This condition usually presents with nonspecific dyspnea, particularly with exertion, and is definitively diagnosed through direct measurement of pulmonary artery pressures on right heart cauterization. Progressive increases in pulmonary arterial pressure (PAP) and vascular stiffness ultimately culminate in right ventricular (RV) failure and premature death (72). Although the exact prevalence is unknown, it is estimated that one in 200,000 adults in the United States (U.S.) has PH (166). The fibrotic remodeling cascade seen in PH upon vascular injury is driven by dysregulation of normal cellular processes in the pulmonary vasculature such as changes in proliferation, differentiation, inflammation, and cell death programs involving endothelial cells (EC), smooth muscle cells (SMC) and fibroblasts (162, 175). Many studies, including our own, have revealed that the activated vascular cell phenotypes observed in PH are highly dependent on cues from the surrounding microenvironment, including structure, stiffness, and composition (18, 39, 103, 136, 154).
On the cellular level, several in vitro studies have elucidated the role of matrix stiffness as a critical regulator of cellular activity and/or metabolism in PH. For instance, pulmonary artery SMCs and pulmonary artery ECs cultured on 2D polyacrylamide substrates approaching fibrotic stiffness exhibited increased proliferation and excessive ECM production (103). Building on this work, Bertero et al. (15) have presented evidence that matrix remodeling induces YAP/TAZ-miR-130/301 early in PH, which promotes a mechanoactive feedback loop that drives pulmonary vascular cellular activation by using collagen-coated hydrogels (Matrigen) with varying stiffnesses (1–50 kPa). Likewise, a study by Kudryashova et al. (95) showed how inactivation of large tumor suppressor 1 (LATS1), a central component of the Hippo pathway that is in part driven by increased ECM stiffness, promoted a YAP-fibronectin-integrin-linked kinase-1 signaling loop providing self-sustaining proliferation and resistance to apoptosis in pulmonary arterial vascular smooth muscle cells. Recently, the role of matrix stiffness in 3D hydrogels, a system more relevant to studying cellular mechanosensing, was investigated for human aortic adventitial fibroblasts. Culturing adventitial fibroblasts in soft (5–10 kPa) 3D PEG-based hydrogels elicited greater proliferation, elevated monocyte chemoattractant protein-1 secretion (MCP-1), and enhanced monocyte recruitment compared with stiff 3D hydrogel analogs (154). Surprisingly, many cellular events reported in these 3D microenvironments deviated from the commonly accepted results observed in 2D model systems, suggesting that cellular mechanics in 3D are complex and unresolved and highlighting the need for high-fidelity in vitro models to study the cellular and molecular mechanisms underlying PH.
Modeling COPD
COPD is a group of progressive, debilitating respiratory conditions, including chronic bronchitis and emphysema, that will rise to the third leading cause of death in the U.S. by 2020 (23, 139). It is characterized clinically by the presence of persistent respiratory symptoms and chronic airflow limitation on pulmonary function testing due to airway and/or alveolar abnormalities (139). COPD is thought to be secondary to innate and adaptive immune responses to long-term exposure to noxious particles and gases, in particular cigarette smoke domestically or biomass fuel exposure used in indoor cooking internationally (54, 108). These exposures drive chronic inflammation that affects the proximal and distal airways, lung parenchyma, and pulmonary vasculature in different ways. There are also noted imbalances between oxidants and antioxidants (oxidative stress) and proteases and antiproteases in the ECM compartment observed in COPD patients (108). These heterogeneous effects account for the large clinical spectrum seen among patients (23). The pathological changes in COPD ECM are primarily due to extensive destruction of elastin fibers by ECM-degrading enzymes released by inflammatory cells, mainly macrophages, neutrophils, and T cells (25). There is also evidence to suggest abnormal collagen remodeling and alterations in production of proteoglycans (60). This combination of tissue destruction and ECM remodeling leads to disorganization and reduced matrix cross-linking, causing significantly decreased tissue stiffness and increased tissue compliance in the lung parenchyma. Currently there are no therapies available for patients that stabilize or reverse progression of COPD.
Several human tissue or cell-based models have been applied to studying COPD, including ex vivo tissue explants (142), lung slices (176), decellularization (180), and in vitro cultures of primary cells (116). Bucchieri et al. (22), for example, showed that bronchial tissue embedded in Matrigel could form outgrowth cultures with multicellular sheets including motile cilia that were viable for more than one year in culture. Repetitive exposure to cigarette smoke extract revealed epithelial damage, loss of cilia, and ECM remodeling within these platforms. Similarly, as highlighted in the work of Shronska-Wasek et al. ex vivo 3D lung tissue cultures (lung slices) showed that reduced frizzled receptor 4 expression in COPD prevents WNT/β-catenin-driven alveolar lung repair. Although these models show promising utility for drug discovery and preclinical testing of airway remodeling in COPD, insufficient viability post-dissection, reduced preservation of essential structural integrity, and degradation of cell-cell and cell-matrix interactions for longer duration experiments are significant limitations (22, 158). Protocols for decelluarlization of pulmonary tissues through the application of a variety of detergents have been developed and implemented by several research groups to investigate cell-matrix interactions ex vivo (135, 174). Interestingly, many studies have demonstrated the ability to recellularize these scaffolds derived from healthy tissue, and recently, one of these bioengineered lungs was proven to maintain functionality upon implantation into a porcine model (122). Unfortunately, recellularization of tissues derived from COPD patients has yet to be realized but could be an important ex vivo model (180). To overcome these limitations, bioengineered synthetic cell culture platforms (94) have recently emerged as alternatives for creating COPD scaffolds in vitro. Poly(ε-caprolactone) (PCL), for example, is a widely used, biodegradable material that is known to produce fewer acidic products after hydrolysis than other commonly used substrates. This feature makes PCL an attractive biomaterial for tissue engineering. Kosmala et al. (94) demonstrated for the first time the ability to modify the relatively hydrophobic nature of PCL by grafting gelatin to the surface to improve attachment of lung epithelial cells with the goal of modeling COPD in vitro. While PCL may not be the ideal material for this application, this study is one of the very few reports that highlight the application of synthetic biomaterials to study COPD and thus leads the way to explore precision biomaterials in the development of more biomimetic models of this chronic pulmonary disease.
Given the complex interplay between genetic and environmental stimuli that drive disease progression in COPD, modeling of the air-liquid interface has been shown to be particularly important. Organ-on-chip technology allows for integrating multiple interdependent cell lines present in human tissues within a controlled environment (19, 129, 168). Organ-on-chip systems are most often based on microfluidic fabrication techniques that create fluid channels within PDMS, an optically transparent and biologically inert polymer. Cells can be cultured and maintained within the micropatterned features either directly on PDMS or on ECM coatings that preferentially allow for attachment of specific cell lines. In this way, multiple cell types can be patterned within single flow channels of organ-on-chip systems by templating ECM for cell attachment (16, 47, 78). This soft-lithographic technique was used to create the first lung-on-chip device in 2010 (70), which is probably the best known bioengineered lung analog to date. The membrane within this system supports human alveolar epithelial cells and microvascular endothelial cells attached to opposing sides and is flexible, allowing for cyclic straining of the adhered cell layers. After cells were seeded and grown to confluence, the epithelial channel was perfused with air, placing the cells in direct contact with the gas. The epithelial cells secreted surfactant, as they do in vivo. In subsequent studies, this lung-on-chip technology was used to investigate lung inflammation and drug responses (14), including the impact of smoking on alveolar membranes (13). The entire system was exposed to whole cigarette smoke to study ciliary micropathologies, COPD-specific molecular signatures, and epithelial responses to smoke generated by electronic cigarettes (13). Although this system has been used to study a number of airway-related pathologies, it is also somewhat limited by its reliance on a relatively thick porous polymer membrane and PDMS to mimic the lung ECM (69). Recently, Horvath et. al. (65) published an engineered air-blood barrier that was fabricated using a Matrigel basement membrane that more closely mimics in situ physiology. These engineering approaches demonstrate the significant progress that has been made in modeling the complexity of COPD; however, these microenvironments still lack many of the hallmarks of COPD ECM. Ultimately, incorporating a variety of human cell types into the models described here could bridge the gap between preclinical testing and human clinical trial outcomes.
Even though current engineered models of pulmonary diseases exist at the leading edge of technology, a tremendous amount of work remains to be accomplished in integrating the outputs of advanced characterization with precision biomaterials and biomanufacturing to create the next generation of more organotypic reproductions of human disease in vitro. Next, we explore the challenges and opportunities on the road toward translation of tissue-informed engineering strategies into precision medical treatments.
CHALLENGES AND OPPORTUNITIES FOR ENGINEERING TISSUE-INFORMED MODELS OF CHRONIC PULMONARY DISEASES
There is compelling evidence that microenvironmental changes in pathological ECM play a key role in disease progression. Advanced characterization techniques have revealed that the cellular and microenvironmental remodeling events that alter pulmonary tissue structure, mechanics, and composition during the development of chronic pulmonary diseases are inherently heterogeneous and dynamic. While engineered in vitro models of human pulmonary diseases have been essential to studying the cellular and molecular mechanisms underlying these conditions, lungs are formidable to recapitulate in vitro, and several key challenges remain. Looking forward, there are equally many opportunities to further tissue-informed engineering that will facilitate the realization of the full potential of these models to serve as the foundation for precision medical treatments (17, 77).
Challenge I: Recreating Microenvironmental Changes in Chronic Pulmonary Diseases
It is widely recognized that the ECM not only provides structural support for cells but is also a critical mediator of cellular phenotype and function (18, 24, 57, 171, 188). Although this framework is critical for guiding cell shape and orientation to form specialized structures that are adapted to the particular function of the tissue (137), it is not static. Instead, the ECM is dynamically degraded, synthesized, and remodeled by cells in both healthy and diseased tissues (179). Although it has been particularly challenging to recreate the dynamic biophysical and biochemical changes that occur in ECM during disease progression, there are several promising precision biomaterial-based strategies that have been proven in other healthcare applications to overcome this barrier to progress.
Opportunity: precision biomaterials for controlled modification of microenvironmental mechanics.
Modern polymer chemistry technologies provide the foundation for unprecedented control over biomaterial structure, mechanics, and composition, which is crucial for creating more physiologically relevant models of human disease. Dynamic responsiveness within these sophisticated biomaterials can be initiated by user- or cell-controlled mechanisms through incorporation into the hydrogel backbone, into the crosslinking macromers, or as pendant functional groups. User-controlled stimuli, such as light (147), temperature, ultrasound, and magnetic fields, can be exploited to improve our understanding of how cells interact with and receive information from the extracellular microenvironment, while materials that respond to endogenous signals from cells (e.g., enzyme secretion, pH changes, protein interactions, and cell-generated forces) provide a framework for observing how cells remodel the extracellular space. The ability to trigger these responses affords temporal and spatial control over microenvironmental mechanics and composition (85).
One design criterion that is particularly important for control within in vitro models of chronic pulmonary diseases is the microenvironmental modulus. Multiple studies have linked IPF and PH with ECM stiffening (20, 103, 109), while others have associated COPD with decreased matrix stiffness in regions of the lung parenchyma (96, 148). Dual-stage polymerization systems are emerging as a novel way to recreate ECM stiffening in vitro. Guvendiren et al. (56) implemented one of the first protocols for in situ hydrogel stiffening in the presence of cells. Briefly, a hyaluronic acid (HA) polymer backbone was first reacted with a short dithiothreitol (DTT) cross-linker to create a soft hydrogel (~3 kPa) for cellular encapsulation. Next, excess materials were sequentially cross-linked and stiffened (~30 kPa) by light-initiated polymerization. Rosales et al. (146) recently advanced this technology by adding in light-sensitive moieties that allow researchers to reverse the stiffening process through either photodegradation of a cross-linker or photoisomerization of reversible binding pairs connected to a HA backbone (145). Human mesenchymal stem cells cultured on these dynamic substrates were shown to activate or deactivate mechanosensitive pathways as measured by YAP/TAZ nuclear localization and differentiation in response to temporal stiffening and softening (56, 146). Light-initiated reactions represent a valuable class of biomanufacturing techniques that, when controlled using two-photon lithography, can achieve submicron resolutions (62).
Likewise, Anseth and colleagues [Kloxin et al. (89)] have developed a fully synthetic photocleavable PEG cross-linker that provides spatiotemporal control over microenvironmental softening and have established its use in developing photodegradable hydrogels that can be created with initially high stiffness (~32 kPa), mimicking fibrotic tissue and softened on demand to values that recapitulate healthy tissue (~3 kPa) (84, 88, 99, 100, 146). These materials have become invaluable in studying the mechanosensitive pathways that lead to the activation of valvular interstitial cells during the progression of heart valve disease and have revealed key insights into the mechanical memory of cells cultured on supraphysiologically stiff substrates (184). Although the biomaterials highlighted here facilitate dynamic control over matrix mechanics, user-controlled initiation of modulus changes may limit the viscous behavior of materials that is ultimately necessary to emulate pulmonary tissue mechanics.
To better recapitulate the mechanical properties of native tissues such as lung, the Mooney laboratory recently developed an alginate-based biomaterial system for probing the influence of viscoelastic parameters on cell function and/or the impact of cell-generated forces on the surrounding microenvironment. The natural polymer alginate is a polysaccharide derived from seaweed, which can be cross-linked covalently or ionically. Due to the reversible nature of ionic bonds, the application of stress can unbind and rebind these cross-links, producing hydrogels that demonstrate viscous behavior; by comparison, the covalently cross-linked alginate hydrogels exhibit near-elastic response under stress. Interestingly, when fibroblasts were cultured on soft alginate hydrogels with viscous behavior, cell spreading was increased compared with that observed on elastic substrates of the same modulus (29). Later, using a similar alginate-based hydrogel platform, the authors demonstrated fate control of human mesenchymal stem cells, which formed a mineralized, collagen I-rich matrix similar to bone ECM in hydrogels with rapid stress relaxation (viscous behavior) and initial elastic modulus of 17 kPa (30). Although these studies demonstrate the power of transient mechanics to direct important cellular processes, the natural polymer networks utilized are often limited by lack of tunability and precise modification.
Recently, a completely synthetic approach to designing PEG-based hydrogels with precisely defined mechanics and viscoelastic properties has been reported. The authors (21) introduced thioester-containing cross-linkers into a PEG-thiol-ene network to impart adaptable covalent cross-linking into the fully synthetic biomaterial system. Control of stress relaxation and thus the viscoelastic properties of these materials was demonstrated by uncaging a thiol ester exchange catalyst via light exposure (21). These results highlight for the first time the ability to produce material platforms with control over the transient mechanical properties that capture the dynamic complexities observed in ECM mechanics.
Opportunity: precision biomaterials for controlled modification of microenvironmental composition.
Dynamic control of the microenvironmental composition is also a fundamental design criteria for precision biomaterial platforms. Arguably one of the most significant advantages of utilizing synthetic networks is the capability to incorporate and release biochemical cues with spatiotemporal fidelity. Growth factors (112, 161), peptide sequences (37), protein fragments (150), and other soluble mediators (91) can be conjugated to the extracellular environment via a thiol-containing cysteine group terminating the sequence or by attachment to an enzyme-cleavable linker (55). Thiol-containing biochemical mediators can be reacted into the network via a photoinitiated reaction (58, 59) to achieve precise spatial presentation. Alternatively, light can be used to present biochemical cues that have been prereacted into the network. This is usually achieved by conjugating the biochemical cue to the network with a photoisomerizable or photodegradable molecule (43, 102) or by uncaging cues through ultraviolet (UV) cleavage of a protective group (124, 132).
Recently, the Kloxin group (159) developed a method to conjugate complete ECM proteins to PEG hydrogels by incorporating amine-bearing peptide tethers during hydrogel formation. Using a pendant CGGGK peptide sequence, free amines could be introduced into the hydrogel matrix and subsequent reaction with sulfo-SANPAH [sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate] in the presence of UV light renders the hydrogel reactive to amine-containing proteins such as common ECM components like collagen I and fibronectin. Using this approach, the authors compared the integrin binding of human primary fibroblasts to PEG hydrogels functionalized with complete ECM proteins (collagen I or fibronectin) or respective peptide mimics (GFOGER or RGD). Interestingly, the authors demonstrated that, on the collagen I-mimicking peptide GFOGER, activated clusters of fibroblasts were observed to form over time, reminiscent of fibroblast foci and which were not observed when cultured on the full-length protein collagen I. Such systems highlight the necessity to design precise hydrogel systems to investigate the effects of integrin binding on cell behavior, where the effects of composition can be decoupled from other significant parameters such as matrix structure and stiffness.
An attractive alternative to photomediated ligand presentation is the dynamic release of biochemical signaling factors through cell-controlled, enzymatically mediated cleavage of covalently bound signals such as growth factors (50), ligands (149), or full proteins (4). In vivo liberation of matrix-bound growth factors occurs in a highly localized manner. In an effort to replicate this native cell-growth factor interaction, seminal work from the Hubbell research group exploited the natural proteolytic programs of tissue repair, i.e., MMP secretion, to release vascular endothelial growth factor retained in a synthetic PEG matrix (189). Upon implantation into murine models, these matrices were remodeled with vascularized tissue in areas of cell-initiated growth factor release (189). In a similar study, the fibronectin-derived cell-adhesive peptide sequence RGD was incorporated into a PEG-based hydrogel network through attachment to an MMP-13 cleavable linker (149). Human mesenchymal stem cells encapsulated in these hydrogels exhibited a 10- fold increase in glycosaminoglycan production compared with cells encapsulated in a non-cleavable RGD containing network (149). Precision biomaterials in which bioactive chemical mediators are retained until local release is triggered by cells are powerful tools for mimicking the reciprocal cell-ECM interactions that drive ECM remodeling in the context of disease. Collectively, these state-of-the-art biomaterial systems are examples of promising candidates that can be verified for tissue-informed engineering of disease models to uncover cellular and molecular mechanisms underlying chronic pulmonary diseases such as IPF, PH, and COPD.
Opportunity: multiresponsive biomaterial systems.
The dynamic biomaterials described above are quickly evolving into sophisticated systems that are increasingly biomimetic in their responses to physiological or externally applied stimuli (90). By employing a modular chemical framework, researchers are imparting hydrogels with precise responsiveness to multiple microenvironmental cues to trigger both biochemical and biophysical reactions that are critical for emulating pathogenesis in disease modeling. To further improve specificity to disease-associated biochemical hallmarks found in vivo, the DeForest research group has produced multi-stimuli-responsive hydrogel biomaterials that are able to perform biocomputation, i.e., simultaneously sense multiple inputs (enzyme, reductant, and light) and follow a user-programmed, Boolean logic-based algorithm to produce a functional output (degradation and therapeutic release) (8). The 17 distinct biomaterial systems created in this work represented all of the possible YES/OR/AND logic outputs from input combinations of three stimuli. By controlling the molecular connectivity of multiple stimuli-responsive chemical moieties, Badeau et al. (8) demonstrated the first sequential and spatiotemporally varied delivery of multiple cell lines from a single biomaterial in response to physiological changes within the microenvironment.
Recently, the Anseth group took a completely synthetic approach to designing multi-stimuli-responsive PEG-based hydrogels with viscoelastic properties that respond to both light and cell-generated forces over several orders of magnitude. Combining PEG-thiol-ene click chemistry with adaptable covalent thiol ester exchange (reversible cross-links), the authors demonstrated stress relaxation of these synthetic networks with the capacity to control the viscoelastic properties by uncaging the thiol ester catalysts via light exposure (21). The benefits of such an approach highlight the ability to produce highly tunable material platforms with transient mechanical parameters mediated by multiple inputs. Platforms with such control of viscoelastic matrix properties will undoubtedly provide the necessary material parameters to capture the complex transient mechanics of lung ECM in both normal and diseased states. The user-programmable and high-resolution tuning of these multiresponsive biomaterials classify them as ideal candidates for tissue-informed design of chronic pulmonary disease models with increasing complexity (8, 90).
Challenge II: Biomanufacturing, Scalability, and Translation
Realizing the full potential of engineered models of chronic pulmonary diseases presents both technical and translational challenges. As mentioned previously, pulmonary tissue is a complex arrangement of cells and ECM that results in a hierarchical macroscopic structure (75, 157). Therefore, models must span a broad length scale from the cell-ECM interactions at the submicrometer level to the spatiotemporal irregularities seen at the tissue level. Manufacturing techniques that recapitulate not only the spatiotemporal heterogeneity but also the vascularization found in pulmonary tissues are critical to the success of future engineering strategies (Fig. 3). Another significant technical challenge involves the standardization and automation of the highly variable advanced manufacturing techniques currently used to model pulmonary diseases that were originally conceived for laboratory research and development. Manufacturing and scalability limitations can be addressed through advances in biomanufacturing procedures, while standardization and translation will be achieved only through collaborations with both industrial and regulatory partners.
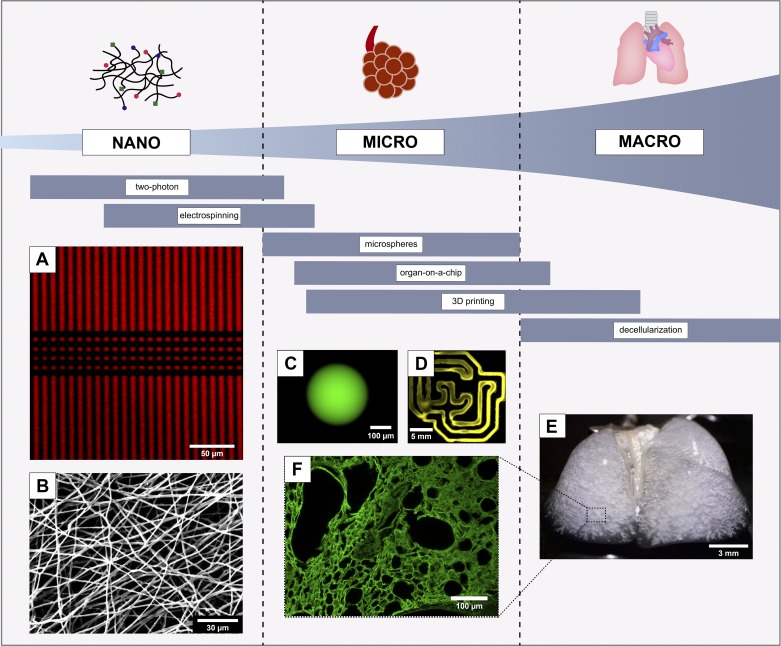
Fig. 3.
Depiction of the spatial resolution capabilities of selected biomanufacturing techniques with representative product images. A: fluorescently labeled polyethylene glycol (PEG)-based hydrogels patterned using two-photon lithography (reproduced in part from Ref (86), with permission of John Wiley & Sons). B: scanning electron microscopy image of electrospun PEG thiol-ene fibers. C: fluorescently-labeled PEG thiol-ene hydrogel microsphere synthesized via inverse emulsion polymerization. 3D bioprinted, fluorescent PEG thiol-ene hydrogel (D), decellularized mouse lung (E), and an immunofluorescently stained image of collagen IV (F) visualizing microstructure of decellularized lung tissue (E and F, courtesy of the Wagner Laboratory).
Opportunity: biomanufacturing techniques to mimic tissue structure and vascularization.
Additive manufacturing of biological materials allows for construction of complex 3D architectures impossible to realize with other methods. Electrospinning and bioprinting are promising additive manufacturing technologies for the precise stereotactic placement of biomaterials for engineering in vitro models of disease. Hydrogels, including collagen, chitosan, alginate, fibrin, HA, PEG, Matrigel, and methylcellulose (79, 119, 156, 187), are the most commonly used materials (127) for electrospinning (2) and bioprinting engineered tissues. While some materials are better suited to particular applications than others, all are biocompatible and provide an engineered matrix suitable for supporting living cells. These biomanufacturing techniques can be used to fabricate hierarchical macroscale models of pulmonary disease with microscale resolution of microenvironmental changes ranging from 5 to 50 μm (118).
Mimicking larger vascular tissue requires integrating both the fibrous nature of the ECM and anisotropic mechanical properties with compartmentalized cellular regions. One approach to producing these fibrous ECM substrates is electrospinning, a method by which a polymer solution is drawn from an extrusion source, typically a syringe, by an electrical potential (2, 169). This technique allows for control of fiber parameters, including structure (geometry and size), mechanics (material modulus and fiber orientation), and composition, which can all be tuned to fabricate structures with physiologically relevant scale and architecture (2, 9). Electrospun scaffolds not only have utility in tissue engineered vascular grafts (2, 80), but their application toward achieving complex cellular or disease models is compelling as well. Recently, Cheng et al. (33) prepared a multi-cell-laden tubular structure comprised of electrospun PCL and poly(lactic-co-glycolic acid) that retained their 3D structure after implantation. These tubular vessels with distinct cellular regions (intima, media, and adventitia) represent a step toward emulating the complex macroscale tissue organization of native vessels. Although the authors proposed these engineered vessels for vascular regeneration in vitro or in vivo, translation of such advanced biomanufacturing methodologies could pave the way for the development of high-fidelity vascular disease models.
Functional vascularization of microscale engineered tissue constructs is essential to further improve our ability to model pulmonary disease (92, 93, 97, 115). Multiple research groups have developed novel ways to embed an active vasculature in 3D constructs by use of bioprinting (93, 97). Bioprinting encompasses three main technologies: extrusion-, inkjet-, and laser-based bioprinting. Extrusion-based bioprinting, the most frequently used technique of the three, employs a robotically controlled syringe tip that extrudes a thin, continuous bioink filament (127, 128). Pioneering work by Kolesky et al. demonstrated a method to bioprint pluronic F127 as a sacrificial material to recreate blood vessels within a cell-laden hydrogel. The sacrificial pluronic F127 material was printed as interpenetrating tubes of 200 μm diameter within a gelatin methacrylate (GelMA) bioink, and the whole construct was cast in pure GelMA. Once cured, the pluronic F127 was removed by cooling the device to 4°C to liquify and eliminate the sacrificial material. The blood vessels thus created were endothelialized using a suspension of human umbilical vein endothelial cells, which remained viable and continued to proliferate while being cultured for 7 days (93). The integration of high-resolution biomanufacturing techniques with innovations in multi-stimuli-responsive polymer chemistries will likely drive the realization of vascularized disease models that recapitulate the macro- to-microscale organization of pulmonary vasculature and lung tissues.
Opportunity: collaboration with industry and regulatory agencies.
Although animal studies can emulate a variety of physiologically complex interactions that occur during disease pathogenesis, the scientific validity and translatability of these results to humans has been inconsistent over the years (126). Engineered models of chronic pulmonary diseases have the potential to enable safety and efficacy testing for new drug candidates as well as identification of novel therapeutic targets in vitro. Incorporating these innovative technologies early in the research and development pipeline for commercial drug discovery may increase the likelihood of success of subsequent clinical trials. High-throughput screening (HTS) is an example of a process traditionally employed in pharmacological studies that can be applied to narrow design criteria for biomaterial (53) and microenvironment specification (11) in cases where the level of tunable parameters is large enough to prohibit performing the same experiments with conventional techniques. Indeed, in our hands we have employed a HTS method to successfully interrogate engineered microenvironments, with tunable matrix structure, mechanics, and composition, against EC and SMC (38, 39) as well as various other cell lines (46, 156). In doing so, we have identified specifications that produce ideal microenvironments for endothelial integrity (38), smooth muscle contractility (39), and vascular differentiation of stem cells (46). Application of HTS technologies early in disease model development will enable efficient identification of design and process parameters necessary for scale-up of well-defined model systems that emulate complex phenomena in pathogenesis.
Furthermore, successful translation of in vitro models of chronic pulmonary diseases from proof of concept on the benchtop to commercial screening platforms at the bedside will require assays to be reliable, robust, and compliant with regulatory guidance. Achieving this milestone will require basic translational researchers to form partnerships with leaders in industry as well as regulatory agencies. Together, these partnerships will streamline the commercialization process. Incorporating design and process controls, as well as analytical, bench, preclinical verification, and clinical validation testing recommended by regulatory agencies into research and development plans at an early stage will expedite the commercialization of these much-needed advanced technologies (134).
OUTLOOK
Over the past three decades, advances in tissue characterization and in vitro modeling have revealed substantial insight into the structure, mechanics, and composition of the ECM as well as the critical roles it plays in morphogenesis, tissue homeostasis, and disease progression (188). Consider the case of IPF, for example. Diagnosis of this disease is based on recognition of aggregate structures of proliferating fibroblasts and myofibroblasts surrounded by unusually dense ECM, called “fibroblast foci”, on high-resolution chest CT or surgical lung biopsy. Historically, these lesions were proposed to have been linked in a complex, highly interconnected reticulum that extends from the pleura into the underlying parenchyma (35). In 2016, Jones et al. (75) presented state-of-the-art 3D characterization results that suggested that fibroblast foci form a constellation of heterogeneous structures with large variations in size, geometry, and volume that change over time and with no apparent connectivity. To illustrate the power of the opportunities currently available for engineering more organotypic models of human pulmonary diseases, we propose that multi-stimuli-responsive biomaterials capable of spatiotemporally controlled stiffening and release of soluble factors like TGFβ could be combined with advanced biomanufacturing tools (two-photon lithography) to emulate progression of IPF in vitro (Fig. 4). Many of the precise biomaterials and biomanufacturing techniques described as opportunities in this review are either relatively new or not yet used at all in the field of pulmonary disease research but have the potential to initiate a transformative shift in the preclinical translational research efforts underway in this area. Future tissue-informed designs will benefit from the ability of precision biomaterials and advanced biomanufacturing techniques to increase the complexity of our models, adding dynamic microenvironments, high-resolution control of structure, and improved cellular diversity. The ability to translate complex in vivo events into in vitro models, investigate subsequent cell-matrix interactions, and use these findings to elucidate mechanisms underlying pathogenesis will have transformative effects in pulmonary regenerative medicine. These efforts are fundamental in our progress to overcome current translational barriers with innovative solutions that embody the principles of precision medicine.
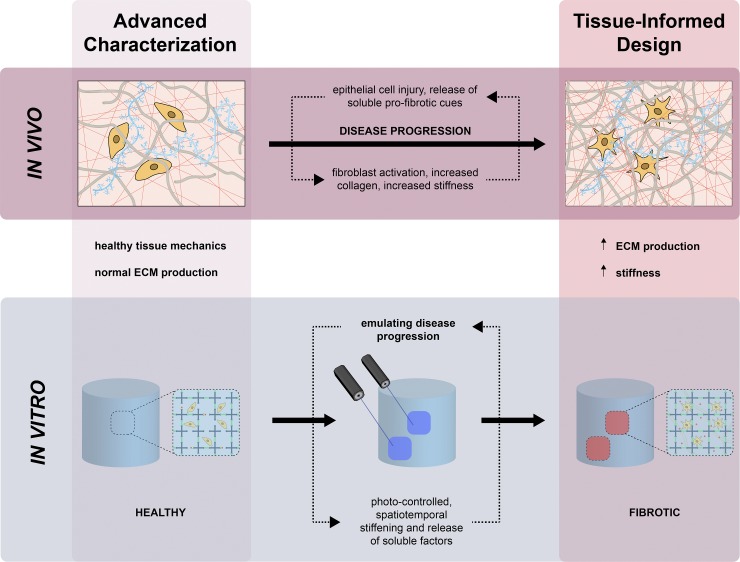
Fig. 4.
Application of the integrated framework for engineering more organotypic models of idiopathic pulmonary fibrosis (IPF) in vitro. 1) advanced characterization reveals localized regions of fibrotic activity, distinguished by increased extracellular matrix (ECM) deposition and matrix stiffness. 2) dynamic multiresponsive biomaterial systems impart photo-controlled spatiotemporal stiffening and release of soluble factors to emulate disease progression. 3 and 4) Preclinical verification and clinical validation expedite the translation and commercialization of precision therapeutic interventions for IPF.
GRANTS
This work was supported by funding from the American Thoracic Society Foundation Research Program (to C. M. Magin and T. J. D’Ovidio) and the National Heart, Lung, and Blood Institute Grants T32-HL-07171-39 (to M. L. Floren) and T32-HL-007085-43 (to K. E. Bailey).
DISCLOSURES
C. M. Magin is a consultant for Sharklet Technologies, Inc., although there is no perceived conflict of interest. No conflicts of interest, financial or otherwise, are declared by the other authors.
AUTHOR CONTRIBUTIONS
K.E.B., M.L.F., T.J.D., and C.M.M. prepared figures; K.E.B., M.L.F., T.J.D., S.R.L., and C.M.M. drafted manuscript; K.E.B., M.L.F., T.J.D., S.R.L., K.R.S., and C.M.M. edited and revised manuscript; K.E.B., M.L.F., T.J.D., S.R.L., K.R.S., and C.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Darcy Wagner and laboratory, in particular Deniz Bölukbas and Martina De Santis, for the decellularized images of lung tissue found in Fig. 3.
REFERENCES
- 1.Abraham T, Hogg J. Extracellular matrix remodeling of lung alveolar walls in three dimensional space identified using second harmonic generation and multiphoton excitation fluorescence. J Struct Biol 171: 189–196, 2010. doi: 10.1016/j.jsb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Ahn H, Ju YM, Takahashi H, Williams DF, Yoo JJ, Lee SJ, Okano T, Atala A. Engineered small diameter vascular grafts by combining cell sheet engineering and electrospinning technology. Acta Biomater 16: 14–22, 2015. doi: 10.1016/j.actbio.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Åhrman E, Hallgren O, Malmström L, Hedström U, Malmström A, Bjermer L, Zhou XH, Westergren-Thorsson G, Malmström J. Quantitative proteomic characterization of the lung extracellular matrix in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. J Proteomics 189: 23–33, 2018. doi: 10.1016/j.jprot.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Aimetti AA, Machen AJ, Anseth KS. Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials 30: 6048–6054, 2009. doi: 10.1016/j.biomaterials.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alsafadi HN, Staab-Weijnitz CA, Lehmann M, Lindner M, Peschel B, Königshoff M, Wagner DE. An ex vivo model to induce early fibrosis-like changes in human precision-cut lung slices. Am J Physiol Lung Cell Mol Physiol 312: L896–L902, 2017. doi: 10.1152/ajplung.00084.2017. [DOI] [PubMed] [Google Scholar]
- 6.Anseth KS, Bowman CN, Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 17: 1647–1657, 1996. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 7.Ayala P, Lopez JI, Desai TA. Microtopographical cues in 3D attenuate fibrotic phenotype and extracellular matrix deposition: implications for tissue regeneration. Tissue Eng Part A 16: 2519–2527, 2010. doi: 10.1089/ten.tea.2009.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat Chem 10: 251–258, 2018. doi: 10.1038/nchem.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater 14: 1262–1268, 2015. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol 4: 410–421, 2012. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 11.Barata D, van Blitterswijk C, Habibovic P. High-throughput screening approaches and combinatorial development of biomaterials using microfluidics. Acta Biomater 34: 1–20, 2016. doi: 10.1016/j.actbio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Belair DG, Le NN, Murphy WL. Design of growth factor sequestering biomaterials. Chem Commun (Camb) 50: 15651–15668, 2014. doi: 10.1039/C4CC04317K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benam KH, Novak R, Nawroth J, Hirano-Kobayashi M, Ferrante TC, Choe Y, Prantil-Baun R, Weaver JC, Bahinski A, Parker KK, Ingber DE. Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip. Cell Syst 3: 456–466.e4, 2016. doi: 10.1016/j.cels.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee H-H, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, Hamilton GA, Ingber DE. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods 13: 151–157, 2016. doi: 10.1038/nmeth.3697. [DOI] [PubMed] [Google Scholar]
- 15.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang Y-Y, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, Chan SY. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Reports 13: 1016–1032, 2015. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 32: 760–772, 2014. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 17.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, Brown KK, Chapman HA, Collard HR, Cosgrove GP, Deterding R, Doyle R, Flaherty KR, Garcia CK, Hagood JS, Henke CA, Herzog E, Hogaboam CM, Horowitz JC, King TE Jr, Loyd JE, Lawson WE, Marsh CB, Noble PW, Noth I, Sheppard D, Olsson J, Ortiz LA, O’Riordan TG, Oury TD, Raghu G, Roman J, Sime PJ, Sisson TH, Tschumperlin D, Violette SM, Weaver TE, Wells RG, White ES, Kaminski N, Martinez FJ, Wynn TA, Thannickal VJ, Eu JP. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189: 214–222, 2014. doi: 10.1164/rccm.201306-1141WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med 186: 866–876, 2012. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18: 240–249, 2013. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J Pathol 229: 25–35, 2013. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown TE, Carberry BJ, Worrell BT, Dudaryeva OY, McBride MK, Bowman CN, Anseth KS. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178: 496–503, 2018. doi: 10.1016/j.biomaterials.2018.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucchieri F, Pitruzzella A, Fucarino A, Gammazza AM, Bavisotto CC, Marcianò V, Cajozzo M, Lo Iacono G, Marchese R, Zummo G, Holgate ST, Davies DE. Functional characterization of a novel 3D model of the epithelial-mesenchymal trophic unit. Exp Lung Res 43: 82–92, 2017. doi: 10.1080/01902148.2017.1303098. [DOI] [PubMed] [Google Scholar]
- 23.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AMB, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E; BOLD Collaborative Research Group . International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 370: 741–750, 2007. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 24.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol 240: 397–409, 2016. doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 50: 1601805, 2017. doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 26.Byron A, Humphries JD, Humphries MJ. Defining the extracellular matrix using proteomics. Int J Exp Pathol 94: 75–92, 2013. doi: 10.1111/iep.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calle EA, Hill RC, Leiby KL, Le AV, Gard AL, Madri JA, Hansen KC, Niklason LE. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater 46: 91–100, 2016. doi: 10.1016/j.actbio.2016.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol 4: 173, 2014. doi: 10.3389/fphar.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ. Substrate stress relaxation regulates cell spreading. Nat Commun 6: 6365, 2015. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15: 326–334, 2016. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Nadiarynkh O, Plotnikov S, Campagnola PJ. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7: 654–669, 2012. doi: 10.1038/nprot.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y-W, Huang SX, de Carvalho ALRT, Ho S-H, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova J-L, Bhattacharya J, Liang AF, Palermo LM, Porotto M, Moscona A, Snoeck H-W. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19: 542–549, 2017. doi: 10.1038/ncb3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Jin Y, Wang N, Cao F, Zhang W, Bai W, Zheng W, Jiang X. Self-adjusting, polymeric multilayered roll that can keep the shapes of the blood vessel scaffolds during biodegradation. Adv Mater 29: 29, 2017. doi: 10.1002/adma.201700171. [DOI] [PubMed] [Google Scholar]
- 34.Choi YS, Vincent LG, Lee AR, Dobke MK, Engler AJ. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials 33: 2482–2491, 2012. doi: 10.1016/j.biomaterials.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. Am J Respir Crit Care Med 174: 654–658, 2006. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 73: 1721–1732, 2013. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem 3: 925–931, 2011. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Y, Floren M, Tan W. High-throughput screening of vascular endothelium-destructive or protective microenvironments: cooperative actions of extracellular matrix composition, stiffness, and structure. Adv Healthc Mater 6: 1601426, 2017. doi: 10.1002/adhm.201601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding Y, Xu X, Sharma S, Floren M, Stenmark K, Bryant SJ, Neu CP, Tan W. Biomimetic soft fibrous hydrogels for contractile and pharmacologically responsive smooth muscle. Acta Biomater 74: 121–130, 2018. doi: 10.1016/j.actbio.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle AD, Yamada KM. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp Cell Res 343: 60–66, 2016. doi: 10.1016/j.yexcr.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 42.Epa AP, Thatcher TH, Pollock SJ, Wahl LA, Lyda E, Kottmann RM, Phipps RP, Sime PJ. Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2. PLoS One 10: e0135266, 2015. doi: 10.1371/journal.pone.0135266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater 21: 5005–5010, 2009. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 11: 404–406, 2014. doi: 10.1513/AnnalsATS.201311-405PS. [DOI] [PubMed] [Google Scholar]
- 45.Fiore VF, Strane PW, Bryksin AV, White ES, Hagood JS, Barker TH. Conformational coupling of integrin and Thy-1 regulates Fyn priming and fibroblast mechanotransduction. J Cell Biol 211: 173–190, 2015. doi: 10.1083/jcb.201505007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Floren M, Tan W. Three-dimensional, soft neotissue arrays as high throughput platforms for the interrogation of engineered tissue environments. Biomaterials 59: 39–52, 2015. [Erratum in: Biomaterials 67: 204, 2015.] doi: 10.1016/j.biomaterials.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folch A, Ayon A, Hurtado O, Schmidt MA, Toner M. Molding of deep polydimethylsiloxane microstructures for microfluidics and biological applications. J Biomech Eng 121: 28–34, 1999. doi: 10.1115/1.2798038. [DOI] [PubMed] [Google Scholar]
- 48.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol (1985) 67: 2408–2419, 1989. doi: 10.1152/jappl.1989.67.6.2408. [DOI] [PubMed] [Google Scholar]
- 49.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujimoto K, Takahashi T, Miyaki M, Kawaguchi H. Cell activation by the micropatterned surface with settling particles. J Biomater Sci Polym Ed 8: 879–891, 1997. doi: 10.1163/156856297X00065. [DOI] [PubMed] [Google Scholar]
- 51.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 1840: 2506–2519, 2014. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gocheva V, Naba A, Bhutkar A, Guardia T, Miller KM, Li CM, Dayton TL, Sanchez-Rivera FJ, Kim-Kiselak C, Jailkhani N, Winslow MM, Del Rosario A, Hynes RO, Jacks T. Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc Natl Acad Sci USA 114: E5625–E5634, 2017. doi: 10.1073/pnas.1707054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groen N, Guvendiren M, Rabitz H, Welsh WJ, Kohn J, de Boer J. Stepping into the omics era: Opportunities and challenges for biomaterials science and engineering. Acta Biomater 34: 133–142, 2016. doi: 10.1016/j.actbio.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu BH, Madison MC, Corry D, Kheradmand F. Matrix remodeling in chronic lung diseases. Matrix Biol 73: 52–63, 2018. doi: 10.1016/j.matbio.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo C, Kim H, Ovadia EM, Mourafetis CM, Yang M, Chen W, Kloxin AM. Bio-orthogonal conjugation and enzymatically triggered release of proteins within multi-layered hydrogels. Acta Biomater 56: 80–90, 2017. doi: 10.1016/j.actbio.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun 3: 792, 2012. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 57.Haak AJ, Tan Q, Tschumperlin DJ. Matrix biomechanics and dynamics in pulmonary fibrosis. Matrix Biol 73: 64–76, 2018. doi: 10.1016/j.matbio.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv Mater 18: 2679–2684, 2006. doi: 10.1002/adma.200600647. [DOI] [Google Scholar]
- 59.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials 27: 2519–2524, 2006. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 60.Hallgren O, Nihlberg K, Dahlbäck M, Bjermer L, Eriksson LT, Erjefält JS, Löfdahl CG, Westergren-Thorsson G. Altered fibroblast proteoglycan production in COPD. Respir Res 11: 55, 2010. doi: 10.1186/1465-9921-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton DW, Wong KS, Brunette DM. Microfabricated discontinuous-edge surface topographies influence osteoblast adhesion, migration, cytoskeletal organization, and proliferation and enhance matrix and mineral deposition in vitro. Calcif Tissue Int 78: 314–325, 2006. doi: 10.1007/s00223-005-0238-x. [DOI] [PubMed] [Google Scholar]
- 62.Heintz KA, Bregenzer ME, Mantle JL, Lee KH, West JL, Slater JH. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Adv Healthc Mater 5: 2153–2160, 2016. doi: 10.1002/adhm.201600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest 128: 45–53, 2018. doi: 10.1172/JCI93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinz B. Mechanical aspects of lung fibrosis: a spotlight on the myofibroblast. Proc Am Thorac Soc 9: 137–147, 2012. doi: 10.1513/pats.201202-017AW. [DOI] [PubMed] [Google Scholar]
- 65.Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep 5: 7974, 2015. doi: 10.1038/srep07974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huan C, Yang T, Liang J, Xie T, Cheng L, Liu N, Kurkciyan A, Monterrosa Mena J, Wang C, Dai H, Noble PW, Jiang D. Methylation-mediated BMPER expression in fibroblast activation in vitro and lung fibrosis in mice in vivo. Sci Rep 5: 14910, 2015. doi: 10.1038/srep14910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang W, Yen RT, McLaurine M, Bledsoe G. Morphometry of the human pulmonary vasculature. J Appl Physiol (1985) 81: 2123–2133, 1996. doi: 10.1152/jappl.1996.81.5.2123. [DOI] [PubMed] [Google Scholar]
- 68.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47: 340–348, 2012. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4: 159ra147, 2012. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 328: 1662–1668, 2010. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15: 802–812, 2014. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38: 581–587, 2001. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 73.Janson IA, Putnam AJ. Extracellular matrix elasticity and topography: material-based cues that affect cell function via conserved mechanisms. J Biomed Mater Res A 103: 1246–1258, 2015. doi: 10.1002/jbm.a.35254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joannes A, Brayer S, Besnard V, Marchal-Sommé J, Jaillet M, Mordant P, Mal H, Borie R, Crestani B, Mailleux AA. FGF9 and FGF18 in idiopathic pulmonary fibrosis promote survival and migration and inhibit myofibroblast differentiation of human lung fibroblasts in vitro. Am J Physiol Lung Cell Mol Physiol 310: L615–L629, 2016. doi: 10.1152/ajplung.00185.2015. [DOI] [PubMed] [Google Scholar]
- 75.Jones MG, Fabre A, Schneider P, Cinetto F, Sgalla G, Mavrogordato M, Jogai S, Alzetani A, Marshall BG, O’Reilly KM, Warner JA, Lackie PM, Davies DE, Hansell DM, Nicholson AG, Sinclair I, Brown KK, Richeldi L. Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. JCI Insight 1: e86375, 2016. doi: 10.1172/jci.insight.86375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonkman J, Brown CM. Any way you slice it—a comparison of confocal microscopy techniques. J Biomol Tech 26: 54–65, 2015. doi: 10.7171/jbt.15-2602-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kadyk LC, DeWitt ND, Gomperts B. Proceedings: regenerative medicine for lung diseases: a CIRM workshop report. Stem Cells Transl Med 6: 1823–1828, 2017. doi: 10.1002/sctm.17-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials 20: 2363–2376, 1999. doi: 10.1016/S0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 79.Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication 8: 032002, 2016. doi: 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 80.Khorshidi S, Solouk A, Mirzadeh H, Mazinani S, Lagaron JM, Sharifi S, Ramakrishna S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J Tissue Eng Regen Med 10: 715–738, 2016. doi: 10.1002/term.1978. [DOI] [PubMed] [Google Scholar]
- 81.Kim EJ, Boehm CA, Mata A, Fleischman AJ, Muschler GF, Roy S. Post microtextures accelerate cell proliferation and osteogenesis. Acta Biomater 6: 160–169, 2010. doi: 10.1016/j.actbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 83.King TEJ Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 84.Kirschner CM, Alge DL, Gould ST, Anseth KS. Clickable, photodegradable hydrogels to dynamically modulate valvular interstitial cell phenotype. Adv Healthc Mater 3: 649–657, 2014. doi: 10.1002/adhm.201300288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirschner CM, Anseth KS. Hydrogels in healthcare: from static to dynamic material microenvironments. Acta Mater 61: 931–944, 2013. doi: 10.1016/j.actamat.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirschner CM, Anseth KS. In situ control of cell substrate microtopographies using photolabile hydrogels. Small 9: 578–584, 2013. doi: 10.1002/smll.201201841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31: 1–8, 2010. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324: 59–63, 2009. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc 5: 1867–1887, 2010. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knipe JM, Peppas NA. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen Biomater 1: 57–65, 2014. doi: 10.1093/rb/rbu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koehler KC, Alge DL, Anseth KS, Bowman CN. A Diels-Alder modulated approach to control and sustain the release of dexamethasone and induce osteogenic differentiation of human mesenchymal stem cells. Biomaterials 34: 4150–4158, 2013. doi: 10.1016/j.biomaterials.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci USA 113: 3179–3184, 2016. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26: 3124–3130, 2014. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 94.Kosmala A, Fitzgerald M, Moore E, Stam F. Evaluation of a gelatin-modified poly(ε-Caprolactone) film as a scaffold for lung disease. Anal Lett 50: 219–232, 2017. doi: 10.1080/00032719.2016.1163363. [DOI] [Google Scholar]
- 95.Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL, Morelli AE, Zhao J, Ihida-Stansbury K, Chang B, DeLisser H, Tuder RM, Kawut SM, Silljé HH, Shapiro S, Zhao Y, Goncharova EA. HIPPO-integrin-linked kinase cross-talk controls self-sustaining proliferation and survival in pulmonary hypertension. Am J Respir Crit Care Med 194: 866–877, 2016. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kulkarni T, O’Reilly P, Antony VB, Gaggar A, Thannickal VJ. Matrix remodeling in pulmonary fibrosis and emphysema. Am J Respir Cell Mol Biol 54: 751–760, 2016. doi: 10.1165/rcmb.2015-0166PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee VK, Lanzi AM, Haygan N, Yoo SS, Vincent PA, Dai G. Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng 7: 460–472, 2014. doi: 10.1007/s12195-014-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehtonen ST, Veijola A, Karvonen H, Lappi-Blanco E, Sormunen R, Korpela S, Zagai U, Sköld MC, Kaarteenaho R. Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis. Respir Res 17: 14, 2016. doi: 10.1186/s12931-016-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewis KJ, Tibbitt MW, Zhao Y, Branchfield K, Sun X, Balasubramaniam V, Anseth KS. In vitro model alveoli from photodegradable microsphere templates. Biomater Sci 3: 821–832, 2015. doi: 10.1039/C5BM00034C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis KJ, Hall JK, Kiyotake EA, Christensen T, Balasubramaniam V, Anseth KS. Epithelial-mesenchymal crosstalk influences cellular behavior in a 3D alveolus-fibroblast model system. Biomaterials 155: 124–134, 2018. doi: 10.1016/j.biomaterials.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li CX, Talele NP, Boo S, Koehler A, Knee-Walden E, Balestrini JL, Speight P, Kapus A, Hinz B. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nat Mater 16: 379–389, 2017. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 102.Liu D, Xie Y, Shao H, Jiang X. Using azobenzene-embedded self-assembled monolayers to photochemically control cell adhesion reversibly. Angew Chem Int Ed Engl 48: 4406–4408, 2009. doi: 10.1002/anie.200901130. [DOI] [PubMed] [Google Scholar]
- 103.Liu F, Haeger CM, Dieffenbach PB, Sicard D, Chrobak I, Coronata AM, Suárez Velandia MM, Vitali S, Colas RA, Norris PC, Marinković A, Liu X, Ma J, Rose CD, Lee SJ, Comhair SA, Erzurum SC, McDonald JD, Serhan CN, Walsh SR, Tschumperlin DJ, Fredenburgh LE. Distal vessel stiffening is an early and pivotal mechanobiological regulator of vascular remodeling and pulmonary hypertension. JCI Insight 1: e86987, 2016. doi: 10.1172/jci.insight.86987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol 308: L344–L357, 2015. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190: 693–706, 2010. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu F, Tschumperlin DJ. Micro-mechanical characterization of lung tissue using atomic force microscopy. J Vis Exp 54: 2911, 2011. doi: 10.3791/2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X, Wong SS, Taype CA, Kim J, Shentu TP, Espinoza CR, Finley JC, Bradley JE, Head BP, Patel HH, Mah EJ, Hagood JS. Thy-1 interaction with Fas in lipid rafts regulates fibroblast apoptosis and lung injury resolution. Lab Invest 97: 256–267, 2017. doi: 10.1038/labinvest.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacNee W. ABCs of chronic obstructive pulmonary disease pathology, pathogenesis, and pathophysiology. BMJ 332: 1202–1204, 2006. doi: 10.1136/bmj.332.7551.1202. [DOI] [Google Scholar]
- 109.Marinković A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol 48: 422–430, 2013. doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marinković A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol 303: L169–L180, 2012. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 3: 17074, 2017. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 112.McCall JD, Luoma JE, Anseth KS. Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv Transl Res 2: 305–312, 2012. doi: 10.1007/s13346-012-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Paré PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 365: 1567–1575, 2011. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, Murawski K, Kingham E, Oreffo ROC, Dalby MJ. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 10: 637–644, 2011. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 115.Melchiorri AJ, Fisher JP. Bioprinting of blood vessels. In: Essentials of 3D Biofabrication and Translation (Atala A, Yoo JJ, editors). Boston, MA: Academic Press, 2015, chapt 20, p. 337–350. doi: 10.1016/B978-0-12-800972-7.00020-7 [DOI] [Google Scholar]
- 116.Meng A, Zhang X, Wu S, Wu M, Li J, Yan X, Kopec-Harding K, Wu J. In vitro modeling of COPD inflammation and limitation of p38 inhibitor - SB203580. Int J Chron Obstruct Pulmon Dis 11: 909–917, 2016. doi: 10.2147/COPD.S99810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moore BB, Lawson WE, Oury TD, Sisson TH, Raghavendran K, Hogaboam CM. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol 49: 167–179, 2013. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 32: 773–785, 2014. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 119.Murphy SV, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A 101A: 272–284, 2013. doi: 10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 120.Naba A, Pearce OMT, Del Rosario A, Ma D, Ding H, Rajeeve V, Cutillas PR, Balkwill FR, Hynes RO. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J Proteome Res 16: 3083–3091, 2017. doi: 10.1021/acs.jproteome.7b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ngalim SH, Magenau A, Le Saux G, Gooding JJ, Gaus K. How do cells make decisions: engineering micro- and nanoenvironments for cell migration. J Oncol 2010: 1–7, 2010. doi: 10.1155/2010/363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nichols JE, La Francesca S, Niles JA, Vega SP, Argueta LB, Frank L, Christiani DC, Pyles RB, Himes BE, Zhang R, Li S, Sakamoto J, Rhudy J, Hendricks G, Begarani F, Liu X, Patrikeev I, Pal R, Usheva E, Vargas G, Miller A, Woodson L, Wacher A, Grimaldo M, Weaver D, Mlcak R, Cortiella J. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med 10: eaao3926, 2018. doi: 10.1126/scitranslmed.aao3926. [DOI] [PubMed] [Google Scholar]
- 123.Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, Richter J, Gundersen HJ. The number of alveoli in the human lung. Am J Respir Crit Care Med 169: 120–124, 2004. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 124.Ohmuro-Matsuyama Y, Tatsu Y. Photocontrolled cell adhesion on a surface functionalized with a caged arginine-glycine-aspartate peptide. Angew Chem Int Ed Engl 47: 7527–7529, 2008. doi: 10.1002/anie.200802731. [DOI] [PubMed] [Google Scholar]
- 125.Olsen KC, Sapinoro RE, Kottmann RM, Kulkarni AA, Iismaa SE, Johnson GV, Thatcher TH, Phipps RP, Sime PJ. Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med 184: 699–707, 2011. doi: 10.1164/rccm.201101-0013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol 32: 56–67, 2000. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 127.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76: 321–343, 2016. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 128.Ozbolat IT, Moncal KK, Gudapati H. Evaluation of bioprinter technologies. Additive Manufacturing 13: 179–200, 2017. doi: 10.1016/j.addma.2016.10.003. [DOI] [Google Scholar]
- 129.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8: 839–845, 2007. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 130.Parker MW, Rossi D, Peterson M, Smith K, Sikström K, White ES, Connett JE, Henke CA, Larsson O, Bitterman PB. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest 124: 1622–1635, 2014. doi: 10.1172/JCI71386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pena AM, Fabre A, Débarre D, Marchal-Somme J, Crestani B, Martin JL, Beaurepaire E, Schanne-Klein MC. Three-dimensional investigation and scoring of extracellular matrix remodeling during lung fibrosis using multiphoton microscopy. Microsc Res Tech 70: 162–170, 2007. doi: 10.1002/jemt.20400. [DOI] [PubMed] [Google Scholar]
- 132.Petersen S, Alonso JM, Specht A, Duodu P, Goeldner M, del Campo A. Phototriggering of cell adhesion by caged cyclic RGD peptides. Angew Chem Int Ed Engl 47: 3192–3195, 2008. doi: 10.1002/anie.200704857. [DOI] [PubMed] [Google Scholar]
- 133.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43, Suppl S: S25–S32, 2004. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 134.Prestwich GD, Bhatia S, Breuer CK, Dahl SL, Mason C, McFarland R, McQuillan DJ, Sackner-Bernstein J, Schox J, Tente WE, Trounson A. What is the greatest regulatory challenge in the translation of biomaterials to the clinic? Sci Transl Med 4: 160cm14, 2012. doi: 10.1126/scitranslmed.3004915. [DOI] [PubMed] [Google Scholar]
- 135.Price AP, Godin LM, Domek A, Cotter T, D’Cunha J, Taylor DA, Panoskaltsis-Mortari A. Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng Part C Methods 21: 94–103, 2015. doi: 10.1089/ten.tec.2013.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 308: L229–L252, 2015. doi: 10.1152/ajplung.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials Science: An Introduction to Materials in Medicine. San Diego: Elsevier Academic Press, 2004. [Google Scholar]
- 138.Raymond S, Weintraub L. Acrylamide gel as a supporting medium for zone electrophoresis. Science 130: 711, 1959. doi: 10.1126/science.130.3377.711. [DOI] [PubMed] [Google Scholar]
- 139.Rennard SI. COPD: overview of definitions, epidemiology, and factors influencing its development. Chest 113, Suppl: 235S–241S, 1998. doi: 10.1378/chest.113.4_Supplement.235S. [DOI] [PubMed] [Google Scholar]
- 140.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 389: 1941–1952, 2017. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 141.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 142.Rimington TL, Hodge E, Billington CK, Bhaker S, KC B, Kilty I, Jelinsky S, Hall IP, Sayers I. Defining the inflammatory signature of human lung explant tissue in the presence and absence of glucocorticoid. F1000 Res 6: 460, 2017. doi: 10.12688/f1000research.10961.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Lung Cell Mol Physiol 301: L625–L635, 2011. doi: 10.1152/ajplung.00105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roberts J, Lunn K, Tear V, Jones M, Davies D, Cao L, Jarolimek W, Monk P. Inhibition of collagen cross-links by novel LOXL2 selective inhibitors in an in-vitro model of fibroblastic foci of IPF. Eur Respir J PA4035: 48, 2016. doi: 10.1183/13993003.congress-2016.PA4035. [DOI] [Google Scholar]
- 145.Rosales AM, Rodell CB, Chen MH, Morrow MG, Anseth KS, Burdick JA. Reversible control of network properties in azobenzene-containing hyaluronic acid-based hydrogels. Bioconjug Chem 29: 905–913, 2018. doi: 10.1021/acs.bioconjchem.7b00802. [DOI] [PubMed] [Google Scholar]
- 146.Rosales AM, Vega SL, DelRio FW, Burdick JA, Anseth KS. Hydrogels with reversible mechanics to probe dynamic cell microenvironments. Angew Chem Int Ed Engl 56: 12132–12136, 2017. doi: 10.1002/anie.201705684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ruskowitz ER, DeForest CA. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat Rev Mat 3: 17087, 2018. doi: 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- 148.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163: 1304–1309, 2001. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 149.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29: 2370–2377, 2008. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sargeant TD, Desai AP, Banerjee S, Agawu A, Stopek JB. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater 8: 124–132, 2012. doi: 10.1016/j.actbio.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 151.Sava P, Ramanathan A, Dobronyi A, Peng X, Sun H, Ledesma-Mendoza A, Herzog EL, Gonzalez AL. Human pericytes adopt myofibroblast properties in the microenvironment of the IPF lung. JCI Insight 2: e96352, 2017. doi: 10.1172/jci.insight.96352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schiller HB, Fernandez IE, Burgstaller G, Schaab C, Scheltema RA, Schwarzmayr T, Strom TM, Eickelberg O, Mann M. Time- and compartment-resolved proteome profiling of the extracellular niche in lung injury and repair. Mol Syst Biol 11: 819, 2015. doi: 10.15252/msb.20156123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schulte VA, Díez M, Möller M, Lensen MC. Surface topography induces fibroblast adhesion on intrinsically nonadhesive poly(ethylene glycol) substrates. Biomacromolecules 10: 2795–2801, 2009. doi: 10.1021/bm900631s. [DOI] [PubMed] [Google Scholar]
- 154.Scott RA, Kharkar PM, Kiick KL, Akins RE. Aortic adventitial fibroblast sensitivity to mitogen activated protein kinase inhibitors depends on substrate stiffness. Biomaterials 137: 1–10, 2017. doi: 10.1016/j.biomaterials.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3: 364–372, 2006. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 156.Sharma S, Floren M, Ding Y, Stenmark KR, Tan W, Bryant SJ. A photoclickable peptide microarray platform for facile and rapid screening of 3-D tissue microenvironments. Biomaterials 143: 17–28, 2017. doi: 10.1016/j.biomaterials.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 157.Sicard D, Haak AJ, Choi KM, Craig AR, Fredenburgh LE, Tschumperlin DJ. Aging and anatomical variations in lung tissue stiffness. Am J Physiol Lung Cell Mol Physiol 314: L946–L955, 2018. doi: 10.1152/ajplung.00415.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Skronska-Wasek W, Mutze K, Baarsma HA, Bracke KR, Alsafadi HN, Lehmann M, Costa R, Stornaiuolo M, Novellino E, Brusselle GG, Wagner DE, Yildirim AO, Königshoff M. Reduced frizzled receptor 4 expression prevents Wnt/β-catenin-driven alveolar lung repair in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 196: 172–185, 2017. doi: 10.1164/rccm.201605-0904OC. [DOI] [PubMed] [Google Scholar]
- 159.Smithmyer ME, Spohn JB, Kloxin AM. Probing fibroblast activation in response to extracellular cues with whole protein- or peptide-functionalized step-growth hydrogels. ACS Biomat Sci Eng 4: 3304–3316, 2018. doi: 10.1021/acsbiomaterials.8b00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Society FoIRSWH The Global Impact of Respiratory Disease. Sheffield, UK: European Respiratory Society, 2017. [Google Scholar]
- 161.Sridhar BV, Doyle NR, Randolph MA, Anseth KS. Covalently tethered TGF-β1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J Biomed Mater Res A 102: 4464–4472, 2014. doi: 10.1002/jbm.a.35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 164.Sundarakrishnan A, Chen Y, Black LD, Aldridge BB, Kaplan DL. Engineered cell and tissue models of pulmonary fibrosis. Adv Drug Deliv Rev 129: 78–94, 2018. doi: 10.1016/j.addr.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 165.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J 11: 51–59, 1997. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 166.The Lancet Respiratory Medicine Pulmonary hypertension: still a rare disease? Lancet Respir Med 4: 241, 2016. doi: 10.1016/S2213-2600(16)00103-X. [DOI] [PubMed] [Google Scholar]
- 167.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev 97: 4–27, 2016. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 103: 655–663, 2009. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Torres-Giner S, Pérez-Masiá R, Lagaron JM. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym Eng Sci 56: 500–527, 2016. doi: 10.1002/pen.24274. [DOI] [Google Scholar]
- 170.Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, O’Donnell CJ, Hoffmann U. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 5: 147–154, 2012. doi: 10.1161/CIRCIMAGING.111.968610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tschumperlin DJ, Ligresti G, Hilscher MB, Shah VH. Mechanosensing and fibrosis. J Clin Invest 128: 74–84, 2018. doi: 10.1172/JCI93561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One 6: e15978, 2011. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, McDonald J, Liu X, El-Chemaly S, Perrella MA, Rosas IO. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J Respir Cell Mol Biol 58: 208–215, 2018. doi: 10.1165/rcmb.2017-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tsuchiya T, Mendez J, Calle EA, Hatachi G, Doi R, Zhao L, Suematsu T, Nagayasu T, Niklason LE. Ventilation-based decellularization system of the lung. Biores Open Access 5: 118–126, 2016. doi: 10.1089/biores.2016.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med 28: 23–42, 2007. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Uhl FE, Vierkotten S, Wagner DE, Burgstaller G, Costa R, Koch I, Lindner M, Meiners S, Eickelberg O, Königshoff M. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J 46: 1150–1166, 2015. doi: 10.1183/09031936.00183214. [DOI] [PubMed] [Google Scholar]
- 177.Uhl FE, Wagner DE, Weiss DJ. Preparation of decellularized lung matrices for cell culture protein analysis. In: Fibrosis: Methods in Molecular Biology, edited by Rittie L. New York: Humana, 2017, vol. 1627. doi: 10.1007/978-1-4939-7113-8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Verleden SE, Vasilescu DM, Willems S, Ruttens D, Vos R, Vandermeulen E, Hostens J, McDonough JE, Verbeken EK, Verschakelen J, Van Raemdonck DE, Rondelet B, Knoop C, Decramer M, Cooper J, Hogg JC, Verleden GM, Vanaudenaerde BM. The site and nature of airway obstruction after lung transplantation. Am J Respir Crit Care Med 189: 292–300, 2014. doi: 10.1164/rccm.201310-1894OC. [DOI] [PubMed] [Google Scholar]
- 179.Wade RJ, Burdick JA. Engineering ECM signals into biomaterials. Mater Today 15: 454–459, 2012. doi: 10.1016/S1369-7021(12)70197-9. [DOI] [Google Scholar]
- 180.Wagner DE, Bonenfant NR, Parsons CS, Sokocevic D, Brooks EM, Borg ZD, Lathrop MJ, Wallis JD, Daly AB, Lam YW, Deng B, DeSarno MJ, Ashikaga T, Loi R, Weiss DJ. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 35: 3281–3297, 2014. doi: 10.1016/j.biomaterials.2013.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wilkinson DC, Alva-Ornelas JA, Sucre JM, Vijayaraj P, Durra A, Richardson W, Jonas SJ, Paul MK, Karumbayaram S, Dunn B, Gomperts BN. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med 6: 622–633, 2017. doi: 10.5966/sctm.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9: 157–179, 2014. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yan Z, Kui Z, Ping Z. Reviews and prospectives of signaling pathway analysis in idiopathic pulmonary fibrosis. Autoimmun Rev 13: 1020–1025, 2014. doi: 10.1016/j.autrev.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 184.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater 13: 645–652, 2014. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang Y, Kusano K, Frei H, Rossi F, Brunette DM, Putnins EE. Microtopographical regulation of adult bone marrow progenitor cells chondrogenic and osteogenic gene and protein expressions. J Biomed Mater Res A 95A: 294–304, 2010. doi: 10.1002/jbm.a.32838. [DOI] [PubMed] [Google Scholar]
- 186.Yuan H, Kononov S, Cavalcante FS, Lutchen KR, Ingenito EP, Suki B. Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol (1985) 89: 3–14, 2000. doi: 10.1152/jappl.2000.89.1.3. [DOI] [PubMed] [Google Scholar]
- 187.Zhang HB, Xing TL, Yin RX, Shi Y, Yang SM, Zhang WJ. Three-dimensional bioprinting is not only about cell-laden structures. Chin J Traumatol 19: 187–192, 2016. doi: 10.1016/j.cjtee.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, Bitterman PB, Corley RA, Ding B-S, Engler AJ, Hansen KC, Hagood JS, Kheradmand F, Lin QS, Neptune E, Niklason L, Ortiz LA, Parks WC, Tschumperlin DJ, White ES, Chapman HA, Thannickal VJ. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol 73: 77–104, 2018. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, Rizzi SC, Davies N, Schmökel H, Bezuidenhout D, Djonov V, Zilla P, Hubbell JA. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J 17: 2260–2262, 2003. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 190.Zych B, Garcia-Saez D, Simon AR. Lung donor shortage—how to overcome it? Kardiochir Torakochirurgia Pol 13: 195–197, 2016. doi: 10.5114/kitp.2016.62603. [DOI] [PMC free article] [PubMed] [Google Scholar]