Abstract
Inflammation from airborne microbes can overwhelm compensatory mucociliary clearance mechanisms, leading to mucous cell metaplasia. Toll-like receptor (TLR) activation via myeloid differentiation factor 88 (MyD88) signaling is central to pathogen responses. We have previously shown that agricultural organic dust extract (ODE), with abundant microbial component diversity, activates TLR-induced airway inflammation. With the use of an established model, C57BL/6J wild-type (WT) and global MyD88 knockout (KO) mice were treated with intranasal inhalation of ODE or saline, daily for 1 wk. ODE primarily increased mucin (Muc)5ac levels relative to Muc5b. Compared with ODE-challenged WT mice, ODE-challenged, MyD88-deficient mice demonstrated significantly increased Muc5ac immunostaining, protein levels by immunoblot, and expression by quantitative PCR. The enhanced Muc5ac levels in MyD88-deficient mice were not explained by differences in the differentiation program of airway secretory cells in naïve mice. Increased Muc5ac levels in MyD88-deficient mice were also not explained by augmented inflammation, IL-17A, or neutrophil elastase levels. Furthermore, the enhanced airway mucins in the MyD88-deficient mice were not due to defective secretion, as the mucin secretory capacity of MyD88-KO mice remained intact. Finally, ODE-induced Muc5ac levels were enhanced in MyD88-deficient airway epithelial cells in vitro. In conclusion, MyD88 deficiency enhances airway mucous cell metaplasia under environments with high TLR activation.
Keywords: agriculture, airway, epithelial, lung, Muc5ac, Muc5b, mucin, MyD88, organic dust
INTRODUCTION
Mucin overproduction and secretion are hallmark characteristics of chronic inflammatory airway diseases, including asthma, chronic bronchitis, and chronic obstructive lung disease. Occupational organic dust exposure in agricultural environments can lead to these diseases, with increases noted in neutrophil influx, inflammatory cytokine release, airway hyper-responsiveness, and lung function loss over time (15, 45, 46). The prominent reported respiratory symptoms of organic dust-exposed persons include chronic cough and sputum production (27, 29, 46). Increased production of respiratory mucins contributes to airway obstruction and decline of lung function in chronic inflammatory airway diseases, including asthma and chronic obstructive lung disease (17, 25).
The barrier epithelium of the airway is lined with ciliated and secretory cells that combine to sweep the airway via coordinated mucociliary clearance. Mucin glycoproteins are organized in granules at the trans-Golgi and are transported to the apical surface where secretion is regulated independently of production (43). Baseline mucin secretion dominates normal airway physiology, interspersed with agonist-stimulated mucin secretion that is predominately triggered by extracellular triphosphate nucleotides, such as ATP (9, 17, 34). The two principle gel-forming mucins secreted in the airway are mucin (Muc)5ac and Muc5b. In the mouse, airway secretory cells predominately produce secretoglobin 1a1 (Scgb1a1), in addition to Muc5b at baseline. During inflammation, the secretory cells assume a goblet cell phenotype, and the dominant mucin produced from airway epithelial cells is Muc5ac (18). Muc5b is produced constitutively and is primarily responsive for mucociliary clearance and is less responsive to inflammatory signals. In particular, loss of Muc5b leads to reduced innate immunity and spontaneous airway infection (44). In human allergy and allergic mouse models, which are driven by T helper type 2 (Th2) and eosinophilic inflammatory responses (33), Muc5ac overproduction is dependent on the IL-13/IL-4 receptor α complex and the EGF receptor (EGFR) (17). Various stimuli, including cigarette smoke, allergens, viruses, bacterial and fungal products, and proinflammatory cytokines, can induce mucin expression and secretion (1, 18, 28, 36). Bacteria infection models activate both Muc5ac and Muc5b (49).
Compared with the gastrointestinal tract, less is known about the potential impact of innate immunity and Toll-like receptor (TLR) signaling on airway epithelial cell mucin production. Recent studies with gut epithelial cells demonstrated a role for adaptor protein myeloid differentiation factor 88 (MyD88) in the regulation of epithelial responses to bacterial products. Targeted deletion of MyD88 in the gut epithelium led to loss of epithelial integrity, reduced colonic Muc2 expression, and increased bacteria in the adherent inner-mucus layers (21). Moreover, MyD88-dependent signaling through TLR2/1, TLR4, and TLR5 recognition by microbial motifs was recently demonstrated to mediate the Muc2 section by sentinel goblet cells in the colon of mice (4). Thus MyD88 signaling may play a role in the regulation of secretory cells in the airway in response to bacterial ligands.
Based on these collective studies, we hypothesized that the use of a swine confinement facility organic dust extract (ODE), rich in diverse, microbial TLR-activating motifs, would increase airway mucus production and secretion and that this response would be dependent on MyD88. Our findings demonstrate that MyD88 deficiency leads to upregulation of mucus production, providing a layer of control in conditions of broad TLR activation. This suggests that MyD88 negatively regulates mucous cell metaplasia during microbial-mediated airway inflammation. Unlike the gut, we do not find a role for MyD88 in directly mediating mucus secretion in the airways.
METHODS
Animals.
C57BL/6J wild-type (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice with global MyD88 gene interruption on the same C57BL/6J background were provided by S. Akira (Osaka University, Osaka, Japan). Confirmation of the MyD88 knockout (KO) genotype was independently assured by Transnetyx genotyping services (data not shown; Cordova, TN). Male and female mice were used between 10 and 12 wk of age for all experimental studies. Food and water were provided ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center or the MD Anderson Cancer Center and were conducted in accordance with the U.S. National Institutes of Health guidelines for the use of rodents.
Organic dust extract.
Aqueous ODE was prepared from settled surface dust collected from horizontal surfaces (3 ft above floor) of swine confinement feeding operations, and extracts were batch prepared, as previously described (41). Briefly, dust (1 g) was dissolved into Hanks’ balanced salt solution (10 ml; Sigma, St. Louis, MO), incubated at room temperature for 1 h, and centrifuged for 20 min at 2,000 g, and the final supernatant was filter sterilized (0.22 μm), a process that also removes coarse particles. Stock ODE was batch prepared and diluted to a final concentration of 5% (vol/vol) and 12.5% (vol/vol) in sterile PBS (pH 7.4; diluent) for cell-culture and animal studies, respectively. The 12.5% ODE has been previously shown to elicit optimal lung inflammation in mice and is well tolerated (41). A 12.5% ODE extract contained 3–4 mg/ml total protein, as measured by NanoDrop spectrophotometry (NanoDrop Technologies, Wilmington, DE), and endotoxin levels ranged from 22.1 to 91.1 EU/ml, as assayed using the limulus amebocyte lysate assay, according to the manufacturer’s instruction (Sigma). Muramic acid levels, a chemical marker of bacterial cell-wall peptidoglycans, were ~400 pmol/mg, as previously determined by mass spectrometry (38). Concentrations of fungal components, as previously determined by mass spectroscopy, were low and similar to that found in domestic homes (38). The 5% ODE concentration was selected for in vitro work to minimize nonspecific cell toxicity, while maximizing signaling, leading to mucous cell metaplasia. A 5% ODE equates to 8.8–36.4 EU/ml endotoxin. This concentration is also consistent with our previously published work in epithelial cells, whereby the 5% ODE concentration induces inflammation in vitro (42, 52, 54). Prior work using shotgun metagenomics analysis of DNA pyrosequencing techniques demonstrated an abundant and wide diversity of gram-positive and -negative bacteria in the organic dust (5).
Murine model of exposure to ODE, ATP, ovalbumin, and Pam2-ODN.
Mice were lightly anesthetized by isoflurane inhalation before intranasal inhalation of 50 μl sterile saline or 12.5% ODE, daily for 3 days, 1 wk (eight challenges), or 3 wk (15 challenges), per the established protocol (41). Animals were euthanized 5 h following final exposure for experimental end points. To assess the role of MyD88 in the secretory response of intracellular mucins to ATP, a subset of WT and MyD88-KO mice was placed in a plexiglass chamber and exposed to nebulized ATP (100 mM diluted in sterile water), 30–60 min before the animal was euthanized, as previously described (37). To test further the role of MyD88 in mucin secretory responses, mucous metaplasia was first induced by intraperitoneal immunization with ovalbumin (OVA) and aerosol exposure to OVA, as described (19). A subset of mice was exposed to nebulized oligodeoxynucleotide M362 (ODN) and Pam2CSK [Pam2 (Pam2-ODN); InvivoGen, San Diego, CA], as described (8), 30–60 min before euthanasia.
Bronchoalveolar lavage fluid studies.
Bronchoalveolar lavage fluid (BALF) was collected using 3 × 1 ml instillations of PBS. From cell-free supernatant of the first lavage, cytokines implicated in the regulation of mucin production, including IL-17A, IL-13, and IL-33, were quantitated by ELISA (eBioscience through Thermo Fisher Scientific, Waltham, MA) with a lower limit of detections of 4, 4, and 33 pg/ml, respectively. Neutrophil elastase from the first lavage fraction was also quantitated by ELISA (R&D Systems, Minneapolis, MN), with a lower limit of detection of 12.5 ng/ml. After the three lavages were pooled, the total number of cells per mouse was enumerated and differential cell counts performed using cytospin-prepared slides (Cytopro Cytocentrifuge; Wescor, Logan, UT), stained with DiffQuick (Siemens, Newark, DE).
Mouse and human tracheal epithelial cell culture.
Mouse tracheal epithelial cells (mTECs) were isolated after pronase digestion of mouse tracheas and grown on supported membrane inserts, as previously described (10, 11). Nondiseased human tracheobronchial epithelial cells (hTECs) were derived for culture from excess trachea tissue, donated for lung transplantation. After 5–7 days, air–liquid interface (ALI) conditions were established, and cells were fed with PneumaCult ALI media (Catalog No. 05001; StemCell Technologies, Cambridge, MA). After 18–21 days in ALI conditions, media ± 5% ODE was added to the basolateral compartment for 5 days for mTEC and 7 days for hTEC in vitro experiments.
Immunostaining and microscopy of mouse lungs.
After euthanasia, the right heart was cannulated, and sterile PBS with heparin sulfate (0.67 U/ml) was infused to remove blood from the pulmonary vasculature. The whole lungs were then excised and slowly inflated with 10% formalin and hung under 20 cm H2O pressure for 24 h. Fixed lung tissue was embedded in paraffin, and 5 μm lung sections were cut. Mucous cells were detected by staining either with periodic acid Schiff (PAS) or fluorescent PAS (19). Antibody- and lectin-specific staining was performed on embedded lung-tissue slides, as previously described (11). Lectin [Ulex europaeus agglutinin I (UEA-1)] specifically recognizes fucosylated-containing glycans that are upregulated in Muc5ac mucins (37, 43). Mucin-detection methods in mouse lungs included lectin UEA-1 for Muc5ac conjugated with fluorophore 555 nm (1:500 vol/vol dilution) and mouse anti-Muc5b (3E1) antibody (1:250 vol/vol dilution) (37, 43). Staining for Scgb1a1, also termed club cell secretory protein, was done with a rabbit polyclonal antibody (No. WRAB-3950; Seven Hills Bioreagents, Cincinnati, OH). Fluorophore-labeled donkey secondary species-specific antibodies were Alexa Fluor 488 or 555 (Nos. A-31570, A21202, A21206, and A31572; Thermo Fisher Scientific). To counterstain nuclei and quantify the number of cells per airway, DNA, with 4′,6-diamidino-2-phenylindole (DAPI), was included in Vectashield mounting medium (H-1200; Vector Laboratories, Burlingame, CA). Images were obtained using a Zeiss Axio Observer Z.1 microscope and analyzed by Zen software (Zeiss, Thornwood, NY). Intracellular mucin was assessed by morphometric analysis using ImageJ software and quantified from images of multiple fields at ×20 magnification, as previously described (10). Briefly, with the use of a common signal threshold among all images, monochromatic images were used to measure individual mucin-staining area per secretory cell, as well as number of mucin-positive secretory cells per DAPI, plus cells from a given airway. Values less than this threshold were recorded at a value of zero.
In vitro mTEC/hTEC staining was performed in both cross-sections of paraffin-embedded formalin inserts and fixed whole membranes. First, mTECs were embedded in warmed, 37°C, 1% agarose. After being cooled, the inserts were fixed with 10% formalin and embedding in paraffin. Cross-sections from the inserts were then cut and fixed on slides for immunostaining, as previously described (11). With the use of the same antigen retrieval and blocking method described above for lung slides, mTECs were stained with (1:100 vol/vol dilution) rabbit polyclonal antibody to MyD88 (ab135693; Abcam, Cambridge, MA) or UEA-1. Nonembedded membranes with mTEC/hTEC inserts were fixed with 4% paraformaldehyde for 15 min, followed by three, 5-min PBS washes. Cells were blocked by a solution with 5% donkey serum (D9663; Sigma) and 0.1% BSA in PBS with 0.1% Triton X-100 (BP151; Thermo Fisher Scientific). mTEC staining was then performed with lectin UEA-1 conjugated with fluorophore 555 nm for Muc5ac (1:500 vol/vol dilution) or mouse Muc5b MAb (37, 43) hTEC staining was performed with mouse MAb (45M1 epitope) for Muc5ac, as previously described (10, 11), or the mouse Muc5b MAb. Nuclear counterstaining was performed with DNA with DAPI. Mucin-staining area and number of positive cells per total cell number were quantified by ImageJ, as described above and previously (10).
Mucin immunoblots.
Total mucin content from lungs was performed, as previously described (37). In brief, after excision, lungs were snap frozen in liquid nitrogen. Lungs were then homogenized in guanidium chloride buffer (pH 8.0) containing 6 M guanidium chloride, 0.1 M Tris·HCl, 5 mM ethylenediamine tetraacetic acid, and protease inhibitors (Roche, Mannheim, Germany), using a gentleMACS dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). Subsequently, the lysates were centrifuged for 30 min at 16,000 rpm at 4°C. Lung homogenate supernatants were then dialyzed against 6 M urea buffer (6 M urea, 0.1 M Tris·HCl, 5 mM EDTA, pH 8.0) using Slide-A-Lyzer MINI Dialysis, Units 2–10 K, molecular-weight cut-off (Thermo Fisher Scientific) for 16 h at 4°C. After dialysis, 14 U DNAse 1 was added to 50 μg sample and incubated at 37°C for 15 min. Loading buffer (10× buffer: 25 mg bromophenol blue, 1% wt/vol SDS, 50% vol/vol glycerol, and 50% vol/vol 6 M urea buffer), containing 5 mM DTT for Muc5b detection or no DTT for Muc5ac by UEA-1 detection, was added with water. Samples were denatured for 10 min at 95°C, and lysates were loaded on a 1% agarose gel, along with Precision Plus Protein dual-color standards (Bio-Rad, Hercules, CA), and run at 120 mV for 2 h at room temperature using SDS gel electrophoresis buffer. The gel was rinsed with water, then incubated in 10 mM DTT in saline sodium citrate (SCC) buffer (3.0 M sodium chloride, 0.3 M sodium citrate, pH 7.0) at room temperature for 20 min, and then rinsed in water for Muc5b detection. Gels with Muc5ac by UEA-1 detection were rinsed with SCC buffer without DTT and proceeded directly to transfer. The protein was then transferred onto a nitrocellulose membrane (Bio-Rad) using a vacuum blotter (model 785; Bio-Rad). During transfer, the gel was covered in SCC buffer for 2 h at 10 mmHg constant pressure at room temperature. The blot was collected into PBS and stained with REVERT total protein stain with conjugated 700 nm fluorochrome (LI-COR, Lincoln, NE) for 5 min. Blots were rinsed with deionized H2O and imaged wet using an Odyssey CLx Imager (LI-COR) for total protein detection. Blots were rinsed with PBS and blocked using blocking buffer (PBS with 5% nonfat milk) for 1 h at room temperature and then incubated with mouse MAb for Muc5b (1:1,000 vol/vol) or lectin UEA-1 L8146 1 mg/ml (1:1,000 vol/vol; Sigma) overnight at 4°C. For the detection of Muc5b, the membranes were washed with PBS for 5 min, three times. IRDye 800CW goat anti-mouse (LI-COR) at a 1:10,000 dilution for Muc5b or rabbit anti-lectin UEA-1 (U4754; Sigma) at a 1:1,000 dilution in blocking buffer for Muc5ac was added to the mucin blot and incubated at room temperature for 1 h with constant rocking. Muc5b membranes were washed with PBS for 5 min, three times, and then imaged wet using an Odyssey CLx Imager. IRDye 800CW goat anti-rabbit (LI-COR) at a 1:10,000 dilution in blocking buffer was added to the Muc5ac blot and incubated at room temperature for 1 h with constant rocking. Muc5ac membranes were washed and imaged as above. Mucin blots were quantified by the determination of the mucin signal in the 700-nm channel, and the division of the total protein signal with an 800-nm signal in each lane using the Odyssey CLx Imager (LI-COR) was previously described (30).
Quantitative PCR.
RNA was isolated from lung tissue using the RNeasy Spin column kit (No. 75144; Qiagen, Germantown, MD) and then reverse transcribed using the cDNA RT Kit (No. 4308228; Thermo Fisher Scientific). cDNAs were amplified using Fast SYBR Green Master Mix (330511; Qiagen) in a Lightcycler 480 (Roche). Sequence-specific primers for mouse Muc5ac, Muc5b, Scgb1a1, and forkhead box j1 (Foxj1) and human MUC5AC and MUC5B were used (Table 1). Gene expression was reported as fold change over the WT–saline condition, normalized to GAPDH in mouse lung tissues, and calculated using the double-delta comparative threshold cycle method, as previously described (10, 11).
Table 1.
Sequence-specific primers for mouse Muc5ac, Muc5b, Scgb1a1, and Foxj1 and human MUC5AC and MUC5B
| Target Gene | Forward and Reverse Sequence |
|---|---|
| Human MUC5AC | F: 5′-AGGCCAGCTACCGGGCCGGCCAGACCA-3′ |
| R: 5′-GTCCCCGTACACGGCGCAGGTGGCCAG-3′ | |
| Human MUC5B | F: 5′-GCTGCTGCTACTCCTGTGAGG-3′ |
| R: 5′-AGGTGATGTTGACCTCGGTCTC-3′ | |
| Human OAZ1 | F: 5′-GAGGGGAGCAAGGACAGC-3′ |
| R: 5′-GGTTCTTGTGGAAGCAAATGA-3′ | |
| Mouse Foxj1 | F: 5′-CACGGACAACTTCTGCTACTTCC-3′ |
| R: 5′-AGGACAGGTTGTGGCGGAT-3′ | |
| Mouse Gapdh | F: 5′-TGTGTCCGTCGTGGATCTGA-3′ |
| R: 5′-CCTGCTTCACCACCTTCTTGAT-3′ | |
| Mouse Muc5ac | F: 5′-TACCACTCCCTGCTTCTGCAGCGTGTCA-3′ |
| R: 5′-ATAGTAACAGTGGCCATCAAGGTCTGTCT-3′ | |
| Mouse Muc5b | F: 5′-AGGAAGACCAGTGTGTTTGTC-3′ |
| R: 5′-GTCCTCATTGAAGAAGGGCTG-3′ | |
| Mouse Scgb1a1 | F: 5′-TGCTGCAGCTCAGCTTCTTCGG-3′ |
| R: 5′-GCCAGGGTTGAAAGGCTTCAGGG-3′ |
F, forward; Foxj1, forkhead box j1; MUC5AC/MUC5B, mucin 5AC/B; OAZ1, ornithine decarboxylase antizyme 1; R, reverse; Scgb1a1, secretoglobin 1a1.
Statistical methods.
Data are presented as means and SE. To detect significant changes among groups, a one-way ANOVA was used, and a nonparametric Mann-Whitney test was performed to determine the difference between groups if the ANOVA P values were <0.05. Mann-Whitney was performed for comparison of two groups. Prism software (version 7.0c; GraphPad Software, La Jolla, CA) was used. Significance was accepted at values <0.05.
RESULTS
MyD88-deficient mice have enhanced ODE-induced mucous cell metaplasia.
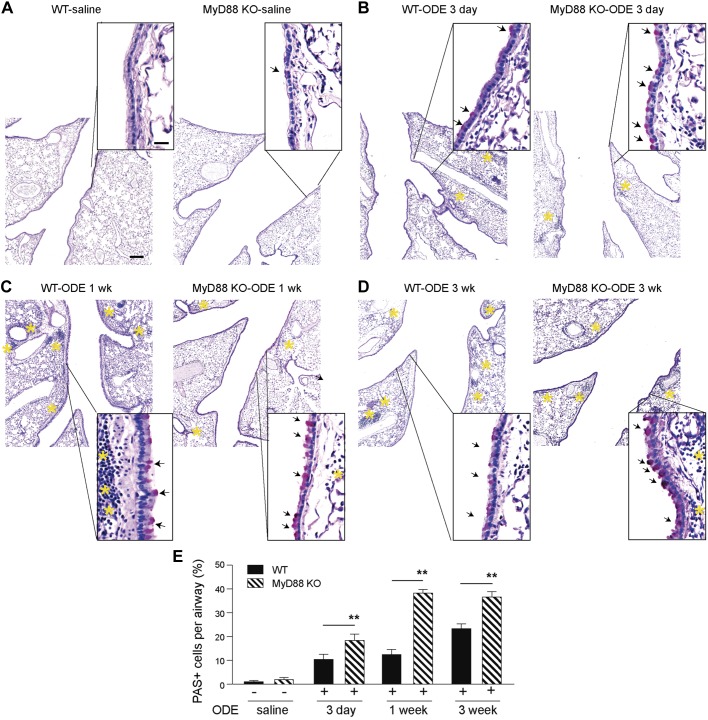
Agricultural-derived ODE consists of a complex mixture of bacterial components and other organic materials known to activate TLR2, -4, and -9 in the lungs (3, 38, 40). To assess the role of MyD88 in the regulation of mucus in the airway epithelium following broad TLR activation with ODE, mouse lung airways were stained with PAS. To determine the optimal time point for the examination of mucous cell metaplasia following ODE airway inflammation, a time course experiment was performed with 3-day (three airway challenges), 1-wk (eight airway challenges), and 3-wk (15 airway challenges) ODE intranasal challenges. We found that there were only rare PAS-positive cells concentrated in the proximal bronchi of mice challenged with saline. The number of PAS-positive cells increased in a time-dependent fashion at 3 days, 1 wk, and 3 wk of daily ODE challenges (Fig. 1, A–D). These were located mainly in the primary bronchi. The MyD88-deficient mice had significantly increased PAS-positive cells, extending from the tracheal bifurcation down to the subsegmental airways (Fig. 1, A–D) at all time points. Despite the increased number of PAS-positive cells in the airways, there were relatively fewer and smaller regions of inflammatory cells in the lungs in the MyD88-deficient mice following ODE exposure. The greatest difference in PAS staining between WT and MyD88-deficient mice occurred at the 1-wk time point (Fig. 1E). Therefore, this time period was chosen for future experiments to balance mucous cell metaplasia without excessive inflammation seen in the 3-wk model. We next sought to characterize ODE-mediated inflammation.
Fig. 1.
Myeloid differentiation factor 88 (MyD88)-deficient mice have reduced lung inflammation and increased airway periodic acid Schiff (PAS) staining following organic dust extract (ODE)-mediated inflammation. Mice were challenged with either intranasal saline for 3 days or intranasal 12.5% ODE for 3 days, 1 wk (8 airway challenges), or 3 wk (15 airway challenges; A–D). The day of the last challenge, mouse lungs were fixed with formalin and stained by PAS. Insets: low-power images were shown with higher magnification images. Arrows indicate PAS-positive cells. Yellow asterisks indicate regions of inflammatory cell infiltrate. Original scale bar, 100 μm for low-power images and 20 μm for high-magnification insets. E: quantification of percent PAS-positive cells per airway for each group (n = 1 for saline mice, and n = 2 mice for ODE-challenged groups, with 10–13 airways per mouse). **P < 0.05, significant difference between wild-type (WT) and MyD88 knockout (KO) mice.
ODE-induced neutrophil influx, neutrophil elastase release, and IL-17 are dependent on MyD88.
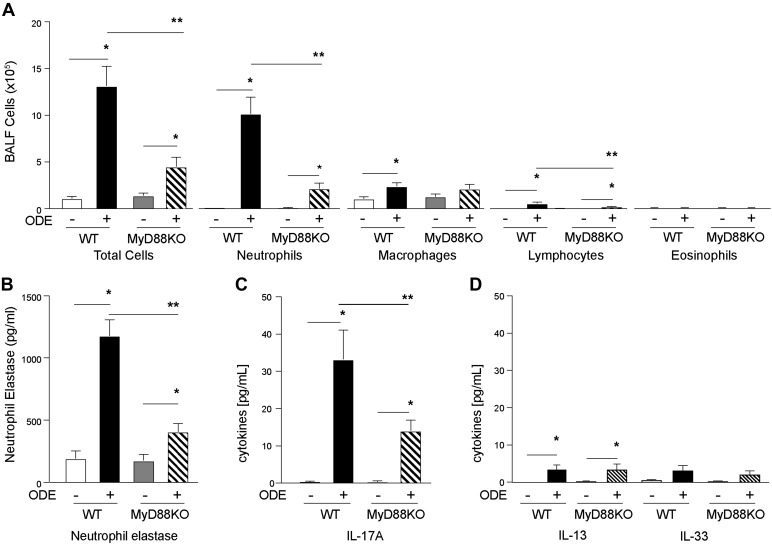
MyD88 regulates inflammatory neutrophil chemotactin and proinflammatory responses following TLR activation, which may, in turn, be responsible for the observed changes in PAS staining. To characterize the type of ODE inflammation in the context of mouse airway mucous cell metaplasia, we next examined inflammatory cells in ODE-challenged WT and MyD88-KO mice. Consistent with previous studies on neutrophil chemotactins (3, 24), total cell count and neutrophil influx were significantly reduced in ODE-challenged MyD88-KO mice compared with ODE-challenged WT mice (Fig. 2A). There was also a decrease in ODE-induced lymphocyte influx in MyD88-KO mice compared with WT. No significant levels of eosinophils were detected. Neutrophil elastase and IL-17A are well-recognized activators of airway mucous cell metaplasia during neutrophil-mediated inflammatory disease (22, 51). ODE-induced neutrophil elastase levels were significantly reduced in MyD88-KO mice compared with WT mice (Fig. 2B). Cytokines involved in mucin expression, including IL-17A, IL-13, and IL-33, were also investigated. ODE-induced IL-17A release in the BALF was significantly reduced in MyD88-KO mice compared with WT (Fig. 2C). Levels of IL-13 and IL-33 were nominally increased (3–5 pg/ml) with ODE in both WT and MyD88-KO mice (Fig. 2D). In comparison, we previously found an ~1,500 pg/ml IL-13 after OVA stimulation in Balb/c mice (10, 11). Thus the increase in PAS staining in the MyD88-KO mice (Fig. 1) is not driven by Th2 cytokines and cannot be explained by augmented neutrophil- or cytokine-mediated inflammation. We next sought to identify the specific mucin response to ODE-mediated airway inflammation.
Fig. 2.
Organic dust extract (ODE)-induced neutrophil and lymphocyte influx, neutrophil elastase, and IL-17A levels are reduced in myeloid differentiation factor 88 (MyD88) knockout (KO) animals. C57BL/6 MyD88 wild-type (WT) and KO mice were treated with intranasal inhalation of saline or ODE daily for 1 wk, and bronchoalveolar lavage fluid (BALF) was collected 5 h after final treatment. Graphs depict mean value and SE of total cells and cell differential (A). B: neutrophil elastase levels were quantified in BALF. IL-17A (C) and IL-13 and IL-33 (D) levels were quantified by ELISA from cell-free BALF (n = 8 mice per group). *P < 0.05, significant difference between ODE and saline. **P < 0.05 between WT and MyD88-KO mice.
MyD88-deficient mice have enhanced ODE-induced Muc5ac levels.
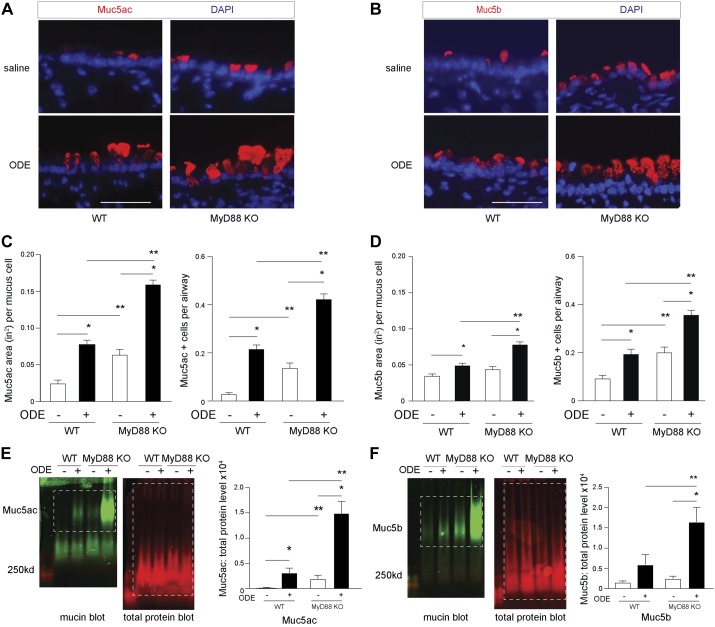
Muc5ac and Muc5b are gel-forming secretory mucins that are produced and secreted from airway epithelial cells. We examined airway mucin staining in WT and MyD88-KO mice by affinity fluorescence. Lectin UEA-1 marks fuctose carbohydrate residues that are highly specific to Muc5ac (7, 37) and was used to assess Muc5ac levels in mouse airways by immunofluorescence. Under inflammatory conditions, secretory cells transition to mucus-producing cells (19). Consistent with prior reports (55), we found Scgb1a1 within apical granules that also contained mucin costaining with Muc5b monoclonal and UEA-1 lectin. These Scgb1a1-positive granules were enlarged and apically concentrated following ODE-induced inflammation (data not shown). We found a modest increase in the Muc5ac-staining area and the number of Muc5ac positive per airway (metaplasia) in WT mice exposed to ODE (Fig. 3, A and C). However, Muc5ac staining was significantly enhanced in ODE-challenged, MyD88-deficient mice compared with ODE-challenged WT mice. In addition, MyD88-deficient mice had increased Muc5b staining compared with the ODE-challenged WT mice (Fig. 3, B and D). The increased staining of these mucins in the airway epithelium of ODE-challenged MyD88-KO mice was confirmed by immunoblot analysis in whole-lung homogenates (Fig. 3, E and F). Not unexpectedly, there was little detectable Muc5ac using lectin UEA-1 by immunoblot in saline control mice of either WT or MyD88-KO groups, due to few numbers of Muc5ac-positive cells in saline-challenged airways. Collectively, these studies demonstrate that ODE challenge increases Muc5ac compared with saline. Notably, MyD88-deficient mice had increased levels of Muc5ac, and to a lesser extent, Muc5b with ODE mediated airway inflammation compared with WT mice. To confirm enhanced airway mucin levels, we next evaluated gene expression from lung homogenates.
Fig. 3.
Myeloid differentiation factor 88 (MyD88)-deficient mice have increased mucin levels with organic dust extract (ODE)-mediated inflammation. C57BL/6 wild-type (WT) or MyD88 knockout (KO) mice received intranasal saline or ODE 12.5% daily for 1 wk. Representative images from immunostaining of lung tissues for mucin (Muc)5ac [Ulex europaeus agglutinin I (UEA-1) lectin; A] and Muc5b (B) are shown (original scale bars, 20 μm). Bar graphs depict mean value of Muc5ac (C)- and Muc5b (D)-staining area per mucous cell and number of positive Muc5ac/Muc5b cells per airway with SE bars (n = 7 mice per ODE condition, and n = 5 mice per saline condition, with 8–12 airway images per mouse). Representative immunoblotting for UEA-1 (Muc5ac; E) or Muc5b (F), with corresponding quantification of band densitometry from whole-lung lysate homogenates (n = 8–9 mice/group). Dashed boxes indicate area of quantification. *P < 0.05, significant difference between ODE and saline. **P < 0.05, significant difference between WT and MyD88-KO animals. DAPI, 4′,6-diamidino-2-phenylindole.
MyD88-deficient mouse lungs have increased Muc5ac expression in response to ODE-induced inflammation.
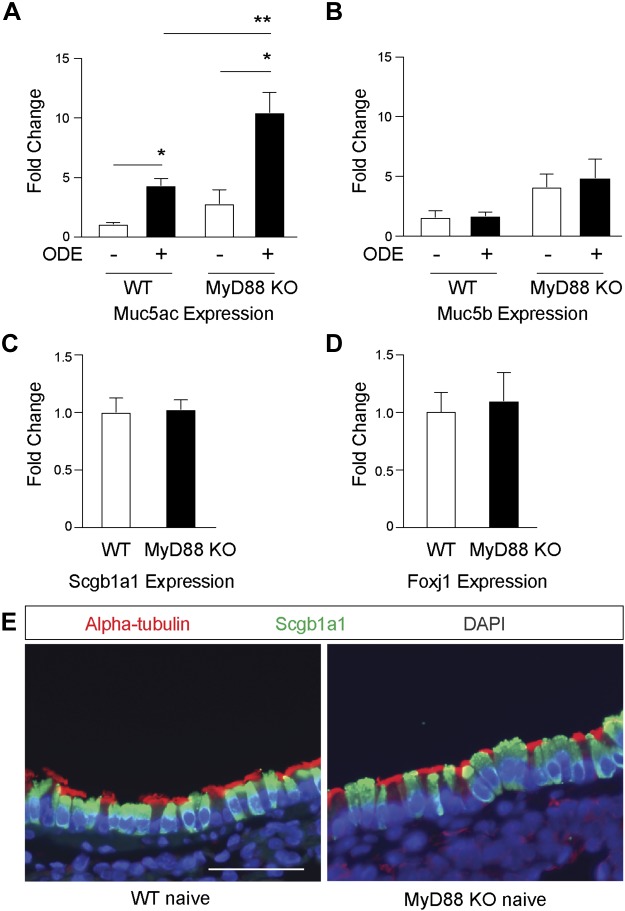
Intracellular mucin staining content in the airway is dependent on ongoing mucin production, storage of granules, and secretion (43). To assess the relative level of control of mucin production by MyD88 as an explanation of the observed staining patterns, we next investigated mRNA levels by quantitative PCR (qPCR) from lung-tissue homogenates. ODE-treated WT mice demonstrate a modest four- to fivefold increase in Muc5ac gene expression (Fig. 4A) compared with saline control mice. As with protein levels, there was significant augmentation of Muc5ac gene expression in the ODE-challenged, MyD88-deficient mice compared with WT ODE-challenged mice. Muc5b expression did not change with ODE challenge in WT mice compared with saline controls, and there was only a nonsignificant trend toward higher amounts of Muc5b expression in the ODE-challenged, MyD88-deficient mice compared with WT ODE-challenged mice (Fig. 4B). The observed differences in MyD88-dependent Muc5ac transcripts may reflect fundamental changes to the epithelial differentiation program, resulting in an increased number of secretory cells at baseline. Therefore, we assessed Scgb1a1 expression as a marker of mouse secretory cells and Foxj1 expression as a marker of ciliated cells in whole-lung preparations from naïve WT and MyD88-KO mice (Fig. 4, C–E). There was no difference in gene expression of these differentiation markers (n = 4 mice per group), consistent with previous findings in isolated mouse airway epithelial cells (26). These findings suggest that MyD88 negatively regulates the transcription program of Muc5ac in existing secretory cells and that the accumulation of mucins in the airways in ODE-challenged MyD88-KO mice is explained by increased production during broad TLR-activating conditions.
Fig. 4.
Myeloid differentiation factor 88 (MyD88)-deficient mice have enhanced organic dust extract (ODE)-mediated mucin (Muc)5ac expression. Wild-type (WT) and MyD88 knockout (KO) mice were treated with intranasal inhalation of saline or ODE for 1 wk, whereupon lung homogenates were collected. Bar graphs depict mean value and SE for Muc5ac (A) and Muc5b (B) mRNA expression, as measured by quantitative PCR (qPCR). Mucin expression was normalized to GAPDH and reported as fold change relative to mean WT saline condition (n = 12–14 mice per group from 6 separate experiments). C and D: secretoglobin 1a1 (Scgb1a1) and forkhead box j1 (Foxj1) expression in lung homogenates was measured by qPCR from naïve WT and MyD88-KO mice not challenged with either saline or ODE (n = 4 mice per group). E: representative images of naïve WT and MyD88-KO mouse airways with α-tubulin for cilia and Scgb1a1 immunostaining. Original scale bar, 50 μm. *P < 0.05, significant difference between ODE and saline. **P < 0.05, significant difference between WT and MyD88-KO mice. DAPI, 4′,6-diamidino-2-phenylindole.
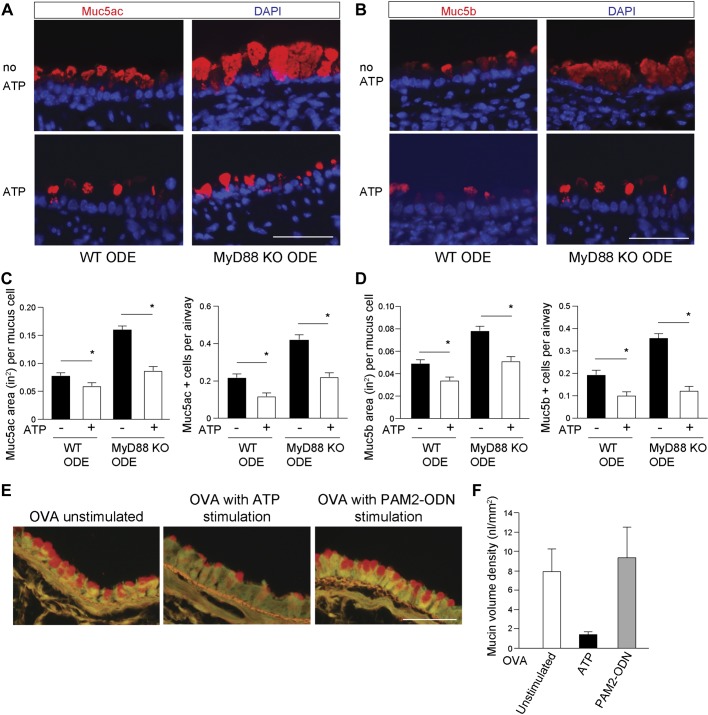
MyD88-deficient mice secrete mucin normally in response to ATP stimulation, and WT mice fail to secrete mucin in response to ligands upstream of MyD88.
It was recently found that MyD88 mediates secretion of Muc2 in sentinel goblet cells of the intestinal crypt (4). Therefore, we explored the role of MyD88 on mucin secretion in two contexts: first, we measured the response to ATP in MyD88-deficient mice. Second, we measured the effect on secretion of MyD88-dependent TLR agonists in WT mice. The airway mucins, Muc5ac and Muc5b, undergo ATP-stimulated secretion through P2Y2 purinergic receptors on the apical epithelial surface to signal intracellular calcium influx and induce mucin granule exocytosis (34, 55). WT and MyD88-KO mice, challenged with ODE daily for 1 wk, received nebulized ATP, 30–60 min before euthanasia. We then assessed differences in levels of pre- and post-ATP intracellular mucin staining between ODE-treated WT and MyD88-KO mice in terms of mucin-staining area and number of mucin-positive cells. We found the expected decrease in Muc5ac and Muc5b staining after ATP treatment in both WT and MyD88-KO mice (Fig. 5, A–D). These studies demonstrate that the ATP responses required for activated mucin secretion are intact in MyD88-deficient mice and did not account for the observed intracellular mucin increases. We then used a combination of TLR agonists, Pam2-ODN (8), which powerfully activates airway epithelial cells in a MyD88-dependent manner, to test whether stimulated mucin secretion could occur by MyD88 signaling. We found no induction of stimulated mucin secretion after a single Pam2-ODN-nebulized application (Fig. 5, E and F). These findings indicate that in the mouse airway, MyD88 does not mediate stimulated mucin secretion.
Fig. 5.
Myeloid differentiation factor 88 (MyD88) is not required for ATP-activated mucin secretion in vivo. Representative images of mucin (Muc)5ac detected by fluorescent-labeled Ulex europaeus agglutinin I lectin (A) and Muc5b with mouse MAb (B) in organic dust extract (ODE)-challenged wild-type (WT) and MyD88 knockout (KO) mouse airways. Bottom: representative images of mucin staining, followed by ATP in vivo stimulation. Top: images of staining without ATP (no ATP). Bar graphs depict mean value and SE of Muc5ac (C)- and Muc5b (D)-staining area and number of positive mucous cells per airway, with and without ATP stimulation. Values for no ATP are taken from Fig. 2 quantification (n = 5 mice per condition; 8–12 images per mouse). Original scale bars, 50 μm. *P < 0.05, significant difference between ODE and saline challenges. E: mucous metaplasia was induced by intraperitoneal immunization to ovalbumin (OVA), followed by aerosol exposure to OVA. Mice were then exposed to aerosolized ATP or Pam2CSK (Pam2)-oligodeoxynucleotide M362 (ODN) to induce mucin secretion, then mice were euthanized after 30–60 min, and excised lungs were stained with fluorescent periodic acid Schiff to examine intracellular mucin content. Representative images are shown of mice with OVA-induced mucous metaplasia but not challenged with an aerosolized secretagogue (left), challenged with ATP (middle), or challenged with Pam2-ODN (right). F: bar graphs depict mean values and SE. DAPI, 4′,6-diamidino-2-phenylindole.
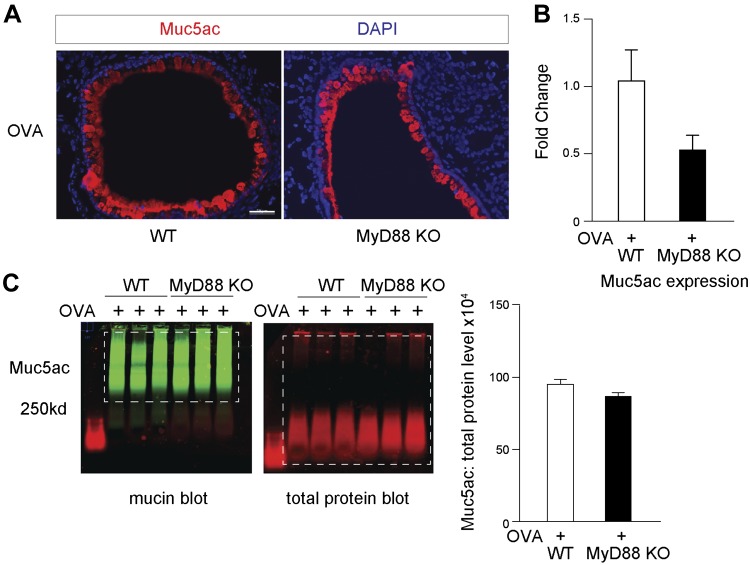
MyD88 deficiency has no effect on Muc5ac levels during Th2-mediated inflammation.
We next addressed whether global MyD88 deficiency affected mucous cell metaplasia during Th2 inflammation. WT and MyD88-deficient mice were sensitized and challenged with OVA. As expected, OVA challenge induced massive amounts of Muc5ac. We found, however, no significant difference in Muc5ac by mRNA expression or total protein levels using UEA-1 immunoblotting between WT or MyD88-deficient mouse lungs after OVA challenge (Fig. 6, A–C). This indicates that MyD88 selectively controls Muc5ac production under broad TLR activation conditions from ODE but not indiscriminately during other airway insults, such as Th2-mediated inflammation.
Fig. 6.
Myeloid differentiation factor 88 (MyD88)-deficient mice have no difference in mucous cell metaplasia in response to ovalbumin (OVA)-mediated T helper cell type 2 inflammation. A: representative images of Ulex europaeus agglutinin I (UEA-1) for mucin (Muc)5ac staining from wild-type (WT) and MyD88 knockout (KO) mouse lungs following OVA-mediated inflammation. Original scale bar, 50 μm. B: Muc5ac expression by mRNA from WT or MyD88-KO mouse lung (n = 3 mice per group). C: immunoblotting for UEA-1 and total protein lysates (B) with corresponding quantification (n = 3 mice per group). Dashed boxes indicate areas of quantification. DAPI, 4′,6-diamidino-2-phenylindole.
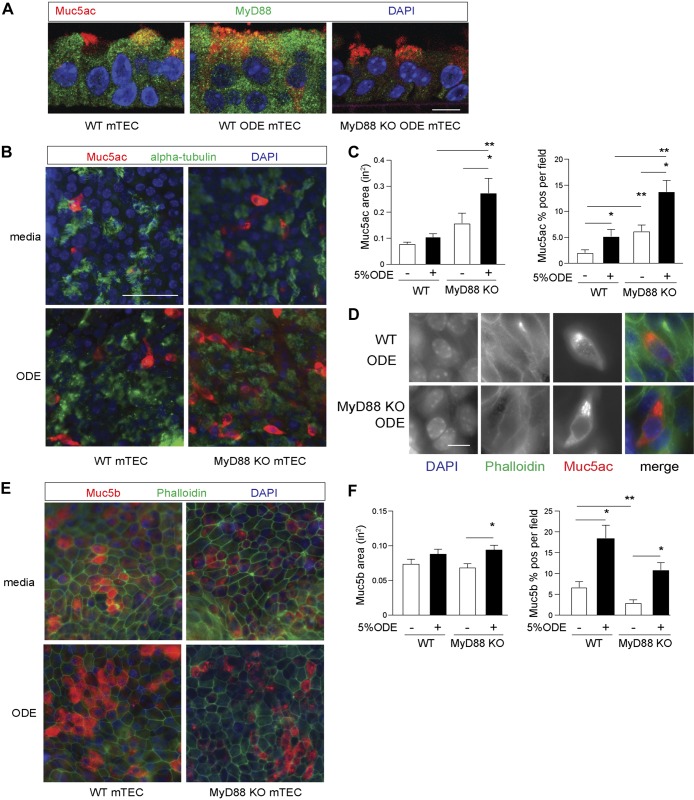
MyD88-deficient airway epithelial cells have enhanced Muc5ac staining with ODE challenge in vitro.
These studies have focused on the role of MyD88 in regulating mucins in an in vivo animal model that is globally deficient in MyD88. Nonepithelial cells could have impacted the observed changes in mucous cell metaplasia. In these next studies, we used isolated primary-culture airway epithelial cells to characterize the response to ODE with direct in vitro activation and determine the epithelial cell-specific role of MyD88 in regulating mucin. Agricultural workers exposed to ODE develop increased cough and sputum production (16). As there are important differences between mouse and human airways, our observations in the mouse led us to determine if the same findings developed in human airway epithelial models. Mouse airways have fewer mucus-producing goblet cells than human airways at baseline (19) and instead, have higher club cell populations that can functionally change to mucus-producing goblet cells following inflammation. To verify the effect of the ODE intranasal challenge observed in mouse airways, normal hTECs were cultured under ALI conditions to generate differentiated ciliated and secretory cells and then treated with 5% ODE in the basal chamber media for 7 days. There was an increase in MUC5AC and MUC5B levels with ODE (Fig. 7). To assess the role of MyD88 in epithelial cells, mTECs were collected from naive WT and MyD88-KO mice and differentiated under ALI conditions for 3 wk, whereupon ±5% ODE was added to the basal chamber media every other day for 5 days. The staining for MyD88 was diffuse across the WT airway epithelium with an apical cytoplasmic predominance, and the staining for MyD88 increased with ODE challenge (Fig. 8A). MyD88 was not specific for secretory cells in WT airway epithelial cells, as it was present in all cells. Consistent with the in vivo model, Muc5ac staining by UEA-1 was increased in the MyD88-deficient mTEC after ODE challenge (Fig. 8, B and C). Higher magnification-representative images of ODE-challenged mTEC confirmed that the UEA-1 stain for Muc5ac cells contained the expected intracellular mucin granules (Fig. 8D). Muc5b staining increased with ODE–in vitro challenge in both WT mTEC and MyD88-deficient mTEC. However, unlike Muc5ac, we found no significant difference in Muc5b staining between ODE-challenged WT mTEC and MyD88-deficient mTEC, and these in vitro studies confirm the in vivo findings that MyD88 deficiency during robust TLR activation by ODE leads to enhanced Muc5ac production in well-differentiated airway epithelial cells.
Fig. 7.
Organic dust extract (ODE) increases both mucin (MUC)5AC and MUC5B in primary-culture human airway epithelial cells. A: representative images of MUC5AC and MUC5B immunostaining by mouse MAb (red) with phalloidin (green) to mark the plasma membrane of human tracheobronchial epithelial cells (hTEC) under air–liquid interface (ALI) condition ±5% ODE for 7 days. Original scale bar, 50 μm. B: fold change of mucin gene expression by quantitative PCR (qPCR) in hTEC (n = 5). Fold change measured by paired Mann-Whitney’s U-test. *P < 0.05, significant difference between media and ODE conditions. DAPI, 4′,6-diamidino-2-phenylindole.
Fig. 8.
Organic dust extract (ODE) increase in mucin (Muc)5ac is augmented on myeloid differentiation factor 88 (MyD88)-deficient airway epithelial cells in vitro. A: MyD88 immunostaining (green) with Ulex europaeus agglutinin I (UEA-1) localization of Muc5ac (red) of mouse tracheobronchial epithelial cell (mTEC) isolated from wild-type (WT) and MyD88 knockout (KO) mice and grown under air–liquid interface (ALI) conditions ±5% ODE for 5 days. Original scale bar, 10 μ. B: representative images by UEA-1 staining for Muc5ac and acetylated α-tubulin for cilia in WT or MyD88-KO mTECs under ALI conditions challenged ±5% ODE for 5 days. Original scale bar, 50 μm. C: quantification of Muc5ac-staining area and percent-positive Muc5ac per cellular field from B (n = 4 per condition with 5 images per sample). D: representative higher magnification images demonstrating intracellular UEA-1- Muc5ac (red) staining with phalloidin (green) to mark plasma membrane borders. Original scale bar, 10 μm. E: representative images of Muc5b immunostaining with phalloidin to mark plasma membrane borders in WT or MyD88-KO mouse-derived mTEC under ALI conditions as, in part, A. F: quantification of Muc5b staining from A (n = 3 per condition with 5 images per sample condition). *P < 0.05, significant difference between ODE and saline. **P < 0.05, significant difference between WT and MyD88-KO mice. DAPI, 4′,6-diamidino-2-phenylindole.
DISCUSSION
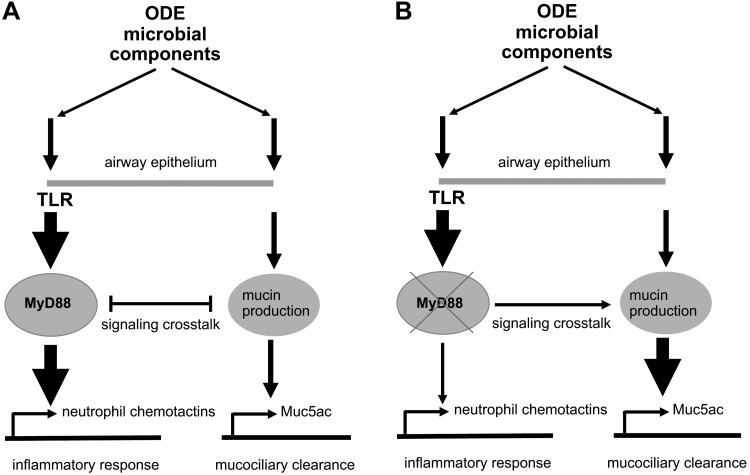
We have previously reported that ODE challenge broadly activates TLR2, -4, and -9 in mouse lungs and signals via the MyD88 adaptor protein (3, 40). Here, we show that in contrast to previous work demonstrating that airway inflammatory sequelae were reduced in ODE-challenged MyD88-KO mice (3, 42), MyD88 deficiency amplified mucous cell metaplasia, especially for enhanced Muc5ac levels (Fig. 9). This observation of a negative regulatory role for MyD88 on Muc5ac was demonstrated in both in vivo and in vitro systems by immunostaining, immunoblotting, and qPCR. The enhancement in ODE-induced airway epithelial Muc5ac levels occurred, despite a decrease in airway inflammation (i.e., neutrophil influx, neutrophil elastase, and IL-17 release) in the MyD88-KO mice. The observed increase in Muc5a, following ODE in MyD88-KO mice, was not due to an alteration in the epithelial differentiation program at baseline. MyD88-KO and WT mice had similar expression of Foxj1 and Scgb1a1 markers of ciliated and secretory cell lineages, respectively. The accumulation of mucins in the airway was also not due to an inherent ATP-activated secretory defect, as MyD88-KO mouse airways were responsive to ATP. Furthermore, a combined TLR (8) agonist did not induce mucin secretion in vivo in WT mice. These two key observations indicate that the accumulation of airway mucins in the MyD88-KO mice, observed in Fig. 2, is not due to abnormal mucin secretion but rather, due to increased production. These are the first studies to establish that inflammation from agricultural ODE, consisting primarily of bacterial-wall components (5)—a complex that broadly activates TLR—induced mucous cell metaplasia. Our findings in the airways expand on an earlier report, indicating that MyD88 deficiency led to increased submucosal gland formation in naïve trachea and increased mucin staining in the chemically injured mouse trachea with 2% polidocanol (26). Collectively, these data suggest a negative regulatory role for MyD88 in Muc5ac expression induced following inflammation in response to a nonallergenic, microbial, component-enriched bioaerosol.
Fig. 9.
Proposed mechanism for myeloid differentiation factor 88 (MyD88) control of mucin (Muc)5ac responses in the airway epithelium during broad Toll-like receptor (TLR) activation from organic dust extract (ODE). A: ODE consists of gram-positive and -negative bacterial cell-wall components that induce innate-immune pathways through the parallel activation of TLR-MyD88 leading to neutrophil recruitment and second, a modest mucus production leading to enhanced mucociliary clearance. MyD88 signaling dampens mucin production in favor of neutrophil chemotaxis following ODE-mediated inflammation. B: in MyD88-deficient airway epithelium, mucus production is enhanced to compensate for lack of an inflammatory response. Thin arrows, modest activation; thick arrows, significant activation; X, MyD88-KO; →, transcriptional regulation.
Inhalation of ODE in mice induces features of airway inflammatory disease similar to that observed in exposed agricultural workers. Increased respiratory symptoms of cough and chronic phlegm production have been well documented in agricultural workers, particularly workers in large-scale swine operations (14, 16, 27, 29, 45). There have been no prior studies investigating the underlying mechanisms of mucus production and/or secretion in the context of exposure to dust in agricultural settings. The modest induction of Muc5ac mRNA (approximately four- to fivefold), induced by repetitive ODE exposure in WT C57BL/6J mice, was less robust than what we observed in experimental allergic asthma mouse models, such as OVA challenge in Balb/c mice (11). We also demonstrated a similar fold increase in Muc5ac in human airway epithelial cells during in vitro ODE. ODE-induced inflammation relied on neutrophil influx and a Th1/Th17-skewed lymphocyte response, as opposed to an allergic eosinophilic and Th2 response. Our findings on the effect of ODE on WT mice mucous cell metaplasia are in agreement with previous work that used a combined airway challenge of both LPS and elastase, which caused a fivefold increase in Muc5ac and no significant change in Muc5b (23), as well as chronic airway challenge of human elastase, which demonstrated an 8- to 13-fold increase in Muc5ac (49). This modest ODE-induced mucous cell metaplasia occurred independent of MyD88-TLR activation, meaning a non-MyD88 signaling ODE component increases mucus levels. Yet, the response is dampened by MyD88, as seen in globally deficient MyD88 mice or mTECs.
How MyD88 regulates Muc5ac production during ODE-mediated airway inflammation is not known. ODE consists primarily of microbial components from gram-positive bacteria but also to a lesser degree, gram-negative bacteria (5). Previous work by our group (38) did not find significant levels of the fungal component, ergosterol, levels by gas chromatography. Evidence for allergic inflammation following ODE exposures is lacking, as only small amounts of serum IgE are observed (39). Furthermore, in this study, eosinophil influx and increased IL-13 or IL-33 levels are not demonstrated. Upregulation of mucin levels in the context of nonallergic airway inflammatory stimuli has been described following exposure to cigarette smoke and gram-negative and -positive bacterial-wall components (i.e., LPS and lipotechoic acid; TLR agonists) (1, 2, 13, 33, 47). These stimuli primarily increase mucins via the EGFR (2, 13, 33). ODE has been shown to activate EGFR phosphorylation and downstream signaling events (12). One possibility to investigate in future studies is crosstalk between MyD88 and EGFR signaling during ODE-mediated inflammation. A second potential mechanism for MyD88 control of Muc5ac is via SAM-pointed domain-containing Ets-like factor (SPDEF). SPDEF is part of a transcription network in the epithelial cells that signals Muc5ac production (6). Whitsett and coworkers (6, 31) provide evidence that during respiratory viral illness and Th2 inflammation, the mucous cell metaplasia transcription factor SPDEF negatively regulates MyD88-directed innate-immune responses to bacterial coinfection. However, the lack of Th2 inflammation observed in our model makes SPDEF involvement less likely. Furthermore, we found no significant increase in SPDEF expression following ODE (data not shown). Other potential regulators of mucous cell metaplasia in vivo include myristoylated alanine-rich C-kinase substrate (32, 35, 48) and Erb2 (20, 50). Exploratory studies have not revealed an expression signal for these pathways in this model (data not shown). It is possible that during repetitive and broad TLR activation, as in ODE-mediated inflammation, MyD88 dampens the signals in the epithelium that lead to Muc5ac production (Fig. 9). MyD88 deficiency unveils the degree of crosstalk between the parallel signaling arms of the innate-immune response, neutrophil influx, and mucociliary clearance. In MyD88-deficient conditions, the epithelium compensates for the lack of inflammation by increasing mucous cell metaplasia. Future work will investigate the mechanism of how broad TLR activation alters the transcription program for Muc5ac and Muc5b in the presence and absence of MyD88.
MyD88 has a broad role in a number of hematopoietic cells, as well as the epithelium. However, the observed mucus phenotype was likely regulated at the level of the epithelial cell, independent of inflammatory cells. First, we have previously reported epithelial-specific, MyD88-dependent phenotypes. MyD88 regulates the ODE-mediated reduction in cilia beat frequency (53). Our previous MyD88 bone marrow chimeric studies were used to delineate epithelial vs. hematopoietic roles for MyD88 signaling in the context of ODE (42). Airway hyper-responsiveness following acute ODE exposure was reduced when MyD88 was absent in the lung structural (i.e., epithelial) cells but not when absent in hematopoietic cells. Second, we demonstrated in this current study that Muc5ac is increased in mTEC derived from MyD88-KO mice in response to ODE in vitro. Third, we found that there was enhanced Muc5ac levels in MyD88-deficient mice, despite a reduction in neutrophils and IL-17. Finally, there was no difference in crucial Th2 cytokines (i.e., IL-13 or IL-33) that regulate Muc5ac in the airway between WT and MyD88-KO mice. Our focus on the airway epithelial role of MyD88 is supported by recent work highlighting the importance MyD88 in bacteria-derived TLR ligands in Muc2 secretion in the colonic epithelium localized to specific goblet cells at the opening of crypts (4). In our study, however, we found that MyD88-deficient mouse airways respond normally to ATP and secrete mucins.
Taken together, MyD88 is critical to the regulation of mucus in airway epithelial cells to complex, TLR ligand-enriched, agriculture organic dust exposures. This current study demonstrated a novel finding, where MyD88 deficiency augments mucous cell metaplasia in airway epithelial cells. Future studies will focus on the determination of the signaling mechanism of MyD88 dampening of Muc5ac production and exploit this pathway for potential preventative and therapeutic strategies.
GRANTS
This work was supported by awards from the National Institutes of Health Grants R01-ES-019325 (to J. A. Poole), 1K08-HL-131992 (to J. D. Dickinson), and R01-HL-129795 (to B. F. Dickey) and, in part, by the Central States Center for Agricultural Safety and Health (CS-CASH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.D. conceived and designed research; J.D.D., J.M.S., E.B.S., A.J.N., K.W., A.M.J., and J.A.P. performed experiments; J.D.D., J.M.S., E.B.S., K.L.B., K.W., A.M.J., B.F.D., and J.A.P. analyzed data; J.D.D., K.L.B., A.M.J., and B.F.D. interpreted results of experiments; J.D.D., J.M.S., E.B.S., A.J.N., A.M.J., B.F.D., and J.A.P. prepared figures; J.D.D., K.L.B., A.M.J., B.F.D., and J.A.P. drafted manuscript; J.D.D., J.M.S., E.B.S., A.J.N., K.L.B., K.W., A.M.J., B.F.D., and J.A.P. edited and revised manuscript; J.D.D., J.M.S., E.B.S., A.J.N., K.L.B., K.W., A.M.J., B.F.D., and J.A.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the University of Nebraska Medical Center Tissue Science Facility in the Department of Pathology and Microbiology for assistance with lung-tissue processing, sectioning, and digital microscopy images prepared for the manuscript. We also thank Dr. Tammy Kielian for assistance with obtaining MyD88-KO mice and Zoulikha Azzegagh for performance of the secretion experiment with Pam2-ODN. In addition, we thank Lisa Chudomelka for manuscript preparation assistance and Steven L. Brody and Chris M. Evans for critical evaluations of the data during manuscript preparation.
REFERENCES
- 1.Baginski TK, Dabbagh K, Satjawatcharaphong C, Swinney DC. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am J Respir Cell Mol Biol 35: 165–174, 2006. doi: 10.1165/rcmb.2005-0259OC. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H. Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR). Novartis Found Symp 248: 171–176, 2002. [PubMed] [Google Scholar]
- 3.Bauer C, Kielian T, Wyatt TA, Romberger DJ, West WW, Gleason AM, Poole JA. Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am J Respir Cell Mol Biol 48: 781–789, 2013. doi: 10.1165/rcmb.2012-0479OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birchenough GM, Nyström EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 352: 1535–1542, 2016. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boissy RJ, Romberger DJ, Roughead WA, Weissenburger-Moser L, Poole JA, LeVan TD. Shotgun pyrosequencing metagenomic analyses of dusts from swine confinement and grain facilities. PLoS One 9: e95578, 2014. doi: 10.1371/journal.pone.0095578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Korfhagen TR, Karp CL, Impey S, Xu Y, Randell SH, Kitzmiller J, Maeda Y, Haitchi HM, Sridharan A, Senft AP, Whitsett JA. Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 189: 301–313, 2014. doi: 10.1164/rccm.201306-1181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119: 2914–2924, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleaver JO, You D, Michaud DR, Pruneda FA, Juarez MM, Zhang J, Weill PM, Adachi R, Gong L, Moghaddam SJ, Poynter ME, Tuvim MJ, Evans SE. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol 7: 78–88, 2014. doi: 10.1038/mi.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 10.Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ, Stappenbeck TS, Brody SL. IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy 12: 397–409, 2016. doi: 10.1080/15548627.2015.1056967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson JD, Sweeter JM, Warren KJ, Ahmad IM, De Deken X, Zimmerman MC, Brody SL. Autophagy regulates DUOX1 localization and superoxide production in airway epithelial cells during chronic IL-13 stimulation. Redox Biol 14: 272–284, 2018. doi: 10.1016/j.redox.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodmane PR, Schulte NA, Heires AJ, Band H, Romberger DJ, Toews ML. Airway epithelial epidermal growth factor receptor mediates hogbarn dust-induced cytokine release but not Ca2+ response. Am J Respir Cell Mol Biol 45: 882–888, 2011. doi: 10.1165/rcmb.2010-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta 1406: 251–259, 1998. doi: 10.1016/S0925-4439(98)00010-6. [DOI] [PubMed] [Google Scholar]
- 14.Donham KJ, Reynolds SJ, Whitten P, Merchant JA, Burmeister L, Popendorf WJ. Respiratory dysfunction in swine production facility workers: dose-response relationships of environmental exposures and pulmonary function. Am J Ind Med 27: 405–418, 1995. doi: 10.1002/ajim.4700270309. [DOI] [PubMed] [Google Scholar]
- 15.Dosman JA, Fukushima Y, Senthilselvan A, Kirychuk SP, Lawson JA, Pahwa P, Cormier Y, Hurst T, Barber EM, Rhodes CS. Respiratory response to endotoxin and dust predicts evidence of inflammatory response in volunteers in a swine barn. Am J Ind Med 49: 761–766, 2006. doi: 10.1002/ajim.20339. [DOI] [PubMed] [Google Scholar]
- 16.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: the role of biological agents. Chest 136: 716–725, 2009. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 17.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 15: 4–11, 2009. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, McGing MA, McElwee MM, Williams OW, Sanchez E, Roy MG, Kindrachuk KN, Wynn TA, Eltzschig HK, Blackburn MR, Tuvim MJ, Janssen WJ, Schwartz DA, Dickey BF. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun 6: 6281, 2015. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 31: 382–394, 2004. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer BM, Cuellar JG, Byrd AS, Rice AB, Bonner JC, Martin LD, Voynow JA. ErbB2 activity is required for airway epithelial repair following neutrophil elastase exposure. FASEB J 19: 1374–1376, 2005. doi: 10.1096/fj.04-2675fje. [DOI] [PubMed] [Google Scholar]
- 21.Frantz AL, Rogier EW, Weber CR, Shen L, Cohen DA, Fenton LA, Bruno ME, Kaetzel CS. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol 5: 501–512, 2012. doi: 10.1038/mi.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol 183: 6236–6243, 2009. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganesan S, Faris AN, Comstock AT, Sonstein J, Curtis JL, Sajjan US. Elastase/LPS-exposed mice exhibit impaired innate immune responses to bacterial challenge: role of scavenger receptor A. Am J Pathol 180: 61–72, 2012. doi: 10.1016/j.ajpath.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 117: 3786–3799, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh A, Boucher RC, Tarran R. Airway hydration and COPD. Cell Mol Life Sci 72: 3637–3652, 2015. doi: 10.1007/s00018-015-1946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giangreco A, Lu L, Mazzatti DJ, Spencer-Dene B, Nye E, Teixeira VH, Janes SM. Myd88 deficiency influences murine tracheal epithelial metaplasia and submucosal gland abundance. J Pathol 224: 190–202, 2011. doi: 10.1002/path.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulec Balbay E, Cakiroglu EB, Arbak P, Balbay O, Avcioğlu F, Belada A. Respiratory symptoms and functions in barn workers. Ann Agric Environ Med 21: 25–28, 2014. [PubMed] [Google Scholar]
- 28.Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T, Caramori G, Zhu J, Edbrooke MR, Stanciu LA, Kon OM, Papi A, Jeffery PK, Edwards MR, Johnston SL. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-κB and EGFR pathways. Eur Respir J 36: 1425–1435, 2010. doi: 10.1183/09031936.00026910. [DOI] [PubMed] [Google Scholar]
- 29.Hoppin JA, Umbach DM, Long S, Rinsky JL, Henneberger PK, Salo PM, Zeldin DC, London SJ, Alavanja MC, Blair A, Beane Freeman LE, Sandler DP. Respiratory disease in United States farmers. Occup Environ Med 71: 484–491, 2014. doi: 10.1136/oemed-2013-101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirshner ZZ, Gibbs RB. Use of the REVERT® total protein stain as a loading control demonstrates significant benefits over the use of housekeeping proteins when analyzing brain homogenates by Western blot: an analysis of samples representing different gonadal hormone states. Mol Cell Endocrinol 473: 156–165, 2018. doi: 10.1016/j.mce.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korfhagen TR, Kitzmiller J, Chen G, Sridharan A, Haitchi HM, Hegde RS, Divanovic S, Karp CL, Whitsett JA. SAM-pointed domain ETS factor mediates epithelial cell-intrinsic innate immune signaling during airway mucous metaplasia. Proc Natl Acad Sci USA 109: 16630–16635, 2012. doi: 10.1073/pnas.1208092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langlet C, Springael C, Johnson J, Thomas S, Flamand V, Leitges M, Goldman M, Aksoy E, Willems F. PKC-alpha controls MYD88-dependent TLR/IL-1R signaling and cytokine production in mouse and human dendritic cells. Eur J Immunol 40: 505–515, 2010. doi: 10.1002/eji.200939391. [DOI] [PubMed] [Google Scholar]
- 33.Lemjabbar H, Li D, Gallup M, Sidhu S, Drori E, Basbaum C. Tobacco smoke-induced lung cell proliferation mediated by tumor necrosis factor alpha-converting enzyme and amphiregulin. J Biol Chem 278: 26202–26207, 2003. doi: 10.1074/jbc.M207018200. [DOI] [PubMed] [Google Scholar]
- 34.Lethem MI, Dowell ML, Van Scott M, Yankaskas JR, Egan T, Boucher RC, Davis CW. Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am J Respir Cell Mol Biol 9: 315–322, 1993. doi: 10.1165/ajrcmb/9.3.315. [DOI] [PubMed] [Google Scholar]
- 35.Mancek-Keber M, Bencina M, Japelj B, Panter G, Andrä J, Brandenburg K, Triantafilou M, Triantafilou K, Jerala R. MARCKS as a negative regulator of lipopolysaccharide signaling. J Immunol 188: 3893–3902, 2012. doi: 10.4049/jimmunol.1003605. [DOI] [PubMed] [Google Scholar]
- 36.Moghaddam SJ, Clement CG, De la Garza MM, Zou X, Travis EL, Young HW, Evans CM, Tuvim MJ, Dickey BF. Haemophilus influenzae lysate induces aspects of the chronic obstructive pulmonary disease phenotype. Am J Respir Cell Mol Biol 38: 629–638, 2008. doi: 10.1165/rcmb.2007-0366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piccotti L, Dickey BF, Evans CM. Assessment of intracellular mucin content in vivo. Methods Mol Biol 842: 279–295, 2012. doi: 10.1007/978-1-61779-513-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, Mehaffy J, Reynolds SJ. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A 73: 684–700, 2010. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole JA, Mikuls TR, Duryee MJ, Warren KJ, Wyatt TA, Nelson AJ, Romberger DJ, West WW, Thiele GM. A role for B cells in organic dust induced lung inflammation. Respir Res 18: 214, 2017. doi: 10.1186/s12931-017-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, Golden G, West WW, Sisson JH, Romberger DJ. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol 45: 711–719, 2011. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 296: L1085–L1095, 2009. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole JA, Wyatt TA, Romberger DJ, Staab E, Simet S, Reynolds SJ, Sisson JH, Kielian T. MyD88 in lung resident cells governs airway inflammatory and pulmonary function responses to organic dust treatment. Respir Res 16: 111, 2015. doi: 10.1186/s12931-015-0272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren B, Azzegagh Z, Jaramillo AM, Zhu Y, Pardo-Saganta A, Bagirzadeh R, Flores JR, Han W, Tang YJ, Tu J, Alanis DM, Evans CM, Guindani M, Roche PA, Rajagopal J, Chen J, Davis CW, Tuvim MJ, Dickey BF. SNAP23 is selectively expressed in airway secretory cells and mediates baseline and stimulated mucin secretion. Biosci Rep 35: e00220, 2015. doi: 10.1042/BSR20150004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senthilselvan A, Chénard L, Grover V, Kirychuk SP, Hagel L, Ulmer K, Hurst TS, Dosman JA. Excess longitudinal decline in lung function in grain farmers. J Agromed 15: 157–165, 2010. doi: 10.1080/10599241003634686. [DOI] [PubMed] [Google Scholar]
- 46.Senthilselvan A, Chénard L, Ulmer K, Gibson-Burlinguette N, Leuschen C, Dosman JA. Excess respiratory symptoms in full-time male and female workers in large-scale swine operations. Chest 131: 1197–1204, 2007. doi: 10.1378/chest.06-2323. [DOI] [PubMed] [Google Scholar]
- 47.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol 287: L420–L427, 2004. doi: 10.1152/ajplung.00019.2004. [DOI] [PubMed] [Google Scholar]
- 48.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med 10: 193–196, 2004. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 49.Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol 287: L1293–L1302, 2004. doi: 10.1152/ajplung.00140.2004. [DOI] [PubMed] [Google Scholar]
- 50.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 172: 1013–1018, 2005. doi: 10.1164/rccm.200410-1398OC. [DOI] [PubMed] [Google Scholar]
- 51.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest 135: 505–512, 2009. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 52.Wyatt TA, Poole JA, Nordgren TM, DeVasure JM, Heires AJ, Bailey KL, Romberger DJ. cAMP-dependent protein kinase activation decreases cytokine release in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 307: L643–L651, 2014. doi: 10.1152/ajplung.00373.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wyatt TA, Sisson JH, Von Essen SG, Poole JA, Romberger DJ. Exposure to hog barn dust alters airway epithelial ciliary beating. Eur Respir J 31: 1249–1255, 2008. doi: 10.1183/09031936.00015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyatt TA, Slager RE, Heires AJ, Devasure JM, Vonessen SG, Poole JA, Romberger DJ. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. Am J Respir Cell Mol Biol 42: 706–715, 2010. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CM, Vasquez PA, Forest MG, Lethem MI, Dickey BF, Davis CW. Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS One 10: e0127267, 2015. doi: 10.1371/journal.pone.0127267. [DOI] [PMC free article] [PubMed] [Google Scholar]