Abstract
Measurements of aldosterone for diagnosis of primary aldosteronism are usually made from blood sampled in the morning when aldosterone typically peaks. We tested the relative contributions and interacting influences of the circadian system, ongoing behaviors, and prior sleep to this morning peak in aldosterone. To determine circadian rhythmicity and separate effects of behaviors on aldosterone, 16 healthy participants completed a 5-day protocol in dim light while all behaviors ranging from sleep to exercise were standardized and scheduled evenly across the 24-h circadian period. In another experiment, to test the separate effects of prior nocturnal sleep or the inactivity that accompanies sleep on aldosterone, 10 healthy participants were studied across 2 nights: 1 with sleep and 1 with maintained wakefulness (randomized order). Plasma aldosterone was measured repeatedly in each experiment. Aldosterone had a significant endogenous rhythm (P < 0.001), rising across the circadian night and peaking in the morning (~8 AM). Activity, including exercise, increased aldosterone, and different behaviors modulated aldosterone differently across the circadian cycle (circadian phase × behavior interaction; P < 0.001). In the second experiment, prior nocturnal sleep and prior rested wakefulness both increased plasma aldosterone (P < 0.001) in the morning, to the same extent as the change in circadian phases between evening and morning. The morning increase in aldosterone is due to effects of the circadian system plus increased morning activities and not prior sleep or the inactivity accompanying sleep. These findings have implications for the time of and behaviors preceding measurement of aldosterone, especially under conditions of shift work and jet lag.
Keywords: aldosterone, chronotherapy, endogenous circadian rhythm, hyperaldosteronism, sleep
INTRODUCTION
Aldosterone is a mineralocorticoid hormone that is produced in the outer zona glomerulosa of the adrenal glands and is primarily regulated by the renin-angiotensin system to in turn regulate extracellular fluid volume, electrolyte balance, and blood pressure (9). Specifically, aldosterone binds to the mineralocorticoid receptors in the renal epithelial cells resulting in potassium excretion, sodium reabsorption, and water retention, thereby resulting in increased volume load and cardiac output (18). Excess secretion to aldosterone leads to low renin levels, hypokalemia, and hypertension: a syndrome called “primary aldosteronism” (39). Patients with primary aldosteronism can cause secondary hypertension and increased risk of adverse cardiovascular events compared with patients with essential hypertension (20). The American College of Cardiology and the American Heart Association recommend screening for primary aldosteronism in any person with resistant hypertension, hypokalemia, an incidentally discovered adrenal mass, a family history of early onset hypertension, or stroke at a young age (<40 yr; 35). Clinical measurement of plasma aldosterone, used as part of the diagnosis of primary aldosteronism, is typically performed in the morning, likely because of the commonly observed morning peak in aldosterone (3, 11, 19). Theoretically, the morning rise in plasma aldosterone could be a result of prior behaviors such as sleep, the inactivity that accompanies sleep, food intake (15, 27), and/or the endogenous circadian system independent of these other behaviors (25). Using two distinct protocols, one in which sleep was shifted to the daytime and a second in which participants underwent sleep deprivation, Charloux and colleagues concluded that the morning rise in aldosterone is due to the prior sleep and that sleep deprivation blunts aldosterone secretion (6, 7). However, these studies did not test or control for any simultaneous effects of the endogenous circadian system. If the circadian system substantially influences aldosterone, this would have important implications for the time of measurement of aldosterone levels and for full interpretation of aldosterone levels in the clinic especially under conditions of jet lag or shift work when the sleep-wake cycle occurs at unusual circadian phases, which has a prevalence in the United States of ~20% of the adult working population (34). Using two separate protocols conducted in dim light (to avoid circadian phase shift induced by light), we tested the hypothesis that the morning rise in aldosterone is due to the effects of the endogenous circadian system. It is well known that different postures and levels of physical activity affect aldosterone levels (2). However, how these behaviors interact with the circadian system to produce absolute levels of aldosterone is not known. Thus, we also tested how common behaviors that could happen around the time of aldosterone measurement in the clinic, such as prior sleep, postural changes (supine vs. seated), and exercise, affect aldosterone across the circadian cycle.
METHODS
Participants.
We studied 16 participants (10 female) during an established circadian “forced desynchrony” (FD) protocol (5, 26) and 10 participants (5 female) during a sleep inactivity-versus-wake inactivity (SI/WI) protocol (for participant characteristics, see Table 1). Four volunteers participated in both protocols. Participants were healthy except for mild untreated hypertension in one participant in the FD protocol and moderate untreated obstructive sleep apnea in one participant in the SI/WI protocol. Studies were approved by the institutional review board for human subject protection at Oregon Health & Science University, and all participants provided written informed consent. These data were collected as part of a larger study of the effects of the circadian system, sleep, and activity on cardiovascular function in humans, and vascular function measurements taken in the second protocol have already been reported (30).
Table 1.
Participant information
| Protocol 1 (FD) | Protocol 2 (SI/WI) | |
|---|---|---|
| n | 16 | 10 |
| Sex (M/F), n | 6/10 | 5/5 |
| Age, yr | 51 ± 2 | 46.4 ± 3 |
| Body mass index, kg/m2 | 28.5 ± 1 | 26 ± 1 |
| Resting HR, beats/min | 64 ± 2 | 64 ± 3 |
| Systolic BP, mmHg | 119 ± 3 | 119 ± 4 |
| Diastolic BP, mmHg | 66 ± 3 | 69 ± 3 |
| Fasting glucose, mg/dl | 92 ± 1 | 90 ± 2 |
| Plasma sodium, meq/l | 139 ± 1 | 138 ± 0.05 |
| Plasma potassium, meq/l | 4 ± 0.05 | 4 ± 0.1 |
Values are means ± SE; n = no. of participants. In protocol 1, for ethnicity, 15 participants identified themselves as non-Hispanic or Latino, and 1 participant declined to answer; for race, 14 participants identified themselves as white, 1 as Asian, and 1 as American Indian or Alaskan native or black or African American. In protocol 2, for ethnicity, all 10 participants identified themselves as non-Hispanic or Latino; for race, 7 participants identified themselves as white, 2 as Asian, and 1 as black or African American. For chronotype, using the Morningness-Eveningness Questionnaire, in protocol 1, 3 participants were moderate evening type, 4 were intermediate type, 7 were moderate morning type, and 2 were definite morning type. In protocol 2 the chorotype questionnaire was not administered. BP, blood pressure; F, female; FD, forced desynchrony; HR, heart rate; M, male; SI/WI, sleep inactivity vs. wake inactivity.
Clinical screening.
For the FD study, health status was based on self-report and physical and psychological examination by a physician including a 12-lead electrocardiogram, 3 repeated blood pressure measurements in the laboratory plus 48-h blood pressure monitoring (Spacelabs Healthcare), at-home sleep apnea screening (WatchPAT; Itamar Medical), and laboratory testing of hematological and metabolic measures (i.e., hemoglobin and hematocrit levels, basic blood chemistry, and blood glucose levels). Exclusion criteria included pregnancy, history of chronic disease or smoking, and use of any prescription or nonprescription medications (Drugsmart 12-panel cup; Speares Medical) or cotinine (NicAlert; Nymox). The recruitment and screening procedures were identical in the SI/WI protocol except for the absence of a physical exam in the SI/WI protocol, as previously published (30).
Home routine.
To ensure stability of circadian rhythmicity before the laboratory study, a history of travel across >3 time zones in the prior 3 mo or shift work in the past 6 mo was exclusionary. In addition, for at least 1 wk before entering the laboratory, participants maintained a constant self-selected 8-h sleep schedule to stabilize sleep-wake patterns and circadian rhythmicity (verified by actigraphy; ActiGraph wGT3X-BT; ActiGraph; phone calls to a time-stamped mailbox at bedtime and upon awakening and a written sleep diary). For this at-home period, participants refrained from any medication, food supplements, caffeinated food or beverages, alcohol, and light-intensity physical activity for >45 min per day. All participants adhered to the self-selected sleep-wake patterns judged as within 1 h of scheduled sleep-wake time from call-in logs and actigraphy.
Circadian FD protocol.
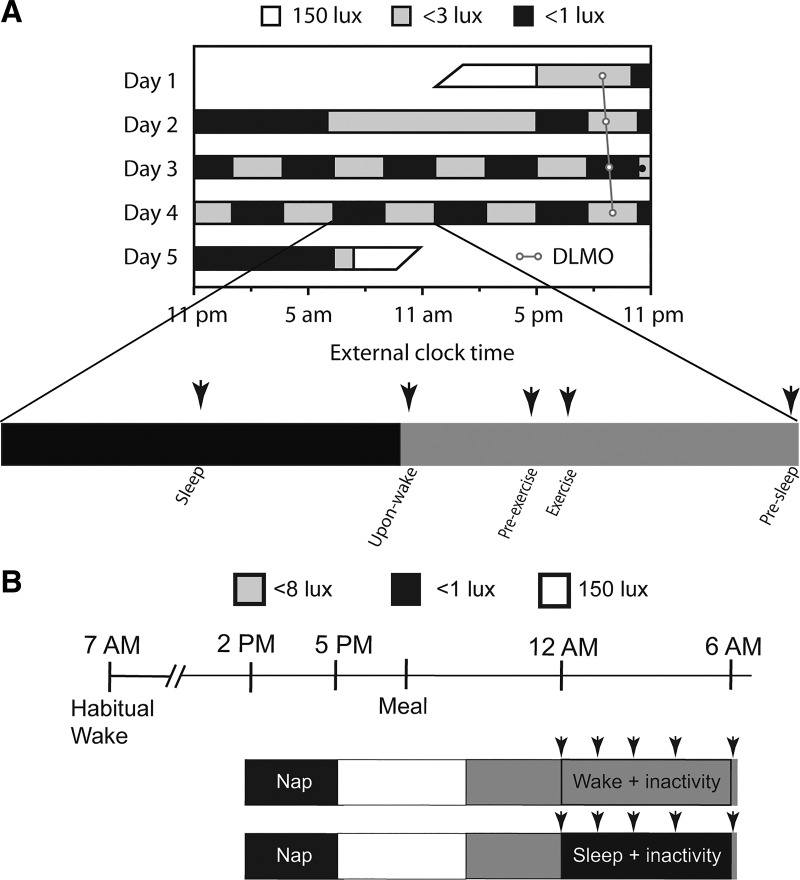
Upon admission to the Oregon Clinical & Translational Research Institute laboratory, a drug-screening test and a pregnancy test (in premenopausal women) were performed to ensure adherence to preadmission instructions. Subsequently, participants were instrumented for full polysomnography (except leg electromyograms) according to the guidelines of the American Academy of Sleep Medicine (12), and an intravenous catheter was inserted in the nondominant arm. To study endogenous circadian rhythmicity of physiological variables including circulating aldosterone concentrations, participants completed an FD protocol in a time-isolated and temperature-controlled environment with all physiological measurements repeated across the entire circadian cycle (Fig. 1A; 5). Light levels were <3 lux at the horizontal angle of gaze during scheduled wakefulness to prevent light-induced phase shifts of the circadian clock (28) and were <0.1 lux during scheduled sleep periods. The FD protocol consisted of a baseline 8-h sleep opportunity at the participant’s habitual sleep time and a baseline day followed by 10 identical recurring 5-h 20-min sleep-wake cycles (2-h 40-min sleep opportunity and 2-h 40-min scheduled wakefulness) to desynchronize the behaviors from the endogenous circadian system (31). For the ten 5-h 20-min behavioral cycles there was a 2-h 40-min sleep opportunity, and then participants were gently awoken in a standardized fashion by use of a mild auditory stimulus. Thereafter, the following standardized activities were scheduled with the same sequence: assessment of vascular endothelial function, a short cognitive test battery, a 15-min period of mild-intensity cycle ergometer exercise [<50% maximum predicted heart rate (13)], and consumption of a small balanced meal representing 22% of the 24-h total isocaloric requirement. On average, participants consumed 579 ± 24 (SE) mg of sodium and 789 ± 34 mg of potassium per isocaloric meal, totaling to ~2,629 mg sodium and ~3,589 mg potassium over a 24-h period. Within each 5-h 20-min FD episode, venous blood was sampled across five different behaviors: 1) immediately upon awakening (supine rest); 2) preexercise (seated rest); 3) during exercise (seated on a bike); 4) presleep (supine rest); and 5) during sleep (rest), as depicted in Fig. 1A. Blood samples were taken via a 12-ft catheter tube, which permitted samples to be taken without disturbing the participants during behaviors 1, 4, and 5. Samples during behaviors 2 and 3 were taken through the same 12-ft catheter but while in the same room as the participant.
Fig. 1.
Forced desynchrony (FD) protocol (A) and sleep inactivity-vs.-wake inactivity protocol (B) schemes. In both protocols, represent instances of blood draws. In the FD protocol, ○ represents an individual’s circadian period. DLMO, dim-light melatonin onset.
SI/WI protocol.
Detailed methods for the SI/WI protocol have been published elsewhere (30). To understand the effects of nocturnal sleep on plasma aldosterone, participants completed two trials in a randomized order (Fig. 1B). For each trial, after completion of the same home routine as described for the FD protocol, participants reported to the Oregon Clinical and Translational Research Institute laboratory in the early afternoon. To help reduce any sleep “debt” before starting an overnight study, in both SI and WI trials, participants had a 3-h sleep opportunity (7 h after habitual wake) in darkness. Participants were then provided with a standardized snack and, later, a standardized dinner 4.5 h before the commencing the SI or WI trial. In the SI trial, participants were provided a 6-h sleep opportunity (<0.1 lux), beginning 1 h later and ending 1 h earlier than their habitual sleep period. In the WI trial, all procedures were the same as in the SI trial except that participants remained awake and inactive in bed in dim light (<8 lx), which is bright enough for vision but not so bright as to affect the phase of the internal circadian clock (40). During the WI trial an experimenter was constantly present in the room to monitor and ensure wakefulness via occasional verbal interaction. Participants were encouraged to remain still and were always in a recumbent posture. Scheduled voiding breaks were provided, and if needed, participants used a bedside commode/urinal. Blood was sampled immediately before beginning sleep/supine rest (0 h), at 1.5, 3, and 4.5 h into the trials, and at the end of sleep/supine rest (6 h). In all participants, trials were conducted at least 1 wk apart. In premenopausal female participants (n = 2), trials were conducted 1 mo apart during the follicular phase of the menstrual cycle.
Measurements.
For this report, the primary independent variables of interest were circadian phase (derived from salivary melatonin assays) and five standardized behaviors: 1) immediately upon awakening (supine rest), 2) preexercise (seated rest), 3) during exercise (seated on a bike), 4) presleep (supine rest), and 5) during sleep (rest), as depicted in Fig. 1A. The dependent variable of interest was plasma aldosterone.
Polysomnography.
Standard full polysomnography was acquired including electroencephalograms, electrooculograms, submental electromyogram, an electrocardiogram, oronasal airflow, intranasal pressure, chest and abdominal breathing movements, snoring, and arterial oxygen saturation via pulse oximetry. Leg electromyograms were not recorded to avoid unnecessarily increasing participant burden. Sleep and wakefulness were scored according to guidelines of the American Academy of Sleep Medicine (12).
Circadian phases.
In the FD protocol, saliva was regularly collected in a Salivette cotton swab (Sarstedt), spun, and then frozen until assays were performed. Salivary melatonin was analyzed using a radioimmunoassay from Bühlmann Laboratories (Schönenbuch, Switzerland) employing the Kennaway G-280 anti-melatonin antibody. The in-house interassay coefficient of variation was 11%. Dim-light melatonin onset (DLMO) was used as the circadian phase marker for alignment of aldosterone data (4). For each day we determined the DLMO as the linear interpolated time point when salivary melatonin exceeded 3 pg/ml (4). In one participant whose salivary melatonin never dipped below 3 pg/ml, 4 pg/ml was used as the threshold (4). Circadian period was calculated from the average differences between consecutive DLMOs.
Aldosterone.
Blood was spun down, and plasma was aliquoted and stored at −81°C until aldosterone assays were performed using ELISA (IBL-America, Minneapolis, MN). The sensitivity of this assay is <5.7 pg/ml.
Statistical analyses.
Unless otherwise noted, data are reported as means ± SE. In the FD protocol, for each individual, DLMO was assigned a circadian phase of 0°, and each individual’s data were assigned circadian phases (from 0 to 359°) on the basis of their circadian period and time relative to the DLMO. To determine whether resting aldosterone has an endogenous circadian rhythm with a morning peak, mixed-model cosinor analyses were performed on all upon-awakening supine measurements (behavior 1) to test for any systematic rhythmicity having an underlying sinusoidal shape across the 24-h period. These cosinor analyses included the fixed factors of circadian phase (2-harmonic parameterization; 10) and time into experiment of each measurement and the random factor of subject. To determine the effect of posture, physical activity, and sleep (behaviors 1–5) across the circadian cycle, cosinor analyses similar to those described above were run on all data along with a circadian phase × behavior interaction term. Significance for all statistical analyses was set at P < 0.05 for two-tailed tests. Confidence intervals (CIs) are shown, where appropriate, at the 95% level.
From data collected in the SI/WI protocol, 1) differences in plasma aldosterone before and after the nocturnal period, 2) differences in plasma aldosterone between SI and WI, and 3) any interacting effects were tested using mixed-model analysis with subject as the random factor.
To compare the change in aldosterone across the biological night in the FD study to the change in aldosterone across the night in the SI/WI study, we ran individual cosinor analyses on resting upon-awakening data and, using the DLMO for each participant, calculated the change in aldosterone levels at the circadian degrees corresponding to 12 AM and 6 AM (i.e., matching the premeasurement and postmeasurement times in the SI/WI protocol). A paired t-test was performed to test whether aldosterone increased across the biological night, and unpaired t-tests were run (FD vs. SI and FD vs. WI) to test whether the change across the night was different between these studies.
RESULTS
Sleep.
In the circadian protocol, complete baseline polysomnography data were available on 12 of the 16 participants. During the baseline night, participants obtained an average sleep duration of 6.5 ± 0.35 h yielding an average sleep efficiency of 84.7 ± 2.2% and sleep onset latency of 6 ± 1.2 min. Across the FD protocol, participants obtained an average sleep duration equivalent of 7.3 ± 0.4 h per 24 h and sleep onset latency of 18 ± 2.6 min. In the SI/WI study, the average lights-off time in the laboratory was 11:46 PM ±17 min, and the lights-on time was 5:45 AM ±18 min. From polysomnography in the SI trial (data available for 9 participants), the average sleep duration was 4.69 ± 0.18 h of the 6-h sleep opportunity, yielding an average sleep efficiency of 80.3 ± 2.5% and sleep onset latency of 14.4 ± 2.9 min (30).
Effect of the circadian system on morning peak in plasma aldosterone.
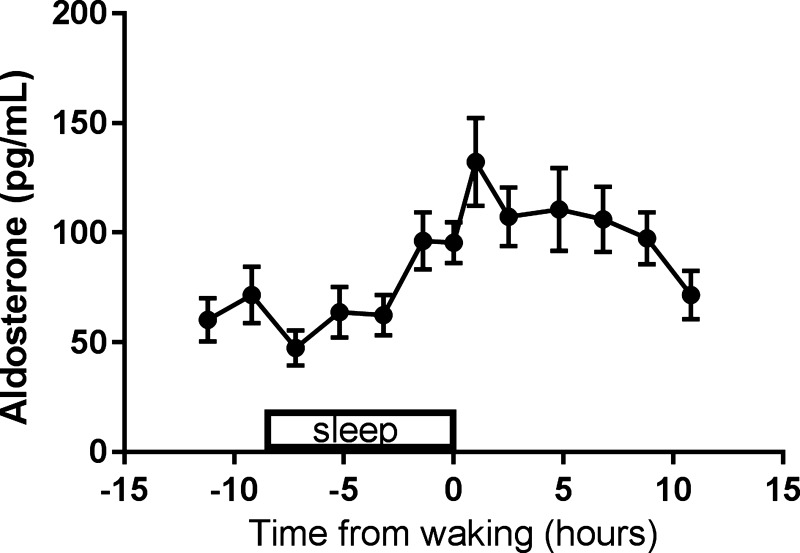
The baseline day-night aldosterone profile immediately preceding the FD protocol in which sleep occurred across the night and wakefulness occurred across the day (Fig. 2) showed a trough during sleep and a peak in the morning hours, as expected from prior work (14).
Fig. 2.
Baseline profile of aldosterone from the circadian study. The baseline profile from the circadian study shows a trough in aldosterone during sleep and a peak in the morning upon awakening. Data were standardized to each participant’s time of sleep and then averaged. Data are plotted as means ± SE.
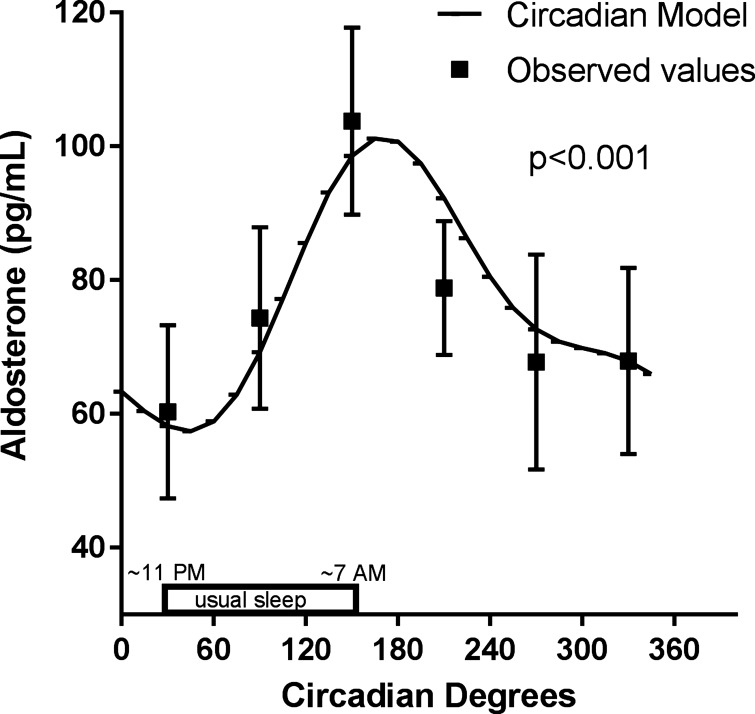
From analysis of the FD data (Fig. 3), plasma aldosterone, measured during standardized relaxed wakefulness while supine immediately after scheduled awakenings, displays a large and significant endogenous circadian rhythm [F(5,119) = 25, P < 0.001], with aldosterone doubling across the usual sleep period. Specifically, the cosinor model peak was 101 pg/ml (CI: 73–129 pg/ml) at circadian phase 165° (corresponding to ~8 AM), and the model trough (57 pg/ml; CI: 29–86 pg/ml) occurred during the biological night at circadian phase 45° (corresponding to approximately midnight). The cosinor analysis also detected and accounted for any linear trend in the data related to time in the laboratory: there was a small (0.3 pg·ml−1·h−1) but significant decrease in the mean aldosterone level across the protocol (P < 0.001).
Fig. 3.
Endogenous circadian rhythm in plasma aldosterone during relaxed wakefulness while supine. Plasma aldosterone measured during standardized relaxed wakefulness while supine immediately after scheduled awakenings displays a large and significant endogenous circadian rhythm, with aldosterone doubling across the usual sleep period and peaking in the biological morning at ~8 AM (n = 16, P < 0.001 using mixed-model cosinor analyses). The circadian model is plotted from the cosinor analyses. Raw observed values were binned by circadian phase within subject and then averaged within group and are plotted as means ± SE.
SI/WI protocol.
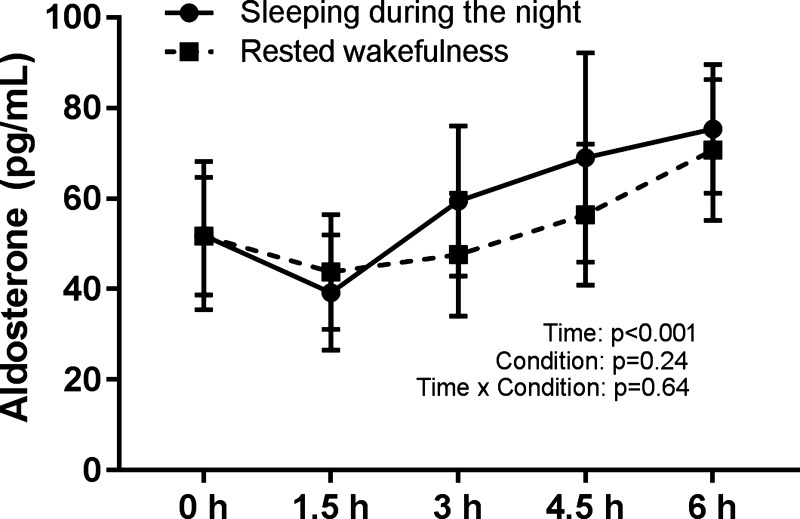
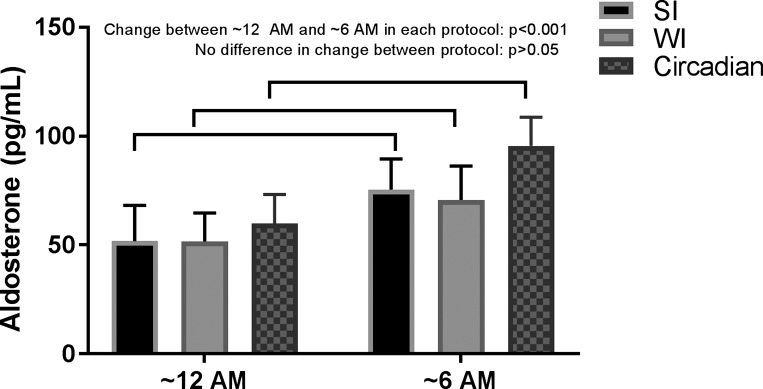
Figure 4 shows that there was a significant increase in aldosterone across the night in both SI and WI protocols [F(4,67) = 9.8, P < 0.001], from 53 pg/ml at approximately midnight (CI: 21–84 pg/ml) to 73 pg/ml at ~6 AM (CI: 42–104 pg/ml). There was no systematic difference between SI (mean 58 pg/ml; CI: 27–89 pg/ml) and WI (mean 54 pg/ml; CI: 23–85 pg/ml) (P = 0.24) and no visit × measurement interaction (P = 0.64). Aldosterone significantly increased across this circadian FD study across phases corresponding to approximately midnight and ~6 AM (P < 0.001).
Fig. 4.
Effects of sleep inactivity (SI) and wake inactivity (WI) on plasma aldosterone. There was a significant increase in aldosterone across both trials (n = 9, P < 0.001 using mixed-model analyses). There was no difference between trials or any trial × time interaction. Values are means ± SE. The 0-h measurement occurred at the beginning of the SI/WI trial, and the 6-h measurement occurred upon awakening.
The comparison of measurements across the circadian night in all protocols (FD, WI, and SI) with measurements made in the same standardized state of relaxed wakefulness while supine, Fig. 5 shows that aldosterone significantly increased across the circadian night in all three protocols and that the increases between these protocols were not significantly different. Thus, the increase across the circadian night is likely caused by the circadian system rather than sleep or the inactivity that accompanies sleep.
Fig. 5.
Similar changes in aldosterone across the circadian night in forced desynchrony (Circadian), sleep inactivity (SI), and wake inactivity (WI) protocols. Aldosterone significantly increased across the circadian night in all three protocols when measured in the same standardized state [relaxed wakefulness while supine; n = 16 for protocol 1 (Circadian) and n = 9 for protocol 2 (SI/WI); P < 0.001 using 1-sample t-test for each protocol]. The increase between these protocols was not significantly different (P > 0.05 using independent-sample t-tests); thus, the increase across the circadian night is likely caused by the circadian system rather than sleep or the inactivity that accompanies sleep.
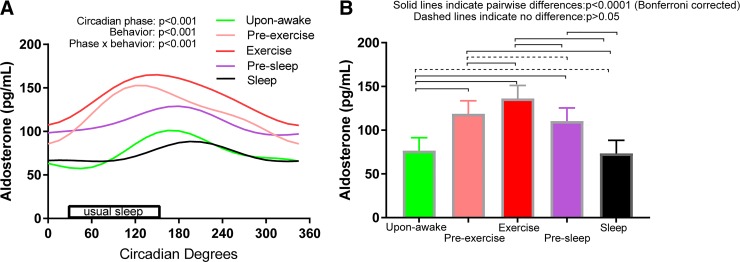
Effect of standardized behaviors on plasma aldosterone and interaction with the circadian cycle.
Figure 6 shows that 1) there were significant endogenous circadian rhythms in plasma aldosterone in all five standardized behaviors (P < 0.001); 2) there was a significant difference in mean aldosterone level between these five behaviors (measured across all circadian phases), with lowest aldosterone level during sleep or immediately after awakening and highest aldosterone level during exercise (P < 0.001); and 3) there were complex differences in circadian rhythmicity (amplitudes, phases, or overall shapes) between behaviors (i.e., a significant circadian phase × behavior interaction; P < 0.001). The peaks ranged from circadian phase 120° (corresponding to ~5 AM) in behavior 2 (preexercise seated rest) to circadian phase 195° (corresponding to ~10 AM) in behavior 5 (sleep). Time into protocol was not significant in this overall model (P = 0.13).
Fig. 6.
Effects on plasma aldosterone of the circadian cycle, standardized behaviors, and their interaction. There were significant endogenous circadian rhythms (n = 16, P < 0.001 using mixed-model cosinor analyses) in plasma aldosterone in all five standardized behaviors (A), significant differences in mean aldosterone level between these five behaviors (B), and a significant interaction between these effects (see different circadian amplitudes, phases, and shapes between behaviors in A).
DISCUSSION
Increases in plasma aldosterone in the morning.
Our main findings are as follows: 1) the primary mechanism for the rise in aldosterone in the morning hours appears to be the endogenous circadian system and likely not nocturnal sleep or the inactivity that accompanies sleep, 2) different standardized behaviors systematically affect aldosterone, and 3) different behaviors influence aldosterone differently across the circadian cycle (i.e., a circadian phase × behavior interaction). Our finding that the endogenous circadian system increases aldosterone in the morning (independent of behaviors) may have clinical implications for the measurement of aldosterone in populations such as night shift workers. Approximately 20% of the adult US population participates in shift work with exposure to bright light beyond the daytime (34). Light exposure has been shown to either advance or delay the circadian clock depending on when the exposure happens (17), and it is possible that the change in circadian phase may change the time of peak aldosterone concentrations. For instance, the peak in aldosterone may be missed if an individual with a delayed circadian phase is measured early in the morning, when the concentration of aldosterone would be rising but would not have peaked. Similarly, the phases of the centrally regulated circadian rhythms such as core body temperature and cortisol are delayed in evening-type individuals compared with morning-type individuals (1). It is possible that the circadian peak in aldosterone is also different between these groups, and this is an important factor to consider during measurement and clinical interpretation of aldosterone levels. Finally, in common conditions such as social jet lag, where there is a mismatch between the circadian time and social time, this degree of social jet lag ought to be considered in the interpretation of aldosterone levels (36). We have discovered that sleep is unlikely to cause a rise in plasma aldosterone. First, there was no difference in mean aldosterone levels between the SI and WI conditions, suggesting that plasma aldosterone levels were not different in the presence or absence of sleep. Second, when we compared the changes in aldosterone across the biological night corresponding to approximately midnight to ~6 AM, we found that aldosterone increased across this time in all three protocols to a similar degree (Fig. 5).
Our finding that it is likely the circadian system, and not sleep, that is responsible for the morning rise in aldosterone is different from the findings of Charloux and colleagues, who concluded that sleep was essential for the morning peak in aldosterone (6, 7). These studies were very comprehensive but caused sleep deprivation and did not control for light exposure and hence did not control for the effects of the endogenous circadian system (40). Participants in these studies also went to bed at the same time (11:00 PM to 7:00 AM), and their history of sleep has not been described. In our studies, sleep episodes for each time were individualized to the participant’s habitual sleep time. In addition, before commencing the trials the participants were likely not sleep deprived because of the regular sleep routine at home in both protocols plus the 3-h nap in the SI/WI protocol.
Impact of behaviors on aldosterone across the circadian cycle.
This is perhaps the first study to characterize the response of plasma aldosterone to common daily behaviors across the entire circadian cycle. We have discovered that there is a complex circadian phase × behavior interaction. Of note, the FD protocol enables us to examine circadian rhythms and behaviors somewhat independently. Thus, Fig. 6A examines circadian variations across independent behaviors, and Fig. 6B examines effects of behaviors evenly balanced across all circadian phases; these ranges between circadian phases and varied behaviors were of similar magnitudes. However, in real life, behaviors change at the same time as changes in circadian phases; thus, effects on aldosterone of circadian rhythms and behaviors are summated. Such summations presumably occurred naturally in the day-night profile (Fig. 2), where we see the largest daily range in plasma aldosterone level compared with the isolated circadian effects (Fig. 6A) and isolated behavioral effects (Fig. 6B). Although we cannot easily predict absolute levels because of the complex statistically significant interaction between circadian effects and the behavioral effects noted above, we can begin to think about some clinically relevant scenarios. For instance, examining Fig. 6A, the difference between a supine resting measurement upon awakening (behavior 2) and a seated resting measurement ~30 min after awakening (behavior 3) is greatest (~72 pg/ml) at the circadian phase corresponding to ~4 AM, and the difference is least (~19 pg/ml) at the circadian phase corresponding to ~8 PM. At the circadian phase corresponding to ~9 AM, a time when blood samples for aldosterone measurement typically would be made in the clinic, the difference between these resting measurements in different postures is 30 pg/ml. This suggests that whether a person is supine or seated in the morning can make a clinically significant difference in the interpretation of aldosterone results. During the same time in the morning, a resting supine measurement made after ~1.5 h of light physical activity (presleep measurement) is ~28 pg/ml higher compared with a supine measurement made without prior physical activity. This suggests that the behaviors of a patient before coming in for measurement of plasma aldosterone can have a significant bearing on the clinical interpretation and perhaps should be standardized.
Potential implications for chronotherapy.
In patients with heart failure, aldosterone is a target for drug therapy: aldosterone antagonists cause diuresis and effectively reduce mortality and hospitalizations in this population (29). Recent findings suggest that the aldosterone antagonist eplerenone targets products of the circadian gene nuclear receptor 3c2 (Nr3c2; 24). Owing to the short half-life of eplerenone (~4–6 h; 29), it could be postulated that the efficacy of eplerenone could be improved by timing the dose to target the peak of aldosterone concentration based on a patient’s circadian rhythm. This is an important area of work that could benefit from well-controlled randomized trials.
Aldosterone and morning cardiovascular risk.
The circadian peak in aldosterone falls within the time period when adverse cardiovascular events most commonly occur (21). In humans, aldosterone can affect the autonomic nervous system by blunting the baroreflex (37). Aldosterone can cause constriction of the resistance vessels (23) and potentiate catecholamines to cause vascular smooth muscle constriction (33). We have previously shown that the circadian system drives plasminogen activator inhibitor-1 to peak in the morning hours (25) when vascular endothelial function is the most impaired (22). Furthermore, the morning hours are known for the transition between sleep and wakefulness, a change in posture from supine to sitting and standing, and a transient increase in physical activity that is associated with a surge in blood pressure (16). The additional stress of a hypercoagulable state in addition to aldosterone-induced vasoconstriction could, in theory, increase the risk of adverse cardiovascular events in vulnerable populations (32). There is some evidence to support this hypothesis; for instance, in people with heart failure, spironolactone-induced aldosterone blockade improves heart rate variability and QT dispersion most favorably between 6 and 10 AM (38).
Strengths and limitations.
There are many important features of this study. First, we used the gold standard FD circadian protocol to discover whether there exists any underlying endogenous circadian rhythm in plasma aldosterone. We conducted this multiday protocol in constant dim light (to avoid circadian phase changes), with constant temperature, time isolation, and evenly distributed isocaloric food consumption. All behaviors were distributed evenly across the endogenous circadian cycle. We also studied the effects of standardized behaviors on aldosterone across the circadian cycle. We strictly controlled all baseline preadmission conditions and experimental conditions including history of sleep and activity, light exposure, meal size and timing, activity, and sleep in the laboratory. We also encouraged participants to avoid light exposure during the at-home sleep periods. Such controls could be instigated before measurement of aldosterone in the clinic to aid accurate interpretation of the results. Despite these strengths, our study had several limitations. These studies were part of larger studies and not designed to primarily to measure the renin-angiotensin-aldosterone system, and therefore we do not have the repeated measurements of potassium, sodium, or renin. However, our participants underwent a rigorous screening process and were ostensibly healthy with plasma sodium and potassium concentrations within normal limits (see Table 1). In our SI/WI protocol, we provided participants with a 3-h nap opportunity on admission day before the trials started to avoid any sleep debt during the baseline measurement. However, we do not know how this affected aldosterone measurements during the subsequent night. We also did not measure sleep during these naps, but all participants were in bed and in darkness. We controlled for the effects of the endogenous circadian system by controlling the intensity of light, specifically allowing the central pacemaker to “free run” (8). Although this experimental control is paramount in circadian protocols, this does not reflect normal daily life, where people may be in bright light before and immediately following sleep. Although our results suggest that aldosterone increases across the night and peaks in the morning because of the endogenous circadian system, we have not analyzed electroencephalographic delta power from polysomnography, nor do we have the high frequency of blood sampling that occurred in some investigations (6, 7). Furthermore, to establish clinical relevance, this would have to be verified in vulnerable individuals rather than the healthy individuals in our study group. Therefore, for improved generalization or specific population-based recommendation we believe similar studies need to be conducted in diverse large-population groups (e.g., shift workers; people with primary aldosteronism due to adrenal adenomas, bilateral hyperplasia, or familial syndromes; individuals with obesity; and people with obstructive sleep apnea).
Perspectives and Significance
In ostensibly healthy adults, the rise in plasma aldosterone during the morning is due to the effect of the endogenous circadian system plus ongoing activities and is unlikely to be due to the prior night of sleep. Thus, differences in the circadian phases at which blood samples are taken (as can occur with varying degrees of jet lag, social jet lag, or night shift work) can be clinically meaningful. If the endogenous circadian system similarly modulates aldosterone in vulnerable populations such as shift workers and people with primary aldosteronism or resistant hypertension, it could have important implications for finding the optimal time of measurements during the morning hours to avoid underdiagnosis of adrenal gland disorders. Effects of changes in posture and physical activity are also clinically meaningful during the morning hours, and strict control of these behaviors preceding and during blood sampling ought to be considered for full clinical interpretation of resultant values.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-HL-125893, R01-HL-125893-03S1, R01-HL-142064, R01-HL-140577, and F32-HL-131308; National Space Biomedical Research Institute through Cooperative Agreement NCC 9-58; the Ford Foundation Fellowship Program; Oregon Institute of Occupational Health Sciences at Oregon Health & Science University via funds from the Division of Consumer and Business Services of the State of Oregon (Grant ORS 656.630); and NIH Clinical Translational Science Award UL1-TR-000128.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.T., M.R.L., N.A.C., J.S.E., and S.A.S. conceived and designed research; S.S.T., A.M.B., M.X.H., N.A.C., S.A.R., N.P.B., J.S.E., and S.A.S. performed experiments; S.S.T., A.M.B., M.R.L., M.X.H., and S.A.R. analyzed data; S.S.T., J.F.R., M.R.L., N.P.B., D.H.E., and S.A.S. interpreted results of experiments; S.S.T. and N.P.B. prepared figures; S.S.T. drafted manuscript; S.S.T., J.F.R., A.M.B., M.R.L., M.X.H., N.P.B., D.H.E., and S.A.S. edited and revised manuscript; S.S.T., J.F.R., A.M.B., N.A.C., S.A.R., N.P.B., J.S.E., D.H.E., and S.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Michael Stowasser for advice on this work.
REFERENCES
- 1.Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int 18: 249–261, 2001. doi: 10.1081/CBI-100103189. [DOI] [PubMed] [Google Scholar]
- 2.Balikian HM, Brodie AH, Dale SL, Melby JC, Tait JF, Faire A, Flood C, Willoughby S, Wilson TE. Effect of posture on the metabolic clearance rate, plasma concentration and blood production rate of aldosterone in man. J Clin Endocrinol Metab 28: 1630–1640, 1968. doi: 10.1210/jcem-28-11-1630. [DOI] [PubMed] [Google Scholar]
- 3.Bartter FC, Delea CS, Halberg F. A map of blood and urinary changes related to circadian variations in adrenal cortical function in normal subjects. Ann NY Acad Sci 98: 969–983, 1962. doi: 10.1111/j.1749-6632.1962.tb30612.x. [DOI] [PubMed] [Google Scholar]
- 4.Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL, Revell VL. Measuring melatonin in humans. J Clin Sleep Med 4: 66–69, 2008. [PMC free article] [PubMed] [Google Scholar]
- 5.Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep (Basel) 38: 1793–1801, 2015. doi: 10.5665/sleep.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charloux A, Gronfier C, Chapotot F, Ehrhart J, Piquard F, Brandenberger G. Sleep deprivation blunts the night time increase in aldosterone release in humans. J Sleep Res 10: 27–33, 2001. doi: 10.1046/j.1365-2869.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- 7.Charloux A, Gronfier C, Lonsdorfer-Wolf E, Piquard F, Brandenberger G. Aldosterone release during the sleep-wake cycle in humans. Am J Physiol Endocrinol Metab 276: E43–E49, 1999. doi: 10.1152/ajpendo.1999.276.1.E43. [DOI] [PubMed] [Google Scholar]
- 8.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284: 2177–2181, 1999. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 9.Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology 151: 5098–5102, 2010. doi: 10.1210/en.2010-0465. [DOI] [PubMed] [Google Scholar]
- 10.Hu K, Scheer FA, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 123: 961–970, 2011. doi: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurwitz S, Cohen RJ, Williams GH. Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol (1985) 96: 1406–1414, 2004. doi: 10.1152/japplphysiol.00611.2003. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (1st ed.). Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 13.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate: a longitudinal study. Ann Med Exp Biol Fenn 35: 307–315, 1957. [PubMed] [Google Scholar]
- 14.Katz FH, Romfh P, Smith JA, Roper EF, Barnes JS, Boyd JB. Diurnal variation of plasma aldosterone, cortisol and renin activity in supine man. J Clin Endocrinol Metab 40: 125–134, 1975. doi: 10.1210/jcem-40-1-125. [DOI] [PubMed] [Google Scholar]
- 15.Lamarre-Cliche M, de Champlain J, Lacourcière Y, Poirier L, Karas M, Larochelle P. Effects of circadian rhythms, posture, and medication on renin-aldosterone interrelations in essential hypertensives. Am J Hypertens 18: 56–64, 2005. doi: 10.1016/j.amjhyper.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Leary AC, Struthers AD, Donnan PT, MacDonald TM, Murphy MB. The morning surge in blood pressure and heart rate is dependent on levels of physical activity after waking. J Hypertens 20: 865–870, 2002. doi: 10.1097/00004872-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int 15: 71–83, 1998. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson C, Young WF Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol 2: 198–208, 2006. doi: 10.1038/ncpneph0151. [DOI] [PubMed] [Google Scholar]
- 19.Michelakis AM, Horton R. The relationship between plasma renin and aldosterone in normal man. Circ Res 27, Suppl 1: S185–S194, 1970. [PubMed] [Google Scholar]
- 20.Milliez P, Girerd X, Plouin P, Blacher J, Safar ME, Mourad J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45: 1243–1248, 2005. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation 75: 131–138, 1987. doi: 10.1161/01.CIR.75.1.131. [DOI] [PubMed] [Google Scholar]
- 22.Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V. Early morning attenuation of endothelial function in healthy humans. Circulation 109: 2507–2510, 2004. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- 23.Romagni P, Rossi F, Guerrini L, Quirini C, Santiemma V. Aldosterone induces contraction of the resistance arteries in man. Atherosclerosis 166: 345–349, 2003. doi: 10.1016/S0021-9150(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 24.Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, Anafi RC, Hogenesch JB. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med 10: eaat8806, 2018. doi: 10.1126/scitranslmed.aat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 123: 590–593, 2014. doi: 10.1182/blood-2013-07-517060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spengler CM, Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med 162: 1038–1046, 2000. doi: 10.1164/ajrccm.162.3.9911107. [DOI] [PubMed] [Google Scholar]
- 27.Steegers EA, Benraad TJ, Jongsma HW, Tan AC, Hein PR. Effects of dietary sodium restriction and posture on plasma levels of atrial natriuretic peptide, aldosterone and free aldosterone in normal human pregnancy. J Endocrinol 124: 507–513, 1990. doi: 10.1677/joe.0.1240507. [DOI] [PubMed] [Google Scholar]
- 28.St Hilaire MA, Gooley JJ, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a 1 h pulse of bright white light. J Physiol 590: 3035–3045, 2012. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol 31: 153–158, 2008. doi: 10.1002/clc.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thosar SS, Berman AM, Herzig MX, Roberts SA, Lasarev MR, Shea SA. Morning impairment in vascular function is unrelated to overnight sleep or the inactivity that accompanies sleep. Am J Physiol Regul Integr Comp Physiol 315: R986–R993, 2018. doi: 10.1152/ajpregu.00143.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thosar SS, Herzig MX, Roberts SA, Berman AM, Clemons NA, McHill AW, Bowles NP, Morimoto M, Butler MP, Emens JS. Lowest perceived exertion in the late morning due to effects of the endogenous circadian system. Br J Sports Med 52: 1011–1012, 2018. doi: 10.1136/bjsports-2018-099148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, Gleason RE, Williams GH, Muller JE. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med 316: 1514–1518, 1987. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- 33.Weber MA, Purdy RE. Catecholamine-mediated constrictor effects of aldosterone on vascular smooth muscle. Life Sci 30: 2009–2017, 1982. doi: 10.1016/0024-3205(82)90441-6. [DOI] [PubMed] [Google Scholar]
- 34.Weibel L, Brandenberger G. Disturbances in hormonal profiles of night workers during their usual sleep and work times. J Biol Rhythms 13: 202–208, 1998. doi: 10.1177/074873098129000048. [DOI] [PubMed] [Google Scholar]
- 35.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 71: e127–e248, 2018. [Erratum in J Am Coll Cardiol 71: 2275–2279, 2018.] doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int 23: 497–509, 2006. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 37.Yee KM, Struthers AD. Aldosterone blunts the baroreflex response in man. Clin Sci (Lond) 95: 687–692, 1998. doi: 10.1042/cs0950687. [DOI] [PubMed] [Google Scholar]
- 38.Yee KM, Pringle SD, Struthers AD. Circadian variation in the effects of aldosterone blockade on heart rate variability and QT dispersion in congestive heart failure. J Am Coll Cardiol 37: 1800–1807, 2001. doi: 10.1016/S0735-1097(01)01243-8. [DOI] [PubMed] [Google Scholar]
- 39.Young WF Jr, Klee GG. Primary aldosteronism. Diagnostic evaluation. Endocrinol Metab Clin North Am 17: 367–395, 1988. doi: 10.1016/S0889-8529(18)30425-0. [DOI] [PubMed] [Google Scholar]
- 40.Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526: 695–702, 2000. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]