Abstract
Fluorescent protein reporter genes are widely used to identify and sort murine pancreatic β-cells. In this study, we compared use of the MIP-GFP transgene, which exhibits aberrant expression of human growth hormone (hGH), with a newly derived Ins2Apple allele that lacks hGH expression on the expression of sex-specific genes. β-Cells from MIP-GFP transgenic mice exhibit changes in the expression of 7,733 genes, or greater than half of their transcriptome, compared with β-cells from Ins2Apple/+ mice. To determine how these differences might affect a typical differential gene expression study, we analyzed the effect of sex on gene expression using both reporter lines. Six hundred fifty-seven differentially expressed genes were identified between male and female β-cells containing the Ins2Apple allele. Female β-cells exhibit higher expression of Xist, Tmed9, Arpc3, Eml2, and several islet-enriched transcription factors, including Nkx2-2 and Hnf4a, whereas male β-cells exhibited a generally higher expression of genes involved in cell cycle regulation. In marked contrast, the same male vs. female comparison of β-cells containing the MIP-GFP transgene revealed only 115 differentially expressed genes, and comparison of the 2 lists of differentially expressed genes revealed only 17 that were common to both analyses. These results indicate that 1) male and female β-cells differ in their expression of key transcription factors and cell cycle regulators and 2) the MIP-GFP transgene may attenuate sex-specific differences that distinguish male and female β-cells, thereby impairing the identification of sex-specific variations.
Keywords: gene expression, growth hormone, pancreatic β-cells, RNA-Seq, sex
INTRODUCTION
Pancreatic β-cells play an essential role in maintaining blood glucose homeostasis. These cells develop functional impairments in response to the combined effects of genetic susceptibility and metabolic stress that may result in type 2 diabetes (T2D). In some populations, T2D is more common among men despite a higher prevalence of obesity in women (8, 13, 21, 24, 26), and men tend to develop T2D at a lower body mass index than women (22). Consistent with these sex-specific differences in disease prevalence, ex vivo glucose-stimulated insulin secretion (GSIS) is higher in human islets from women than in those from men (16). In addition, several T2D-associated genes that influence both insulin secretion and expression of specific microRNAs are differentially expressed in male and female islets (16). In an attempt to explain these sex-based differences, it has been postulated that women may be partially protected from β-cell failure in response to metabolic stress, due to effects of estrogen receptor signaling (19, 34, 37, 44), and that sex-specific differences in islet DNA methylation status, by altering gene expression patterns in males and females, may contribute to differences in the susceptibility to T2D (16).
The use of transgenic fluorescent protein (FP) reporter lines has greatly enhanced our understanding of both β-cell development and function. However, when transgenic mice that express FPs and Cre in a β-cell-specific manner were first produced, intronic and polyadenylation sequences from genes, including human growth hormone (hGH)-encoding minigene, were used to overcome variegated patterns of transgene expression (3, 28). Although the precise mechanisms remain uncertain, inclusion of these sequences improved transgene expression, presumably by stabilizing the expressed RNA or promoting a more open chromatin structure (3). More importantly, although the hGH minigene contains coding sequences for hGH, the sequences were inserted into these transgenes as a second cistron, thereby presumably preventing the production of hGH. However, beginning in 2014, Brouwers et al. (4) and others (2, 6, 18, 29) proved this assumption wrong when it was reported that several lines of hGH minigene containing transgenic mice, including the MIP-GFP transgenic mouse, ectopically express hGH. Gene expression analysis of β-cells from an hGH-expressing Pdx1-Cre transgenic line by DNA microarray revealed a pregnancy-like gene expression profile, suggesting that the ectopically expressed hGH, through low-affinity binding to the prolactin receptor (4), activates a lactogenic signaling pathway and causes an increase in β-cell proliferation and an impairment in glucose tolerance (2, 4).
To overcome deficiencies of the MIP-GFP mice, and to determine the extent to which aberrant transgene-mediated hGH expression affects gene expression in β-cells, we derived mice containing an Ins2Apple allele. Although this new allele also contains an hGH minigene, something we found essential for achieving penetrant expression of histone 2B (H2B)-Apple, it does not ectopically express any detectable hGH. With the use of both MIP-GFP transgenic and Ins2Apple/+ mice, we performed RNA sequencing (RNA-Seq) using FACS-purified β-cells from male and female mice to identify sex-specific gene expression differences in β-cells. Our results indicate that aberrant hGH expression from the MIP-GFP transgene not only profoundly perturbs β-cell gene expression, but also attenuates the ability to discriminate gene expression changes based on sex. Analysis of sex-based gene expression differences using the Ins2Apple allele also revealed significant differences in gene expression between male and female β-cells that may lead to a greater understanding of sex-based risk of developing T2D in humans.
MATERIALS AND METHODS
Mice and husbandry.
Mice were fed a standard chow diet (PicoLab, 5L0D), maintained on a 12:12-h light-dark cycle, and housed in a specific-pathogen-free facility at Vanderbilt University (Nashville, TN). MIP-GFP mice [Tg(Ins1-EGFP/GH1)1Hara] were acquired from Jackson (stock no. 006864), maintained on a C57BL/6J background, and genotyped as previously described (17). All animal experimentation was approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Gene targeting.
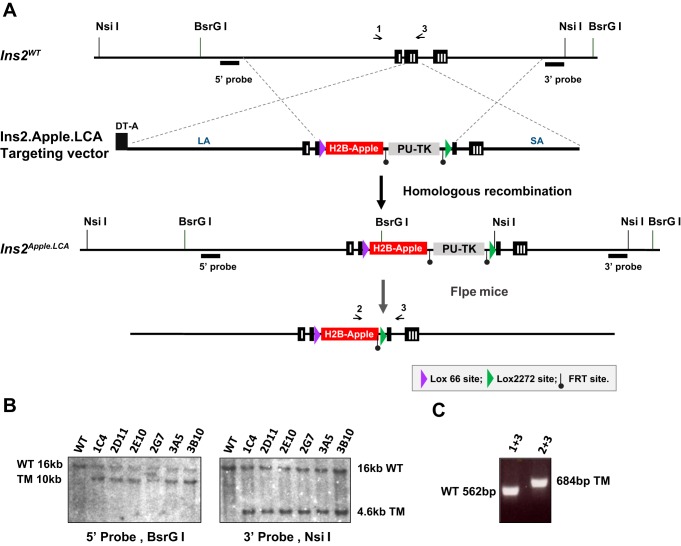
pSP72.Ins2.GFP.LNL (41), a gift from Lori Sussel (Barbara Davis Center, University of Colorado), was modified by inserting a Lox66 site, H2B-Apple sequences, an FRT-flanked puromycin resistance-Δ-thymidine kinase (PU-ΔTK) selection cassette, and a Lox2272 site. Inclusion of these heteromeric Lox sites and the PU-ΔTK cassette enables the targeted allele to serve also as a cassette acceptor allele (Fig. 1A). After electroporation of the targeting vector pIns2.H2B.Apple.LCA into TL-1 mouse embryonic stem (ES) cells (mESCs), 162 puromycin-resistant mESC clones were obtained and were screened by Southern blot hybridization using region-specific 5′- and 3′-probes. Six correctly targeted clones were identified, one of which (1C4) was injected into mouse blastocysts to generate chimeric mice (Fig. 1B). After germline transmission, Ins2Apple.LCA/+ mice were genotyped using the following primers: Ins2F1 (5′-GAGGTGTTGACGTCCAATGAG-3′) and Ins2R1 (5′-GAACTCACCTTGTGGGTCCTC-3′), which produce a wild-type band of 562 bp; Ins2F and AppleR1 (5′-CATGTTATTCTCCTCGCCCTTG-3′), which produce an Ins2LCA.Apple allele-specific band of 876 bp; and Cherry 2F (5′-CAGTTCATGTACGGCTCC-3′) and InsSeqR1 (5′-CAGTGGCAGAACTCACCTTG-3′), which produce an Ins2LCA.Apple allele-specific band of 684 bp after PU-ΔTK is deleted by Flpe (Fig. 1C).
Fig. 1.
Generation of Ins2Apple.LCA mice. A: schematic of the Ins2.Apple.LCA targeting vector, which contains a Lox66 site, the histone 2B (H2B)-Apple sequence, an FRT-flanked puromycin resistance-Δ-thymidine kinase (PU-ΔTK) selection marker, and a Lox2272 site. Ins2WT represents the wild-type Ins2 allele. The Ins2LCA allele was created by homologous recombination in mouse embryonic stem (ES) cells. To generate the final allele, mice expressing the Ins2Apple.LCA allele were crossed with mice expressing FLPe to excise the PU-ΔTK cassette. Primer binding sites are represented by arrows above the schemes. DT-A, diphtheria toxin A; LA, long homology arm; SA, short homology arm. B: Southern blot analyses using either BsrGI-digested ES cell clone DNA and the 5′-probe or NsiI-digested ES cell clone DNA and the 3′-probe. Clone 1C4 was injected into mouse blastocysts to generate live mice. TM, targeted mutation; WT, wild-type. C: PCR analysis used to distinguish between the wild-type and targeted Ins2 alleles.
Recombinase-mediated cassette exchange.
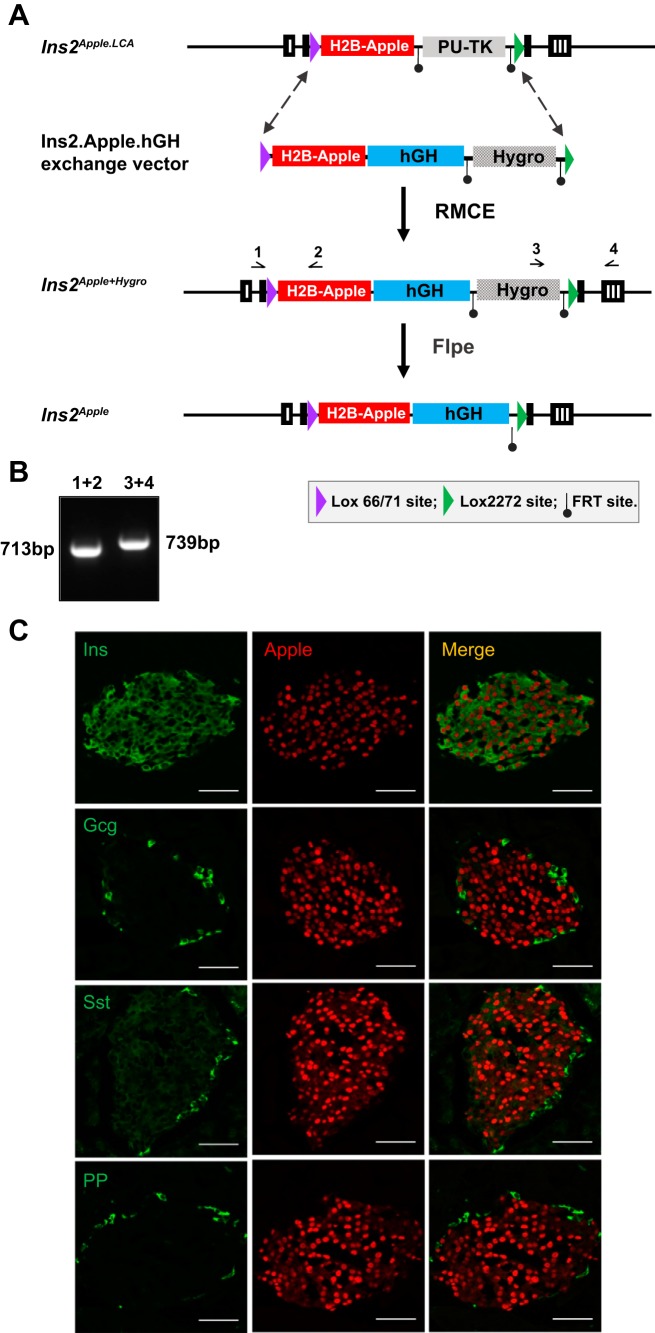
The Ins2Apple allele was made by inserting hGH genomic sequences downstream of H2B-Apple using recombinase-mediated cassette exchange (RMCE). First, an exchange vector (pIns2.H2B-Apple.hGH) was made based on the pMCS.71/2272.hygro vector (7). This plasmid contains a Lox71 site, H2B-Apple, a 3-kb fragment of the hGH gene, a Lox2272 site, and an FRT-flanked pgk-Hygro positive selection cassette (Fig. 2A). Ins2LCA.Apple mESCs (clone 1C4) were coelectroporated with the exchange plasmid and pBS185, a Cre-expression plasmid, as previously described (7). Of 90 hygro-resistant clones, 9 survived ganciclovir selection, all of which were determined by PCR to be correctly exchanged. Subclone 1A1 of colony 1C4 was injected into mouse blastocysts to achieve germ-line transmission. Microinjections were performed by the Vanderbilt Transgenic Mouse/ES Cell Shared Resource. To genotype the Ins2Apple/+ mice, the following primers were used: Ins2F1 and AppleR1, which produce a band of 713 bp, and Hygro.3R (5′-ACCGATGGCTGTGTAGAAGTACT-3′) and Ins2R1, which produce a band of 739 bp (Fig. 2B). The pgk-Hygro selection cassette was removed by interbreeding with mice containing an FLPe-expressing transgene (33) and then backcrossed for at least five generations into a C57BL/6J background before use.
Fig. 2.
Generation of Ins2Apple/+ mice. A: schematic of the Ins2.Apple.hGH exchange vector, which contains a Lox71 site, the histone 2B (H2B)-Apple sequence, a 3-kb fragment of human growth hormone (hGH), an FRT-flanked pgk-Hygro selection marker, and a Lox2272 site. The Ins2Apple+Hygro allele was generated by recombinase-mediated cassette exchange (RMCE) in mouse embryonic stem cells expressing the Ins2Apple.LCA allele. To generate the final Ins2Apple allele, mice with the Ins2Apple+Hygro allele were interbred with animals that express FLPe to excise the pgk-Hygro cassette. Primer binding sites are represented by arrows above the schemes. B: PCR analysis used to identify mice expressing the targeted allele and mice in which pgk-HygroR resistance cassette has been excised. C: representative images from frozen pancreatic sections from Ins2Apple/+ mice subjected to immunofluorescence using antibodies against insulin (Ins), glucagon (Gcg), somatostatin (Sst), pancreatic polypeptide (PP), and Apple. Scale bar = 50 μm. PU-TK, puromycin resistance-Δ-thymidine kinase.
Glucose homeostasis measurements.
Both fed and 16-h fasting blood glucose measurements were performed in mice at 11–12 wk of age. Glucose tolerance was assessed by the intraperitoneal injection of d-glucose (2 mg/g body wt) and measuring glucose concentrations at 0, 15, 30, 60, and 120 min using a BD Logic glucometer.
Immunofluorescence microscopy.
Whole pancreata were fixed for 4 h in 4% paraformaldehyde, incubated overnight at 4°C in 30% sucrose, embedded in optimum cutting temperature compound (Tissue-Tek), frozen on dry ice, and sectioned at a depth of 8 μm. Antibodies used were guinea pig anti-insulin (1:1,000; Invitrogen), rabbit anti-glucagon (1:1,000; Linco Research), goat anti-somatostatin (1:1,000; Santa Cruz Biotechnology), guinea pig anti-pancreatic polypeptide (1:1,000; Linco Research), rabbit anti-red FP (1:1,000; Rockland Immunochemicals), and rabbit anti-serotonin (1:1,000; ImmunoStar). After antibody staining, slides were mounted with ProLong Gold with 4′,6′-diamidino-2-phenylindole (DAPI; Invitrogen). Images were acquired using either a Zeiss Axioplan 2 upright microscope or an Olympus FV1000 inverted confocal microscope. Images were pseudocolored using ImageJ and are representative of the phenotype observed in at least three different animals per genotype.
hGH radioimmunoassay.
Islets were isolated from male mice (n = 3 for each genotype) at 9–10 wk of age. Islets were lysed in the TETG buffer (20 mM Tris·HCl, pH 8.0, 1% Triton X-100, 10% glycerol, 140 mM NaCl, 2 mM EGTA) with 1× protease inhibitors (P8340; Sigma) by repeated freezing and thawing cycles. Final lysates were cleared by centrifugation and used for hGH radioimmunoassay (07151102; MP Biomedicals) performed in triplicates by Vanderbilt Hormone Assay and Analytical Services Core. Results were normalized to total protein content.
Islet isolation and perifusion.
Islet isolation and perifusion experiments were performed by the Vanderbilt Islet Procurement and Analysis Core. Islets were isolated by injection of 0.6 mg/ml Collagenase P (Roche) into the pancreatic bile duct, and partially dissociated tissue was fractionated using a Histopaque-1077 (Sigma) gradient followed by hand-picking of islets. For FACS and RNA-Seq, islets from 3 to 7 mice were pooled for each sample. Glucose-stimulated insulin secretion was measured using islets from 4 wild-type male C57BL/6J mice (acquired directly from Jackson) and 4 Ins2Apple/+ male mice bred at Vanderbilt (99.6% C57BL/6J) that were both 12 wk of age. A parallel, 4-column apparatus, maintained at 37°C by water immersion, was used to perifuse 50 size-matched islets from 4 different mice simultaneously. Islets were perifused with low (5.6 mM) glucose perifusion media (DMEM without glucose, pH 7.4, 38.1 mM sodium bicarbonate, 4 mM l-glutamine, 1 mM sodium pyruvate, 0.5% phenol red, 5.0 mM HEPES, and 0.1% BSA) for a 30-min equilibration period (baseline) before fractions were collected for 9 min in low (5.6 mM) glucose, 30 min in high (16.7 mM) glucose, 21 min in low glucose, 9 min in 20 mM KCl + 5.6 mM glucose, and 21 min in 5.6 mM glucose. Insulin was measured using a radioimmunoassay kit (RI-13K; Millipore Sigma). Data were normalized to islet equivalent number. Area under the curve was quantified using GraphPad Prism software.
Real-time quantitative PCR.
RNA was purified from whole islets using the Maxwell 16 LEV simplyRNA Tissue Kit (TM351; Promega). Reverse transcription was done using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher). Two nanograms of cDNA were used in real-time quantitative PCR with Power SYBR Green PCR Master Mix (Thermo Fisher) using CFX96 Real-Time PCR Detection System (Bio-Rad). Primers for detection of Tph1, Slc2a2, hGH, and Hprt were as previously published (4). Relative expression in comparison with wild-type was determined using the comparative cycle threshold method, using Hprt expression for normalization.
Cell isolation.
Mouse β-cells were purified as previously described (36). Briefly, islets were dispersed in an Accumax (Sigma) and 1 U/ml DNase (AM2222; Invitrogen) solution at 37°C. Flow Cytometry Staining Buffer (FCB; FC001; R&D Systems) supplemented with DNase and 0.5 M EDTA was added to the cell suspension, and cells were filtered using a 35-μm cell strainer. Cell pellets were resuspended in FCB supplemented with DNase and EDTA. DAPI (final concentration 5 μg/ml; D21490; Thermo Fisher), for Ins2Apple/+ samples, or 7-AAD (final concentration 1 μg/ml; A1310; Thermo Fisher), for MIP-GFP samples, was added to the sorting media for exclusion of dead cells. Live cells expressing the fluorescent reporter were sorted with a 100-μm nozzle using the FACSAria II (BD Biosciences) instrument. Cells were collected in the Maxwell 16 LEV simplyRNA Tissue Kit Homogenization Solution supplemented with 1-thioglycerol.
RNA purification and quality control.
RNA was isolated from FACS-purified β-cells using the Maxwell 16 LEV simplyRNA Tissue Kit. After extraction, RNasin (40 U/μl; Promega) was added to the RNA samples before storage at −80°C. RNA samples were analyzed using the Agilent 2100 Bioanalyzer, and only those samples with an RNA integrity number >6 were used for sequencing.
Library assembly and sequencing.
RNA samples from FACS-purified β-cells were amplified using the SMART-Seq v4 Ultra Low Input RNA Kit for Sequencing (Clontech) using 8 cycles of PCR. cDNA libraries were constructed using the Low Input Library Prep Kit (Clontech). An Illumina HiSeq 3000 instrument was then used to produce paired-end, 75-nucleotide reads for each RNA sample.
Bioinformatics analysis.
Raw sequencing reads were processed using Trim Galore! 0.4.0 (which relies on cutadapt 1.9.dev2) to remove adapter sequences and pairs that were either <20 bp or that had Phred scores <20. The Spliced Transcripts Alignment to a Reference (STAR) application (10) was used to perform sequence alignments to the mm10 (GRCm38) mouse genome reference and GENCODE comprehensive gene annotations (Release M8). The two-pass mapping approach in STAR was used to increase the detection of reads mapping to novel junctions identified during the first mapping pass. Thirty to seventy-six million uniquely mapped reads were acquired per sample (mean = forty-four million). HTSeq was used for counting reads mapped to genomic features (1), and DESeq2 was employed for differential gene expression analysis (23), using the Advanced Computing Center for Research and Education (ACCRE) at Vanderbilt University. RNA-Seq data are available in ArrayExpress (https://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-6329.
Pathway analysis and upstream regulator prediction.
DAVID Bioinformatics Resource version 6.8 was used to identify Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that were significantly up- or downregulated in different conditions using differentially expressed genes generated from RNA-Seq data (9). False discovery rate-adjusted P value of 0.05 was used as a cutoff to declare significance.
Statistical analysis.
Statistical significance was determined using two-tailed Student’s t-test. Data are represented as means ± SE. A threshold of P < 0.05 was used to declare significance.
RESULTS
Generation of Ins2Apple/+ mice.
To improve on the shortcomings of the MIP-GFP mice, we generated a novel line of mice that expresses a nuclear-localized, histone 2B (H2B)-Apple fusion protein under control of the endogenous Ins2 gene locus (Ins2Apple/+; Figs. 1 and 2). Apple, a red FP, was used instead of Cherry, another red FP, since it is brighter when measured by both immunofluorescence microscopy and FACS and since Cherry is known to exhibit nonuniform, punctate expression in cells (7). With the use of gene targeting, we first derived a knockin allele (Ins2Apple.LCA) in which coding sequences in the Ins2 gene were replaced with those for the nuclear-localized H2B-Apple fusion protein (Fig. 1A). Two heterotypic LoxP sites were engineered into the locus to enable other variant alleles to be created by RMCE. The Ins2Apple.LCA allele relies on the Ins2 gene to provide the 3′-untranslated and polyadenylation signal sequences of the resulting mRNA transcript. However, we were surprised to find that only 11.6% ± 8.7% of insulin-expressing cells in mice containing this allele visibly expressed H2B-Apple.
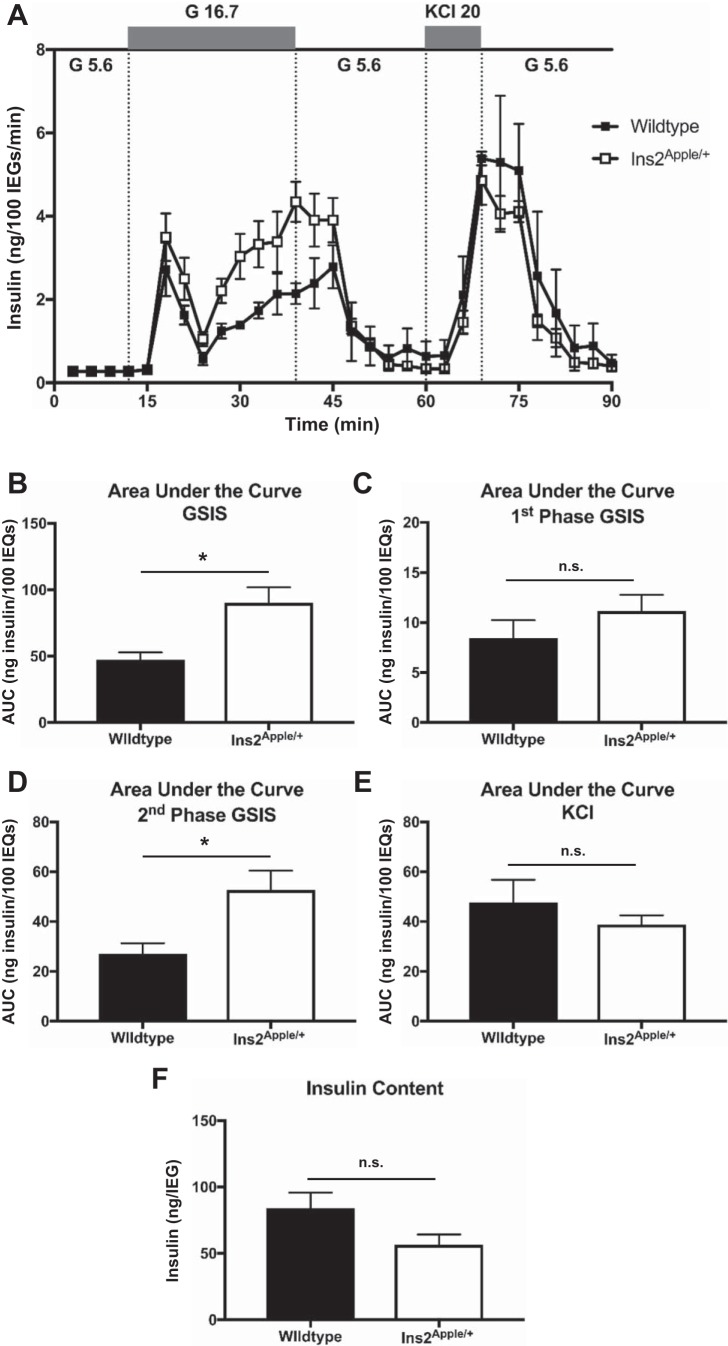
To increase the penetrance of red FP expression, we used RMCE to generate a variant allele in which an hGH minigene, lacking promoter sequences, was placed immediately downstream of H2B-Apple coding sequences (Fig. 2A). Since the hGH minigene lacks a promoter, both the H2B-Apple and hGH cistrons are contained within a single mRNA transcript. In agreement with prior reports (3) that describe an hGH minigene-mediated increase in transgene expression, immunostaining revealed that 91.9 ± 2.8% of insulin-expressing β-cells coexpressed H2B-Apple in the Ins2Apple/+ animals (Fig. 2C), a nearly 8-fold increase in penetrance over the Ins2Apple.LCA allele. Further immunostaining confirmed that H2B-Apple expression is specific to β-cells within mouse islets (Fig. 2C). As expected, an hGH-encoding cistron was readily detected in both Ins2Apple/+ and MIP-GFP islets (Fig. 3A). However, hGH protein was only detected in MIP-GFP islets with neither the Ins2Apple/+ nor wild-type islets exhibiting any detectable protein (Fig. 3B). Tph1 mRNA, an established target of prolactin and hGH signaling (4), although slightly upregulated in Ins2Apple/+ compared with wild-type islets, was >1,000-fold lower than in MIP-GFP islets (Fig. 3C) and was not associated with a detectable amount of serotonin (Fig. 3D). Additionally, Slc2a2, another known target of hGH signaling (4), was unchanged in Ins2Apple/+ islets, whereas it is downregulated in MIP-GFP mice (Fig. 3E). Although Ins2Apple/+ animals showed no impairments in blood glucose homeostasis or change in body weight (Fig. 4), islet perifusion analysis revealed a modest enhancement in the second phase of glucose-stimulated insulin secretion in Ins2Apple/+ animals compared with wild-type controls (Fig. 5, A and D). However, there was no change in either first-phase or KCl-stimulated secretion (Fig. 5, C and E), and insulin content of the islets was also unchanged (Fig. 5F).
Fig. 3.
Ins2Apple/+ mice do not exhibit ectopic human growth hormone (hGH) protein expression. A, C, and E: real-time quantitative PCR for hGH (A), Tph1 (C), and Slc2a2 (E) using whole islet RNA from wild-type, Ins2Apple/+, and MIP-GFP adult males. N = 4 mice per genotype. B: measurement of hGH protein concentration by radioimmunoassay in adult male wild-type, Ins2Apple/+, and MIP-GFP islets. N = 3 mice per genotype. Results are presented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.0001, n.s. = not significant (Student’s t-test, 2-tailed, equal variance). D: coimmunostaining of pancreatic sections from Ins2Apple/+ and MIP-GP mice for either insulin or green fluorescent protein (GFP) and serotonin (5-HT). Scale bar = 50 μm. 2-ΔΔCt, comparative cycle threshold method.
Fig. 4.
Ins2Apple/+ mice exhibit normal glycemic control. Random fed (A) and fasted (B) blood glucose measurements of male and female Ins2Apple/+ mice were compared with littermate Ins2+/+ controls at 11–12 wk of age. C and D: intraperitoneal glucose tolerance tests (IPGTTs) using Ins2Apple/+ males compared with littermate Ins2+/+ males (C) and Ins2Apple/+ females compared with littermate Ins2+/+ females (D). E: area under the curve calculations for glucose tolerance tests shown in C and D. F: body weight of male and female Ins2Apple/+ mice compared with littermate Ins2+/+ controls at 8–9 wk of age. N = 9 mice per group. Results are presented as means ± SE. n.s., Not significant.
Fig. 5.
Perifusion of islets from Ins2Apple/+ mice. A: insulin secretion was measured from islets isolated from Ins2Apple/+ or wild-type C57BL/6J males at 12 wk of age in response to 5.6 mM glucose (G 5.6), 16.7 mM glucose (G 16.7), or 5.6 mM glucose + 20 mM KCl (KCl 20) by perifusion. IEQ, islet equivalent. B–E: area under the curve (AUC) calculations for the time periods representing glucose-stimulated insulin secretion (GSIS; B), the 1st phase of GSIS alone (C), the 2nd phase of GSIS alone (D), and KCl-stimulated insulin secretion (E). F: islet insulin content from Ins2Apple/+ or wild-type males at 12 wk of age. N = 4 mice per group. n.s., Not significant. *P < 0.05.
Effects of ectopic hGH on β-cell gene expression.
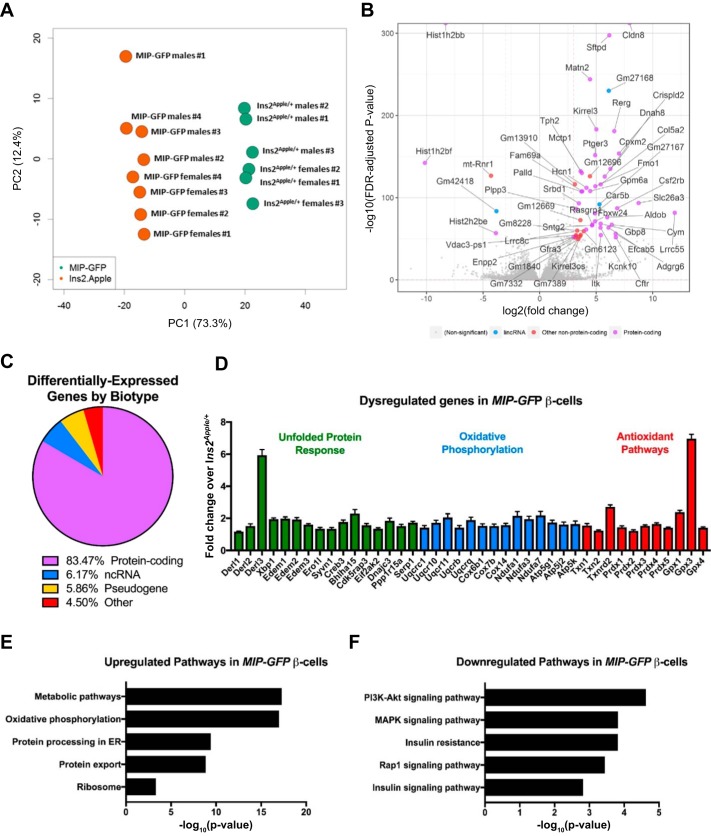
With the use of both male and female mice that contain either the Ins2Apple allele or MIP-GFP transgene, we performed RNA-Seq on 14 different samples of postnatal day 60 β-cells. This collection of data sets that enabled us to perform a series of pairwise RNA expression analyses is summarized in Table 1. First, to determine the effects of ectopic hGH on β-cell gene expression, we combined the male and female data sets and compared, in aggregate, the gene expression profile of cells from Ins2Apple/+ (n = 6) and MIP-GFP (n = 8) mice. As expected, principal component analysis indicated that the samples clustered by genotype (Fig. 6A). Differential expression analysis revealed a total of 7,733 genes (3,878 upregulated and 3,855 downregulated) that varied between the MIP-GFP and Ins2Apple/+ β-cells (based on false discovery rate-adjusted P value <0.05; Fig. 6B; Supplemental Table S1, available in the data supplement online at the AJP-Endocrinology and Metabolism Web site). Of the transcripts identified, 83.5% were protein-coding, 6.2% were noncoding RNA, 5.9% were pseudogenes, and 4.5% were other types of transcripts (Fig. 6C). As previously observed, glucose transporter GLUT2, encoded by Slc2a2, is significantly downregulated in MIP-GFP compared with Ins2Apple/+ β-cells (1.8-fold; P = 9.02 × 10−14; Supplemental Table S1; Ref. 4). Additionally, expression of genes associated with a pregnancy-like phenotype known to be induced by ectopic hGH expression including Tph1, Tph2, Gbp8, Neb, Cish, Cldn8, Matn2, Tnfrsf11b, Lonrf3, Car8, Ehhadh, Ivd, and Prlr (4) were all significantly reduced in β-cells from the Ins2Apple/+ mice compared with those from MIP-GFP animals (Supplemental Table S1).
Table 1.
Summary of pairwise comparisons performed
| Description of Comparison | No. Dysregulated Genes | Corresponding Figure |
|---|---|---|
| Ins2Apple/+ males and females (pooled) vs. MIP-GFP males and females (pooled) | 7,733 | Fig. 6 |
| Ins2Apple/+ males vs. Ins2Apple/+ females | 657 | Fig. 7 |
| MIP-GFP males vs. MIP-GFP females | 115 | Fig. 8 |
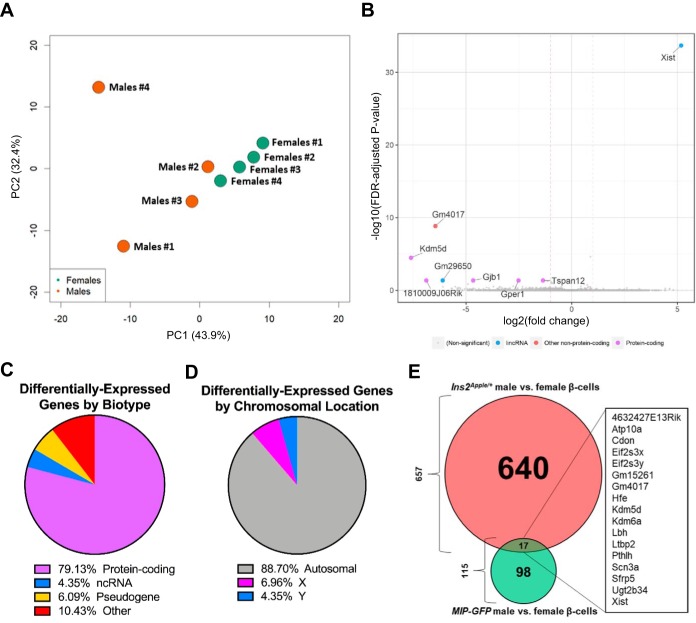
Using the 14 RNA sequencing data sets obtained from FACS-purified pancreatic β-cells, we performed 3 different pairwise comparisons. First, we compared pooled Ins2Apple/+ males and females with pooled MIP-GFP males and females. This comparison is summarized in Fig. 6 and identified 7,733 differentially regulated genes. Second, we compared Ins2Apple/+ males with Ins2Apple/+ females. This comparison is summarized in Fig. 7 and identified 657 differentially regulated genes. Finally, we compared MIP-GFP males with MIP-GFP females. This comparison is summarized in Fig. 8 and identified 115 differentially regulated genes.
Fig. 6.
Dysregulated genes in MIP-GFP β-cells. We performed RNA sequencing of FACS-purified β-cells from Ins2Apple/+ and MIP-GFP animals at postnatal day 60. A: principal component analysis shows that the 14 samples used for RNA sequencing cluster by genotype, with some variation in the 2nd principal component (PC2). B: volcano plot showing the most differentially expressed genes in MIP-GFP β-cells based on the −log10 [false discovery rate (FDR)-adjusted P value] and the log2 (fold change). Genes with a log2 (fold change) >3 are labeled and grouped into categories based on biotype characterization. lincRNA, long intervening noncoding RNA. C: pie chart showing the percentage of differentially regulated genes that fall into biotype categories of protein-coding, noncoding RNA (ncRNA), other RNA, pseudogene, and other types of transcripts. D: selected differentially expressed genes, grouped in categories identified by Database for Annotation, Visualization, and Integrated Discovery (DAVID), in MIP-GFP β-cells. All genes shown have FDR-adjusted P values <0.05. Results are presented as means ± SE. E and F: we used the DAVID Bioinformatics Resource (version 6.8) to identify enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway categories among the top 3,000 genes that were significantly up- (E) or downregulated (F) in MIP-GFP β-cells. ER, endoplasmic reticulum; PI3K, phosphatidylinositol 3-kinase.
Next, we used an unsupervised gene ontology approach (DAVID version 6.8; Ref. 9) to categorize the potential functional defects in MIP-GFP β-cells using the top 3,000 up- or downregulated genes. This analysis revealed that pathways involved in cell metabolism, oxidative phosphorylation, endoplasmic reticulum (ER) protein processing, and protein export were upregulated in MIP-GFP β-cells, whereas phosphatidylinositol 3-kinase-Akt signaling, MAPK signaling, Rap1 signaling, and insulin signaling pathways were downregulated (Fig. 6, E and F). Further examination of these individual genes also revealed some known to be involved in the unfolded protein response (Fig. 6D). Interestingly, in addition to increasing the expression of genes involved in mitochondrial oxidative phosphorylation, such as ubiquinol-cytochrome c reductases, cytochrome c oxidases, NADH dehydrogenases, and ATP synthase subunits, MIP-GFP β-cells also upregulate several genes involved in detoxifying reactive oxygen species, natural by-products of the electron transport chain, including thioredoxins, peroxiredoxins, and glutathione peroxidases (Fig. 6D). These results indicate that the MIP-GFP transgene causes the upregulation of genes in β-cells that are associated with both ER and oxidative stress.
Sex-based gene expression differences in Ins2Apple/+ mice.
To understand better how sex affects β-cell gene expression and influences T2D risk, we next compared the sex-segregated β-cell samples using the Ins2Apple/+ data sets. Male and female β-cells clustered by sex (Fig. 7A) and differed by the expression of 657 genes (Fig. 7B; Supplemental Table S2). The vast majority of the dysregulated genes were protein-coding (96%; Fig. 7C). Not surprisingly, most of the dysregulated genes were located on autosomal chromosomes (94%; Fig. 7D), consistent with the principal cause of differential gene expression between males and females being differences in regulation, not to location on a sex chromosome.
Fig. 7.
Differential expression analysis of male and female InsApple/+ β-cells. We performed RNA sequencing of FACS-purified β-cells from male and female Ins2Apple/+ mice. A: principal component (PC) analysis shows that the 6 samples used for RNA sequencing cluster by sex. B: volcano plot showing the most differentially expressed genes in female compared with male Ins2Apple/+ β-cells based on the −log10 [false discovery rate (FDR)-adjusted P value] and the log2 (fold change). Genes with a log2 (fold change) >1 are labeled and grouped into categories based on biotype characterization. lincRNA, long intervening noncoding RNA. C: pie chart showing the percentage of differentially regulated genes that fall into biotype categories of protein-coding, noncoding RNA (ncRNA), pseudogene, and other. D: pie chart depicting the percentage of differentially regulated genes located on either autosomal chromosomes or on either of the 2 sex chromosomes. E: selected genes encoding critical β-cell transcription factors, which are upregulated in female β-cells, and cell cycle regulators, which are upregulated in male β-cells. All genes shown have FDR-adjusted P values <0.05. Results are presented as means ± SE. F and G: we used the DAVID Bioinformatics Resource (version 6.8) to identify enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway categories among the genes that were significantly upregulated in either Ins2Apple/+ females (F) or males (G). ER, endoplasmic reticulum; PI3K, phosphatidylinositol 3-kinase; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Xist, a long-noncoding RNA responsible for X-chromosome inactivation in females, was the most highly differentially expressed gene (91-fold upregulated in females; Ref. 5). However, excluding Xist, gene expression differences between males and females were generally small, with the next most affected gene, Tmed9, differing by only 3.8-fold. Tmed9 is upregulated in female β-cells (Fig. 7B) and belongs to the p24 family of regulators of secretory protein trafficking (31). Although Tmed9 has not been studied in β-cells, a related family member, Tmed6, is expressed in islets and has been shown to regulate insulin secretion positively (43). Another gene, Arpc3, which is 3.2-fold upregulated in female β-cells (Fig. 7B), is a part of the actin-related protein 2/3 (Arp2/3) complex. Actin cytoskeleton remodeling, which is partially controlled by Arp2/3 (40), is known to influence insulin secretion (39). A third gene, Eml2, belongs to a family of microtubular destabilizers (12). Interestingly, microtubule stabilization is negatively correlated with insulin exocytosis, with microtubule catastrophe enhancing GSIS (46). Thus an increase in the expression of any or all three of these genes in female β-cells, based on the known functions of the proteins they encode, may contribute to the sex-based differences in GSIS (16).
Again, using DAVID, we identified enriched pathways among these differentially expressed genes and found several interesting differences. For example, female Ins2Apple/+ β-cells express Nkx2-2, Hnf4a, Mnx1, Pax6, and Rfx6 (11, 30, 32, 35, 42), transcription factors known to be important for β-cell development and function, at higher levels than in males. On the other hand, male Ins2Apple/+ β-cells exhibited a generally higher expression of genes involved in cell cycle regulation (Fig. 7, E–G). These differences may provide important clues for understanding the higher tendency of male β-cells to fail in response to metabolic stress (16, 19, 34, 37).
Sex-based gene expression differences in MIP-GFP mice.
We next identified genes differentially expressed in male and female β-cells isolated from the MIP-GFP transgenic mice. Although the male and female MIP-GFP samples also clustered by sex, we detected fewer differentially expressed genes than when comparing male and female Ins2Apple/+ samples (115 vs. 657, respectively; Fig. 8B; Supplemental Table S3). Similar to the previous sex comparison, the majority of the dysregulated genes were protein-coding (79%; Fig. 8C) and were located on autosomal chromosomes (89%; Fig. 8D). Although Xist was, again, the most differentially regulated gene (Fig. 8B), its expression level in MIP-GFP females was only 36-fold greater than in males (vs. 91-fold greater in Ins2Apple/+ females over males), and KEGG pathway analysis failed to reveal any significant pathway enrichments. Furthermore, comparison of the sex-driven, differentially expressed genes identified using the Ins2Apple allele with those identified using the MIP-GFP transgene revealed an overlap of just 17 transcripts (Fig. 8E). This number is far smaller than would be expected if the MIP-GFP transgene affected both sexes equally, suggesting that use of the MIP-GFP transgene, compared with the Ins2Apple allele, impairs the ability to elucidate sex-dependent changes in gene expression.
Fig. 8.
Differential expression analysis of male and female MIP-GFP β-cells. We performed RNA sequencing of FACS-purified β-cells from male and female MIP-GFP mice. A: principal component (PC) analysis shows that the 8 samples used for RNA sequencing cluster by sex. B: volcano plot showing the most differentially expressed genes in female compared with male MIP-GFP β-cells based on the −log10 [false discovery rate (FDR)-adjusted P value] and the log2 (fold change). lincRNA, long intervening noncoding RNA. C: pie chart showing the percentage of differentially regulated genes that fall into biotype categories of protein-coding, noncoding RNA (ncRNA), pseudogene, and other. D: pie chart depicting the percentage of differentially regulated genes located on either autosomal chromosomes or on either of the 2 sex chromosomes. E: Venn diagram summarizing the comparison of the differentially expressed genes identified between Ins2Apple/+ males and females with those identified between MIP-GFP males and females. Only 17 genes overlap between the 2 comparisons.
DISCUSSION
Despite the obvious utility of FP reporter transgenes/alleles for studying murine β-cells, the derivation of a FP-expressing line that is uniformly expressed in pancreatic β-cells has been a challenge for the field. Many widely used transgenic lines, including the MIP-GFP mice, have been reported to express hGH in β-cells ectopically (4, 18). In at least one of these lines, ectopic expression of hGH activates STAT5 signaling, causing both an increase in serotonin biosynthesis and β-cell mass, decreased glucose-stimulated insulin secretion, and impaired glucose tolerance. In addition, Slc2a2 expression decreases, thereby causing resistance to the β-cell toxin streptozotocin (2, 4, 6, 18, 29). To generate an improved nuclear-localized red FP line for β-cells, we generated Ins2Apple/+ mice, a line in which H2B-Apple was inserted into the endogenous Ins2 gene locus. Interestingly, when H2B-Apple coding sequences were inserted by themselves into the Ins2 gene, red fluorescence was observed in just over 10% of β-cells. However, when we modified the allele also to contain an hGH minigene as a second cistron, expression was achieved in >90% of β-cells. Although the Ins2Apple allele produces a bicistronic mRNA that contains both H2B-Apple and hGH coding sequences, Ins2Apple/+ islets were found not to express a detectable amount of either hGH protein or serotonin and showed normal expression of Slc2a2 (Fig. 3). By quantitative PCR, the Ins2Apple allele exhibits a 14-fold higher expression of the bicistronic mRNA than does the MIP-GFP transgene, consistent with the Ins2 gene having greater relative transcriptional activity than that of the Ins1 gene, as suggested by a 3-fold higher expression of Ins2 compared with Ins1 in our previously generated RNA-Seq data sets (36). Although the reason for these differences is not known, it is possible, perhaps likely, that the MIP-GFP transgene lacks distal enhancer regions that may help drive expression of the Ins2 gene locus (45). Moreover, although Ins2Apple/+ animals exhibited normal glucose tolerance, there is a trend toward slightly improved glucose clearance and slight increase in second-phase insulin secretion. However, this may be the result of a compensatory response to lower insulin production due to the Ins2 gene disruption (20) and not to a subdetectable amount of hGH protein expression.
Although it is well-established that hGH-expressing transgenes adversely affect β-cell gene expression and function, it is not known to what extent this may interfere with differential expression analysis, particularly when the effect of sex is being analyzed. For this reason, we obtained FACS-purified β-cells from both male and female animals containing either the MIP-GFP transgene or Ins2Apple/+ allele. After pooling both the male and female data sets to maximize the number of individual replicates, we were surprised to find that 7,733 genes, or more than half of the transcriptome (estimated size of 15,200 transcripts after filtering out genes with <10 counts in ≥3 samples), were affected.
Although the adverse effects of transgene-mediated hGH expression in β-cells was previously determined using DNA microarrays (4), our RNA-Seq analysis indicates that the impact of leaky hGH expression on β-cells is greater than previously reported. Indeed, the large number of differentially expressed genes we have observed suggests that hGH may not simply activate STAT5 signaling, but that it may also activate signaling pathways associated with ER and oxidative stress and the unfolded protein response, both of which are known to contribute to the development of T2D (15). Although ectopic hGH is likely the primary cause of the differences in gene expression identified between Ins2Apple/+ and MIP-GFP β-cells, we cannot rule out the potential contributions of 1) differences in the FP used, 2) differences in relative expression levels of the FPs, 3) differences due to the localization of the FP, and 4) differences in the promoter used to drive the FP. Moreover, the reason why hGH protein is expressed from the MIP-GFP transgene but not the Ins2Apple allele is not known. We speculate that a cryptic splice acceptor site in the hGH gene may capture transcription from an unknown nearby gene when present as a transgene but not when it has been precisely inserted downstream of the Ins2 promoter, as is the case for the Ins2Apple allele.
Sex has long been known to influence T2D risk, with development of the disease being more common among men (8, 13, 21, 24, 26). Several reports have suggested that female β-cells may be partially protected from oxidative and metabolic stress due to signaling through the estrogen receptor (19, 34, 37), and gonadal sex steroids regulate energy metabolism, insulin secretion, and β-cell growth in a manner that is different between males and females (14, 25, 27). In addition, glucose-stimulated insulin secretion in human islets may be higher in women than men (16). By using the Ins2Apple allele to perform RNA-Seq of male and female β-cells, we identified 657 sexually dimorphic genes, many of which could lead to sex-specific functional differences. For instance, female animals express higher levels of Nkx2-2, Hnf4a, Mnx1, Pax6, and Rfx6 than males, and all of these transcription factors are important for endocrine cell function (11, 30, 32, 35, 42). Conversely, male β-cells exhibit greater expression of genes known to regulate β-cell proliferation. These results suggest that sex-specific differences in the gene expression profiles of β-cells may directly affect the risk of developing T2D. Indeed, if taken at face value, our results suggest that male β-cells may not be able to adapt as well as female cells to metabolic stress due to the decreased expression of key genes regulating β-cell function.
Remarkably, when we compared male and female β-cells from MIP-GFP mice, we were only able to resolve 115 differentially expressed genes, a much smaller number than obtained when comparing both sexes of Ins2Apple/+ β-cell, and side-by-side comparison of the 2 lists of differentially expressed genes revealed only 17 genes in common. Moreover, and probably because of the lower number of statistically significant differences, KEGG pathway analysis failed to identify any significant pathway enrichment among differentially regulated genes between MIP-GFP male and female β-cells. We speculate that these differences are due to the inappropriate activation of STAT3/5 signaling in male mice. It has been previously shown that gonadal estrogen-mediated changes in gene expression are due, in part, to the activation of STAT3/5 signaling (25, 38). If so, the ectopic expression of hGH in male β-cells, by increasing STAT3/5 signaling, may shift the gene expression of male β-cells toward more of a female profile. In support of this idea, principal component analysis using MIP-GFP male and female samples revealed less separation between the sexes than observed between Ins2Apple/+ males and females (compare Figs. 7A and 8A). Two MIP-GFP male samples, in particular, appear almost indistinguishable from the female samples (Fig. 8A). If so, use of the MIP-GFP transgene to determine sex-specific gene expression differences becomes a nearly futile activity.
A recent report using human islets has shown sex-specific differences in DNA methylation status and gene expression that may also contribute to differences in susceptibility to T2D (16). Eighteen of the dysregulated genes identified in this report are located on autosomal chromosomes, whereas sixty-one are on the X chromosome. This distribution contrasts markedly with our analyses, which found that most the differentially regulated genes are autosomal (Figs. 7D and 8D). Interestingly, and again in contrast to our results, XIST was not identified as being differentially methylated or expressed between human islets from men and women. With the use of the mouse homologs to the genes identified in this published study, only six genes were also differentially expressed between Ins2Apple/+ male and female β-cells. Ddx3x, Eif2s3x, Kdm5c, Kdm6a, and Mid2 are X-linked, and Slfn9 (SLFN13 in humans) is autosomal. Two of those genes (Eif2s3x and Kdm6a) were also dysregulated between MIP-GFP males and females. The lack of concordance among this published data set and the data sets generated in the current study may stem from species-specific differences or could reflect the use of whole islets rather than purified β-cells.
In conclusion, our results indicate that the MIP-GFP transgene not only greatly alters the gene expression profile of β-cells, but also that it may lessen the gene expression differences of male and female β-cells and interfere with the detection of sex-based differences in gene expression.
GRANTS
These studies were supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants DK-72473 and DK-89523 to M. A. Magnuson. Use of the Vanderbilt Transgenic Mouse/ES Cell Shared Resource and Cell Imaging Shared Resource was supported NIH Grants DK-020593 and CA-68485, and use of the Vanderbilt Islet Procurement and Analysis Core and Hormone Assay Core was supported by NIDDK Grant DK-020593.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.M. conceived and designed research; A.B.O. and M.A.M. designed and generated Ins2Apple.LCA and Ins2Apple alleles; J.S.S. and A.B.O. performed experiments; J.S.S., A.B.O., J.-P.C., and M.A.M. analyzed data; J.-P.C. performed RNA-Seq data processing, alignment, and differential expression analyses; J.S.S., A.B.O., J.-P.C., and M.A.M. interpreted results of experiments; J.S.S. and J.-P.C. prepared figures; J.S.S. and M.A.M. drafted manuscript; J.S.S., A.B.O., and M.A.M. edited and revised manuscript; J.S.S., A.B.O., and M.A.M. approved final version of manuscript; M.A.M. supervised and funded the experiments.
Supplemental Data
ACKNOWLEDGMENTS
We thank Rama Gangula and Susan Hipkens for their outstanding technical assistance, the Vanderbilt Transgenic Mouse/ES Cell Shared Resource for performing blastocyst microinjection of ES cells, the Vanderbilt Islet Procurement and Analysis Core for performing islet isolations and perifusions, Vanderbilt Technologies for Advanced Genomics (VANTAGE) for performing cell sorting and RNA-Seq, the Vanderbilt Cell Imaging Shared Resource for helping acquire images, and the Vanderbilt Hormone Assay Core for performing hGH radioimmunoassay.
REFERENCES
- 1.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169, 2015. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baan M, Kibbe CR, Bushkofsky JR, Harris TW, Sherman DS, Davis DB. Transgenic expression of the human growth hormone minigene promotes pancreatic β-cell proliferation. Am J Physiol Regul Integr Comp Physiol 309: R788–R794, 2015. doi: 10.1152/ajpregu.00244.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA 85: 836–840, 1988. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouwers B, de Faudeur G, Osipovich AB, Goyvaerts L, Lemaire K, Boesmans L, Cauwelier EJ, Granvik M, Pruniau VP, Van Lommel L, Van Schoors J, Stancill JS, Smolders I, Goffin V, Binart N, in’t Veld P, Declercq J, Magnuson MA, Creemers JW, Schuit F, Schraenen A. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab 20: 979–990, 2014. doi: 10.1016/j.cmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349: 38–44, 1991. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 6.Carboneau BA, Le TD, Dunn JC, Gannon M. Unexpected effects of the MIP-CreER transgene and tamoxifen on β-cell growth in C57Bl6/J male mice. Physiol Rep 4: e12863, 2016. doi: 10.14814/phy2.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SX, Osipovich AB, Ustione A, Potter LA, Hipkens S, Gangula R, Yuan W, Piston DW, Magnuson MA. Quantification of factors influencing fluorescent protein expression using RMCE to generate an allelic series in the ROSA26 locus in mice. Dis Model Mech 4: 537–547, 2011. doi: 10.1242/dmm.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998–2005. Diabetes Care 32: 2016–2020, 2009. doi: 10.2337/dc08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3, 2003. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 10.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21, 2013. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle MJ, Sussel L. Nkx2.2 regulates β-cell function in the mature islet. Diabetes 56: 1999–2007, 2007. doi: 10.2337/db06-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichenmüller B, Everley P, Palange J, Lepley D, Suprenant KA. The human EMAP-like protein-70 (ELP70) is a microtubule destabilizer that localizes to the mitotic apparatus. J Biol Chem 277: 1301–1309, 2002. doi: 10.1074/jbc.M106628200. [DOI] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375: 2215–2222, 2010. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gannon M, Kulkarni RN, Tse HM, Mauvais-Jarvis F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol Metab 15: 82–91, 2018. doi: 10.1016/j.molmet.2018.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. β-Cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 37: 1751–1758, 2014. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall E, Volkov P, Dayeh T, Esguerra JL, Salö S, Eliasson L, Rönn T, Bacos K, Ling C. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biol 15: 522, 2014. doi: 10.1186/s13059-014-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol Endocrinol Metab 284: E177–E183, 2003. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Kim H, Kim K, German MS, Kim H. Ectopic serotonin production in β-cell specific transgenic mice. Biochem Biophys Res Commun 495: 1986–1991, 2018. doi: 10.1016/j.bbrc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA 103: 9232–9237, 2006. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leroux L, Desbois P, Lamotte L, Duvillié B, Cordonnier N, Jackerott M, Jami J, Bucchini D, Joshi RL. Compensatory responses in mice carrying a null mutation for Ins1 or Ins2. Diabetes 50, Suppl 1: S150–S153, 2001. doi: 10.2337/diabetes.50.2007.S150. [DOI] [PubMed] [Google Scholar]
- 21.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995–2005: a population-based study. Lancet 369: 750–756, 2007. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 22.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, Wild SH, Sattar N; Scottish Diabetes Research Network Epidemiology Group . Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 54: 3003–3006, 2011. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav 187: 20–23, 2018. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauvais-Jarvis F. Role of sex steroids in β cell function, growth, and survival. Trends Endocrinol Metab 27: 844–855, 2016. doi: 10.1016/j.tem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauvais-Jarvis F, Sobngwi E, Porcher R, Riveline JP, Kevorkian JP, Vaisse C, Charpentier G, Guillausseau PJ, Vexiau P, Gautier JF. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of β-cell dysfunction and insulin resistance. Diabetes 53: 645–653, 2004. doi: 10.2337/diabetes.53.3.645. [DOI] [PubMed] [Google Scholar]
- 27.Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab 23: 837–851, 2016. doi: 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci USA 89: 6861–6865, 1992. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oropeza D, Jouvet N, Budry L, Campbell JE, Bouyakdan K, Lacombe J, Perron G, Bergeron V, Neuman JC, Brar HK, Fenske RJ, Meunier C, Sczelecki S, Kimple ME, Drucker DJ, Screaton RA, Poitout V, Ferron M, Alquier T, Estall JL. Phenotypic characterization of MIP-CreERT1Lphi mice with transgene-driven islet expression of human growth hormone. Diabetes 64: 3798–3807, 2015. doi: 10.2337/db15-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan FC, Brissova M, Powers AC, Pfaff S, Wright CV. Inactivating the permanent neonatal diabetes gene Mnx1 switches insulin-producing β-cells to a δ-like fate and reveals a facultative proliferative capacity in aged β-cells. Development 142: 3637–3648, 2015. doi: 10.1242/dev.126011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pastor-Cantizano N, Montesinos JC, Bernat-Silvestre C, Marcote MJ, Aniento F. p24 family proteins: key players in the regulation of trafficking along the secretory pathway. Protoplasma 253: 967–985, 2016. doi: 10.1007/s00709-015-0858-6. [DOI] [PubMed] [Google Scholar]
- 32.Piccand J, Strasser P, Hodson DJ, Meunier A, Ye T, Keime C, Birling MC, Rutter GA, Gradwohl G. Rfx6 maintains the functional identity of adult pancreatic β cells. Cell Rep 9: 2219–2232, 2014. doi: 10.1016/j.celrep.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140, 2000. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 34.Russo GT, Giorda CB, Cercone S, Nicolucci A, Cucinotta D; BetaDecline Study Group . Factors associated with beta-cell dysfunction in type 2 diabetes: the BETADECLINE study. PLoS One 9: e109702, 2014. doi: 10.1371/journal.pone.0109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 11: 1662–1673, 1997. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 36.Stancill JS, Cartailler JP, Clayton HW, O’Connor JT, Dickerson MT, Dadi PK, Osipovich AB, Jacobson DA, Magnuson MA. Chronic β-cell depolarization impairs β-cell identity by disrupting a network of Ca2+-regulated genes. Diabetes 66: 2175–2187, 2017. doi: 10.2337/db16-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, Mauvais-Jarvis F. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. J Clin Invest 121: 3331–3342, 2011. doi: 10.1172/JCI44564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiano JP, Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol 8: 342–351, 2012. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 39.Tomas A, Yermen B, Min L, Pessin JE, Halban PA. Regulation of pancreatic β-cell insulin secretion by actin cytoskeleton remodelling: role of gelsolin and cooperation with the MAPK signalling pathway. J Cell Sci 119: 2156–2167, 2006. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 40.Tran DT, Masedunskas A, Weigert R, Ten Hagen KG. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat Commun 6: 10098, 2015. doi: 10.1038/ncomms10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakae-Takada N, Xuan S, Watanabe K, Meda P, Leibel RL. Molecular basis for the regulation of islet beta cell mass in mice: the role of E-cadherin. Diabetologia 56: 856–866, 2013. doi: 10.1007/s00125-012-2824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Hepatocyte nuclear factor 4α regulates the expression of pancreatic β-cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem 275: 35953–35959, 2000. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Yang R, Jadhao SB, Yu D, Hu H, Glynn-Cunningham N, Sztalryd C, Silver KD, Gong DW. Transmembrane emp24 protein transport domain 6 is selectively expressed in pancreatic islets and implicated in insulin secretion and diabetes. Pancreas 41: 10–14, 2012. doi: 10.1097/MPA.0b013e318223c7e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu B, Allard C, Alvarez-Mercado AI, Fuselier T, Kim JH, Coons LA, Hewitt SC, Urano F, Korach KS, Levin ER, Arvan P, Floyd ZE, Mauvais-Jarvis F. Estrogens promote misfolded proinsulin degradation to protect insulin production and delay diabetes. Cell Rep 24: 181–196, 2018. doi: 10.1016/j.celrep.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Z, Lefevre GM, Felsenfeld G. Chromatin structure, epigenetic mechanisms and long-range interactions in the human insulin locus. Diabetes Obes Metab 14, Suppl 3: 1–11, 2012. doi: 10.1111/j.1463-1326.2012.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu X, Hu R, Brissova M, Stein RW, Powers AC, Gu G, Kaverina I. Microtubules negatively regulate insulin secretion in pancreatic β cells. Dev Cell 34: 656–668, 2015. doi: 10.1016/j.devcel.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.