Abstract
The purpose of this study was to better understand the role obesity plays in the inflammatory response during sepsis, specifically regarding the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway in the liver. We hypothesized that inhibiting STAT3 would lead to an increase in the inflammatory response and that obesity would amplify this effect. To investigate this, we inhibited STAT3 in two ways: pharmacological systemic inhibition and genetic hepatic-specific inhibition. In pharmacological inhibition studies, male C57BL/6 mice were randomized to a high-fat (60% kcal fat) or normal (16% kcal fat) diet for 6–7 wk and pretreated with Stattic before inducing sepsis by cecal ligation and puncture. In genetic inhibition studies, mice were randomized by genotype before induction of sepsis. To investigate obesity in mice with hepatic-specific STAT3 inhibition, we randomized mice to a high-fat or normal diet as described above for 6 mo before induction of sepsis. Body composition was analyzed using EchoMRI. We found that systemic STAT3 inhibition by Stattic resulted in an increased inflammatory response and that obesity amplified this effect. We also found that genetically inhibiting STAT3 in the liver resulted in higher mortality, increased inflammation, and liver injury. High-fat-fed mice with hepatic STAT3 inhibition gained more weight and had more fat than control mice on the same diet, and obesity increased neutrophil infiltration to the liver of these mice during sepsis. In conclusion, STAT3 plays an important regulatory role in the inflammatory response during sepsis, and obesity contributes to the dysregulated response observed when STAT3 is inhibited.
Keywords: inflammation, liver, obesity, sepsis, STAT3
INTRODUCTION
Sepsis is a life-threatening syndrome in which the body responds inappropriately to infection, causing damage to its vital organs (19). Mortality rate is very high, with approximately one in four patients with severe sepsis dying during their hospital course (13). During sepsis, both pro- and anti-inflammatory pathways are activated in a dysregulated way as a response to infection (19). One of these pathways is the Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway (11). The STAT3 pathway plays a central role in inflammatory signaling cascades, and its activation plays a critical role in host-bacterial interactions (12). STAT3 activation during sepsis is crucial to survival, as it appears to modulate the inflammatory response (2). Cytokines like interleukin (IL)-6, IL-10, and IL-11 can activate phosphorylation of tyrosine and serine residues of STAT3 by JAK (6). Activated STAT3, in turn, translocates into the nucleus, where it binds to specific promoter sequences and regulates transcription of target genes.
In the United States, ~17% of children and over one-third of adults are considered obese (15). Obesity is one of the world’s greatest health challenges, contributing to chronic diseases and premature mortality while burdening health services. Morbid obesity is independently associated with increased sepsis risk (20). In obesity, adipokines such as leptin and IL-6 also activate STAT3 and initiate its cell signaling pathway (6). Previous work from our laboratory has demonstrated an increased mortality rate in obese septic mice compared with nonobese septic mice (9) and suggests that obesity amplifies sepsis-induced liver inflammation and increases STAT3 expression in the liver (10).
Because both obesity and sepsis are major health problems, and both are affected by the STAT pathway, we explored the impact of obesity on the STAT3 pathway in mice during sepsis. We hypothesized that inhibiting STAT3 would worsen inflammation during sepsis. We investigated the role of STAT3 in obesity-associated inflammation by use of two approaches during sepsis: a generalized pharmacological approach to inhibit STAT3 and a specific deletion of hepatic STAT3.
MATERIALS AND METHODS
Animal model.
Our investigations conformed to the Guide for the Care and Use of Laboratory Animals (14) and were approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Medical Center (CCHMC). Mice were housed in the animal facility at CCHMC. Food and water were provided ad libitum.
Pharmacological STAT3 inhibition in obese or nonobese mice.
Male C57BL/6 mice at 6 wk of age from Charles River Laboratories International, (Wilmington, MA) were randomized to high-fat diet (HFD; TestDiet 58Y1: 60% kcal provided by fat) or normal diet (13) (Formulab no. 5008, 16% kcal provided by fat) for 6–7 wk. After the dietary intervention, mice were randomized by injection of Stattic (6-nitrobenzo[b]thiophene 1) 25 mg/kg; EMD Millipore, Billerica, MA) or vehicle (5 mg/ml DMSO), via intraperitoneal injection, 30 min before cecal ligation and puncture (CLP; described below in the next sections), and tissue was harvested 18 h after CLP. Control mice did not undergo CLP and did not receive vehicle or Stattic treatment. Stattic is a highly specific drug that inhibits STAT3 phosphorylation at Tyr705 by targeting the STAT3-SH2 domain.
Survival study in mice treated with Stattic.
Male C57BL6 mice at 6 wk of age were randomized to HFD or normal diet for 6 wk and at 12 wk of age underwent CLP with double puncture using a 22-gauge needle. Mice received Stattic (25 mg/kg) or vehicle 30 min before CLP. Animals were fluid resuscitated with normal saline (1 ml) injected subcutaneously and received imipenem (25 mg/kg body wt) for antimicrobial coverage. Mice were monitored for survival.
Polymicrobial sepsis model.
Mice underwent CLP to induce polymicrobial sepsis, as previously described (8, 22). Mice were anesthetized with isoflurane, the abdomen was opened, and the cecum was exteriorized and ligated with a 6.0 ligature at its base. For Stattic-treated mice and their controls, a double-puncture technique was performed with a 23-gauge needle, and fecal material was expressed into the peritoneum. For hepatic-specific STAT3 knockout (H-STAT3 KO) mice and littermate controls, a single-puncture technique was used to induce a less severe model of sepsis. The abdominal incision was closed with liquid topical adhesive. Animals were fluid resuscitated with normal saline (1 ml) injected subcutaneously and received imipenem (25 mg/kg body wt) for antimicrobial coverage. To minimize pain, buprenorphine (0.05 mg/kg body wt) was administered subcutaneously after surgery. Tissue and plasma were harvested from mice at 0 or 18 h after CLP.
H- STAT3 KO mice.
To investigate the effects of STAT3 inhibition, genetically modified Cre-lox mice were generated to have a specific deletion of H-STAT3. Albumin promoter Cre transgenic mice [B6.Cg-Tg(Alb-cre)21Mgn/J; Jackson Laboratory, Bar Harbor, ME] were crossed with STAT3flox/flox (B6.129S1-Stat3tm1Xyfu/J) mice. The resulting genetically modified mice had deletions on exons 18, 19, and 20, which coincide with the SH2 domain important for phosphorylation of STAT3. The two genotypes used for experimentation were STAT3f/f_Alb-Cre+ (H-STAT3 KO) and STAT3f/WT_Alb-Cre+ (control). These genotypes were confirmed by polymerase chain reaction (PCR). Male and female H-STAT3 KO and control mice were weighed once a week, and at 13 wk of age they underwent CLP as described above.
Body composition.
To quantify fat mass, we analyzed body composition using NMR imaging by EchoMRI body composition analyzers, as previously described (EchoMRI, Houston, TX) (10).
Survival study.
In separate studies, H-STAT3 KO mice ~4 mo of age underwent CLP to induce polymicrobial sepsis and were monitored for survival for 72 h. Animals were fluid resuscitated with normal saline (1 ml) injected subcutaneously and received imipenem (25 mg/kg body wt) for antimicrobial coverage. To minimize pain, buprenorphine (0.05 mg/kg body wt) was administered subcutaneously after surgery. Mice were monitored every 6–8 h over the 72-h study. An injury severity scale was used to assess the severity of symptoms. Mice were graded on a scale of 0 to 3 for lethargy, diarrhea, ocular discharge, and piloerection. A score of 0 referred to no signs of illness, with 1, 2, and 3 referring to minimum, mild, and severe symptoms. The scores were tallied for the four categories for each mouse, and if the total score was 8 or higher, the mouse was euthanized and declared dead. STAT3f/WT_Alb-Cre+, STAT3f/f, and STAT3f/WT_Alb-Cre- mice were used as controls for H-STAT3 KO mice.
H-STAT3 KO mice on HFD or normal diet.
To investigate the effects of high-fat feeding in mice with hepatic STAT3 inhibition, we placed 6- to 8-wk-old H-STAT3 KO mice and their littermates on a HFD or normal diet as described above. Body weights were monitored once a week throughout the diet phase. Mice remained on the diet for 6 mo, and body composition was analyzed using EchoMRI.
Myeloperoxidase activity.
Myeloperoxidase (MPO) activity was determined as an index of neutrophil accumulation in liver and lung, as previously described (23).
Alanine aminotransferase assays.
Plasma alanine aminotransferase (ALT) was measured using standard enzyme assay kits (Sekisui Diagnostics, Lexington, MA).
Adipokine and cytokine analysis.
Plasma levels of leptin, IL-6, and tumor necrosis factor-α (TNFα) were measured with a multiplex assay kit (EMD Millipore) using the manufacturer’s protocol.
Quantitative RT-PCR.
mRNA was reversely transcribed to cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Grand Island, NY). Comparative quantitative (q)PCR was performed using TaqMan Gene Expression Master Mix (Applied Biosystems) with the following primers and probes: GAPDH (Mm99999915_gl) and TNFα (Mm00443258_ml). The reaction was performed and analyzed using a qPCR system (QuantStudio-6Flex machine, Applied Biosystems). Samples were run in duplicate. The change in TNFα gene expression was normalized to GAPDH.
Subcellular fractionation and nuclear protein extraction.
Tissue samples were prepared as previously described (9). The amount of protein was quantified by Bradford assay.
Western blot analysis.
The Invitrogen NuPAGE gel electrophoresis system (Invitrogen) was used for all Western blotting. NuPAGE 10% Bis-Tris gels were used with NuPAGE MOPS buffer and Invitrogen Novex Mini-Cell, Bio-Rad PowerPac 300. Membranes imaged using the Bio-Rad ChemiDoc XRS+ gel documentation system and analyzed using ImageLab v. 5.1 software (Bio-Rad, Hercules, CA). The following antibodies were used for nuclear extracts: STAT3, phospho (p-)STAT3 (Tyr705) (Cell Signaling Technology, Danvers, MA), and β-actin (Santa Cruz Biotechnology, Dallas, TX).
Statistical analysis.
Data were analyzed using SigmaStat for Windows v. 13 (SysStat Software, San Jose, CA). For data comparison among only two groups, statistical analysis was performed using Student’s t-test. For data comparison among three or more groups, statistical analysis was performed using two-way ANOVA with the Holm-Sidak method for parametric data and the Kruskal-Wallis ANOVA with the Dunn post hoc test for nonparametric data. Data are expressed as means and SD for parametric data and median and interquartile range for nonparametric data in the text and figures. Survival analysis was performed by log rank test. A value of P ≤ 0.05 was considered significant.
RESULTS
Pharmacological inhibition of STAT3 phosphorylation increases lung and liver neutrophil infiltration in obese septic mice.
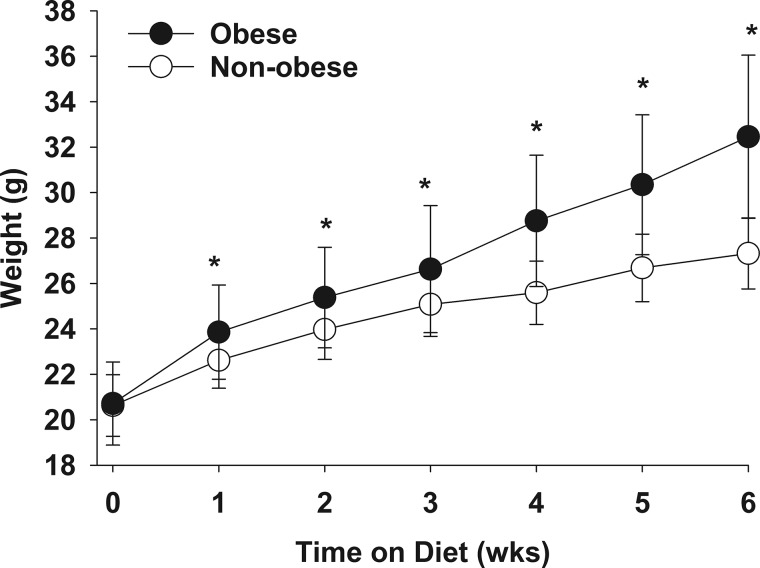
Six-week-old C57BL/6 mice were placed on a HFD or normal diet and weighed once a week. There was no difference in weight between the two groups at baseline (before the dietary intervention). HFD-fed mice had higher weights than normal diet-fed mice starting 1 wk after initiation of the diet, and weights remained higher after 6 wk of feeding (Fig. 1). On the basis of these results, we refer to the HFD-fed mice as “obese” and normal diet-fed mice as “nonobese.”
Fig. 1.
Mice gained significantly more weight on a high-fat diet than on a normal diet. Six-week-old mice were placed on normal or high-fat diet and weighed once a week for 6 wk. Data are presented as means and SD and were analyzed by t-test. *P ≤ 0.05 vs. normal-diet group (n = 31–32/group).
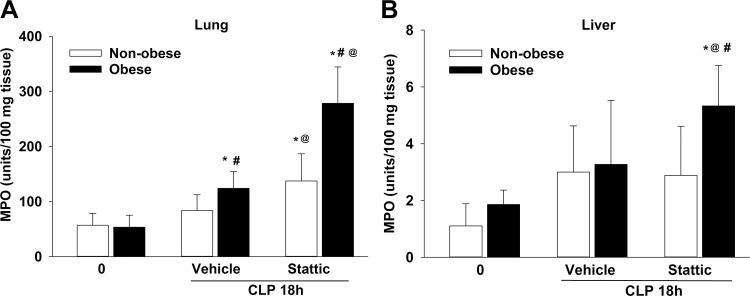
Previous data from our laboratory demonstrated that obesity enhances hepatic STAT3 activation (10). To determine the importance of STAT3 activation in organ injury of obese mice during sepsis, we treated mice with the STAT3 inhibitor Stattic or vehicle and measured myeloperoxidase activity as a marker of neutrophil infiltration in the liver and lung after CLP. Lung MPO activity was higher in obese vehicle-treated mice after CLP compared with baseline (CLP 0 h: 124 ± 31 vs. 53 ± 22 U/100 mg tissue, P ≤ 0.05) and compared with the nonobese vehicle-treated mice after CLP (84 ± 29, P ≤ 0.05) (Fig. 2A). Both Stattic-treated groups had higher lung MPO activity compared with vehicle and baseline. Lung MPO activity was higher in Stattic-treated obese septic mice than in Stattic-treated nonobese septic mice (278 ± 66 and 137 ± 50 U/100 mg tissue, respectively, P ≤ 0.05). A dietary effect was also evident in the liver, in which MPO activity was higher in obese Stattic-treated mice during sepsis compared with all other treatment groups (Fig. 2B).
Fig. 2.
Effects of pharmacological signal transducer and activator of transcription 3 (STAT3) inhibition on lung (A) and liver (B) neutrophil infiltration during sepsis. Lung and liver myeloperoxidase (MPO) activity was determined in STAT3 inhibitor Stattic-treated nonobese and obese mice at 0 and 18 h after cecal ligation and puncture (CLP). Data represent means and SD and were analyzed by two-way ANOVA. *P ≤ 0.05 vs. 0-h CLP by diet; #P ≤ 0.05 vs. nonobese mice within treatment group; @P ≤ 0.05 vs. vehicle by diet. Open bars, nonobese; filled bars, obese (n = 7–10/group).
Pharmacological inhibition of STAT3 phosphorylation does not influence liver injury during sepsis.
To determine the effect of STAT3 inhibition on hepatic injury, we examined plasma ALT levels. Septic vehicle-treated obese and nonobese mice had similar ALT levels (102 ± 45 and 118 ± 33 U/l, respectively) compared with septic Stattic-treated obese and nonobese mice (99 ± 38 and 114 ± 21 U/l, respectively). There was no detectable dietary or treatment effect. These results suggest that STAT3 inhibition does not cause hepatocyte injury.
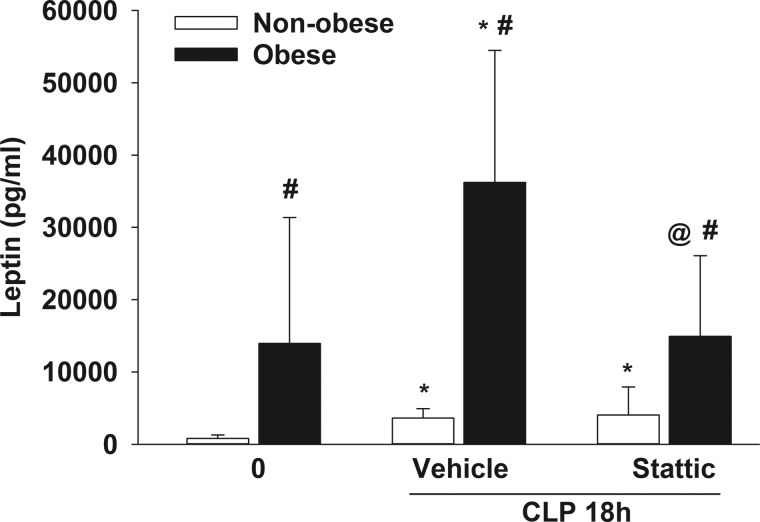
Pharmacological inhibition of STAT3 phosphorylation decreases plasma leptin in obese mice during sepsis.
Because leptin is an activator of STAT3 (6), this adipokine was analyzed in the plasma of Stattic-treated and vehicle-treated mice with or without dietary intervention. As expected, obese mice had higher levels of leptin at baseline than nonobese mice (P ≤ 0.05; Fig. 3). Leptin increased in both nonobese and obese vehicle-treated mice during sepsis but was ~10-fold higher in obese mice. Stattic-treated obese septic mice had lower leptin levels than vehicle-treated obese septic mice, but Stattic treatment had no effect on leptin levels in nonobese mice.
Fig. 3.
Signal transducer and activator of transcription 3 (STAT3) inhibitor Stattic treatment decreased leptin in obese mice during sepsis. Plasma leptin was measured in obese and nonobese mice with vehicle or Stattic treatment at 0 and 18 h after cecal ligation and puncture (CLP). Data represent means and SD and were analyzed by two-way ANOVA. *P ≤ 0.05 vs. 0-h CLP by diet; #P ≤ 0.05 vs. nonobese mice within treatment group; @P ≤ 0.05 vs. vehicle by diet. Open bars, nonobese; filled bars, obese (n = 7–10/group).
Pharmacological inhibition of STAT3 increases mortality.
To determine the effects of hepatic STAT3 inhibition on outcomes during sepsis, we performed a survival study in mice with pharmacological inhibition of STAT3 or vehicle-treated mice. At 72 h after CLP, Stattic-treated mice had significantly lower survival compared with vehicle-treated mice (0 vs. 11%, P = 0.021 by log rank test), but mortality was high in both groups.
H-STAT3 KO mice have higher mortality during sepsis.
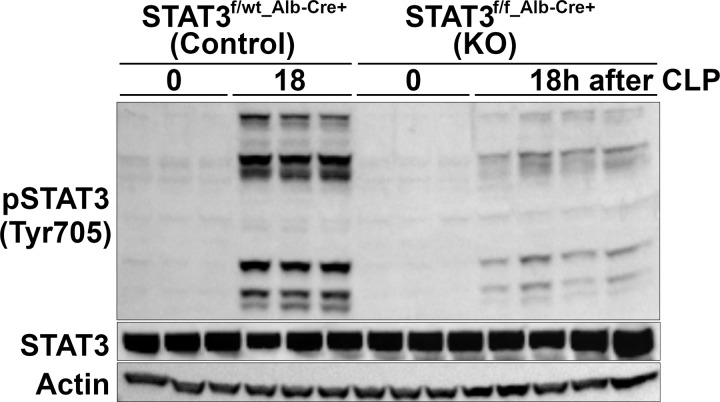
Previous work from our laboratory demonstrated that obesity enhances sepsis-induced liver inflammation and injury in mice and that this may be, in part, secondary to hepatic STAT3 activation (10). We sought to investigate whether hepatic STAT3 inhibition would worsen inflammation and outcomes during sepsis, especially in obese mice. We generated Cre-lox mice with a hepatocyte-specific STAT3 inhibition. We confirmed hepatic STAT3 inhibition in mice with and without sepsis (Fig. 4). As shown in Fig. 4, there was an increase in phosphorylation of STAT3 at Tyr705 in septic control (STAT3f/wt Alb-Cre+) mice, whereas STAT3 phosphorylation was inhibited in H-STAT3 KO mice.
Fig. 4.
Hepatic expression of phosphorylated signal transducer and activator of transcription 3 (p-STAT3 (Tyr705) in knockout (KO) mice and littermate controls. Representative Western blot analysis for hepatic p-STAT3 (Tyr705), STAT3, and actin in KO mice (STAT3f/f Alb-Cre+) and littermate controls (STAT3f/wt Alb-Cre+) at 0 and 18 h after cecal ligation and puncture (CLP) (n = 3–4/group).
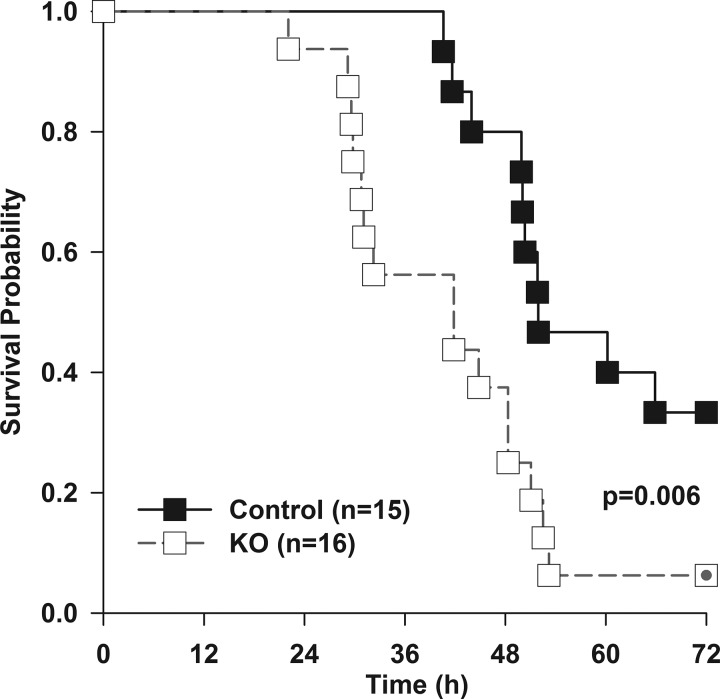
Given the high mortality in Stattic- and vehicle-treated mice, we chose a less severe model of sepsis to investigate effects in H-STAT3 KO mice. Survival in H-STAT3 KO mice was significantly lower than that of control mice, with 6% (1/16) of H-STAT3 KO mice alive compared with 33% (5/15) alive in the control group (Fig. 5). To determine the factors that contributed to early deaths in the H-STAT3 KO mice, we focused on changes that occurred early in sepsis, at 18 h after CLP.
Fig. 5.
Hepatic signal transducer and activator of transcription 3 knockout (H-STAT3 KO) mice have increased mortality during sepsis. H-STAT3 KO mice (STAT3f/f Alb-Cre+) and littermate controls (STAT3f/wt Alb-Cre+) at 15–20 wk of age underwent cecal ligation and puncture and were monitored for survival. Mice were censored at 72 h. Survival analysis was determined by log-rank test (n = 15–16/group).
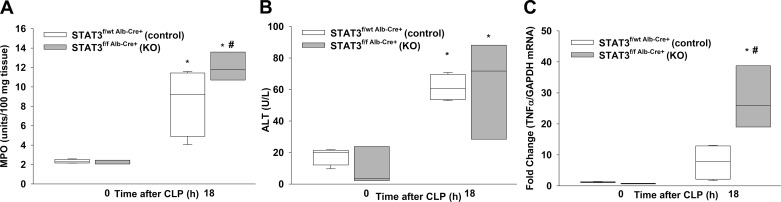
Hepatic STAT3 inhibition increases liver inflammation and injury during sepsis.
We investigated the effects of inhibiting STAT3 activation on liver inflammation by measuring MPO activity in the liver of H-STAT3 KO mice during sepsis. We did not observe a difference in baseline liver MPO levels between H-STAT3 KO mice and littermate controls (Fig. 6A). After CLP, both H-STAT3 KO and control mice had increased levels of MPO activity. However, H-STAT3 KO mice had significantly higher levels of MPO activity than controls after CLP. To determine whether hepatic STAT3 inhibition affected liver injury, we measured plasma ALT levels. ALT levels were higher in septic mice, but there was no difference between genotypes (Fig. 6B). These data suggest that sepsis induces hepatic injury and that inhibiting STAT3 activation in the liver worsens hepatic inflammation and injury during sepsis. We investigated the effects of hepatic STAT3 inhibition on hepatic inflammation by analyzing TNFα gene expression in the liver. Septic H-STAT3 KO mice had significantly higher hepatic TNFα expression than control septic mice and compared with baseline (Fig. 6C).
Fig. 6.
Effects of hepatic signal transducer and activator of transcription 3 (STAT3) inhibition on liver inflammation and injury. A: liver neutrophil infiltration as measured by myeloperoxidase (MPO) activity. B: plasma alanine amonotransfrase (ALT) levels. C: relative liver tumor necrosis factor-α (TNFα) gene expression as measured by RT-PCR for knockout (KO; STAT3f/f Alb-Cre+) and littermate controls (STAT3f/wt Alb-Cre+) at 0 and 18 h after cecal ligation and puncture (CLP). Vertical box represents 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values; whiskers represent 10th and 90th percentiles. White box, control; gray box, KO. Data were analyzed by two-way ANOVA (n = 3–4/group). *P ≤ 0.05 vs. CLP 0 h within genotype; #P ≤ 0.05 vs. control at time after CLP.
Hepatic STAT3 inhibition does not affect plasma systemic cytokine levels during sepsis.
We analyzed plasma IL-6 and TNFα levels in H-STAT3 KO and control mice during sepsis. We found that both H-STAT3 KO mice and control mice had higher levels of IL-6 and TNFα in the plasma during sepsis compared with baseline, but there was no difference between the two genotypes (data not shown).
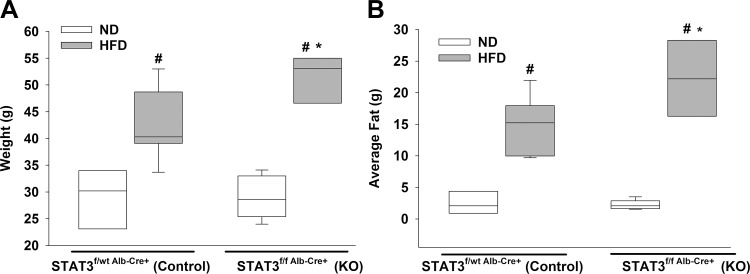
Mice with hepatic STAT3 inhibition gain more weight and fat mass on high-fat diet.
Our laboratory is interested in the effects of obesity during sepsis, so we investigated the impact of long-term HFD or normal diet feeding on H-STAT3 KO and control mice. Mice were randomized to the dietary intervention for 6 mo. We found that both HFD-fed control and HFD-fed H-STAT3 KO mice had higher weights and more average fat compared with normal diet-fed mice within the same genotype (Fig. 7). We also observed that HFD-fed H-STAT3 KO mice had higher weights and more fat than control mice on the same diet. These findings suggest that hepatic STAT3 plays a role in adipose tissue accumulation although the exact mechanisms are unknown.
Fig. 7.
Effects of high-fat diet (HFD) feeding on hepatic signal transducer and activator of transcription 3 knockout (H-STAT3 KO) mice. Weight (A) and fat mass (B) of KO (STAT3f/f Alb-Cre+) and littermate controls (STAT3f/wt Alb-Cre+) were measured by EchoMRI at the conclusion of dietary intervention. Vertical box represents 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values; whiskers represent 10th and 90th percentiles. White box, normal diet ND); gray box, HFD. Data were analyzed by two-way ANOVA (n = 3–7/group). *P ≤ 0.05 vs. control within diet; #P ≤ 0.05 vs. ND within genotype.
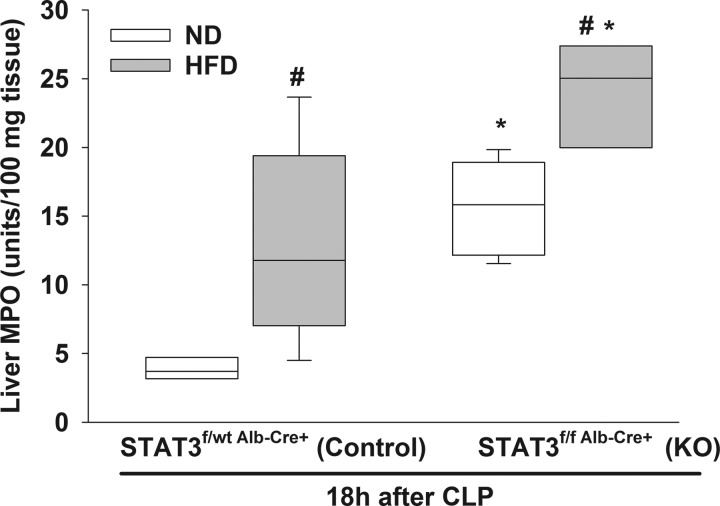
High-fat feeding increases liver neutrophil infiltration in H-STAT3 KO mice during sepsis.
To investigate the combined effect of high-fat feeding and hepatic STAT3 inhibition, we subjected H-STAT3 KO mice and littermate controls to HFD or normal diets. After the dietary intervention, mice underwent CLP to induce polymicrobial sepsis. Liver MPO activity was higher in HFD-fed septic control mice compared with normal diet-fed septic control mice (Fig. 8). We also found that liver MPO activity was significantly higher in HFD-fed septic H-STAT3 KO mice compared with both HFD-fed septic littermate controls and normal diet-fed septic H-STAT3 KO mice. Additionally, leptin levels were higher in obese control (27,659 ± 15,825 pg/ml) and obese H-STAT3 KO septic mice (28,945 ± 16,807 pg/ml) compared with nonobese control and nonobese H-STAT3 KO septic mice (4,438 ± 2,736 and 6,818 ± 2,397 pg/ml, respectively). However, there was no difference between the two genotypes.
Fig. 8.
Obesity enhances neutrophil infiltration to the liver in hepatic signal transducer and activator of transcription 3 knockout (H-STAT3 KO) mice during sepsis. Liver myeolperoxidase (MPO) activity was measured in normal (ND) and high-fat diet (HFD) control (STAT3f/wt Alb-Cre+) and KO (STAT3f/f Alb-Cre+) mice during sepsis. Vertical box represents 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values; whiskers represent 10th and 90th percentiles. White box, ND; gray box, HFD. Data were analyzed by two-way ANOVA (n = 3–6/group). *P ≤ 0.05 vs. control within diet; #P ≤ 0.05 vs. ND within genotype.
DISCUSSION
To understand the role of STAT3 and the effect of obesity during sepsis, we utilized two methods of STAT3 inhibition, pharmacological inhibition and genetic liver-specific inhibition. We found that pharmacological STAT3 inhibition and specific hepatic STAT3 inhibition resulted in higher mortality, increased inflammation, and liver injury during sepsis. Furthermore, obesity amplified the inflammatory response of mice deficient in activated STAT3.
In the current studies, pharmacological inhibition of STAT3 using Stattic increased the inflammatory response in septic mice, as evidenced by increased neutrophil infiltration in the lung and liver of Stattic-treated septic mice compared with vehicle-treated septic mice. This is in contrast to a study by Pena et al. (17), who found that STAT3 inhibition prevented a hyperactive systemic immune response and that pretreatment with Stattic rescued mice during sepsis. Pena et al. used BALB/c mice, whereas we used C57BL/6 mice; therefore, the differences in STAT3 activation may be strain specific.
The liver plays an important role in the acute-phase response (3) and is a target of multiple organ failure during sepsis (21). We therefore focused on the role of hepatic STAT3 during sepsis. We found that with inhibition of activated hepatic STAT3 neutrophil infiltration and liver injury worsened during sepsis. We also observed higher mortality in H-STAT3 KO mice compared with control mice. Our results align with findings by Sakamori et al. (18), who also observed an increase in inflammation, liver injury, and mortality in H-STAT3 KO mice (18). However, Sakamori et al. saw a more pronounced systemic proinflammatory cytokine response in H-STAT3 KO mice during sepsis compared with our study. We found that, although sepsis causes an increase in systemic proinflammatory cytokines, the hepatocyte-specific inhibition of STAT3 did not have a significant systemic effect. One possible explanation for why H-STAT3 KO mice in our study had a more site-specific inflammatory response compared with a systemic response observed in the study of Sakamori et al. may be related to the difference in duration of sepsis, since Sakamori et al. used a longer duration of sepsis.
We observed an increase in hepatic TNFα expression in H-STAT3 KO mice compared with control mice during sepsis. Although an increase in neutrophil infiltration in obese mice during sepsis was observed in previous studies in our laboratory (10), it is evident that STAT3 inhibition enhances this inflammatory response. Obese Stattic-treated mice had more neutrophil infiltration in the liver during sepsis than nonobese Stattic-treated mice as well as the corresponding vehicle-treated groups. We further confirmed this in studies with genetically altered mice deficient in active STAT3 in the liver. We found with hepatic STAT3 inhibition that neutrophil infiltration increased in the liver during sepsis and was affected by obesity, as obese H-STAT3 KO mice had increased neutrophil infiltration in the liver compared with other treatment groups. The increase in hepatic TNFα coupled with the increase in neutrophil infiltration to the liver suggests that, when deficient in activated STAT3, the liver experiences a hyperactive inflammatory response during sepsis. These findings support the conclusion that STAT3 activation plays an important role in the regulation of inflammation during sepsis and is affected by obesity.
Our current study is distinct from others’ in that we tested whether obesity was affected by STAT3 activation. Previous work in our laboratory showed that obese septic mice have more inflammation, organ injury, and STAT3 expression in the liver (10). We found that HFD-fed H-STAT3 KO mice gained more weight and had more adipose tissue than HFD-fed control mice. These findings suggest that activated STAT3 plays a regulatory role in adiposity. A study by Inoue et al. (7) saw similar results in which inhibiting hepatic STAT3 activation led to higher weights, more fat mass, and insulin resistance. They also observed that these differences were ameliorated when STAT3 was restored. Although leptin was not a focus of their study, it is possible that an increase in leptin after feeding was responsible for the increased STAT3 activation.
One possible explanation for the mechanism by which adiposity is altered in H-STAT3 KO mice is the interaction between STAT3 and leptin. Leptin is a hormone that activates the JAK/STAT3 pathway and leads to phosphorylation of STAT3 (4). Leptin also plays an important role in metabolism and weight gain (16) as well as the regulation of glucose (5) and overall food consumption (1). Leptin helps suppress appetite and is found in higher concentrations in obese mice (9). Buettner et al. (4) found that STAT3 signaling is crucial to the regulation of leptin and its various roles in feeding and metabolism. In their study, an injection of leptin resulted in suppression of appetite and reduced body weight in rats. However, an injection of leptin with a STAT3 peptide inhibitor eliminated the functionality of leptin with no change in appetite or body weight. Taken together with our findings that STAT3 inhibition reduced leptin expression in obese mice during sepsis, it suggests that STAT3 and leptin are involved in weight control and are affected during sepsis. It is unclear whether H-STAT3 KO mice have greater food intake or whether there are differences in energy expenditure. Future studies in our laboratory will focus on these etiologies.
In conclusion, the results of our study show that both STAT3 inhibition and obesity play an important role in the inflammatory response during sepsis. STAT3 inhibition leads to an increased inflammatory response in mice, and obesity amplifies this effect. STAT3 inhibition increases organ injury and mortality in mice after CLP. Future studies are necessary to understand the metabolic impact on STAT3 inhibition during sepsis.
GRANTS
This work was supported by NIH Grants R01 GM-126551 (J. Kaplan) and P30 DK-078392.
DISCLOSURES
J. Kaplan reports financial support to Cincinnati Children’s Hospital Medical Center for work on the Data and Safety Monitoring Board of a clinical trial (Eli Lilly) not related in any way to the work in this paper. No conflicts of interest, financial or otherwise, are declared by the other authors.
AUTHOR CONTRIBUTIONS
J.K. conceived and designed research; J.K. analyzed data; J.K. prepared figures; L.W. and H.S. performed experiments; L.W. and J.K. interpreted results of experiments; L.W., I.A., and J.K. drafted manuscript; L.W., I.A., and J.K. edited and revised manuscript; L.W., I.A., and J.K. approved final version of manuscript.
REFERENCES
- 1.Ahima RS, Flier JS. Leptin. Annu Rev Physiol 62: 413–437, 2000. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 2.Andrejko KM, Chen J, Deutschman CS. Intrahepatic STAT-3 activation and acute phase gene expression predict outcome after CLP sepsis in the rat. Am J Physiol Gastrointest Liver Physiol 275: G1423–G1429, 1998. doi: 10.1152/ajpgi.1998.275.6.G1423. [DOI] [PubMed] [Google Scholar]
- 3.Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol 91: 496–505, 2012. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab 4: 49–60, 2006. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 1: 63–72, 2005. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 93: 6231–6235, 1996. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue H, Ogawa W, Ozaki M, Haga S, Matsumoto M, Furukawa K, Hashimoto N, Kido Y, Mori T, Sakaue H, Teshigawara K, Jin S, Iguchi H, Hiramatsu R, LeRoith D, Takeda K, Akira S, Kasuga M. Role of STAT-3 in regulation of hepatic gluconeogenic genes and carbohydrate metabolism in vivo. Nat Med 10: 168–174, 2004. doi: 10.1038/nm980. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan J, Nowell M, Chima R, Zingarelli B. Pioglitazone reduces inflammation through inhibition of NF-kappaB in polymicrobial sepsis. Innate Immun 20: 519-528, 2014. doi: 10.1177/1753425913501565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan JM, Nowell M, Lahni P, O’Connor MP, Hake PW, Zingarelli B. Short-term high-fat feeding increases organ injury and mortality after polymicrobial sepsis. Obesity (Silver Spring) 20: 1995–2002, 2012. doi: 10.1038/oby.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan JM, Nowell M, Lahni P, Shen H, Shanmukhappa SK, Zingarelli B. Obesity enhances sepsis-induced liver inflammation and injury in mice. Obesity (Silver Spring) 24: 1480–1488, 2016. doi: 10.1002/oby.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy DE, Lee CK. What does Stat3 do? J Clin Invest 109: 1143–1148, 2002. doi: 10.1172/JCI0215650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu R, Zhang YG, Sun J. STAT3 activation in infection and infection-associated cancer. Mol Cell Endocrinol 451: 80–87, 2017. doi: 10.1016/j.mce.2017.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence 5: 4–11, 2014. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals. 8th edition. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 15.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311: 806–814, 2014. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 17.Peña G, Cai B, Liu J, van der Zanden EP, Deitch EA, de Jonge WJ, Ulloa L. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol 40: 2580–2589, 2010. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K, Akira S, Hayashi N. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology 46: 1564–1573, 2007. doi: 10.1002/hep.21837. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HE, Griffin R, Judd S, Shapiro NI, Safford MM. Obesity and risk of sepsis: a population-based cohort study. Obesity (Silver Spring) 21: E762–E769, 2013. doi: 10.1002/oby.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang P, Chaudry IH. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol Regul Integr Comp Physiol 270: R927–R938, 1996. doi: 10.1152/ajpregu.1996.270.5.R927. [DOI] [PubMed] [Google Scholar]
- 22.Zingarelli B, Piraino G, Hake PW, O’Connor M, Denenberg A, Fan H, Cook JA. Peroxisome proliferator-activated receptor delta regulates inflammation via NF-kappaB signaling in polymicrobial sepsis. Am J Pathol 177: 1834–1847, 2010. doi: 10.2353/ajpath.2010.091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zingarelli B, Sheehan M, Hake PW, O’Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol 171: 6827–6837, 2003. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]