Abstract
Renal fibrosis is the pathological hallmark of chronic kidney disease (CKD) and manifests as glomerulosclerosis and tubulointerstitial fibrosis. Reactive oxygen species contribute significantly to renal inflammation and fibrosis, but most research has focused on superoxide and hydrogen peroxide (H2O2). The animal heme peroxidases myeloperoxidase (MPO), eosinophil peroxidase (EPX), and peroxidasin (PXDN) uniquely metabolize H2O2 into highly reactive and destructive hypohalous acids, such as hypobromous and hypochlorous acid. However, the role of these peroxidases and their downstream hypohalous acids in the pathogenesis of renal fibrosis is unclear. Our study defines the contribution of MPO, EPX, and PXDN to renal inflammation and tubulointerstitial fibrosis in the murine unilateral ureteral obstruction (UUO) model. Using a nonspecific inhibitor of animal heme peroxidases and peroxidase-specific knockout mice, we find that loss of EPX or PXDN, but not MPO, reduces renal fibrosis. Furthermore, we demonstrate that eosinophils, the source of EPX, accumulate in the renal interstitium after UUO. These findings point to EPX and PXDN as potential therapeutic targets for renal fibrosis and CKD and suggest that eosinophils modulate the response to renal injury.
Keywords: eosinophil peroxidase, hypohalous acid, peroxidasin, myeloperoxidase, reactive oxygen species, renal fibrosis, unilateral ureteral obstruction
INTRODUCTION
Renal fibrosis, the aberrant accumulation of extracellular matrix, is the pathological hallmark of chronic kidney disease (CKD) and manifests as glomerulosclerosis and tubulointerstitial fibrosis. Podocyte loss and dysfunction in the glomerulus and tubular epithelial cell atrophy and loss also contribute to CKD (4). Renal inflammation, the accumulation of interstitial leukocytes, often accompanies tubulointerstitial fibrosis, and a complex interplay between renal tubular epithelial cells, interstitial fibroblasts, and leukocytes is thought to drive the initiation and progression of renal cell loss and fibrosis (15, 30).
In addition to a host of growth factors, cytokines, and signaling molecules, reactive oxygen species (ROS) contribute to renal cell injury, inflammation, and fibrosis (35). Much of the research on ROS has focused on superoxide (O2•−) and hydrogen peroxide (H2O2) primarily generated by mitochondrial metabolism and NADPH/dual oxidase family members within renal epithelial cells, fibroblasts, and leukocytes. O2•− and H2O2 oxidatively modify proteins and lipids to alter their function and cell behavior in an autocrine or paracrine manner to promote renal fibrosis (9, 35). Animal heme peroxidases uniquely metabolize H2O2 into hypohalous acids, such as hypobromous acid (HOBr) and hypochlorous acid (HOCl), which are more reactive and destructive oxidants compared with H2O2 (10). Thus, conversion of H2O2 to HOBr and HOCl significantly amplifies downstream oxidative damage and signaling. However, the role of these hypohalous acids in kidney injury is unclear. Most work on hypohalous acids in renal disease has focused on myeloperoxidase (MPO), found within neutrophils and macrophages, as an autoantibody target and effector mechanism in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (7, 20). In ANCA vasculitis, activated neutrophils release MPO, which generates HOCl. HOCl oxidatively damages glomerular basement membrane proteins and promotes glomerular injury (21). Because increased levels of MPO and chlorinated proteins are found in murine unilateral ureteral obstruction (UUO) and 5/6th nephrectomy, and human membranous and diabetic nephropathy, MPO may play a broader role in renal inflammation and fibrosis (5, 11, 16, 24, 26).
Aside from MPO, the animal heme peroxidases eosinophil peroxidase (EPX) and peroxidasin (PXDN) may also contribute to renal cell injury and fibrosis. EPX, found within eosinophils, generates HOBr, which may cause oxidative injury similar to MPO-derived HOCl (40). Although interstitial eosinophils are found in human CKD, little is known on whether eosinophils or EPX contribute to renal disease pathogenesis (8). Our laboratory has recently identified peroxidasin (PXDN), a novel peroxidase found within basement membranes in nearly all tissues, as an endogenous source of HOBr (3, 29). In normal tissue development, PXDN generates HOBr to form sulfilimine cross-links within the collagen IV network of basement membranes (3). These cross-links reinforce basement membranes and contribute to their mechanical stability (2, 3, 39). However, recent work has found increased PXDN deposition within accumulating interstitial matrix in the UUO injury model, raising the question of whether this peroxidase promotes renal fibrosis after injury (36).
In this work, we defined the contribution of MPO, EPX, and PXDN to the pathogenesis of renal inflammation and fibrosis in the murine UUO model. We found that nonspecific pharmacological inhibition of peroxidases and genetic loss of Epx and Pxdn, but not Mpo, reduced renal fibrosis. In support of a role for EPX, we found increased renal eosinophil numbers after UUO, raising the possibility that eosinophils may contribute to renal fibrosis.
MATERIALS AND METHODS
Animal models.
The Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved all animal procedures that were consistent with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Peroxidasin knockout (Pxdn-KO) mice were generated as previously stated (2). Wild-type (WT) C57BL/6J and myeloperoxidase knockout (Mpo-KO) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Eosinophil peroxidase knockout (Epx-KO) mice were kindly provided by Dr. James J. Lee (Mayo Clinic, Scottsdale, AZ). All knockout mice were backcrossed to the C57BL/6J background for >10 generations. WT and knockout mice were used at 8–12 wk of age for all studies.
Unilateral ureteral obstruction.
Experimental UUO was performed on Pxdn-KO, Mpo-KO, Epx-KO, and WT C57BL/6J male and female mice. WT mice were given daily intraperitoneal injections of phloroglucinol (PHG; 1 mg/kg) or saline solution beginning 1 day before surgery until the mice were sacrificed either 3 or 7 days postsurgery. During surgery, the left ureter of each animal was ligated at the ureteropelvic junction with nonabsorbable surgical sutures. Sham animals had their kidneys externalized without any manipulation of the ureter. Kidneys were harvested either 3 or 7 days postsurgery.
Renal histology.
Mouse kidneys were fixed for 24 h in 3.7% paraformaldehyde in acidified phosphate buffer (40 mM sodium phosphate, 1% acetic acid, and 40 mM sodium periodate), paraffin embedded, cut into 5-μm sections, and stained with hematoxylin and eosin and Masson’s trichrome blue using standard techniques.
Mouse kidney sections were probed with antibodies to F4/80 (Abcam, Cambridge, MA), collagen I (Abcam), α-smooth muscle actin (α-SMA; Sigma Aldrich, St. Louis, MO), and major basic protein (MBP; courtesy of Dr. James J. Lee, Mayo Clinic). For F4/80 and collagen I, paraffin sections were deparaffinized by standard procedure, incubated with acidic urea (6 M urea, 0.1 M glycine, pH 3.5) for 30 min, and then washed with phosphate-buffered saline (PBS). Next, the sections were incubated with 3% H2O2 for 15 min, washed with PBS, and blocked for 1 h at room temperature in PowerBlock (Biogenex Laboratories, Fremont, CA). Sections were incubated with primary antibody at 4°C overnight, washed with PBS, and incubated with biotinylated anti-rat or rabbit IgG (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. After being washed, sections were incubated with the ABC peroxidase kit (Vector Laboratories) per the manufacturer’s instructions, counterstained with Gill’s hematoxylin, dehydrated, and mounted with a cover slip. For F4/80 quantification, five high-powered fields were acquired from the medullary tubulointerstitium of each animal per experimental group. The number of F4/80-positive cells was determined in each image using the ImageJ cell counter tool (FIJI v1.46; NIH) and then averaged to yield an estimate of F4/80-positive cells per high-power field.
For α-SMA staining, paraffin sections were deparaffinized by standard procedure, and a standard antigen retrieval method with citrate buffer (pH 6.0) was performed. After being washed with PBS, sections were incubated with Cy3-conjugated anti-α-SMA antibody for 1 h at room temperature. Next, sections were washed with PBS and mounted with Vectashield antifade mounting medium containing DAPI (Vector Laboratories). For α-SMA quantification, five high-powered fields from each animal per experimental group were acquired, and staining was scored as percent positive area using ImageJ (FIJI v1.46; NIH).
For MBP staining, paraffin sections were deparaffinized by standard procedure followed by incubation with dual endogenous enzyme blocking reagent (Agilent Technologies, Wilmington, DE) for 10 min. Next, the sections were incubated with digest-All 3 solution (Life Technologies, Grand Island, NY) for 10 min and blocked for 20 min at room temperature in PowerBlock (Biogenex Laboratories). After being blocked, sections were incubated with primary antibody at 4°C overnight, washed with PBS, and incubated with alkaline phosphatase-conjugated anti-rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. After being washed, sections were incubated with ImmPACT Vector Red (Vector Laboratories) and permanent red (Agilent Technologies) substrate solutions per the manufacturer’s instructions, counterstained with 0.1% methyl green, rinsed with water, air-dried, and mounted with a cover slip. For MBP quantification, five high-powered fields from each animal per experimental group were acquired. The number of MBP-positive cells in each image was determined using the ImageJ cell counter tool (FIJI v1.46; NIH) and then averaged to yield an estimate of MBP-positive cells per high-power field for a given animal.
TUNEL assay.
Mouse kidney samples were probed with terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) for localization of apoptotic DNA fragmentation. Paraffin sections from kidneys harvested 3 days post-UUO or sham surgery were deparaffinized by standard procedure and then incubated with 3% H2O2 for 15 min and washed with PBS. Next, the sections were incubated with proteinase K (Takara Bio USA, Mountain View, CA) in TE-CaCl2 buffer (10 mM Tris·HCl and 1 mM EDTA in PBS with 1 mM CaCl2 and 0.5 mM MgCl2, pH 8.0) for 15 min at room temperature and washed with PBS. The sections were then incubated with TdT (Promega, Madison, WI) and biotinylated 14-dATP (Life Technologies) for 1 h at 37°C, washed with PBS, and then blocked with 2% bovine serum albumin at room temperature for 10 min. After being blocked, sections were washed with PBS and incubated with the ABC peroxidase kit (Vector Laboratories) per the manufacturer’s instructions, counterstained with Gill’s hematoxylin, dehydrated, and mounted with a cover slip. For TUNEL-positive cell quantification, 20 high-powered fields from each animal per experimental group were acquired. The number of TUNEL-positive cells in each image was determined using the ImageJ cell counter tool (FIJI v1.46; NIH) and then averaged to yield an estimate of TUNEL-positive cells per high-power field for a given animal.
Solubilization of kidneys.
One-half mouse kidney from sham and UUO animals was snap-frozen in liquid nitrogen, crushed into a powder with a mortar and pestle, and added to 1 ml of deoxycholate buffer (1% sodium deoxycholate, 10 mM Tris·Cl, pH 7.5, and 1 mM EDTA-Na) including protease inhibitors (0.5 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstatin) and 50 μM PHG. The kidney samples were then sonicated and centrifuged at 14,000 rpm in a refrigerated centrifuge for 15 min. The lysate supernatants were used to determine protein concentration by bicinchoninic acid assay (Thermo Fisher, Waltham, MA) for gel loading. Laemmli sample buffer with 50 mM dithiothreitol was added to the remaining insoluble pellet, sonicated briefly to resuspend, and heated at 80°C for 15 min.
Gel electrophoresis and immunoblots.
Kidney lysates and solubilized matrix pellets were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using either 4–20% gradient or 7.5% polyacrylamide gels (Bio-Rad, Hercules, CA), transferred to nitrocellulose membranes, and blocked with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) for 1 h. Lysate samples were normalized to total protein with REVERT Total Protein Stain (LI-COR Biosciences) per the manufacturer’s instructions. The solubilized matrix pellet samples were probed overnight with either rabbit anti-collagen I (MD Biosciences, Oakdale, MN), rabbit anti-EPX (Aviva Systems Biology, San Diego, CA), mouse anti-MPO (Life Technologies), or mouse anti-Pxdn (clone 10H7) antibodies at 4°C. Bound antibody was detected using the appropriate secondary antibodies conjugated with IR Dye 680 or IR Dye 800 (LI-COR Biosciences) and visualized using LI-COR’s Odyssey Infrared Imaging System. Quantification and analysis of bands were performed using Odyssey software (version 3.0).
Statistical analysis.
Statistical analysis was conducted using GraphPad Prism version 5.04 (GraphPad Software, La Jolla, CA). In studies involving two groups, unpaired t-tests were conducted. In experiments involving more than two groups, one-way ANOVA followed by pairwise comparisons using Bonferroni correction for multiple comparisons was used.
RESULTS
PXDN, MPO, and EPX are upregulated after UUO.
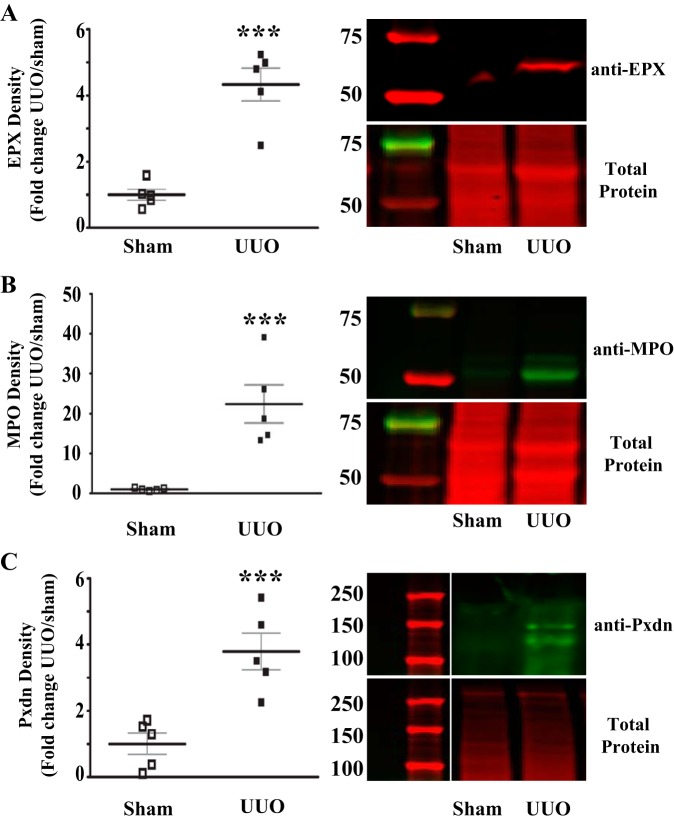
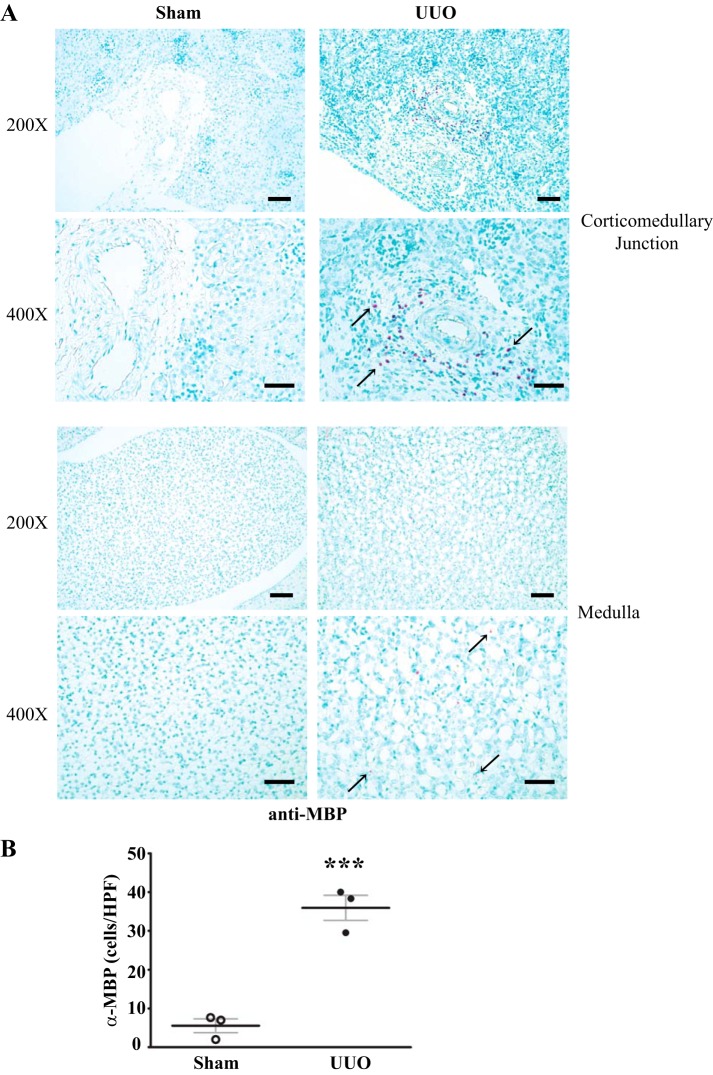
To investigate a possible role for PXDN, MPO, and EPX in the development of renal inflammation and fibrosis, we initially determined whether these enzymes are upregulated in the UUO model. Prior work by others suggested that PXDN and MPO are upregulated after UUO, whereas expression of EPX in UUO has not been investigated. Quantitative immunoblotting revealed a significant increase in PXDN, MPO, and EPX protein in UUO kidney compared with sham-operated kidney, suggesting a possible role for these enzymes in the development of renal fibrosis in this model (Fig. 1). Neutrophils and macrophages, found in increased numbers after UUO, express MPO, whereas renal tubular and interstitial cells have been found to express PXDN. Eosinophils, the only cellular source of EPX (40), have not been investigated after UUO. Therefore, we immunostained for MBP, a well-described eosinophil marker, since murine eosinophils are difficult to identify based on classic granule morphology (1, 14, 23). Eosinophils are rarely present in WT sham-operated mice while MBP-positive cells increased dramatically in the kidney following UUO (Fig. 2). Thus, eosinophils are clearly present after UUO and may contribute to renal injury and fibrosis via secretion of EPX.
Fig. 1.
Animal heme peroxidases are upregulated in the unilateral ureteral obstruction (UUO)-injured kidney. A: quantitative analysis of anti-eosinophil peroxidase (EPX) immunoblot data for UUO mice normalized to sham surgery mice. Representative immunoblot (top right) and total protein stain (bottom right) from sham surgery and UUO mice. B: quantitative analysis of anti-myeloperoxidase (MPO) immunoblot data for UUO mice normalized to sham surgery mice. Representative immunoblot (top right) and total protein stain (bottom right) from sham surgery and UUO mice. C: quantitative analysis of anti-peroxidasin (Pxdn) immunoblot data for UUO mice normalized to sham surgery mice. Representative immunoblot (top right) and total protein stain (bottom right) from sham surgery and UUO mice (n = 5 for all groups, ***P < 0.005).
Fig. 2.
Eosinophils accumulate in renal interstitium after unilateral ureteral obstruction (UUO). A: representative kidney sections immunostained for major basic protein (MBP), an eosinophil marker, from wild-type (WT) mice 7 days after sham surgery or UUO depicting medulla and corticomedullary junction regions. MBP-positive cells within the corticomedullary region tended to accumulate around vessels, whereas medulla MBP-positive cells were diffusely present. B: quantification of the no. of MBP-positive cells per high-power field in WT mice after sham surgery or UUO (n = 3 in both groups, ***P < 0.005). Scale bar = 50 μm for ×200 images and 20 μm for ×400 images.
Pharmacological inhibition of peroxidases reduces renal fibrosis and inflammation after UUO.
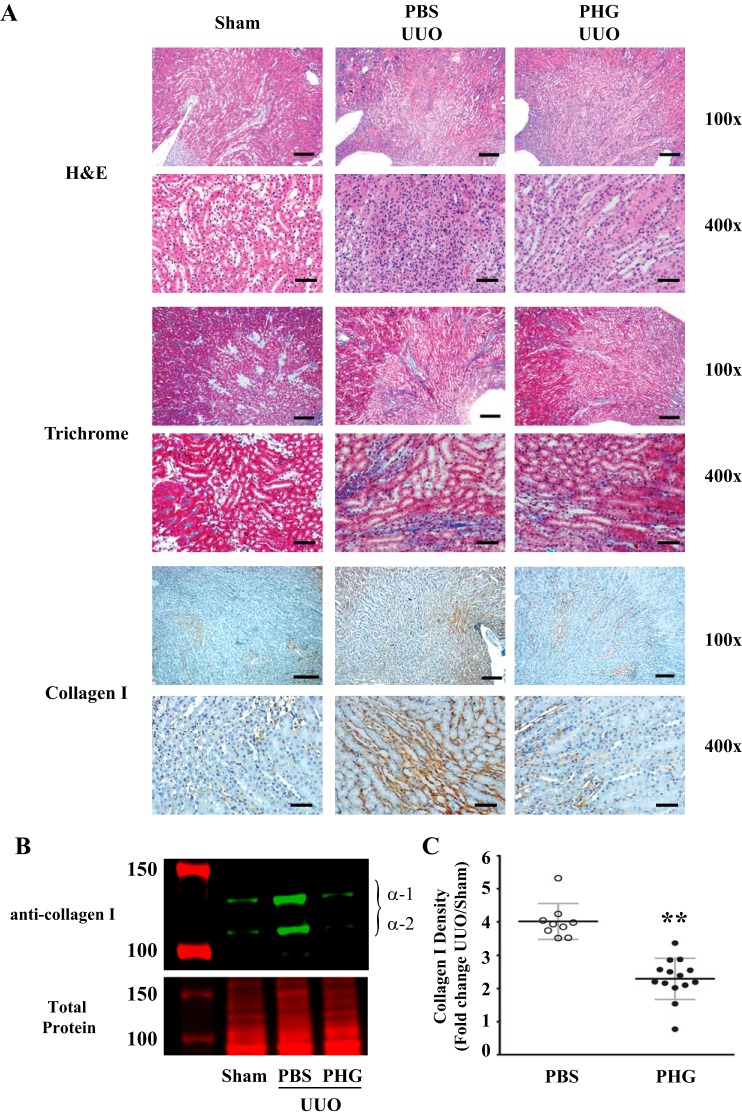
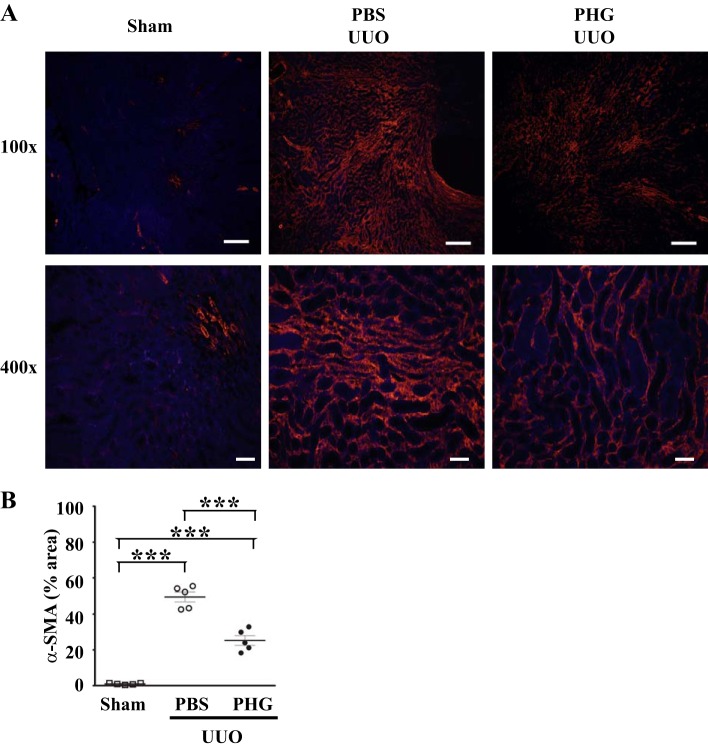
Utilizing the UUO model of kidney injury, we set out to establish if peroxidases play a critical role in renal fibrosis. To do this, we administered PHG, a nonspecific peroxidase inhibitor, to mice at the onset of UUO and continued for 7 days during the course of renal injury (3, 13, 27, 32). Sham-operated mice demonstrated little tubular atrophy and fibrosis, whereas vehicle-treated UUO animals demonstrated significant tubular atrophy and accumulation of collagen by trichrome blue staining and collagen I immunohistochemistry (Fig. 3A). This collagen accumulation in UUO-injured mice was significantly decreased by treatment with PHG (Fig. 3A). To quantify the effect of PHG treatment on UUO-induced fibrosis, we immunoblotted kidney tissue for collagen I and performed densitometry. PBS-treated mice demonstrated about a fourfold increase in collagen I accumulation after UUO compared with sham-operated mice, and this increase was significantly blunted in mice treated with PHG (Fig. 3, B and C). Expression of α-SMA, a marker of activated fibroblasts that is upregulated in fibrosis (18), was significantly attenuated in UUO mice treated with PHG compared with vehicle-treated obstructed mice (Fig. 4).
Fig. 3.
Phloroglucinol (PHG), a nonspecific peroxidase inhibitor, reduces collagen accumulation after unilateral ureteral obstruction (UUO). A: representative kidney sections from wild-type C57BL/6J mice 7 days after sham surgery or UUO. UUO mice were treated daily with PBS vehicle or PHG. Sections were stained with hematoxylin and eosin (H&E) or Masson’s trichrome blue (Trichrome) or immunostained for collagen I (Collagen I). B: representative collagen I immunoblot (top) and total protein stain (bottom) from sham surgery and UUO mice treated with PBS vehicle or PHG. C: quantitative analysis of immunoblot data normalized to sham surgery mice from PBS-treated (n = 9) and PHG-treated (n = 14) UUO mice (**P < 0.01). Scale bar = 100 μm for ×100 images and 20 μm for ×400 images.
Fig. 4.
Peroxidase inhibition reduces α-smooth muscle actin (α-SMA) expression after unilateral ureteral obstruction (UUO)-induced renal injury. A: representative kidney sections after immunofluorescent staining for α-SMA from wild-type mice that underwent sham surgery or UUO with either PBS vehicle or phloroglucinol (PHG) treatment. B: quantification of α-SMA-positive area (e.g., red fluorescence) is shown as a percentage of medullary interstitial area from sham surgery mice, PBS vehicle-treated UUO mice, or PHG-treated UUO mice (n = 5 for all groups, ***P < 0.005). Scale bar = 100 μm for ×100 images and 20 μm for ×400 images.
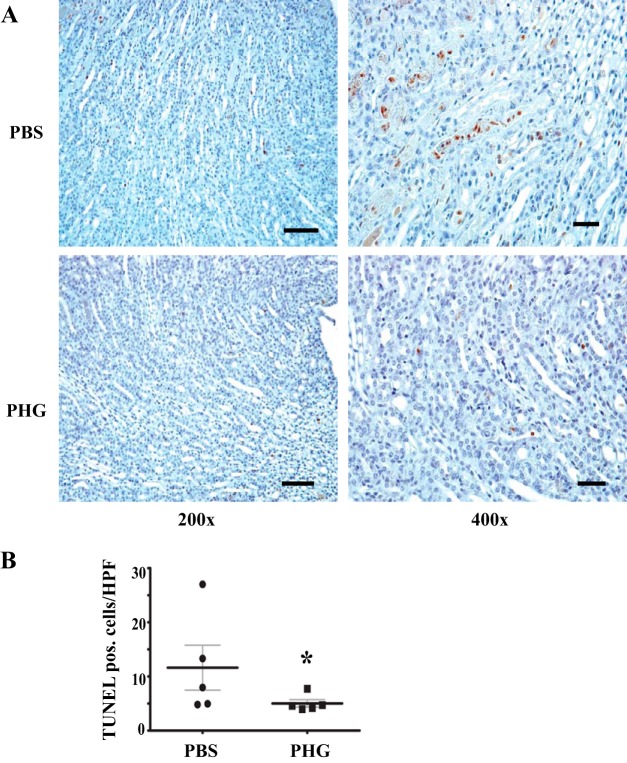
In addition to fibroblast activation and fibrosis, tubular cell injury and apoptotic loss also contribute to deterioration of renal function after renal injury. To determine whether peroxidase blockade ameliorates tubular apoptosis, we conducted TUNEL to detect apoptotic tubular epithelial cells in vehicle- and PHG-treated mice after UUO. We found that PHG treatment reduced tubular apoptotic cells, suggesting that peroxidases promote tubular cell loss (Fig. 5).
Fig. 5.
Peroxidase inhibition reduces apoptosis after unilateral ureteral obstruction (UUO). A: representative terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining of kidney sections from wild-type mice 3 days after sham surgery or UUO treated daily with PBS vehicle or phloroglucinol (PHG). B: quantitative analysis of TUNEL-positive cells per high-power field in kidneys from PBS-treated (n = 5) and PHG-treated (n = 5) UUO mice (*P < 0.05). Scale bar = 50 μm for ×200 images and 20 μm for ×400 images.
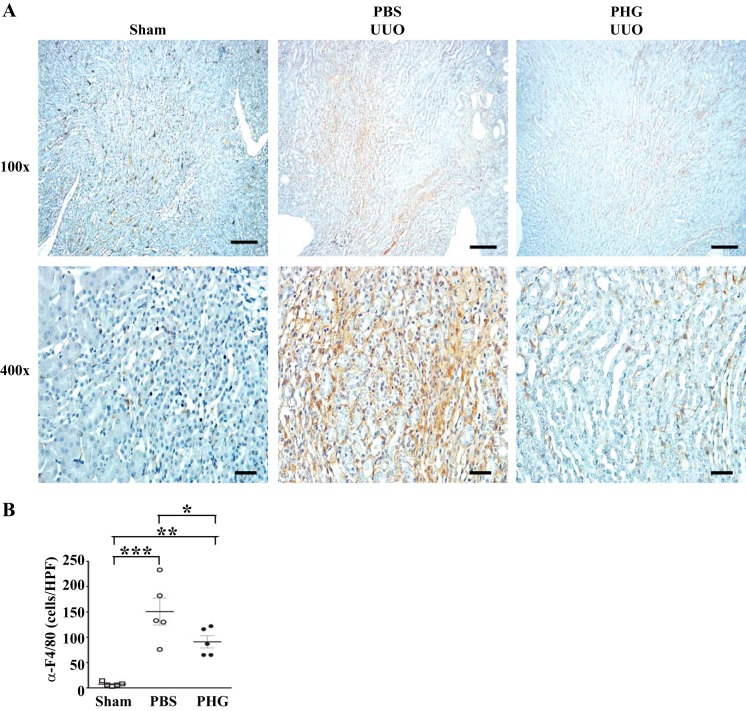
Inflammation within the renal interstitium, comprised mainly of macrophages, commonly precedes and coincides with the development of renal fibrosis in many models, including UUO (25, 30, 31). To determine the effect of peroxidase inhibition on macrophage infiltration after UUO, we stained vehicle or PHG-treated obstructed kidneys for F4/80. Treatment of UUO mice with PHG significantly reduced the number of interstitial F4/80-positive cells (Fig. 6). Taken together, inhibition of peroxidases with PHG reduces renal fibrosis, tubular cell apoptosis, and inflammation in the murine UUO model.
Fig. 6.
Peroxidase inhibition reduces F4/80-positive cells in the unilateral ureteral obstruction (UUO)-injured kidney. A: representative kidney sections stained with F4/80 antibody from wild-type mice that underwent sham surgery or UUO and treated with PBS vehicle or phloroglucinol (PHG). B: no. of F4/80-positive cells per high-power field in sham surgery and UUO mice treated either with PBS vehicle or PHG (n = 5 for all groups, *P < 0.05, **P < 0.01, and ***P < 0.005). Scale bar = 100 μm for ×100 images and 20 μm for ×400 images.
PXDN and EPX, but not MPO, contribute to renal fibrosis after UUO.
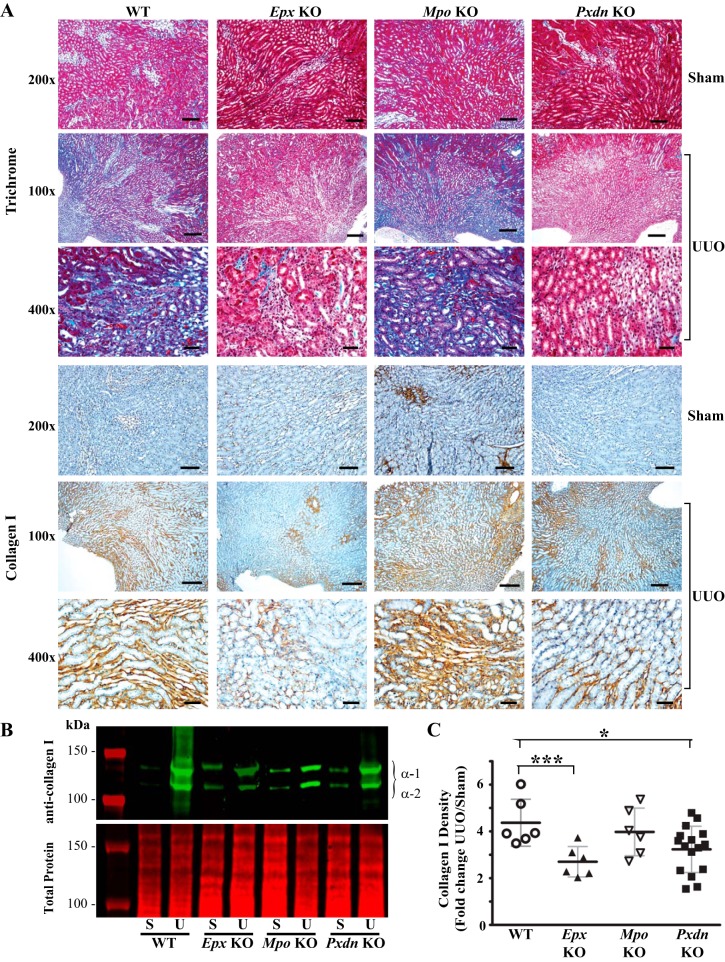
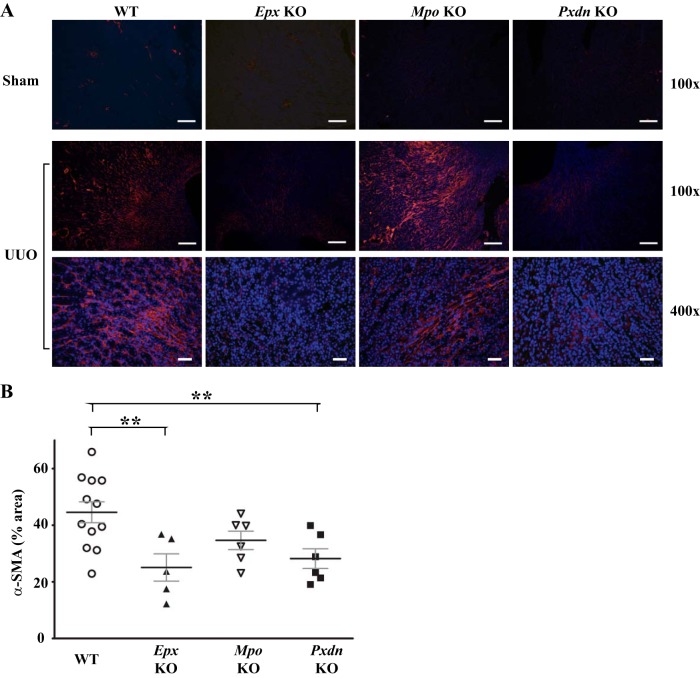
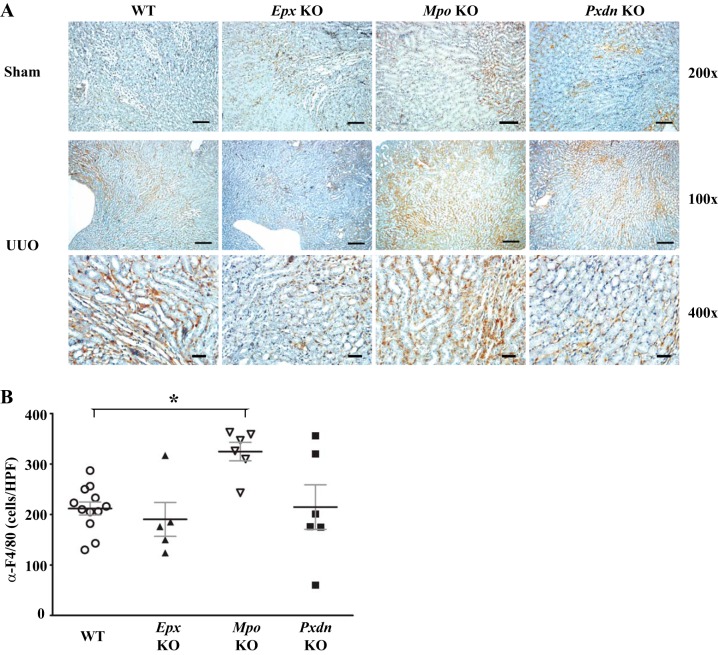
Our data with PHG suggest a role for peroxidases in the pathogenesis of renal inflammation and fibrosis after UUO but do not identify the specific peroxidase(s) involved. Additionally, the findings may reflect unknown off-target effects of PHG. To address the role of different animal heme peroxidases, we used Mpo, Epx, and Pxdn knockout mice and compared their response to UUO against WT mice. Interestingly, Pxdn and Epx knockout mice, but not Mpo knockout mice, demonstrated qualitatively decreased collagen accumulation judged by trichrome blue staining and collagen I immunohistochemistry (Fig. 7A). Based on quantitative immunoblotting, Epx and Pxdn knockout mice demonstrated reduced collagen I expression compared with WT mice (Fig. 7, B and C). Conversely, Mpo knockout mice exhibited no significant difference in collagen I expression compared with WT mice (Fig. 7, B and C). Similarly, α-SMA expression was decreased in Epx and Pxdn knockout mice compared with WT mice while Mpo-deficient mice did not demonstrate a significant difference (Fig. 8). With PHG treatment, we observed a decrease in F4/80-positive macrophages in the renal interstitium and therefore examined F4/80-positive cell numbers in Mpo, Pxdn, and Epx knockout mice. Compared with WT mice, Pxdn and Epx knockout mice failed to demonstrate a significant change in F4/80-positive cells. However, Mpo knockout mice demonstrated an increased accumulation of F4/80 cells (Fig. 9). Taken together, these data demonstrate that PXDN and EPX contribute to the pathogenesis of renal fibrosis in the UUO model. Presumably, the antifibrotic effect of PHG, a nonspecific peroxidase inhibitor, was predominantly driven by inhibition of PXDN and EPX. Surprisingly, MPO loss of function had little effect on renal fibrosis and worsened renal inflammation, based on F4/80-positive cell infiltration.
Fig. 7.
Peroxidasin knockout (Pxdn-KO) and eosinophil peroxidase knockout (Epx-KO), but not myeloperoxidase knockout (Mpo-KO), mice exhibit reduced fibrosis after unilateral ureteral obstruction (UUO)-induced renal injury. A: representative kidney sections from wild-type (WT), Epx-KO, Mpo-KO, and Pxdn-KO after UUO stained with Masson’s trichrome blue or immunostained for collagen I. B: representative immunoblot of collagen I (top) and total protein (bottom) from kidney tissue obtained from WT, Epx-KO, Mpo-KO, and Pxdn-KO mice. C: densitometry analysis of collagen I immunoblots as fold change in UUO compared with surgery in WT (n = 6), Epx-KO (n = 6), Mpo-KO (n = 6), and Pxdn-KO (n = 16) mice (***P < 0.005 and *P < 0.05). Scale bar = 50 μm for ×200 images, 100 μm for ×100 images, and 20 μm for ×400 images.
Fig. 8.
Peroxidasin knockout (Pxdn-KO) and eosinophil peroxidase knockout (Epx-KO), but not myeloperoxidase knockout (Mpo-KO), mice reduce α-smooth muscle actin (α-SMA) expression in unilateral ureteral obstruction (UUO)-induced renal injury. A: representative kidney sections from wild-type (WT), Epx-KO, Mpo-KO, and Pxdn-KO mice 7 days after UUO immunostained for α-SMA. B: quantification of α-SMA-positive area as a percent of medullary interstitial area in WT (n = 12), Epx -KO (n = 5), Mpo-KO (n = 6), and Pxdn-KO (n = 6) mice (**P ≤ 0.01). Scale bar = 100 μm for ×100 images and 20 μm for ×400 images.
Fig. 9.
Differential effects of peroxidase-specific knockout mice on renal F4/80 macrophage accumulation after unilateral ureteral obstruction (UUO). A: representative kidney sections immunostained for F4/80 from wild-type (WT), eosinophil peroxidase knockout (Epx-KO), myeloperoxidase knockout (Mpo-KO), and peroxidasin knockout (Pxdn-KO) mice after UUO. B: quantification of F4/80-positive cells per high-power field in stained sections from WT (n = 12), Epx-KO (n = 5), Mpo-KO (n = 6), and Pxdn-KO (n = 6) mice (*P < 0.05). Scale bar = 100 μm for ×100 images, 50 μm for ×200 images, and 20 μm for ×400 images.
DISCUSSION
In the UUO model of renal fibrosis, we found that a nonspecific peroxidase inhibitor, PHG, reduced macrophages (i.e., F4/80-positive cells), α-SMA expression, tubular cell apoptosis, and collagen I accumulation. Using specific peroxidase gene knockout mice, we found that Epx and Pxdn knockout mice demonstrated decreased α-SMA expression and collagen I deposition but had no effect on F4/80-positive macrophage accumulation. Conversely, Mpo knockout mice demonstrated no change in α-SMA and collagen I expression and paradoxically increased F4/80 interstitial macrophages.
The reduction in renal fibrosis, but not accumulation of F4/80 macrophages, in Epx and Pxdn knockout mice may suggest an effect on renal tubular epithelial cells or fibroblast activation that occurs independent of interstitial leukocytes. We did find that peroxidase inhibition with PHG reduced tubular cell apoptosis. Similarly, recent work by others has suggested that peroxidases may posttranslationally enhance fibroblast collagen deposition (12). Alternatively, deleting murine Epx and Pxdn may not change macrophage number but could alter macrophage phenotype toward a reparative subtype (25, 31). Future studies with detailed characterization of the interstitial leukocyte infiltrate in Epx and Pxdn knockout mice will address this possibility.
Prior studies have shown that MPO can exacerbate renal disease, presumably by chlorination and oxidation of glomerular basement membrane (GBM) proteins and other targets to drive renal injury (16, 21). Exogenous renal artery infusion of MPO and H2O2 causes significant GBM damage and glomerular injury (21). In the early heterologous phase of the anti-GBM antibody model of glomerulonephritis, Mpo knockout mice demonstrate significantly diminished glomerular injury and proteinuria (33). Similarly, Mpo knockout mice exhibit reduced glomerular pathology and albuminuria and decreased interstitial accumulation of macrophages and lymphocytes in the 5/6th nephrectomy model of renal fibrosis and CKD (24). However, MPO also attenuates T cell immunity in crescentic glomerulonephritis. Mpo knockout mice demonstrated increased accumulation of T lymphocytes and macrophages in the late autologous phase of the sheep anti-GBM antibody model, with little effect on glomerular injury or proteinuria (33, 34). These findings, albeit in a different form of renal injury, are consistent with our findings in Mpo knockout mice, which exhibited increased accumulation of macrophages but no significant effect on renal fibrosis. Taken together, MPO exhibits model-specific effects on renal inflammation and fibrosis.
Eosinophils have been detected in human renal biopsies in cases of drug-induced interstitial nephritis but also in several glomerular diseases, raising the possibility that these cells may more broadly contribute to CKD and renal fibrosis (8, 37). Eosinophils have classically been considered detrimental in inflammatory states in other organs, such as allergic pulmonary disease (19, 28). Along with other injurious granule proteins, EPX contributes to eosinophilic injury via HOBr-mediated oxidative modification and damage (40). However, some recent studies have found eosinophils to promote resolution of tissue injury and repair (17, 22). Little data exist regarding a pathogenic role for eosinophils in renal disease. A recent study suggests that eosinophils may have a beneficial role in adriamycin-induced nephropathy, since eosinophil depletion abrogated the IL-33-dependent improvement in proteinuria and glomerular injury (38). Our work, however, suggests that eosinophils accumulating in the renal interstitium promote renal fibrosis via EPX activity. Eosinophils and EPX may modulate the inflammatory response in a time- and disease-specific manner. Future work will examine the role of eosinophils in models of renal disease with varying types of injury and time course.
Our recent work on PXDN, a multidomain peroxidase found in extracellular matrix, showed that HOBr mediates a beneficial structural role rather than a destructive oxidizing function (3, 6, 29). PXDN generates HOBr as a reactive intermediate to form sulfilimine cross-links in the collagen IV network of basement membranes. These cross-links mechanically stabilize basement membranes, pointing to an important role for both PXDN and bromine in tissue development and homeostasis (2, 3, 29, 39). We have shown that Pxdn knockout mice demonstrate significantly reduced sulfilimine cross-links, leading to reduced stiffness of renal tubular basement membranes (2). Thus, in the UUO model, we initially predicted that Pxdn knockout mice would exhibit enhanced renal inflammation or fibrosis driven by increased mechanical stretch and injury to the tubular epithelium. However, we found the opposite effect, with Pxdn knockout mice demonstrating reduced renal fibrosis. These data might suggest that a less strong but more pliable basement membrane deficient of collagen IV cross-links reduces epithelial cell apoptosis or maladaptive signaling to renal fibroblasts and leukocytes. Alternatively, the increased generation of HOBr-dependent protein oxidation with excess PXDN following renal injury may outweigh the beneficial effects of collagen IV cross-links in the UUO model. In support of this notion, Péterfi and colleagues demonstrated that PXDN is disproportionately increased in regions of interstitial fibrosis after UUO (36). Because EPX and PXDN primarily generate HOBr, while MPO solely forms HOCl, an intriguing hypothesis regarding the divergent effects of MPO, EPX, and PXDN loss of function may lie in different oxidative targets for HOCl vs. HOBr, with the latter leading to maladaptive alterations promoting renal fibrosis.
Taken together, our work examined the role of the animal heme peroxidases, MPO, EPX, and PXDN, in the development of renal inflammation and fibrosis in the UUO model. We found that loss of EPX and PXDN reduced renal fibrosis, whereas loss of MPO did not. Future work in other animal models of renal fibrosis will determine whether EPX and PXDN are potential therapeutic targets for the treatment of renal fibrosis and CKD.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08-DK-097306 (G. Bhave), R01-DK-116964-01 (G. Bhave), 3R01-DK-116964-01S1 (S. Colon), and R01-DK-108968-01 (L. Gewin), a Burroughs Wellcome Fund Career Award for Medical Scientists (13030995) to G. Bhave, and developmental funds from the Vanderbilt University Medical Center Division of Nephrology (G. Bhave and L. Gewin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.B. conceived and designed research; S.C., H.L., Y.L., and C.M. performed experiments; S.C., H.L., Y.L., and G.B. analyzed data; S.C., L.G., and G.B. interpreted results of experiments; S.C. and Y.L. prepared figures; S.C. and G.B. drafted manuscript; S.C., L.G., and G.B. edited and revised manuscript; S.C., H.L., Y.L., L.G., and G.B. approved final version of manuscript.
REFERENCES
- 1.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem 289: 17406–17415, 2014. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhave G, Colon S, Ferrell N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. Am J Physiol Renal Physiol 313: F596–F602, 2017. doi: 10.1152/ajprenal.00096.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, Kang J-S, Pedchenko V, Fessler LI, Fessler JH, Hudson BG. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8: 784–790, 2012. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 5.Brown KL, Darris C, Rose KL, Sanchez OA, Madu H, Avance J, Brooks N, Zhang M-Z, Fogo A, Harris R, Hudson BG, Voziyan P. Hypohalous acids contribute to renal extracellular matrix damage in experimental diabetes. Diabetes 64: 2242–2253, 2015. doi: 10.2337/db14-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colon S, Page-McCaw P, Bhave G. Role of hypohalous acids in basement membrane homeostasis. Antioxid Redox Signal 27: 839–854, 2017. doi: 10.1089/ars.2017.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couser WG, Johnson RJ. What is myeloperoxidase doing in ANCA-associated glomerulonephritis? Kidney Int 88: 938–940, 2015. doi: 10.1038/ki.2015.259. [DOI] [PubMed] [Google Scholar]
- 8.Dai D-F, Sasaki K, Lin MY, Smith KD, Nicosia RF, Alpers CE, Najafian B. Interstitial eosinophilic aggregates in diabetic nephropathy: allergy or not? Nephrol Dial Transplant 30: 1370–1376, 2015. doi: 10.1093/ndt/gfv067. [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta 1703: 93–109, 2005. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal 10: 1199–1234, 2008. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 11.Demirbilek S, Emre MH, Aydin EN, Edali MN, Aksoy RT, Akin M, Gürünlüoğlu K, Tas E, Ay S, Yilmaz Z. Sulfasalazine reduces inflammatory renal injury in unilateral ureteral obstruction. Pediatr Nephrol 22: 804–812, 2007. doi: 10.1007/s00467-006-0416-8. [DOI] [PubMed] [Google Scholar]
- 12.DeNichilo MO, Panagopoulos V, Rayner TE, Borowicz RA, Greenwood JE, Evdokiou A. Peroxidase enzymes regulate collagen extracellular matrix biosynthesis. Am J Pathol 185: 1372–1384, 2015. doi: 10.1016/j.ajpath.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Divi RL, Doerge DR. Mechanism-based inactivation of lactoperoxidase and thyroid peroxidase by resorcinol derivatives. Biochemistry 33: 9668–9674, 1994. doi: 10.1021/bi00198a036. [DOI] [PubMed] [Google Scholar]
- 14.Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, Foster PS, Hogan SP. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol 172: 5664–5675, 2004. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 15.Gewin L, Zent R, Pozzi A. Progression of chronic kidney disease: too much cellular talk causes damage. Kidney Int 91: 552–560, 2017. doi: 10.1016/j.kint.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gröne H-J, Gröne EF, Malle E. Immunohistochemical detection of hypochlorite-modified proteins in glomeruli of human membranous glomerulonephritis. Lab Invest 82: 5–14, 2002. doi: 10.1038/labinvest.3780390. [DOI] [PubMed] [Google Scholar]
- 17.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153: 376–388, 2013. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobsen EA, Lee NA, Lee JJ. Re-defining the unique roles for eosinophils in allergic respiratory inflammation. Clin Exp Allergy 44: 1119–1136, 2014. doi: 10.1111/cea.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol 10: 463–473, 2014. doi: 10.1038/nrrheum.2014.103. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Couser WG, Chi EY, Adler S, Klebanoff SJ. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest 79: 1379–1387, 1987. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy 40: 563–575, 2010. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol 130: 572–584, 2012. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehners A, Lange S, Niemann G, Rosendahl A, Meyer-Schwesinger C, Oh J, Stahl R, Ehmke H, Benndorf R, Klinke A, Baldus S, Wenzel UO. Myeloperoxidase deficiency ameliorates progression of chronic kidney disease in mice. Am J Physiol Renal Physiol 307: F407–F417, 2014. doi: 10.1152/ajprenal.00262.2014. [DOI] [PubMed] [Google Scholar]
- 25.Lin SL, Castaño AP, Nowlin BT, Lupher ML Jr, Duffield JS. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183: 6733–6743, 2009. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- 26.Malle E, Buch T, Grone H-J. Myeloperoxidase in kidney disease. Kidney Int 64: 1956–1967, 2003. doi: 10.1046/j.1523-1755.2003.00336.x. [DOI] [PubMed] [Google Scholar]
- 27.Mayeno AN, Curran AJ, Roberts RL, Foote CS. Eosinophils preferentially use bromide to generate halogenating agents. J Biol Chem 264: 5660–5668, 1989. [PubMed] [Google Scholar]
- 28.McBrien CN, Menzies-Gow A. The biology of eosinophils and their role in asthma. Front Med (Lausanne) 4: 93, 2017. doi: 10.3389/fmed.2017.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 157: 1380–1392, 2014. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X-M, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10: 493–503, 2014. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 31.Nelson PJ, Rees AJ, Griffin MD, Hughes J, Kurts C, Duffield J. The renal mononuclear phagocytic system. J Am Soc Nephrol 23: 194–203, 2012. doi: 10.1681/ASN.2011070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J 13: 3438–3447, 1994. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odobasic D, Kitching AR, Semple TJ, Holdsworth SR. Endogenous myeloperoxidase promotes neutrophil-mediated renal injury, but attenuates T cell immunity inducing crescentic glomerulonephritis. J Am Soc Nephrol 18: 760–770, 2007. doi: 10.1681/ASN.2006040375. [DOI] [PubMed] [Google Scholar]
- 34.Odobasic D, Kitching AR, Yang Y, O’Sullivan KM, Muljadi RCM, Edgtton KL, Tan DSY, Summers SA, Morand EF, Holdsworth SR. Neutrophil myeloperoxidase regulates T-cell-driven tissue inflammation in mice by inhibiting dendritic cell function. Blood 121: 4195–4204, 2013. doi: 10.1182/blood-2012-09-456483. [DOI] [PubMed] [Google Scholar]
- 35.Okamura DM, Pennathur S. The balance of powers: redox regulation of fibrogenic pathways in kidney injury. Redox Biol 6: 495–504, 2015. doi: 10.1016/j.redox.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Péterfi Z, Donkó A, Orient A, Sum A, Prókai A, Molnár B, Veréb Z, Rajnavölgyi E, Kovács KJ, Müller V, Szabó AJ, Geiszt M, Donkó Á, Orient A, Sum A, Prókai Á, Molnár B, Veréb Z, Rajnavölgyi É, Kovács KJ, Müller V, Szabó AJ, Geiszt M. Peroxidasin is secreted and incorporated into the extracellular matrix of myofibroblasts and fibrotic kidney. Am J Pathol 175: 725–735, 2009. doi: 10.2353/ajpath.2009.080693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raghavan R, Shawar S. Mechanisms of drug-induced interstitial nephritis. Adv Chronic Kidney Dis 24: 64–71, 2017. doi: 10.1053/j.ackd.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Riedel J-H, Becker M, Kopp K, Düster M, Brix SR, Meyer-Schwesinger C, Kluth LA, Gnirck A-C, Attar M, Krohn S, Fehse B, Stahl RAK, Panzer U, Turner J-E. IL-33-mediated expansion of type 2 innate lymphoid cells protects from progressive glomerulosclerosis. J Am Soc Nephrol 28: 2068–2080, 2017. doi: 10.1681/ASN.2016080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanacore R, Ham A-JL, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG. A sulfilimine bond identified in collagen IV. Science 325: 1230–1234, 2009. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Slungaard A. Role of eosinophil peroxidase in host defense and disease pathology. Arch Biochem Biophys 445: 256–260, 2006. doi: 10.1016/j.abb.2005.10.008. [DOI] [PubMed] [Google Scholar]