Abstract
Here, we aimed to explore sex differences and the impact of sex hormones on cardiac contractile properties in doxorubicin (DOX)-induced cardiotoxicity. Male and female Sprague-Dawley rats were subjected to sham surgery or gonadectomy and then treated or untreated with DOX (2 mg/kg) every other week for 10 wk. Estrogen preserved maximum active tension (Tmax) with DOX exposure, whereas progesterone and testosterone did not. The effects of sex hormones and DOX correlated with both altered myosin heavy chain isoform expression and myofilament protein oxidation, suggesting both as possible mechanisms. However, acute treatment with oxidative stress (H2O2) or a reducing agent (DTT) indicated that the effects on Tmax were mediated by reversible myofilament oxidative modifications and not only changes in myosin heavy chain isoforms. There were also sex differences in the DOX impact on myofilament Ca2+ sensitivity. DOX increased Ca2+ sensitivity in male rats only in the absence of testosterone and in female rats only in the presence of estrogen. Conversely, DOX decreased Ca2+ sensitivity in female rats in the absence of estrogen. In most instances, this mechanism was through altered phosphorylation of troponin I at Ser23/Ser24. However, there was an additional DOX-induced, estrogen-dependent, irreversible (by DTT) mechanism that altered Ca2+ sensitivity. Our data demonstrate sex differences in cardiac contractile responses to chronic DOX treatment. We conclude that estrogen protects against chronic DOX treatment in the heart, preserving myofilament function.
NEW & NOTEWORTHY We identified sex differences in cardiotoxic effects of chronic doxorubicin (DOX) exposure on myofilament function. Estrogen, but not testosterone, decreases DOX-induced oxidative modifications on myofilaments to preserve maximum active tension. In rats, DOX exposure increased Ca2+ sensitivity in the presence of estrogen but decreased Ca2+ sensitivity in the absence of estrogen. In male rats, the DOX-induced shift in Ca2+ sensitivity involved troponin I phosphorylation; in female rats, this was through an estrogen-dependent mechanism.
Keywords: cardiotoxicity, myofilament Ca2+ activation, oxidative modification, sex differences
INTRODUCTION
In women, a lower premenopausal risk but an increased postmenopausal risk of cardiovascular disease compared with men suggests differential regulatory and protective actions of female and male sex hormones on the heart (5, 31, 44, 58). Expression of both estrogen (23, 25) and androgen (34) receptors in cardiac tissues of both sexes supports the differential impact of sex hormones. The physiological significance of female and male sex hormones on cardiac contractile function have been well documented (14, 18, 20, 54, 56, 57). While both gonadectomized female and male rats show reductions in maximum cardiac contractile activation, myofilament Ca2+ hypersensitivity is only observed in the ovariectomized (OVX) rat heart (54). In contrast, changes in the Ca2+ transient, including suppressed amplitude and prolonged decay time, are similarly detected in both female and male rat hearts after sex hormone deprivation (9, 18, 32, 56).
Sex differences in cardioprotective actions have also been reported in many pathological conditions. In myocardial infarction, female sex hormones increase ejection fraction and reduce left ventricular dimension (15), but male sex hormones suppress cardiac contractility as well as the Ca2+ transient amplitude (43). Male sex hormones have also been found to increase the cardiac rupture rate and infarct expansion index (16). In an ischemia-reperfusion (I/R) model, female sex hormones reduce the Ca2+ transient amplitude (7) but induce recovery of aortic flow, cardiac output, and contractility (28) as well as prevent cardiomyocyte apoptosis (27). On the other hand, male sex hormones increase the Ca2+ transient amplitude but desensitize cardiac myofilament Ca2+ sensitivity (7).
Besides differences in cardiac physiology and pathology, sex differences in drug-induced cardiotoxicity, especially by certain chemotherapeutic drugs, have also been identified (1, 24, 36, 37). Clinical observations have noted that adult men develop more functional toxicity to antracycline therapy [doxorubicin (DOX)] than age-matched women (6, 39). Conversely, prepubertal girls and postmenopausal women who have low levels of female sex hormones are more sensitive to DOX-induced cardiotoxicity than age-matched male patients with cancer. This notion supports the cardioprotective effect of female sex hormones (13, 29, 30, 46). The sex difference effects on cardiac physiological and pathological actions as well as drug-induced toxicity may be attributable to changes at the levels of myofilament and/or Ca2+ handling.
In the present study, we aimed to study sex differences and the protective effect of sex hormones on cardiac contractile responses to chronic DOX treatment. We used gonadectomized female and male rats with and without hormone supplementation for 10 wk. Changes in cardiac contractile functions were analyzed using skinned fiber and myocyte preparations. Our results demonstrate the potential for female sex hormones, especially estrogen, to protect against DOX-induced depression of myofilament function, whereas testosterone does not.
MATERIALS AND METHODS
Animal experiments.
Female Sprague-Dawley rats weighing between 180 and 200 g (7–8 wk old) and male Sprague-Dawley rats weighing between 250 and 300 g (7–8 wk old) were housed in a plastic shoebox cage under a 12:12 light-dark cycle with controlled temperature and humidity. They were fed ad libitum with laboratory rat chow (C. P.) during the whole period of the experiment. Female and male rat hearts were examined under physiological and chronic DOX treatment. To investigate the impact of sex hormones as a key modulator underlying the differences in responses, female rats were subdivided into the following four groups: 1) sham-operated (sham) control, 2) OVX, 3) OVX with estrogen (E) supplementation, and 4) OVX with progesterone (P) supplementation. Male rats were subdivided into the following three groups: 1) sham control, 2) orchidectomy (ORX), and 3) ORX with testosterone supplementation. The animal experimental protocol was approved by the Experimental Animal Committee of the Faculty of Science of Mahidol University in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011).
Hormone replacement and DOX injection.
The hormone supplementation groups of OVX rats were subcutaneously injected with either 5 µg of 17β-estradiol or 1 mg of progesterone using corn oil as a solvent in a fixed volume of 0.1 ml for 3 times/wk for 10 wk starting 2 days after the operation, as previously described (42). An equal volume of corn oil was injected in the control group of female rats. In the supplementation group of male rats, ORX rats were subcutaneously injected with testosterone propionate in ethyl oleate (2 mg/kg body wt) for 3 times/wk adjusted from 2.5 mg/kg used in a previous study (56). Ethyl oleate was injected in the control groups. The groups with DOX treatment received an intraperitoneal injection of 2.0 mg/kg body wt every other week in 10 wk. Thus, the accumulative dose of DOX was 10 mg/kg body wt (8, 17) throughout the period of study. In a vehicle-treated group, rats received normal saline solution in the same volume as that injected in DOX-treated groups.
Measurement of myofilament Ca2+ activation.
Left ventricular papillary muscle was placed in ice-cold high relaxing buffer containing 10 mM EGTA, 2 mM free Mg2+, 5 mM MgATP, 79.2 mM KCl, 12 mM creatine phosphate, 20 mM MOPS (pH 7.0) (ionic strength: 0.15 M), 2.5 µg/ml pepstatin A, 1 µg/ml leupeptin, and 50 µM PMSF. Small muscle bundles were dissected for force measurements as previously described (55). Briefly, stripped papillary fibers were skinned in high relaxing buffer containing 1% Triton X-100 for 1 h at 25°C. The skinned fiber bundle was attached using aluminum T clips at one end to a displacement generator and at the other end to a force transducer (KG-7). Active tension was measured at a fixed sarcomere length of 2.2 μm in a solution containing various Ca2+ concentrations ranging from pCa 7.0 to 4.5 at pH 7.0 and 20°C (26). For the H2O2 treatment experiment, a new skinned fiber bundle was directly introduced to 5 mM H2O2 in the active buffer containing various Ca2+ concentrations ranging from pCa 7.0 to 4.5. The cross-sectional area of the fiber bundle was calculated based on an elliptical model. pCa-active tension relationships were fit to a modified Hill equation using nonlinear least-squares regression analysis (GraphPad Prism, version 5) to derive maximum tension (Tmax), half-maximal activating Ca2+ concentration (pCa50; an index representing myofilament Ca2+ responsiveness), and the Hill coefficient.
Measurement of myofilament Ca2+ activation under DTT treatment.
DTT treatment was performed in single skinned cells. Briefly, left ventricular tissue was placed in ice-cold high relaxing buffer containing 1 mM DTT and homogenate to generate single cell fragments as previously described (51). Cells were next placed in ice-cold high relaxing buffer containing 1% Triton X-100 for 30 min at 25°C (55). The skinned cells continued to stay in ice-cold high relaxing buffer with 1 mM DTT, and active force was then measured by attaching one end to a displacement generator and at the other end to a force transducer. Active tension was measured at a fixed sarcomere length of 2.2 μm in a solution containing various Ca2+ concentrations ranging from pCa 7.0 to 4.5 at room temperature. Isometric tension was recorded on a chart recorder. Isometric tension measurements were plotted as a function of pCa and fit by a nonlinear least-squares regression analysis to the Hill equation using GraphPad Prism 5. From this fitted curve, we derived the Tmax, pCa50, and the Hill coefficient.
OxyBlot procedure.
Oxidized myofilament proteins were determined using Western blot analysis and anti-dinitrophenyl (DNP) antibodies. The protein carbonyl side chains were derivatized to DNP by reaction with 2,4-dinitrophenylhydrazine. Samples were run on 12% SDS-PAGE at 4°C with dinitrophenylated protein molecular weight standards (Intergen). Proteins were transferred onto 0.45-µm pore size polyvinylidene difluoride membranes. Membranes were blocked and incubated with specific anti-DNP (1:100, OxyBlot kit, Millipore). Blots were washed, incubated with peroxidase-labeled anti-rabbit IgGs (1:300 dilution), and developed using a chemiluminescence detection system. The ratio between densitometric values of the OxyBlot bands and those of the corresponding bands stained with Coomassie blue was used as an index of oxidized proteins.
Immunoblot analysis.
The ratio of phosphorylated troponin I (pTnI) to total troponin I (TnI) was evaluated using immunoblot near-infrared fluorescence analysis. Myofilament proteins were purified, run on 10% SDS-PAGE, and then transferred to the nitrocellulose membrane. The membrane was blocked for 1 h in Odyssey blocking buffer and incubated overnight with specific antibodies to pTnI (Ser23/Ser24). Protein quantification was performed using a Li-Cor Odyssey automated infrared imaging system according to the manufacturer’s instructions. Quantification of pTnI and TnI was accomplished using Image Studio (version 4.0). Myosin heavy chain (MHC) isoforms were separated electrophoretically with a 6.5% SDS-polyacrylamide gel as previously described (35). After samples had been stained with Coomassie blue dye, the relative amount of α-MHC to total MHC was analyzed.
Statistical analysis.
Data are presented as means ± SE. Two-way ANOVA was used to determine the global effects of sex and DOX-induced cardiotoxicity followed by a Student-Newman-Keuls post hoc test (GraphPad PRISM7). A paired t-test was applied to study direct effects of H2O2 on the tension development of cardiac myofilaments. Values of P < 0.05 were considered as a significant difference between and among groups.
RESULTS
Sex differences in DOX-induced changes in myofilament function.
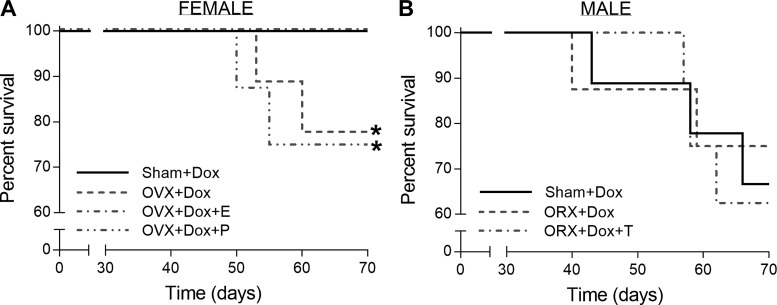
The in vivo characteristics of the female and male groups are shown in Table 1 and Table 2, respectively. Of the male rats treated with DOX, 80% had ascites, enlarged and pale livers, swollen kidneys, and spleens, and half suffered from diarrhea. Interestingly, these symptoms only occurred in 15% of female rats treated with DOX. While there was a protective effect of estrogen on body weight after DOX exposure, neither progesterone nor testosterone had any protective effect. However, there was no change in the percentage of heart weight normalized to body weight in rats treated with DOX compared with control rats. While all DOX-untreated groups of both sexes survived well throughout the term of study, DOX treatment decreased the percent survival in both groups (Fig. 1). DOX treatment induced later onset of mortality in female rats than in male rats. In addition, mortality in DOX-exposed groups of female OVX rats and female OVX rats supplemented with progesterone was ~25%, whereas ~40% mortality was observed in all male groups.
Table 1.
Body, heart, uterine, and lung weights from sham control and OVX rats without and with estrogen or progesterone supplementation of the DOX-untreated/treated rat set
| Without DOX | With DOX | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Sham | OVX | OVX + E | OVX + P | Sham | OVX | OVX + E | OVX + P |
| Body weight, g | 272 ± 3 | 370 ± 9* | 240 ± 5 | 367 ± 7* | 270 ± 4 | 320 ± 7* | 236 ± 5 | 323 ± 6* |
| Heart weight, g | 1.04 ± 0.03 | 1.33 ± 0.03* | 0.92 ± 0.03 | 1.31 ± 0.04* | 1.05 ± 0.04 | 1.19 ± 0.04 | 0.93 ± 0.03 | 1.17 ± 0.03 |
| Heart weight/body weight, % | 0.38 ± 0.01 | 0.36 ± 0.01 | 0.38 ± 0.01 | 0.36 ± 0.01 | 0.39 ± 0.01 | 0.37 ± 0.02 | 0.39 ± 0.01 | 0.36 ± 0.01 |
| Uterine weight, g | 0.55 ± 0.05 | 0.15 ± 0.01* | 0.57 ± 0.03 | 0.14 ± 0.01* | 0.55 ± 0.03 | 0.13 ± 0.01* | 0.57 ± 0.03 | 0.14 ± 0.01* |
| Lung dry/wet weight, % | 19.3 ± 0.4 | 20.7 ± 0.8 | 19.3 ± 0.7 | 19.8 ± 0.4 | 20.1 ± 0.4 | 20.4 ± 0.4 | 20.1 ± 0.5 | 20.7 ± 0.4 |
Values are means ± SE; n = 7–8 rats/group. DOX, doxorubicin; sham, sham operated; E, estrogen; P, progesterone; OVX, ovarectomized.
Significantly different (P < 0.05) from the sham control group of the same treatment using a Student-Newman-Keuls test after ANOVA.
Table 2.
Body, heart, uterine, and lung weights from sham control and ORX rats without and with testosterone supplementation of the DOX-untreated/treated rat set
| Without DOX | With DOX | |||||
|---|---|---|---|---|---|---|
| Parameters | Sham | ORX | ORX + T | Sham | ORX | ORX + T |
| Body weight, g | 498 ± 11 | 482 ± 9 | 482 ± 11 | 436 ± 8† | 424 ± 9† | 422 ± 11† |
| Heart weight, g | 1.73 ± 0.04 | 1.69 ± 0.06* | 1.69 ± 0.03 | 1.45 ± 0.02† | 1.41 ± 0.03† | 1.38 ± 0.02† |
| Heart weight/body weight, % | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.33 ± 0.01 |
| Seminal vesicular weight, g | 0.72 ± 0.01 | 0.09 ± 0.01* | 0.73 ± 0.02 | 0.40 ± 0.01† | 0.04 ± 0.01* | 0.53 ± 0.04† |
| Lung dry/wet weight, % | 21.5 ± 0.4 | 21.2 ± 0.3 | 21.1 ± 0.3 | 21.4 ± 0.1 | 21.9 ± 0.3 | 21.6 ± 0.3 |
Values are means ± SE; n = 8 rats/group. DOX, doxorubicin; sham, sham operated; T, testosterone; ORX, orchidectomized.
P < 0.05 from the sham control group of the same treatment;
P < 0.05 from the DOX-untreated sham group using a Student-Newman-Keuls test after ANOVA.
Fig. 1.
Effects of sex hormone deprivation and doxorubicin (DOX) treatment on percent survival. A: percent survival of female DOX-treated sham-operated (sham) control and ovarectomized (OVX) rats with and without estrogen (E)/progesterone (P) supplementation. B: percent survival of male DOX-treated sham control and orchidectomized (ORX) rats with and without testosterone (T) supplementation. *P < 0.05 from the DOX-treated sham control group by Kaplan-Meier analysis.
To elucidate sex differences in cardiac contractile function, active tension-Ca2+ relationships were examined in skinned papillary fibers. In female rats, Tmax was not significantly reduced by DOX exposure, although after OVX (which reduced Tmax by 31% on its own), DOX decreased Tmax by 55% (Fig. 2A). This suggests that estrogen protects against DOX-induced depression of Tmax. This was not the case in male rats, where the effect of ORX, DOX, and ORX + DOX on Tmax were all similar (40%, 49%, and 42% decreases, respectively; Fig. 2B). The protective effect of estrogen, but not testosterone, on DOX-induced changes in Tmax was further supported by treatment with sex hormones after gonadectomy. Reintroduction of estrogen was able to recover Tmax (Fig. 2C), whereas testosterone was not (Fig. 2D). Summary data for Tmax are shown in Fig. 2, E and F, and also show that progesterone in female rats, like testosterone in male rats, had no protective effect.
Fig. 2.
Effects of sex hormone deprivation and doxorubicin (DOX) treatment on skinned papillary function. A: mean active tension as a function of pCa and fitted curves from skinned left ventricular (LV) fibers from female sham-operated (sham) and ovarectomized (OVX) rats with and without DOX treatment. B: mean active tension as a function of pCa and fitted curves from skinned LV fibers from male sham and orchidectomized (ORX) rats with and without DOX treatment. C: mean active tension as a function of pCa and fitted curves from skinned LV fibers from female sham, OVX + DOX, and OVX + DOX groups with estrogen treatment (OVX + DOX + E2). D: mean active tension as a function of pCa and fitted curves from skinned LV fibers from male sham, ORX + DOX, and ORX + DOX groups with testosterone treatment (ORX + DOX + T). E and F: summary data for maximum active tension (Tmax). G and H: summary data for Ca2+ sensitivity (pCa50). Data are means ± SE of 10–16 fibers from 7–8 hearts/group. *P < 0.05 from the sham control groups of the same treatment; #P < 0.05 from the DOX-untreated sham groups using a Student-Newman-Keuls test after two-way ANOVA.
In addition, there were sex differences in DOX-induced effects on Ca2+ sensitivity in rat hearts. In female rats, DOX and OVX both increased Ca2+ sensitivity independently (Fig. 2G); however, in combination, OVX and DOX treatment had no effect on Ca2+ sensitivity. Thus, the DOX-induced increase in Ca2+ sensitivity is dependent on the presence of either estrogen or progesterone. Male rats showed the opposite, where DOX only caused an increase in Ca2+ sensitivity in the absence of the sex hormone (Fig. 2H).
From these results, we identified sex differences in the impact of DOX exposure on myofilament function, specifically Tmax and Ca2+ sensitivity. We explored the mechanisms of these differences independently, focusing first on Tmax and then on Ca2+ sensitivity.
Mechanisms of sex differences in the DOX-induced decrease in Tmax.
A shift in the MHC isoform from the predominant α-isoform toward more β-isoforms (the slow isoform) may be one mechanism underlying the suppressed Tmax in both hormone deprivation and DOX treatment conditions. Female sex hormone deprivation and DOX treatment significantly decreased the ratio of α-MHC to β-MHC in ventricular tissue compared with control animals (Fig. 3A). Estrogen prevents the isoform switching of MHC and preserves the suppressive effect of female sex hormone deficiency and DOX-induced toxicity on Tmax development of the heart. Male sex hormone deprivation also decreased the ratio of α-MHC to β-MHC, which could be prevented by testosterone supplementation. However, the protective effect of testosterone on MHC isoform switching disappeared under DOX treatment (Fig. 3B). The correlation between Tmax and MHC isoform switching (compare Fig. 2, E and A, with Fig. 2, F and B) suggests a role for this mechanism.
Fig. 3.
Effects of sex hormone deprivation and DOX treatment on expression of myosin heavy chain (MHC) isoforms. A: relative amount of α-MHC as a percentage of total (α+β) MHC of left ventricular homogenates from each female group. Inset: example gel for each of the eight groups. B: same as in A but for male groups. E, estrogen; P, progesterone; T, testosterone; OVX, ovarectomized; ORX, orchidectomized. Data are means ± SE from 4–5 hearts/group. *P < 0.05 from the sham control group of the same treatment; #P < 0.05 from the doxorubicin (DOX)-untreated sham group using a Student-Newman-Keuls test after two-way ANOVA.
However, DOX induces significant oxidative stress as well, so we also examined the oxidation level of the myofilaments using OxyBlot analysis. Myofilament oxidation was higher in OVX and DOX-exposed OVX rats (Fig. 4, A and B), a result that implies a higher level of oxidative stress in both groups. Progesterone did not reduce oxidative modifications in DOX-exposed OVX rats. In contrast, estrogen reduced myofilament protein oxidation in both OVX and DOX-exposed OVX groups. The oxidation level was not changed in male groups without DOX treatment (Fig. 4, C and D). However, the myofilament oxidation level was enhanced in DOX-treated male groups. These findings indicate that testosterone does not prevent oxidative modifications of myofilament proteins, whereas estrogen protects against myofilament oxidative modifications. Therefore, similar to MHC isoform shifting, these findings also show a correlation between Tmax and oxidative modifications of myofilament proteins.
Fig. 4.
Effects of sex hormone deprivation and doxorubicin (DOX) treatment on carbonylation levels of cardiac myofilament proteins. A: representative OxyBlot (left) and Coomassie-stained (right) gels demonstrating carbonylation of various myofilament proteins in left ventricular homogenates from each group of female rats. B: summary data showing the relative amount of carbonylated proteins to total protein from each group of female rats. C and D: same as in A and B but for male rat groups. MW, molecular weight standard; E, estrogen; P, progesterone; T, testosterone; OVX, ovarectomized; ORX, orchidectomized; MHC, myosin heavy chain; MyBP, myosin-binding protein; TnT, troponin T; Tm, tropomyosin; MLC, myosin light chain. Data are means ± SE from 4–5 hearts/group. *P < 0.05 from the sham control group of the same treatment; #P < 0.05 from the DOX-untreated sham group using a Student-Newman-Keuls test after two-way ANOVA.
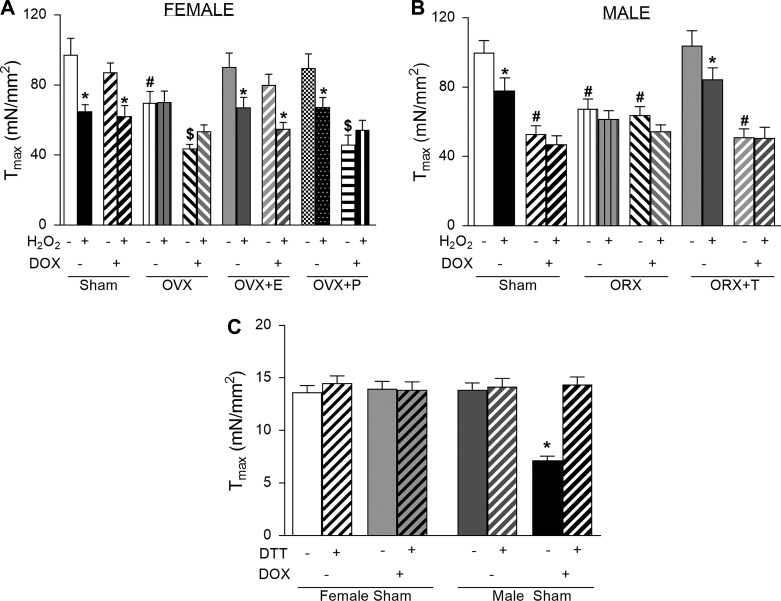
We next wanted to determine the contribution of each of these mechanisms (MHC isoform shifting or myofilament oxidative modifications) on the observed functional impact of gonadectomy and DOX on Tmax. To assess whether the impairment of myofilament function is due to a direct action of reactive oxygen species (ROS), we measured myofilament tension-Ca2+ relationships with and without 5 mM H2O2. As shown in Fig. 5, A and B, Tmax was decreased by H2O2 exposure in both female and male rats without DOX treatment. There was no further decrease in Tmax in both OVX and ORX groups with and without DOX treatment. This implies that oxidative modifications of myofilament proteins resulting in decreased Tmax had already occurred before the addition of H2O2 in those groups.
Fig. 5.
Direct effects of H2O2 and DTT on maximum active contraction in rat hearts. A: maximum tension (Tmax) in skinned left ventricular fibers in the absence and presence of 5 mM H2O2 from each group of female rats. B: same as in A but for the male rat groups. DOX, doxorubicin; E, estrogen; P, progesterone; T, testosterone; OVX, ovarectomized; ORX, orchidectomized. Data are means ± SE of 10–16 fibers from 7–8 hearts/group. *P < 0.05 from the H2O2-untreated group; #P < 0.05 from the sham control group of the same treatment; $P < 0.05 from the DOX-untreated sham group using a Student-Newman-Keuls test after two-way ANOVA. C: Tmax in skinned cardiac cells from sham groups of female and male rats in the absence/presence of 1 mM DTT treatment. Data are means ± SE of 15–16 single skinned cells from 5 hearts/group. *P < 0.05 from the DOX-untreated sham control group using a Student-Newman-Keuls test after two-way ANOVA.
To further confirm whether the observed decreases in Tmax were caused by oxidative modifications of myofilament proteins, we exposed single skinned myocytes to 1 mM DTT to determine whether the effects on Tmax could be reversed by a reducing agent. The suppression of Tmax from 13.9 ± 0.7 mN/mm2 in the male sham control group to 6.6 ± 0.3 mN/mm2 in the DOX-treated group could be completely reversed by DTT (14.4 ± 0.7 mN/mm2; Fig. 5C). As female rats were already protected from oxidative modifications, DTT showed no additional effect.
Together, these results suggest that the observed changes in Tmax induced by DOX exposure, sex hormone deprivation, or a combination are directly due to oxidative modifications of myofilament proteins.
Mechanisms of sex differences in DOX induces effects on myofilament Ca2+ sensitivity.
Interestingly, H2O2 (Fig. 6, A and B) and DTT (Fig. 6C) did not alter Ca2+ sensitivity compared with control fibers in both male and female groups. This suggests that the alteration in myofilament Ca2+ sensitivity induced by DOX treatment are caused by other modifications of myofilament proteins.
Fig. 6.
Effects of H2O2 and DTT on myofilament Ca2+ sensitivity in rat hearts. A: pCa50 in skinned left ventricular fibers in the absence and presence of 5 mM H2O2 from each group of female rats. B: same as in A but for the male rat groups. DOX, doxorubicin; E, estrogen; P, progesterone; T, testosterone; OVX, ovarectomized; ORX, orchidectomized. Data are means ± SE; n = 12–16 fibers from 7–8 hearts/group. #P < 0.05 from the DOX-untreated sham group using a Student-Newman-Keuls test after two-way ANOVA. C: pCa50 of skinned cardiac cells from sham groups of female and male rats with and without 1 mM DTT. Data are means ± SE of 15–16 cells from 5 hearts/group. *P < 0.05 from the sham control group of the same treatment using a Student-Newman-Keuls test after two-way ANOVA.
Myofilament protein phosphorylation is a well-established modulator of Ca2+ sensitivity, and so we investigated this mechanism in myofilament protein fractions of heart tissue from each group. We used Western blot analysis to probe TnI phosphorylation directly. In female rats, we observed an increase in Ser23/Ser24 TnI phosphorylation in the OVX + DOX group; in male rats, we observed increases in DOX and DOX + ORX + testosterone groups (Fig. 7, A and B). There were no changes in TnI phosphorylation status in the absence of DOX exposure. Thus, the TnI phosphorylation status explains the effects of DOX and ORX in male rats but only the effect of DOX in female rats in the absence of estrogen. There is therefore an additional, estrogen-dependent mechanism by which DOX alters Ca2+ sensitivity in female rats.
Fig. 7.
Effects of sex hormone deprivation and doxorubicin (DOX) treatment on cardiac troponin I (TnI) phosphorylation. A: quantification and representative immunoblot (above) of phosphorylated TnI at Ser23/Ser24 (pS23/24 TnI) and total TnI in female groups. B: same as in A but for the male groups. p/T TnI, phosphorylated to total TnI; E, estrogen; P, progesterone; T, testosterone; OVX, ovarectomized; ORX, orchidectomized. Data are means ± SE from 5 hearts/group. *P < 0.05 from sham control group of the same treatment; #P < 0.05 from the DOX-untreated sham group using a Student-Newman-Keuls test after two-way ANOVA.
DISCUSSION
In the present study, we revealed sex differences in cardiac contractile responses to chronic DOX-induced cardiotoxicity. First, specifically, estrogen, but not testosterone or progesterone, protects against DOX-induced depression of myofilament maximum Ca2+-activated tension. Second, while these changes correlated with shifting MHC isoforms, the primary mechanism by which estrogen protects against DOX-induced depressed Tmax is by inhibiting or reversing oxidative modifications of myofilament proteins. Third, DOX increased Ca2+ sensitivity in female rats only in the presence of estrogen and progesterone but increased Ca2+ sensitivity in male rats only in the absence of testosterone. Finally, DOX alters Ca2+ sensitivity in a phospho-Ser23/phospho-Ser24 TnI-dependent manner in male rats but only in the absence of estrogen in female rats (the mechanism is phosphorylation independent in the presence of estrogen).
In agreement with previous studies from our group and others (18, 20, 52, 54, 56), a reduction in Tmax was observed with both male and female sex hormone deprivation. However, with chronic DOX treatment, only estrogen could maintain Tmax. Thus, in male rats, Tmax decreased by 40% after 10 wk of DOX treatment, in agreement with a previous study (8). A proposed mechanism for this decrease in Tmax is a change in the relative levels of α-MHC and β-isoform of MHC (α-MHC and β-MHC, respectively) (14, 40, 52, 57), which also decreases the rate of force redevelopment (ktr) (19). However, our data here suggest that MHC isoform shifting is only one component, and we propose an alternative or additional mechanism: oxidative modifications of myofilament proteins in OVX female and male groups with DOX treatment.
It is unclear, however, whether the protective effects of estrogen on Tmax occur by abrogating the oxidative stress component of DOX exposure or by reversing the oxidative modifications of the proteins themselves. Metabolism of quinone-containing compounds like DOX generally leads to generation of free radicals in the microsomal NADPH-oxidase system (50). Due to the quinone moiety in the tetracyclic aglycone molecule, DOX can form many free radicals by cycling from quinone to semiquinone and then back to quinone, leading to the production of superoxide anions from oxygen. DOX has been reported to increase the peroxidation of polyunsaturated fatty acids within membranes as well as free oxygen radical activity. Estrogen has also been revealed as an effective antioxidant in fatty acid and cholesterol peroxidation (4) as well as in maintaining glutathione peroxidase, catalase, and SOD levels (38). Thus, estrogen might then be able to attenuate oxidative stress-induced cardiotoxicity directly.
The redox-dependent changes in contractile properties are determined by the source and type of the oxidant species, the level of oxidative stress, and the residues modified in individual myofilament proteins (48). There are many types of oxidative modifications of sarcomeric proteins induced by I/R or end-state human heart failure, including the reaction of a cysteine thiolate anion with H2O2 (S-sulfhydration), with nitric oxide (S-nitrosylation), or with GSH (S-glutathionylation). Moreover, ROS and peroxynitrite (ONOO−) can promote protein carbonylation, the addition of a carbonyl group to Lys, Arg, or Pro residues (11, 49). However, few of these have been studied with DOX exposure.
There are several oxidative modifications that have been shown to affect Tmax, which may be involved here. A study in skinned rat cardiac trabeculae found that superoxide anion (O2−) (33) and H2O2 (2) suppress Tmax without altering Ca2+ sensitivity, similar to what we found here. S-nitrosocysteine can also significantly decrease maximal isometric force, although also with a concomitant decrease myofilament Ca2+ sensitivity (21). Conversely, nitroxyl (HNO) increased force production and Ca2+ sensitivity by inducing the formation of dimers between MHC and Cys81 in myosin light chain-1 (22). S-nitrosylation of α-tropomyosin at Cys190 (in the overlap region) in human heart failure yields an interchain disulfide cross-link and alters its flexibility and interferes with its interaction with other thin filament proteins (10, 12, 53). These previous findings suggest that we are observing an oxidative modification that reversibly impacts Tmax but does not interfere with protein phosphorylation, such as S-glutathionylation of cardiac myosin-binding protein C (cMyBP-C), which attenuates cMyBP-C phosphorylation by protein kinases (47).
In addition to sex differences in Tmax, we observed differences in myofilament Ca2+ sensitivity, although the effect was complicated. DOX reduced Ca2+ sensitivity in OVX rat hearts concurrent with an increase in TnI Ser23/Ser24 phosphorylation. However, estrogen enhanced Ca2+ sensitivity in response to DOX-induced oxidative stress independently of any change in TnI phosphorylation. This suggests an estrogen-dependent, DOX-induced, irreversible (by DTT) oxidative modification that increases Ca2+ sensitivity. Previous studies have identified specific oxidant species and oxidative modifications that increase Ca2+ sensitivity that could be responsible for the changes observed here. For example, they are MHC and Cys81 in myosin light chain-1 dimer formation (induced by HNO) (22), tropomyosin oxidation at Cys190 detected in female mice early postmyocardial infarction (3), and S-glutathionylation of cMYBP-C at Cys479, Cys627, and Cys655 (identified in female mice treated with GSSG) (41). Moreover, sex-dependent myocardial S-nitrosothiol proteomes after I/R have recently been reported. Estrogen increases endothelial nitric oxide synthase expression and phosphorylation, resulting in increased S-nitrosylated proteins in female hearts, which likely contribute to sex-dependent cardioprotection (45). These studies suggest the possibility of female sex hormones modifying some aspects of the contractile machinery in a very specific manner that promotes myofilament Ca2+ sensitivity.
It is also possible that the increase in Ca2+ sensitivity with DOX in the presence of estrogen is a compensatory mechanism to offset the decrease in Tmax. While this would help to maintain force production, it could also provoke arrhythmias and diastolic dysfunction.
In conclusion, the results from the present study reveal sex differences in the cardiac contractile response to chronic DOX treatment. Our results support a cardioprotective role for estrogen but not testosterone. These findings expand our understanding of the impact of sex hormones on cardiac contractile function and provide a basis for further preventive and/or therapeutic approaches to cardiac damage induced by DOX.
GRANTS
This work was supported by grants from Mahidol University (to J. Wattanapermpool), National Heart, Lung, and Blood Institute Grants HL-62426 (to P. P. de Tombe) and HL-136737 (to J. A. Kirk), Royal Golden Jubilee PhD Program Grant PHD/0233/2553 (to C. Rattanasopa and J. Wattanapermpool), and American Heart Association Grant 14SDG20380148 (to J. A. Kirk).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R., J.A.K., and J.W. conceived and designed research; C.R. and M.P. performed experiments; C.R. and J.A.K. analyzed data; C.R., J.A.K., P.P.d.T., and J.W. interpreted results of experiments; C.R. and J.A.K. prepared figures; C.R. and J.A.K. drafted manuscript; C.R., J.A.K., T.B.-I., M.P., P.P.d.T., and J.W. edited and revised manuscript; J.A.K. and J.W. approved final version of manuscript.
REFERENCES
- 1.Altieri P, Barisione C, Lazzarini E, Garuti A, Bezante GP, Canepa M, Spallarossa P, Tocchetti CG, Bollini S, Brunelli C, Ameri P. Testosterone antagonizes doxorubicin-induced senescence of cardiomyocytes. J Am Heart Assoc 5: e002383, 2016. doi: 10.1161/JAHA.115.002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avner BS, Hinken AC, Yuan C, Solaro RJ. H2O2 alters rat cardiac sarcomere function and protein phosphorylation through redox signaling. Am J Physiol Heart Circ Physiol 299: H723–H730, 2010. doi: 10.1152/ajpheart.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avner BS, Shioura KM, Scruggs SB, Grachoff M, Geenen DL, Helseth DL Jr, Farjah M, Goldspink PH, Solaro RJ. Myocardial infarction in mice alters sarcomeric function via post-translational protein modification. Mol Cell Biochem 363: 203–215, 2012. doi: 10.1007/s11010-011-1172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayres S, Tang M, Subbiah MT. Estradiol-17β as an antioxidant: some distinct features when compared with common fat-soluble antioxidants. J Lab Clin Med 128: 367–375, 1996. doi: 10.1016/S0022-2143(96)80008-4. [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation 95: 252–264, 1997. doi: 10.1161/01.CIR.95.1.252. [DOI] [PubMed] [Google Scholar]
- 6.Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail 9: 409–414, 2007. doi: 10.1016/j.ejheart.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Bell JR, Curl CL, Harding TW, Vila Petroff M, Harrap SB, Delbridge LMD. Male and female hypertrophic rat cardiac myocyte functional responses to ischemic stress and β-adrenergic challenge are different. Biol Sex Differ 7: 32, 2016. doi: 10.1186/s13293-016-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottone AE, Voest EE, de Beer EL. Impairment of the actin-myosin interaction in permeabilized cardiac trabeculae after chronic doxorubicin treatment. Clin Cancer Res 4: 1031–1037, 1998. [PubMed] [Google Scholar]
- 9.Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol 291: H1101–H1108, 2006. doi: 10.1152/ajpheart.00660.2005. [DOI] [PubMed] [Google Scholar]
- 10.Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S. Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol 57: 300–309, 2011. doi: 10.1016/j.jacc.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Canton M, Neverova I, Menabò R, Van Eyk J, Di Lisa F. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286: H870–H877, 2004. doi: 10.1152/ajpheart.00714.2003. [DOI] [PubMed] [Google Scholar]
- 12.Canton M, Skyschally A, Menabò R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J 27: 875–881, 2006. doi: 10.1093/eurheartj/ehi751. [DOI] [PubMed] [Google Scholar]
- 13.Caram MEV, Guo C, Leja M, Smerage J, Henry NL, Giacherio D, Rubenfire M, Schott A, Davis M, Hayes DF, Van Poznak C, Cooney KA, Hertz DL, Banerjee M, Griggs JJ. Doxorubicin-induced cardiac dysfunction in unselected patients with a history of early-stage breast cancer. Breast Cancer Res Treat 152: 163–172, 2015. doi: 10.1007/s10549-015-3454-8. [DOI] [PubMed] [Google Scholar]
- 14.Carnes CA, Geisbuhler TP, Reiser PJ. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats. J Appl Physiol (1985) 97: 446–453, 2004. doi: 10.1152/japplphysiol.00439.2003. [DOI] [PubMed] [Google Scholar]
- 15.Cavasin MA, Sankey SS, Yu A-L, Menon S, Yang X-P. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 284: H1560–H1569, 2003. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 16.Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol 290: H2043–H2050, 2006. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology 115: 155–162, 2010. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curl CL, Delbridge LM, Canny BJ, Wendt IR. Testosterone modulates cardiomyocyte Ca2+ handling and contractile function. Physiol Res 58: 293–297, 2009. [DOI] [PubMed] [Google Scholar]
- 19.de Beer EL, Bottone AE, van Der Velden J, Voest EE. Doxorubicin impairs crossbridge turnover kinetics in skinned cardiac trabeculae after acute and chronic treatment. Mol Pharmacol 57: 1152–1157, 2000. [PubMed] [Google Scholar]
- 20.Eleawa SM, Sakr HF, Hussein AM, Assiri AS, Bayoumy NM, Alkhateeb M. Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta Physiol (Oxf) 209: 136–147, 2013. doi: 10.1111/apha.12158. [DOI] [PubMed] [Google Scholar]
- 21.Figueiredo-Freitas C, Dulce RA, Foster MW, Liang J, Yamashita AM, Lima-Rosa FL, Thompson JW, Moseley MA, Hare JM, Nogueira L, Sorenson MM, Pinto JR. S-nitrosylation of sarcomeric proteins depresses myofilament Ca2+ sensitivity in intact cardiomyocytes. Antioxid Redox Signal 23: 1017–1034, 2015. doi: 10.1089/ars.2015.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res 111: 1002–1011, 2012. doi: 10.1161/CIRCRESAHA.112.270827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grohé C, Kahlert S, Löbbert K, Stimpel M, Karas RH, Vetter H, Neyses L. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett 416: 107–112, 1997. doi: 10.1016/S0014-5793(97)01179-4. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y, Aihara K, Akaike M, Sato T, Ishikawa K, Ise T, Yagi S, Iwase T, Ueda Y, Yoshida S, Azuma H, Walsh K, Tamaki T, Kato S, Matsumoto T. Androgen receptor counteracts doxorubicin-induced cardiotoxicity in male mice. Mol Endocrinol 24: 1338–1348, 2010. doi: 10.1210/me.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingegno MD, Money SR, Thelmo W, Greene GL, Davidian M, Jaffe BM, Pertschuk LP. Progesterone receptors in the human heart and great vessels. Lab Invest 59: 353–356, 1988. [PubMed] [Google Scholar]
- 26.Janssen PM, de Tombe PP. Protein kinase A does not alter unloaded velocity of sarcomere shortening in skinned rat cardiac trabeculae. Am J Physiol Heart Circ Physiol 273: H2415–H2422, 1997. doi: 10.1152/ajpheart.1997.273.5.H2415. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem 281: 6760–6767, 2006. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 28.Kolodgie FD, Farb A, Litovsky SH, Narula J, Jeffers LA, Lee SJ, Virmani R. Myocardial protection of contractile function after global ischemia by physiologic estrogen replacement in the ovariectomized rat. J Mol Cell Cardiol 29: 2403–2414, 1997. doi: 10.1006/jmcc.1997.0476. [DOI] [PubMed] [Google Scholar]
- 29.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 332: 1738–1744, 1995. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 30.Lipshultz SE, Sambatakos P, Maguire M, Karnik R, Ross SW, Franco VI, Miller TL. Cardiotoxicity and cardioprotection in childhood cancer. Acta Haematol 132: 391–399, 2014. doi: 10.1159/000360238. [DOI] [PubMed] [Google Scholar]
- 31.Luczak ED, Leinwand LA. Sex-based cardiac physiology. Annu Rev Physiol 71: 1–18, 2009. doi: 10.1146/annurev.physiol.010908.163156. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald JK, Pyle WG, Reitz CJ, Howlett SE. Cardiac contraction, calcium transients, and myofilament calcium sensitivity fluctuate with the estrous cycle in young adult female mice. Am J Physiol Heart Circ Physiol 306: H938–H953, 2014. doi: 10.1152/ajpheart.00730.2013. [DOI] [PubMed] [Google Scholar]
- 33.MacFarlane NG, Miller DJ. Depression of peak force without altering calcium sensitivity by the superoxide anion in chemically skinned cardiac muscle of rat. Circ Res 70: 1217–1224, 1992. doi: 10.1161/01.RES.70.6.1217. [DOI] [PubMed] [Google Scholar]
- 34.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK, Green GE, Schiebinger RJ. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 98: 256–261, 1998. doi: 10.1161/01.CIR.98.3.256. [DOI] [PubMed] [Google Scholar]
- 35.Martin AF, Phillips RM, Kumar A, Crawford K, Abbas Z, Lessard JL, de Tombe P, Solaro RJ. Ca2+ activation and tension cost in myofilaments from mouse hearts ectopically expressing enteric γ-actin. Am J Physiol Heart Circ Physiol 283: H642–H649, 2002. doi: 10.1152/ajpheart.00890.2001. [DOI] [PubMed] [Google Scholar]
- 36.Morrissy S, Xu B, Aguilar D, Zhang J, Chen QM. Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell 9: 799–809, 2010. doi: 10.1111/j.1474-9726.2010.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moulin M, Piquereau J, Mateo P, Fortin D, Rucker-Martin C, Gressette M, Lefebvre F, Gresikova M, Solgadi A, Veksler V, Garnier A, Ventura-Clapier R. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circ Heart Fail 8: 98–108, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001180. [DOI] [PubMed] [Google Scholar]
- 38.Muñoz-Castañeda JR, Montilla P, Muñoz MC, Bujalance I, Muntané J, Túnez I. Effect of 17-β-estradiol administration during adriamycin-induced cardiomyopathy in ovariectomized rat. Eur J Pharmacol 523: 86–92, 2005. doi: 10.1016/j.ejphar.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 39.Myrehaug S, Pintilie M, Yun L, Crump M, Tsang RW, Meyer RM, Sussman J, Yu E, Hodgson DC. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood 116: 2237–2240, 2010. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 40.Pandit S, Woranush W, Wattanapermpool J, Bupha-Intr T. Significant role of female sex hormones in cardiac myofilament activation in angiotensin II-mediated hypertensive rats. J Physiol Sci 64: 269–277, 2014. doi: 10.1007/s12576-014-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel BG, Wilder T, Solaro RJ. Novel control of cardiac myofilament response to calcium by S-glutathionylation at specific sites of myosin binding protein C. Front Physiol 4: 336, 2013. doi: 10.3389/fphys.2013.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rattanasopa C, Phungphong S, Wattanapermpool J, Bupha-Intr T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J Steroid Biochem Mol Biol 147: 1–9, 2015. doi: 10.1016/j.jsbmb.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro Júnior RF, Ronconi KS, Jesus ICG, Almeida PWM, Forechi L, Vassallo DV, Guatimosim S, Stefanon I, Fernandes AA. Testosterone deficiency prevents left ventricular contractility dysfunction after myocardial infarction. Mol Cell Endocrinol 460: 14–23 2018. doi: 10.1016/j.mce.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Schnabel RB, Wild PS, Prochaska JH, Ojeda FM, Zeller T, Rzayeva N, Ebrahim A, Lackner KJ, Beutel ME, Pfeiffer N, Sinning CR, Oertelt-Prigione S, Regitz-Zagrosek V, Binder H, Münzel T, Blankenberg S; Gutenberg Health Study Investigators . Sex differences in correlates of intermediate phenotypes and prevalent cardiovascular disease in the general population. Front Cardiovasc Med 2: 15, 2015. doi: 10.3389/fcvm.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao Q, Fallica J, Casin KM, Murphy E, Steenbergen C, Kohr MJ. Characterization of the sex-dependent myocardial S-nitrosothiol proteome. Am J Physiol Heart Circ Physiol 310: H505–H515, 2016. doi: 10.1152/ajpheart.00681.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silber JH, Jakacki RI, Larsen RL, Goldwein JW, Barber G. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol 21: 477–479, 1993. doi: 10.1002/mpo.2950210704. [DOI] [PubMed] [Google Scholar]
- 47.Stathopoulou K, Wittig I, Heidler J, Piasecki A, Richter F, Diering S, van der Velden J, Buck F, Donzelli S, Schröder E, Wijnker PJ, Voigt N, Dobrev D, Sadayappan S, Eschenhagen T, Carrier L, Eaton P, Cuello F. S-glutathiolation impairs phosphoregulation and function of cardiac myosin-binding protein C in human heart failure. FASEB J 30: 1849–1864, 2016. doi: 10.1096/fj.201500048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinberg SF. Oxidative stress and sarcomeric proteins. Circ Res 112: 393–405, 2013. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiago T, Palma PS, Gutierrez-Merino C, Aureliano M. Peroxynitrite-mediated oxidative modifications of myosin and implications on structure and function. Free Radic Res 44: 1317–1327, 2010. doi: 10.3109/10715762.2010.502170. [DOI] [PubMed] [Google Scholar]
- 50.Vásquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA Jr, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry 36: 11293–11297, 1997. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 51.Vives-Bauza C, Yang L, Manfredi G. Assay of mitochondrial ATP synthesis in animal cells and tissues. Methods Cell Biol 80: 155–171, 2007. doi: 10.1016/S0091-679X(06)80007-5. [DOI] [PubMed] [Google Scholar]
- 52.Vutthasathien P, Wattanapermpool J. Regular exercise improves cardiac contractile activation by modulating MHC isoforms and SERCA activity in orchidectomized rats. J Appl Physiol (1985) 119: 831–839, 2015. doi: 10.1152/japplphysiol.00224.2015. [DOI] [PubMed] [Google Scholar]
- 53.Walsh TP, Wegner A. Effect of the state of oxidation of cysteine 190 of tropomyosin on the assembly of the actin-tropomyosin complex. Biochim Biophys Acta 626: 79–87, 1980. doi: 10.1016/0005-2795(80)90199-3. [DOI] [PubMed] [Google Scholar]
- 54.Wattanapermpool J. Increase in calcium responsiveness of cardiac myofilament activation in ovariectomized rats. Life Sci 63: 955–964, 1998. doi: 10.1016/S0024-3205(98)00353-1. [DOI] [PubMed] [Google Scholar]
- 55.Witayavanitkul N, Ait Mou Y, Kuster DW, Khairallah RJ, Sarkey J, Govindan S, Chen X, Ge Y, Rajan S, Wieczorek DF, Irving T, Westfall MV, de Tombe PP, Sadayappan S. Myocardial infarction-induced N-terminal fragment of cardiac myosin-binding protein C (cMyBP-C) impairs myofilament function in human myocardium. J Biol Chem 289: 8818–8827, 2014. doi: 10.1074/jbc.M113.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witayavanitkul N, Woranush W, Bupha-Intr T, Wattanapermpool J. Testosterone regulates cardiac contractile activation by modulating SERCA but not NCX activity. Am J Physiol Heart Circ Physiol 304: H465–H472, 2013. doi: 10.1152/ajpheart.00555.2012. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res 57: 388–394, 2003. doi: 10.1016/S0008-6363(02)00705-8. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Fang J, Gillespie C, Wang G, Hong Y, Yoon PW. Age-specific gender differences in in-hospital mortality by type of acute myocardial infarction. Am J Cardiol 109: 1097–1103, 2012. doi: 10.1016/j.amjcard.2011.12.001. [DOI] [PubMed] [Google Scholar]