Abstract
The progression of coronary artery diseases in premenopausal women is lower than in age-matched men; however, its probability increases rapidly after menopause. The aim of our study was to investigate the postconditioning-like effects of voluntary physical exercise on postmenopausal cardiovascular outcomes after myocardial infarction. We used fertile Wistar females [control (CTRL)] and pharmacologically induced estrogen-deficient (POVX; 750 µg/kg triptorelin im, every 4th week) rats. CTRL and POVX animals were randomly assigned to receive an injection of 0.1 mg isoproterenol (ISO)/kg. At the 20th hour after ISO injection, serum markers of myocardial injury, such as lactate dehydrogenase (LDH) and myoglobin, were measured. After a 3-wk resting period, ISO-treated and untreated animals were further divided into subgroups on the basis of 6 wk of physical exercise. At the end of the experiment, cardiac activity and content of the antioxidative heme oxygenase (HO) enzyme, levels of GSH and GSH + GSSG, activity of myeloperoxidase, as well as the concentration of TNF-α were determined. At the end of the experimental period, we observed a significant decrease in the activity and content of HO enzymes in POVX and POVX/ISO rats, whereas physical exercise significantly improved HO and GSH values in both CTRL and POVX rats. Furthermore, our training protocol significantly reduced the pathological levels of myeloperoxidase and TNF-α. Our findings clearly demonstrate that modulation of the HO system by voluntary physical exercise is a key process to decrease inflammatory parameters and ameliorate the antioxidative status in estrogen-deficient conditions postmyocardial injury.
NEW & NOTEWORTHY We used a noninvasive rat model of estrogen deficiency and myocardial infarction. The long-term effects of isoproterenol treatment revealed reduced heme oxygenase enzyme activity and expression and decreased glutathione levels. Isoproterenol treatment enhanced the myeloperoxidase enzyme activity. Voluntary physical exercise ameliorated the antioxidative status by increasing of the heme oxygenase enzyme system. Voluntary physical exercise is a potential therapeutic tool to improve cardiac antioxidant status in menopausal women postmyocardial injury.
Keywords: estrogen deficiency, heme oxygenase, inflammation, myocardial infarction, physical exercise
INTRODUCTION
In premenopausal women, the incidence and progression of coronary artery diseases are lower compared with age-matched men; however, they increase rapidly after the onset of menopause (3, 34). While female sex hormones have been proven to be antioxidative and to modulate vasodilator system, loss of estrogen can be associated with increasing oxidative stress and cardiovascular risk factors (30). Among the main factors that contribute to maintaining physiological antioxidant/oxidant homeostasis, the heme oxygenase (HO) enzyme system plays a key role against oxidative stress-induced processes. The disruption of the antioxidant/oxidant balance related to estrogen deficiency may lead to pathological changes in women postmyocardial infarction (post-MI). Studies using animal models have been conducted to better understand the mechanisms that occur during and after MI.
Isoproterenol (ISO) is a synthetic β-adrenoceptor agonist that induces myocardial injury in rats. Therefore, the animal model of ISO-induced MI provides a noninvasive technique for investigating the pathological processes and dysfunctions in the myocardium (23). Recent studies have proven that β-adrenergic receptor activation plays a key role in the production of reactive oxygen species (ROS), which initiate the development of MI (12). ISO-induced acute ROS generation can be associated with low reduced glutathione-to-oxidized glutathione ratios (GSH/GSSG) as well as superoxide dismutase, catalase, and GSH values (28, 39). Moreover, it induces the expression of proinflammatory cytokines, which cause structural and functional changes in the myocardium (12).
As a result of estrogen deprivation, postmenopausal women suffer from adverse cardiovascular outcomes post-MI (9, 29). Hence, the assessment of risk stratification and therapeutic approaches are necessary to improve cardiac prognosis after MI. In our previous studies, we verified that voluntary physical exercise is a potential preventive strategy to ameliorate cardiometabolic parameters in experimental menopause (33), and it resulted in positive effects on cardiac remodeling and a significant decrease in ischemia-reperfusion injury (41).
Based on our earlier investigations, the aim of the present study was to examine the long-term effects of ISO-induced cardiac damage on antioxidant and inflammatory profiles in fertile and estrogen-deficient rat models. We hypothesized that voluntary physical exercise could be an effective therapeutic strategy to ameliorate the ISO-induced cardiac abnormalities.
MATERIALS AND METHODS
Animals.
Female Wistar rats weighing 180–200 g (Toxi-Coop) were used in our research. Animals were kept in cages with free access to water and phytoestrogen-free rat chow (Table 1) at controlled temperature (20–23°C) and a 12:12-h light-dark cycle. All procedures were approved by the Institutional Ethical Committee and were performed in accordance with the standards of European Community guidelines on the care and use of laboratory animals (I.74-40/2017. MÁB).
Table 1.
Nutrient composition of phytoestrogen-free rat chow
| Nutrient Composition | Phytoestrogen-Free Chow |
|---|---|
| Sorghum | 58.4% |
| Potato protein | 15% |
| Locust bean sleet | 13% |
| Nonfat sweet whey powder | 5.5% |
| Wheat straw | 5.0% |
| Monocalcium phosphate | 1.5% |
| l-lysine-HCl | 0.7% |
| NaCl | 0.4% |
| Vitamins and microelement mixture | 0.5% |
| Total | 100% |
Experimental protocol.
After a 1-wk acclimatization period, rats were divided into control (CTRL) and pharmacologically induced estrogen-deficient (POVX) animal groups. To achieve estrogen-depleted conditions, POVX animals received 750 µg/kg triptorelin im (Decapeptyl depot, Ferring) every fourth week. Rats in the CTRL group received an intramuscular injection of physiological saline (every fourth week). After two injection treatments with triptorelin (56 days) to verify POVX-induced menopause, serum estradiol levels were checked using an estradiol quantitative ELISA (Rat E2 ELISA Kit, SunRed Biological Technology, Shanghai, China) (30).
The induction of MI was performed in CTRL and POVX animals through subcutaneous administration of ISO (Sigma Chemicals) at a single dose of 0.1 mg/kg diluted in 1 ml physiological saline solution. False induction of MI was performed by a single subcutaneous administration of 1 ml saline in both CTRL and POVX groups. To ensure that ISO treatment caused myocardial injury, serum lactate dehydrogenase (LDH) and myoglobin values were determined 20 h after ISO induction, and cardiac damage was proven by 1% 2,3,5-triphenyltetrazolium chloride (TTC) staining (10 min at 37°C).
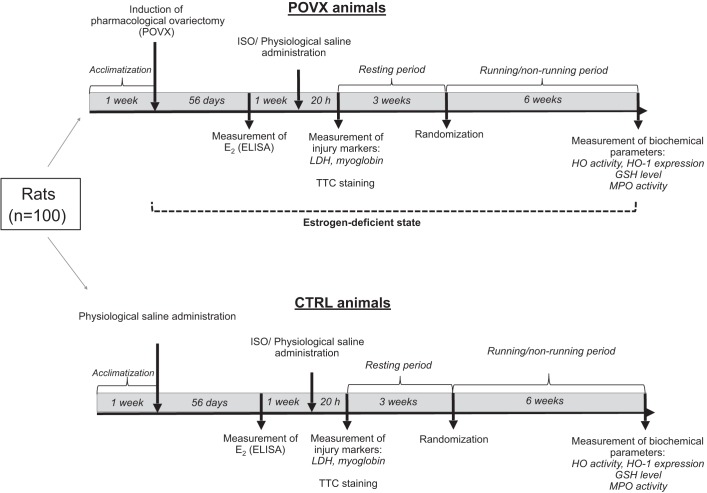
Three weeks after ISO/saline treatment, CTRL and POVX animals were randomly divided into running and sedentary subgroups. During our experimental period, rats in the exercise subgroups were placed into special cages in pairs to avoid stress-induced effects and to provide social relationships. These cages were equipped with a running wheel. Free access to the wheel was provided 24 h/day for 6 wk. The training protocol was defined as a voluntary wheel-running model and was selected in an effort to isolate the effects of exercising from the additional stress associated with forced exercise protocols (31). The average running distance was 4.0 km ± 10.0%·day−1·rat−1. Animals from the sedentary subgroups were housed for the same amount of time in standard holding cages. Considering the potential weight gain, three rats were placed in each cage to provide 350 cm2 territory/rat (Directive 2010/63/EU). In our experiment, all efforts were made to minimize the number of animals as well as animal suffering. One hundred female rats were uniformly divided into eight subgroups based on the different treatments (POVX, ISO, and exercise). The number of animals was n = 9–12 rats/subgroup; however, POVX and ISO treatments resulted in a decrease in sample size. At the end of the experimental protocol, heart tissues were dissected immediately. After dissection, hearts were either used for TTC staining or were clamped, milled, and then stored at −80°C until biochemical measurements. The experimental protocol of the study is shown in Fig. 1.
Fig. 1.
The experimental protocol of the study. POVX, pharmacologically induced estrogen deficiency; CTRL, control; E2, estradiol; GSH, glutathione; HO, heme oxygenase; ISO, isoproterenol; LDH, lactate dehydrogenase; MPO, myeloperoxidase enzyme; TTC, 2,3,5-triphenyltetrazolium chloride.
Measurement of serum LDH and myoglobin.
Blood samples were collected from vena safena 20 h after the induction of ISO. Serum samples were centrifuged at 2,000 rpm for 20 min at 4°C, and the supernatant was assayed with commercial kits (GenAsia Biotech, Shanghai, China) at 450 nm (Benchmark Microplate reader, Bio-Rad, Hercules, CA). LDH values are expressed in units per liter, and myoglobin values are expressed in nanograms per liter.
Measurement of serum glutamic oxaloacetic transaminase, glutamic-pyruvic transaminase, and alkaline phosphatase.
Serum levels of glutamic oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), and alkaline phosphatase (ALP) were measured on a Biolis 24i Premium system (Siemens) 20 h after ISO treatment. Reactions of GOT and GPT were monitored by measuring the rate of decrease in absorbance at 340 nm due to the oxidation of NADH to NAD. The method of the ALP measurement used 4-nitrophenylphosphate as a substrate, and ALP activity was measured at 405 nm. GOT, GPT, and ALP values are expressed in units per liter (16, 17).
Measurement of HO activity.
Rat heart tissues were homogenized in ice-cold buffer containing 10.0 mM HEPES, 32.0 mM sucrose, 1.0 mM DTT, 0.10 mM EDTA, 10.0 μg/ml trypsin inhibitor, 10.0 μg/ml leupeptin, and 2.0 μg/ml aprotinin (pH 7.4). After centrifugation at 15,000 g for 20 min at 4°C, we collected the supernatant. The reaction mix contained the following compounds in a total volume of 1.50 ml: 2.0 mM glucose-6-phosphate, 0.14 U/ml glucose-6-phosphate dehydrogenase, 15.0 μM hemin, 120.0 μg/ml rat liver cytosol as a source of biliverdin reductase, 2.0 mM MgCl2·6H2O, 100.0 mM KH2PO4, and 150.0 μl supernatant. The reaction was started by the addition of 100.0 μl reduced β-NADPH and incubation in the dark for 60 min at 37°C. The reaction was stopped by ice cooling. Bilirubin content was determined as follows: optical density was measured at 465 and 530 nm, and the difference between the two densities was calculated. HO activity was defined as the amount of bilirubin (in nmol) produced per hour per milligram of protein.
Measurement of HO-1 content.
Cardiac tissues were homogenized in RIPA buffer and then sonicated for 10 min. Homogenates were centrifuged at 12,000 rpm at 4°C for 10 min and then boiled for 5 min. Equal amounts of protein (80 µg) were separated (90 V/2 gels) by 10% polyacrylamide gel and transferred (35 V/2 gels for 2.5 h) to nitrocellulose membranes. In the presence of 0.10% Ponceau red, we could determine the equivalence of protein loading. To increase the final HO-1 signal sensitivity, membranes were incubated with Pierce Western Blot Signal Enhancer (ThermoFisher Scientific) and then blocked for overnight in 5% nonfat dry milk in Tris-buffered saline-Tween 20. In our previous studies, anti-HO-1 (ab82219, Abcam) and anti-β-actin antibodies (ab20272, Abcam) have been tested in cardiac samples. Due to the adequate probes, these antibodies were used in the present work. Proteins were detected by incubating the membranes for 4 h at room temperature with anti-HO-1 rabbit polyclonal primary antibody (1:500, ab82219, Abcam). After being washed, membranes were incubated for 1 h at room temperature with bovine anti-rabbit IgG-horseradish peroxidase (1:5,000, sc-2370, Santa-Cruz Biotechnology). For our HO-1 immunoblot, β-actin served as the loading control to show that similar amounts of protein were loaded in each lane. For β-actin detection, membranes were blocked for overnight in 5% BSA. After being washed, membranes were probed with mouse anti-β-actin antibody (1:4,000, 2 h, ab20272, Abcam) (21) and then incubated with polyclonal rabbit anti-mouse horseradish peroxidase (1:2,000, 1 h, Dako). MagicMark XP Western protein standard (Invitrogen, ThermoFisher Scientific) was used for convenient protein determination. The standard consists of nine recombinant proteins ranging in molecular mass from 20 to 220 kDa and promotes the identification of both HO-1 and β-actin proteins. Furthermore, the specificity of the HO-1 antibody was evaluated by loading recombinant HO-1 protein (ADI-SPP-730, Enzo). Bands were visualized by Uvi Chemi Pro and analyzed by Quantity One software (Bio-Rad).
Measurement of cardiac GSH + GSSG content.
Rat cardiac tissues were homogenized in a solution of 0.25 M sucrose, 1 mM DTT, and 20 mM Tris and then centrifuged at 15,000 g for 30 min at 4°C. Supernatant fractions were collected, and 0.1 M CaCl2, 0.25 M sucrose, 20 mM Tris, and 1 mM DTT were pipetted to the samples. After an incubation at 0°C for 30 min, samples were further centrifuged at 21,450 g for 60 min at 4°C. As a diluent buffer, a mixture of 125 mM Na phosphate and 6.0 mM EDTA was used for the stock solution of GSH, GSH reductase, 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB), and β-NADPH. A total volume of 40 µl of each blank, standard, or heart sample and equal volumes of DTNB stock solution (20 µl) and β-NADPH (140 µl) were added to each well and then incubated at 25°C for 5 min. A 10-µl volume of GSH reductase was used to start the reaction, and absorbance was measured at 405 nm in a microplate reader after 10 min from the initiation of the reaction.
In the spectrophotometric assay for total GSH, GSH was sequentially oxidized by DTNB and reduced by NADPH in the presence of GSH reductase. Total GSH values are expressed in nanomoles per milligram of protein (41).
Determination of cardiac GSH and TNF-α.
Cardiac samples were homogenized (Ultra-Turrax T8, 2 × 30 s) in phosphate buffer (pH 7.4) and then centrifuged at 2,000 rpm for 20 min at 4°C. Cardiac GSH and TNF-α were assayed with commercial kits purchased from GenAsia, and optical density was measured at 450 nm (Benchmark Microplate reader, Bio-Rad). GSH levels are expressed in milligrams per liter, and TNF-α values are expressed in picograms per milligram of protein.
Measurement of myeloperoxidase activity.
Rat heart samples were homogenized in a solution composed of ice-cold PBS and 0.5% hexadecyltrimethylammoniumbromide (pH 6.0). After three repeats of freezing and melting, samples were centrifuged at 15,000 g for 15 min at 4°C. The supernatant was removed, and a 12-µl aliquot was pipetted to a mixture of 280 µl PBS (pH 6.0) and 0.167 mg/ml of O-dianisidine dihydrochloride. The reaction was stated with 10 µl of 0.03% hydrogen peroxide and shaken for 90 s. Cardiac myeloperoxidase (MPO) activity was measured spectrophotometrically at 490 nm and is expressed in microunits per milligram of protein (30).
Protein determination.
With the use of a commercial protein assay kit (Bio-Rad Laboratories), aliquots (20 μl) of the diluted samples were mixed with 980 μl distilled water with 200 μl Bradford reagent added to each sample. After being mixing and after 10 min of incubation, samples were assayed spectrophotometrically at 595 nm. Protein levels are expressed in milligrams of protein per milliliter.
Statistical analysis.
Results are expressed as means ± SE. The normality of data was verified with a Sharipo-Wilk test. Differences between groups were calculated using one-way ANOVA followed by a Tukey posttest. P ≤ 0.05 was considered as significant. Furthermore, the interaction of the paired combinations of the three parameters (ISO treatment, estrogen status, and exercise) was investigated with two-way ANOVA with a Tukey posttest.
RESULTS
Changes in serum estradiol.
Serum estradiol levels were determined after two injection treatments with triptorelin (56 days). We detected significantly diminished estrogen values in POVX rats compared with fertile CTRL rats (CTRL: 290.92 ± 3.10 ng/l vs. POVX: 160.90 ± 2.98 ng/l).
Effects of ISO treatment on myocardial injury marker enzymes.
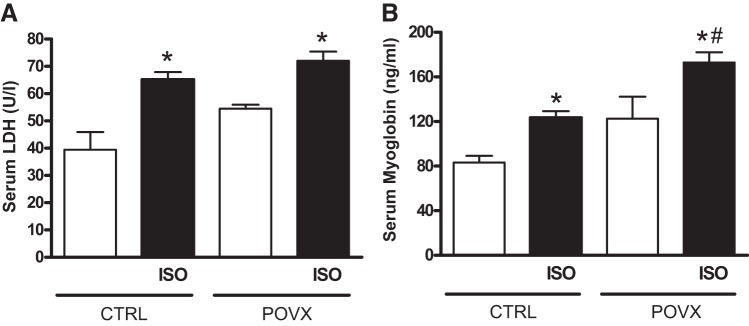
Figure 2 shows serum LDH activities and myoglobin contents 20 h after ISO treatment. ISO administration produced a significant increase in LDH values of both CTRL and POVX animals. Similarly, myoglobin levels were also elevated in the two ISO groups. POVX animals treated with ISO exhibited the highest level of myoglobin, which was twofold higher than in CTRL animals.
Fig. 2.
A: changes in serum lactate dehydrogenase (LDH) concentrations at 20 h after isoproterenol (ISO) treatment. Results are shown as means ± SE; n = 7–14. B: changes in serum myoglobin concentrations at 20 h after ISO treatment. Results are shown as means ± SE; n = 4–11. *P < 0.05, statistical significance between ISO vs. the respective non-ISO groups; #P < 0.05, statistical significance between pharmacologically induced estrogen-deficient (POVX) group vs. the respective control (CTRL) groups.
Whereas 0.1 mg/kg ISO treatment resulted in a significant increase in GOT enzymes in CTRL and POVX rats, neither POVX nor ISO altered serum GPT activities (Table 2).
Table 2.
Changes in serum concentrations of GOT, GPT, and ALP at 24 h after ISO treatment
| CTRL | CTRL + ISO | POVX | POVX + ISO | |
|---|---|---|---|---|
| GOT, U/l | 93.66 ± 2.89 | 130.78 ± 9.85* | 97.40 ± 3.01 | 137.69 ± 9.97* |
| GPT, U/l | 36.82 ± 1.29 | 41.87 ± 2.28 | 43.11 ± 1.60 | 45.36 ± 1.09 |
| ALP, U/l | 268.12 ± 15.60 | 244.46 ± 11.40 | 329.34 ± 19.34 | 415.28 ± 22.06*† |
Results are shown as means ± SE; n = 5–10. CTRL, control; ISO, isoproterenol; POVX, pharmacologically induced estrogen deficiency; GOT, glutamic oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase; ALP, alkaline phosphatase.
P < 0.05, statistical significance between ISO vs. the respective non-ISO groups;
P < 0.05, statistical significance between POVX vs. the respective CTRL groups.
Serum ALP significantly increased in POVX rats compared with their CTRL counterparts. Moreover, ISO produced further increases in ALP activities in POVX but not CTRL rats (Table 2).
Evaluation of cardiac HO enzyme activity and HO-1 content.
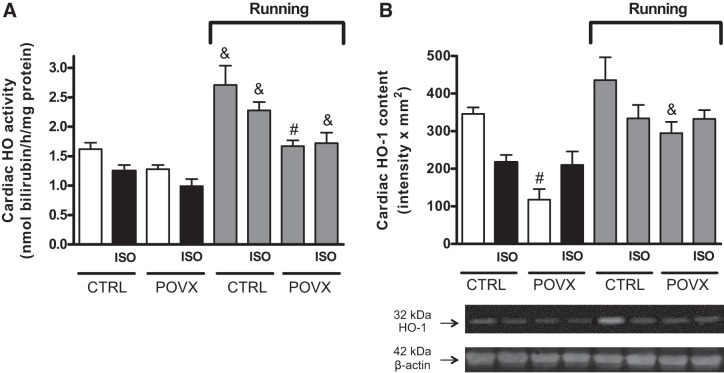
To evaluate the potential therapeutic effects of physical exercise on antioxidant homeostasis, the activity and content of HO in the myocardium were analyzed after 6 wk of running exercise or nonrunning control. We detected a reduction in HO activity in ISO-treated CTRL (CTRL/ISO) animals, which was significant in POVX/ISO animals compared with CTRL animals (CTRL animals served as an “absolute” control). Six weeks of physical exercise caused a significant enhancement in running groups. In addition to this, the most notable elevation was observed in the running CTRL and CTRL/ISO groups. Data are shown in Fig. 3A. Both exercise and estrogen status significantly influenced cardiac HO activity without any significant interaction among them.
Fig. 3.
A: effects of isoproterenol (ISO) administration and 6-wk voluntary wheel-running exercise on cardiac heme oxygenase (HO) activity in control (CTRL) and pharmacologically induced estrogen-deficient (POVX) groups. Result are shown as means ± SE; n = 5–12. B: effects of ISO administration and 6-wk voluntary wheel-running exercise on cardiac HO-1 content in CTRL and POVX groups. Results are shown as means ± SE; n = 3–6. #P < 0.0, statistical significance between POVX vs. the respective CTRL groups; &P < 0.05, statistical significance between running vs. the respective nonrunning groups.
HO-1 protein content was significantly reduced in POVX and POVX/ISO rats compared with CTRL rats, whereas wheel-running exercise ameliorated the antioxidant’s status by enhancing the content of HO-1 in running groups. Compared with POVX animals, we found a significant increase in the running CTRL, CTRL/ISO, and POVX/ISO groups. The results are shown in Fig. 3B. When we analyzed the interaction between different effects, we observed that exercise and estrogen status in itself affected the cardiac HO-1 content, although ISO treatment did not show any significant effect on the enzyme content. However, the interaction between ISO treatment and estrogen status reached the level of statistical significance (P = 0.0236).
Measurement of cardiac GSH + GSSG content and GSH concentration.
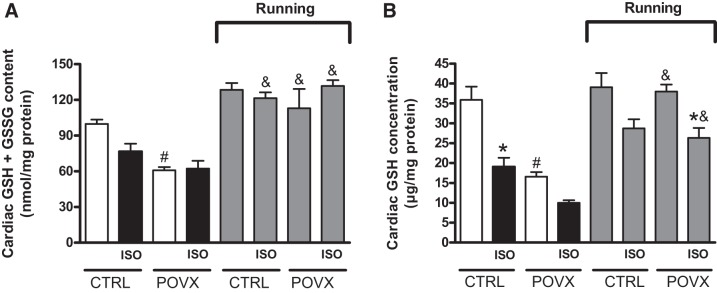
As shown in Fig. 4A, cardiac levels of GSH + GSSG were significantly decreased in POVX and POVX/ISO groups compared with the CTRL group. As a result of voluntary wheel-running exercise, a significant improvement was detected in the levels of GSH in both CTRL and POVX rats, which were diminished by estrogen depletion and ISO treatment. Nevertheless, we found a significant interaction (P = 0.0176) between exercise and estrogen status.
Fig. 4.
A: effects of isoproterenol (ISO) administration and 6-wk voluntary wheel-running exercise on cardiac glutathione (GSH) + glutathione disulfide (GSSG) content in control (CTRL) and pharmacologically induced estrogen-deficient (POVX) groups. Results are shown as means ± SE; n = 5–8. B: effects of ISO administration and 6-wk voluntary wheel-running exercise on cardiac GSH concentration in CTRL and POVX groups. Result are shown as means ± SE; n = 6–7. *P < 0.05, statistical significance between ISO vs. the respective non-ISO groups; #P < 0.05, statistical significance between POVX vs. the respective CTRL groups; &P < 0.05, statistical significance between running vs. the respective nonrunning groups.
The concentration of cardiac GSH was determined to analyze the antioxidant status of the myocardium. A single dose of ISO treatment resulted in reduced levels of cardiac GSH in each ISO-treated group, which was significant in CTRL/ISO and running POVX/ISO groups compared with the respective non-ISO groups. Similar to ISO treatment, a significant decrease in GSH concentration was observed in estrogen-deficient animals relative to animals in the absolute CTRL group. Our results clearly show that 6 wk of exercise was able to ameliorate the reduced GSH values in POVX and POVX/ISO rats. Data are shown in Fig. 4B. Our analysis showed a significant interaction between exercise and estrogen status on GSH concentration (P = 0.0164).
Cardiac TNF-α concentration.
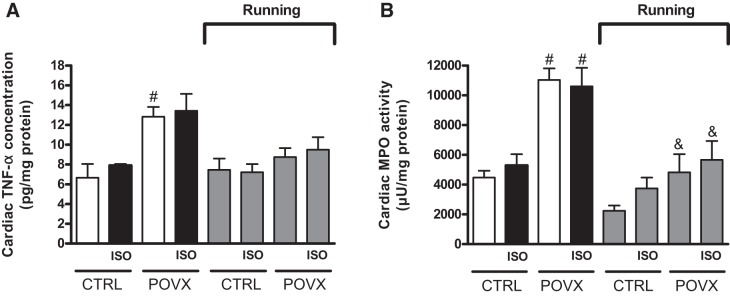
Figure 5A shows the concentration of cardiac TNF-α. As expected, TNF-α levels were increased in estrogen-deficient animals compared with animals in the respective CTRL groups. Nevertheless, these POVX-induced elevated values were moderated by 6 wk of physical exercise. The interaction between exercise and estrogen status reached a significant level (P = 0.011).
Fig. 5.
A: effects of isoproterenol (ISO) administration and 6-wk voluntary wheel-running exercise on cardiac TNF-α in control (CTRL) and pharmacologically induced estrogen-deficient (POVX) groups. Results are shown as means ± SE; n = 4–6. B: effects of ISO administration and 6-wk voluntary wheel-running exercise on cardiac myeloperoxidase (MPO) activity in CTRL and POVX groups. Results are shown as means ± SE; n = 4–7. #P < 0.05, statistical significance between POVX vs. the respective CTRL groups; &P < 0.05, statistical significance between running vs. the respective nonrunning groups.
Cardiac MPO enzyme activity.
Cardiac MPO enzyme activity was determined at the end of the experimental protocol. As expected, POVX and POVX/ISO animals exhibited the highest MPO activity, which was significantly higher than in CTRL animals. However, 6 wk of exercise was able to restore the pathological values of MPO in POVX and POVX/ISO animals compared with their nonrunning counterparts. The efficacy of exercise training in the reduction of inflammatory processes was verified by diminishing of MPO activity. Data are presented in Fig. 5B. Both exercise and estrogen status significantly affected cardiac MPO activity with a significant interaction (P = 0.0077) between them.
DISCUSSION
Cardiovascular diseases are the leading causes of morbidity and mortality in postmenopausal women, since estrogen deficiency causes detrimental effects on metabolism and cardiovascular function (8, 36). Menopause is associated with changes in body fat distribution, abnormal lipid and glucose metabolism, hypertension, and inflammation, which increase the risk for the development of cardiac abnormalities, including MI. Experimental studies have demonstrated that the loss of estrogen has detrimental effect on cardiovascular and inflammatory status: it reduces nitric oxide bioavailability and increases aortic reactivity, the inflammatory response, and oxidative stress, which can negatively influence the life expectancy of postmenopausal women post-MI (4, 40).
The majority of human and animal studies have demonstrated that physical exercise training is a beneficial therapeutic strategy in the prevention of MI (33); however, fewer studies have been conducted to examine the effects of physical exercise post-MI. Thus, our aim was to investigate the effects of voluntary physical exercise on antioxidant/oxidant homeostasis and inflammatory status post-MI. We used a noninvasive experimental model in which estrogen deficiency was induced by the administration of triptorelin and MI was induced by a single dose of ISO. In contrast to coronary ligation surgery, which has a higher incidence of morbidity and mortality, induction of MI by ISO in rats results in reliable and effective changes on the cardiovascular system as well as a lower mortality rate of the animals (23). To determine the most efficacious dosage, five different dosages of ISO were tested: 10, 1, 0.1, and 0.01 mg/kg body wt. Regarding mortality rate, 0.1 mg/kg was found to be optimal dosage to achieve myocardial damage in estrogen-deficient female animals. In our survival rat model, subcutaneous administration of ISO caused a significant increase in serum markers of myocardial injury, such as LDH, myoglobin, GOT, and ALP. This observation is in agreement with earlier studies that proved that elevated levels of these enzymes are diagnostic markers of myocardial damage (11, 22, 23). In addition to blood parameters, the extent of myocardial injury was assessed by TTC staining in ISO-treated CTRL and POVX hearts. A single administration of 0.1 mg/kg ISO caused 15.83% infarct size in CTRL/ISO animals, whereas there was a 23.54% necrotic ratio in hearts from POVX/ISO animals. A growing number of studies have examined the acute effects of ISO administration on the myocardium and the potential preventive effects of various compounds on ISO-induced MI (11, 14, 26). To the best of our knowledge, this is the first study to describe long-term effects of ISO treatment and examine the role of physical exercise in a noninvasive rat model of MI. It has been well established that ISO administration causes a significant increase in inflammation and activates the free radical generating system; thus, the questions of when to begin exercise and at what intensity imply a significant impact on exercise-induced cardiac outcomes after MI. Garza et al. (13) summarized the data from the literature, which proved that exercising immediately after MI may aggravate the necrotic damage, early exercise (<1 wk) after MI may further exacerbate left ventricular remodeling, and exercise initiated late (>3 wk) after moderate MI does not aggravate cardiac outcomes. In the present study, rats performed a voluntary wheel-running training 3 wk after ISO treatment. The results clearly show that the cardioprotective effect of exercise training is mediated by increased activity and expression of the HO enzyme system in both CTRL and POVX animals. HO and its products have both short-term and long-term protective effects on the cardiovascular system. HO degrades heme into biologically active reaction products, such as carbon monoxide (CO), free iron, and biliverdin, which converted to bilirubin by cytosolic biliverdin reductase (20). These cytoprotective products mitigate apoptosis and inflammation, regulate vasomotor tone, and exert antioxidant and immunomodulatory properties (1, 2, 15). While estrogen withdrawal and/or earlier ISO administration resulted in a remarkable reduction in HO enzyme activity and expression, 6 wk of voluntary exercise restored the antioxidant/oxidant balance. In spontaneously hypertensive rats, Ren et al. (35) demonstrated that moderate-intensity aerobic exercise significantly increased the activity and expression of HO enzyme in both aortic and heart tissue. They concluded that exercise can upregulate HO activity, which increases endogenous CO generation and improves cGMP levels to regulate vascular tone. Cardiac-specific activation of the HO enzyme, either pharmacologically with different compounds (6, 7, 7a) or by exercise, improves postischemic cardiac functions and reduces cardiac apoptosis, inflammatory cell infiltration, and oxidative damage (31, 42). Wang et al. (43) demonstrated that HO-1 overexpression improved post-MI survival, ameliorated left ventricular dilatation, and reduced apoptosis, fibrosis, and oxidative stress in mice.
Oxidative stress is enhanced by an unbalance between augmented ROS production and an impaired antioxidant system. Lobo Filho et al. (23) detected a progressive decrease in the concentrations of antioxidant enzymes such as catalase and GSH in the early stages of MI. During MI, superoxide radicals modulate the activity of antioxidants and lead to damage to the myocardium. We found that administration of ISO resulted a long-term effect on cardiac GSH content. It caused a diminished level of GSH in CTRL animals and especially in POVX animals compared with absolute CTRL fertile animals. Similar to the HO enzyme, GSH values were also augmented by physical exercise training. GSH protects the myocardium against oxidative stress by regulating the redox status of proteins (38). Both GSH and HO pathways have important antioxidant activities and provide physiological cytoprotection of a complementary nature. Bilirubin, which is an end product of heme metabolism, largely protects against lipid peroxidation, whereas GSH primarily prevents the oxidation of water-soluble proteins (37). The increasing antioxidant capacity by physical exercise can be associated with the improved ROS scavenging ability of GSH in the myocardium (10). Numerous studies have supported the involvement of HO-1 in the maintenance of oxidant/antioxidants homeostasis. In the complex transcriptional regulation of HO-1, nuclear factor E2-related factor 2 (Nrf2) is the most important regulator of the antioxidant response. Nrf2 has a critical role in the regulation of cysteine uptake, which leads to protection against oxidative stress by maintaining intracellular cysteine and GSH levels (18). Yu et al. (44) reported the role of the Nrf2/HO-1 pathway in cardiovascular pathologies. They demonstrated that Nfr2/HO-1 signaling activation reduces MI, inflammation, and oxidative stress. Examination of this regulatory pathway provides more comprehensive evidence between physical exercise and its antioxidant effect for our future research. In addition to the antioxidant effects, exercise-induced enhancement in HO activity offers protection against inflammatory processes. It can induce upregulation of HO-1 activity by increasing the production of biliverdin and endogenous CO. CO inhibits lipopolysaccharide-mediated expression of proinflammatory mediators such as TNF-α and IL-1β while simultaneously increasing the expression of anti-inflammatory IL-10. A number of studies have demonstrated a strong correlation between physical inactivity and low-grade inflammatory state, which further augments in aging and age-associated diseases (30). In our study, estrogen deficiency triggered by POVX resulted in the highest inflammatory response via increasing TNF-α and MPO activity. MPO is stored in azurophilic granules of neutrophils and macrophages and catalyzes the oxidative modification of lipoproteins and consumes endothelium-derived nitric oxide, thereby resulting impaired vasodilative properties in the cardiovascular system. MPO is an early biomarker of inflammation, and it plays a role in the initiation and progression of cardiovascular diseases (25, 27). In our earlier work, we demonstrated that treatment with 30.0 µg/kg tin protoporphyrin IX (SnPP), which is a potent competitive inhibitor of HO, resulted in a significant increase of cardiac MPO enzyme activity in both ovary-intact and estrogen-treated ovariectomized female rats (32). In addition to chemical inhibitors, the HO-1 knockout mouse model also demonstrates the role of HO-1 in inflammation. Kapturczak et al. (19) noted that animals lacking HO-1 developed progressive inflammatory disease with increased production of proinflammatory cytokines. Six weeks of wheel-running exercise with its anti-inflammatory capacity decreased the cardiac activity of MPO and concentration of TNF-α compared with estrogen-deficient POVX animals and thus contributes to the amelioration of the cardiac inflammatory state.
We can conclude that 6 wk of voluntary wheel-running exercise resulted in improvements in cardiac antioxidant and inflammatory status in a noninvasive rat model of MI. The possible mechanisms involved in the protective effects of physical exercise are related to the increase in the activity and expression of the HO enzyme as well as in the GSH level, which contributes to a reduction in the inflammatory response. Therefore, voluntary physical exercise could be a potential therapeutic target to improve cardiac outcomes in menopausal women post-MI.
GRANTS
This work was supported by the New National Excellence Program of the Ministry of Human Capacities Grants UNKP-17-3 (to S. Renáta), UNKP-17-4 ( to P. Anikó), and UNKP-17-2 (B. Denise), GINOP-2.3.2-15-2016-00062, and Ministry of Human Capacities, Hungary Grant 20391-3/2018/FEKUSTRAT. Furthermore, this work was supported by the European Union, cofinanced by the European Social Fund (EFOP-3.6.2-16-2017-00009).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.P. conceived and designed research; R.S., D.B., Z.K., K.N., A.M.B., M.V., S.T., K.K., and A.P. performed experiments; R.S., R.G., S.T., B.J., and A.P. analyzed data; R.S., R.G., A.M.B., M.V., and B.J. interpreted results of experiments; R.S., D.B., and K.N. prepared figures; R.S., D.B., Z.K., and R.G. drafted manuscript; K.K., C.V., B.J., and A.P. edited and revised manuscript; C.V. and A.P. approved final version of manuscript.
REFERENCES
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 2.Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br J Pharmacol 172: 1587–1606, 2015. doi: 10.1111/bph.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett-Connor E. Menopause, atherosclerosis, and coronary artery disease. Curr Opin Pharmacol 13: 186–191, 2013. doi: 10.1016/j.coph.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi PR, Gumz BP, Giuberti K, Stefanon I. Myocardial infarction increases reactivity to phenylephrine in isolated aortic rings of ovariectomized rats. Life Sci 78: 875–881, 2006. doi: 10.1016/j.lfs.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 6.Csepanyi E, Czompa A, Haines D, Lekli I, Bakondi E, Balla G, Tosaki A, Bak I. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol Res 100: 148–156, 2015. doi: 10.1016/j.phrs.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Csepanyi E, Czompa A, Szabados-Furjesi P, Lekli I, Balla J, Balla G, Tosaki A, Bak I. The effects of long-term, low- and high-dose beta-carotene treatment in zucker diabetic fatty rats: the role of HO-1. Int J Mol Sci 19: E1132, 2018. doi: 10.3390/ijms19041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Czompa A, Szoke K, Prokisch J, Gyongyosi A, Bak I, Balla G, Tosaki A, Lekli I. Aged (black) versus raw garlic against ischemia/reperfusion-induced cardiac complications. Int J Mol Sci 19: E1017, 2018. doi: 10.3390/ijms19041017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosi R, Bhatt N, Shah P, Patell R. Cardiovascular disease and menopause. J Clin Diagn Res 8: 62–64, 2014. doi: 10.7860/JCDR/2014/6457.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fioretti F, Tavani A, Gallus S, Franceschi S, La Vecchia C. Menopause and risk of non-fatal acute myocardial infarction: an Italian case-control study and a review of the literature. Hum Reprod 15: 599–603, 2000. doi: 10.1093/humrep/15.3.599. [DOI] [PubMed] [Google Scholar]
- 10.Frasier CR, Sloan RC, Bostian PA, Gonzon MD, Kurowicki J, Lopresto SJ, Anderson EJ, Brown DA. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol 111: 1751–1759, 2011. doi: 10.1152/japplphysiol.01214.2010. [DOI] [PubMed] [Google Scholar]
- 11.Ganesan B, Buddhan S, Anandan R, Sivakumar R, AnbinEzhilan R. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol Biol Rep 37: 1319–1327, 2010. doi: 10.1007/s11033-009-9508-4. [DOI] [PubMed] [Google Scholar]
- 12.Garg M, Khanna D. Exploration of pharmacological interventions to prevent isoproterenol-induced myocardial infarction in experimental models. Ther Adv Cardiovasc Dis 8: 155–169, 2014. doi: 10.1177/1753944714531638. [DOI] [PubMed] [Google Scholar]
- 13.Garza MA, Wason EA, Zhang JQ. Cardiac remodeling and physical training post myocardial infarction. World J Cardiol 7: 52–64, 2015. doi: 10.4330/wjc.v7.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal SN, Sharma C, Mahajan UB, Patil CR, Agrawal YO, Kumari S, Arya DS, Ojha S. Protective effects of cardamom in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci 16: 27457–27469, 2015. doi: 10.3390/ijms161126040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haines DD, Lekli I, Teissier P, Bak I, Tosaki A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol (Oxf) 204: 487–501, 2012. doi: 10.1111/j.1748-1716.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 16.Haussament TU. Quantitative determination of serum alkaline phosphatase. Clin Chim Acta 35: 271–273, 1977. [Google Scholar]
- 17.Henley KS. IFCC methods for the measurement of catalytic concentrations of enzymes. Part 3, IFCC. Method for alanine aminotransferase (l-alanine 2-oxoglutarate aminotransferase, ec 2.6.1.2). Clin Chim Acta 105: 145F–172F, 1980. [PubMed] [Google Scholar]
- 18.Ishii T, Mann GE. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol 2: 786–794, 2014. doi: 10.1016/j.redox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol 165: 1045–1053, 2004. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakkisto P, Csonka C, Fodor G, Bencsik P, Voipio-Pulkki LM, Ferdinandy P, Pulkki K. The heme oxygenase inducer hemin protects against cardiac dysfunction and ventricular fibrillation in ischaemic/reperfused rat hearts: role of connexin 43. Scand J Clin Lab Invest 69: 209–218, 2009. doi: 10.1080/00365510802474392. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Ruiz-Velasco A, Wang S, Khan S, Zi M, Jungmann A, Dolores Camacho-Muñoz M, Guo J, Du G, Xie L, Oceandy D, Nicolaou A, Galli G, Müller OJ, Cartwright EJ, Ji Y, Wang X. Metabolic stress-induced cardiomyopathy is caused by mitochondrial dysfunction due to attenuated Erk5 signaling. Nat Commun 8: 494, 2017. doi: 10.1038/s41467-017-00664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu YT, Jia HM, Chang X, Ding G, Zhang HW, Zou ZM. The metabolic disturbances of isoproterenol induced myocardial infarction in rats based on a tissue targeted metabonomics. Mol Biosyst 9: 2823–2834, 2013. doi: 10.1039/c3mb70222g. [DOI] [PubMed] [Google Scholar]
- 23.Lobo Filho HG, Ferreira NL, Sousa RB, Carvalho ER, Lobo PL, Lobo Filho JG. Experimental model of myocardial infarction induced by isoproterenol in rats. Rev Bras Cir Cardiovasc 26: 469–476, 2011. doi: 10.5935/1678-9741.20110024. [DOI] [PubMed] [Google Scholar]
- 25.Loria V, Dato I, Graziani F, Biasucci LM. Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm 2008: 135625, 2008. doi: 10.1155/2008/135625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murugesan M, Revathi R, Manju V. Cardioprotective effect of fenugreek on isoproterenol-induced myocardial infarction in rats. Indian J Pharmacol 43: 516–519, 2011. doi: 10.4103/0253-7613.84957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olza J, Aguilera CM, Gil-Campos M, Leis R, Bueno G, Martínez-Jiménez MD, Valle M, Cañete R, Tojo R, Moreno LA, Gil A. Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care 35: 2373–2376, 2012. doi: 10.2337/dc12-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padmanabhan M, Mainzen Prince PS. S-allylcysteine ameliorates isoproterenol-induced cardiac toxicity in rats by stabilizing cardiac mitochondrial and lysosomal enzymes. Life Sci 80: 972–978, 2007. doi: 10.1016/j.lfs.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Parashar S, Reid KJ, Spertus JA, Shaw LJ, Vaccarino V. Early menopause predicts angina after myocardial infarction. Menopause 17: 938–945, 2010. doi: 10.1097/gme.0b013e3181e41f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pósa A, Szabó R, Csonka A, Veszelka M, Berkó AM, Baráth Z, Ménesi R, Pávó I, Gyöngyösi M, László F, Kupai K, Varga C. Endogenous estrogen-mediated heme oxygenase regulation in experimental menopause. Oxid Med Cell Longev 2015: 429713, 2015. doi: 10.1155/2015/429713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pósa A, Szabó R, Kupai K, Baráth Z, Szalai Z, Csonka A, Veszelka M, Gyöngyösi M, Radák Z, Ménesi R, Pávó I, Berkó AM, Varga C. Cardioprotective effects of voluntary exercise in a rat model: role of matrix metalloproteinase-2. Oxid Med Cell Longev 2015: 876805, 2015. doi: 10.1155/2015/876805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posa A, Szabó R, Kupai K, Berkó AM, Veszelka M, Szűcs G, Börzsei D, Gyöngyösi M, Pávó I, Deim Z, Szilvássy Z, Juhász B, Varga C. Cardioprotective effect of selective estrogen receptor modulator raloxifene are mediated by heme oxygenase in estrogen-deficient rat. Oxid Med Cell Longev 2017: 2176749, 2017. doi: 10.1155/2017/2176749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pósa A, Szabó R, Kupai K, Csonka A, Szalai Z, Veszelka M, Török S, Daruka L, Varga C. Exercise training and calorie restriction influence the metabolic parameters in ovariectomized female rats. Oxid Med Cell Longev 2015: 787063, 2015. doi: 10.1155/2015/787063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu H, Depre C, Vatner SF, Vatner DE. Sex differences in myocardial infarction and rupture. J Mol Cell Cardiol 43: 532–534, 2007. doi: 10.1016/j.yjmcc.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren C, Qi J, Li W, Zhang J. The effect of moderate-intensity exercise on the expression of HO-1 mRNA and activity of HO in cardiac and vascular smooth muscle of spontaneously hypertensive rats. Can J Physiol Pharmacol 94: 448–454, 2016. doi: 10.1139/cjpp-2015-0122. [DOI] [PubMed] [Google Scholar]
- 36.Rosano GM, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric 10, Suppl 1: 19–24, 2007. doi: 10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
- 37.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA 106: 5171–5176, 2009. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanely Mainzen Prince P, Priscilla H, Devika PT. Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in Wistar rats. Eur J Pharmacol 615: 139–143, 2009. doi: 10.1016/j.ejphar.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Sudha M, Rajkumar D, Felix JW. Protective effect of glutathione against isoproterenol induced myocardial injury in rats. Indian J Physiol Pharmacol 57: 132–137, 2013. [PubMed] [Google Scholar]
- 40.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res 81: 482–490, 2009. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szabó R, Karácsonyi Z, Börzsei D, Juhász B, Al-Awar A, Török S, Berkó AM, Takács I, Kupai K, Varga C, Pósa A. Role of exercise-induced cardiac remodeling in ovariectomized female rats. Oxid Med Cell Longev 2018: 6709742, 2018. doi: 10.1155/2018/6709742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol 283: H688–H694, 2002. doi: 10.1152/ajpheart.00133.2002. [DOI] [PubMed] [Google Scholar]
- 43.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation 121: 1912–1925, 2010. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H, Shi L, Zhao S, Sun Y, Gao Y, Sun Y, Qi G. Triptolide attenuates myocardial ischemia/reperfusion injuries in rats by inducing the activation of Nrf2/HO-1 defense pathway. Cardiovasc Toxicol 16: 325–335, 2016. doi: 10.1007/s12012-015-9342-y. [DOI] [PubMed] [Google Scholar]