Abstract
A hallmark of chronic heart failure (HF) with low ejection fraction (HFrEF) is exercise intolerance. We hypothesized that reduced expression of nuclear factor E2-related factor 2 (Nrf2) in skeletal muscle contributes to impaired exercise performance. We further hypothesized that curcumin, a Nrf2 activator, would preserve or increase exercise capacity in HF. Experiments were carried out in mice with coronary artery ligation-induced HFrEF. Curcumin was deliveried by a subcutaneous osmotic minipump at a dose of 50 mg·kg−1·day−1 for 8 weeks. In vivo, in situ, and in vitro experiments were employed to evaluate exercise capacity, muscle function, and molecular mechanisms. We found that: 1) the maximal speed, running distance to exhaustion, and limb grip force were significantly lower in HFrEF mice compared with sham. Curcumin-treated HF mice displayed enhanced exercise performance compared with vehicle-treated HF mice; 2) both soleus (Sol) and extensor digitorum longus (EDL) muscles of HFrEF mice exhibited reduced force and rapid fatigue, which were ameliorated by curcumin; and 3) protein expression of Nrf2, hemeoxygenase-1, SOD2, myogenin, and MyoD were significantly lower, but total ubiquitinated proteins, MURF1, and atrogen-1 were higher in Sol and EDL of HFrEF compared with sham mice, whereas these alterations in Nrf2 signaling and antioxidant defenses in HFrEF were attenuated by curcumin, which had no effect on cardiac function per se in mice with severe HFrEF. These data suggest that impaired Nrf2 signaling intrinsic to skeletal muscle contributes to exercise intolerance in HFrEF. Skeletal muscle Nrf2 should be considered as a novel therapeutic target in severe HF.
NEW & NOTEWORTHY These studies suggest that impaired nuclear factor E2-related factor 2 (Nrf2) signaling is a critical mechanism underlying the enhanced oxidative stress in skeletal muscle in heart failure with low ejection fraction (HFrEF). Curcumin prevents the decline in running performance in HFrEF mice by upregulating antioxidant defenses in skeletal muscle, likely mediated by activating Nrf2 signaling. These findings suggest a novel therapeutic target for the improvement of exercise capacity and quality of life in HFrEF patients.
Keywords: exercise intolerance, HFrEF, myopathy, oxidative stress
INTRODUCTION
Heart failure (HF) is one of the leading causes of death in the United States, affecting ~6.5 million people and claiming one in eight deaths (6). Exercise intolerance represents a major clinical manifestation of this syndrome, profoundly impacting patients’ quality of life and prognosis (38). A low exercise capacity was initially attributed to hemodynamic dysfunction-induced reduction of blood flow to skeletal muscles during exercise. However, exercise capacity in a majority of HF patients are not remarkably improved even after left ventricular function and hemodynamics are improved (52). Some HF patients display a paradox between exercise performance and cardiac output (34). In contrast, a number of studies reveal a close relationship between exercise intolerance and intrinsic muscle abnormalities, such as atrophy, a shift from type I to type II fibers, metabolic dysfunction, impaired excitation-contraction coupling, and low force capacity in HF patients (25). By affecting large and small muscles involved in posture, locomotion, and respiration, these pathological alterations play a major role in early fatigue, and exercise intolerance in HF patients (9). In addition, loss of muscle mass in HF patients also results in weakness, fatigue, prolonged bedrest, and delayed ambulation, which increase the risk for thromboembolic complications and accelerate the degradation of muscle proteins, thus creating a vicious cycle. HF patients can be categorized as having low or preserved ejection fraction (EF). Skeletal muscle alterations and responses to therapeutic interventions are different between HFrEF and HFpEF (1). A recent study showed that skeletal myopathy is more severe in HFrEF than HFpEF (44). The present study focused exclusively on mice with severe HFrEF.
Enhanced oxidative stress is an important determinant of disease severity (24) and has been shown to be responsible for exercise intolerance (35) in HFrEF patients. Excessive reactive oxygen species (ROS) not only exacerbates the failing heart but also underlies abnormalities of skeletal muscle (48). While redox imbalance in skeletal muscle in HFrEF may contribute to the overproduction of ROS by NADPH oxidase (5) and/or mitochondrial oxidases (47), impaired antioxidant enzyme mechanisms may play an important role. It has been demonstrated that, in skeletal muscles from HF patients and animals, superoxide dismutase (SODs), catalase (Cat), glutathione peroxidase, and other antioxidant enzymes are significantly downregulated (29). Skeletal muscle-specific overexpression of SOD3 induced by somatic gene transfer or transgenesis in HFrEF mice significantly attenuated muscle atrophy and dysfunction (36). On the other hand, upregulation of antioxidant enzymes in skeletal muscle is a principle mechanism underlying the improved exercise capacity following chronic exercise training in both HF patients (28) and animals (17). However, it is unclear why such endogenous antioxidants are downregulated. To our knowledge, there has not been an evaluation of nuclear factor E2-related factor 2 (Nrf2), a master transcription factor regulating antioxidant enzyme expression, in skeletal muscle of animals or humans with HF. In the present study, we hypothesized that suppressed Nrf2 signaling contributes to the impaired antioxidant defenses in skeletal muscle of mice with HFrEF. We further hypothesized that activation of Nrf2 by a linear diarylheptanoid, curcumin (a known Nrf2 activator) improves exercise performance in mice with HF.
MATERIALS AND METHODS
Animal Preparation
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and were carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sixty male C57BL/6 mice 10 wk of age were used in this study. Eighteen mice underwent sham surgery and one died from excessive bleeding during surgery. Forty-two mice underwent coronary artery ligation, among which 19 mice died from acute HF during surgery or within one week after surgery (45.24% mortality). The surviving 40 mice were assigned to four groups: sham-vehicle (Sh-Veh, n = 8), sham-curcumin (Sh-Cur, n = 9), HF-vehicle (HF-Veh, n = 12), and HF-curcumin (HF-Cur, n = 11). The mice were housed in standard Micro-Isolator mouse cages with five mice per cage.
Induction of HF.
The HFrEF model was produced by permanent coronary artery ligation, as previously described (53) with modifications for this study. Although exercise intolerance is a common symptom in HF patients, skeletal myopathy and sarcopenia usually emerge in an advanced stage of the disease (e.g., NYHA Class IV). Therefore, we aimed to generate HFrEF mice with a large myocardial infarction (>70%) and markedly low EF (<25%). Briefly, while under isoflurane anesthesia (~2%, 98% O2), mice were intubated and ventilated by a mouse ventilator (tidal volume: 150−250 μl; frequency: 200 breaths/min; Mouse Ventilator MiniVent, model no. 845; Hugo Sachs Elektronik, March-Hugstetten, Germany). The hearts were exposed through a left thoracotomy by an incision in the fourth left intercostal space. Instead of ligating the anterior descending branch of the left coronary artery, a permanent ligation was made with 6.0 suture as high as possible near the aorta to produce a large myocardial infarction. Sham mice underwent thoracotomy and manipulation of the heart, but no coronary artery ligation was performed. This modified method generated very severe HF (as showed in Table 1) compared with those in our previous studies (53) and that of other groups (12). To improve survival rate, the mice were placed in a recovery cage for three days after surgery. A regular Micro-Isolator mouse cage (16 × 30 cm with a micro-isolator cap) was placed on a 30–32°C heating pad and received a continuous delivery of 100% oxygen (2.5 l/min) by a vinyl tube. This postoperative care remarkably reduced mortality from more than 90% to ~45%.
Table 1.
Anatomic, hemodynamic, and echocardiographic measurements associated with failing hearts
| Sh-Veh | Sh-Cur | HF-Veh | HF-Cur | |
|---|---|---|---|---|
| n | 8 | 9 | 12 | 11 |
| Whole heart weight, g | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.26 ± 0.04* | 0.28 ± 0.06‡ |
| Atria weight, mg | 15.0 ± 1.0 | 15.5 ± 0.7 | 43.1 ± 3.4* | 46.8 ± 2.6‡ |
| RVFW weight, mg | 26.95 ± 2.6 | 27.85 ± 2.2 | 64.8 ± 6.1* | 69.3 ± 7.3‡ |
| Septum weight, mg | 51.9 ± 2.0 | 50.7 ± 3.2 | 77.9 ± 9.5* | 86.3 ± 8.3‡ |
| LVFW weight, mg | 46.1 ± 3.7 | 45.96 ± 2.8 | 74.3 ± 8.6* | 74.66 ± 8.0‡ |
| Infarct size, % of LV | 0 | 0 | 79.4 ± 8.9† | 81.6 ± 6.4§ |
| Ejection fraction, % | 68.6 ± 8.4 | 67.3 ± 7.9 | 21.3 ± 2.1* | 19.5 ± 3.3‡ |
| Fractional shortening, % | 34.8 ± 5.5 | 36.1 ± 4.8 | 10.1 ± 2.1* | 11.3 ± 1.8‡ |
| LVEDP, mmHg | 1.3 ± 1.4 | 0.9 ± 2.1 | 29.8 ± 7.8† | 27.4 ± 6.3§ |
| dP/dtmax, mmHg/s | 12,138 ± 919 | 11,198 ± 1001 | 2,101 ± 315† | 2,198 ± 333§ |
| dP/dtmin, - mmHg/s | 12,952 ± 867 | 12,166 ± 981 | 2,540 ± 441† | 2,719 ± 462§ |
Values are means ± SE. RVFW, right ventricular free wall; LV, left ventricle; LVFW, LV free wall; LVEDP, LV end-diastolic pressure.
P < 0.01 and
P < 0.001 vs. Sh-Veh;
P < 0.01 and
P < 0.001 vs. Sh-Cur.
Echocardiography.
Echocardiograms were carried out on all mice at four weeks postmyocardial infarction (post-MI) surgery to validate the HF model and at eight weeks after curcumin treatment to evaluate the effects of curcumin on cardiac function. Mice were imaged on a model Vevo 3100 ultrasound; Visual Sonics,Toronto, ON, Canada, using a 40-MHz probe. Under light isoflourane anesthesia (0.5–1%) 2D B-mode images were acquired in the long- and short-parasternal axis. M-Mode images were acquired at the level of the left ventricular papillary muscles. Left ventricular volumes and diameters were measured. EF was calculated by a standard formula [(LVEDV−LVESV)/LVEDV] × 100. Fractional shortening (FS) was calculated as [(LVEDD−LVESD)/LVEDD] × 100. The echocardiographer (BH) was blinded to the animal groups.
Curcumin administration.
Curcumin (50 mg·kg−1·day−1) was continuously supplied by two osmotic minipumps (model no. AP1004; Alzet, Cupertino, CA). The first pump was implanted subcutaneously in the midscapular area at 12 weeks post-MI surgery and replaced with a new pump after four weeks corresponding to the capacity of the pump. The dose of curcumin in the present study was adopted based on studies where curcumin was reported to improve skeletal muscle function and exercise performce in mice and rats (21, 40). Curcumin was dissolved in dimethyl sulfoxide, and the same volume of dimethyl sulfoxide was used as the vehicle control.
In Vivo Experiments
Maximal exercise performance.
Exercise capacity was tested on a mouse treadmill (model no. Exer-3/6 Treadmill; Columbus Instruments, Columbus, OH) as described by others (30) with some modifications. Briefly, mice were exposed to the treadmill for 20 min, once a day, for three days before the first running test. On the day of test, mice were placed on the treadmill supplied with a shocking grid at the rear. The treadmill was run at an inclination of 15° starting at a speed of 6 m/min for 6 min, followed by an increase of 3 m/min every 3 min until exhaustion, defined as when mice remained on the shocking grid for 20 s without attempting to reengage the treadmill.
Whole-body tension.
Whole-body tension was used to evaluate muscle function in vivo, as previously described (10). Briefly, mice were placed in a solid polyvinyl chloride (PVC) tube, which was 1 foot in length and 1.5 inch in diameter with the interior lined by aluminum mesh. The tail of mouse was attached to a force transducer using a no.1 suture that was 5–7 cm in length. The force exerted on the transducer was measured and recorded using a Powerlab system (ADInstruments, Colorado Springs, CO) and Chart 7 software. To motivate the mouse to move forward, the tail was stroked with a serrated forceps and the forward pulling tension was measured. Typically, each mouse received 10–13 strokes in a period of 50 s at ~2- to 4-s intervals. The mean value of the five peak readings was used for statistical analysis.
In Situ Muscle Function
Functional assessment of in situ muscles was performed on the left soleus (Sol) and extensor digitorum longus (EDL) based on a modified method used for the tibialis anterior muscle (45). Under ~2% isoflurane anesthesia, mice were placed on a metal heating pad in the prone position. A small incision on the skin above the calf was made and the Sol and EDL were identified. The proximal tendon of the Sol and the distal tendon of the EDL were isolated, cut, and sutured with a no. 6 silk suture, by which the tendon was attached to a force transducer (model no. MLT1030/A; ADInstruments, Duneden, New Zealand). The muscle belly was carefully kept intact with normal vasculature and innervation. A silver bipolar electrode was inserted into the muscle belly and an intermittent tetanic stimulation with trains of square wave pulses (2.5 V, 0.3 s at 50 Hz per 3 s for a total of 20 min) was delivered by a pulse generator (model no. A310, WPI; Sarasota, FL). During the experiment, the mice were kept warm by an isothermal pad and heat lamp, while the muscles and tendons were moisturized by periodic administration of warm saline. Once the functional assessment was completed, mice were euthanized by inhalation of CO2. The right Sol and EDL were harvested for the following ex vivo analyses.
Ex Vivo Experiments
Immunohistochemistry.
Muscle fiber types and morphology were analyzed by using immunofluorescence staining to detect myosin heavy chain (MHC) expression as previously described (7). In brief, the Sol and EDL were embedded in OCT compound side by side, and cut into 10-μm thick cryosections with a cryostat (model no. CM1850; Leica, Buffalo Grove, IL) maintained at −20 C. The sections of the midbelly were used for immunofluorescence staining. First, the sections were blocked with 10% goat serum in PBS for 60 min, followed by 60 min incubation with a primary antibody cocktail [BA-F8 to MHCI (1:50), SC-71 to MHCIIa (1:600), and BF-F3 to MHCIIb (1:100); Developmental Studies Hybridoma Bank, University of Iowa]. After being washed three times with PBS, the sections were incubated 60 min with a secondary antibody cocktail [Alexa Fluor 350 IgG2b (1:500), Alexa Fluor 555 IgG1 (1:500), and Alexa Fluor 488 M (1:500); Invitrogen, Waltham, MA], followed by three PBS washes. The slices were then mounted with an Aqua-Mount mounting medium (VWR, Radno, PA), and then were examined with a laser confocal microscope (model no. TSC STED; Leica). Cross-sectional area (CSA) was determined for each fiber separately using ImageJ software, as described by Papadopulos, et al. (37). Eight slices per muscle were assessed to obtain an average CSA for each type of myofiber/muscle/mouse, which was then used for statistical analysis. The minimal numbers of fibers analyzed in Sol/EDL were 208/24 for FI, 224/136 for FIIa, and 32/616 for FIIb.
Western blot analyses.
The skeletal muscle tissues were homogenized in RIPA buffer (50 mM Tris·HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS) with 1% protease inhibitor cocktail (ab65621; Abcam, Cambridge, MA) from which total protein was extracted by centrifuging at 20,000 g. The protein concentration of extract was measured using a protein assay kit (Pierce; Rockford, IL) and then adjusted to an equal volume in all samples with 2× 4% SDS sample buffer. The samples were boiled for five minutes and then loaded on a 7.5% SDS-PAGE gel (30 μg protein/10 μl per well) followed by electrophoresis using a Bio-Rad (Hercules, CA) mini gel apparatus at 40 mA/gel for 45 min. The fractionized protein on the gel was electrically transferred onto a polyvinyl difluoride membrane (Millipore, Burlington, MA). The membrane was first probed with the primary antibodies: Nrf2 ab-137550, Kelch-like ECH-associated protein 1 (Keap1) sc-33569, hemeoxygenase (HO-1) ab-68477, NQO1 ab-80588, SOD2 sc-30080, Cat sc-50508, glycosylated 91-kDa glycoprotein component of flavocytochrome b558 (gp 91) BD-611414, ubiquitinated proteins BML-PW0930, myogenin sc-576, MyoD sc-760, MuRF1 sc-398608, and atrogen-1 ab-168372 (Abcam; Santa Cruz Biotechnology, Dallas, TX; BD Biosciences, San Jose, CA; and Enzo Life Sciences, Farmingdale, NY) and then the secondary antibody (HRP goat anti-rabbit IgG antibody and HRP goat anti-mouse IgG, HRP; Thermo-Fisher Scientific, Waltham, MA). After three washes with tris-buffered saline-Tween 20, the membrane was treated with enhanced chemiluminescence substrate (Pierce; Rockford, IL) for five minutes. The blots on the membrane were visualized and analyzed using a UVP bioImaging system (Epi Chemi II Darkroom, Analytik Jena US LLC, Upland, CA). The membranes were then treated with Restore Western blot stripping buffer (Thermo Scientific) to remove the blots, followed by probing with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibodies (sc-32233), or α-tubulin (ab-64332) to get GAPDH/α-tubulin blots as an internal control. The final reported data are the normalized target protein band densities divided by α-tubulin. We did not test the linearity of target or loading control proteins. The Western blot was completed by a technician blinded to the groups.
Statistical Analyses
Data are means ± SE. A two-way repeated measures ANOVA with the Student-Newman-Keuls test was used for analyzing the differences among the four groups, with the aid of SigmaPlot software. A P value of < 0.05 was taken as indicative of statistical significance.
RESULTS
HF Generation and Cardiac Function
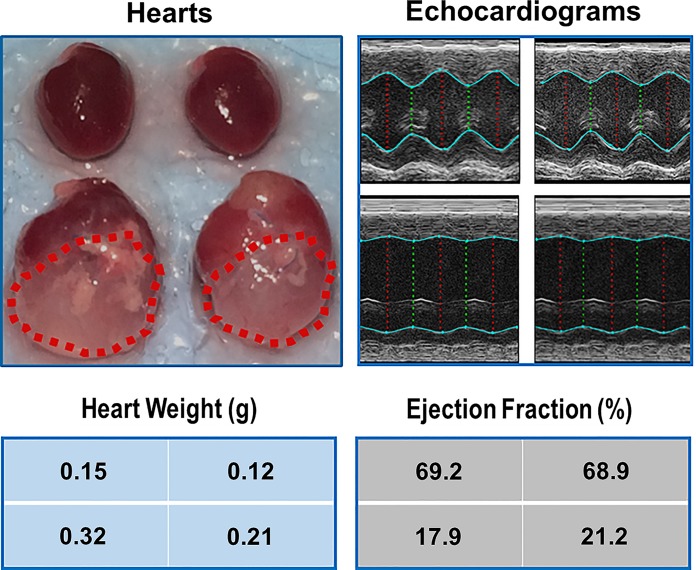
Although exercise intolerance is a common symptom in HF patients, skeletal myopathy and sarcopenia usually emerge in advanced stages of the disease. Figure 1 shows representative gross anatomy, heart weight, representative echocardiograms, and EF from sham and HF mice. When compared with sham hearts, the failing hearts were remarkably dilated, with a ~2-fold increase in the weight and size. Correspondingly, these hearts displayed significantly impaired function as compared with the hearts in sham mice.
Fig. 1.
Representative hearts and echocardiograms for sham-vehicle (Sh-Veh; top, left), sham-curcumin (Sh-Cur; top, right), heart failure-vehicle (HF-Veh; bottom, left), and heart failure-curcumin (HF-Cur; bottom, right) group. Dotted lines outline the infarct areas where the myocardium is replaced by scars. Position of data blocks corresponds to position of hearts.
Table 1 summarizes anatomic, hemodynamic, and echocardiographic measurements at the end of experiment in the four groups. The HF-Veh group exhibited significantly increased heart weight, decreased EF and FS, elevated left ventricular end diastolic pressure, and reduced maximal and minimal dP/dt, as compared with the Sh-Veh group. However, there were no differences in these parameters between Sh-Veh and Sh-Cur or HF-Veh and HF-Cur, suggesting that curcumin had no effects on cardiac function in healthy or in failing hearts. The increased weights of all cardiac components in HF groups suggests global cardiac hypertrophy, including the residual myocardium of the free wall of the left ventricle.
Exercise Capacity and Limb Grip Force
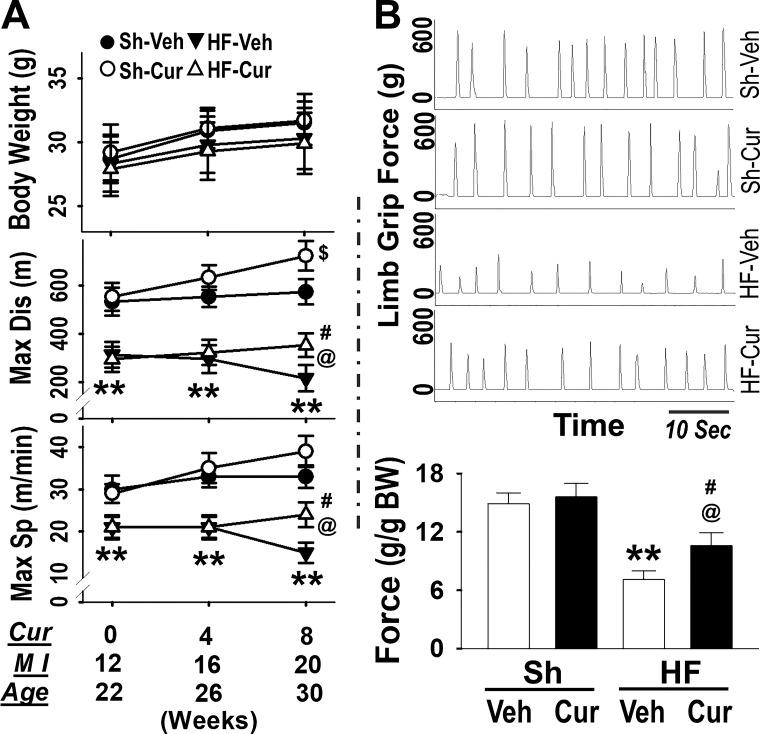
Exercise capacity was evaluated on a treadmill at three time points: 0- (pre-), 4-, and 8-week postcurcumin treatments, and correspondingly at 12-, 16-, and 20-week post-MI or at the ages of 22, 26, and 30 weeks old. HF-Veh mice displayed a significantly shorter maximum running distance and slower speed compared with Sh-Veh mice in all tests, validating exercise intolerance (Fig. 2). Sh-Cur mice exhibited an increase in maximum running distance at eight weeks as compared with their baseline at zero weeks and Sh-Veh at eight weeks, suggesting that curcumin enhanced exercise tolerance in the healthy state. On the other hand, distance and speed at eight weeks was decreased in HF-Veh, but there was no significant change in the HF-Cur group as compared with the baseline at zero weeks. Although no difference was observed between zero and eight weeks, the HF-Cur group exhibited significantly better exercise performance (distance and speed) at eight weeks as compared with the HF-Veh group, suggesting that curcumin prevented the decline in running performance in HF. During this period, no difference in body weight was found among these groups. Figure 2B shows whole-body tension at eight weeks postcurcumin treatment. HF-Veh displayed a significantly lower limb grip force than Sh-Veh, whereas HF-Cur had a significantly greater limb grip force than HF-Veh.
Fig. 2.
A: exercise performance [top: body weight (BW); middle: Max Dis, maximum distance; bottom: Max Sp, maximum speed; Cur: weeks postcurcumin treatment; MI (myocardial infarction) weeks post-MI surgery]. B: whole-limb grip force generation (top: original recording; bottom: mean data). Groups are sham-vehicle (Sh-Veh, n = 8), sham-curcumin (Sh-Cur, n = 9), heart failure-vehicle (HF-Veh, n = 12), and HF-curcumin (HF-Cur, n = 11). **P < 0.01 HF-Veh vs. Sh-Veh; @P < 0.05 HF-Cur vs. HF-Veh; #P < 0.05 HF-Cur vs. Sh-Veh; $P < 0.05 Sh-Cur vs. Sh-Cur at 0 wk and Sh-Veh at 8 weeks.
In Situ Muscle Function
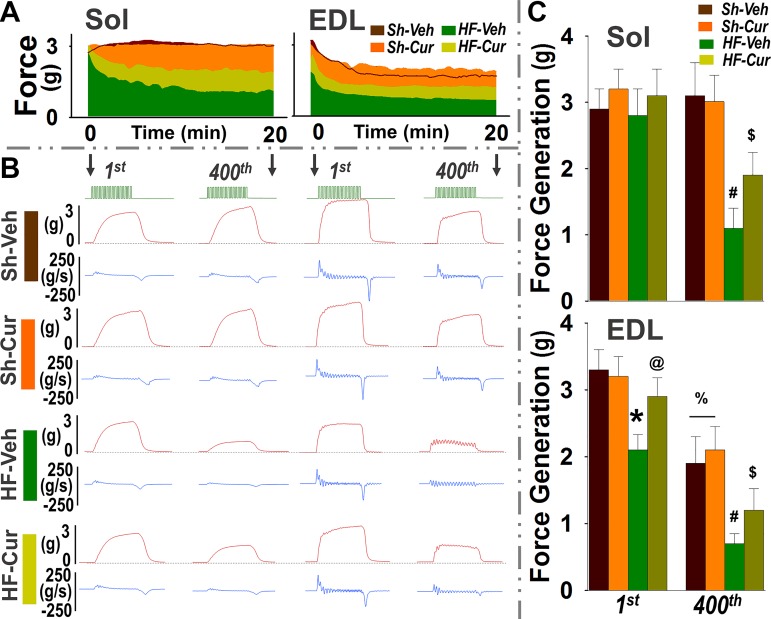
Sol and EDL muscles were selected for determination of the muscle function in situ because they represent typical oxidative (FI and FIIa in Sol) and glycolytic (FIIb in EDL) muscles, respectively. Figure 3A shows a representative time course profile of maximal force during tetanic stimulation. Figure 3B shows representative traces of force and force generation rate at the first and 400th tetanic stimulus. Figure 3C shows the mean data. In Sh-Veh mice, the force developed by the Sol was maintained during the entire period of stimulation, whereas the force of the EDL rapidly declined with time, confirming the fatigue resistance of oxidative muscles (Sol) and fatigability of glycolytic muscles (EDL). Curcumin had no effects on this parameter in sham mice (Sh-Cur). The Sol of the HF-Veh mice displayed a similar force during the first several stimuli, but significantly decreased with time, as compared with the Sol of the Sh-Veh. The EDL of the HF-Veh mice exhibited a significantly lower force in both initial and latter tetanic stimuli compared with the EDL of Sh-Veh, suggesting dysfunction of Sol and EDL in HF. This muscle dysfunction in HFrEF was attenuated by curcumin (HF-Cur).
Fig. 3.
In situ soleus (Sol) and extensor digitorum longus (EDL) force. A: representative time course profiles of maximal contractile response. B: representative tracings of force (red) and force generation rate (blue) of first and last tetanus. Light green horizontal trace is the stimuli markers (300 ms, 50 Hz). C: mean data of force generation of the first and last tetanus. Groups are sham-vehicle (Sh-Veh, n = 8), sham-curcumin (Sh-Cur, n = 9), heart failure-vehicle (HF-Veh, n = 12), and HF-curcumin (HF-Cur, n = 11). *P < 0.05 compared with first of Sh-Veh @P < 0.05 compared with first of HF-Veh; #P < 0.05 compared with 400th of Sh-Veh; $P < 0.05 compared with 400th of HF-Veh; %P < 0.05 compared with first of Sh-Veh and Sh-Cur.
Morphological Assessment
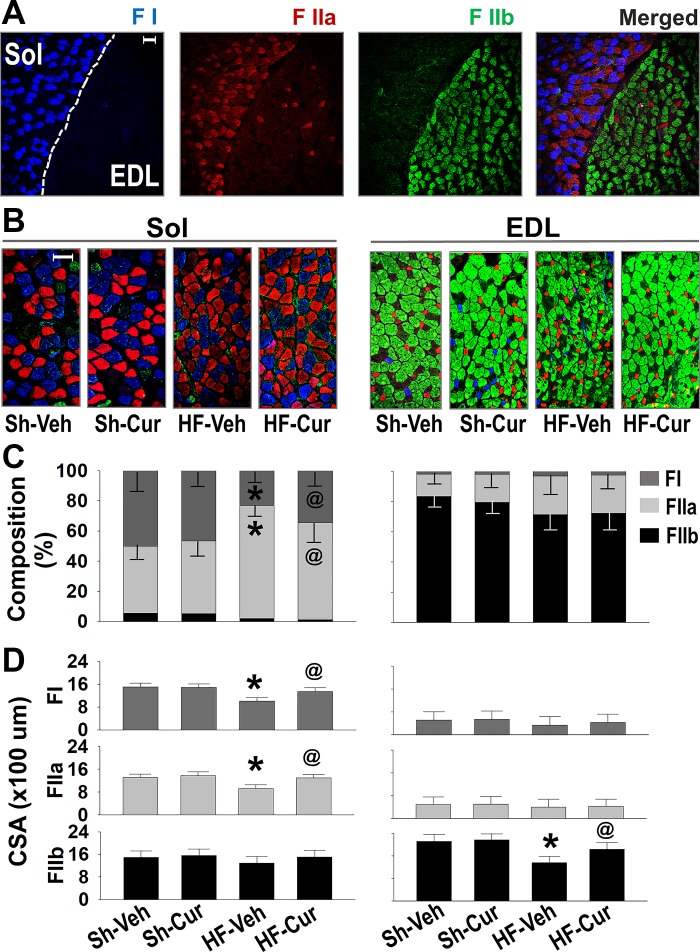
Figure 4A shows that distinct fiber distribution in Sol and EDL was clearly different. The Sol contained almost equal numbers of type I and IIa fibers, both of which are the oxidative fibers, whereas EDL was composed primarily of glycolytic type IIb fibers. Type IIa fibers in the EDL were smaller in size compared with the IIa fibers in the Sol. These differences in fiber composition form the well-characterized basis of functional characteristics in Sol and EDL. Figure 4B shows muscle fiber composition and size for each group. HF-Veh mice exhibited a significant increase in type IIa fibers and decrease in type I in the Sol and decrease in IIb fibers in the EDL as compared with Sh-Veh. In addition, fiber CSA was significantly reduced in both Sol and EDL of HF-Veh as compared with Sh-Veh. These morphologic alterations in HF were attenuated by curcumin.
Fig. 4.
Immunofluorescence staining assessment of fiber type and size in situ soleus (Sol) and extensor digitorum longus (EDL) muscles. A: distribution of type I fibers (blue), IIa (red), and IIb (green) in Sol and EDL of a normal mouse. Bar represents 100 μm. Representative images (B), fiber composition (C), and fiber size (D) of Sol and EDL in the four groups of mice. Fiber-type specific cross-sectional areas (CSAs) in a given muscle of individual animal were provided by averaging CSAs of fibers in Sol/EDL with the numbers of FI in 208–264/24–96, FIIa in 224–560/136–392, and FIIb in 32–64/616–872. Groups are sham-vehicle (Sh-Veh, n = 8), sham-curcumin (Sh-Cur, n = 9), heart failure-vehicle (HF-Veh, n = 12), and HF-curcumin (HF-Cur, n = 11). *P < 0.05 vs. Sh-Veh; @P < 0.05 vs. HF-Veh.
Biochemical and Molecular Biological Analyses
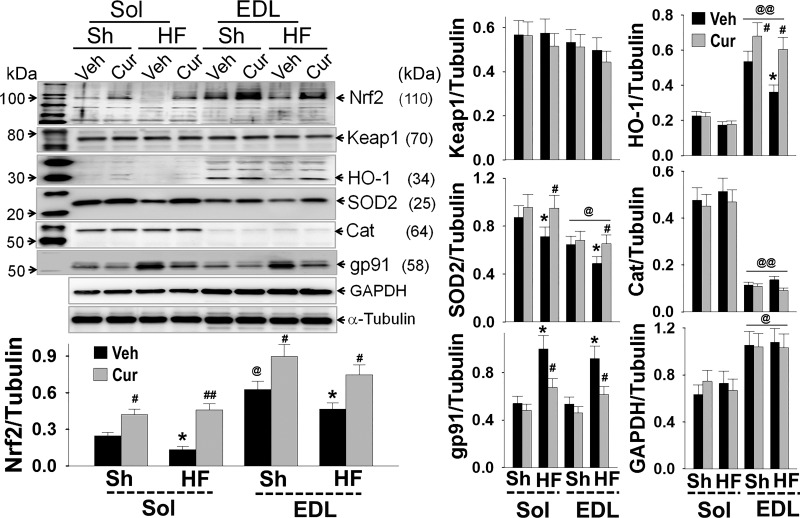
Figure 5 shows expression of Nrf2 and its target proteins in both muscles. Sol and EDL exhibited differential expression profiles: Nrf2, HO-1, and GAPDH were highly expressed in EDL while SOD2 and Cat were largely expressed in Sol. This finding suggests differential redox status and metabolic characteristics of oxidative and glycolytic muscles. There was no difference in the levels of Keap1, gp91, and α-tubulin between these two muscles types. As compared with Sh-Veh mice, HF-Veh mice expressed lower Nrf2, HO-1, and SOD2 in both Sol and EDL, which were increased by curcumin, suggesting an impaired antioxidant defense in HFrEF and a potential beneficial effect of Nrf2 activation. In addition, curcumin upregulated Nrf2, HO-1, and SOD2 expression in sham mice, suggesting an enhancement of curcuminon antioxidant defenses even in healthy muscle. However, curcumin had no effect on Keap1, GAPDH, and α-tubulin expression in sham or HF mice.
Fig. 5.
Western blots show the expression of Nrf2 and associated proteins in soleus (Sol) and extensor digitorum longus (EDL) of sham and heart failure with low ejection fraction (HFrEF) mice. Downregulated nuclear factor E2-related factor 2 (Nrf2) and target proteins in muscles of HFrEF mice were attenuated by curcumin. Cat, catalase; Keap1, Kelch-like ECH associated protein 1; HO-1 hemeoxygenase; gp91, glycosylated 91-kDa glycoprotein component of flavocytochrome b558. Groups are sham-vehicle (Sh-Veh, n = 8), sham-curcumin (Sh-Cur, n = 9), heart failure-vehicle (HF-Veh, n = 12), and HF-curcumin (HF-Cur, n = 11). *P < 0.05 and **P < 0.01 vs. Sh; #P < 0.05 and ##P < 0.01 vs. Veh; @P < 0.05 and @@P < 0.01 vs. Sol. Both bands of gp91 blot were desitometrically analyzed.
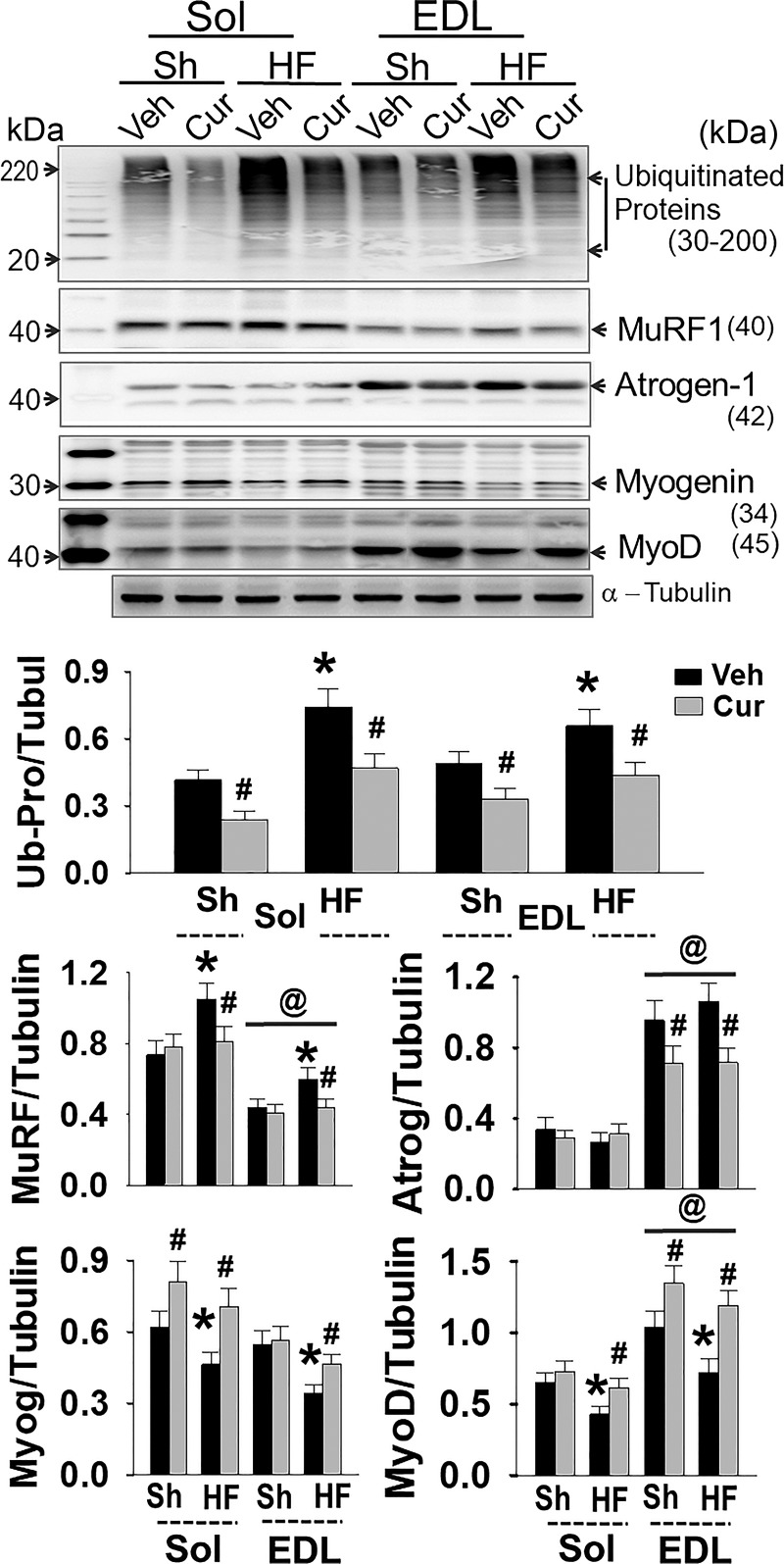
Protein expression of important muscle proteins related to muscle atrophy are shown in Fig. 6. In both Sol and EDL of HF animals, total ubiquitinated proteins were significantly elevated, suggesting that the UPS was activated. This was further confirmed by upregulated MuRF1 and atrogen-1, two muscle-specific E3 ubiquitin ligases. Interestingly, MuRF1 was highly expressed in the Sol, while atrogen-1 was highly expressed in the EDL, implying different mechanisms of protein degradation between oxidative and glycolytic muscles. As the targets of MuRF1/atrogen-1, two muscle-specific transcription factors, myogenin/MyoD, were significantly downregulated in the muscle of HF mice. These alterations were attenuated by curcumin.
Fig. 6.
Expression of ubiquitinated-proteins, myogenin, and MyoD. Groups are sham-vehicle (Sh-Veh, n = 8), sham-curcumin (Sh-Cur, n = 9), heart failure-vehicle (HF-Veh, n = 12), and HF-curcumin (HF-Cur, n = 11). *P < 0.05 vs. Sh; #P < 0.05 vs. Veh; @P < 0.05 vs. Sol.
DISCUSSION
HF with reduced EF is a complex clinical syndrome characterized by a decrease in cardiac output, resulting in an insufficient perfusion of peripheral tissues. Exercise intolerance is a major clinical manifestations in HF that profoundly impacts patient quality of life and prognosis. Although hemodynamic abnormalities and reduced blood flow to working muscles were previously considered as the sole contributor to exercise intolerance, performance of HFrEF patients does not necessarily improve when cardiac function and hemodynamics are significantly improved (34). Instead, reduced exercise capacity in HFrEF closely correlates with abnormal skeletal muscle per se (32). This includes muscle atrophy and a shift from type I to type II fibers, metabolic dysfunction, impaired excitation-contraction coupling, and decreased muscle force. By affecting large and small muscles that are involved in posture, locomotion, and respiration, these intrinsic pathological changes in skeletal muscles play a critical role in the symptoms of HFrEF patients, including shortness of breath, early fatigue, and exercise intolerance (51). However, the underlying mechanisms for these abnormalities remain to be elucidated, and pharmacological therapies are unavailable. In the present study, we assessed oxidative stress-associated pathogenesis of skeletal muscle and the therapeutic potential of an antioxidant intervention on exercise intolerance in mice with coronary artery ligation-induced HFrEF. We found that HFrEF mice exhibited markedly impaired exercise capacity, and whole-body pulling tension, reduced muscle force and fatigue resistance, increased ratio of type II to I fibers, and decreased fiber size. We further demonstrated that Nrf2/antioxidant enzymes and myogenin/MyoD were significantly downregulated, while total ubiquitinated proteins and MURF1/atrogen-1 were increased in the skeletal muscle of HF mice. These functional impairments, morphological abnormalities, and biochemical alterations were remarkably attenuated by chronic administration of curcumin. These data suggest, for the first time to our knowledge, that impaired Nrf2 signaling contributes to skeletal myopathy and that activation of Nrf2 is a promising therapeutic strategy to improve exercise capacity in the setting of HFrEF. While we provide evidence suggesting a strong correlation between ROS and Nrf2 signaling and the effects of curcumin, skeletal muscle function, and exercise performance, we did not evaluate the oxidative status per se in animals and tissues in the present study, thus limiting our conclusions concerning the role of ROS.
ROS increases with muscle contractions. During aerobic exercise, skeletal muscles produce 50- to 100-fold more ROS as compared with the resting condition (49), including O2·−, H2O2, and ·OH (22). These unstable molecules and ions are extremely reactive and highly detrimental to skeletal myocytes by promoting oxidation of proteins, lipids, and DNA. Even in the healthy condition, muscle contraction generates ROS during chronic exercise training, or acute intense exercise may result in potential oxidative damage to skeletal myocyte constituents (41).
It is well established that oxidative stress is systemically increased in the HFrEF state (48). Excessive free radicals not only exacerbate the failing heart but also injure peripheral organs, skeletal muscle being one of the major victims. Indeed, in skeletal muscles from HFrEF animals, redox homeostasis is poorly maintained and excessive ROS accumulates, leading to contractile dysfunction and muscle atrophy and subsequent fatigue and exercise intolerance (5). In skeletal muscle from HFrEF patients (28) and in animal models (29), the mRNA and proteins of free radical scavenging enzymes have been demonstrated to be significantly low. However, it is not clear why these antioxidant defenses fail to be activated in this pathological condition.
One of the principle mechanisms to enhance endogous antioxidant capacity is Nrf2-induced transcriptional activation of antioxidant response elements located within the promoter region of antioxidant genes (19). The function of Nrf2 and its downstream targets suggest that this transcriptional factor plays a central role in protecting cells and tissues against damage from oxidative stress (26). Increasing evidence suggests that Nrf2 participates in the maintainance of redox hemostasis of skeletal muscle and plays a critical role in skeletal muscle function. For instance, acute treadmill exercise leads to Nrf2 release from Keap1, translocation to the nucleus, and upregulation of SOD1, SOD2, Cat, HO-1, γ-glutamyl cysteine ligase-catalytic, and γ-glutamyl cysteine ligase-modulatory in skeletal muscles (27). In cultured myocytes, electrical stimulation activated Nrf2 and upregulated antioxidant enzymes, which was abolished by Nrf2 siRNA (20). In addition, the baseline expression of antioxidant enzymes in skeletal muscle of Nrf2, knockout mice (KO) was significantly lower than wild-type (WT) mice (33). Nrf2 deficiency exacerbates age-related contractile dysfunction and skeletal muscle atrophy (2). Chronic exercise training improved exercise capacity in WT mice, whereas it impaired exercise capacity in Nrf2 KO mice (31). An in situ study further demonstrated that the gastrocnemius of Nrf2 KO mice developed more rapid fatigue and less force generation as compared with the WT (14). In the present study, we found that Nrf2 expression in Sol and EDL muscles was significantly lower in HF mice as compared with sham, suggesting that the above-mentioned dysfunction of endogenous antioxidant defenses in skeletal muscle in HFrEF is, at least partially, due to impairement of Nrf2 signaling.
Enhanced oxidative stress induces muscle atrophy in a wide variety of pathological conditons, including HF. Some patients or animal models with mild/moderate HFrEF may not display this alteration; whereas, it does exist in most advanced HFrEF patients and animals (16, 36). To ensure muscle atrophy in the present study, we modified the coronary ligation surgery to generate HF with EF < 25%, a very severe condition likely mimicking NYHA Class III-IV patients. Indeed, these mice clearly showed a significant increase in the ratio of fiber type II to I and decrease in the CSA in both Sol and EDL. Evidence from HFrEF rats suggested that skeletal muscle atrophy is attributed to the ROS-induced hyperactivation of ubiquitin proteasome system (UPS) (5). UPS not only increases turnover of sarcomeric proteins but also degrades the transcription factors crucial for muscle protein synthesis (8). In skeletal muscle of HFrEF rats, two muscle-specific E3 ubiquitin ligases, MuRF-1 and atrogin-1, are significantly upregulated (11). Administration of bortezomib, a proteasome inhibitor, in HFrEF rats improves diaphragm function by restoring myosin content via suppressing MuRF-1/atrogin-1 expression and UPS activity (50). Interestingly, apocynin, an antioxidant reagent, normalized UPS activity and prevented skeletal muscle atrophy in HFrEF rats (5), suggesting a critical role of ROS in the UPS-induced skeletal myopathy. In the present study, we found a significant increase in total ubiquitinated proteins, upregulated MuRF-1/atrogin-1, and downregulated myogenin/MyoD in the muscle of HFrEF mice, confirming the contribution of UPS to muscle atrophy in HFrEF state. We further found that these molecular alterations were attenuated and acompanied by an upregulated Nrf2 after curcumin administration, suggesting Nrf2 as a potential therapeutic target of skeletal muscle atrophy in HFrEF.
Treadmill testing revealed a significantly decreased maximal distance and speed in HF mice as compared with sham. We also found a progressive decrease in exercise capacity of HF-Veh mice from 12 to 20 weeks after MI surgery, suggesting that skeletal muscle and/or cardiac function were deteriorating over time. In contrast, during this period, the distance and speed were increased in HF-Cur mice, resulting in a significantly enhanced exercise capacity as compared with HF-Veh at eight weeks of postcurcumin treatment. These data suggest that curcumin prevents the decline of skeletal muscle and/or cardiac dysfunction in the HFrEF mice. Although Nrf2-mediated signaling in the heart was not evaluated, it is unlikely that differences in cardiac function contributed to the improved exercise performance because no significant changes were noticed between groups of mice. Accordingly, we believe that the enhanced exercise performance in HF-Cur mice is primarily derived from the beneficial effects of curcumin on skeletal muscle per se, although the influence on microvessel and nerves cannot be completely excluded. Indeed, by using the whole-body tension test, an in vivo technique to evaluate transitory force of skeletal muscle with less influence by cardiopulmonary adjustments, we found an increased limb grip force in HF-Cur mice as compared with HF-Veh mice. In addition, the data from in situ muscle experiments also support this speculation. Finally, our data show that Sh-Cur mice display an improvement in exercise capacity as compared with Sh-Veh, suggesting the effects of curcuminon skeletal muscle is not exclusive to the HFrEF state.
Employing an in situ muscle preparation, we assessed force generation and fatigue resistance of Sol and EDL muscles. For the Sol, there was no difference in the initial force (evoked by the first stimulation) between HF-Veh and Sh-Veh mice, whereas the later force (evoked by the 400th stimulation) in HF-Veh mice was significantly reduced as compared with Sh-Veh. For the EDL, however, both initial and later force of HF-Veh mice were significantly lower than those in Sh-Veh mice. These abnormalities were found to be ameliorated in HF-Cur mice. These data suggest that both oxidative and glycolytic muscles from HFrEF mice exhibit a reduced resistance to fatigue, a mechanism underlying the exercise intolerance in HF. Curcumin enhanced the resistance to fatigue of the Sol and EDL from HF mice measured in situ, and, therefore, ameliorated the impaired exercise performance of HFrEF mice. In this study, however, we were not able to observe a reduction of initial force generation in the Sol of HF mice although its CSA was decreased. This contradiction may have been due to the fact we used a submaximal stimulation, which could lead to a shift of the force-frequency relationship and therefore to similar forces in atrophic muscle.
Curcumin is a low-molecular weight polyphenol derived from the dietary spice turmeric, a traditional Asian medicine used for centuries to promote wound healing (13). It has been suggested that curcumin exerts antioxidant effects and protects mitochondria by activating the Nrf2 pathway (46). A growing body of evidence indicates that curcumin can limit skeletal muscle atrophy in several pathological conditions (3) via enhancing antioxidant defense by stimulating the Nrf2 signaling pathway (42). In rats with sepsis-induced wasting, curcumin blocked the increase in muscle protein breakdown by inhibiting proteasome-dependent protein degradation (39). In lipopolysaccharide-induced wasting mice, curcumin abolished atrogin-1/MAFbx upregulation and muscle protein loss (23). In addition, curcumin protects skeletal muscle against ischemia/reperfusion injury by increasing SOD, Cat, and GSH (4). Curcumin also ameliorates exercise-induced fatigue and extends exercise endurance in healthy mice (21). Although curcumin has been evaluated in several clinical trials for multiple diseases (18), its therapeutic potential on skeletal muscle dysfunction in the HFrEF state has not been tested in animal models or in patients. In the present study, we demonstrated that eight weeks of curcumin treatment improved exercise performance, increased whole-body pulling tension, and ameliorated the skeletal myopathy in mice with HFrEF, suggesting a potential application of curcumin in skeletal muscle dysfunction associated with HF.
In summary, we show here that Nrf2 was downregulated in skeletal muscle of mice with HFrEF, and that curcumin treatment ameliorated exercise intolerance in HF mice accompanied by upregulated Nrf2 and antioxidant enzymes. These data suggest that activation of Nrf2 in skeletal muscle may represent a novel therapeutic strategy to improve HFrEF patients’ quality of life. Given that many Nrf2 activators are being developed for clinical applications (15, 43), we believe this strategy is practicable.
GRANTS
This study was supported by NIH Grant P01-HL62222.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.W., H.S., I.Z., and L.G. conceived and designed research; A.W., J.H., T.R., L.Y., B.H., and L.G. performed experiments; A.W. and L.G. analyzed data; H.W., H.S., I.Z., and L.G. interpreted results of experiments; A.W. and L.G. prepared figures; A.W. and L.G. drafted manuscript; H.W., H.S., I.Z., and L.G. edited and revised manuscript; A.W., J.H., T.R., L.Y., B.H., H.W., H.S., I.Z., and L.G. approved final version of manuscript.
REFERENCES
- 1.Adams V, Linke A, Winzer E. Skeletal muscle alterations in HFrEF vs. HFpEF. Curr Heart Fail Rep 14: 489–497, 2017. doi: 10.1007/s11897-017-0361-9. [DOI] [PubMed] [Google Scholar]
- 2.Ahn B, Pharaoh G, Premkumar P, Huseman K, Ranjit R, Kinter M, Szweda L, Kiss T, Fulop G, Tarantini S, Csiszar A, Ungvari Z, Van Remmen H. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol 17: 47–58, 2018. doi: 10.1016/j.redox.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alamdari N, O’Neal P, Hasselgren PO. Curcumin and muscle wasting: a new role for an old drug? Nutrition 25: 125–129, 2009. doi: 10.1016/j.nut.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avci G, Kadioglu H, Sehirli AO, Bozkurt S, Guclu O, Arslan E, Muratli SK. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J Surg Res 172: e39–e46, 2012. doi: 10.1016/j.jss.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Bechara LR, Moreira JB, Jannig PR, Voltarelli VA, Dourado PM, Vasconcelos AR, Scavone C, Ramires PR, Brum PC. NADPH oxidase hyperactivity induces plantaris atrophy in heart failure rats. Int J Cardiol 175: 499–507, 2014. doi: 10.1016/j.ijcard.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation 137: e67–e492, 2018. [Erratum in Circulation 137: e493, 2018]. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 7.Bloemberg D, Quadrilatero J. Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7: e35273, 2012. doi: 10.1371/journal.pone.0035273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 6: 25–39, 2013. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brum PC, Bacurau AV, Medeiros A, Ferreira JC, Vanzelli AS, Negrão CE. Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function. Braz J Med Biol Res 44: 827–835, 2011. doi: 10.1590/S0100-879X2011007500075. [DOI] [PubMed] [Google Scholar]
- 10.Carlson CG, Rutter J, Bledsoe C, Singh R, Hoff H, Bruemmer K, Sesti J, Gatti F, Berge J, McCarthy L. A simple protocol for assessing inter-trial and inter-examiner reliability for two noninvasive measures of limb muscle strength. J Neurosci Methods 186: 226–230, 2010. doi: 10.1016/j.jneumeth.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho RF, Castan EP, Coelho CA, Lopes FS, Almeida FL, Michelin A, de Souza RW, Araújo JP Jr, Cicogna AC, Dal Pai-Silva M. Heart failure increases atrogin-1 and MuRF1 gene expression in skeletal muscle with fiber type-specific atrophy. J Mol Histol 41: 81–87, 2010. doi: 10.1007/s10735-010-9262-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Ceholski DK, Liang L, Fish K, Hajjar RJ. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 313: H275–H282, 2017. doi: 10.1152/ajpheart.00127.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell 130: 769–774, 2007. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crilly MJ, Tryon LD, Erlich AT, Hood DA. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J Appl Physiol (1985) 121: 730–740, 2016. doi: 10.1152/japplphysiol.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, Ghezzi P, León R, López MG, Oliva B, Pajares M, Rojo AI, Robledinos-Antón N, Valverde AM, Guney E, Schmidt HHHW. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev 70: 348–383, 2018. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 16.Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 34: 512–519, 2013. doi: 10.1093/eurheartj/ehs381. [DOI] [PubMed] [Google Scholar]
- 17.Gomes MJ, Martinez PF, Campos DH, Pagan LU, Bonomo C, Lima AR, Damatto RL, Cezar MD, Damatto FC, Rosa CM, Garcia CM, Reyes DR, Fernandes AA, Fernandes DC, Laurindo FR, Okoshi K, Okoshi MP. Beneficial effects of physical exercise on functional capacity and skeletal muscle oxidative stress in rats with aortic stenosis-induced heart failure. Oxid Med Cell Longev 2016: 8695716, 2016. doi: 10.1155/2016/8695716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15: 195–218, 2013. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39: 199–218, 2014. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Horie M, Warabi E, Komine S, Oh S, Shoda J. Cytoprotective role of Nrf2 in electrical pulse stimulated C2C12 myotube. PLoS One 10: e0144835, 2015. doi: 10.1371/journal.pone.0144835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang WC, Chiu WC, Chuang HL, Tang DW, Lee ZM, Wei L, Chen FA, Huang CC. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 7: 905–921, 2015. doi: 10.3390/nu7020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal 15: 2477–2486, 2011. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin B, Li YP. Curcumin prevents lipopolysaccharide-induced atrogin-1/MAFbx upregulation and muscle mass loss. J Cell Biochem 100: 960–969, 2007. doi: 10.1002/jcb.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol 31: 1352–1356, 1998. doi: 10.1016/S0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 25.Kinugawa S, Takada S, Matsushima S, Okita K, Tsutsui H. Skeletal muscle abnormalities in heart failure. Int Heart J 56: 475–484, 2015. doi: 10.1536/ihj.15-108. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Li J, Johnson DA, Stein TD, Kraft AD, Calkins MJ, Jakel RJ, Johnson JA. Nrf2, a multi-organ protector? FASEB J 19: 1061–1066, 2005. doi: 10.1096/fj.04-2591hyp. [DOI] [PubMed] [Google Scholar]
- 27.Li T, He S, Liu S, Kong Z, Wang J, Zhang Y. Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic Res 49: 1269–1274, 2015. doi: 10.3109/10715762.2015.1066784. [DOI] [PubMed] [Google Scholar]
- 28.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Möbius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation 111: 1763–1770, 2005. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 29.Mangner N, Weikert B, Bowen TS, Sandri M, Höllriegel R, Erbs S, Hambrecht R, Schuler G, Linke A, Gielen S, Adams V. Skeletal muscle alterations in chronic heart failure: differential effects on quadriceps and diaphragm. J Cachexia Sarcopenia Muscle 6: 381–390, 2015. doi: 10.1002/jcsm.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medeiro A, Vanzelli AS, Rosa KT, Irigoyen MC, Brum PC. Effect of exercise training and carvedilol treatment on cardiac function and structure in mice with sympathetic hyperactivity-induced heart failure. Braz J Med Biol Res 41: 812–817, 2008. doi: 10.1590/S0100-879X2008000900012. [DOI] [PubMed] [Google Scholar]
- 31.Merry TL, Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol 594: 5195–5207, 2016. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail 3: 537–546, 2010. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, Symons JD, Paine R III, Hoidal JR, Rajasekaran NS. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim Biophys Acta 1822: 1038–1050, 2012. doi: 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson KR Jr, Duscha BD, Hranitzky PM, Kraus WE. Chronic heart failure and exercise intolerance: the hemodynamic paradox. Curr Cardiol Rev 4: 92–100, 2008. doi: 10.2174/157340308784245757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiyama Y, Ikeda H, Haramaki N, Yoshida N, Imaizumi T. Oxidative stress is related to exercise intolerance in patients with heart failure. Am Heart J 135: 115–120, 1998. doi: 10.1016/S0002-8703(98)70351-5. [DOI] [PubMed] [Google Scholar]
- 36.Okutsu M, Call JA, Lira VA, Zhang M, Donet JA, French BA, Martin KS, Peirce-Cottler SM, Rembold CM, Annex BH, Yan Z. Extracellular superoxide dismutase ameliorates skeletal muscle abnormalities, cachexia, and exercise intolerance in mice with congestive heart failure. Circ Heart Fail 7: 519–530, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, Pasquinelli G. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastruct Pathol 31: 401–407, 2007. doi: 10.1080/01913120701719189. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Richardson RS, Haykowsky MJ, Hirai DM, Musch TI. Exercise limitations in heart failure with reduced and preserved ejection fraction. J Appl Physiol (1985) 124: 208–224, 2018. doi: 10.1152/japplphysiol.00747.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poylin V, Fareed MU, O’Neal P, Alamdari N, Reilly N, Menconi M, Hasselgren PO. The NF-kappaB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediators Inflamm 2008: 317851, 2008. doi: 10.1155/2008/317851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray Hamidie RD, Yamada T, Ishizawa R, Saito Y, Masuda K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 64: 1334–1347, 2015. doi: 10.1016/j.metabol.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol (1985) 73: 1797–1804, 1992. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 42.Sahin K, Pala R, Tuzcu M, Ozdemir O, Orhan C, Sahin N, Juturu V. Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: an in vivo model. J Inflamm Res 9: 147–154, 2016. doi: 10.2147/JIR.S110873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satoh T, Lipton S. Recent advances in understanding NRF2 as a druggable target: development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000 Res 6: 2138, 2017. doi: 10.12688/f1000research.12111.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seiler M, Bowen TS, Rolim N, Dieterlen MT, Werner S, Hoshi T, Fischer T, Mangner N, Linke A, Schuler G, Halle M, Wisloff U, Adams V. Skeletal muscle alterations are exacerbated in heart failure with reduced compared with preserved ejection fraction: mediated by circulating cytokines? Circ Heart Fail 9: e003027, 2016. doi: 10.1161/CIRCHEARTFAILURE.116.003027. [DOI] [PubMed] [Google Scholar]
- 45.Tamayo T, Eno E, Madrigal C, Heydemann A, García K, García J. Functional in situ assessment of muscle contraction in wild-type and mdx mice. Muscle Nerve 53: 260–268, 2016. doi: 10.1002/mus.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trujillo J, Chirino YI, Molina-Jijón E, Andérica-Romero AC, Tapia E, Pedraza-Chaverrí J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol 1: 448–456, 2013. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsutsui H, Ide T, Hayashidani S, Suematsu N, Shiomi T, Wen J, Nakamura Ki, Ichikawa K, Utsumi H, Takeshita A. Enhanced generation of reactive oxygen species in the limb skeletal muscles from a murine infarct model of heart failure. Circulation 104: 134–136, 2001. doi: 10.1161/01.CIR.104.2.134. [DOI] [PubMed] [Google Scholar]
- 48.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: H2181–H2190, 2011. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 49.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 189: 41–54, 2003. doi: 10.1016/S0300-483X(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 50.van Hees HW, Li YP, Ottenheijm CA, Jin B, Pigmans CJ, Linkels M, Dekhuijzen PN, Heunks LM. Proteasome inhibition improves diaphragm function in congestive heart failure rats. Am J Physiol Lung Cell Mol Physiol 294: L1260–L1268, 2008. doi: 10.1152/ajplung.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Haehling S, Anker SD. Cachexia vs obesity: where is the real unmet clinical need? J Cachexia Sarcopenia Muscle 4: 245–246, 2013. doi: 10.1007/s13539-013-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson JR, Martin JL, Ferraro N. Impaired skeletal muscle nutritive flow during exercise in patients with congestive heart failure: role of cardiac pump dysfunction as determined by the effect of dobutamine. Am J Cardiol 53: 1308–1315, 1984. doi: 10.1016/0002-9149(84)90085-7. [DOI] [PubMed] [Google Scholar]
- 53.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 58: 1057–1065, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]