Abstract
Aerobic exercise may acutely prime the brain to be more responsive to rehabilitation, thus facilitating neurologic recovery from conditions like stroke. This aerobic priming effect could occur through multiple mechanisms, including upregulation of circulating brain-derived neurotrophic factor (BDNF), increased corticospinal excitability, and decreased intracortical inhibition. However, optimal exercise parameters for targeting these mechanisms are poorly understood. This study tested the effects of exercise intensity on acute BDNF and neurophysiological responses. Sixteen ambulatory persons >6 mo poststroke performed three different 20-min exercise protocols in random order, approximately 1 wk apart, including the following: 1) treadmill high-intensity interval training (HIT-treadmill); 2) seated-stepper HIT (HIT-stepper); and 3) treadmill moderate-intensity continuous exercise (MCT-treadmill). Serum BDNF and transcranial magnetic stimulation measures of paretic lower limb excitability and inhibition were assessed at multiple time points during each session. Compared with MCT-treadmill, HIT-treadmill elicited significantly greater acute increases in circulating BDNF and corticospinal excitability. HIT-stepper initially showed BDNF responses similar to HIT-treadmill but was no longer significantly different from MCT-treadmill after decreasing the intensity in reaction to two hypotensive events. Additional regression analyses showed that an intensity sufficient to accumulate blood lactate appeared to be important for eliciting BDNF responses, that the interval training approach may have facilitated the corticospinal excitability increases, and that the circulating BDNF response was (negatively) related to intracortical inhibition. These findings further elucidate neurologic mechanisms of aerobic exercise and inform selection of optimal exercise-dosing parameters for enhancing acute neurologic effects.
NEW & NOTEWORTHY Acute exercise-related increases in circulating BDNF and corticospinal excitability are thought to prime the brain for learning. Our data suggest that these responses can be obtained among persons with stroke using short-interval treadmill high-intensity interval training, that a vigorous aerobic intensity sufficient to generate lactate accumulation is needed to increase BDNF, that interval training facilitates increases in paretic quadriceps corticospinal excitability, and that greater BDNF response is associated with lesser intracortical inhibition response.
Keywords: aerobic exercise, brain-derived neurotrophic factor, high-intensity interval training, locomotion, transcranial magnetic stimulation
INTRODUCTION
Aerobic exercise has well-established cardiovascular health benefits and has also been shown to have positive effects on brain function and neuroplasticity, which is especially relevant to neurologic rehabilitation (15, 54). It has been proposed that the acute effects of aerobic exercise could prime the brain to be more responsive to rehabilitation therapies, thus facilitating neurologic recovery from conditions like stroke (29). This hypothesis is largely based on studies among animals and neurologically intact humans, showing that cognitive learning, motor learning, and neuroplasticity processes are enhanced when practice is immediately preceded or followed by aerobic exercise (30, 31, 42, 48, 50, 51, 56, 57, 63). The aerobic priming effect on motor learning has also recently been observed among persons with stroke (37). Aerobic priming may occur through multiple potentially related mechanisms, including upregulation of brain-derived neurotrophic factor (BDNF) and modulation of corticospinal excitability (54).
BDNF is expressed in the cerebral cortex, basal forebrain, and hippocampus and plays key roles in neuroplasticity, motor learning, and cognitive function (29, 58). In animals, exercise has been shown to upregulate brain levels of BDNF (3, 40, 41). In humans, exercise-induced learning enhancement has been correlated with acute increases in circulating BDNF (in addition to lactate and catecholamines) (48, 63). It remains unclear how well changes in circulating BDNF reflect central processes (48), but the human brain appears to contribute 70–80% of circulating BDNF, both at rest and during exercise (41).
During motor learning, corticospinal excitability typically increases and motor intracortical inhibition typically decreases (9, 17). Aerobic exercise has been shown to elicit similar acute neurophysiological effects for both exercised (12, 32, 45, 48, 51, 58, 63) and nonexercised muscles (13, 33, 46, 49, 52, 59, 64). Thus it is possible that aerobic priming may enhance motor learning by augmenting these acute neurophysiological responses (54). However, sustained aerobic exercise often elicits no significant corticospinal excitability change (28, 33, 38, 45, 49, 52), with a trend toward decreased excitability in exercised muscles (28, 45). Thus the acute effect of aerobic exercise on corticospinal excitability appears to depend on the balance between increased central motor activation and the inhibitory effects of fatigue (59). Given that BDNF regulates the balance between neural excitation and inhibition and enhances synaptic γ-amino butyric acid (GABA) clearance, it may mediate the effects of aerobic exercise on corticospinal excitability and intracortical inhibition (16). However, no previous studies have evaluated the association between acute circulating BDNF and intracortical inhibition responses to exercise.
Exercise intensity appears to be a key factor influencing aerobic priming effects. Among healthy adults, higher aerobic intensity has been shown to elicit greater enhancement of motor learning (57). In addition, the circulating BDNF response to exercise seems to occur most reliably in studies that have used an intensity above ~70% of maximal heart rate (HR) (24). This approximate threshold is consistent with the onset of blood lactate accumulation (32), and lactate infusion at rest has also been shown to increase circulating BDNF (44). Thus achieving an acute circulating BDNF response with exercise may require a vigorous exercise intensity to generate lactate accumulation. Yet, the two previous studies comparing BDNF response between vigorous- and moderate-intensity exercise did not find a significant difference (19, 63). Thus it remains unclear if there is an intensity threshold for eliciting an acute circulating BDNF response, and no previous studies have tested for such a threshold among persons with stroke.
While neurological impairments from stroke make it more difficult to achieve and sustain a vigorous exercise intensity (5), this has been shown to be feasible using high-intensity interval training (HIT) on a treadmill (7, 10). A seated exercise mode may also further increase the feasibility of achieving lactate accumulation poststroke (12), but seated HIT has not been previously tested in this population. The purpose of this study was to determine the effect of exercise intensity on acute circulating BDNF and neurophysiological responses poststroke. We hypothesized that vigorous aerobic exercise, whether performed on a treadmill (HIT-treadmill) or seated stepper (HIT-stepper), would elicit significantly greater acute increases in serum BDNF and corticospinal excitability and a significantly greater acute decrease in intracortical inhibition compared with moderate-intensity continuous exercise (MCT-treadmill). We also sought to evaluate relationships among exercise intensity, BDNF, and neurophysiological responses.
MATERIALS AND METHODS
Participants
This study was approved by the University of Cincinnati Institutional Review Board. Participants were recruited from the community between April and August of 2015 and provided written informed consent before participation. Inclusion criteria were age 21–80 yr; unilateral paresis from stroke experienced >6 mo before enrollment; residual gait impairment; able to walk with assistive devices as needed and no physical assistance; stable cardiovascular condition, i.e., American Heart Association class B, not including aerobic capacity criterion (1); discharged from formal rehabilitation; and able to communicate with investigators and correctly answer consent comprehension questions. Exclusion criteria were evidence of significant arrhythmia or myocardial ischemia on treadmill graded exercise test (GXT) (1); hospitalization for cardiopulmonary disease within 3 mo; pacemaker or implanted defibrillator; lower extremity (LE) claudication; severe LE spasticity, i.e., Ashworth >2 (2); LE weight-bearing pain >4/10; and pregnancy. To be eligible for TMS testing, participants also had to be MRI compliant with no history of seizures or surgery over the motor cortex.
Study Design
A within-participant crossover design was employed. Each participant had a clinical examination and a symptom-limited GXT with electrocardiography (ECG) to determine eligibility and baseline characteristics. This was followed by a repeated GXT with gas exchange analysis (on a separate day) to determine peak oxygen consumption rate (V̇o2peak) and heart rate (HRpeak). Each participant then performed single sessions of three different exercise protocols in random order with approximately 1 wk between sessions. Within participants, each session was scheduled at the same time of day to minimize random error from diurnal fluctuations.
Graded Exercise Test
The GXT followed a stroke-specific symptom-limited protocol (26). Briefly, speed was predetermined using a treadmill screening test and was held constant while incline was increased 2–4% every 2 min until the participant drifted backward and could not recover, reached a cardiovascular safety limit (1, 20) or exhibited severe gait instability, defined as tripping without immediate recovery, toe drag persisting into midswing, stepping laterally off the treadmill belt, excessive joint instability (e.g., ankle inversion or knee hyperextension), or another form of gait instability judged to pose an imminent safety risk by the testing physical therapist, who was experienced in stroke rehabilitation.
Exercise Protocols
Participants held a height-adjusted handrail and wore a harness secured to an overhead support system for fall protection (not body weight support) during all treadmill walking. During each session, participants wore their habitual orthotic devices and no physical assistance was provided. Safety monitoring included ECG activity, blood pressure, and other signs or symptoms of cardiorespiratory insufficiency, worsening neurological impairments, or orthopedic injury, using accepted stopping criteria (1, 20). Participants were also questioned at the end of the session about pain, lightheadedness, and nausea and were questioned the following session about delayed onset effects. Adverse events (AEs) were categorized and graded using the Common Terminology Criteria for Adverse Events (36). Each of the following protocols included a 3-min warm up at 30–50% heart rate reserve (HRR), 20 min of exercise, and a 2-min cool down.
Treadmill high-intensity interval training.
HIT-treadmill involved repeated 30-s bursts at maximum tolerated speed (0% incline), alternated with recovery periods where the treadmill was stopped. Recovery duration was decreased from 60 to 30 s after the first 5 min (7, 8). Initial burst speed was determined by a rapid acceleration test where speed was increased by 0.1 mph every 5 s until reaching the limit where the participant drifted backward or exhibited gait instability. Burst speed started at 0.1 mph below this limit and was continuously progressed, maintained, or regressed between bursts based on performance criteria to maintain maximal safe motor intensity (7, 8). Recovery duration was extended if fastest safe burst speed decreased below the initial speed, if the participant requested to sit and was not able to stand back up in time, or if the participant was too short of breath to talk when a burst was scheduled to start.
Seated stepper high-intensity interval training.
HIT-stepper used the same burst and recovery durations as HIT-treadmill but on a seated stepper (NuStep T5XR). Bursts were performed at maximum possible cadence against 50% of maximal resistance (rounded down). Maximal resistance was predetermined with a steep ramp test where resistance was increased by one level every 15 s until the participant could not maintain a cadence of 80 steps/min (40 cycles/min). During bursts, visual feedback about cadence and verbal encouragement were provided to increase or maintain maximal cadence. If participants were able to achieve higher cadence without using their upper limb(s), they used lower limb(s) only (this was the case for all participants). If end-burst cadence was <80 or >200 steps/min, resistance was decreased or increased for the next burst, respectively. Recovery duration was extended if the participant was too short of breath to talk when a burst was scheduled to start.
Treadmill moderate-intensity continuous exercise.
MCT-treadmill involved continuous treadmill walking with speed continuously adjusted to achieve a mean aerobic intensity of 45 ± 5% HRR (27).
Exercise intensity measures.
Blood lactate was measured during each exercise session (see Blood Collection, Processing, and Analysis for details). Oxygen consumption rate (V̇o2) was measured from expired air using a Parvomedics TrueOne 2400 metabolic cart with a facemask interface (18). HR responses were captured by an ECG. V̇o2 and HR were recorded in 20-s averages by the metabolic cart software. Data were processed to obtain mean and maximum exercise V̇o2/HR and time spent above different V̇o2peak/HRR thresholds, including the lower limits of moderate (≥40%)-, vigorous (≥60%)-, and very hard (≥85%)-intensity zones (1). Rating of perceived exertion (RPE) was recorded using the Borg 6–20 scale at 10 min and at the end of each 20-min exercise protocol. Treadmill speeds and HIT-stepper step count were recorded from device displays. Treadmill step counts were recorded with a StepWatch Activity Monitor placed on the nonparetic ankle (34).
Blood collection, Processing, and Analysis
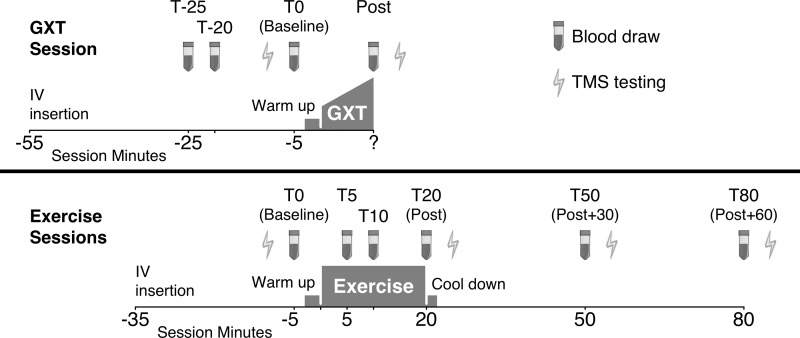
Blood collection, processing, and storage procedures were performed by experienced research nurse phlebotomists and technicians. An antecubital intravenous line was placed in the nonparetic arm upon participant arrival for each session, using ultrasound guidance if needed. Baseline samples were taken 30 min after intravenous insertion, to minimize any potential effect of intravenous insertion-related stress. A waste sample was obtained before each blood draw and the line was flushed after each draw. For the GXT session, 10-ml blood samples were obtained at three baseline time points (T-25, T-20, and T0) and 1 min after the GXT. For each of the exercise sessions, 10-ml blood samples were taken before the warm up (T0); after 5, 10, and 20 min of exercise (T5, T10, and T20); and then 30 and 60 min after finishing the exercise (T50 and T80) (Fig. 1).
Fig. 1.
Timing (T) of blood collection and transcranial magnetic stimulation (TMS) testing. GXT, graded exercise test; IV, intravenous line.
One drop from each sample was immediately analyzed with an EPOC point of care analysis system to measure blood lactate and hematocrit (53). The remaining sample clotted for 30 min at room temperature in a serum separator tube and then was centrifuged at 1,000 g for 15 min. The resultant serum was then aliquoted into multiple 250-μl cryovials (to avoid repeated freeze-thaw cycles during testing), transported on dry ice, stored in a −80°C freezer, and batch analyzed in duplicate to determine BDNF concentration using Quantikine sandwich enzyme-linked immunosorbent assay kits (R&D Systems; Minneapolis, MN). Laboratory staff performing the BDNF analysis were blinded to the study aims, design, and exercise protocol associated with the samples.
Neurophysiological Testing
For TMS-eligible participants, MRI-navigated single-pulse TMS testing was performed with a Nexstim eXimia 3.2 system and a figure 8 coil (43) to measure the corticospinal activation threshold of the paretic quadriceps femoris (vastus lateralis) and the cortical silent period (CSP). For the GXT session, this testing was performed before and after the GXT. For the exercise sessions, this testing was performed before the warm up (T0) and after the exercise (T20) and then 30 and 60 min later (T50 and T80) (Fig. 1). Participants sat in a semireclined chair with knees flexed 90° with the paretic ankle attached to a Biodex computerized robotic dynamometer, set in isometric mode with continuous visual feedback on knee extension torque to provide standardized quadriceps activation during testing. This procedure has previously shown excellent test-retest reliability for measuring paretic quadriceps active motor threshold poststroke (intraclass correlation coefficient = 0.975–0.978) (61). During TMS testing, the participant was given a torque target that produced a peak-to-peak EMG amplitude of ~100 μV. The amplified (×500) electromyographic (EMG) signals were sampled at 3 kHz, bandpass filtered (10–500 Hz), and stored for offline analysis.
During the first baseline test (from the GXT session), the cortical hotspot was identified as the three-dimensional coil location that reliably elicited the highest motor-evoked potentials (MEPs) in the paretic quadriceps. This location was then saved and repeated for all subsequent testing. Active motor threshold was defined as the minimal TMS intensity needed to elicit MEPs >200 μV in >50% of trials. This threshold was determined using adaptive Bayesian parameter estimation by sequential testing, with the default prior distribution and a 95% posterior probability interval stopping criterion (39).
To measure the CSP, 10 pulses at 120% of the most recent active motor threshold were delivered ≥3 s apart. Offline, rectified EMG activity from −200 to +500 ms was averaged across these 10 pulses. The CSP was defined as the immediate post-MEP drop in EMG amplitude below the mean rectified prepulse amplitude. CSP onset and offset were determined with a semiautomated method, using a MATLAB script supplemented with visual inspection, which was performed by a study team member who was blinded to all non-CSP study data. CSP area was calculated using the following formula: [(mean prepulse EMG amplitude × CSP duration) – area under the CSP EMG signal] (38), and it was normalized against the prepulse EMG level by the following: [CSP area/(mean prepulse EMG amplitude × CSP duration) × 100] (55).
Statistical Analysis
Test-retest reliability testing.
To assess the test-retest reliability of the serum BDNF and neurophysiological measures across days, we compared the T0 results across the four testing sessions (GXT and 3 exercise sessions). Separate general linear models were constructed for each variable with a fixed effect for protocol and an unstructured covariance matrix to account for the within-participant repeated measures (without making assumptions about the covariance structure). To assess within-session test-retest reliability for BDNF, we compared the three repeated baselines from the GXT session (T-25, T-20, and T0) using a model with a fixed effect for time and an unstructured covariance matrix. To estimate the individual BDNF response needed to be confident that a participant’s within-session change exceeded the limits of measurement error, we also calculated minimal detectable change upper confidence limits (MDC-UCLs):
where is the mean test-retest difference (calculated from T-25 to T0), is the confidence interval normal quantile (1.28, 1.44, 1.64, and 1.96 for 80, 85, 90, and 95% confidence intervals, respectively), and is the SD of the test-retest difference (60).
Primary hypothesis testing.
The primary analysis used a separate fixed-effects general linear model for each dependent variable (BDNF, active motor threshold, and CSP) to compare responses between protocols. The model included fixed effects for protocol, time, protocol × time, session order, session × time, hemolysis (for BDNF), and participant, with an unstructured covariance matrix to account for within-participant, within-session repeated measures (23). Protocol × time interaction contrasts were constructed to test the hypothesis that HIT-treadmill and HIT-stepper would each elicit significantly different changes compared with MCT-treadmill from T0 to the average of T5, T10, and T20 (for BDNF) or from T0 to T20 (for motor threshold and CSP).
Sample size determination.
GLIMMPSE (21) was used to estimate power to detect a significant BDNF protocol × time interaction with the above model, based on a two-tailed significance level of 0.05. The expected effect size and covariance parameters were estimated from the lactate data and a smaller preliminary BDNF data subset. This analysis indicated an expected power of 0.90 with 15 participants and no missing samples. Target sample size was set at 16 to account for potential missing samples and loss to follow up.
Planned sensitivity analyses.
Since measured BDNF concentrations could increase during exercise simply due to a decrease in circulating plasma volume (i.e., increase in hematocrit) (24), we performed a sensitivity analysis with adjustment for hematocrit. We performed a second sensitivity analysis without adjustment for hemolysis and a third that tested the effect of different cofactors on the BDNF response, including: age, sex, stroke chronicity, body mass index, comfortable and fast gait speed, V̇o2peak, V̇o2, and an unstructured covariance matrix. We also tested for any cofactor × protocol interactions.
Intensity measure associations with BDNF and neurophysiological responses.
Each exercise intensity measure was compared between protocols using a separate model with the intensity measure as the dependent variable, a fixed effect for protocol, and an unstructured covariance matrix. To test exercise intensity associations with BDNF and neurophysiological responses, we used separate models with the BDNF or neurophysiological exercise response as the dependent variable, fixed effects for the intensity measure, protocol and the T0 level of the dependent variable, and an unstructured covariance matrix. We also tested for intensity × protocol interactions.
With the use of the MDC-UCLs from the test-retest analysis to define “true” BDNF responses for each participant and session, receiver operating characteristic curves were constructed for each intensity measure to identify the threshold that maximized (sensitivity + specificity) for discriminating a BDNF response. The area under the receiver operating characteristic curve (area under the curve), ranging from 0 to 1, indexes how well the measure as a whole discriminates between dependent variable responses and nonresponses, with values significantly >0.5 indicating better than chance discrimination (14). Area under the curve values of 0.7–0.8 are considered acceptable and values of 0.8–0.9 are considered excellent (14).
Associations between BDNF and neurophysiological responses.
To test for associations between BDNF and neurophysiological responses, separate models were constructed using each TMS exercise response (motor threshold and CSP) as the dependent variable, with fixed effects for BDNF response, protocol, T0 BDNF and the T0 neurophysiological measure, and an unstructured covariance matrix.
Exploratory mediation analyses.
Based on the associations identified in the above analysis, we also performed exploratory mediation analyses to better understand potential causal relationships (25, 35) among protocol, intensity, BDNF, and neurophysiological responses. More specifically, we tested 1) whether effects of protocol on BDNF and neurophysiological responses were mediated through exercise intensity; and 2) whether protocol and exercise intensity associations with neurophysiological responses were mediated through the BDNF response.
RESULTS
Participant Flow and Data Completeness
All 16 eligible participants (Table 1) completed the study with no loss to follow up. Participants completed each 20-min protocol with the following exceptions: one participant requested to stop after 18.8 min of HIT-treadmill and after 19 min of HIT-stepper, while a different participant requested to stop after 17.6 min of HIT-stepper. In each case, the T20 data collection started immediately after the participant stopped exercising and the T50 and T80 data collections were adjusted to be 30 and 60 min after T20, as these were planned as time postexercise.
Table 1.
Participant flow and data completeness
| Characteristics and Results | |
|---|---|
| Participant characteristics (n = 16) | |
| Age, yr | 57.4 (9.7) [37.7–72.1] |
| Women, n (%) | 7 (43.8%) |
| Ischemic stroke type, n (%) | 12 (75%) |
| Years poststroke | 6.5 (4.1) [0.5–16.1] |
| Body mass index, kg/m2 | 27.6 (3.7) [21.4–35.5] |
| Comfortable gait speed, m/s | 0.72 (0.33) [0.07–1.13] |
| %Predicted | 54.2 (25.3) [5.2–91.4] |
| Fastest gait speed, m/s | 0.90 (0.41) [0.07–1.37] |
| β-Blocker use, n (%) | 3 (18.8%) |
| Age-predicted maximal HR,* beat/min | 160 (20) [118–182] |
| Resting HR, beat/min | 69 (11) [49–85] |
| Baseline GXT results | |
| Time to exhaustion, min | 7.9 (3.2) [3.3–12.9] |
| V̇o2peak, ml·kg−1·min−1 | 17.2 (3.3) [11.3–22.2] |
| %Predicted | 65.8 (13.7) [39.6–87.5] |
| V̇o2-VT, ml·kg−1·min−1 | 11.6 (2.5) [7.2–15.0] |
| HRpeak, beats/min | 139 (21) [98–174] |
| %Predicted | 87.1 (8.6) [75.4–102.9] |
| HR-VT, beats/min | 110 (20) [72–153] |
| Peak respiratory exchange ratio | 1.07 (0.09) [0.93–1.25] |
Data are presented as means (SD) [range] or n (%). HR, heart rate; GXT, graded exercise testing; V̇o2-VT, V̇o2 at ventilatory threshold; V̇o2peak. peak oxygen consumption rate; HRpeak, peak heart rate; HR-VT, heart rate at ventilatory threshold.
Adjusted for β-blocker use (see materials and methods).
We successfully obtained 332 (94.3%) out of 352 planned blood collections. The primary reason for missed blood collection was insufficient blood flow from the intravenous at the designated draw time. When this occurred, we attempted to obtain a drop of blood by finger stick for measurement of lactate and hematocrit with the point of care device. Thus we successfully obtained 340 (96.6%) out of 352 planned point of care blood measurements. Signs of hemolysis were observed in 72 (21.8%) out of 352 obtained serum collections. Among the 16 participants, 10 (62.5%) were eligible for TMS testing. Among these 10 participants, we successfully completed 133 (95%) out of 140 planned motor threshold assessments and 132 (94%) out of 140 CSP assessments.
Adverse Events
Among the first nine participants to complete HIT-stepper, two participants exhibited symptomatic hypotension and near syncope during recovery. One of these AEs was grade 2. The other was a serious AE (grade 3) that led to temporary hospitalization, where it was discovered that dehydration and malnourishment were likely contributing factors. Therefore, we began providing increased education to study participants on hydration and nourishment. We also revised the HIT-stepper protocol by removing the resistance and by using a mean intensity target of 70% HRR, with a limit of 85% HRR. These changes resulted in significant differences in intensity between the original and revised HIT-stepper protocols, so we analyzed them separately in a sensitivity analysis. The final seven participants completed the revised HIT-stepper protocol without any hypotensive events.
Eight other nonserious AEs occurred that were related or possibly related to testing or training. There was one grade 2 ankle pain AE associated with the treadmill GXT; four different grade 1 pain AEs associated with the treadmill GXT (one AE) and each exercise protocol (three AEs); two grade 1 lightheadedness AEs associated with the treadmill GXT (one AE) and MCT-treadmill (one AE); and one grade 1 skin abrasion AE associated with both the HIT-treadmill and MCT-treadmill protocols.
Test-Retest Reliability Results
There were no significant differences in T0 BDNF, active motor threshold, or CSP values across the four testing sessions and test-retest intraclass correlation coefficients ranged from 0.70 to 0.90 (Table 2).
Table 2.
Test-retest reliability of blood testing and transcranial magnetic stimulation testing
| n/No. Time Points/Total No. Tests | ICC (2, 1) [95% CI] | Omnibus P For Test-Retest Differences | Significant (P < 0.05) Pairwise Test-Retest Differences | |
|---|---|---|---|---|
| Serum brain-derived neurotrophic factor, ng/ml | ||||
| T-25, T-20, and T0 from GXT session | 16/3/48 | 0.77 [0.55, 0.90] | 0.1258 | T-20 < T-25 |
| T-25 to T0 from GXT session | 16/2/32 | 0.87 [0.67, 0.95] | 0.7927 | None |
| T0s from GXT and 3 exercise sessions | 15/4/60 | 0.70 [0.49, 0.87] | 0.6714 | None |
| Paretic quad active motor threshold, %maximum stimulator output | ||||
| T0s from GXT and 3 exercise sessions | 10/4/40 | 0.90 [0.77, 0.97] | 0.5056 | None |
| Cortical silent period normalized area, % | ||||
| T0s from GXT and 3 exercise sessions | 9/4/36 | 0.73 [0.45, 0.92] | 0.2460 | None |
T-25, T-20, and T0, three baseline time points; ICC, intraclass correlation coefficient; CI, confidence interval; GXT, graded exercise test; MCT, moderate intensity continuous training; HIT, high-intensity interval training.
During the BDNF multiple baseline testing from the treadmill GXT session (before the GXT), serum BDNF significantly decreased from T-25 to T-20 (−3.8 ng/ml [95% confidence interval: −7.5, −0.1]) and then increased back to near T-25 levels from T-20 to T0 (+3.5 ng/ml [−0.1, +7.0]). The significant serum BDNF drop from T-25 to T-20 resembled a similar drop seen in all three exercise protocols from T5 to T10 (Fig. 2). These two time points with BDNF drops (T-20 from the GXT session and T10 from the exercise sessions) were the only blood draws performed only 5 min after a previous blood draw (T0 and T5 were separated by a transfer onto the treadmill and a warm up plus 5 min). Therefore, we believe these BDNF drops may be a data collection artifact (e.g., insufficient time between blood draws for systemic circulation to replenish the blood draw site). Consequently, we omitted the T-20 and T10 time points from subsequent BDNF analyses, except for the a priori primary analysis. There was no significant BDNF change from T-25 to T0 (−0.3 ng/ml, SDchange 4.8 [−2.9, 2.3]). Calculated from these time points, the BDNF MDC-UCLs were as follows: MDC80, +5.87 ng/ml; MDC85, +6.64; MDC90, +7.61; and MDC95, +9.15.
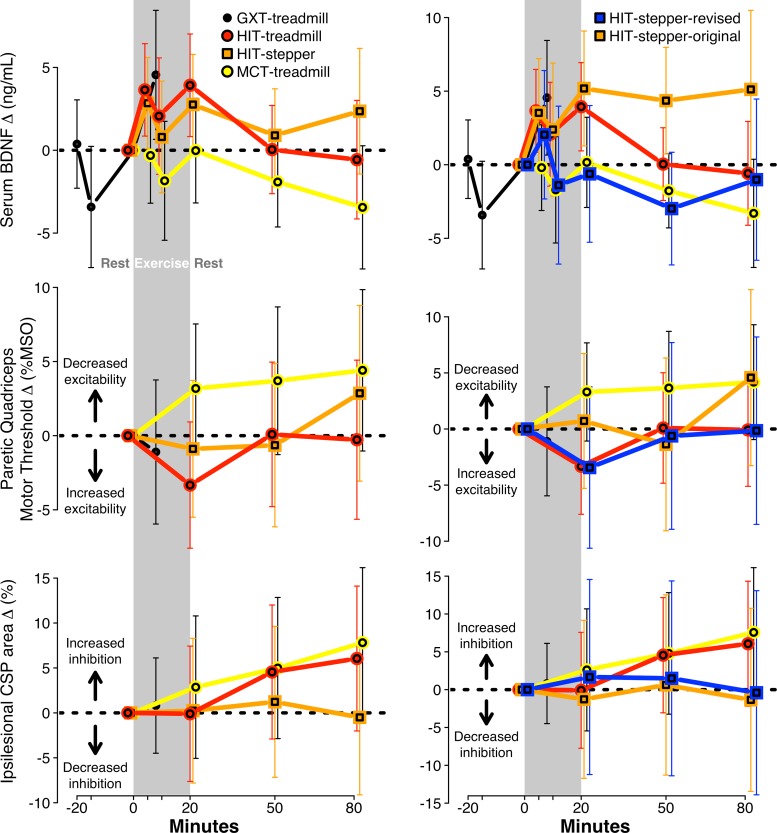
Fig. 2.
Time course of changes in serum brain-derived neurotrophic factor (BDNF) and neurophysiological measures by protocol. Results from the primary analysis (left) and a sensitivity analysis separating HIT-stepper into original and revised protocols (right), presented as change from T0, with 95% confidence interval error bars. Sample sizes for BDNF, active motor threshold and cortical silent period (CSP) were n = 16 (332 samples), n = 10 (133 tests), and n = 10 (132 tests). GXT, graded exercise test; HIT, high-intensity interval training; MCT, moderate-intensity continuous training; MSO, maximum stimulator output.
Circulating BDNF differences between protocols
Serum BDNF significantly increased during the treadmill GXT (+4.6 ng/ml [95% confidence interval: 0.7, 8.4]) (Fig. 2; top left). In the a priori primary analysis (with adjustment for session order and hemolysis), serum BDNF response from T0 to the mean of T5, T10, and T20 for HIT-treadmill, HIT-stepper, and MCT-treadmill was +3.2 [0.5, 5.9], +2.1 [−0.5, 4.7], and −0.7 [−3.4, 2.0]. The increase was significantly greater for HIT-treadmill compared with MCT-treadmill (+3.9 [0.1, 7.8]) but not for HIT-stepper compared with MCT-treadmill (+2.9 [−1.0, 6.7]).
After T10 (which oddly showed a BDNF drop for all 3 protocols) was omitted in a post hoc sensitivity analysis, these BDNF responses were +3.8 [1.2, 6.3], +2.8 [0.3, 5.3], and −0.2 [−2.8, 2.5] and the significance of between protocol differences remained unchanged. BDNF at T0 was a significant predictor of BDNF response from T0 to T5, T10, and T20 (β = −0.58 [−0.82, −0.33]), but adjusting for T0 BDNF in another post hoc sensitivity analysis did not change the significance of the between protocol differences. Neither did adjusting for hematocrit (changes in plasma volume) or removing hemolysis adjustment from the model. There were no significant associations between BDNF response and any of the tested cofactors (age, sex, stroke chronicity, body mass index, and comfortable and fast gait speed, V̇o2peak, V̇o2VT, HRpeak, and HR-VT) and no significant cofactor × protocol interactions.
In a post hoc sensitivity analysis (Fig. 2, top right), we compared BDNF responses between protocols with HIT-stepper separated into the original (n = 9) and revised (n = 7) protocols. HIT-stepper-original showed a significant BDNF response from T0 to T5 and T20 (+4.4 [1.1, 7.6]), but HIT-stepper-revised did not (+0.7 [−3.2, 4.6]). The HIT-stepper-original response was not significantly different from HIT-stepper-revised (+3.6 [−1.5, 8.8]), but it was significantly greater than MCT-treadmill (+4.4 [0.2, 8.5].
Neurophysiological Differences Between Protocols
Paretic quadriceps active motor threshold and CSP normalized area each showed nonsignificant changes during the treadmill GXT (Fig. 2, middle and bottom left). In the a priori primary analysis (with adjustment for session order), active motor threshold response from T0 to T20 for HIT-treadmill, HIT-stepper and MCT-treadmill was −3.3% MSO [−7.6, 0.9], −0.9 [−5.5, 3.7], and +3.2 [−1.2, 7.5] (Fig. 2, middle left). The decrease was significantly greater for HIT-treadmill compared with MCT-treadmill (−6.5 [−12.6, −0.4]) but not for HIT-stepper compared with MCT-treadmill (−4.1 [−10.6, 2.4]). CSP response from T0 to T20 for HIT-treadmill, HIT-stepper, and MCT-treadmill was −0.1% [−7.6, 7.4], +0.2 [−7.8, 8.3], and +2.9 [−5.1, 10.8] (Fig. 2, bottom left). The response was not significantly different for HIT-treadmill compared with MCT-treadmill (−3.0 [−13.9, 8.0]) nor for HIT-stepper compared with MCT-treadmill (−2.6 [−14.2, 8.9]).
T0 for active motor threshold was not a significant predictor of T0 to T20 response (β = +0.0 [−0.1, 0.2]), but T0 for CSP area was a significant predictor of the T0 to T20 response (β = −0.3 [−0.5, −0.1]). In a post hoc sensitivity analysis, adjusting motor threshold response for T0 motor threshold increased the precision of the model estimates, making the HIT-treadmill decrease statistically significant (−3.3%MSO [−6.5, −0.0]) while maintaining the significance of the HIT-treadmill versus MCT-treadmill comparison. Adjusting CSP response for T0 CSP area did not change the (non-) significance of the within-protocol effects or between-protocol differences.
Exercise Intensity Differences Between Protocols
Compared with MCT-treadmill, HIT-treadmill and HIT-stepper (with the original and revised protocols combined) each elicited significantly greater responses for blood lactate, maximum V̇o2, time spent ≥85% V̇o2peak, almost every HR-based intensity measure, and end-session RPE (Table 3). The revised HIT-stepper (n = 7) had significantly lower responses than the original HIT-stepper (n = 9) for all lactate, V̇o2, and HR measures except maximum HR expressed as %HRR (P = 0.09).
Table 3.
Intensity differences between protocols and association with BDNF/TMS responses
| Intensity Measure | Mean Intensity by Protocol | βintensity Association with BDNF/TMS Responses [95% CI]Adjusted for Protocol and Baseline BDNF/TMS Variable | |||||
|---|---|---|---|---|---|---|---|
| HITTreadmill | HIT*stepper | MCTtreadmill | P | BDNF, ng/ml | Paretic quad MT, %MSO | CSP normalized area, % | |
| Blood lactate measures | |||||||
| Mean lactate, mmol/l | 4.6b | 6.8a (8.5/3.3) | 2.0c | <0.0001 | 0.90 [0.01, 1.78] | 1.2 [0.1, 2.3] | −1.0 [−3.1, 1.1] |
| T5 lactate, mmol/l | 3.0 | 3.9a (5.5/2.3) | 1.8b | 0.0095 | 1.71 [0.30, 3.12] | 1.2 [−0.6, 3.0] | 0.1 [−2.8, 3.0] |
| T10 lactate, mmol/l | 4.0b | 6.2a (8.2/3.0) | 1.8c | 0.0005 | 0.99 [0.21, 1.77] | 0.5 [−1.3, 2.4] | DC |
| T20 lactate, mmol/l | 5.9b | 8.9a (11.5/4.1) | 1.9c | <0.0001 | 0.59 [−0.07, 1.25] | 0.8 [−0.2, 1.8] | −1.5 [−3.4, 0.5] |
| V̇o2-based measures | |||||||
| Mean V̇o2, %V̇o2peak | 64.0 | 69.3a (77.7/54.5) | 56.6b | 0.0269 | 0.18 [0.05, 0.31] | −0.0 [−0.3, 0.2] | −0.2 [−0.2, −0.1] |
| Max V̇o2, %V̇o2peak | 100.5a | 96.1a (106.8/77.6) | 72.1b | <0.0001 | 0.12 [0.03, 0.22] | −0.0 [−0.2, 0.2] | −0.1 [-[−0.2, −0.1] |
| Minutes 40–60% V̇o2peak | 7.3 | 6.0b (2.5/9.7) | 9.9a | 0.0678 | −0.48 [−0.97, 0.01] | −0.2 [−0.9, 0.5] | −1.0 [−1.6, −0.3] |
| Minutes 60–85% V̇o2peak | 6.5 | 7.4 (10.4/4.3) | 6.8 | 0.8361 | 0.61 [0.12, 1.10] | −0.3 [−1.0, 0.4]† | −0.8 [−1.8, 0.1] |
| Minutes ≥85% V̇o2peak | 3.2a | 4.5a (6.4/1.4) | 0.9b | 0.0070 | 0.65 [0.07, 1.24] | 0.6 [−0.6, 1.7] | −1.0 [−1.8, −0.2] |
| HR-based measures | |||||||
| Mean HR, %HRpeak | 79.8a | 83.9a (88.5/73.7) | 72.3b | 0.0140 | 0.22 [0.06, 0.39] | 0.3 [−0.1, 0.8] | −0.1 [−0.4, 0.2] |
| Mean HR, %APmaxHR | 72.1a | 75.3a (78.3/65.3) | 64.1b | 0.0027 | 0.20 [0.02, 0.39] | 0.3 [0.0, 0.5] | −0.1 [−0.4, 0.2] |
| Mean HR, %HRRpeak | 59.0a | 67.5a (79.1/43.6) | 43.8b | 0.0121 | 0.13 [0.05, 0.22] | 0.2 [0.1, 0.3] | −0.1 [−0.3, 0.1] |
| Mean HR, %APmaxHR | 48.4a | 54.9a (60.5/37.5) | 35.5b | 0.0038 | 0.15 [0.04, 0.26] | 0.2 [0.0, 0.3] | −0.1 [−0.2, 0.1] |
| Max HR, %HRpeak | 98.1a | 101.8a (106.8/93.4) | 79.8b | <0.0001 | 0.12 [−0.04, 0.27] | 0.3 [0.2, 0.4] | −0.2 [−0.5, 0.1] |
| Max HR, %APmaxHRR | 88.8a | 91.2a (95.1/81.8) | 71.4b | <0.0001 | 0.11 [−0.06, 0.28] | 0.2 [0.0, 0.4] | −0.1 [−0.3, 0.1] |
| Max HR, %HRRpeak | 95.2a | 103.8a (112.6/88.7) | 58.3b | <0.0001 | 0.05 [−0.02, 0.13] | 0.1 [0.1, 0.2] | −0.1 [−0.2, 0.1] |
| Max HR, %APmaxHRR | 78.9a | 83.9a (90.8/68.0) | 49.7b | <0.0001 | 0.07 [−0.03, 0.16] | 0.1 [0.0, 0.2] | −0.1 [−0.2, 0.1] |
| Minutes 40–60%HRRpeak | 3.8b | 5.3b (3.7/6.9) | 12.2a | <0.0001 | −0.02 [−0.55, 0.51]‡ | −0.2 [−1.1, 0.8] | 0.3 [−1.0, 1.5] |
| Minutes 60–85%HRRpeak | 6.9a | 4.2b (4.4/3.6) | 1.0c | <0.0001 | −0.23 [−0.81, 0.35] | −0.2 [−1.4, 1.0] | 0.7 [−0.9, 2.3] |
| Minutes ≥85%HRRpeak | 2.0 | 5.0a (8.5/0.5) | 0.4b | 0.0372 | 0.57 [0.19, 0.95] | 0.7 [0.2, 1.3] | −0.4 [−1.1, 0.3] |
| RPE | |||||||
| Midsession RPE (6–20) | 13.1b | 14.3a (14.9/13.8) | 12.0b | 0.0015 | 0.25 [−0.65, 1.15] | 2.9 [2.6, 3.2] | −1.3 [−4.1, 1.5] |
| Whole session RPE (6–20) | 15.1a | 15.7a (16.5/15.2) | 13.1b | 0.0050 | −0.10 [−1.08, 0.89] | 1.3 [0.1, 2.4] | −0.6 [−2.6, 1.3] |
| Neuromotor intensity measures | |||||||
| Peak speed, m/s | 1.30a | N/A | 0.68b | <0.0001 | −0.45 [−9.44, 8.54] | DC | DC |
| Peak speed, %FGS | 165.6a | N/A | 77.7b | <0.0001 | 0.01 [−0.02, 0.03] | 0.0 [−0.1, 0.1] | DC |
| Consistent speed, m/s | 1.16a | N/A | 0.74b | 0.0013 | −1.27 [−16.7, 14.1] | 3.1 [−8.2, 14.3] | DC |
| Consistent speed, %FGS | 155.0a | N/A | 77.6b | <0.0001 | 0.01 [−0.00, 0.01] | 0.0 [-0.1, 0.1] | DC |
| Step count | 1441b | 1,821a (1,732/1890) | 1,750a | 0.0032 | 0.00 [−0.01, 0.01] | 0.0 [−0.1, 0.1] | 0.0 [−0.0, 0.0] |
BDNF response is mean of timepoints T5 and T20 minus T0. Mean lactate is mean of timepoints T5, T10, and T20. BDNF, brain-derived neurotrophic factor; TMS, transcranial magnetic stimulation; HIT, high-intensity interval training; MCT, moderate-intensity continuous training; MT, active motor threshold; MSO, maximum stimulator output; CSP, cortical silent period; DC, did not converge; HR, heart rate; HRR, heart rate reserve; peak, maximum value reached during symptom-limited, graded exercise test; APmax, age-predicted maximum; FGS, fastest (floor) gait speed; RPE, rating of perceived excertion; V̇o2, oxygen consumption rate.
Data are reported as HIT-stepper protocols combined (original protocol/revised protocol).
βintensity Significantly higher for HIT-treadmill vs. MCT-treadmill.
βintensity Significantly lower for HIT-treadmill and HIT-stepper vs. MCT-treadmill.
P < 0.05, differing letters indicate significant differences between protocols. Bold font indicates an association with P < 0.05.
Exercise Intensity Associations with BDNF and Neurophysiological Responses
BDNF response from T0 to the mean of T5 and T20 was positively associated with blood lactate during exercise and V̇o2/HR responses (Table 3). Time spent at vigorous and time spent at very hard aerobic intensities (60–85% V̇o2peak; ≥85% V̇o2peak/HRR) were each positively associated with BDNF response, but time spent at moderate intensity (40–60% V̇o2peak /HRR) was not. Paretic quadriceps active motor threshold response from T0 to T20 was positively associated with mean blood lactate during exercise, HR responses, and RPE (Table 3). CSP response was negatively associated with VO2 responses.
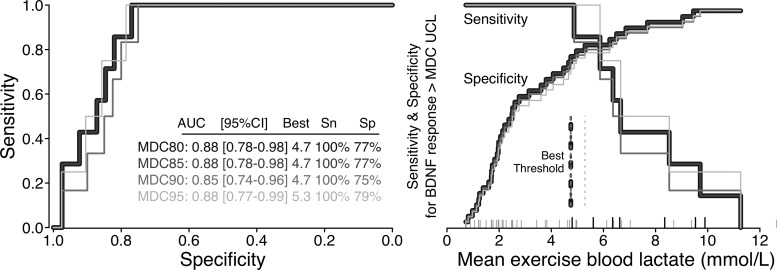
Individual BDNF responses from T0 to T5 and T20 exceeded the MDC80 and MDC85 UCLs for 7 (15%) out of 46 participant sessions with available data (3 during HIT-treadmill, 3 during HIT-stepper-original and 1 during HIT-stepper-revised). Six of these BDNF responses also exceeded the MDC90-UCL and four exceeded the MDC95-UCL. Optimal intensity thresholds for discriminating BDNF responses were fairly consistent across the four MDC confidence thresholds and especially for MDC80–90 (Fig. 3 and Table 4). No sessions with intensity below the following thresholds had a BDNF response greater than the MDC80-UCL (i.e., these were the highest thresholds with 100% sensitivity): mean exercise blood lactate of 4.7; T10 blood lactate of 4.5; mean V̇o2 of 67% V̇o2peak; 6 min spent above 60% V̇o2peak; mean HR of 84% HRpeak, 74% APmaxHR, 72% HRRpeak, and 53% APmaxHRR; and 6 min above 85%HRR.
Fig. 3.
Example analysis to identify optimal intensity threshold for discriminating brain-derived neurotrophic factor (BDNF) responses (n = 16; 48 sessions). Receiver operating characteristic curve (left) and threshold visualization (right). A BDNF response was defined as an increase during exercise (from T0 to the average of T5 and T20) that exceeded the minimal detectable change upper confidence limit (MDC-UCL). Different shaded lines are results from using different MDC confidence limits to define a BDNF response. MDC80 and MDC85 had perfect overlap. The optimal intensity threshold for discriminating a BDNF response was defined as the one that maximized (sensitivity + specificity). These “best” thresholds for each MDC-UCL are reported in the left panel table and are shown as vertical dotted lines in the right panel. The right panel x-axis tick marks show the distribution of mean exercise blood lactate for each session. Sessions with a BDNF response > MDC80 UCL are indicated by longer black ticks. AUC, area under the curve; Sn, sensitivity; Sp, specificity.
Table 4.
Intensity thresholds most discriminative of BDNF responses exceeding MDC-UCL
| Intensity Measure/BDNF MDC-UCL | AUC [95% CI] | Best Threshold | Sensitivity, % | Specificity, % |
|---|---|---|---|---|
| Blood lactate mean, mmol/l | ||||
| MDC80&85 | 0.88 [0.78, 0.98] | 4.7 | 100 | 77 |
| MDC90 | 0.85 [0.74, 0.96] | 4.7 | 100 | 75 |
| MDC95 | 0.88 [0.77, 0.99] | 5.3 | 100 | 79 |
| Blood lactate T5, mmol/l | ||||
| MDC80&85 | 0.87 [0.75, 0.99] | 4.1 | 86 | 84 |
| MDC90 | 0.83 [0.70, 0.97] | 4.1 | 83 | 82 |
| MDC95 | 0.89 [0.78, 1.00] | 4.1 | 100 | 80 |
| Blood lactate T10, mmol/l | ||||
| MDC80&85 | 0.90 [0.80, 1.00] | 4.5 | 100 | 76 |
| MDC90 | 0.88 [0.76, 0.99] | 4.5 | 100 | 74 |
| MDC95 | 0.94 [0.86, 1.00] | 7.3 | 100 | 90 |
| Mean V̇o2, %V̇o2peak | ||||
| MDC80&85 | 0.92 [0.84, 1.00] | 67 | 100 | 82 |
| MDC90 | 0.91 [0.82, 1.00] | 67 | 100 | 80 |
| MDC95 | 0.90 [0.80, 0.90] | 72 | 100 | 81 |
| Max V̇o2, %V̇o2peak | ||||
| MDC80&85 | 0.87 [0.72, 1.00] | 116 | 71 | 97 |
| MDC90 | 0.85 [0.68, 1.00] | 117 | 67 | 98 |
| MDC95 | 0.89 [0.71, 1.00] | 120 | 75 | 98 |
| Minutes 60–85% V̇o2peak | ||||
| MDC80&85 | 0.65 [0.46, 0.84] | 6 | 100 | 44 |
| MDC90 | 0.66 [0.46, 0.87] | 6 | 100 | 43 |
| MDC95 | 0.46 [0.20, 0.71] | 7 | 75 | 55 |
| Minutes ≥85% V̇o2peak | ||||
| MDC80&85 | 0.89 [0.78, 1.00] | 4 | 86 | 85 |
| MDC90 | 0.88 [0.74, 1.00] | 4 | 83 | 83 |
| MDC95 | 0.90 [0.80, 1.00] | 4 | 100 | 81 |
| Mean HR, %HRpeak | ||||
| MDC80&85 | 0.94 [0.87, 1.00] | 84 | 100 | 89 |
| MDC90 | 0.95 [0.89, 1.00] | 90 | 100 | 95 |
| MDC95 | 0.94 [0.87, 1.00] | 90 | 100 | 90 |
| Mean HR, %APmaxHRR | ||||
| MDC80&85 | 0.93 [0.85, 1.00] | 74 | 100 | 84 |
| MDC90 | 0.91 [0.81, 1.00] | 74 | 100 | 82 |
| MDC95 | 0.83 [0.71, 0.96] | 74 | 100 | 78 |
| Mean HR, %HRRpeak | ||||
| MDC80&85 | 0.94 [0.87, 1.00] | 72 | 100 | 92 |
| MDC90 | 0.95 [0.88, 1.00] | 76 | 100 | 92 |
| MDC95 | 0.95 [0.88, 1.00] | 80 | 100 | 93 |
| Mean HR, %APmaxHRR | ||||
| MDC80&85 | 0.92 [0.83, 1.00] | 53 | 100 | 86 |
| MDC90 | 0.89 [0.79, 0.99] | 53 | 100 | 84 |
| MDC95 | 0.83 [0.71, 0.96] | 53 | 100 | 80 |
| Minutes ≥85%HRR | ||||
| MDC80&85 | 0.94 [0.86, 1.00] | 6 | 100 | 92 |
| MDC90 | 0.93 [0.85, 1.00] | 6 | 100 | 89 |
| MDC95 | 0.93 [0.84, 1.00] | 8 | 100 | 88 |
Receiver operating characteristic analysis of intensity variables with significant association to brain-derived neurotrophic factor (BDNF) response (see Table 2). The “best” threshold was the one that maximized (sensitivity + specificity). MDC-UCL, minimal detectable change upper confidence limits; AUC, area under the curve; V̇o2, oxygen consumption rate; HR, heart rate; HRR, heart rate reserve; peak, maximum value reached during symptom-limited, graded exercise test; APmax, age-predicted maximum.
Associations Between BDNF and Neurophysiological Responses
When testing for associations between BDNF and neurophysiological responses, T0 BDNF and the T0 neurophysiological measure were included in the models based on previous analyses above. BDNF response was not significantly associated with active motor threshold response (β = −0.1 [95% confidence interval: −0.6, 0.5]) but was negatively associated with CSP response (−0.7 [−1.2, −0.2]).
Exploratory Mediation Results
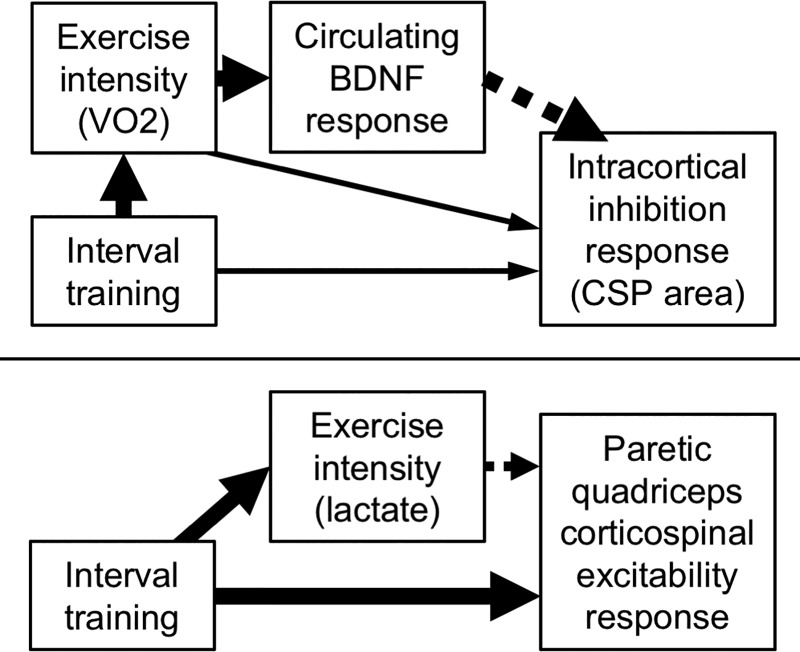
For the first mediation analysis, we tested whether the observed positive effect of HIT-treadmill on BDNF response was mediated by the observed positive association of mean exercise V̇o2 with BDNF response. When tested in the same model, HIT-treadmill no longer had a significantly greater effect on BDNF response than MCT (+3.1 [−1.2, 7.4]) and mean exercise V̇o2 remained positively associated with BDNF response (β = 0.2 [0.1,0.3]). Thus the effect of HIT-treadmill on BDNF response appeared to be completely mediated through exercise intensity (Fig. 4, top).
Fig. 4.
Hypothesized causal models based on exploratory mediation analysis. Causal models for the effect of training protocol and intensity on ipsilesional intracortical inhibition response (top) and for the effect of training protocol and intensity on paretic quadriceps corticospinal excitability (assessed by active motor threshold) response (bottom). Solid and dashed arrows indicate positive and negative associations, respectively. Arrow size roughly indicates the relative effect magnitude. V̇o2, oxygen consumption rate; BDNF, brain-derived neurotrophic factor; CSP, cortical silent period.
For the second mediation analysis, we tested whether the observed negative association of mean exercise V̇o2 with CSP response was mediated by the observed negative association of BDNF response with CSP response. When tested in the same model, mean exercise V̇o2 became positively (rather than negatively) associated with CSP response (β = 0.2 [0.1, 0.2]) and BDNF response became even more negatively associated with CSP response (β = −1.2 [−1.3, −1.1]). Thus the overall negative association of V̇o2 intensity with CSP response appeared to be driven by a negative indirect effect mediated through BDNF response, which exceeded the positive direct effect (Fig. 4, top).
For the third mediation analysis, we tested whether the observed null effect of protocol on CSP response was mediated by the observed negative overall association of mean exercise V̇o2 with CSP response. With adjustment for mean exercise V̇o2, each HIT protocol had a greater CSP response than MCT (HIT-treadmill +1.9 [0.3, 3.4]; HIT-stepper +1.9 [0.5, 3.4]). Thus the null overall effect of HIT on CSP response appeared to be due to a net cancellation between a positive direct effect and a negative indirect effect mediated through V̇o2 intensity and BDNF response (Fig. 4, top).
For the final mediation analysis, we tested whether the observed negative effect of HIT-treadmill on active motor threshold response was mediated by the observed positive association of lactate response with motor threshold response. When tested together in the same model, both protocol and lactate remained significantly associated with motor threshold response (protocol omnibus P = 0.0182; mean lactate β = 1.2 [0.1, 2.3]), indicating partial mediation. In this model, motor threshold response from T0 to T20 for HIT-treadmill, HIT-stepper, and MCT-treadmill was −2.9%MSO [−6.5, 0.7], −8.8 [−13.7, −3.8], and +5.6 [1.4, 9.8]. The between-protocol difference became more significant for HIT-treadmill versus MCT-treadmill (−8.5 [−14.4, −2.6]), and became significant for HIT-stepper versus MCT-treadmill (−14.3 [−22.2, −6.4]). Thus the overall negative effect of HIT (versus MCT) on active motor threshold response appeared to be driven by a large direct negative effect that exceeded the positive indirect effect mediated through lactate response (Fig. 4).
DISCUSSION
This study tested the effect of exercise intensity on acute circulating BDNF and neurophysiological responses among stroke survivors. We report for the first time that vigorous-intensity exercise elicited significantly greater acute increases in circulating BDNF and corticospinal excitability compared with moderate-intensity exercise and that greater circulating BDNF responses were associated with decreased intracortical inhibition responses. Significant BDNF increases were observed for both treadmill HIT and seated stepper HIT, but the increase was no longer significant for seated stepper HIT after modifying the protocol for safety reasons. Independent of exercise protocol, the BDNF response was positively correlated with lactate, V̇o2, and HR measures of intensity. Optimal mean intensity thresholds for discriminating BDNF responses were largely found to be around the onset of blood lactate accumulation (mean lactate >4.7 mmol/l, mean V̇o2 >67% V̇o2peak, mean HR >84% HRpeak, >74% APmaxHR, >72% HRRpeak, or >53% APmaxHRR).
These findings are mostly consistent with previous studies among healthy adults who have generally shown significant BDNF increases with vigorous-intensity exercise but not with lower intensity (24). In contrast, a recent study that tested a short 5-min vigorous seated stepper protocol among stroke survivors found no significant acute BDNF increases despite a mean blood lactate of 6.1 mmol/l (12). Thus it is possible that an exercise duration longer than 5 min and a more sustained lactate accumulation is required to acutely upregulate circulating BDNF. This may seem to be contradicted by the significant increase in serum BDNF we observed after only 5 min of HIT-treadmill, when mean blood lactate was only 3.0 mmol/l. However, the T5 testing time point was also after a 3-min warm-up (8 min total) and the optimal lactate threshold for discriminating individual BDNF responses at T5 was still >4.0 mmol/l.
With little exception (13), aerobic exercise usually shows no significant acute effects on TMS measures of corticospinal excitability (28, 33, 38, 45, 49, 52), likely due to a cancellation between increased central motor activation and the inhibitory effects of fatigue (59). Yet, here we observed a significantly greater acute increase in corticospinal excitability after HIT-treadmill compared with MCT-treadmill. This HIT-treadmill protocol was designed to maximize motor activation with short 30-s bursts of maximal speed walking, while mitigating fatigue with alternating 30- to 60-s recovery periods (7). Potential proxies of fatigue (higher lactate, HR, and RPE measures) were associated with a decreased corticospinal excitability response (Table 3). In our exploratory mediation analysis, adjusting for the indirect negative effect of mean lactate on corticospinal excitability revealed an even greater direct positive effect of HIT versus MCT. Thus the HIT-treadmill protocol seems to have successfully shifted the balance of positive and negative influences on corticospinal excitability by achieving greater central motor activation.
Conversely, a recent poststroke study using a different treadmill HIT protocol (2.2-min bursts and recovery until reaching warm-up HR) found a nonsignificant trend toward decreased corticospinal excitability of the paretic tibialis anterior (28). The HIT protocol used in the current study involved much shorter bursts (30 s), which typically enables faster walking speeds and could contribute to increased corticospinal excitability (22). The current HIT protocol also involved higher aerobic intensity [max HR of 89% age-predicted maximum versus ~68% (stimated from HR figure and mean age)] and more trials of locomotor practice (12–18 bursts versus 4–6 bursts) (28). Previous studies have shown that aerobic priming before the induction of motor learning-like plasticity enhances the corticospinal excitability response (30, 47). By involving both vigorous aerobic intensity and intensive motor practice, the current HIT-treadmill protocol could be increasing corticospinal excitability by driving both aerobic priming and motor learning simultaneously.
To our knowledge, this is the first study to report an association between acute exercise-induced increases in circulating BDNF and a measure of central nervous system physiology (decreased intracortical inhibition). Given that motor learning has also been associated with decreased intracortical inhibition (17), this finding provides some support for acute circulating BDNF changes as a potential marker of central neuroplasticity processes. In exploratory analyses, greater exercise intensity (mean V̇o2) was found to directly increase the intracortical inhibition response (CSP area) but to indirectly decrease it even more through upregulation of circulating BDNF. These results are consistent with the role of BDNF in enhancing synaptic GABA clearance (16) and with the hypothesis that exercise-induced learning enhancement (i.e., the aerobic priming effect) may be mediated through upregulation of BDNF (29).
Limitations and Future Study
The primary limitation to this study is that we did not assess learning effects. Learning has been previously correlated with acute increases in circulating BNDF and corticospinal excitability of similar or lesser magnitude than we found for HIT-TM) (9, 17, 48, 63). However, these relationships are difficult to test for causality in humans. Thus interpretation of these acute biomarker changes should be done cautiously. Furthermore, it was recently reported that aerobic priming may only facilitate certain types of learning after stroke (11). Thus future studies are needed to better understand mechanistic interactions between aerobic priming biomarkers and learning.
Another limitation is that there were some variations from the initially expected exercise intensities. Most notably, the original “all-out” HIT-stepper protocol did not appear to be safe enough for continued use, and the protocol changes we implemented to decrease intensity also seemed to eliminate the acute BDNF response. Future study should consider testing the safety and physiologic effects of a poststroke HIT-stepper protocol with intensity between our original and revised protocols to see if it is feasible to safely elicit acute BDNF responses with this exercise mode. The need for prolonged postexercise stillness during blood draws and TMS testing may have also contributed to the hypotensive responses and could be decreased. However, it is important to note that there were no serious AEs associated with HIT-treadmill and only two AEs related to this protocol, both of which were anticipated and mild in severity. This AE profile was similar to both MCT-treadmill and the treadmill GXT, both of which are widely accepted and recommended in stroke rehabilitation guidelines (4, 62).
The intensive data collection likely also contributed to the HIT-treadmill aerobic intensity being slightly lower than previously reported with a similar protocol (7). Conversely, peak intensity was slightly higher than expected for MCT-treadmill, because intensity was increased above the target toward the end of the MCT session if needed to achieve a mean HR of 45% HRR. Interestingly, despite the relatively small differences in mean aerobic intensity between HIT-treadmill and MCT-treadmill, there was still a significant difference in the acute BDNF response, thus providing additional evidence of an intensity threshold. Some reported intensity differences between protocols were also slightly decreased by the handling of within-participant correlations in the statistical model. For example, no participants exceeded 85% HRRpeak during MCT-treadmill despite the modeled mean of 0.4 min spent in this zone (Table 3).
Another possible concern is that protocols were not matched for total work performed to control for potential confounding effects. However, this type of protocol matching would have created between-protocol differences in exercise duration, which could also be confounding, especially when collecting data at specific time points during and after exercise. In clinical stroke rehabilitation, exercise duration is often limited by therapy reimbursement and other factors (6). Therefore, we chose to control for this factor to increase clinical relevance and allow consistent timing of data collection across protocols.
Finally, the lack of sustained postexercise biomarker changes (Fig. 2) should be interpreted with caution. Animal studies indicate that exercise-induced BDNF upregulation initiates a molecular cascade in the brain that outlasts the acute increase in circulating BDNF (3, 40). It is also important to note that the timing of postexercise TMS measures was not exact. It took 10–15 min to initiate the T20 TMS assessment due to the need for a cool down, the transfer from the treadmill to the TMS chair and setup. Furthermore, each TMS assessment took ~10 min to complete. Thus the differences in active motor threshold change observed at T20 were actually ~15–20 min postexercise.
In conclusion, this study tested the effects of exercise intensity on several aerobic priming biomarkers among stroke survivors. Vigorous aerobic intensity sufficient to generate lactate accumulation appears to be required for eliciting an acute increase in circulating BDNF. This biomarker response has been previously correlated with enhanced learning. We report for the first time that acute increases in circulating BDNF can be obtained among persons with stroke using a 20-min treadmill HIT protocol involving 30-s bursts of maximum speed walking alternated with 30- to 60-s recovery periods. This treadmill HIT protocol additionally appears to increase corticospinal excitability of the paretic lower limb significantly more than moderate intensity continuous exercise, which could also facilitate motor learning. Furthermore, higher metabolic exercise intensity and acute circulating BDNF increases seem to be associated with decreased intracortical inhibition, another motor learning correlate. A similar HIT protocol on a seated stepper enabled stroke survivors to achieve even higher intensities but needs further testing to balance safety and benefit.
GRANTS
This work was supported by the American Heart Association Grant 17MCPRP33670446, National Center for Advancing Translational Sciences Grants UL1-TR-000077 and KL2-TR-001426), Foundation for Physical Therapy (Promotion of Doctoral Studies Scholarship), and University of Cincinnati Neuroscience Institute (Pilot Research Award).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.B., K.H., D.S.R., D. Cunningham, D. Carl, J.C.K., M.G., B.K., and K.D. conceived and designed research; P.B., C.M., J.W., D.W., and K.H. performed experiments; P.B., D. Cunningham, C.J., and J.C.K. analyzed data; P.B., D.S.R., D. Cunningham, D. Carl, J.C.K., M.G., and K.D. interpreted results of experiments; P.B. prepared figures; P.B. drafted manuscript; P.B., C.M., J.W., D.W., K.H., D.S.R., D. Cunningham, D. Carl, C.J., J.C.K., M.G., B.K., and K.D. edited and revised manuscript; P.B., C.M., J.W., D.W., K.H., D.S.R., D. Cunningham, D. Carl, C.J., J.C.K., M.G., B.K., and K.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the staff of the Clinical Translational Research Center, Schubert Research Clinic and Biochemistry Core Laboratory at Cincinnati Children’s Hospital Medical Center for their assistance with blood collection, processing, and analysis. We also thank the staff of the University of Cincinnati Medical Center Cardiovascular Stress Laboratory for assistance with participant screening.
REFERENCES
- 1.American College of Sports Medicine ACSM’s Guidelines for Exercise Testing and Prescription. Philadephia, PA: Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 2.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 192: 540–542, 1964. [PubMed] [Google Scholar]
- 3.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133: 853–861, 2005. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, Shaughnessy M, Tang A; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology . Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 2532–2553, 2014. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 5.Billinger SA, Boyne P, Coughenour E, Dunning K, Mattlage A. Does aerobic exercise and the FITT principle fit into stroke recovery? Curr Neurol Neurosci Rep 15: 519, 2015. doi: 10.1007/s11910-014-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyne P, Billinger S, MacKay-Lyons M, Barney B, Khoury J, Dunning K. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of US physical therapists. J Neurol Phys Ther 41: 119–128, 2017. doi: 10.1097/NPT.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Kissela B. Within-session responses to high-intensity interval training in chronic stroke. Med Sci Sports Exerc 47: 476–484, 2015. doi: 10.1249/MSS.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 8.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Rockwell B, Keeton G, Westover J, Williams A, McCarthy M, Kissela B. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Phys Ther 96: 1533–1544, 2016. doi: 10.2522/ptj.20150277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97: 3661–3665, 2000. doi: 10.1073/pnas.97.7.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carl DL, Boyne P, Rockwell B, Gerson M, Khoury J, Kissela B, Dunning K. Preliminary safety analysis of high-intensity interval training (HIIT) in persons with chronic stroke. Appl Physiol Nutr Metab 42: 311–318, 2017. doi: 10.1139/apnm-2016-0369. [DOI] [PubMed] [Google Scholar]
- 11.Charalambous CC, Alcantara CC, French MA, Li X, Matt KS, Kim HE, Morton SM, Reisman DS. A single exercise bout and locomotor learning after stroke: physiological, behavioural, and computational outcomes. J Physiol 596: 1999–2016, 2018. doi: 10.1113/JP275881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charalambous CC, Helm EE, Lau KA, Morton SM, Reisman DS. The feasibility of an acute high-intensity exercise bout to promote locomotor learning after stroke. Top Stroke Rehabil 25: 83–89, 2018. doi: 10.1080/10749357.2017.1399527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coco M, Alagona G, Rapisarda G, Costanzo E, Calogero RA, Perciavalle V, Perciavalle V. Elevated blood lactate is associated with increased motor cortex excitability. Somatosens Mot Res 27: 1–8, 2010. doi: 10.3109/08990220903471765. [DOI] [PubMed] [Google Scholar]
- 14.Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 7: 541–546, 2007. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30: 464–472, 2007. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Coxon JP, Cash RFH, Hendrikse JJ, Rogasch NC, Stavrinos E, Suo C, Yücel M. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J Physiol 596: 691–702, 2018. doi: 10.1113/JP274660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coxon JP, Peat NM, Byblow WD. Primary motor cortex disinhibition during motor skill learning. J Neurophysiol 112: 156–164, 2014. doi: 10.1152/jn.00893.2013. [DOI] [PubMed] [Google Scholar]
- 18.Crouter SE, Antczak A, Hudak JR, DellaValle DM, Haas JD. Accuracy and reliability of the ParvoMedics TrueOne 2400 and MedGraphics VO2000 metabolic systems. Eur J Appl Physiol 98: 139–151, 2006. doi: 10.1007/s00421-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 19.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 39: 728–734, 2007. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention . Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 128: 873–934, 2013. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Logan HL, Glueck DH, Muller KE. Selecting a sample size for studies with repeated measures. BMC Med Res Methodol 13: 100, 2013. doi: 10.1186/1471-2288-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornby TG, Straube DS, Kinnaird CR, Holleran CL, Echauz AJ, Rodriguez KS, Wagner EJ, Narducci EA. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil 18: 293–307, 2011. doi: 10.1310/tsr1804-293. [DOI] [PubMed] [Google Scholar]
- 23.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. Boca Raton, FL: CRC, 2015. [Google Scholar]
- 24.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity–exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med 40: 765–801, 2010. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Lamb SE. Advancing physical therapist interventions by investigating causal mechanisms. Phys Ther 97: 1119–1121, 2017. doi: 10.1093/ptj/pzx095. [DOI] [PubMed] [Google Scholar]
- 26.Macko RF, Ivey FM, Forrester LW. Task-oriented aerobic exercise in chronic hemiparetic stroke: training protocols and treatment effects. Top Stroke Rehabil 12: 45–57, 2005. doi: 10.1310/PJQN-KAN9-TTVY-HYQH. [DOI] [PubMed] [Google Scholar]
- 27.Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, Silver KH, Goldberg AP. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke 36: 2206–2211, 2005. doi: 10.1161/01.STR.0000181076.91805.89. [DOI] [PubMed] [Google Scholar]
- 28.Madhavan S, Stinear JW, Kanekar N. Effects of a single session of high intensity interval treadmill training on corticomotor excitability following stroke: implications for therapy. Neural Plast 2016: 1686414, 2016. doi: 10.1155/2016/1686414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther 93: 1707–1716, 2013. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol (1985) 117: 1325–1336, 2014. doi: 10.1152/japplphysiol.00498.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mang CS, Snow NJ, Wadden KP, Campbell KL, Boyd LA. High-intensity aerobic exercise enhances motor memory retrieval. Med Sci Sports Exerc 48: 2477–2486, 2016. doi: 10.1249/MSS.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 32.Mezzani A, Hamm LF, Jones AM, McBride PE, Moholdt T, Stone JA, Urhausen A, Williams MA; European Association for Cardiovascular Prevention and Rehabilitation; American Association of Cardiovascular and Pulmonary Rehabilitation; Canadian Association of Cardiac Rehabilitation . Aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European Association for Cardiovascular Prevention and Rehabilitation, the American Association of Cardiovascular and Pulmonary Rehabilitation, and the Canadian Association of Cardiac Rehabilitation. J Cardiopulm Rehabil Prev 32: 327–350, 2012. doi: 10.1097/HCR.0b013e3182757050. [DOI] [PubMed] [Google Scholar]
- 33.Mooney RA, Coxon JP, Cirillo J, Glenny H, Gant N, Byblow WD. Acute aerobic exercise modulates primary motor cortex inhibition. Exp Brain Res 234: 3669–3676, 2016. doi: 10.1007/s00221-016-4767-5. [DOI] [PubMed] [Google Scholar]
- 34.Mudge S, Stott NS. Test-retest reliability of the StepWatch Activity Monitor outputs in individuals with chronic stroke. Clin Rehabil 22: 871–877, 2008. doi: 10.1177/0269215508092822. [DOI] [PubMed] [Google Scholar]
- 35.Mulroy SJ, Winstein CJ, Kulig K, Beneck GJ, Fowler EG, DeMuth SK, Sullivan KJ, Brown DA, Lane CJ; Physical Therapy Clinical Research Network . Secondary mediation and regression analyses of the PTClinResNet database: determining causal relationships among the International Classification of Functioning, Disability and Health levels for four physical therapy intervention trials. Phys Ther 91: 1766–1779, 2011. doi: 10.2522/ptj.20110024. [DOI] [PubMed] [Google Scholar]
- 36.National Cancer Institute . National Cancer Institute Updates CTCAE to v.4.03. Washington Crossing, PA: Oncology.tv, 2009. [Google Scholar]
- 37.Nepveu JF, Thiel A, Tang A, Fung J, Lundbye-Jensen J, Boyd LA, Roig M. A single bout of high-intensity interval training improves motor skill retention in individuals with stroke. Neurorehabil Neural Repair 31: 726–735, 2017. doi: 10.1177/1545968317718269. [DOI] [PubMed] [Google Scholar]
- 38.Neva JL, Brown KE, Mang CS, Francisco BA, Boyd LA. An acute bout of exercise modulates both intracortical and interhemispheric excitability. Eur J Neurosci 45: 1343–1355, 2017. doi: 10.1111/ejn.13569. [DOI] [PubMed] [Google Scholar]
- 39.Qi F, Wu AD, Schweighofer N. Fast estimation of transcranial magnetic stimulation motor threshold. Brain Stimul 4: 50–57, 2011. doi: 10.1016/j.brs.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Quirié A, Hervieu M, Garnier P, Demougeot C, Mossiat C, Bertrand N, Martin A, Marie C, Prigent-Tessier A. Comparative effect of treadmill exercise on mature BDNF production in control versus stroke rats. PLoS One 7: e44218, 2012. doi: 10.1371/journal.pone.0044218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 94: 1062–1069, 2009. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 42.Roig M, Skriver K, Lundbye-Jensen J, Kiens B, Nielsen JB. A single bout of exercise improves motor memory. PLoS One 7: e44594, 2012. doi: 10.1371/journal.pone.0044594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruohonen J, Karhu J. Navigated transcranial magnetic stimulation. Neurophysiol Clin 40: 7–17, 2010. doi: 10.1016/j.neucli.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Schiffer T, Schulte S, Sperlich B, Achtzehn S, Fricke H, Strüder HK. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett 488: 234–237, 2011. doi: 10.1016/j.neulet.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 45.Sidhu SK, Hoffman BW, Cresswell AG, Carroll TJ. Corticospinal contributions to lower limb muscle activity during cycling in humans. J Neurophysiol 107: 306–314, 2012. doi: 10.1152/jn.00212.2011. [DOI] [PubMed] [Google Scholar]
- 46.Sidhu SK, Lauber B, Cresswell AG, Carroll TJ. Sustained cycling exercise increases intracortical inhibition. Med Sci Sports Exerc 45: 654–662, 2013. doi: 10.1249/MSS.0b013e31827b119c. [DOI] [PubMed] [Google Scholar]
- 47.Singh AM, Neva JL, Staines WR. Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Exp Brain Res 232: 3675–3685, 2014. doi: 10.1007/s00221-014-4049-z. [DOI] [PubMed] [Google Scholar]
- 48.Skriver K, Roig M, Lundbye-Jensen J, Pingel J, Helge JW, Kiens B, Nielsen JB. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem 116: 46–58, 2014. doi: 10.1016/j.nlm.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Smith AE, Goldsworthy MR, Garside T, Wood FM, Ridding MC. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp Brain Res 232: 1875–1882, 2014. doi: 10.1007/s00221-014-3879-z. [DOI] [PubMed] [Google Scholar]
- 50.Snow NJ, Mang CS, Roig M, McDonnell MN, Campbell KL, Boyd LA. The effect of an acute bout of moderate-intensity aerobic exercise on motor learning of a continuous tracking task. PLoS One 11: e0150039, 2016. doi: 10.1371/journal.pone.0150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Statton MA, Encarnacion M, Celnik P, Bastian AJ. A single bout of moderate aerobic exercise improves motor skill acquisition. PLoS One 10: e0141393, 2015. doi: 10.1371/journal.pone.0141393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stavrinos EL, Coxon JP. High-intensity interval exercise promotes motor cortex disinhibition and early motor skill consolidation. J Cogn Neurosci 29: 593–604, 2017. doi: 10.1162/jocn_a_01078. [DOI] [PubMed] [Google Scholar]
- 53.Stotler BA, Kratz A. Analytical and clinical performance of the epoc blood analysis system: experience at a large tertiary academic medical center. Am J Clin Pathol 140: 715–720, 2013. doi: 10.1309/AJCP7QB3QQIBZPEK. [DOI] [PubMed] [Google Scholar]
- 54.Stoykov ME, Corcos DM, Madhavan S. Movement-based priming: clinical applications and neural mechanisms. J Mot Behav 49: 88–97, 2017. doi: 10.1080/00222895.2016.1250716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tazoe T, Perez MA. Selective activation of ipsilateral motor pathways in intact humans. J Neurosci 34: 13924–13934, 2014. doi: 10.1523/JNEUROSCI.1648-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas R, Flindtgaard M, Skriver K, Geertsen SS, Christiansen L, Korsgaard Johnsen L, Busk DV, Bojsen-Møller E, Madsen MJ, Ritz C, Roig M, Lundbye-Jensen J. Acute exercise and motor memory consolidation: does exercise type play a role? Scand J Med Sci Sports 27: 1523–1532, 2017. doi: 10.1111/sms.12791. [DOI] [PubMed] [Google Scholar]
- 57.Thomas R, Johnsen LK, Geertsen SS, Christiansen L, Ritz C, Roig M, Lundbye-Jensen J. Acute exercise and motor memory consolidation: the role of exercise intensity. PLoS One 11: e0159589, 2016. doi: 10.1371/journal.pone.0159589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.VandenBerg PM, Bruneau RM. BDNF is required for maintaining motor map integrity in adult cerebral cortex. Soc Neurosci Abst 681: 5, 2004. [Google Scholar]
- 59.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Intensity-dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. Am J Physiol Regul Integr Comp Physiol 308: R998–R1007, 2015. doi: 10.1152/ajpregu.00021.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 19: 231–240, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Wheaton LA, Villagra F, Hanley DF, Macko RF, Forrester LW. Reliability of TMS motor evoked potentials in quadriceps of subjects with chronic hemiparesis after stroke. J Neurol Sci 276: 115–117, 2009. doi: 10.1016/j.jns.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, Lang CE, MacKay-Lyons M, Ottenbacher KJ, Pugh S, Reeves MJ, Richards LG, Stiers W, Zorowitz RD; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Quality of Care and Outcomes Research . Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 47: e98, 2016. [Erratum in Stroke 48: e78, 2017] doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 63.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem 87: 597–609, 2007. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi T, Fujiwara T, Liu W, Liu M. Effects of pedaling exercise on the intracortical inhibition of cortical leg area. Exp Brain Res 218: 401–406, 2012. doi: 10.1007/s00221-012-3026-7. [DOI] [PubMed] [Google Scholar]