Abstract
Menstrual cycle phase has long been thought to modulate thermoregulatory function. However, information pertaining to the effects of menstrual phase on time-dependent changes in whole body dry and evaporative heat exchange during exercise-induced heat stress and the specific heat load at which menstrual phase modulates whole body heat loss remained unavailable. We therefore used direct calorimetry to continuously assess whole body dry and evaporative exchange in 12 habitually active, non-endurance-trained, eumenorrheic women [21 ± 3 (SD) yr] within the early-follicular, late-follicular, and midluteal menstrual phases during three 30-min bouts of cycling at increasing fixed exercise intensities of 40% (Low), 55% (Moderate), and 70% (High) peak oxygen uptake, each followed by a 15-min recovery, in hot, dry conditions (40°C, 15% relative humidity). This model elicited equivalent rates of metabolic heat production among menstrual phases (P = 0.80) of ~250 (Low), ~340 (Moderate), and ~430 W (High). However, dry and evaporative heat exchange and the resulting changes in net heat loss (dry ± evaporative heat exchange) were similar among phases (all P > 0.05), with net heat loss averaging 216 ± 43 (Low), 287 ± 63 (Moderate), and 331 ± 75 W (High) across phases. Accordingly, cumulative body heat storage (summation of heat production and loss) across all exercise bouts was similar among phases (P = 0.55), averaging 464 ± 122 kJ. For some time, menstrual cycle phase has been thought to modulate heat dissipation; however, we show that menstrual cycle phase does not influence the contribution of whole body dry and evaporative heat exchange or the resulting changes in net heat loss or body heat storage, irrespective of the heat load.
NEW & NOTEWORTHY Menstrual phase has long been thought to modulate thermoregulatory function in eumenorrheic women during exercise-induced heat stress. Contrary to that perception, we show that when assessed in young, non-endurance-trained women within the early-follicular, late-follicular, and midluteal phases during three incremental exercise-induced heat loads in hot, dry conditions, menstrual phase does not modify whole body dry and evaporative heat exchange or the resulting changes in body heat storage, regardless of the heat load employed.
Keywords: calorimetry, exercise, menstrual cycle, sex hormones, sweating, thermoregulation
INTRODUCTION
In eumenorrheic women, increases in circulating estrogen and progesterone following ovulation (midluteal phase) are associated with elevations in basal core temperature (mediated via progesterone) and the threshold core temperature for the activation of heat loss responses of skin blood flow and sweating during resting or exercise-induced heat stress compared with the early- (menses) or late-follicular (preovulation) phases (2, 26, 38, 39, 43). During resting heat exposure, it is generally well established that this postovulatory rise in basal core temperature does not modify the absolute increase (i.e., magnitude of response) or temperature-dependent profile (i.e., thermosensitivity) of these heat loss responses relative to the follicular phase (5, 13, 15, 20, 43). However, during exercise-induced heat stress, several investigators have observed reductions in the magnitude of skin blood flow and local sweat rate, a reduction in the thermosensitivity of the local sweating response, and/or a greater rise in core temperature relative to the midluteal phase (11, 12, 14, 42). Although others have shown that those local heat loss responses are unchanged across the menstrual cycle (1, 6, 26, 38, 39, 41, 44) or even lowered in the midluteal relative to the follicular phase in such conditions (7, 22), these inconsistencies may be owing to multiple factors including differences in the exercise intensity and environmental conditions employed and the endurance-trained status of the population studied, the latter of which has been shown in some instances to lower reproductive hormone fluctuations (22, 23).
Most previous studies on the effects of menstrual cycle phase on thermoregulatory function during exercise-induced heat stress have been directed to evaluating the control of skin blood flow and sweating (1, 6, 11, 12, 14, 21, 22, 38, 44) and/or exercise tolerance (26, 41, 42). To achieve these objectives, skin blood flow was often assessed at the forearm (i.e., laser Doppler or venous occlusion plethysmography), and sweating was measured at single body regions (e.g., ventilated capsule at the forearm or chest) and/or from body mass changes before and after exercise (1, 6, 11, 12, 14, 22, 38, 39, 41, 43, 44), sometimes while participants wore impermeable protective clothing preventing sweat evaporation (38, 42). Although those studies have markedly improved our understanding of the effects of menstrual phase on thermoregulatory function in such conditions, we possess only limited information on the integrated, global effects of any menstrual phase-related differences in skin blood flow and sweating on time-dependent changes in whole body dry and evaporative heat exchange and whether those effects modify the relative contribution of those heat exchanges. Indeed, given that differences in skin blood flow can affect heat transfer between the core and periphery and, when combined with altered sweating, changes in whole body dry and evaporative exchange (16), this represents an important gap in our understanding. Furthermore, since most of those groups focused their experimental paradigm on a single exercise bout (1, 6, 11, 12, 14, 22), information on the exercise-induced heat load (an important determinant of other factors modifying heat exchange including aging, sex, fitness, acclimation state, and others; 10, 24, 28, 32, 37) at which menstrual phase begins to modulate whole body heat exchange is unavailable. Therefore, despite several studies, it remains unclear whether the integrated effects of any menstrual phase-related differences in skin blood flow and sweating cause subsequent changes in whole body dry or evaporative heat exchange during exercise in the heat or whether those effects are heat load dependent.
Given the importance of this information for improving our understanding of factors that influence heat exchange in women and for designing experiments directed at assessing thermoregulatory function in women, we sought to evaluate whether menstrual cycle phase modulates the relative time-dependent contribution of dry and evaporative heat exchange and therefore net whole body heat loss and to identify the exercise-induced heat load and menstrual phase at which those differences occur. To achieve this objective, all participants were tested within the three menstrual phases that evoke physiologically relevant differences in circulating reproductive hormone concentration (3). These included 1) the early-follicular phase, when estrogen and progesterone concentrations are lowest, 2) the late-follicular phase, when there is a peak in estrogen only, and 3) the midluteal phase, when both estrogen and progesterone peak. Moreover, we used direct calorimetry to precisely quantify time-dependent changes in the contribution of dry and evaporative heat exchange to whole body net heat loss and body heat storage (19) in non-endurance-trained, eumenorrheic women, during intermittent exercise eliciting increasing, equivalent rates of metabolic heat production among menstrual cycle phases of ~250 (Low), ~340 (Moderate), and ~430 W (High) in a hot, dry environment. On the basis of prior evidence of reduced local heat loss responses among women in the follicular relative to the midluteal phase (11, 12, 14, 42), we hypothesized that whole body net heat loss would be lowered in both the early- and late-follicular phases relative to the midluteal phase, with the magnitude of that difference being greatest at the highest heat load of ~430 W (High). By using direct calorimetry, as opposed to evaluating skin blood flow and sweating responses at select regions around the body, we were able to quantify the integrated, global effects of any menstrual phase-related differences in those responses on whole body dry and evaporative heat exchange. With this unique approach, we could determine the specific heat load and menstrual phase at which the menstrual cycle modulates whole body net heat loss, while providing mechanistic insights into menstrual phase-related differences in the relative contribution of dry and evaporative heat exchange.
METHODS
Ethical approval.
The experimental protocol was approved by the University of Ottawa Health Sciences and Science Research Ethics Board in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before their participation.
Participants.
Twelve habitually active, but not endurance-trained, young eumenorrheic women participated. All participants were healthy nonsmokers and did not report a history of cardiovascular, metabolic, and/or respiratory disorders. Participants were not taking over-the-counter or prescription medications, including oral contraception. The mean ± SD physical characteristics of the participants were as follows: age, 21 ± 3 yr; height, 164 ± 5 cm; body mass, 59.0 ± 8.3 kg; body surface area, 1.63 ± 0.12 m2; body fat, 21.9 ± 5.3%; and peak oxygen uptake (V̇o2peak), 37.4 ± 3.7 ml·kg−1·min−1.
Experimental design.
Participants completed one preliminary session and three experimental trials. During the preliminary session, measurements of body height (model 2391; Detecto, Webb City, MO) and mass were obtained and used to approximate body surface area (4). Body density was estimated using the hydrostatic weighing technique and used to approximate body fat percentage (36). A maximal incremental semirecumbent cycling protocol was used to determine V̇o2peak (Corival; Lode, Groningen, The Netherlands). Cycling started at a work rate between 40 and 60 W. Then resistance increased by ~20 W each minute until volitional fatigue or participants failed to maintain a cadence of a minimum of 60 rpm.
The experimental sessions were performed at the same time of day during 1) the early-follicular phase (~4 days following menses), 2) the late-follicular phase (after the end of menstruation and before the start of ovulation, ~10 days following menses), and 3) the midluteal phase (~22 days following menses). Although most previous studies on this topic have evaluated thermoregulatory function only between the early-follicular and midluteal phases (1, 2, 5–7, 12, 14, 15, 20–23, 26, 41, 42), there is evidence that rises in circulating 17β-estradiol within the late-follicular phase can modulate thermoregulatory function (primarily due to increases in cutaneous vasodilatation; 3, 30). We therefore chose to evaluate women within the early-follicular, late-follicular, and midluteal phases to provide a thorough evaluation of whole body heat exchange across the menstrual cycle. To ensure that this important experimental design criterion was satisfied, two methods were used. First, participants were required to report the start and end dates of menstrual bleeding 3 mo preceding the experimental sessions to confirm normal and consistent menstrual cycles as well as to schedule experimental sessions. Second, to verify that hormone concentrations were reflective of menstrual cycle phase before experimentation, a venous blood sample was collected into SST Vacutainer tubes (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ) upon arrival to the laboratory following a 30-min seated rest period in a non-heat stress condition. Blood samples were analyzed for concentrations of plasma progesterone and 17β-estradiol using automated chemiluminescent microparticle immunoassays (ARCHITECT system; Abbott Diagnostics, Abbott Park, IL) by an independent external laboratory (Gamma-Dynacare Medical Laboratories, Ottawa, ON, Canada) using appropriate monoclonal antibody-coated microparticles and acridinium-labeled conjugates. All three experimental trials were conducted within the same monthly cycle. The only exceptions were two women who repeated one of their experimental sessions in the following monthly cycle, as plasma concentrations did not correspond to the range of values representative of that menstrual phase (Table 1; 40). Although this meant that repeated trials were conducted 1 mo following the others within this cycle, this approach ensured that physiologically relevant differences in reproductive hormone concentration could be evaluated.
Table 1.
Progesterone and estradiol reference ranges and measured values within the early-follicular, late-follicular, and midluteal phases of the menstrual cycle
| Progesterone, nmol/l |
Estradiol, pmol/l |
|||
|---|---|---|---|---|
| Reference | Measured | Reference | Measured | |
| Early-follicular | 0.6–4.7 | 1.9 ± 0.6* | 60–854 | 98 ± 20 |
| Late-follicular | 0.6–4.7 | 1.7 ± 0.8* | 60–854 | 273 ± 24† |
| Midluteal | 5.3–86.0 | 21.2 ± 3.2 | 82–1,251 | 354 ± 42† |
Reference values are ranges (40), and measured values are means ± SD (n = 12); n = no. of participants. Significance level was set at P < 0.05.
Significantly different from the midluteal phase;
significantly different from the early-follicular phase.
Participants were asked to consume a light meal 2 h before their arrival and refrain from alcohol, caffeine, and antipyretics for 12 h before the session. Furthermore, participants were asked to abstain from performing strenuous physical activity for 24 h before each session. To ensure that all participants arrived at the laboratory in a euhydrated state, they were asked to drink ~500 ml of water the night before, as well as the morning of, each experimental trial. Upon arrival to the laboratory, participants changed into athletic shorts, sports bra, socks, and running shoes and were instrumented (~30 min) in an upright seated posture at an ambient room temperature of ~24°C. Participants were then moved to the whole body direct air calorimeter regulated to an ambient temperature of 40°C and relative humidity of 15%, where they rested in a semirecumbent position for 15 min (baseline). Participants then performed three successive bouts of cycling at progressively greater, fixed intensities equal to 40% (Low), 55% (Moderate), and 70% (High) of each individual’s predetermined V̇o2peak, each followed by 15-min resting recovery. This exercise model elicited three incremental metabolic heat production rates of ~250 (Low), ~340 (Moderate), and ~430 W (High), which were matched across phases, making it possible to determine the heat load at which menstrual phase modulates whole body heat loss.
Measurements.
The modified Snellen direct air calorimeter was used to directly measure rates of evaporative heat loss and dry heat exchange (19). Absolute humidity was measured using high-precision dew point hygrometry (model 373-H; RH Systems, Albuquerque, NM), whereas air temperature was measured using high-precision temperature sensors (±0.002°C, Black Stack model 1560; Hart Scientific, American Fork, UT). A known heat source placed in the effluent airstream was used to determine air mass flow through the calorimeter. Real-time data for absolute humidity, air temperature, and air mass flow were displayed and recorded on a personal computer with LabVIEW software (version 7.0; National Instruments, Austin, TX). The rate of evaporative heat loss was calculated using the calorimeter outflow-inflow difference in absolute humidity, multiplied by the air mass flow (kg/s) and the latent heat of vaporization of sweat (2,426 J/g). Rate of dry heat exchange was calculated using the calorimeter outflow-inflow difference in air temperature, multiplied by the air mass flow and specific heat capacity of air (1,005 J·kg air−1·°C−1). Dry and evaporative heat losses were expressed as positive values, with a negative value for dry heat loss representing environmental heat gain (i.e., when ambient temperature exceeds skin temperature). It is important to note that these heat exchanges represent the integrated effects of any menstrual phase-related differences in skin blood flow and sweating and thus were assessed with the aim of providing mechanistic information on global changes in those heat exchanges across the menstrual cycle.
Oxygen consumption, carbon dioxide production, and minute ventilation were derived continuously from measures of expired gases and airflows (MOXUS modular metabolic system; AEI Technologies, Bastrop, TX) and used to approximate metabolic energy expenditure (19). Expired air was recycled back into the calorimeter chamber to account for respiratory dry and evaporative heat loss. Metabolic heat production during exercise was subsequently calculated as metabolic energy expenditure minus external work.
Esophageal temperature was measured by inserting a general-purpose thermocouple temperature probe (Mon-a-therm General Purpose Temperature Probe; Mallinckrodt Medical, St. Louis, MO) 40 cm past the entrance of the nostril while the participant sipped water through a straw. The esophageal probe could not be inserted in three of the participants; therefore, core temperature was measured at the rectum by inserting the same general-purpose thermocouple 12 cm beyond the anal sphincter. Skin temperature was measured on the chest, bicep, thigh, and calf using T-type thermocouples (Concept Engineering, Old Saybrook, CT) and used to calculate mean skin temperature (chest, 30%; bicep, 30%; thigh, 20%; and calf, 20%; 34). All temperature data were continuously measured (model 2497-A; HP Agilent) and simultaneously displayed and recorded in a spreadsheet format on a personal computer with LabVIEW software (version 7.0; National Instruments).
Heart rate was also continuously measured using a Polar coded WearLink and transmitter (Polar M400 watch, Polar H7 WearLink; Polar Electro Oy, Kempele, Finland). Because of technical difficulties in one trial, a reduced sample size was used for statistical analysis of heart rate (n = 11). A urine sample was collected before the experimental sessions for the measurement of urine specific gravity (Reichert TS 400 total solids refractometer; Reichert, Depew, NY).
Data analysis.
Minute averages were calculated for all continuously measured variables. Statistical analyses were performed using an average of the last 5 min of baseline and each exercise period. The change in body heat storage (i.e., amount of heat stored in the body) was calculated as the temporal summation of metabolic heat production and net heat loss (dry heat loss + evaporative heat loss) for each exercise bout. The cumulative change in body heat storage across all exercise bouts was calculated by summing the change in the amount of heat stored in the body measured within each exercise bout. Change in core temperature was determined as the difference from preexercise rest. The thermosensitivity of the whole body evaporative heat loss response was determined as the slope of the relationship between mean body temperature (coefficient weighting: 0.9 esophageal temperature and 0.1 mean skin temperature) and evaporative heat loss for each exercise period (n = 9). However, since sweating commenced at the start of exercise in all participants (i.e., likely because of nonthermal effects of central command, muscle metaboreceptor, and/or mechanoreceptor activation; 16), the body temperature at the onset of the evaporative heat loss response was not determined.
Statistical analysis.
Rates of metabolic heat production and dry, evaporative, and net heat loss, as well as body heat storage, core and skin temperatures, and heart rate were analyzed using a two-way repeated-measures analysis of variance with the repeated factors of exercise bout (Low, Moderate, or High) and menstrual phase (early-follicular, late-follicular, or midluteal phases). A one-way repeated-measures analysis of variance was performed to determine menstrual phase-related differences in data collected at single time points (estrogen and progesterone concentrations, pretrial urine specific gravity, baseline data, and the cumulative change in body heat storage). When a significant interaction or main effect was observed, post hoc comparisons were conducted using the Bonferroni procedure. The level of significance (α) was set at P ≤ 0.05. An a priori power analysis was performed using previously reported between-phase differences in local sweat rate (12), since data for the primary variable of interest (whole body net heat loss) were unavailable. On the basis of the effect size (Cohen’s d = 1.38) associated with those differences in local sweating, a minimum of nine subjects would be required to detect differences in whole body net heat loss among phases of this effect size with at least 80% statistical power after correcting for multiple comparisons (i.e., α/3). Therefore, with the present sample (n = 12), these analyses were adequately powered (>80%). All statistical tests were performed using SPSS Statistics 24 for Windows (IBM, Armonk, NY). Data are presented as means ± SD.
RESULTS
Measured levels of 17β-estradiol were lower during the early-follicular compared with the late-follicular (P = 0.01) and midluteal (P < 0.01) phases, whereas measured progesterone levels in the midluteal phase were higher than in both the early-follicular (P = 0.03) and late-follicular (P = 0.03) phases (Table 1). Pretrial urine specific gravity was similar among menstrual phases (P = 0.86), averaging 1.008 ± 0.006 across phases. These outcomes confirmed that all participants were assessed during the early-follicular, late-follicular, and midluteal phases and were similarly hydrated before experimentation.
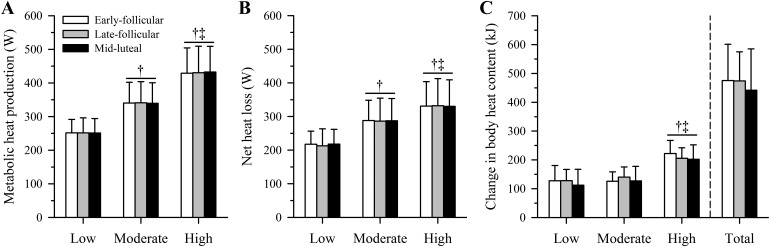
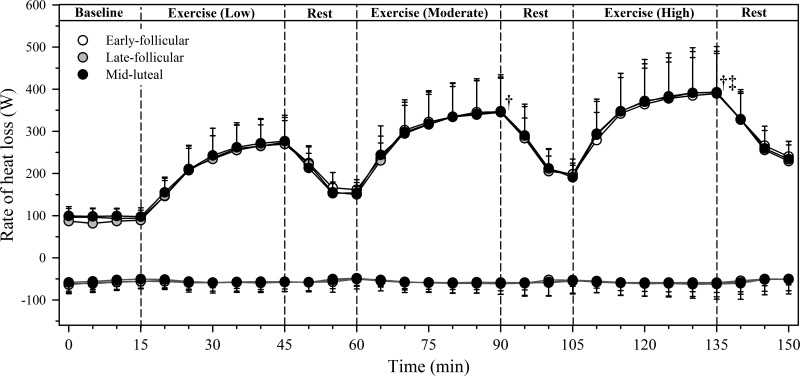
Baseline metabolic heat production and dry heat gain as well as evaporative and net heat loss did not differ significantly among menstrual cycle phases (all P > 0.05), averaging 83 ± 10, −54 ± 16, 96 ± 19, and 61 ± 17 W, respectively, across phases. The exercise intensity and therefore rate of metabolic heat production increased over each exercise bout (main effect of time: P < 0.01) but did not differ across menstrual cycle phases (P = 0.80), averaging 252 ± 41 W (Low), 340 ± 60 W (Moderate), and 431 ± 75 W (High) across phases (Fig. 1A). Dry heat gain did not differ among exercise bouts (P = 0.45) and was also similar among phases (P = 0.87; Fig. 2). Evaporative heat loss (Fig. 2) and the subsequent change in net heat loss (Fig. 1B) increased across exercise bouts (both P < 0.01) but were both similar across menstrual phases (P = 0.88 and P = 0.94, respectively). The thermosensitivity of the evaporative heat loss response decreased across exercise bouts (P < 0.01) but did not differ among menstrual phases (P = 0.56; Table 2). However, whereas there was a menstrual phase-by-time interaction for the change in body heat storage during exercise (P = 0.05), no significant among-phase differences were noted within each exercise bout (all P > 0.05; Fig. 1C). As such, the cumulative change in body heat storage across the three exercise bouts (total) was similar among phases (P = 0.55; Fig. 1C).
Fig. 1.
Metabolic heat production (A) and net heat loss (dry heat gain + evaporative heat loss; B) as well as the change in body heat storage (C) in young, non-endurance-trained women (n = 12) within the early-follicular (open bars), late-follicular (gray bars), and midluteal (closed bars) phases of the menstrual cycle during three 30-min bouts of cycling at increasing fixed heat loads of ~250 (Low), ~340 (Moderate), and ~430 W (High) {equivalent to low-intensity [40% peak oxygen uptake (V̇o2peak)], moderate-intensity (55% V̇o2peak), and high-intensity (70% V̇o2peak) exercise, respectively} in hot, dry conditions (40°C, 15% relative humidity). Metabolic heat production and net heat loss (means ± SD) are averages obtained during the final 5 min of each exercise bout. Change in body heat storage (means ± SD) represents the temporal summation of metabolic heat production and net heat loss within each exercise bout and across all exercise bouts (total; bars to the right of the vertical dashed line). Significance level was set at P < 0.05. No significant among-phase differences were observed (P > 0.05). †Significantly different from Low. ‡Significantly different from Moderate.
Fig. 2.
Evaporative (black lines) and dry heat loss (dark gray lines) in young, non-endurance-trained women (n = 12) within the early-follicular (○), late-follicular (gray circles), and midluteal (●) phases of the menstrual cycle during three 30-min bouts of cycling at increasing fixed heat loads of ~250 (Low), ~340 (Moderate), and ~430 W (High) {equivalent to low-intensity [40% peak oxygen uptake (V̇o2peak)], moderate-intensity (55% V̇o2peak), and high-intensity (70% V̇o2peak) exercise, respectively} in hot, dry conditions (40°C, 15% relative humidity). Data are 5-min averages (means ± SD), with data from the final 5 min of each exercise bout being used for statistical analysis. Negative values for dry heat loss represent environmental heat gain. Significance level was set at P < 0.05. No significant among-phase differences were observed (P > 0.05). †Significantly different from Low. ‡Significantly different from Moderate.
Table 2.
Thermosensitivity of whole body evaporative heat loss response throughout the menstrual cycle during three 30-min bouts of cycling at increasing fixed metabolic heat production rates
| Exercise-Induced Heat Load |
|||
|---|---|---|---|
| Low | Moderate | High | |
| Whole body thermosensitivity, W·m−2·°C−1 | |||
| Early-follicular | 444 ± 193 | 326 ± 170 | 207 ± 77†‡ |
| Late-follicular | 419 ± 166 | 296 ± 161 | 189 ± 68†‡ |
| Midluteal | 413 ± 158 | 371 ± 220 | 197 ± 85†‡ |
Values are means ± SD. The thermosensitivity of the whole body evaporative heat loss response (n = 9) throughout the menstrual cycle during three 30-min bouts of cycling at increasing fixed metabolic heat production rates of ~250 (Low), ~340 (Moderate), and ~430 W (High) in hot, dry conditions (40°C, 15% relative humidity). Significance level was set at P < 0.05. No significant among-phase differences were observed (P > 0.05).
Significantly different from Low.
Significantly different from Moderate.
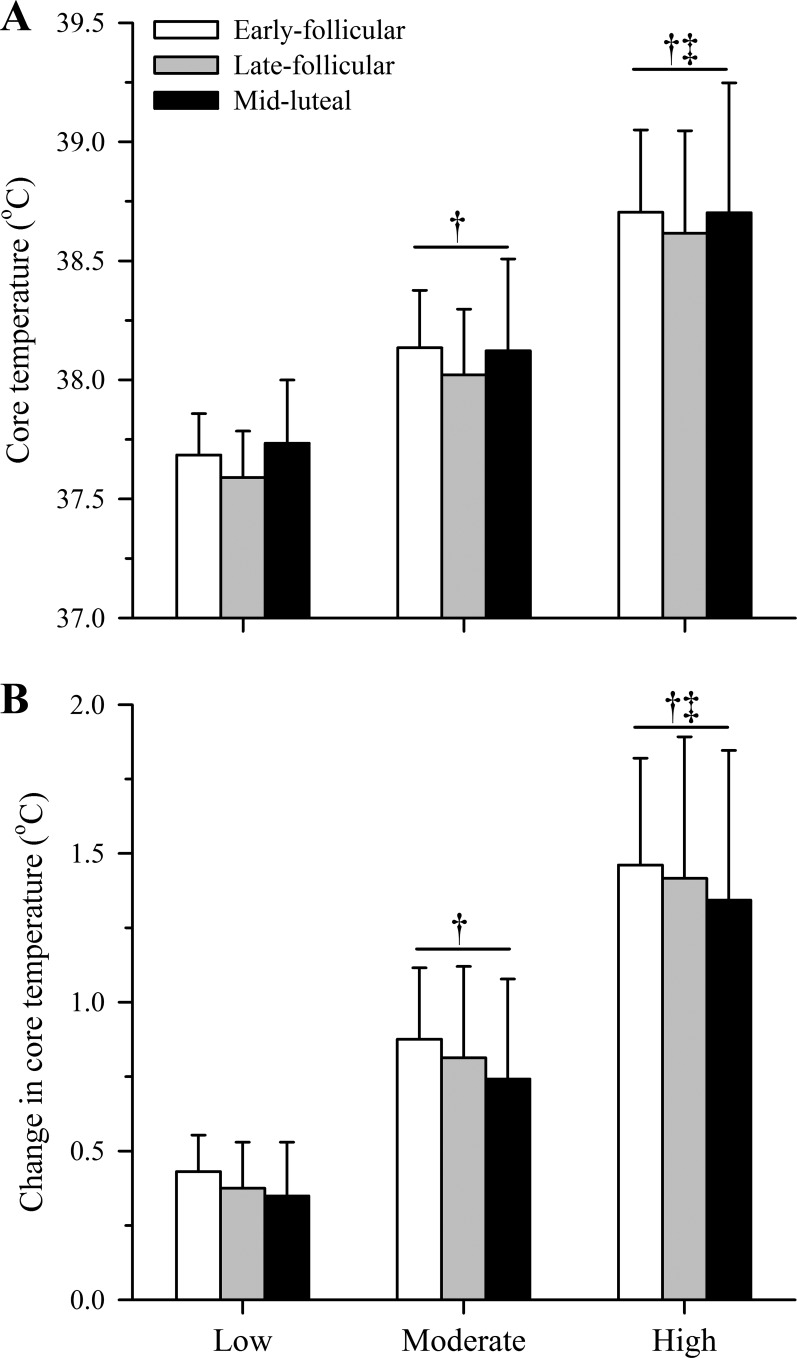
As anticipated, baseline core temperature in the midluteal phase (37.4 ± 0.2°C) was higher than both the early- (37.2 ± 0.1°C) and late-follicular (37.2 ± 0.2°C) phases (both P < 0.05). However, whereas core temperature (Fig. 3A) and the change in core temperature (Fig. 3B) increased over time (both P < 0.01), they did not differ significantly among phases (P = 0.42 and P = 0.31, respectively). Mean skin temperature increased across exercise bouts (P < 0.01) but was similar among phases at baseline (P = 0.27) and during exercise (P = 0.57), averaging 36.0 ± 0.3°C (baseline), 36.2 ± 0.3°C (Low), 36.4 ± 0.4°C (Moderate), and 36.8 ± 0.5°C (High) across phases. Heart rate also increased across exercise bouts (P < 0.01) but was comparable among phases at baseline (P = 0.42) and during exercise (P = 0.62), averaging 87 ± 16 beats/min (baseline), 126 ± 14 beats/min (Low), 151 ± 15 beats/min (Moderate), and 173 ± 14 beats/min (High) across phases.
Fig. 3.
Core temperature (A) and core temperature change (B) in young, non-endurance-trained women (n = 12) within the early-follicular (open bars), late-follicular (gray bars), and midluteal (closed bars) phases of the menstrual cycle during three 30-min bouts of cycling at increasing fixed heat loads of ~250 (Low), ~340 (Moderate), and ~430 W (High) {equivalent to low-intensity [40% peak oxygen uptake (V̇o2peak)], moderate-intensity (55% V̇o2peak), and high-intensity (70% V̇o2peak) exercise, respectively} in hot, dry conditions (40°C, 15% relative humidity). Data (means ± SD) are averages obtained during the final 5 min of each exercise bout. Significance level was set at P < 0.05. No significant among-phase differences were observed (P > 0.05). †Significantly different from Low. ‡Significantly different from Moderate.
DISCUSSION
In the present study, time-dependent changes in whole body heat loss and body heat storage were assessed in habitually active, but not endurance-trained, eumenorrheic women during exercise in the heat at increasing heat loads within the early-follicular, late-follicular, and midluteal menstrual cycle phases. With this novel design, it was possible to determine the specific heat load at which any menstrual phase-related differences in relative contribution of skin blood flow and sweating modulate whole body dry and evaporative heat exchange. However, whereas we observed greater baseline core temperature in the midluteal compared with both the early- and late-follicular phases of the menstrual cycle, changes in whole body dry and evaporative heat exchange and the thermosensitivity of the whole body evaporative heat loss response did not differ across menstrual phases, irrespective of the heat load employed. This was paralleled by similar changes in body heat storage and core temperature, indicating that menstrual phase does not appear to modulate the body’s physiological capacity to dissipate heat or modify the relative contribution of dry and evaporative heat exchange to a given net heat loss in non-endurance-trained women during exercise in hot, dry conditions. Most importantly, our findings provide the most definitive evidence to date that refutes the long-standing assumption that menstrual cycle phase modulates heat dissipation during exercise-induced heat stress.
Although thermoregulatory function during exercise in the heat has been studied extensively across the menstrual cycle (26, 38, 39, 41–43), the effect of menstrual phase on heat loss responses of skin blood flow and sweating in such conditions remained equivocal, and no study to our knowledge had been designed to evaluate whether such differences caused corresponding time-dependent changes in whole body dry and evaporative heat exchange or identify the heat load at which menstrual cycle begins to modulate whole body heat loss. We therefore used direct calorimetry to assess time-dependent changes in whole body dry and evaporative heat exchange in young, non-endurance-trained, eumenorrheic women using an incremental heat load model (i.e., consisting of low, moderate, and high exercise intensities and therefore exercise-induced heat loads) within the early-follicular, late-follicular, and midluteal phases. This approach provided an unfiltered overview of the impact of potential menstrual phase-related differences in skin blood flow and sweat production on the relative changes in dry and evaporative heat exchange, while also establishing the specific heat load and menstrual phase at which such differences may occur during exercise-induced heat stress.
It is well established that increases in circulating estrogen and progesterone following ovulation (midluteal phase) are associated with elevations in basal core temperature compared with the early- (menses) or late-follicular (preovulation) phases (38, 39, 43), and this was confirmed in the present study. These reductions in hormone concentration and basal core temperature during the early-follicular phase have also been shown to be paralleled by reduced local skin blood flow and sweating responses, a reduction in the thermosensitivity of the local sweating response, and/or greater rises in core temperature relative to the midluteal phase during exercise in temperate or hotter environments (11, 12, 14, 42). It was therefore anticipated that these impairments would compromise whole body heat loss and exacerbate body heat storage in the early- and late-follicular phases relative to the midluteal phase, particularly at the highest heat load employed (~430 W; High: 70% V̇o2peak). However, whole body heat exchange (Fig. 2) and the thermosensitivity (slope) of the relationship between mean body temperature and evaporative heat loss (Table 2) were similar across menstrual phases during all exercise bouts. This was coupled with similar changes in body heat storage (Fig. 1C) and core temperature (Fig. 3B) across menstrual phases throughout the incremental exercise protocol. Therefore, contrary to our working hypothesis, menstrual phase did not exert a modulating effect on the body’s physiological capacity to dissipate heat, irrespective of the heat load employed (and therefore level of exercise intensity). This is both novel and important as thermoregulation across the menstrual cycle during exercise-induced heat stress has not, to our knowledge, been viewed previously in terms of whole body heat exchange.
Whereas our findings are consistent with the similarities in local sweat rate across the menstrual cycle during exercise in the heat reported in several studies (6, 38, 39, 44), others have demonstrated that local sweat rate is either reduced (11, 12, 14) or increased (7, 22) in the follicular phase relative to the luteal phase in such conditions. In addition to differences in the exercise and environmental conditions employed among those studies, these contradictory observations may be explained, at least in part, by the methodology used to assess the sweating response. In contrast to the present study, where whole body direct calorimetry was used to quantify time-dependent changes in evaporative heat dissipation from sweat secreted by the ≥2 million sweat glands heterogeneously distributed across the body surface, previous investigators have typically assessed sweat rate using the ventilated capsule technique from a small area of the skin (≤7 cm2). It is possible, therefore, that the modifications to local sweating reported across the menstrual cycle in previous studies may originate from intraregional and/or interregional variations that do not translate into meaningful differences in whole body evaporative heat loss. This discrepancy has been observed previously when assessing changes in sweating following heat acclimation (33) and indicates that local sweat rate measurements may not, in some instances, be representative of whole body evaporative heat loss.
Our findings also provide novel insights into the relative contribution of dry and evaporative heat exchange to a given net heat loss across the menstrual cycle as a function of increasing heat load (and therefore heat stress). Several investigators have observed a greater skin blood flow response in the midluteal phase relative to the follicular phase during heat stress (14, 21, 31). This has been ascribed to increases in circulating 17β-estradiol following ovulation that enhance nitric oxide-dependent vasodilation (3, 30). Although the present study was performed in a hot, dry environment (i.e., ambient temperature exceeding that of skin temperature), which places a reliance on sweat evaporation to restore heat balance, it is possible that increases in skin blood flow in the midluteal phase (21, 31) may translate into comparatively greater blood-borne heat delivery to the skin surface. This may, in turn, be associated with a greater rise in skin temperature and a subsequent reduction in the thermal gradient for dry heat gain from the environment relative to the early- or late-follicular phases. However, contrary to that perception, both skin temperature and dry heat gain were similar across menstrual phases, irrespective of the heat load employed (Fig. 2). Although further studies, ideally conducted in temperate environments that facilitate dry heat loss (i.e., air temperature below that of the skin), are required to fully evaluate this possibility, our findings indicate that the relative contribution of dry and evaporative heat exchange to a given net heat loss is not modulated by menstrual cycle phase in hot, dry conditions.
Perspectives.
The outcomes from the present study have important implications for the design of experiments directed at evaluating thermoregulatory function in women. For some time, within-subject comparisons of women, as well as between-subject comparisons of men versus women, have been restricted to the first 7 days following the onset of menses (early-follicular phase) to minimize the confounding influence of menstrual cycle phase on heat loss responses and core temperature (8, 9, 15, 29). The observed differences in baseline core temperature confirm that this design consideration may be important for mechanistic research on thermoregulatory function. However, since whole body dry and evaporative heat exchange (Fig. 2) as well as the resulting changes in body heat storage (Fig. 1C) and core temperature (Fig. 3) were not significantly modified by menstrual phase, this practice may be unnecessary for more applied research aimed at evaluating heat loss responses or assessing heat strain during exercise in hot, dry conditions.
Considerations.
A constituent not considered in the present study is the effects of commonly used single-phase oral contraceptives, which provide a constant dose of estrogen and progesterone for 21 days followed by a 7-day placebo period where menstruation occurs. Like eumenorrheic women, oral contraceptive users display an upward shift in body core temperature during both rest and exercise during active pill consumption (i.e., quasi-luteal phase) relative to placebo consumption (i.e., quasi-follicular phase; 12, 27, 35, 41, 42), an effect that has recently been shown to occur even between quasi-middle-late luteal and quasi-middle-late follicular phases occurring during active pill consumption (25). This rise in resting core temperature has been associated with increases in the threshold body temperature for the initiation of sweat secretion during exercise-induced heat stress (12, 35). Moreover, although the absolute increase in sweat rate has been reported to be similar between eumenorrheic women and oral contraceptive users in such conditions (41, 42), others have demonstrated that sweat rate is attenuated in oral contraceptive users (12, 25). As such, further research is needed to confirm whether the outcomes from the present study hold for oral contraceptive users and to identify whether whole body heat exchange differs between eumenorrheic women and oral contraceptive users during exercise-induced heat stress.
To identify potential menstrual phase-related differences in evaporative heat loss capacity, we chose to evaluate whole body heat exchange during exercise in hot, dry conditions, which permitted full sweat evaporation. It therefore remains unclear whether menstrual phase modulates whole body heat exchange and body heat content in non-endurance-trained, eumenorrheic women during exercise in more humid environments that are common in many parts of the world (e.g., northern Europe, North and South America, Asia, and other regions) and are often experienced by women employed in physically demanding occupations (e.g., military operations, mining, electric utilities, and others). This represents an important area of future research, as there is evidence that menstrual phase may modulate thermoregulatory function during exercise in hot, humid conditions (11, 12, 14) and when exercise is performed while wearing impermeable protective clothing that creates a hot-humid microclimate around the wearer (42).
Finally, to identify whether menstrual cycle phase modulates whole body heat loss capacity as a function of the exercise-induced heat load, an incremental exercise model [an established model used to assess differences in the body’s physiological capacity as a function of various factors (e.g., aging, sex, fitness, acclimation state, and others); 10, 24, 28, 32, 37] was employed involving three 30-min bouts of cycling at increasing fixed metabolic heat production rates of ~250 (Low), ~340 (Moderate), and ~430 W (High), each followed by 15-min rest. Although there is no evidence that menstrual cycle modulates thermoregulatory function during postexercise recovery (17), whole body heat exchange and the subsequent change in body heat storage during exercise in the heat can be modified when preceded by a bout of exercise and recovery (18). We therefore cannot discount the possibility that separating this incremental exercise model into three independent bouts of exercise (i.e., 3 trials performed on separate days), perhaps of longer duration, may have revealed menstrual phase-related differences in whole body heat exchange. Although a study of this nature may have important mechanistic implications, conducting these three experimental trials within each of the three distinct phases of the menstrual cycle (early-follicular, late-follicular, and midluteal) would likely require that the study be conducted over two or more monthly cycles to allow sufficient rest and recovery (≥48 h) among trials. This may therefore introduce confounding effects that independently modify thermoregulatory function (e.g., seasonal changes in acclimatization state and others).
Conclusion.
The present study was designed to provide what is believed to be the first evaluation of whole body heat exchange during incremental exercise in the heat across the three distinct phases of the menstrual cycle. Despite the long-standing belief that menstrual phase has a profound effect on thermoregulatory function, our observations indicate that menstrual phase does not significantly modulate whole body dry and evaporative heat exchange or the subsequent changes in body heat storage and core temperature during exercise in hot, dry conditions.
GRANTS
This study was supported by Natural Sciences and Engineering Research Council Discovery Grant RGPIN-06313-2014 and Discovery Grants Program-Accelerator Supplements Grant RGPAS-462252-2014 (funds held by G. P. Kenny). S. R. Notley is supported by a postdoctoral fellowship from the Human and Environmental Physiology Research Unit. S. Dervis was supported by a Mitacs Accelerate doctoral fellowship. M. P. Poirier is supported by the Human and Environmental Physiology Research Unit and the University of Ottawa.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by authors.
AUTHOR CONTRIBUTIONS
G.P.K. conceived and designed research; S.D. performed experiments; S.D. analyzed data; S.R.N., S.D., M.P.P., and G.P.K. interpreted results of experiments; S.R.N. and S.D. prepared figures; S.R.N. and G.P.K. drafted manuscript; S.R.N., S.D., M.P.P., and G.P.K. edited and revised manuscript; S.R.N., S.D., M.P.P., and G.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all the volunteers for participating in this study. We express our appreciation to the Human and Environmental Physiology Research Unit team for its contribution to this project. We also thank Dr. Daniel Gagnon for valuable input in the initial development of this study.
REFERENCES
- 1.Avellini BA, Kamon E, Krajewski JT. Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol 49: 254–261, 1980. doi: 10.1152/jappl.1980.49.2.254. [DOI] [PubMed] [Google Scholar]
- 2.Bittel J, Henane R. Comparison of thermal exchanges in men and women under neutral and hot conditions. J Physiol 250: 475–489, 1975. doi: 10.1113/jphysiol.1975.sp011066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci 196: 75–80, 2016. doi: 10.1016/j.autneu.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 4.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med (Chic) 17: 863–871, 1916. doi: 10.1001/archinte.1916.00080130010002. [DOI] [Google Scholar]
- 5.Frascarolo P, Schutz Y, Jéquier E. Influence of the menstrual cycle on the sweating response measured by direct calorimetry in women exposed to warm environmental conditions. Eur J Appl Physiol Occup Physiol 64: 449–454, 1992. doi: 10.1007/BF00625066. [DOI] [PubMed] [Google Scholar]
- 6.Frye AJ, Kamon E, Webb M. Responses of menstrual women, amenorrheal women, and men to exercise in a hot, dry environment. Eur J Appl Physiol Occup Physiol 48: 279–288, 1982. doi: 10.1007/BF00422988. [DOI] [PubMed] [Google Scholar]
- 7.Fukuoka Y, Kaneko Y, Takita C, Hirakawa M, Kagawa H, Nakamura Y. The effects of exercise intensity on thermoregulatory responses to exercise in women. Physiol Behav 76: 567–574, 2002. doi: 10.1016/S0031-9384(02)00781-3. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon D, Crandall CG, Kenny GP. Sex differences in postsynaptic sweating and cutaneous vasodilation. J Appl Physiol (1985) 114: 394–401, 2013. doi: 10.1152/japplphysiol.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagnon D, Kenny GP. Does sex have an independent effect on thermoeffector responses during exercise in the heat? J Physiol 590: 5963–5973, 2012. doi: 10.1113/jphysiol.2012.240739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagnon D, Kenny GP. Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J Appl Physiol (1985) 113: 746–757, 2012. doi: 10.1152/japplphysiol.00637.2012. [DOI] [PubMed] [Google Scholar]
- 11.Garcia AM, Lacerda MG, Fonseca IA, Reis FM, Rodrigues LO, Silami-Garcia E. Luteal phase of the menstrual cycle increases sweating rate during exercise. Braz J Med Biol Res 39: 1255–1261, 2006. doi: 10.1590/S0100-879X2006005000007. [DOI] [PubMed] [Google Scholar]
- 12.Grucza R, Pekkarinen H, Titov EK, Kononoff A, Hänninen O. Influence of the menstrual cycle and oral contraceptives on thermoregulatory responses to exercise in young women. Eur J Appl Physiol Occup Physiol 67: 279–285, 1993. doi: 10.1007/BF00864229. [DOI] [PubMed] [Google Scholar]
- 13.Haslag SW, Hertzman AB. Temperature regulation in young women. J Appl Physiol 20: 1283–1288, 1965. doi: 10.1152/jappl.1965.20.6.1283. [DOI] [Google Scholar]
- 14.Hessemer V, Brück K. Influence of menstrual cycle on thermoregulatory, metabolic, and heart rate responses to exercise at night. J Appl Physiol (1985) 59: 1911–1917, 1985. doi: 10.1152/jappl.1985.59.6.1911. [DOI] [PubMed] [Google Scholar]
- 15.Inoue Y, Tanaka Y, Omori K, Kuwahara T, Ogura Y, Ueda H. Sex- and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur J Appl Physiol 94: 323–332, 2005. doi: 10.1007/s00421-004-1303-2. [DOI] [PubMed] [Google Scholar]
- 16.Kenny GP, Jay O. Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol 3: 1689–1719, 2013. doi: 10.1002/cphy.c130011. [DOI] [PubMed] [Google Scholar]
- 17.Kenny GP, Leclair E, Sigal RJ, Journeay WS, Kilby D, Nettlefold L, Reardon FD, Jay O. Menstrual cycle and oral contraceptive use do not modify postexercise heat loss responses. J Appl Physiol (1985) 105: 1156–1165, 2008. doi: 10.1152/japplphysiol.00194.2008. [DOI] [PubMed] [Google Scholar]
- 18.Kenny GP, McGinn R. Restoration of thermoregulation after exercise. J Appl Physiol (1985) 122: 933–944, 2017. doi: 10.1152/japplphysiol.00517.2016. [DOI] [PubMed] [Google Scholar]
- 19.Kenny GP, Notley SR, Gagnon D. Direct calorimetry: a brief historical review of its use in the study of human metabolism and thermoregulation. Eur J Appl Physiol 117: 1765–1785, 2017. doi: 10.1007/s00421-017-3670-5. [DOI] [PubMed] [Google Scholar]
- 20.Kolka MA, Stephenson LA. Control of sweating during the human menstrual cycle. Eur J Appl Physiol Occup Physiol 58: 890–895, 1989. doi: 10.1007/BF02332224. [DOI] [PubMed] [Google Scholar]
- 21.Kolka MA, Stephenson LA. Effect of luteal phase elevation in core temperature on forearm blood flow during exercise. J Appl Physiol (1985) 82: 1079–1083, 1997. doi: 10.1152/jappl.1997.82.4.1079. [DOI] [PubMed] [Google Scholar]
- 22.Kuwahara T, Inoue Y, Abe M, Sato Y, Kondo N. Effects of menstrual cycle and physical training on heat loss responses during dynamic exercise at moderate intensity in a temperate environment. Am J Physiol Regul Integr Comp Physiol 288: R1347–R1353, 2005. doi: 10.1152/ajpregu.00547.2004. [DOI] [PubMed] [Google Scholar]
- 23.Kuwahara T, Inoue Y, Taniguchi M, Ogura Y, Ueda H, Kondo N. Effects of physical training on heat loss responses of young women to passive heating in relation to menstrual cycle. Eur J Appl Physiol 94: 376–385, 2005. doi: 10.1007/s00421-005-1329-0. [DOI] [PubMed] [Google Scholar]
- 24.Lamarche DT, Notley SR, Poirier MP, Kenny GP. Fitness-related differences in the rate of whole-body total heat loss in exercising young healthy women are heat-load dependent. Exp Physiol 103: 312–317, 2018. doi: 10.1113/EP086752. [DOI] [PubMed] [Google Scholar]
- 25.Lei TH, Cotter JD, Schlader ZJ, Stannard SR, Perry BG, Barnes MJ, Mündel T. On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J Physiol. 597: 71–88. doi: 10.1113/JP276233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei TH, Stannard SR, Perry BG, Schlader ZJ, Cotter JD, Mündel T. Influence of menstrual phase and arid vs. humid heat stress on autonomic and behavioural thermoregulation during exercise in trained but unacclimated women. J Physiol 595: 2823–2837, 2017. doi: 10.1113/JP273176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin JG, Buono MJ. Oral contraceptives elevate core temperature and heart rate during exercise in the heat. Clin Physiol 17: 401–408, 1997. doi: 10.1046/j.1365-2281.1997.04444.x. [DOI] [PubMed] [Google Scholar]
- 28.Notley SR, Meade RD, D’Souza AW, Friesen BJ, Kenny GP. Heat loss is impaired in older men on the day after prolonged work in the heat. Med Sci Sports Exerc 50: 1859–1867, 2018. doi: 10.1249/MSS.0000000000001643. [DOI] [PubMed] [Google Scholar]
- 29.Notley SR, Park J, Tagami K, Ohnishi N, Taylor NAS. Variations in body morphology explain sex differences in thermoeffector function during compensable heat stress. Exp Physiol 102: 545–562, 2017. doi: 10.1113/EP086112. [DOI] [PubMed] [Google Scholar]
- 30.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286: R233–R249, 2004. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 31.Petrofsky J, Lee H, Khowailed IA. Sudomotor and vasomotor activity during the menstrual cycle with global heating. Clin Physiol Funct Imaging 37: 366–371, 2017. doi: 10.1111/cpf.12309. [DOI] [PubMed] [Google Scholar]
- 32.Poirier MP, Gagnon D, Friesen BJ, Hardcastle SG, Kenny GP. Whole-body heat exchange during heat acclimation and its decay. Med Sci Sports Exerc 47: 390–400, 2015. doi: 10.1249/MSS.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 33.Poirier MP, Gagnon D, Kenny GP. Local versus whole-body sweating adaptations following 14 days of traditional heat acclimation. Appl Physiol Nutr Metab 41: 816–824, 2016. doi: 10.1139/apnm-2015-0698. [DOI] [PubMed] [Google Scholar]
- 34.Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol 19: 531–533, 1964. doi: 10.1152/jappl.1964.19.3.531. [DOI] [PubMed] [Google Scholar]
- 35.Rogers SM, Baker MA. Thermoregulation during exercise in women who are taking oral contraceptives. Eur J Appl Physiol Occup Physiol 75: 34–38, 1996. doi: 10.1007/s004210050123. [DOI] [PubMed] [Google Scholar]
- 36.Siri WE. The gross composition of the body. Adv Biol Med Phys 4: 239–280, 1956. doi: 10.1016/B978-1-4832-3110-5.50011-X. [DOI] [PubMed] [Google Scholar]
- 37.Stapleton JM, Poirier MP, Flouris AD, Boulay P, Sigal RJ, Malcolm J, Kenny GP. Aging impairs heat loss, but when does it matter? J Appl Physiol (1985) 118: 299–309, 2015. doi: 10.1152/japplphysiol.00722.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol (1985) 86: 22–28, 1999. doi: 10.1152/jappl.1999.86.1.22. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Regul Integr Comp Physiol 249: R186–R191, 1985. doi: 10.1152/ajpregu.1985.249.2.R186. [DOI] [PubMed] [Google Scholar]
- 40.Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med 44: 883–887, 2006. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 41.Sunderland C, Nevill M. Effect of the menstrual cycle on performance of intermittent, high-intensity shuttle running in a hot environment. Eur J Appl Physiol 88: 345–352, 2003. doi: 10.1007/s00421-002-0722-1. [DOI] [PubMed] [Google Scholar]
- 42.Tenaglia SA, McLellan TM, Klentrou PP. Influence of menstrual cycle and oral contraceptives on tolerance to uncompensable heat stress. Eur J Appl Physiol Occup Physiol 80: 76–83, 1999. doi: 10.1007/s004210050561. [DOI] [PubMed] [Google Scholar]
- 43.Wells CL, Horvath SM. Heat stress reponses related to the menstrual cycle. J Appl Physiol 35: 1–5, 1973. doi: 10.1152/jappl.1973.35.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Wells CL, Horvath SM. Responses to exercise in a hot environment as related to the menstrual cycle. J Appl Physiol 36: 299–302, 1974. doi: 10.1152/jappl.1974.36.3.299. [DOI] [PubMed] [Google Scholar]