Abstract
Left ventricular hypertrophy (LVH) is an adaptive response to physiological or pathological stimuli, and distinguishing between the two has obvious clinical implications. However, asymmetric septal hypertrophy and preserved cardiac function are noted in early stages in both cases. We characterized the early anatomic and functional changes in a mouse model of physiological and pathological stress using serial echocardiography-based morphometry and tissue velocity imaging. Weight-matched CF-1 male mice were separated into Controls (n = 10), treadmill Exercise 1 h daily for 5 days/wk (n = 7), and transverse aortic constriction (TAC, n = 7). Hypertrophy was noted first in the left ventricle basal septum compared with other segments in Exercise (0.84 ± 0.02 vs. 0.79 ± 0.03 mm, P = 0.03) and TAC (0.86 ± 0.05 vs. 0.77 ± 0.04 mm, P = 0.02) at 4 and 3 wk, respectively. At 8 wk, eccentric LVH was noted in Exercise and concentric LVH in TAC. Septal E/E′ ratio increased in TAC (32.6 ± 3.7 vs. 37 ± 6.2, P = 0.002) compared with the Controls and Exercise (32.3 ± 5.2 vs. 32.8 ± 3.8 and 31.2 ± 4.9 vs. 28.2 ± 5.0, respectively, nonsignificant for both). Septal s′ decreased in TAC (21 ± 3.6 vs. 17 ± 4.2 mm/s, P = 0.04) but increased in Exercise (19.6 ± 4.1 vs. 29.2 ± 2.3 mm/s, P = 0.001) and was unchanged in Controls (20.1 ± 4.2 vs. 20.9 ± 5.1 mm/s, nonsignificant). With similar asymmetric septal hypertrophy and normal global function during the first 4–8 wk of pathological and physiological stress, there is an early marginal increase with subsequent decrease in systolic tissue velocity in pathological but early and progressive increase in physiological hypertrophy. Tissue velocities may help adjudicate between these two states when there are no overt anatomic or functional differences.
NEW & NOTEWORTHY Pathological and physiological stress-induced ventricular hypertrophy have different clinical connotations but present with asymmetric septal hypertrophy and normal global function in their early stages. We observed a marginal but statistically significant decrease in systolic tissue velocity in pathological but progressive increase in velocity in physiological hypertrophy. Tissue velocity imaging could be an important tool in the management of asymmetric septal hypertrophy by adjudicating between these two etiologies when there are no overt anatomic or functional differences.
Keywords: basal septal hypertrophy, early imaging biomarker, left ventricular remodeling, microimaging, pathological stress, physiological stress
INTRODUCTION
Left ventricular hypertrophy (LVH) is an adaptive response to a variety of stimuli, including pressure and/or volume overload with the objective of normalizing left ventricle (LV) wall stress (36). These stimuli may be physiological or pathological, and distinguishing between the two etiologies has significant clinical implications. Histologically, physiological LVH is associated with normal myofiber architecture and minimal or no fibrosis, whereas pathological LVH is more likely to have myofiber disarray and more fibrosis (6, 9). However, clinically, a heterogeneous progression of LVH and predominant septal involvement has been reported in both physiological and pathological LVH (2, 29). Although particular patterns of regional hypertrophy, dysfunction, and dyssynchrony were described in earlier stages, late stage is associated with global LV dysfunction, which supports the pathological process (18, 23). However, early in the disease when there is asymmetric hypertrophy and preserved function, such a separation is challenging. An understanding of the early evolution of structural and functional changes in physiological and pathological LVH using noninvasive technologies could potentially improve the clinical assessment of individuals presenting early in the disease, particularly asymmetric LVH.
Novel imaging techniques that depict regional and global motion, i.e., tissue velocity or displacement (11, 30), have been shown to be sensitive markers of myocardial dysfunction (12, 21, 31). Tissue velocities have been shown to separate physiological from pathological LVH in animals and humans (11, 38). However, the existing literature primarily compares late-stage cohorts; therefore, it is unclear if these paradigms are applicable earlier in the pathological state. Our study is fundamentally an extension of the seminal study by Derumeaux et al. that demonstrated the mechanical differences between physiological and pathological LVH at the 2- and 9-mo intervals (11). Our study fills the knowledge gap by understanding the early (<2 mo) changes in these stress models. We characterized the evolution of anatomic and functional changes in a mouse model of physiological and pathological stress. Serial echo-based morphometry and tissue velocity imaging were compared between mice undergoing chronic treadmill exercise (physiological stress), those with transverse aortic constriction (pathological stress), and age and sex-matched controls (10, 13).
MATERIALS AND METHODS
Study population.
The research conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (30a). All animal studies were approved in accordance with institutional Animal Care Committee guidelines. Male CF-1 mice (3 mo old) weighing 31.5 ± 0.5 g were housed 3–5/cage in a temperature-controlled room (22°C) with a 12:12-h dark-light cycle with free access to standard laboratory chow and tap water. Animals were randomly assigned into the following three groups: group 1, sedentary controls (Controls, n = 10); group 2, treadmill exercise group (Exercise, n = 7); and group 3, transverse aortic constriction group (TAC, n = 7). All mice underwent B-mode and tissue Doppler echocardiography at baseline and weekly for 8 wk.
Exercise protocol.
Treadmill exercise provides a quantifiable method for physiological stress (13). Animals were exercised on a six-lane motor-driven treadmill (Omnitech, Columbus, OH) that had an adjustable belt speed (0–100 m/min), shock bars with adjustable amperage (0–2 mA), and an on-and-off shock switch for each lane. The mice were acclimated to the treadmill using an incline that was incrementally increased from 0 to 7° with a 15-min low-speed (5–7 m/min) session without shock grid and two 15-min sessions with the shock grid (5–7 and 20 m/min). After an acclimation period, the mice were exercised one time daily with incline progressively increased from 0 to 7° at 20 m/min speed for 60 min with the shock grid. Treadmill exercise was performed for 8 wk, 5 times/wk (adapted from Refs. 13 and 15). To avoid differences in nutritional status of the mice, all animals were fed at libitum. Training was continuously monitored by one investigator. If at any point during the exercise a mouse became exhausted, the shock grid for that lane was turned off, and the mouse was allowed to rest.
TAC procedure.
TAC was performed as described previously (10) to generate pressure overload. We selected TAC as our model for pathological stress since it has been a widely published and well-understood model (10, 11, 43). Mice were anesthetized in an induction chamber with 2% isoflurane mixed with 0.5–1.0 l/min 100% O2. They were then intubated orally and placed on mechanical ventilation (125–150 breaths/min and a tidal volume of 0.1–0.3 ml; Harvard rodent ventilator; Harvard Apparatus). The chest was shaved, and residual hair was removed using an depilatory cream (Nair). A thoracotomy was performed via the second intercostal space at the central upper sternal border to display the transverse aorta. The ascending aorta between the right and left carotid arteries was ligated with an overlying 27-gauge needle that was removed soon after ligation (10). After aortic constriction, the chest was closed with vicryl sutures at the fascial and subcutaneous level. After recovery, mice were returned to the vivarium and ad libitum diet.
Echocardiographic protocol.
Cardiac morphology and function were assessed noninvasively using a high-frequency high-resolution echocardiography system consisting of a Vevo 660 ultrasound machine equipped with a 25- to 50-MHz transducer (Visual Sonics, Toronto, Canada) (4, 8, 26). Mice were anesthetized using 3% isoflurane and transferred to an imaging stage equipped with built-in electrocardiography electrodes for continuous heart rate monitoring. The body temperature was maintained at 37°C. Anesthesia was sustained via a nose cone with 1% isoflurane. High-resolution images were obtained in the parasternal and apical orientations. Standard B-mode (2D) images of the heart and pulsed Doppler images of the mitral valve inflow were acquired. LV area, internal LV end-diastolic dimensions (LVEDD), and wall thickness (WT) were measured at the level of the papillary muscles in the parasternal short axis, at end systole, and end diastole. Parasternal long axis was used for measurement of septal and posterior WT, including LV base, mid and midapical regions. LV ejection fraction (LVEF) and mass were determined as described by de Simone et al. (12). LV area and relative wall thickness (RWT) (=2 × WTd of the posterior wall/LVEDD, where WTd is wall thickness in diastole; RWT <0.42 is defined as eccentric LVH, RWT >0.42 is defined as concentric LVH) were measured according to current recommendations (26). Global diastolic hemodynamics were evaluated using transmitral Doppler velocities. An apical four-chamber view of the heart was obtained. A pulsed Doppler sample was placed at the tip of the mitral leaflets, and transmitral velocities were acquired and stored electronically for offline analysis. Transmitral Doppler data were collected only when the conditions of a stable heart rate, regular rhythm, and consistent velocity profile were met. Early (E) and late (A) transmitral diastolic velocities (m/s) and the deceleration time (ms) were measured and used as noninvasive indicators of global diastolic function (33). In the current study, we used the apical view for pulse wave tissue Doppler imaging (TDI) to measure septal mitral annular tissue velocities. Systolic velocity (S′, mm/s), early (E′) and late (A′) diastolic velocity (mm/s), and E′/A′ were determined, and the ratio of E to E′ was also calculated (28, 37, 40).
Statistics.
SPSS software 16.0 (SPSS, Chicago, IL) was used for statistical analysis. Continous data were presented as means and SD. Comparison of data within each group was made with the paired-sample t-test. ANOVA and independent-sample t-test were used for comparison between groups. A P value <0.05 was considered statistically meaningful.
RESULTS
Hypertrophy.
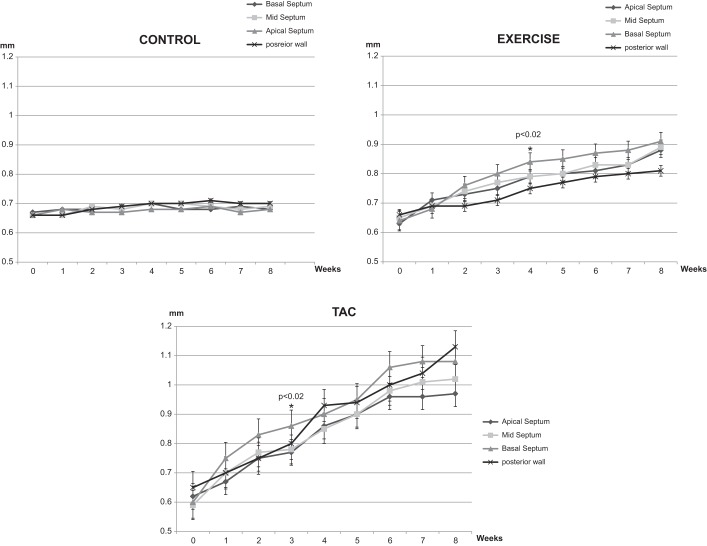
Data are available from 10 Controls, 7 Exercise, and 7 TAC mice. Ten mice underwent TAC, but three died during surgery. All animals were followed weekly for 8 wk; none died during the study period. Mean ejection fraction and LV geometric parameters were similar in the three groups at baseline (Table 1). Whereas no change was detected in Controls (Fig. 1), basal septal hypertrophy was detected at week 4 in the Exercise group (Fig. 1). Significant basal septal hypertrophy was also noted initially in the TAC mice at week 3. Basal septum became significantly thicker than midseptum (0.86 ± 0.05 vs. 0.77 ± 0.04 mm, P = 0.02), thicker than apical septum (0.86 ± 0.05 vs. 0.77 ± 0.04 mm, P = 0.02), and thicker than posterior wall (0.86 ± 0.05 vs. 0.77 ± 0.04 mm, P = 0.02) at week 3 in TAC mice (Fig. 1). Regional hypertrophy located on the LV base progressed in the midapical segment at 8 wk, and posterior wall thickness was significantly increased, but a regional difference was not detected for the posterior wall in either the Exercise or TAC groups at 4 and 8 wk. LV end-diastolic dimension markedly increased in Exercise (Table 1).
Table 1.
Echocardiographic findings of three groups at baseline, after 4 and 8 wk
| Control |
Exercise |
TAC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 wk | P | 8 wk | P | Baseline | 4 wk | P | 8 wk | P | Baseline | 4 wk | P | 8 wk | P | |
| HR, beats/min | 415 ± 41 | 410 ± 20 | NS | 404 ± 20 | NS | 425 ± 47 | 419 ± 35 | NS | 412 ± 35 | NS | 402 ± 39 | 411 ± 22 | NS | 421 ± 22 | NS |
| PW, mm | 0.66 ± 0.03 | 0.7 ± 0.05 | NS | 0.70 ± 0.04 | NS | 0.66 ± 0.05 | 0.75 ± 0.03 | 0.002 | 0.81 ± 0.03 | 0.001 | 0.63 ± 0.05 | 0.93 ± 0.04 | 0.001 | 1.13 ± 0.06 | 0.001 |
| LVEDD, mm | 4.3 ± 0.2 | 4.4 ± 0.3 | NS | 4.4 ± 0.2 | NS | 4.1 ± 0.1 | 4.7 ± 0.2 | 0.002 | 4.7 ± 0.4 | 0.002 | 4.5 ± 0.2 | 4.47 ± 0.3 | NS | 4.47 ± 0.3 | NS |
| RWT, mm | 0.31 ± 0.02 | 0.31 ± 0.04 | NS | 0.31 ± 0.02 | NS | 0.32 ± 0.02 | 0.35 ± 0.03 | NS | 0.39 ± 0.03 | 0.01 | 0.31 ± 0.02 | 0.41 ± 0.03 | 0.002 | 0.5 ± 0.06 | 0.001 |
| LV area, mm2 | 13.8 ± 0.6 | 13.9 ± 0.7 | NS | 13.9 ± 0.7 | NS | 13.5 ± 0.7 | 16.1 ± 0.6 | 0.002 | 19.7 ± 0.9 | 0.001 | 14.4 ± 0.4 | 13.9 ± 0.6 | NS | 13.9 ± 0.6 | NS |
| EF, % | 60.2 ± 0.9 | 60.3 ± 0.4 | NS | 59.1 ± 0.8 | NS | 58.0 ± 0.2 | 59.3 ± 0.3 | NS | 60.5 ± 0.5 | NS | 59.4 ± 0.6 | 53.8 ± 0.5 | NS | 44.6 ± 0.9 | 0.001 |
| E/A | 1.89 ± 0.8 | 1.91 ± 0.6 | NS | 1.92 ± 0.9 | NS | 1.91 ± 0.6 | 2.2 ± 0.7 | NS | 2 ± 0.9 | NS | 1.9 ± 0.7 | 2.1 ± 0.5 | NS | 2.9 ± 0.8 | 0.001 |
| E′/A′ | 1.45 ± 0.2 | 1.64 ± 0.3 | NS | 1.61 ± 0.3 | NS | 1.46 ± 0.3 | 1.65 ± 0.4 | NS | 1.55 ± 0.3 | NS | 1.6 ± 0.6 | 1.57 ± 0.2 | NS | 1.5 ± 0.5 | NS |
| E/E′ | 32.3 ± 5.2 | 32.5 ± 6.1 | NS | 32.8 ± 3.8 | NS | 31.2 ± 4.9 | 30.4 ± 5.7 | NS | 28.2 ± 5.0 | NS | 32.6 ± 3.7 | 33.5 ± 6.3 | NS | 37 ± 6.2 | 0.002 |
| s′, mm/s | 20.1 ± 4.2 | 20.7 ± 3.1 | NS | 20.9 ± 5.1 | NS | 19.6 ± 4.1 | 27.8 ± 5.4 | 0.046 | 29.2 ± 2.3 | 0.001 | 21 ± 3.6 | 18.7 ± 4.6 | NS | 17 ± 4.2 | 0.04 |
Values are means ± SE. HR, heart rate; PW, posterior wall; LVEDD, left ventricular end-diastolic dimension; RWT, relative wall thickness; LV, left ventricle; EF, ejection fraction; NS, nonsignificant.
Fig. 1.
Basal septum, midseptum, apical septum, and posterior wall thicknesses of three groups from baseline to 8 wk. TAC, transverse aortic constriction. *P < 0.05 compared with baseline.
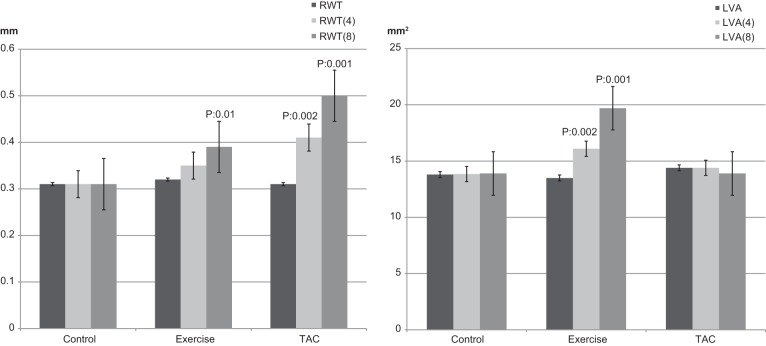
Mean RWT was within the normal range for all three groups at baseline. At week 4, RWT increased distinctly in TAC mice (0.31 ± 0.02 to 0.41 ± 0.03 mm, P = 0.002; Table1 and Fig. 2). Posterior wall thickness increased in Exercise (0.66 ± 0.05 to 0.75 ± 0.03 mm, P = 0.002) and TAC (0.63 ± 0.05 to 0.93 ± 0.04 mm, P = 0.001) mice at week 4 (Table 1). At week 8, there was no LVH pattern in Controls, but there was an eccentric LVH pattern in Exercise mice and a concentric LVH pattern in the TAC mice (mean RWT was 0.31 ± 0.02 in Controls, 0.39 ± 0.03 in Exercise, and 0.5 ± 0.06 in TAC mice; Fig. 2 and Table 1).
Fig. 2.
Relative wall thickness (RWT) and left ventricle area (LVA) at baseline and after 4 and 8 wk.
LV dimensions and function.
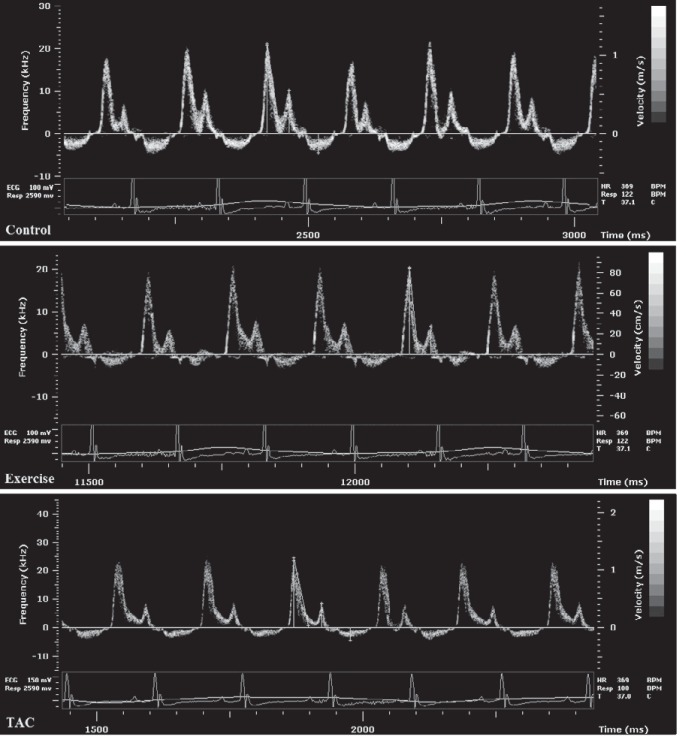
In the Exercise mice from baseline to week 4, LV area increased from 13.5 ± 0.7 to 16.1 ± 0.6 mm2 (P = 0.002), and from week 4 to 8, LV area increased from 16.1 ± 0.6 to 19.7 ± 0.9 mm2 (P = 0.004) (Fig. 2). There were no significant changes in LV area in Controls (13.8 ± 0.6 vs. 13.9 ± 0.7 mm2, nonsignificant) or TAC mice (14.4 ± 0.4 vs. 13.9 ± 0.6 mm2, nonsignificant). Mean LVEF did not change after 8 wk in Controls and Exercise mice (Table 1) but significantly decreased in TAC mice (Fig. 3). Mean LVEF was 59.4 ± 0.6 at baseline, 57.5 ± 0.4 (nonsignificant) at week 2, 53.8 ± 0.5 (nonsignificant) at week 4, and 44.6 ± 0.9 (P = 0.001) at week 8 in TAC mice. Transmitral Doppler measurements demonstrated a significantly increased E/A in the TAC mice from baseline to week 8 (1.9 ± 0.7 to 2.9 ± 0.8, P = 0.001; Fig. 4).
Fig. 3.
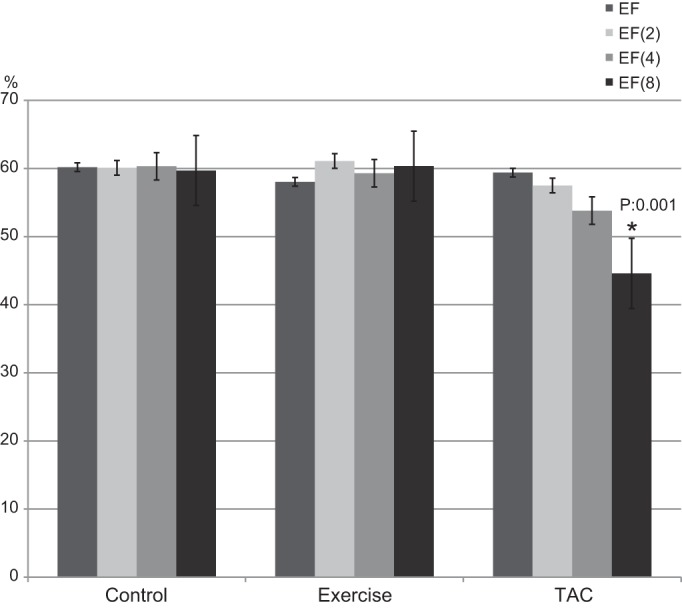
LV ejection fraction (EF) at baseline and after 2, 4, and 8 wk. *P = 0.001.
Fig. 4.
Transmitral Doppler images at 8 wk.
Tissue Doppler results.
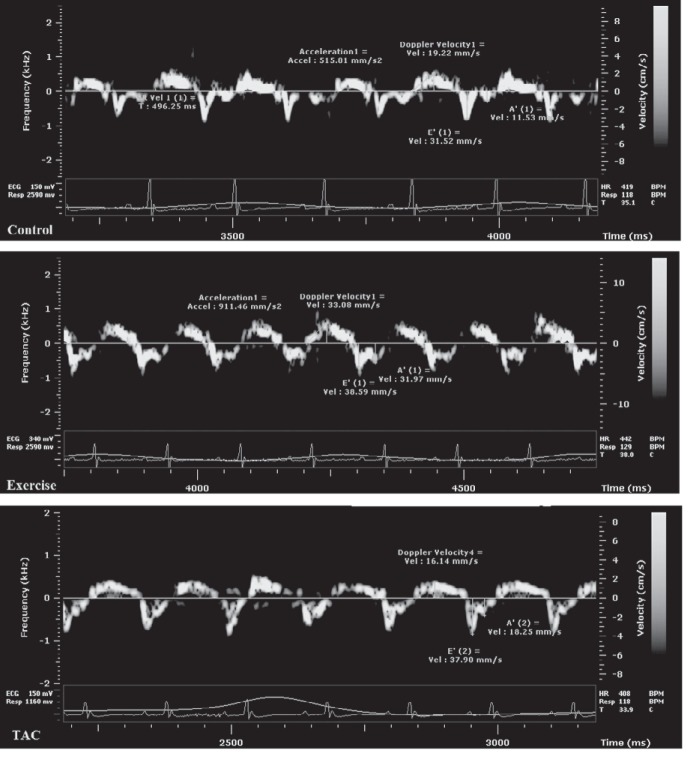
Septal s′ significantly decreased in TAC mice between baseline and week 8 (21 ± 3.6 to 17 ± 4.2 mm/s, P = 0.04), increased in Exercise mice (19.6 ± 4.1 to 29.2 ± 2.3 mm/s, P = 0.02), and was unchanged in Controls (20.1 ± 4.2 to 20.9 ± 5.1 mm/s, nonsignificant) (Fig. 5). Septal s′ was significantly increased in Exercise mice first at week 2 (24 ± 3.4 mm/s, P = 0.05) and thereafter; s′ was 27.8 ± 1.8 mm/s, P = 0.046 at week 4 and 29.2 ± 2.3 mm/s, P = 0.001 at week 8 compared with baseline (Fig. 6).Septal E/E′ increased in TAC from baseline to week 8 (32.6 ± 3.7 to 37 ± 6.2, P = 0.002) compared with the Controls and Exercise mice (32.3 ± 5.2 to 32.8 ± 3.8 and 31.2 ± 4.9 to 28.2 ± 5.0, respectively, nonsignificant; Tables 1 and 2). TDI-derived diastolic velocities (E′, A′) and E′/A′ did not change in any group during the period of 8 wk (Table 1).
Fig. 5.
Tissue Doppler images at 8 wk.
Fig. 6.
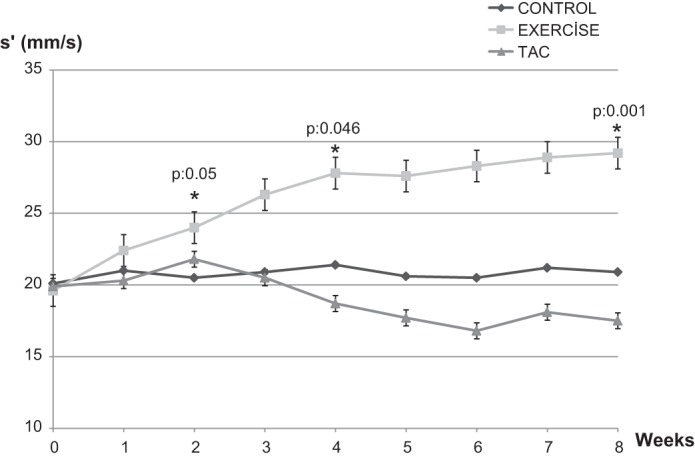
Changes in tissue Doppler s′ velocity from baseline to 8 wk. *P < 0.05 compared with baseline.
Table 2.
Heart weight, left ventricular weight, body weight, and tibial length measurements of three groups (Exercise and TAC groups compared with Control)
| Control | Exercise | P | TAC | P | |
|---|---|---|---|---|---|
| Heart wt, mg | 183 ± 13 | 205 ± 7 | 0.049 | 255 ± 36 | 0.004 |
| LV wt, mg | 121 ± 11 | 145 ± 7 | 0.016 | 196 ± 32 | <0.001 |
| Body wt, g | 43 ± 2.1 | 40 ± 3.2 | NS | 37.33 ± 4.4 | 0.024 |
| Tibial length, mm | 19.2 ± 0.4 | 19.25 ± 0.3 | NS | 18.92 ± 0.1 | NS |
| Heart wt/body wt, mg/g | 4.2 ± 0.3 | 5.1 ± 0.1 | 0.015 | 7.0 ± 0.1 | 0.001 |
| LVW/body wt, mg/g | 2.8 ± 0.2 | 3.6 ± 0.1 | 0.037 | 5.4 ± 0.1 | <0.001 |
| Heart wt/TL, mg/mm | 9.5 ± 0.3 | 10.6 ± 0.4 | 0.035 | 13.4 ± 0.4 | <0.001 |
| LVW/TL, mg/mm | 6.2 ± 0.1 | 7.5 ± 0.3 | 0.009 | 10.3 ± 0.3 | <0.001 |
TAC, transverse aortic constriction; LVW, left ventricular weight; TL, tibial length.
Animals were euthanized at the end of the study, hearts were removed, and LV were dissected and weighed. Tibial lengths (TL) were measured. TL was not different in the groups. Body weight was significantly lower in TAC mice. Heart weight was significantly higher in Exercise and TAC compared with Controls (205 ± 7 vs. 183 ± 13 mg, P = 0.040 in Exercise and 255 ± 36 mg, P = 0.004 in TAC, respectively). Similarly, LV weight (LVW) was significantly higher in Exercise and TAC compared with Controls (145 ± 7 vs. 121 ± 11 mg in Exercise, P = 0.016 and 196 ± 32 mg in TAC, P < 0.001). Heart weight/body weight and LVW/body weight ratios were significantly higher in Exercise and TAC compared with Controls (heart weight/body weight ratio was 4.2 ± 0.3 mg/g in Controls vs. 5.1 ± 0.1 mg/g in Exercise, P = 0.015, 7.0 ± 0.1 mg/g in TAC, P = 0.001; LVW/body weight ratio was 2.8 ± 0.2 mg/g in Controls vs. 3.6 ± 0.1 mg/g in Exercise, P = 0.037, 5.4 ± 0.1 mg/g in TAC, P < 0.001). Similarly, heart weight/TL and LVW/TL ratios were significantly higher in Exercise and TAC compared with Controls (heart weight/TL was 9.5 ± 0.3 mg/mm in Controls vs. 10.6 ± 0.4 mg/mm in Exercise, P = 0.035, 13.4 ± 0.4 mg/mm in TAC, P < 0.001; LVW/TL was 6.2 ± 0.1 mg/mm in Controls vs. 7.5 ± 0.3 mg/mm in Exercise, P = 0.009, 10.3 ± 0.3 mg/mm in TAC, P < 0.001) (Table 2).
DISCUSSION
Our study finds that significant anatomic and functional changes occur in the first few weeks of pathological and physiological stress. Both forms of stress manifest initially with asymmetric septal hypertrophy and preserved global function. However, there is an early separation in systolic tissue velocity that is marginally increased in early and marginally reduced by 4 wk in pathological but progressively increases in physiological remodeling, initially noted as early at 2 wk but prominent at 4 wk after initiation of stress (Fig. 6). These findings may be particularly useful while evaluating patients with asymmetric septal hypertrophy. Our data suggest that serial assessment of regional mechanical function in such patients may help adjudicate whether the hypertrophy is a physiological or pathological response and therefore assist with management.
Management of patients with borderline septal hypertrophy has always been a clinical conundrum. The primary challenge in such cases is adjudicating whether this hypertrophy is a normal variant, response to a physiological stimulus, or the early finding in a pathological process. Anatomic features are similar across these three entities and hence not very useful to the clinician. Similarly, novel imaging techniques such as tissue velocities may be relatively well preserved early in the pathological process.
Our study sought to understand the evolution of anatomic and functional/mechanical changes in response to physiological and pathological stress. Long-term geometric and functional consequences of TAC mice have been documented previously (10). No attempt was made for longer analyses and histology after 8 wk, since our goal was to identify features during an earlier stage that might be useful in separating these two populations. For this purpose, we used clinically relevant and previously validated models and a high-resolution imaging platform and completed the study at 8 wk. The lack of LV dilatation in TAC mice despite decreased LVEF was possibly related to the duration of the study. This well-validated model has enabled significant and innovative advances in the understanding of the molecular basis of pathological LVH and has been instrumental in development of novel therapies (9, 25, 31). It attempts to simulate aortic stenosis and to some extent systemic hypertension, a very prevalent clinical problem worldwide.
The treadmill exercise model has been more recent and has also been similarly validated. It attempts to simulate chronic aerobic exercise (13, 14). Our team has had substantial experience with both models. For imaging, we used a high-resolution high-frequency ultrasound imaging system that would allow detailed high-fidelity assessment of regional and global anatomy and function. Our system is also equipped with tissue velocity interrogation capabilities. This novel imaging system allowed a significantly higher level of anatomic and functional characterization not available in most of the studies reported previously with the same models. The high spatial resolution in our imaging system enabled us to reliably and accurately determine regional differences in hypertrophy.
Several theories have been proposed as to why the septal base is preferentially affected in multiple pathologies. This region may be exposed to relatively greater stress compared with the midapical region. Normally, the internal LV diameter at the LV base is the largest part of the LV cavity, and wall stress is greater in the LV base than in the midapical LV cavity (16). We and others documented decreased regional LV intracavitary volume in the LV base and predominant regional myocardial hypertrophy of the LV base (7, 41). Microneurography and isotope dilution studies have shown that LVH is associated with increased sympathetic activity (36). Interestingly, it has also been documented that the LV base has greater tissue content of norepinephrine and more dominant nerve innervation compared with the apex in animal studies (1, 22), a finding that is consistent with biopsy findings in human hearts (24).
Our finding of asymmetric septal hypertrophy, restricted to the basal septum, recapitulates previously reported findings (3, 5, 6, 29). Asymmetric septal hypertrophy has been documented in young healthy individuals in the setting of intense physical training (27). We have previously suggested that hypertension-mediated pressure overload may potentially contribute to the initial hypertrophy in this group (42). Our current animal study confirms that basal septal hypertrophy occurs early in both physiological and pathological processes. Hypertensive LV remodeling appears to affect the basal septum early in the disease process, manifesting as hypertrophy with mechanical abnormalities of the septal base (18, 21). Functional impairment of the septal base in the early stage may progress more severely in the disease process. In fact, we recently have demonstrated blunted septal mechanical performance compared with the lateral wall in advanced hypertensive disease (39). A clinical study using cardiac magnetic resonance imaging (27) showed that the initial period of LV remodeling under intensive exercise training is associated with asymmetric hypertrophy of the septal base, the closest part of myocardium to increased aterload that is possibly affected by hemodynamic load (2, 3, 5, 29).
Our study findings not only validate these earlier cross-sectional studies but also highlight the critical need for additional methodology to separate pathological and physiological hypertrophy in early disease. Early detection of velocity abnormalities is consistent with clinical studies and may be an appropriate target for preventive strategies (3, 5, 30). A number of studies have shown that pathological LVH has low systolic and early diastolic velocities (11, 19, 28, 35). Most of these models were studied at a late stage (15, 31, 43) and/or had clear anatomic features that would have enabled separation of the entities (type of hypertrophy and low ejection fraction) (11, 38). In our study, we observe an early increment and lack of significant decrement in systolic velocity in TAC animals that we believe is related primarily to compensatory hyperfunction in the early time points after TAC (up to 4 wk). Around 4 wk, there is loss of a hyperdynamic state and appearance of marginal ventricular dysfunction as reflected by a decrease in systolic velocity. However, systolic velocity progressively increases over baseline as early as 1 wk. The lack of examination of early time points in previous studies possibly explains why those studies did not capture these early changes in TAC and exercise-induced hypertrophy. Our results are concordant with the report by Derumeaux et al. (11) demonstrating that TDI-derived myocardial velocity gradients using short-axis view differentiate physiological LVH from pathological LVH. We previously documented that E′ velocity is a relatively independent TDI parameter from preload and increased E/E′ indicates impaired diastolic dysfunction and raised filling pressures (28, 40). In contrast, physiological exercise-induced LVH is associated with increased LV area, normal LVEF, and preserved systolic septal tissue Doppler velocity (13, 14, 32, 34). In the LV remodeling process, myocardial structure is altered with progression of fibrosis that is known to be associated with multiple adverse cardiac outcomes and reduced LV contractility (9, 17, 18, 43).
Notwithstanding these data, there are sparse, if any, longitudinal studies with serial assessment of regional function in such populations. Thus, there exists a knowledge gap on how best to separate physiological and pathological hypertrophy, especially when early assessment shows similar anatomy and function in the two entities. Our study of changes early in the process (<2 mo) therefore covers a period not previously explored by other studies. Our results show that key regional mechanical changes may assist in separating physiological and pathological LVH. However, translation of the present tissue Doppler findings to differentiate between the two conditions in the clinic may be challenging and will need to be validated in clinical studies.
Conclusions
Significant anatomic and functional changes occur early in pathological and physiological stress. Both forms of stress manifest initially with asymmetric septal hypertrophy and preserved global function. However, systolic tissue velocity is reduced in pathological but progressively increases in physiological remodeling starting as early at 4 wk after initiation of stress. These data may assist clinicians in adjudicating between pathological and physiological LVH when there are no overt anatomic or functional differences early in the disease process.
GRANTS
The study was partially supported by National Heart, Lung, and Blood Institute Grant HL-98046. F. Yalcin was supported by a Fulbright scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.Y., N.K., O.H.C., B.M., L.S., and A.P. performed experiments; F.Y. drafted manuscript; F.Y. and T.P.A. edited and revised manuscript; T.P.A. approved final version of manuscript; N.K. analyzed data; N.K. interpreted results of experiments; N.K. prepared figures; M.R.A. and T.P.A. conceived and supervised the research.
REFERENCES
- 1.Angelakos ET. Regional distribution of catecholamines in the dog heart. Circ Res 16: 39–44, 1965. doi: 10.1161/01.RES.16.1.39. [DOI] [PubMed] [Google Scholar]
- 2.Badke FR, Covell JW. Early changes in left ventricular regional dimensions and function during chronic volume overloading in the conscious dog. Circ Res 45: 420–428, 1979. doi: 10.1161/01.RES.45.3.420. [DOI] [PubMed] [Google Scholar]
- 3.Baltabaeva A, Marciniak M, Bijnens B, Moggridge J, He FJ, Antonios TF, MacGregor GA, Sutherland GR. Regional left ventricular deformation and geometry analysis provides insights in myocardial remodelling in mild to moderate hypertension. Eur J Echocardiogr 9: 501–508, 2008. doi: 10.1016/j.euje.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg 44: 372–376, 2006. doi: 10.1016/j.jvs.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Cheng S, Unno K, Lin FC, Liao R. Regional cardiac dysfunction and dyssynchrony in a murine model of afterload stress. PLoS One 8: e59915, 2013. doi: 10.1371/journal.pone.0059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belenkie I, MacDonald RP, Smith ER. Localized septal hypertrophy: part of the spectrum of hypertrophic cardiomyopathy or an incidental echocardiographic finding? Am Heart J 115: 385–390, 1988. doi: 10.1016/0002-8703(88)90486-3. [DOI] [PubMed] [Google Scholar]
- 7.Caselli S, Pelliccia A, Maron M, Santini D, Puccio D, Marcantonio A, Pandian NG, De Castro S. Differentiation of hypertrophic cardiomyopathy from other forms of left ventricular hypertrophy by means of three-dimensional echocardiography. Am J Cardiol 102: 616–620, 2008. doi: 10.1016/j.amjcard.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Chérin E, Williams R, Needles A, Liu G, White C, Brown AS, Zhou YQ, Foster FS. Ultrahigh frame rate retrospective ultrasound microimaging and blood flow visualization in mice in vivo. Ultrasound Med Biol 32: 683–691, 2006. doi: 10.1016/j.ultrasmedbio.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res 89: 265–272, 2011. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 10.deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp 21: 38, 2010. doi: 10.3791/1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, Henry JP, Thuillez C. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation 105: 1602–1608, 2002. doi: 10.1161/01.CIR.0000012943.91101.D7. [DOI] [PubMed] [Google Scholar]
- 12.de Simone G, Wallerson DC, Volpe M, Devereux RB. Echocardiographic measurement of left ventricular mass and volume in normotensive and hypertensive rats. Necropsy validation. Am J Hypertens 3: 688–696, 1990. doi: 10.1093/ajh/3.9.688. [DOI] [PubMed] [Google Scholar]
- 13.Fewell JG, Osinska H, Klevitsky R, Ng W, Sfyris G, Bahrehmand F, Robbins J. A treadmill exercise regimen for identifying cardiovascular phenotypes in transgenic mice. Am J Physiol Heart Circ Physiol 273: H1595–H1605, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes T, Barauna VG, Negrao CE, Phillips MI, Oliveira EM. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol 309: H543–H552, 2015. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman K, Colon-Rivera C, Olsson MC, Moore RL, Weinberger HD, Grupp IL, Vikstrom KL, Iaccarino G, Koch WJ, Leinwand LA. Progression from hypertrophic to dilated cardiomyopathy in mice that express a mutant myosin transgene. Am J Physiol Heart Circ Physiol 280: H151–H159, 2001. doi: 10.1152/ajpheart.2001.280.1.H151. [DOI] [PubMed] [Google Scholar]
- 16.Frielingsdorf J, Franke A, Kühl HP, Hess OM, Flachskampf FA. Evaluation of septal hypertrophy and systolic function in diseases that cause left ventricular hypertrophy: a 3-dimensional echocardiography study. J Am Soc Echocardiogr 14: 370–377, 2001. doi: 10.1067/mje.2001.112674. [DOI] [PubMed] [Google Scholar]
- 17.Gaasch WH, Aurigemma GP. CMR imaging of extracellular volume and myocardial strain in hypertensive heart disease. JACC Cardiovasc Imaging 8: 181–183, 2015. doi: 10.1016/j.jcmg.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 58: 1733–1740, 2011. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Galderisi M, Caso P, Severino S, Petrocelli A, De Simone L, Izzo A, Mininni N, de Divitiis O. Myocardial diastolic impairment caused by left ventricular hypertrophy involves basal septum more than other walls: analysis by pulsed Doppler tissue imaging. J Hypertens 17: 685–693, 1999. doi: 10.1097/00004872-199917050-00013. [DOI] [PubMed] [Google Scholar]
- 21.Hittinger L, Patrick T, Ihara T, Hasebe N, Shen YT, Kalthof B, Shannon RP, Vatner SF. Exercise induces cardiac dysfunction in both moderate, compensated and severe hypertrophy. Circulation 89: 2219–2231, 1994. doi: 10.1161/01.CIR.89.5.2219. [DOI] [PubMed] [Google Scholar]
- 22.Holmgren S, Abrahamsson T, Almgren O. Adrenergic innervation of coronary arteries and ventricular myocardium in the pig: fluorescence microscopic appearance in the normal state and after ischemia. Basic Res Cardiol 80: 18–26, 1985. doi: 10.1007/BF01906740. [DOI] [PubMed] [Google Scholar]
- 23.Katz AM. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med 322: 100–110, 1990. doi: 10.1056/NEJM199001113220206. [DOI] [PubMed] [Google Scholar]
- 24.Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18: 32–39, 2003. doi: 10.1007/s003800300005. [DOI] [PubMed] [Google Scholar]
- 25.Kim HL, Kim YJ, Kim KH, Lee SP, Kim HK, Sohn DW, Oh BH, Park YB. Therapeutic effects of udenafil on pressure-overload cardiac hypertrophy. Hypertens Res 38: 597–604, 2015. doi: 10.1038/hr.2015.46. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography; European Society of Cardiology . Recommendations for chamber quantification. Eur J Echocardiogr 7: 79–108, 2006. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Lee PT, Dweck MR, Prasher S, Shah A, Humphries SE, Pennell DJ, Montgomery HE, Payne JR. Left ventricular wall thickness and the presence of asymmetric hypertrophy in healthy young army recruits: data from the LARGE heart study. Circ Cardiovasc Imaging 6: 262–267, 2013. doi: 10.1161/CIRCIMAGING.112.979294. [DOI] [PubMed] [Google Scholar]
- 28.Liang HY, Cauduro SA, Pellikka PA, Bailey KR, Grossardt BR, Yang EH, Rihal C, Seward JB, Miller FA, Abraham TP. Comparison of usefulness of echocardiographic Doppler variables to left ventricular end-diastolic pressure in predicting future heart failure events. Am J Cardiol 97: 866–871, 2006. doi: 10.1016/j.amjcard.2005.09.136. [DOI] [PubMed] [Google Scholar]
- 29.Maron BJ, Edwards JE, Epstein SE. Disproportionate ventricular thickening in patients with systemic hypertension. Chest 73: 466–470, 1978. doi: 10.1378/chest.73.4.466. [DOI] [PubMed] [Google Scholar]
- 30.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging 2: 382–390, 2009. doi: 10.1161/CIRCIMAGING.108.811620. [DOI] [PubMed] [Google Scholar]
- 30a.National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC: National Academies Press, 2011. [Google Scholar]
- 31.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2: 138–144, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- 32.Pelliccia A, Kinoshita N, Pisicchio C, Quattrini F, Dipaolo FM, Ciardo R, Di Giacinto B, Guerra E, De Blasiis E, Casasco M, Culasso F, Maron BJ. Long-term clinical consequences of intense, uninterrupted endurance training in olympic athletes. J Am Coll Cardiol 55: 1619–1625, 2010. doi: 10.1016/j.jacc.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 33.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA; Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography . Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 15: 167–184, 2002. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 34.Roeske WR, O’Rourke RA, Klein A, Leopold G, Karliner JS. Noninvasive evaluation of ventricular hypertrophy in professional athletes. Circulation 53: 286–291, 1976. doi: 10.1161/01.CIR.53.2.286. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer A, Klein G, Brand B, Lippolt P, Drexler H, Meyer GP. Evaluation of left ventricular diastolic function by pulsed Doppler tissue imaging in mice. J Am Soc Echocardiogr 16: 1144–1149, 2003. doi: 10.1067/S0894-7317(03)00679-5. [DOI] [PubMed] [Google Scholar]
- 36.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation 108: 560–565, 2003. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 37.Shan K, Bick RJ, Poindexter BJ, Shimoni S, Letsou GV, Reardon MJ, Howell JF, Zoghbi WA, Nagueh SF. Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta-adrenergic receptor density in humans. J Am Coll Cardiol 36: 891–896, 2000. doi: 10.1016/S0735-1097(00)00786-5. [DOI] [PubMed] [Google Scholar]
- 38.Vinereanu D, Florescu N, Sculthorpe N, Tweddel AC, Stephens MR, Fraser AG. Differentiation between pathologic and physiologic left ventricular hypertrophy by tissue Doppler assessment of long-axis function in patients with hypertrophic cardiomyopathy or systemic hypertension and in athletes. Am J Cardiol 88: 53–58, 2001. doi: 10.1016/S0002-9149(01)01585-5. [DOI] [PubMed] [Google Scholar]
- 39.Yalçin F, Topaloglu C, Kuçukler N, Ofgeli M, Abraham TP. Could early septal involvement in the remodeling process be related to the advance hypertensive heart disease? Int J Cardiol Heart Vasc 7: 141–145, 2015. doi: 10.1016/j.ijcha.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yalçin F, Kaftan A, Muderrisoğlu H, Korkmaz ME, Flachskampf F, Garcia M, Thomas JD. Is Doppler tissue velocity during early left ventricular filling preload independent? Heart 87: 336–339, 2002. doi: 10.1136/heart.87.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yalçin F, Shiota T, Odabashian J, Agler D, Greenberg NL, Garcia MJ, Lever HM, Thomas JD. Comparison by real-time three-dimensional echocardiography of left ventricular geometry in hypertrophic cardiomyopathy versus secondary left ventricular hypertrophy. Am J Cardiol 85: 1035–1038, 2000. doi: 10.1016/S0002-9149(99)00929-7. [DOI] [PubMed] [Google Scholar]
- 42.Yalçin F, Abraham TP, Gottdiener JS. Letter by Yalcin et al regarding article, “Left ventricular wall thickness and the presence of asymmetric hypertrophy in healthy young army recruits: data from the LARGE Heart Study”. Circ Cardiovasc Imaging 6: e28, 2013. doi: 10.1161/CIRCIMAGING.113.000720. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YJ, Zhang XL, Li MH, Iqbal J, Bourantas CV, Li JJ, Su XY, Muramatsu T, Tian NL, Chen SL. The ginsenoside Rg1 prevents transverse aortic constriction-induced left ventricular hypertrophy and cardiac dysfunction by inhibiting fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol 62: 50–57, 2013. doi: 10.1097/FJC.0b013e31828f8d45. [DOI] [PubMed] [Google Scholar]