Abstract
Objectives
The objective of this study was to characterize the effect of rifampin incorporation into poly(methyl methacrylate) (PMMA) bone cement. While incompatibilities between the two materials have been previously noted, we sought to identify and quantify the cause of rifampin’s effects, including alterations in curing properties, mechanical strength, and residual monomer content.
Methods
Four cement groups were prepared using commercial PMMA bone cement: a control; one with 1 g of rifampin; and one each with equimolar amounts of ascorbic acid or hydroquinone relative to the amount of rifampin added. The handling properties, setting time, exothermic output, and monomer loss were measured throughout curing. The mechanical strength of each group was tested over 14 days. A radical scavenging assay was used to assess the scavenging abilities of rifampin and its individual moieties.
Results
Compared with control, the rifampin-incorporated cement had a prolonged setting time and a reduction in exothermic output during polymerization. The rifampin cement showed significantly reduced strength and was below the orthopaedic weight-bearing threshold of 70 MPa. Based on the radical scavenging assay and strength tests, the hydroquinone structure within rifampin was identified as the polymerization inhibitor.
Conclusion
The incorporation of rifampin into PMMA bone cement interferes with the cement’s radical polymerization. This interference is due to the hydroquinone moiety within rifampin. This combination alters the cement’s handling and curing properties, and lowers the strength below the threshold for weight-bearing applications. Additionally, the incomplete polymerization leads to increased toxic monomer output, which discourages its use even in non-weight-bearing applications.
Cite this article: G. A. Funk, E. M. Menuey, K. A. Cole, T. P. Schuman, K. V. Kilway, T. E. McIff. Radical scavenging of poly(methyl methacrylate) bone cement by rifampin and clinically relevant properties of the rifampin-loaded cement. Bone Joint Res 2019;8:81–89. DOI: 10.1302/2046-3758.82.BJR-2018-0170.R2.
Keywords: Poly(methyl methacrylate) (PMMA), Rifampin, Strength, Radical
Article focus
The purpose of this study was to characterize rifampin-loaded poly(methyl methacrylate) (PMMA) bone cement.
The cause of chemical incompatibility between rifampin and the cement was investigated, and various clinically relevant properties of the rifampin-loaded cement were determined.
Surgical contraindications of rifampin-loaded PMMA bone cement were identified.
Key messages
The addition of rifampin to PMMA bone cement interferes with the radical polymerization mechanism, resulting in delayed cement curing and reduced compressive strength.
The hydroquinone structure within rifampin acts as a radical scavenger.
The resulting incomplete polymerization of the cement causes unreacted toxic monomer to leach from the cement over time.
Strengths and limitations
A multitude of tests were performed to give a full characterization of rifampin-loaded PMMA bone cement.
Compressive properties of, and unreacted monomer loss from, the cement may vary in vivo.
Introduction
Infection associated with orthopaedic surgery, and more specifically total joint arthroplasty (TJA), is a severe complication to patients and burdens the healthcare system.1 While prosthetic joint infection (PJI) occurs in only 1% to 3% of TJA cases, the associated costs to the United States healthcare system are projected to be US$1.62 billion by the year 2020.2-4 Poly(methyl methacrylate) (PMMA) is the major component of commercially available orthopaedic bone cement. It is used to anchor the implant in the bone during primary cases, and in fabricating weight-bearing spacers during revision surgery. Bone cement is routinely loaded with antibiotic(s), both prophylactically and as part of infected revision as a means of delivering high local doses of antibiotics, while avoiding the systemic toxicity associated with intravenous administration.5,6 Staphylococcus aureus (S. aureus) and coagulase-negative Staphylococcus spp. are the most prevalent infectious organisms following orthopaedic surgery.7 Therefore, glycopeptide antibiotics such as vancomycin, and aminoglycosides such as gentamicin, are the most commonly incorporated drugs to treat PJI.8-10
Often, PJI will result in the formation of a biofilm on or near the implant. A biofilm is a collection of bacteria that forms a protective barrier with innate resistance to antibiotics, and has a high propensity to develop on inert surfaces such as implants and post-surgical bone.11,12 The presence of these biofilms can make standard antibiotic treatments ineffective, as both vancomycin and gentamicin are ineffective at clearing biofilms.13 Unlike vancomycin and gentamicin, rifampin is highly effective against staphylococcal biofilms.13,14 Rifampin is used systemically in nearly all orthopaedic infection treatment regimens, especially when the presence of methicillin-resistant S. aureus (MRSA) is suspected.15 Even at easily achievable infection site concentrations of 1 μg/ml to 8 μg/ml, rifampin can lead to a 5 log10 to 9 log10 reduction in bacterial biofilms and suppress bacterial recovery by up to 24 hours post-treatment, which indicates almost complete eradication of the biofilm.16
Recently, there has been increased interest in characterizing the ability of PMMA bone cement to act as a carrier for local delivery of rifampin to fight infection in the presence of biofilms.16-18 In this regard, a number of adverse effects associated with rifampin-loaded PMMA bone cement have been reported.17,19 Rifampin, which was approved in the United States in 1971, was recognized early on as having a detrimental effect on PMMA polymerization and strength, and, as a result, has long been discounted as a possible additive to bone cement. Although clearly recognized, the cause of rifampin’s incompatibility has never been experimentally determined, nor has the effect been quantified.
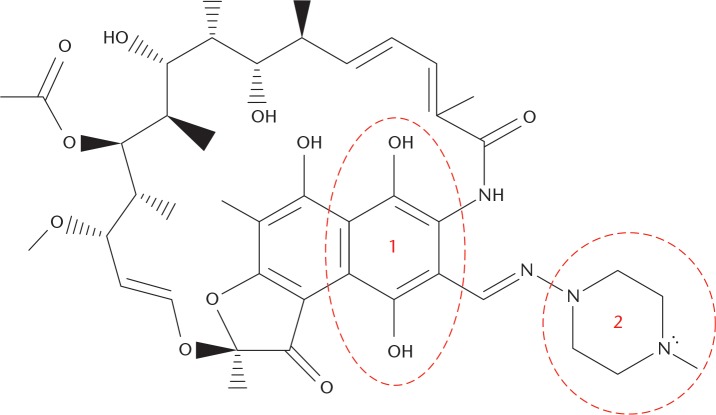
An understanding of possible mechanisms for the effect of rifampin on PMMA bone cement may be obtained by analyzing the chemical structure and properties of the antibiotic. Rifampin contains both a hydroquinone and piperazine moiety within its structure (Fig. 1). Both are known radical scavengers. The ability of rifampin to act as a radical scavenger was noted previously by Tomiyama et al20 in a 1996 study, where they used rifampin to inhibit β protein aggregation in an Alzheimer’s model. Pozdeeva and Denisov21 investigated the mechanism of hydroquinone-mediated oxidation of methyl methacrylate (MMA), and determined its impact on slowing the rate of the reaction. Aware of the scavenging potential of rifampin, McPherson and Portugal19 proposed that rifampin reacted with the cement’s initiators to quench the polymerization process. They reported increases in cement curing time, but this work was a case study and mechanical properties were not determined.
Fig. 1.
The chemical structure of rifampin. The hydroquinone (1) and piperazine (2) moieties have been labelled.
Little experimental work has been performed to understand and overcome rifampin’s effect on PMMA. One paper by Rose et al18 evaluated rifampin release from poly(styrene-co-MMA monomer) (PS-PMMA) films rather than from PMMA bone cement itself. In two separate studies by Sanchez et al16 and Shiels et al,17 the elution and effectiveness of rifampin-loaded PMMA bone cement beads using in vitro bioassays16 and in a rat infection model17 were investigated. While the investigators found reasonable antibacterial effectiveness, they also remarked on the slow and incomplete curing of the cement. Low strength and stiffness of rifampin-loaded cement remain major shortcomings for weight-bearing applications. In the referenced studies, the mechanism of rifampin’s chemical interaction with the cement was not investigated.
The purpose of the present study was to elucidate the interaction between rifampin and a commercially available PMMA bone cement, and to determine a number of clinically relevant properties of the composite material. These characteristics include its biomechanical strength and modulus over time, surface morphology, curing time, and the amount of unreacted monomer still present within the cement. Additionally, we sought to utilize a radical scavenging assay to verify experimentally the mechanism of interaction between rifampin and the cement. We hypothesized that the hydroquinone unit contained within rifampin acts as a radical scavenger, and is responsible for the interaction of rifampin with the cement’s polymerization process. Two known radical scavenging molecules, ascorbic acid and hydroquinone, were also added to PMMA bone cement to further illustrate and verify this chemical interaction.
Materials and Methods
Materials
The PMMA bone cement brand used was SmartSet MV (DePuy Synthes, Blackpool, United Kingdom). The cement was sterile and used before its expiration date. Four groups of cement were prepared: a negative control without any additive (CON); one loaded with 1 g of rifampin (RIF); and two positive controls loaded with either ascorbic acid (AA) or hydroquinone (HQ). In the RIF, AA, and HQ groups, the mass of the additive was selected so that the molar amount was the same (Table I).
Table I.
Cement group composition and study role
| Cement group | Additive | Amount, g (mol) | Purpose |
|---|---|---|---|
| CON | None | N/A | Negative control |
| RIF | Rifampin | 1.00 (1.2 × 10-3) | Experimental |
| AA | Ascorbic acid | 0.21 (1.2 × 10-3) | Positive control |
| HQ | Hydroquinone | 0.13 (1.2 × 10-3) | Positive control |
CON, negative control without any additive; N/A, not applicable; RIF, loaded with 1 g of rifampin; AA, positive control loaded with ascorbic acid; HQ, positive control loaded with hydroquinone
A low dosage of rifampin (1 g) was chosen to differentiate between a ‘filler’ and ‘chemical’ interaction. Hydroquinone and ascorbic acid were chosen based upon their known functions as radical scavengers.21,22 Furthermore, it is to be noted that in many commercially available PMMA bone cement brands (including SmartSet MV), hydroquinone is a constituent of the liquid component and serves to stabilize the polymerization reaction.23
Determination of curing properties
First, the additives were mixed with the 40 g dry cement component, after which the liquid monomer was added, and the material was blended in a polymeric bowl that was open to the ambient laboratory atmosphere. After initial mixing of the cement, dough time (td) was determined as the time at which the dough was probed without adherence, serving as the first checkpoint towards polymerization. An aliquot (10 g) from each group was removed and placed within a polytetrafluoroethylene (PTFE) thermocouple mould based on the ASTM International standard specification for acrylic bone cement, ASTM F451-16.24 The internal temperature of each group was recorded every five seconds throughout curing using K-type thermocouple wires, along with OM-PL series data logger software (OMEGA Engineering, Inc., Stamford, Connecticut). The setting time (tset) of each cement group was calculated by exothermic output, numerically the corresponding time at which the internal temperature was equal to half the sum of the maximum temperature and ambient temperature: tset = (Tmax + Tambient)/2.
Determination of compressive properties
The remainder of the cement dough that was not used in the determination of curing properties was pressed into a PTFE cylindrical mould and allowed to cure at 37°C for one hour, producing specimens with a diameter and height of 6 mm and 12 mm, respectively.24 The specimens were then inspected visually for defects. A total of 30 specimens without defects were selected randomly and placed into individual 15 ml vials. Of the 30 specimens, 20 were immersed in 2.5 ml of 1 × phosphate-buffered saline (PBS) (Ricca Chemical Co., Arlington, Texas), with the solution refreshed every 24 hours. The remaining ten were kept dry.
All compression testing was carried out in accordance with ASTM F451-16 to measure both compressive strength and modulus.24 Testing was performed using an MTS 858 Mini Bionix II hydraulic load frame (MTS Systems Corp., Eden Prairie, Minnesota). The dry specimens from each group were crushed 24 hours after curing, representing ‘Day 1’, while ten specimens from each group were tested after seven and 14 days of soaking.
Radical scavenging assay
α,α-Diphenyl-β-picrylhydrazyl (DPPH) is a molecule with a stabilized free radical, and is often used for testing the antioxidant (radical scavenging) potential of plant extracts and pharmaceuticals. Employed in an assay, it can be robust, highly reproducible, and usable with a wide range of antioxidant compounds.25 The procedure we used was adapted from the study by Blois.26 Six analytes were used for this test: ascorbic acid; hydroquinone; rifampin; vancomycin (VANC); gentamicin (GENT); and 1-methylpiperazine (PIP). Gentamicin and vancomycin were chosen for comparison because they are antibiotics commonly and successfully incorporated into PMMA bone cement. Each analyte was incubated with DPPH in spectroscopy-grade methanol (Thermo Fisher Scientific, Fair Lawn, New Jersey) for 30 minutes in the dark, and the absorption at 515 nm was measured. A loss of absorption at this wavelength indicated radical quenching of the DPPH by the analyte. The absorption of each was compared with a DPPH control solution of the same stock. The efficient concentration value required to quench or reduce the control absorbance by 50%, EC50, was used to quantify the effect of the analyte as proposed by Bondet et al.27 Thus, the lower the EC50, the higher the radical scavenging potency of the analyte. Each analyte was tested in triplicate.
Cement surface morphology
A qualitative surface analysis of both CON and RIF cement pellets was performed via scanning electron microscopy (SEM). The CON and RIF specimens were chosen to determine the influence of rifampin incorporation on the surface morphology of the cement. A single dry specimen from each group was sputter-coated with an 80:20 gold-palladium alloy. Images were taken with 100 × magnification using a scanning electron microscope (Hitachi S-2700; Hitachi, Tokyo, Japan) equipped with a Quartz PCI digital capture (Quartz Imaging Corporation, Vancouver, Canada).
Volatile monomer weight loss quantification
In order to quantify the amount of volatile monomer leaching from the cement, fresh batches of both CON and RIF cement were prepared. Each of these cements was mixed as before until td was reached, at which point the cement was spread throughout the bowl and placed on an enclosed pre-tared scale. The mass of each was recorded over the course of 24 hours.
Statistical analysis
For each of the quantitative properties determined, the results are presented as ± 1 sd. Statistical analysis was performed using one-way analysis of variance (ANOVA) and post hoc unpaired Student’s t-tests (Microsoft Excel; Microsoft, Redmond, Washington). Statistical differences were considered significant if p < 0.05.
Results
Curing properties
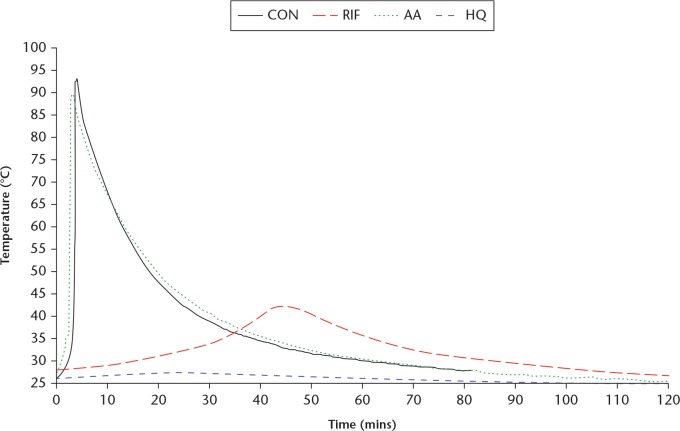
The td, tset, and Tmax of the cement achieved during curing for each cement group are listed in Table II. It is seen that td and tset of RIF are each significantly higher than the corresponding value for CON. Visually, a colour change from bright red to deep red-brown in RIF was observed during cement mixing. For RIF and HQ, a strong acrylic monomer scent was present and persistent throughout mixing and curing. Greater polymerization inhibition was observed in HQ. The AA showed no appreciable changes in td or tset, indicating that ascorbic acid has no effect on the polymerization of the cement. The exothermic output log for each cement group is displayed in Figure 2. The CON and AA exhibited similar exothermic outputs, both achieving Tmax > 90°C within minutes, while tailing off quickly. The RIF had a slow rise in exothermic temperature (Tmax = 42.5°C) over the course of 45 minutes, with slow dissipation thereafter. The HQ showed a minimal increase in temperature throughout curing.
Table II.
Pertinent handling and curing properties of each cement group as defined by the ASTM International standard specification for acrylic bone cement, ASTM F451-1624
| Cement group | td, mins | tset, mins | Tmax, °C |
|---|---|---|---|
| CON | 3.50 | 7.25 | 92.5 |
| RIF | 4.50 | 33.00 | 42.5 |
| AA | 3.50 | 7.50 | 90.1 |
| HQ | 4.75 | 34.75 | 27.6 |
td, dough time; tset, setting time; Tmax, maximum temperature; CON, negative control without any additive; RIF, loaded with 1 g of rifampin; AA, positive control loaded with ascorbic acid; HQ, positive control loaded with hydroquinone
Fig. 2.
Line graph showing a sample curing temperature versus curing time profiles. CON, negative control without any additive; RIF, loaded with 1 g of rifampin; AA, positive control loaded with ascorbic acid; HQ, positive control loaded with hydroquinone.
Compressive properties
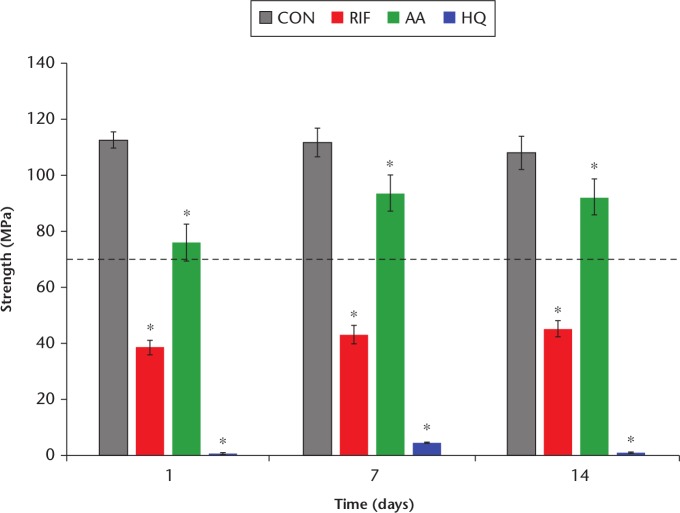
The compressive strengths of the cements are presented in Figure 3. Each group varied significantly from CON during each timepoint (p < 0.05). The AA showed strength above 70 MPa (the minimum required strength, per ASTM F451) throughout the testing period, and increased in strength significantly over the course of seven days. The RIF had significantly reduced strength when compared with CON and stayed well below 70 MPa over the course of 14 days. The HQ exhibited minimal strength and remained elastic, even after 14 days.
Fig. 3.
Chart summarizing the compressive strength values. An asterisk (*) denotes a significant difference from control, p < 0.05. The dashed line indicates the 70 MPa threshold. Ten specimens were tested from each group. CON, negative control without any additive; RIF, loaded with 1 g of rifampin; AA, positive control loaded with ascorbic acid; HQ, positive control loaded with hydroquinone.
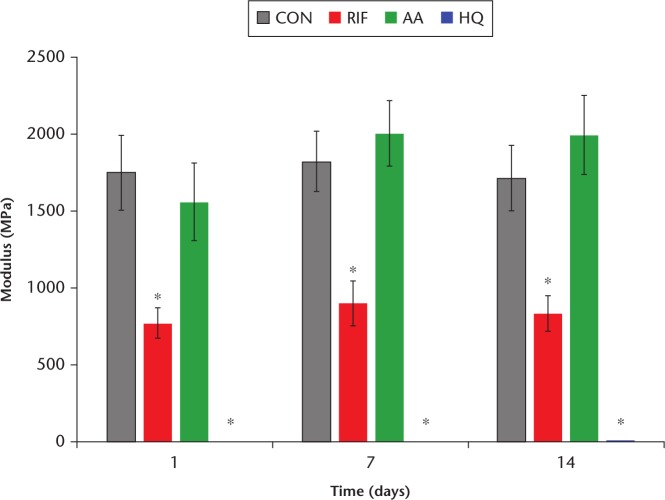
The modulus for each composite group is shown in Figure 4. The moduli of CON and AA were not significantly different from one another over the course of 14 days. The modulus of RIF was significantly lower than that of CON over the course of 14 days. The HQ showed very low moduli over the course of 14 days.
Fig. 4.
Chart summarizing the compressive modulus values. An asterisk (*) denotes a significant difference from control, p < 0.05. Ten specimens were tested from each group. CON, negative control without any additive; RIF, loaded with 1 g of rifampin; AA, positive control loaded with ascorbic acid; HQ, positive control loaded with hydroquinone.
Radical scavenging testing
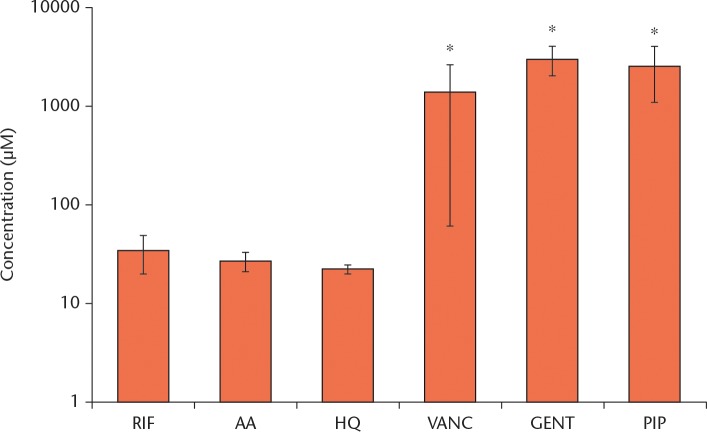
The radical scavenging results for each analyte are presented in Figure 5. Both positive controls (AA and HQ) behaved as potent radical scavengers at low concentrations (EC50). Likewise, RIF exhibited potent scavenging, although not quite as much as AA and HQ. The VANC and GENT were unreactive towards the DPPH radical. 1-methylpiperazine, while known to function as a radical scavenger, did not show an appreciable effect at comparable concentrations.
Fig. 5.
Chart summarizing the computed EC50 results. An asterisk (*) denotes a significant difference from rifampin (RIF). Three specimens were tested from each group. AA, ascorbic acid; HQ, hydroquinone; VANC, vancomycin; GENT, gentamicin; PIP, 1-methylpiperazine.
Surface morphologies
The results for CON and RIF are displayed in Figure 6. Qualitatively, the surface topography of CON appears to be solid without major pores and is generally homogeneous. Conversely, the surface of RIF is inconsistent and contains major structural defects in the form of deep pores and rifts.
Fig. 6.

a) Morphology of the surface of CON (negative control without any additive). Magnified ×100. 115 mm × 89 mm (96 × 96 DPI). b) Morphology of the surface of RIF (loaded with 1 g of rifampin) specimen. Magnified ×100. 114 mm × 89 mm (96 × 96 DPI).
Volatile monomer weight loss
The CON and RIF cements lost 0.40 g and 2.42 g over 24 hours, respectively, representing a 500% increased loss in volatile monomer from RIF. The CON showed no appreciable weight loss after 20 minutes. The RIF weight loss continued over the course of 24 hours, revealing a steady release of monomer (Supplementary Fig. a).
Discussion
The effect of rifampin on the polymerization of PMMA bone cement has been the subject of previous studies.17,19 However, in these studies, only limited attention was given to both elucidating the mechanism involved in the interaction between the materials and determining clinically relevant properties of the rifampin-loaded cement. These aspects are covered in the present work. Regarding the cement curing, td and tset of RIF were each significantly higher than the corresponding value for CON. Over the course of 14 days, the compressive strength of RIF was significantly lower than that of CON. The reduction both in compressive strength and modulus from the addition of 1 g of rifampin to the cement is not merely an artefact of the space occupied by the antibiotic, as this reduction is not seen with similar loading of other antibiotics.28,29 Hydroquinone and rifampin gave similarly low EC50 values, representing potent radical scavenging activity. Thus, the hydroquinone moiety within rifampin acts as a radical scavenger during PMMA polymerization. It is directly responsible for the prolonged curing time, reduced mechanical strength, and observed surface porosity of RIF stemming from incomplete cement polymerization. Furthermore, the effect of partial cement polymerization on toxic MMA release from RIF has not been quantified previously in the literature. Our results show that, over the course of 24 hours, a substantial amount of MMA was released from the RIF specimen.
The addition of ascorbic acid had no appreciable adverse effect on the handling and curing properties of the cement with respect to control. Interestingly, the DPPH assay revealed a similar EC50 value for ascorbic acid compared with both hydroquinone and rifampin, highlighting its known nature as a potent radical scavenger. While AA and CON showed similar curing properties, the compressive strength of AA was significantly lower but remained above 70 MPa. Investigation into this discrepancy revealed solubility issues within the curing cement. Specifically, the high water solubility of the ascorbic acid could have limited its ability to quench the radical chain in the hydrophobic PMMA matrix.
The present study highlights the detrimental effects of incorporating rifampin into PMMA bone cement. Although the systemically administered combination therapy of rifampin and another antibiotic is common, rifampin is seldom used alone.14 Rifampin monotherapy has been linked to a high likelihood of the emergence of resistant pathogens, two of which are the most common orthopaedic infection-causative organisms: S. aureus and S. epidermis.30-32 While the incorporation of a second antibiotic, in addition to rifampin, into PMMA bone cement may alleviate some of the concerns of pathogen resistance, the presence of rifampin may still cause the adverse effects on the cement presented in this study.
Other studies have investigated ways to overcome the deleterious effects of rifampin incorporation in commercial PMMA bone cement. One approach is the encapsulation of rifampin before it is mixed with the cement. Sanz-Ruiz et al33 encapsulated rifampin and reported effective elution of the antibiotic while preserving the mechanical properties of PMMA. Similar results were reported with the use of rifampin-filled cyclodextrin (CD) microparticles. Cyphert et al34 found that the inclusion of CD microparticles in PMMA resulted in similar mechanical properties to the PMMA control and may serve as a refillable antibiotic delivery vehicle. While encapsulation of rifampin is a potential technique that may be used to include this drug in a commercial product, it is not currently an option for the orthopaedic surgeon in the operating theatre unless encapsulated rifampin is made available as a separate product. Additional investigations have included using alternative delivery vehicles, such as bioresorbable carriers like sponges and calcium sulphate, which utilize compositions other than PMMA and alleviate the concern of polymerization inhibition.35,36 While these bioresorbable materials have the potential to release therapeutic levels of rifampin, they have often been limited to non-weight-bearing applications.
In conclusion, the results of the present study demonstrate that the hydroquinone moiety within rifampin interferes with the polymerization of PMMA bone cement, resulting in significantly prolonged cement curing and setting times. This substantial delay represents a severe disadvantage to surgeons by increasing operating time and reducing the cement’s handleability.37 As a result, it impacts the healthcare economy negatively and results in a longer operating time, which is correlated with a higher risk of infection when involving invasive procedures such as joint arthroplasties.38-41 Additionally, compared with the cement without rifampin, the compressive strength and modulus of the rifampin-loaded cement are significantly lower, and the amount of unreacted monomer loss is substantially higher. The lattermost characteristic potentially poses a threat to the health of the patient and of the operating room staff. Toxic occupational exposure to MMA is scarce in orthopaedic literature but common in dental literature, where olfactory lesions and local tissue necrosis are reported.42 These findings suggest that the use of rifampin-incorporated PMMA bone cement in its current form should be discouraged for both weight-bearing and non-weight-bearing applications in orthopaedics.
Acknowledgements
The authors would like to thank Connor Demott for developing custom testing equipment for gathering exothermic data.
Footnotes
Author contributions: G. A. Funk: Designed the study, Collected the data, Wrote and edited the manuscript.
E. M. Menuey: Collected the data, Edited the manuscript.
K. A. Cole: Collected the data, Edited the manuscript.
T. P. Schuman: Edited the manuscript.
K. V. Kilway: Designed the study, Edited the manuscript.
T. E. McIff: Designed the study, Edited the manuscript.
Follow us @BoneJointRes
Supplementary Material
Line graph quantifying the amount of volatile monomer leaching from CON (negative control without any additive) and RIF (loaded with 1 g of rifampin) cements over the course of 24 hours.
Funding statement
This work was supported by the Marc A. and Elinor J. Asher Orthopedic Research Endowment.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Matar WY, Jafari SM, Restrepo C, et al. Preventing infection in total joint arthroplasty. J Bone Joint Surg [Am] 2010;92-A(Suppl 2):36-46. [DOI] [PubMed] [Google Scholar]
- 2. Ong KL, Kurtz SM, Lau E, et al. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty 2009;24(6 Suppl):105-109. [DOI] [PubMed] [Google Scholar]
- 3. Kurtz SM, Ong KL, Lau E, et al. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res 2010;468:52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthoplasty 2012;27(8 Suppl):61-65.e1. [DOI] [PubMed] [Google Scholar]
- 5. Wininger DF, Fass RJ. Antibiotic-impregnated cement and beads. Antimicrob Agents Chemother 1996;40:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic joint infections. N Engl J Med 2004;351:1645-1654. [DOI] [PubMed] [Google Scholar]
- 7. Tsai JC, Sheng WH, Lo WY, Jiang CC, Chang SC. Clinical characteristics, microbiology, and outcomes of prosthetic joint infection in Taiwan. J Microbiol Immunol Infect 2015;48:198-204. [DOI] [PubMed] [Google Scholar]
- 8. Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local antibiotic therapy in osteomyelitis. Semin Plast Surg 2009;23:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLaren AC. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res 2004;427:101-106. [DOI] [PubMed] [Google Scholar]
- 10. Frew NM, Cannon T, Nichol T, Smith T, Stockley I. Comparison of the elution properties of commercially available gentamicin and bone cement containing vancomycin with ‘home-made’ preparations. Bone Joint J 2017;99-B:73-77. [DOI] [PubMed] [Google Scholar]
- 11. Stoodley P, Ehrlich GD, Sedghizadeh PP, et al. Orthopaedic biofilm infections. Curr Orthop Pract 2011;22:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zimmerli W, Sendi P. Orthopaedic biofilm infections. APMIS 2017;125:353-364. [DOI] [PubMed] [Google Scholar]
- 13. Coiffier G, Albert JD, Arvieux C, Guggenbuhl P. Optimizing combination rifampin therapy for staphylococcal osteoarticular infections. Joint Bone Spine 2013;80:11-17. [DOI] [PubMed] [Google Scholar]
- 14. Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev 2010;23:14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013;56:e1-e25. [DOI] [PubMed] [Google Scholar]
- 16. Sanchez CJ, Jr, Shiels SM, Tennent DJ, et al. Rifamycin derivatives are effective against staphylococcal biofilms in vitro and elutable from PMMA. Clin Orthop Relat Res 2015;473:2874-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shiels SM, Tennent DJ, Akers KS, Wenke JC. Determining potential of PMMA as a depot for rifampin to treat recalcitrant orthopaedic infections. Injury 2017;48:2095-2100. [DOI] [PubMed] [Google Scholar]
- 18. Rose WE, Otto DP, Aucamp ME, Miller Z, de Villiers MM. Prevention of biofilm formation by methacrylate-based copolymer films loaded with rifampin, clarithromycin, doxycycline alone or in combination. Pharm Res 2015;32:61-73. [DOI] [PubMed] [Google Scholar]
- 19. McPherson EJ, Portugal D. Deactivation of Palacos R Bone cement with the addition of rifampin antibiotic powder an in-vivo experience -case report. Reconstr Rev 2011;1:34-36. [Google Scholar]
- 20. Tomiyama T, Shoji A, Kataoka K, et al. Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J Biol Chem 1996;271:6839-6844. [DOI] [PubMed] [Google Scholar]
- 21. Pozdeeva NN, Denisov ET. Mechanism of hydroquinone-inhibited oxidation of acrylic acid and methyl methacrylate. Kinet Catal 2011;52:506-512. [Google Scholar]
- 22. Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr 1991;54:1119S-1124S. [DOI] [PubMed] [Google Scholar]
- 23. Vaishya R, Chauhan M, Vaish A. Bone cement. J Orthop Trauma 2013;4:157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. No authors listed. ASTM International. ASTM F451 – 16: Standard specification for acrylic bone cement, 2016. https://www.astm.org/Standards/F451.htm (date last accessed 13 December 2018).
- 25. Shimamura T, Sumikura Y, Yamazaki T, et al. Applicability of the DPPH assay for evaluating the antioxidant capacity of food additives - inter-laboratory evaluation study -. Anal Sci 2014;30:717-721. [DOI] [PubMed] [Google Scholar]
- 26. Blois M. Antioxidant determinations by the use of a stable free radical. Nature 1958;181:1199-1200. [Google Scholar]
- 27. Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. Lebensm Wiss Technol 1997;30:609-615. [Google Scholar]
- 28. Gálvez-López R, Peña-Monje A, Antelo-Lorenzo R, et al. Elution kinetics, antimicrobial activity, and mechanical properties of 11 different antibiotic loaded acrylic bone cement. Diagn Microbiol Infect Dis 2014;78:70-74. [DOI] [PubMed] [Google Scholar]
- 29. Duey RE, Chong ACM, McQueen DA, et al. Mechanical properties and elution characteristics of polymethylmethacrylate cone cement impregnated with antibiotics for various surface area and volume constructs. Iowa Orthop J 2012;32:104-115. [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmerli W, Malisano L, Smitham PJ, Okamoto K, Walsh WR. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections. JAMA 1998;279:1537-1541. [DOI] [PubMed] [Google Scholar]
- 31. Akgün D, Trampuz A, Perka C, Renz N. High failure rates in treatment of streptococcal periprosthetic joint infection. Bone Joint J 2016;99-B:653-659. [DOI] [PubMed] [Google Scholar]
- 32. Wi YM, Greenwood-Quaintance KE, Brinkman CL, et al. Rifampicin resistance in Staphylococcus epidermidis: molecular characterisation and fitness cost of rpoB mutations. Int J Antimicrob Agents 2018;51:670-677. [DOI] [PubMed] [Google Scholar]
- 33. Sanz-Ruiz P, Carbo-Laso E, Del Real-Romero JC, et al. Microencapsulation of rifampicin: a technique to preserve the mechanical properties of bone cement. J Orthop Res 2018;36:459-466. [DOI] [PubMed] [Google Scholar]
- 34. Cyphert EL, Learn GD, Hurley SK, Lu CY, von Recum HA. An additive to PMMA bone cement enables postimplantation drug refilling, broadens range of compatible antibiotics, and prolongs antimicrobial therapy. Adv Healthc Mater 2018;7:e1800812. [DOI] [PubMed] [Google Scholar]
- 35. Wells CM, Beenken KE, Smeltzer MS, et al. Ciprofloxacin and rifampin dual antibiotic-loaded biopolymer chitosan sponge for bacterial inhibition. Mil Med 2018;183(Suppl 1):433-444. [DOI] [PubMed] [Google Scholar]
- 36. Aiken SS, Cooper JJ, Florance H, Robinson MT, Michell S. Local release of antibiotics for surgical site infection management using high-purity calcium sulfate: an in vitro elution study. Surg Infect (Larchmt) 2015;16:54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caraan NA, Windhager R, Webb J, Zentgraf N, Kuehn KD. Role of fast-setting cements in arthroplasty: a comparative analysis of characteristics. World J Orthop 2017;8:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yates P, Serjeant S, Rushforth G, Middleton R. The relative cost of cemented and uncemented total hip arthroplasties. J Arthroplasty 2006;21:102-105. [DOI] [PubMed] [Google Scholar]
- 39. Griffiths EJ, Stevenson D, Porteous MJ. Cost savings of using a cemented total hip replacement. J Bone Joint Surg [Br] 2012;94-B:1032-1035. [DOI] [PubMed] [Google Scholar]
- 40. Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008;466:1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pedersen AB, Svendsson JE, Johnsen SP, Riis A, Overgaard S. Risk factors for revision due to infection after primary total hip arthroplasty: a population-based study of 80,756 primary procedures in the Danish Hip Arthroplasty Registry. Acta Orthop 2010;81:542-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gosavi SS, Gosavi SY, Alla RK. Local and systemic effects of unpolymerised monomers. Dent Res J (Isfahan) 2010;7:82-87. [PMC free article] [PubMed] [Google Scholar]