Abstract
Prostate cancer (PCa) preferentially metastasizes to bone, leading to complications including severe pain, fractures, spinal cord compression, bone marrow suppression, and a mortality of ∼70%. In spite of recent advances in chemo-, hormonal, and radiation therapies, bone-metastatic, castrate-resistant PCa is incurable. PCa is somewhat unique among the solid tumors in its tendency to produce osteoblastic lesions composed of hypermineralized bone with multiple layers of poorly organized type I collagen fibrils that have reduced mechanical strength. Many of the signaling pathways that control normal bone homeostasis are at play in pathologic PCa bone metastases, including the receptor activator of nuclear factor-κB/receptor activator of nuclear factor-κB ligand/osteoprotegerin system. A number of PCa-derived soluble factors have been shown to induce the dysfunctional osteoblastic phenotype. However, therapies directed at these osteoblastic-stimulating proteins have yielded disappointing clinical results to date. One of the soluble factors expressed by PCa cells, particularly in bone metastases, is prostatic acid phosphatase (PAP). Human PAP is a prostate epithelium-specific secretory protein that was the first tumor marker ever described. Biologically, PAP exhibits both phosphatase activity and ecto-5′-nucleotidase activity, generating extracellular phosphate and adenosine as the final products. Accumulating evidence indicates that PAP plays a causal role in the osteoblastic phenotype and aberrant bone mineralization seen in bone-metastatic, castrate-resistant PCa. Targeting PAP may represent a therapeutic approach to improve morbidity and mortality from PCa osteoblastic bone metastases.
Keywords: prostate cancer, osteoblasts, osteoblastic lesions, bone metastases
Prostate cancer (PCa) is the most commonly diagnosed male malignancy in developed countries. Although the prevalence of PCa is high, only a fraction of these cases metastasizes and results in mortality [1]. Although the 5-year survival rate for localized PCa is close to 100%, metastatic, castrate-resistant PCa (mCRPC) has a high mortality rate. Even with the use of newly approved chemo-, hormonal, and radioisotope therapies, ~30,000 deaths will be caused by mCRPC in 2018, according to the American Cancer Society [2]. Ninety percent of men with mCRPC have bone-metastatic disease (bmCRPC) [3].
PCa preferentially metastasizes to bone, leading to complications including severe pain, fractures, spinal cord compression, bone marrow suppression, and a mortality of ∼70% [4, 5]. In spite of recent advances in direct antitumor therapy for advanced disease, bmCRPC is presently an incurable disorder. Over the past several decades, bone-targeted therapies have been used to interrupt the crosstalk between PCa and bone cells. PCa bone lesions have an initial and ongoing osteolytic component similar to other solid tumor-bone metastases. PCa is somewhat unique in its tendency to produce osteoblastic (OB) lesions. Increased OB activity leads to increased bone formation; however, the process is erratic and culminates in a structurally weaker product that is more prone to pathological fractures [6, 7].
Osteoclast-targeting drugs are currently approved for the prevention of skeletal-related events associated with PCa bone metastases [8, 9]. However, these therapies have not been shown to prolong overall survival. A better understanding of the PCa and bone-derived factors that contribute to the lethal phenotype of bmCRPC, particularly those associated with OB lesions, is needed to develop treatments that will reduce morbidity and improve overall survival.
Metastasis involves multiple steps including invasion, colonization, and proliferation of PCa cells in the bone microenvironment. As first described by Stephen Paget [10], the factors that control bone targeting and PCa growth in bone are derived from both the seed (PCa) and the soil (bone cells). Once PCa cells are established in bone, they interact with osteoclasts; osteoblasts and osteocytes set up a vicious cycle that ultimately results in OB lesions composed of hypermineralized bone with multiple layers of poorly organized type I collagen fibrils that have reduced mechanical strength [11]. Activated osteoblasts, in turn, stimulate PCa growth in bone. There are multiple secretory factors, steroid hormones, and steroid hormone receptors that influence this crosstalk [12]. In addition, secretory kinases and phosphatases may affect the aberrant mineralization seen in these lesions by increasing phosphate and calcium availability and/or modulating the activity of key proteins involved in bone mineralization.
1. Methods
A literature search was conducted using PubMed and Google Scholar, up to 1 December 2018, to identify studies that investigated mechanisms of PAP, osteoblastic bone metastases and management in prostate cancer. The following keywords were used in the search: prostate cancer and bone metastases, osteoblastic, osteolytic, animal models, PAP, receptor activator of nuclear factor-κB (RANK) ligand (RANKL), or osteoprotegerin (OPG). Search results were manually curated, evaluated for relevance, and recorded. Publications referenced were selected based on the quality scientific methodology and relevance.
2. Prostate Cancer Osteoblastic Bone Metastases
A. Models of PCa OB Bone Metastases
Preclinical models that faithfully reproduce PCa bone lesions are essential to gaining a functional understanding of bone-PCa interactions. Existing xenograft models of PCa bone metastases have been developed through a variety of injection approaches to study the processes of bone targeting (i.e., lateral tail vein, intracardiac, orthotopic, femoral artery injection) and PCa growth/bone reaction (i.e., intraosseous injection). The model systems that study bone targeting have been less successful than the intraosseous model that recapitulates the final phase of PCa disease progression. For example, PC3 cells have a 20% to 89% take rate in bone after intracardiac injection, and 19% after intravenous injection compared with 60% to 100% after intraosseous injection [13]. The intraosseous technique has proven to be a reproducible approach for studying tumor-bone interactions and factors governing the bone reaction. Table 1 summarizes the distinguishing features of human PCa xenograft models using intraosseous injection of bone metastases [14–20].
Table 1.
Intraosseous Xenograft Models of Prostate
| Cell Line | Origin | Type of Bone Metastasis | Androgen Sensitive | PSA | PAP | CK | Model System | References |
|---|---|---|---|---|---|---|---|---|
| VCaP | Vertebral metastasis from 59-y-old Caucasian male | Osteoblastic | Yes | Yes | Yes | 8 and 18 | Intraosseous SCID models | Korenchuk et al. [14] |
| LNCaP | Supraclavicular lymph node metastasis from 50-y-old Caucasian male | Osteoblastic | Yes | Yes | Yes | 8 and 18 | Intracardiac, intraosseous, orthotopic NOD- SCID, and SCID models | Horoszewicz et al. [15], Thalmann et al. [16], Wu et al. [17], Simmons et al. [18] |
| LNCaP-C4-2B | Highly metastatic variant of LNCaP; isolated from LNCaP xenograft metastatic lesion | Osteoblastic | Yes | Yes | Yes | 8 | Intraosseous and subcutaneous NOD-SCID and SCID models | Thalmann et al. [16], Wu et al., Simmons et al. [18] |
| DU145 | Brain metastasis from 69-y-old Caucasian male | Osteolytic | No | No | Yes | 8 | Intraosseous NOD-SCID and SCID models | Stone et al. [19] |
| PC3 | Bone metastasis from 62-y-old Caucasian male | Osteolytic | No | No | No | 7, 8, 18, 19 | Intracardiac, intraosseous, intravenous, orthotopic, and subcutaneous SCID and NOD-SCID models | Simmons et al. [18], Kaighn et al. [20] |
| PC3M | Highly metastatic variant of PC3; isolated from PC3 xenograft metastatic lesion | Osteolytic | No | No | No | 18 | Intracardiac, orthotopic, and intraosseous NOD-SCID models | Simmons et al. [18] |
Of the available cell lines and xenograft models, the one that most closely reproduces bmCRPC in patients is intratibial inoculation of the VCaP cell line into immunocompromised mice. VCaP cells maintain androgen receptor, prostate-specific antigen (PSA), and prostatic acid phosphatase (PAP) expression, and induce an OB phenotype [14]. In addition, VCaP cells harbor a chromosomal gene rearrangement of transmembrane protease serine 2 (TMPRSS2) that contains an androgen-responsive promoter and the ETS-related gene (ERG). This gene rearrangement leads to androgen-regulated expression of the oncogene ERG that has been demonstrated in >50% of human PCa cases. Several clinical studies suggest that TMPRSS2:ERG fusions are associated both with poorly differentiated samples (Gleason score >6) and with disease recurrence after surgery. Further, TMPRSS2:ERG fusion has been reported to enhance the ability of PCa cell lines to induce OB lesions by stimulating bone formation and inhibiting the osteolytic response [21].
B. The Role of the RANK/RANKL/Osteoprotegerin Signaling Pathway in PCa Bone Metastases
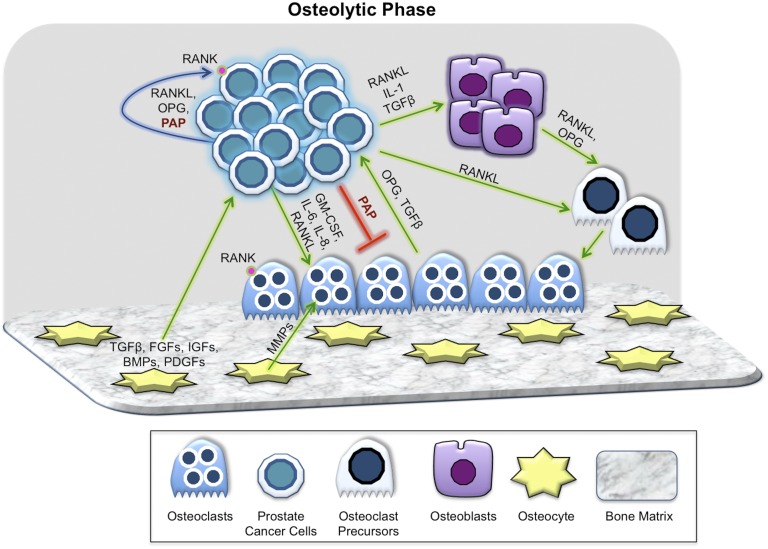
Many of the factors that control bone homeostasis are at play in pathologic PCa bone metastases. In normal bone remodeling, osteoblasts mediate osteoclast differentiation and function by secreting (i) RANKL, which induces osteoclast differentiation when bound to the RANK receptor on osteoclasts; and (ii) OPG, a decoy receptor that binds to RANKL and inhibits its activity. Previous reports demonstrate that PCa cells in bone express RANK, RANKL, and OPG and communicate directly with bone cells via this same signaling pathway [22]. High RANKL in the bone microenvironment favors osteolysis and promotes PCa growth in bone. Osteolysis releases PCa growth-promoting factors from the bone matrix including TGF-β, IGF-1, matrix metalloproteinases (MMPs), fibroblast growth factors (FGFs), bone morphogenic proteins (BMPs), and platelet-derived growth factors (Fig. 1). The RANK/RANKL/OPG pathway is also involved in OB bone metastases. High levels of OPG are associated with end-stage OB bone metastases, and serum OPG levels are the most reliable indicator of such lesions (Fig. 2). Recently, it has been shown that maturing osteoclasts secrete vesicular RANK that binds to OB RANKL and promotes bone formation by triggering RANKL reverse signaling and activating Runt-related transcription factor 2 [23]. It is plausible that vesicular RANK derived from PCa cells in bone has a similar effect on pathologic bone formation in OB PCa metastases.
Figure 1.
Key players in the osteolytic phase of PCa bone metastases. Prostate cancer tumor cells interact with all of the components of bone including osteoblasts, osteoclasts, osteocytes, and bone matrix though paracrine (green arrow), autocrine (blue arrow), and inhibitory (red line) mechanisms. PCa cells secrete osteoclast-activating factors, such as RANKL, that activate bone resorption. Osteolysis releases PCa growth-promoting factors from the bone matrix, including TGF-β, IGF-1, MMPs, FGFs, BMPs, and PDGFs. A variety of other cancer-derived and bone cell–derived secretory factors contribute to the initial and ongoing osteolytic phase of PCa bone metastases. PAP secreted by PCa cells in bone indirectly inhibits osteoclasts by decreasing RANKL, while increasing OPG in the bone niche. PDGF, platelet-derived growth factor.
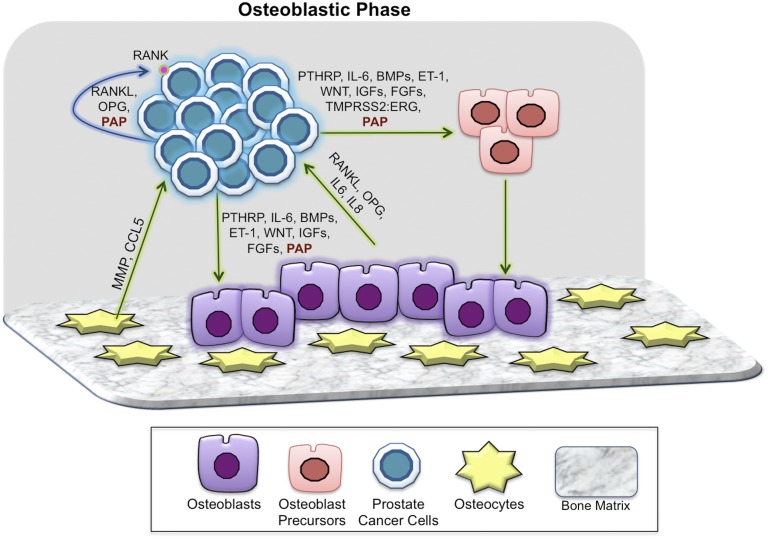
Figure 2.
Key players in the osteoblastic phase of prostate cancer bone metastases. In the osteoblastic phase, PCa cells continue to interact with all of the components of bone, although paracrine (green arrow) and autocrine (blue arrow) mechanisms as noted. A number of PCa-derived soluble factors such as ET-1, WNTs, TGF-β, uPA, IGF-1, FGFs, and BMPs have been shown to induce the dysfunctional osteoblastic phenotype. High levels of OPG are associated with end-stage OB bone metastases. PAP secreted by PCa cells in bone has both autocrine and paracrine effects that coordinately result in higher OPG/RANKL in the bone niche, resulting in osteoblastic lesions. ET-1, endothelin-1.
C. PCa-Derived Factors That Promote the OB Phenotype
A number of PCa-derived soluble factors such as endothelin-1, IGF-1, WNTs, TGF-β, urokinase-type plasminogen activator (uPA), FGFs, and BMPs have been shown to induce the dysfunctional OB phenotype [24–30]. In addition, a high OPG state inhibits osteolysis and promotes PCa cell survival (Fig. 2). However, therapies directed at these OB-stimulating factors have yielded disappointing clinical results to date. Mortality from PCa can be directly linked to these OB lesions, both in terms of the stimulatory effect of activated osteoblasts on PCa growth in bone as well as the destructive effect of hypermineralized bone on the bone marrow.
3. The Role of PAP in Osteoblastic Bone Metastases
A. PAP and Osteoblast Activation
One of the soluble factors expressed by PCa cells, particularly in bone metastases, is PAP. Human PAP is a prostate epithelium-specific secretory protein that is found in large amounts in seminal fluid (1 mg/mL). The precise physiologic function of PAP has not been delineated, although given the very high levels in seminal fluid, it is thought to play a role in male reproduction. PAP was the first human tumor marker ever described in 1936 [31] in a publication that demonstrated high PAP levels in patients with PCa OB bone metastases. Huggins and Hodges [32] reported that castration dramatically reduced PCa OB bone metastases and concomitantly lowered serum PAP levels. Although PSA has largely replaced PAP as a tumor marker, PAP has been shown to be an important prognostic marker in advanced PCa [33, 34]. PAP is a phosphotyrosyl-protein phosphatase [35] with multiple phosphomonoester substrates that functions at an optimum pH range of 4.0 to 6.0 [36]. There are two different isoforms of PAP that result from alternate splicing of the same gene product: (i) a secreted isoform and (ii) a transmembrane isoform [37]. The secreted enzyme PAP is a glycoprotein, nonspecific tyrosine phosphatase, constituted in its active form as a 100-kDa dimer composed of two subunits of 50 kDa [38]. Biologically, PAP exhibits both phosphatase activity [39] and ecto-5′-nucleotidase activity [40], generating extracellular phosphate [41] and adenosine [42] as the final products.
A possible causal role for PAP in OB bone metastases was postulated when it was demonstrated that PAP stimulated collagen synthesis and alkaline phosphatase content of isolated bone cells [43]. Our group demonstrated that PAP is highly expressed in human PCa bone metastases, even after castration therapy, and stimulates preosteoblast proliferation and differentiation [44]. Our data further indicate that PAP effects on bone metastases are mediated in an autocrine and paracrine fashion by modulation of the RANK/RANKL/OPG system. Knockdown or overexpression of PAP in VCaP or PC3M cell lines, respectively, altered the bone phenotype and tumor growth in bone by disrupting the balance between OPG/RANKL in favor of OPG, resulting in an OB bone reaction. These findings suggest that PAP secreted by PCa cells in bone has both autocrine and paracrine effects that coordinately result in OB lesions [45].
B. PAP and Mineralization
The PAP gene, acid phosphatase prostate, is expressed in various tissues, including the testes and oviducts of chickens. Eggshell formation occurs in the specialized shell gland region of the chicken oviduct. The process of shell mineralization in hens is very similar to that of bone mineralization in humans, occurring in stages that include the formation of calcium carbonate depositions, its aggregation as crystals, and its organization by organic matrix. Comparison of gene expression in the shell glands from juvenile and laying hens demonstrated genes for which there was no a priori expectation of differential gene expression including the PAP gene acid phosphatase prostate [46]. In addition, quantitative proteomics to study key stages of shell mineralization revealed that PAP protein expression was overabundant throughout the process of shell formation [47]. Similarly, PAP induced calcium deposit formation in a human osteoblast cell line [48], suggesting that PAP directly stimulates bone mineralization.
C. Possible Mechanisms Underlying PAP Effects on Bone Mineralization
Bone formation involves the synthesis of collagenous organic matrix (by osteoblasts) followed by mineralization of the matrix [49] through formation of hydroxyapatite-based mineral (calcium and phosphate). Small integrin-binding ligand, N-linked glycoproteins (SIBLING) proteins generally serve to inhibit bone mineralization. The SIBLING proteins include osteopontin, bone sialoprotein, dentin matrix protein 1, dentin sialophosphoprotein, and matrix extracellular phosphoglycoprotein [50].
When SIBLING proteins are phosphorylated by the secretory kinase FAM20C [51], mineralization is suppressed. The inactivation of FAM20C kinase leads to dephosphorylation of SIBLING proteins, resulting in abnormal bone mineralization [52]. Inactivation of a secretory kinase (e.g., FAM20C) might produce the same phenotype as increased activity of a secretory phosphatase (e.g., PAP): dephosphorylation of key sites on SIBLING proteins leading to increased bone mineralization. In addition, via its ecto-5′-nucleotidase activity, PAP generates both extracellular phosphate and adenosine as final products. Adenosine has been reported to increase bone formation via G-protein coupled receptors on osteoblasts and osteoclasts, specifically adenosine receptor types 2A and 2B [53, 54].
Taken together, there are at least three possible mechanisms whereby increased PAP in the OB niche might lead to aberrantly mineralized bone: (i) increased generation of extracellular adenosine that stimulates bone formation via adenosine receptor types 2A and 2B; (ii) increased availability of local phosphate; and (iii) dephosphorylation of key sites on SIBLING proteins. All of these would serve to increase formation of hydroxyapatite, inducing bone formation [55] and mineralization [56], and additionally restricting bone resorption [57].
4. Summary and Conclusions
bmCRPC is presently an incurable disease. The majority of bmCRPCs are OB with increased, aberrant bone mineralization. A variety of PCa and bone cell–derived factors have been demonstrated to play a role in the destructive crosstalk in advanced lesions, but targeting them has not led to improvements in overall survival. PAP, the first tumor marker ever described, is highly expressed in bmCRPC. Accumulating evidence demonstrates that PAP plays a causal role in OB bone metastases by increasing bone formation and mineralization. Because both secretory and transmembrane forms of PAP are highly expressed in advanced disease, PAP may be a viable therapeutic target in OB PCa bone metastases.
Acknowledgments
Financial Support: This work was supported by Prostate Action Inc., and the Loan Repayment Program, National Center for Advancing Translational Sciences, National Institutes of Health (to S.I.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- bmCRPC
metastatic, castrate-resistant prostate cancer with bone-metastatic disease
- BMP
bone morphogenic proteins
- ERG
ETS-related gene
- FGF
fibroblast growth factors
- mCRPC
metastatic, castrate-resistant prostate cancer
- MMP
matrix metalloproteinase
- OB
osteoblastic
- OPG
osteoprotegerin
- PCa
prostate cancer
- PAP
prostatic acid phosphatase protein
- PSA
prostate-specific antigen
- RANK
receptor activator of nuclear factor-κB
- RANKL
receptor activator of nuclear factor-κB ligand
- SIBLING
small integrin-binding ligand, N-linked glycoprotein
- TMPRSS2
transmembrane protease serine 2
- uPA
urokinase-type plasminogen activator
References and Notes
- 1. Giles GG. Prostate Cancer, International Encyclopedia of Public Health, 2nd ed, St. Louis: Elsevier; 2017:51–59. [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3. Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–583. [DOI] [PubMed] [Google Scholar]
- 4. Randall RL. A promise to our patients with metastatic bone disease. Ann Surg Oncol. 2014;21(13):4049–4050. [DOI] [PubMed] [Google Scholar]
- 5. Nieder C, Haukland E, Pawinski A, Dalhaug A. Anaemia and thrombocytopenia in patients with prostate cancer and bone metastases. BMC Cancer. 2010;10(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abrams HL. Skeletal metastases in carcinoma. Radiology. 1950;55(4):534–538. [DOI] [PubMed] [Google Scholar]
- 7. Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6213s–6216s. [DOI] [PubMed] [Google Scholar]
- 8. Berry S, Waldron T, Winquist E, Lukka H. The use of bisphosphonates in men with hormone-refractory prostate cancer: a systematic review of randomized trials. Can J Urol. 2006;13(4):3180–3188. [PubMed] [Google Scholar]
- 9. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Netastasus Rev. 1989;8(2):98–101. [PubMed]
- 11. Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies [published correction appears in Nat Rev Clin Oncol. 2011;8(10):568]. Nat Rev Clin Oncol. 2011;8(6):357–368. [DOI] [PubMed] [Google Scholar]
- 12. Liu X-H, Kirschenbaum A, Yao S, Liu G, Aaronson SA, Levine AC. Androgen-induced Wnt signaling in preosteoblasts promotes the growth of MDA-PCa-2b human prostate cancer cells. Cancer Res. 2007;67(12):5747–5753. [DOI] [PubMed] [Google Scholar]
- 13. Berish RB, Ali AN, Telmer PG, Ronald JA, Leong HS. Translational models of prostate cancer bone metastasis. Nat Rev Urol. 2018;15(7):403–421. [DOI] [PubMed] [Google Scholar]
- 14. Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R, Lin DL, Pienta KJ. VCaP, a cell-based model system of human prostate cancer. In Vivo. 2001;15(2):163–168. [PubMed] [Google Scholar]
- 15. Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line--a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 16. Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW.. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44(2):91–103. [DOI] [PubMed]
- 17. Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77(6):887–894. [DOI] [PubMed] [Google Scholar]
- 18. Simmons J, Elshafae S, Keller E, McCauley L, Rosol T. Review of animal models of prostate cancer bone metastasis. Vet Sci. 2014;1(1):16–39. [Google Scholar]
- 19. Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer. 1978;21(3):274–281. [DOI] [PubMed] [Google Scholar]
- 20. Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17(1):16–23. [PubMed] [Google Scholar]
- 21. Delliaux C, Tian TV, Bouchet M, Fradet A, Vanpouille N, Flourens A, Deplus R, Villers A, Leroy X, Clézardin P, de Launoit Y, Bonnelye E, Duterque-Coquillaud M. TMPRSS2:ERG gene fusion expression regulates bone markers and enhances the osteoblastic phenotype of prostate cancer bone metastases. Cancer Lett. 2018;438:32–43. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107(10):1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, Kariya Y, Kato G, Tabata Y, Penninger JM, Udagawa N, Aoki K, Suzuki H. Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561(7722):195–200. [DOI] [PubMed] [Google Scholar]
- 24. Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3(2):110–116. [DOI] [PubMed] [Google Scholar]
- 25. Nordstrand A, Bergström SH, Thysell E, Bovinder-Ylitalo E, Lerner UH, Widmark A, Bergh A, Wikström P. Inhibition of the insulin-like growth factor-1 receptor potentiates acute effects of castration in a rat model for prostate cancer growth in bone. Clin Exp Metastasis. 2017;34(3-4):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hall CL, Kang S, MacDougald OA, Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97(4):661–672. [DOI] [PubMed] [Google Scholar]
- 27. Buijs JT, Stayrook KR, Guise TA. The role of TGF-β in bone metastasis: novel therapeutic perspectives. Bonekey Rep. 2012;1(6):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheng S. The urokinase-type plasminogen activator system in prostate cancer metastasis. Cancer Metastasis Rev. 2001;20(3-4):287–296. [DOI] [PubMed] [Google Scholar]
- 29. Thoma C. Prostate cancer: targeting the FGFR curbs bone metastasis. Nat Rev Urol. 2014;11(11):604. [DOI] [PubMed] [Google Scholar]
- 30. Lai T-H, Fong Y-C, Fu W-M, Yang R-S, Tang C-H. Osteoblasts-derived BMP-2 enhances the motility of prostate cancer cells via activation of integrins. Prostate. 2008;68(12):1341–1353. [DOI] [PubMed] [Google Scholar]
- 31. Gutman EB, Sproul EE, Gutman AB. Significance of increased phosphatase activity of bone at the site of osteoplastic metastases secondary to carcinoma of the prostate gland. Am J Cancer. 1936;28(3):485–495. [Google Scholar]
- 32.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;167(2):948–952. [PubMed] [Google Scholar]
- 33. Taira A, Merrick G, Wallner K, Dattoli M. Reviving the acid phosphatase test for prostate cancer. Oncology (Williston Park). 2007;21(8):1003–1010. [PubMed] [Google Scholar]
- 34. Steineck G, Kelly WK, Mazumdar M, Vlamis V, Schwartz M, Scher HI. Acid phosphatase: defining a role in androgen-independent prostate cancer. Urology. 1996;47(5):719–726. [DOI] [PubMed] [Google Scholar]
- 35. Li HC, Chernoff J, Chen LB, Kirschonbaum A. A phosphotyrosyl-protein phosphatase activity associated with acid phosphatase from human prostate gland. Eur J Biochem. 1984;138(1):45–51. [DOI] [PubMed] [Google Scholar]
- 36. Jakob CG, Lewinski K, Kuciel R, Ostrowski W, Lebioda L. Crystal structure of human prostatic acid phosphatase. Prostate. 2000;42(3):211–218. [DOI] [PubMed] [Google Scholar]
- 37. Araujo CL, Quintero IB, Ovaska K, Herrala AM, Hautaniemi S, Vihko PT. Transmembrane prostatic acid phosphatase (TMPAP) delays cells in G1 phase of the cell cycle. Prostate. 2016;76(2):151–162. [DOI] [PubMed] [Google Scholar]
- 38. Kuciel R, Bakalova A, Mazurkiewicz A, Bilska A, Ostrowski W. Is the subunit of prostatic phosphatase active? Reversible denaturation of prostatic acid phosphatase. Biochem Int. 1990;22(2):329–334. [PubMed] [Google Scholar]
- 39. Luchter-Wasylewska E. Cooperative kinetics of human prostatic acid phosphatase. Biochim Biophys Acta. 2001;1548(2):257–264. [DOI] [PubMed] [Google Scholar]
- 40. Zimmermann H. Prostatic acid phosphatase, a neglected ectonucleotidase. Purinergic Signal. 2009;5(3):273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chuang T-D, Chen S-J, Lin F-F, Veeramani S, Kumar S, Batra SK, Tu Y, Lin M-F. Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ErbB-2 and regulates prostate cancer cell growth. J Biol Chem. 2010;285(31):23598–23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ishibe M, Rosier RN, Puzas JE. Human prostatic acid phosphatase directly stimulates collagen synthesis and alkaline phosphatase content of isolated bone cells. J Clin Endocrinol Metab. 1991;73(4):785–792. [DOI] [PubMed] [Google Scholar]
- 44. Kirschenbaum A, Liu X-H, Yao S, Leiter A, Levine AC. Prostatic acid phosphatase is expressed in human prostate cancer bone metastases and promotes osteoblast differentiation. Ann N Y Acad Sci. 2011;1237(1):64–70. [DOI] [PubMed] [Google Scholar]
- 45. Kirschenbaum A, Izadmehr S, Yao S, O’Connor-Chapman KL, Huang A, Gregoriades EM, Yakar S, Levine AC. Prostatic acid phosphatase alters the RANKL/OPG system and induces osteoblastic prostate cancer bone metastases. Endocrinology. 2016;157(12):4526–4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dunn IC, Wilson PW, Lu Z, Bain MM, Crossan CL, Talbot RT, Waddington D. New hypotheses on the function of the avian shell gland derived from microarray analysis comparing tissue from juvenile and sexually mature hens. Gen Comp Endocrinol. 2009;163(1-2):225–232. [DOI] [PubMed] [Google Scholar]
- 47. Marie P, Labas V, Brionne A, Harichaux G, Hennequet-Antier C, Rodriguez-Navarro AB, Nys Y, Gautron J. Quantitative proteomics provides new insights into chicken eggshell matrix protein functions during the primary events of mineralisation and the active calcification phase. J Proteomics. 2015;126:140–154. [DOI] [PubMed] [Google Scholar]
- 48. Larson SR, Chin J, Zhang X, Brown LG, Coleman IM, Lakely B, Tenniswood M, Corey E, Nelson PS, Vessella RL, Morrissey C. Prostate cancer derived prostatic acid phosphatase promotes an osteoblastic response in the bone microenvironment. Clin Exp Metastasis. 2014;31(2):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(3, Suppl 3):S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zurick KM, Qin C, Bernards MT. Mineralization induction effects of osteopontin, bone sialoprotein, and dentin phosphoprotein on a biomimetic collagen substrate. J Biomed Mater Res A. 2013;101(6):1571–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ishikawa HO, Xu A, Ogura E, Manning G, Irvine KD. The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One. 2012;7(8):e42988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV, Dixon JE. Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science. 2012;336(6085):1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mediero A, Cronstein BN. Adenosine and bone metabolism. Trends Endocrinol Metab. 2013;24(6):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ham J, Evans BAJ. An emerging role for adenosine and its receptors in bone homeostasis. Front Endocrinol (Lausanne). 2012;3:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carroll SH, Wigner NA, Kulkarni N, Johnston-Cox H, Gerstenfeld LC, Ravid K. A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem. 2012;287(19):15718–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murshed M. Mechanism of Bone Mineralization. Cold Spring Harb Perspect Med. 2018;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN. Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum. 2010;62(2):534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]