Abstract
Objective
To determine the effects of varying doses of orally administered BPA on indices of glucose metabolism.
Methods
Eleven college students (21.0 ± 0.8 years; 24.2 ± 3.9 kg/m2) were randomized in a double-blinded, crossover fashion separated by >1 week to placebo (PL), deuterated BPA at 4 µg/kg body weight (BPA-4), and deuterated BPA at 50 µg/kg body weight (BPA-50). Total BPA, glucose, insulin, and C-peptide were assessed at baseline, minutes 15, 30, 45, 60, and every 30 minutes for 2 hours in response to a glucose tolerance test.
Results
There was a significant condition × time interaction for total BPA (P < 0.001) such that BPA increased more rapidly in BPA-50 than BPA-4 and PL (P = 0.003) and increased more rapidly in BPA-4 than PL (P < 0.001). There were no significant condition × time interactions on glucose, insulin, and C-peptide. Significant condition main effects were observed for glucose such that BPA-50 was significantly lower than PL (P = 0.036) and nearly lower for BPA-4 vs PL (P = 0.056). Significant condition main effects were observed such that insulin in BPA-50 was lower than BPA-4 (P = 0.021), and C-peptide in BPA-50 was lower than BPA-4 (t18 = 3.95; Tukey-adjusted P = 0.003). Glucose, insulin, and C-peptide areas under the curve for the 3-hour profile were significantly lower in BPA-50 vs PL (P < 0.05).
Conclusion
Orally administered BPA protocol appeared feasible and has immediate effects on glucose, insulin, and C-peptide concentrations.
Keywords: bisphenol A, diabetes, oral consumption, glucose metabolism, insulin
The prevalence of diabetes is well established, affecting >29 million Americans with 90% to 95% of these individuals diagnosed with type 2 diabetes [1], and linked to several health risks including insulin resistance [2, 3] and cardiovascular disease [4]. Clearly, diet, physical activity, and genetics play roles in the etiology of type 2 diabetes, but only explain 30% to 60% of variance [5]. Emerging data suggest the mass industry-produced chemical bisphenol A (BPA) may play a role in type 2 diabetes and obesity rates [6–17]. BPA exposure is widespread, with an estimated 93% of the US population with detectable urine levels [18]. The National Health and Nutrition Examination Survey, Nurses’ Health Study II, and other cross-sectional data have shown associations between urinary BPA concentrations and type 2 diabetes [16, 17, 19, 20], prediabetes [21], insulin resistance [22], and hemoglobin A1c [23]. BPA appears related to diabetes risk factors independent of weight status and obesity, which has also been correlated with urinary BPA levels in some but not all studies [19, 23]. Animal and in vitro data suggest that BPA has estrogenic activity [7] and disrupts several pathways in the pathogenesis of type 2 diabetes including decreased insulin sensitivity [24], dysregulation of glucose metabolism [25], altered β-cell and hepatic cell functioning [14, 25], and altered adiponectin release from adipose tissue [26].
Animal feeding studies have shown that long-term consumption of BPA induces glucose intolerance, insulin resistance, and ultimately type 2 diabetes [6, 24]. However, in humans, evidence linking BPA exposure with diabetes risk is mainly associative in nature [20, 23]. Previous well-controlled studies have determined the pharmacokinetics of oral consumption of BPA [27–29]. To our knowledge, only one other human study examined the effects of oral BPA consumption on indices of glucose metabolism in men and postmenopausal women without diabetes and showed that BPA consumption of 50 μg/kg body weight (BW) suppressed insulin and C-peptide concentrations in response to glucose infusion [30]. The objective of this pilot study was to determine the effects of varying doses of orally administered BPA on indices of glucose metabolism in nonobese adults using a randomized, double-blinded, crossover design.
1. Materials and Methods
A. Participants
Eleven (eight female and three male) young college students from California Polytechnic State University in San Luis Obispo, CA, were recruited by voluntary approach or flier on campus (Table 1) from 1 September 2016 to 1 March 2018. Eligibility included: (i) 19.7 to 29.9 (but <30) kg/m2 BMI; (ii) 20 to 23 (but <50) years old; (iii) nonsmoking; and (iv) English speaking. Exclusion criteria, assessed by a health history questionnaire, included: (i) type 2 or type 1 diabetes; (ii) cardiovascular disease or any other metabolic disease/complication; (iii) hypertension; and (iv) pregnancy or planned pregnancy. There was no previous history of obesity or other chronic disease and no family history of obesity, diabetes, or cardiovascular disease (assessed by questionnaire). The Institutional Review Board at California Polytechnic State University approved the study, and all participants gave verbal and written consent. This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Participants were compensated $30 for completing each trial for a total of $90.
Table 1.
Participant Characteristics
| Variable | Range | |
|---|---|---|
| N | 11 (8 female, 3 male) | |
| Age, y | 21.0 (0.8) | 20–23 |
| Height, m | 1.7 (0.1) | 1.6–1.8 |
| Weight, kg | 70.6 (10.0) | 59.0–95.2 |
| BMI, kg/m2 | 24.0 (3.4) | 19.7–29.9 |
| Weight status, n (%) | ||
| Normal weight | 7 (64) | |
| Overweight | 4 (36) | |
| Hispanic/Latina, yes/no, n (%) | ||
| Yes | 4 (36) | |
| No | 7 (64) |
Data are mean (SD) unless otherwise indicated.
Abbreviation: BMI, body mass index.
B. Experimental Protocol
After eligibility was determined, participants completed a demographic questionnaire, and weight was collected on digital scale and height by stadiometer. This study adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting randomized trials [31], using a randomized, double-blinded, balanced, crossover study design. The study statistician developed the within-subject randomizations with condition allocations designed as A, B, and C and then provided concealed envelopes to research staff. All research staff administering BPA to participants and collecting data were blinded to condition allocation. Participants arrived at the research center facilities in the morning after an overnight fast (10 hours) to minimize the effects of recent dietary intake on blood and urine BPA [27]. In the morning after the overnight fast, participants consumed 8 ounces of water in a nonplastic container. Participants completed a gastrointestinal distress questionnaire [32] and subjective appetite response questionnaire using a visual analog scale [33]. A catheter was then placed into a forearm vein, and a fasting blood sample was collected. Participants were then randomized, in a double-blinded, balanced, crossover fashion to oral consumption of: (i) placebo (PL), (ii) 4 μg/kg BW of deuterated 6-BPA (d6-BPA; BPA-4), and (iii) 50 μg/kg BW of d6-BPA (BPA-50). Participants rotated through all three conditions with a minimum of 1 week among the three visits. Participants were fed a vanilla wafer cookie containing the corresponding dose of PL, BPA-4, and BPA-50 using d6-BPA (d6-BPA; CDN Isotopes, Pointe-Claire, Quebec, Canada), similar to previous pharmacokinetic studies [27, 29], adjusted for BW. Single-dosing solutions (10 mg/mL) for BPA-4 and BPA-50 were prepared by dissolving d6-BPA in absolute 95% ethanol (Acros Organics, Janssen Pharmaceutical, Beerse, Belgium). For PL, d6-BPA was not included in the ethanol solution. To maintain blinding, a research assistant not involved in any other aspect of this study made the dosing solutions, which were correspondingly labeled A, B, and C. Six to 8 hours before each condition trial, aliquots were passed twice through a sterile microfilter to aid in removal of bacteria and placed onto a vanilla wafer cookie (18 calories, 2.6 g carbohydrate, 0.8 g fat, and 0.1 g protein), allowing the ethanol to dry. The doses of BPA selected in the proposed study were chosen to be consistent with safe doses established by the European Food Safety Authority and the US Environmental Protection Agency (EPA). According to the European Food Safety Authority [34] and the US EPA [35], a BPA value of 50 μg/kg BW-day (50,000 ng/kg-day) is the tolerable daily intake and reference dose considered safe dose throughout a lifetime, although recently the European Food Safety Authority has lowered this dose to 4 μg/kg BW-day [36] extrapolated from animal data. Blood samples, gastrointestinal distress questionnaire, and subjective appetite ratings using a visual analog scale were collected at baseline (prior to consumption of the vanilla wafer cookie), minutes 15, 30, 45, and 60, and then every 30 minutes for the next 2 hours in response to a 75-g oral glucose tolerance test (OGTT; Fisher Scientific, Houston, TX) under each of the three conditions. The primary outcome measures were serum total BPA, glucose, insulin, C-peptide, proinsulin, 17β-estradiol, and triglyceride concentrations. Secondary outcomes measures were gastrointestinal distress and subjective appetite ratings. Based on CONSORT guidelines, there were no participant harms or unintended effects of the three conditions on participants. Besides the vanilla wafer cookie and 75-g glucose drink, participants were not provided any other food during the conditions.
C. Biochemical Analyses
Venous blood samples were collected in sterile syringes and transferred to vacutainers containing sodium fluoride and potassium oxalate for analysis of glucose, EDTA for analysis of insulin, C-peptide, and proinsulin, and serum for analysis of total BPA, 17β-estradiol, and triglycerides. All samples were refrigerated centrifuged (4°C) at (3000g) for 15 minutes, and plasma/serum was aliquoted into polystyrene tubes and stored at −80°C until analysis. Quantitative assessment of total BPA (Detroit R&D, Inc., Detroit, MI) [37], insulin (Thermo Fisher Scientific, Grand Island, NY) [38], C-peptide (Mercodia, Uppsala, Sweden) [39], proinsulin (Mercodia) [40], and 17β-estradiol (Thermo Fisher Scientific) [41] were determined by competitive or sandwich ELISA kits. BPA analysis was consistent with Good Laboratory Practice methodological protocols [42–44] that included direct testing of blood collection and storage apparatus, controls with high and low concentrations (range 1 pg/mL to 100,000 pg/mL), and triplicate sample analysis. The limit of detection for total BPA using the ELISA kit was 0.5 ng/mL. We assessed total BPA in storage apparatus and in the 75-g glucose drink using the established Centers for Disease Control and Prevention protocol [45, 46] by using online solid-phase extraction coupled to HPLC-isotope dilution tandem mass spectrometry with peak focusing as described previously [47]. Total BPA concentrations were nondetectable in all storage apparati and in the 75-g glucose drink. Glucose and triglyceride concentrations were assessed using a glucose oxidase method and triglyceride reagent (Analox Instruments Ltd, Stourbridge, United Kingdom).
D. Calculations
Glucose, insulin, C-peptide, proinsulin, 17β-estradiol, and triglyceride concentrations were used to calculate areas under the curve using the trapezoidal method. Homeostasis model assessment of insulin resistance (HOMA-IR) and Matsuda Index were calculated as previously described [48].
E. Statistical Analysis
JMP Pro 13.2.1 (SAS Institute, Cary, NC) was used for statistical analysis of data. Summary statistics are reported as mean (SD) for participant characteristics, and for clarity purposes, figures are presented as geometric mean without SEs for all outcome measures. Geometric means and asymmetric SEs for all serum/blood markers and raw data for gastrointestinal distress as well as a CONSORT checklist for randomized trials are deposited in the Digital Commons at California Polytechnic State University data repository and freely available for download [49]. All other data are available from the corresponding author upon e-mail request. Nondetectable BPA concentrations were assigned the limit of detection of 0.05 ng/mL [50]. All outcome measures were not normally distributed and were log transformed prior to analysis. A linear mixed model (repeated measures) was used to examine condition, time, and condition × time effects on serum total BPA, glucose, insulin, C-peptide, proinsulin, 17β-estradiol, and triglycerides, adjusting for the covariates study-entry BMI and sex. In secondary analysis for glucose, insulin, and C-peptide only, significance was further explored by comparing the conditions at each single time point in response to the OGTT (minutes 60, 90, 120, 150, 180) using an analysis of covariance. For area under the curve variables, HOMA-IR, and Matsuda Index responses, a linear mixed model for the crossover design was used to compare the responses across conditions, adjusting for the covariates study-entry BMI and sex. A significance level of α = 0.05 was used. Reported P values are unadjusted unless otherwise noted as Tukey honestly significant difference adjusted. All participants completed all three conditions with no missing data.
Our sample size was based on previous pharmacokinetic BPA [27, 29] studies to distinguish three different serum total BPA conditions. Using a total BPA concentration max of 270 ng/mL after consumption of 50 to 100 μg/kg BW of d6-BPA [27, 29], with 11 subjects we had >99% power to detect a 256 ng/mL total BPA concentration maximum difference between oral consumption of 50 μg/kg BW of d6-BPA (BPA-50) and PL using a two-sided t test.
2. Results
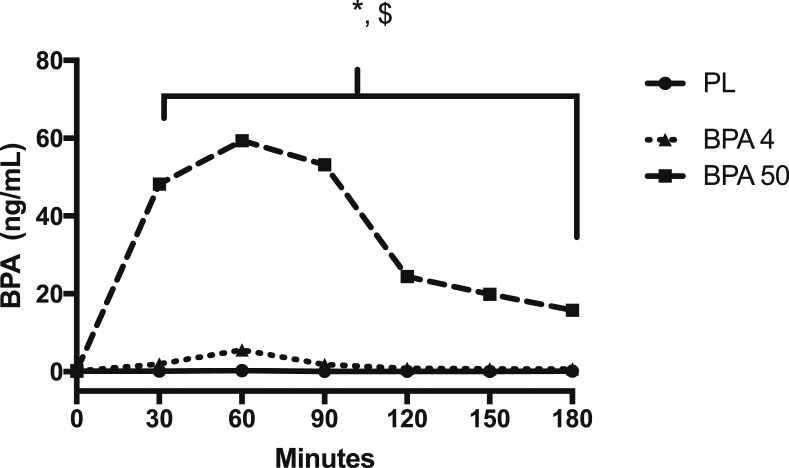
Serum total BPA maximum concentration of 158 ± 50 ng/mL for BPA-50 occurred at 63 ± 11 minutes and a maximum concentration of 25 ± 16 ng/mL for BPA-4 at 60 ± 13 minutes. For serum total BPA concentrations, there was a significant condition main effect and interaction (F2,18 = 46.07, P < 0.001; and F12,178 = 8.20, P < 0.001, respectively). In particular, total BPA concentrations in the BPA-50 condition compared with both PL and BPA-4 increase more rapidly at minutes 30 through 180 (Fig. 1) (t178 = 7.65, P < 0.001; and t178 = 3.65, P = 0.003, respectively). Also, BPA-4 increased more than PL at the same time points (P < 0.001). These data suggest that we were successful at administering BPA at different doses to increase serum total BPA concentrations.
Figure 1.
Serum BPA concentrations in response to orally administered PL, BPA-4, and BPA-50. Values are geometric mean. *BPA-50 significantly (P < 0.05) different than PL and BPA-4 at all time points; $BPA-4 significantly (P < 0.05) different than PL and BPA-50 at all time points.
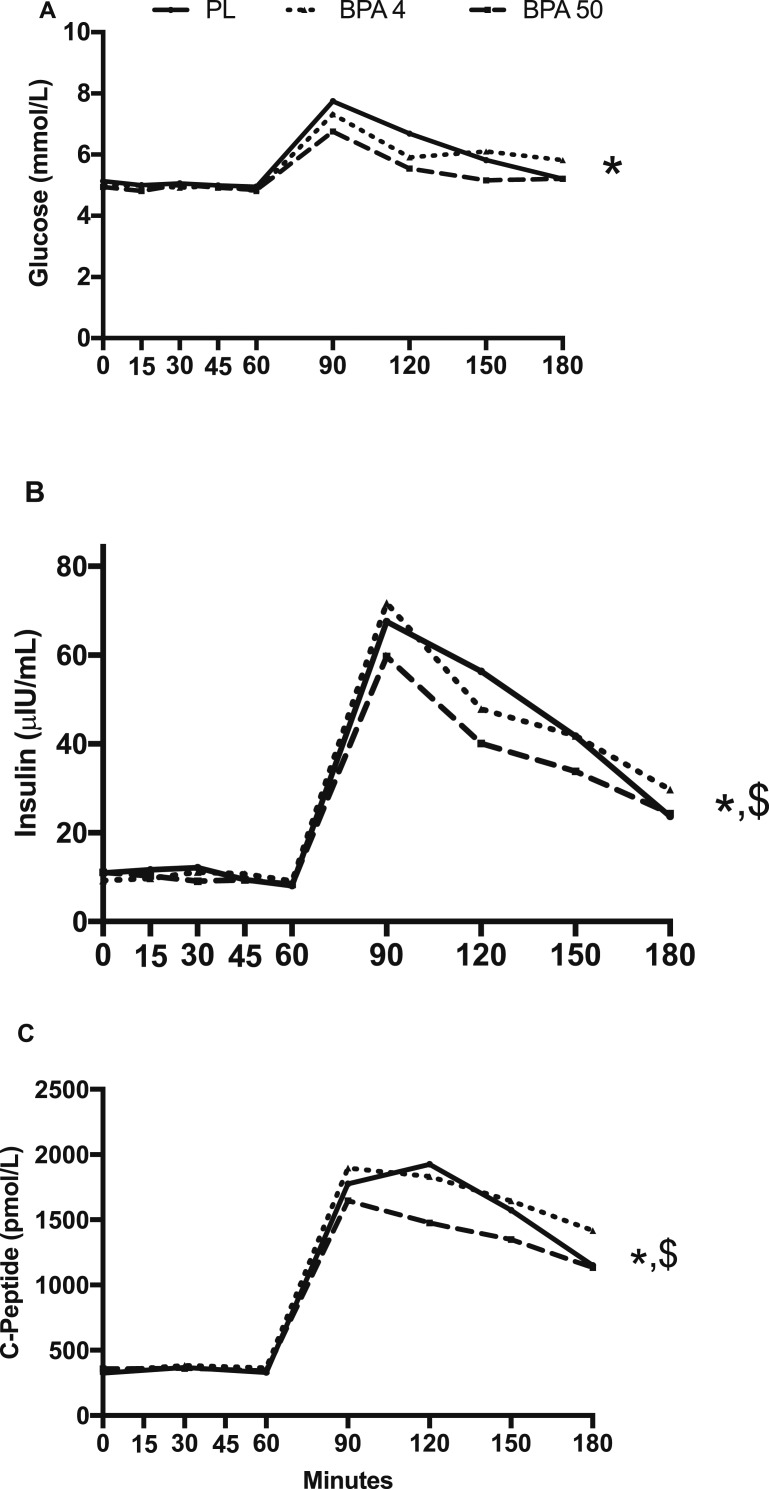
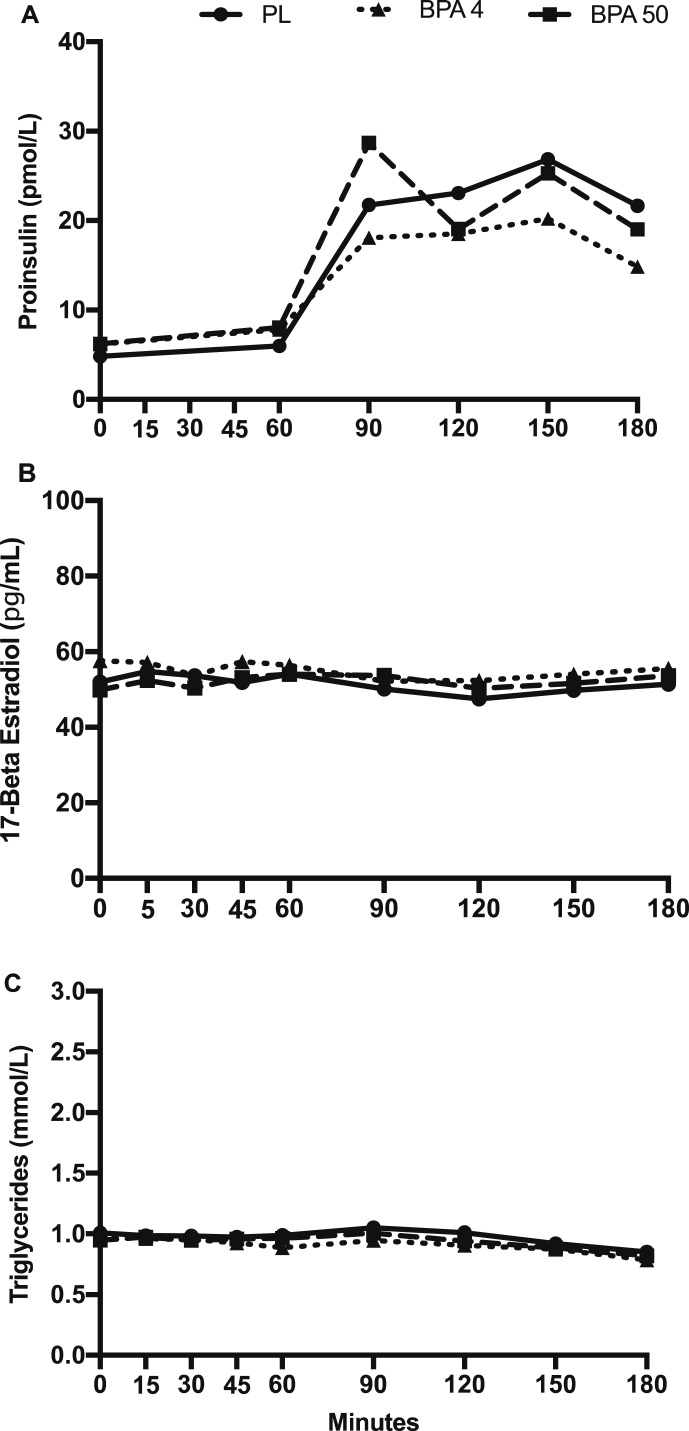
There was no significant condition × time interaction for glucose, insulin, C-peptide, proinsulin, 17β-estradiol, and triglyceride concentrations (P > 0.05; Figs. 2 and 3). However, a significant main effect for condition was observed for glucose, indicating that glucose was significantly lower in the BPA-50 vs PL (t18 = 2.71; Tukey-adjusted P = 0.036) and nearly significant vs BPA-4 (t18 = 2.49; Tukey-adjusted P = 0.056; Fig. 2A). Time-slice post hoc unadjusted comparisons of glucose concentrations across treatment conditions were significantly different at time 120 (F2,251 = 3.88; P = 0.022) and time 150 (F2,251 = 3.42; P = 0.034). In particular, glucose concentrations at time 120 were lower in BPA-50 vs PL (t251 = 2.76; P = 0.006) and suggestively lower vs BPA-4 (t251 = 1.73; P = 0.084). Glucose concentrations at time 150 was suggestively lower in BPA-50 vs PL (t251 = 1.76; P = 0.079) and not evidently different in BPA-4 vs PL (t251 = 0.78; P = 0.430). A significant condition main effect was also observed for insulin (F2,18 = 3.56; P = 0.050), indicating lower insulin concentrations in BPA-50 vs BPA-4 (t18 = 2.53; Tukey-adjusted P = 0.021; Fig. 2B). For C-peptide concentrations, there was a significant condition main effect (F2,18 = 7.84; P = 0.004) such that BPA-50 was significantly lower than BPA-4 (t18 = 3.95; Tukey-adjusted P = 0.003; Fig. 2C), but not vs PL (t18 = 1.71; P = 0.228). For proinsulin, 17β-estradiol, and triglyceride concentrations, there were no significant condition main effects or condition by time interactions (P > 0.05; Fig. 3A–3C).
Figure 2.
(A) Plasma glucose concentrations, (B) insulin concentrations, and (C) C-peptide concentrations in response to orally administered PL, BPA-4, and BPA-50. Values are geometric mean. *BPA-50 condition significantly different than PL condition (P < 0.05); $BPA-50 condition significantly different than BPA-4 condition (P < 0.05).
Figure 3.
(A) Plasma proinsulin concentrations, (B) 17β-estradiol concentrations, and (C) triglyceride concentrations in response to orally administered PL, BPA-4, and BPA-50. Values are geometric mean.
BPA area under the curve was significantly lower in BPA-50 vs PL and BPA-4 (P < 0.001; Table 2), and BPA-4 was significantly lower than PL (P < 0.001; Table 2). Glucose, insulin, and C-peptide area under the curve was significantly lower in BPA-50 vs PL and insulin area under the curve only vs BPA-4 (P < 0.020; Table 2). There was no significant proinsulin, 17β-estradiol, and triglyceride area under the curve difference (P > 0.05) between conditions (Table 2). There was no significant difference (P < 0.05) among PL, BPA-4, and BPA-50 in HOMA-IR (least square mean ± SEM: 2.07 ± 0.26, 2.20 ± 0.26, and 2.19 ± 0.26, respectively; P = 0.91) and Matsuda Index (5.28 ± 0.51, 4.97 ± 0.51, and 5.97 ± 0.51, respectively; P = 0.28).
Table 2.
Calculated Areas Under the Curve for the 3-H Profile
| PL | BPA-4 | BPA-50 | P Value | |
|---|---|---|---|---|
| BPA AUC (ng/mL · min) | 56 (12, 258)a | 571 (126, 2580)b | 10,676 (2319, 49,151)c | <0.001 |
| Glucose AUC (mmol/L · min) | 1088 (978, 1210)a | 1068 (960, 1188)a,b | 998 (897, 1110)b | 0.017 |
| Insulin AUC (μIU/mL · min) | 6036 (5263, 6922)a | 6044 (5271, 6932)a | 5120 (4464, 5871)b | 0.011 |
| C-peptide AUC (pmol/L · min) | 201,637 (171,134, 237,576)a | 210,018 (178,247, 247,451)a | 181,016 (153,632, 213,280)b | 0.003 |
| Proinsulin AUC (pmol/L · min) | 3143 (2510, 3935) | 3078 (2458, 3854) | 3339 (2667, 4181) | 0.749 |
| Estradiol AUC (pg/mL · min) | 8744 (5161, 14,814) | 9608 (5621, 16422) | 8913 (5261, 15,102) | 0.873 |
| TG AUC (mmol/L · min) | 172 (137, 217) | 160 (127, 201) | 165 (131, 208) | 0.612 |
Data are geometric mean (95% CI).
Abbreviations: AUC, area under the curve; Estradiol, 17β-estradiol; TG, triglyceride.
a,b,cWithin each row, geometric means not sharing a similar letter are significantly different (Tukey adjustment, P < 0.05).
There was no significant condition by time interaction or main effect (P > 0.05) in gastrointestinal distress [49] or subjective appetite ratings (data not shown).
3. Discussion
The objective of this pilot study was to determine whether varying doses of orally administered BPA altered indices of glucose metabolism. Findings demonstrated the feasibility of a randomized crossover design, varying dose exposure to BPA, and testing direct effects on indices of glucose metabolism in humans. Although there were no noteworthy condition × time interactions on indices of glucose metabolism, considerable condition main effects suggested that orally administered BPA at 50 μg/kg BW compared with PL or orally administered BPA at 4 μg/kg BW surprisingly lowered glucose, insulin, and C-peptide concentrations. Additionally, calculated areas under the curve for glucose, insulin, and C-peptide were significantly lower with orally administered BPA at 50 μg/kg BW compared with PL. Although these data need to be interpreted with some caution given the relatively small sample size, they provide evidence in humans that orally administered BPA at the US EPA-approved safe dose [35] may have an immediate effect on altering indices of glucose metabolism in humans. These data, which are consistent with the previous human and animal studies [6, 30], provide evidence that the estrogen-mimic BPA may potentially have at least some role in β-cell functioning and insulin resistance.
Previous observational studies have shown positive associations between BPA and indices of glucose metabolism and type 2 diabetes incidence [23, 51, 52]. For example, Tai and Chen [52] found that urinary BPA levels in the third and fourth quartiles, compared with the reference quartile, were significantly associated with increased HbA1c (0.46% and 0.44% increase, respectively), fasting glucose levels (0.092 mmol/L and 0.075 mmol/L increase, respectively), and doctor-diagnosed type 2 diabetes in men. However, these studies were strictly associative in nature, and well-controlled experimental studies are needed to determine the direct effect of orally administered BPA on indices of glucose metabolism and insulin resistance. To our knowledge, only one other human study and one animal study had assessed the immediate effects of a single oral administration of BPA [6, 30]. Recently, Stahlhut et al. [30] showed in men and postmenopausal women without diabetes that oral BPA consumption of 50 μg/kg BW lowered insulin and C-peptide concentrations in response to glucose infusion. Similarly, Alonso-Magdalena et al. [6] showed that in mice, consumption of 10 μg/kg BW of BPA had an acute effect and significantly reduced glycemia (blood glucose concentrations) with a concomitant increase in insulin concentrations. However, after 4 days of injection of 100 μg/kg BW of BPA (double the high dose used in the current study), mice drastically increased glycemia and became hyperinsulinemic. Our results are consistent with these studies, showing that immediate oral administration of BPA (50 μg/kg BW) lowered glucose, insulin, and C-peptide concentrations and reduced calculated areas under the curve for these variables. Surprisingly, there was no concomitant reduction in proinsulin. Previous studies have found that in individuals without diabetes, only 10% of proinsulin is converted to insulin and may take ≥3 hours to observe notable changes in proinsulin [53, 54]. Longer-term (i.e., >1 day) human studies are needed to examine whether an initial acute decline in glucose and insulin is followed by a pattern of elevated glucose and insulin concentrations as seen in the prior mouse study [6].
Previous pharmacokinetic studies in humans have shown that BPA is rapidly metabolized and absorbed with serum total BPA concentrations peaking at ∼1 hour after consumption of BPA and the majority of BPA recovered in urine within 24 hours [27–29, 55]. In the current study, we took advantage of these pharmacokinetic study design models and performed an OGTT at peak BPA levels ~60 minutes after consumption of BPA, all the while assessing glucose, insulin, and C-peptide concentrations. Even though these previous studies have shown that <1% of BPA consumed is unconjugated (bioactive) [27–29], our data and others [30] suggest that the increase in serum total BPA concentrations still has an immediate effect on glucose, insulin, and C-peptide concentrations and calculated areas under the curve.
In the current pilot study, the observed changes in glucose, insulin, and C-peptide concentrations in response to an OGTT and calculated areas under the curve at the US EPA reference dose have clinical implications, as this is the “safe” dose of BPA exposure throughout a lifetime [35]. These data, which are consistent with the one other human and animal study, provide evidence that the estrogen mimic BPA may ultimately play at least some role in insulin resistance. In support, previous animal data have shown that β-cells isolated from BPA-treated mice for 8 days had a greater release of insulin in response to high glucose [56]. In the current study, we used an OGTT, which has clinical advantages; however, hepatic glucose production and muscle insulin sensitivity are not directly measured. Future human studies are needed to determine potential mechanisms using gold standard measurements (e.g., hepatic glucose production via stable isotope infusion, euglycemic-hyperinsulinemic clamp technique, and glucose rate of disposal) through which BPA may act to impact insulin resistance.
Previous human studies have assessed the pharmacokinetics of a single oral consumption of BPA, with a total BPA serum concentration maximum of ∼4 ng/mL for each microgram of BPA ingested per kilogram of BW (150 to 270 ng/mL) occurring at ∼60 minutes [27–29]. The current study used a very similar orally administered BPA dosing protocol, and, although the study was not intended to assess pharmacokinetics, we similarly observed total BPA concentration maximum of 159 ng/mL occurring at ∼60 minutes, with no reported gastrointestinal distress (assessed by questionnaire) or unintended participant harms (based on CONSORT guidelines). The recent oral BPA consumption study by Stahlhut et al. [30] and previous pharmacokinetic studies [27–29] also reported no side effects of BPA consumption. Thus, taken together, these data suggest that in humans, an orally administered BPA protocol, at varying doses, is feasible with no reported side effects. Future large-scale clinical trials are needed to determine the effects of BPA on glucose metabolism and cutoffs needed for human exposure.
There are notable strengths and limitations of the current pilot study. We experimentally tested a randomized, doubled-blinded, balanced trial, consistent with CONSORT guidelines [31], examining varying doses of orally administered BPA on indices of glucose metabolism. We chose to assess total serum BPA (and not unconjugated BPA) with a commercially available ELISA kit that is historically not as reliable [57]. However, the total BPA concentrations observed in the current study were consistent with other pharmacokinetic studies by Thayer et al. [27] and Völkel et al. [29]. Additionally, this study was not specifically designed to assess the pharmacokinetics of BPA, which have been reported elsewhere [27–29]; rather, an objective was to determine the feasibility of recruiting participants in an orally administered BPA protocol on indices of glucose metabolism in which the ELISA total BPA kit was able to distinguish among the three conditions (Fig. 1). Also, even though participants consumed d6-BPA, we assessed total BPA in serum for only 3 hours and did not determine the pharmacokinetics in serum or urine throughout the ensuing days. Thus, it remains unclear whether the observed changes in glucose, insulin, and C-peptide would persist over several days or induce a relative insulin resistance. Finally, we recruited a relatively small, convenient sample of young, nonobese college men and women, and our results are not able to tease apart potential weight status differences and sex differences and may not be generalizable to individuals with higher baseline BPA exposure [19, 20].
4. Conclusion
An orally administered BPA protocol on indices of glucose metabolism appeared feasible in humans with no reported gastrointestinal distress, side effects, or unintended participant harms. Furthermore, results from this pilot study and others [30] provide suggestive evidence that orally administered BPA at the US EPA-approved [35] safe dose of 50 μg kg BW has immediate effects on indices of glucose metabolism in young, nonobese adults. Future larger-scale clinical randomized trials are needed to confirm these findings using gold standard measurements (e.g., hepatic glucose production via stable isotope infusion and insulin sensitivity via hyperinsulinemic-euglycemic clamp technique) and determine the effects of repeated consumption of BPA over several days in humans.
Acknowledgments
We thank Leana Mosesian, Garrett Grant, and Julie Pollard for aiding in data collection, Stuart Chipkin for providing input in the manuscript, and all of the participants in this study. Results of this study were presented at California Polytechnic State University’s College of Science and Mathematics Annual Student Research Conference, 18 and 19 May 2018, and at the American College of Sports Medicine’s 65th Annual Meeting, 29 May–2 June 2018, Minneapolis, MN. This study was partially supported by the Bill and Linda Frost Foundation.
Clinical Trial Information: ClinicalTrials.gov no. NCT03444922 (registered 26 February 2018).
Disclosure Summary: S.P. received a grant from Weight Watchers International unrelated to this work. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BPA
bisphenol A
- BPA-4
deuterated bisphenol A at 4 μg/kg body weight
- BPA-50
deuterated bisphenol A at 50 μg/kg body weight
- BW
body weight
- CONSORT
Consolidated Standards of Reporting Trials
- d6-BPA
deuterated 6-bisphenol A
- EPA
Environmental Protection Agency
- HOMA-IR
homeostasis model assessment of insulin resistance
- OGTT
oral glucose tolerance test
References and Notes
- 1. Centers for Disease Control and Prevention National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 2. Hou X, Liu J, Song J, Wang C, Liang K, Sun Y, Ma Z, Yang W, Li C, Zhang X, Lin P, Gong L, Wang M, Liu F, Li W, Yan F, Qin J, Wang L, Liu J, Zhao R, Chen S, Chen L. Relationship of hemoglobin A1c with β cell function and insulin resistance in newly diagnosed and drug naive type 2 diabetes patients. J Diabetes Res. 2016;2016:8797316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Derakhshan A, Tohidi M, Arshi B, Khalili D, Azizi F, Hadaegh F. Relationship of hyperinsulinaemia, insulin resistance and β-cell dysfunction with incident diabetes and pre-diabetes: the Tehran Lipid and Glucose Study. Diabet Med. 2015;32(1):24–32. [DOI] [PubMed] [Google Scholar]
- 4. Punthakee Z, Werstuck GH, Gerstein HC. Diabetes and cardiovascular disease: explaining the relationship. Rev Cardiovasc Med. 2007;8(3):145–153. [PubMed] [Google Scholar]
- 5. Pandey A, Chawla S, Guchhait P. Type-2 diabetes: Current understanding and future perspectives. IUBMB Life. 2015;67(7):506–513. [DOI] [PubMed] [Google Scholar]
- 6. Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114(1):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109(7):675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Hüppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect. 2009;117(10):1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: results from NHANES. Int J Endocrinol. 2012;2012:598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol. 2012;2012:965243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120(9):1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil Steril. 2015;103(1):11–21. [DOI] [PubMed] [Google Scholar]
- 13. Héliès-Toussaint C, Peyre L, Costanzo C, Chagnon MC, Rahmani R. Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicol Appl Pharmacol. 2014;280(2):224–235. [DOI] [PubMed] [Google Scholar]
- 14. Peyre L, Rouimi P, de Sousa G, Héliès-Toussaint C, Carré B, Barcellini S, Chagnon MC, Rahmani R. Comparative study of bisphenol A and its analogue bisphenol S on human hepatic cells: a focus on their potential involvement in nonalcoholic fatty liver disease. Food Chem Toxicol. 2014;70:9–18. [DOI] [PubMed] [Google Scholar]
- 15. Valvi D, Casas M, Mendez MA, Ballesteros-Gómez A, Luque N, Rubio S, Sunyer J, Vrijheid M. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology. 2013;24(6):791–799. [DOI] [PubMed] [Google Scholar]
- 16. Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA, Kannan K. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ Res. 2018;166:544–552. [DOI] [PubMed] [Google Scholar]
- 17. Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018;61(7):1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Silver MK, O’Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One. 2011;6(10):e26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmadkhaniha R, Mansouri M, Yunesian M, Omidfar K, Jeddi MZ, Larijani B, Mesdaghinia A, Rastkari N. Association of urinary bisphenol a concentration with type-2 diabetes mellitus. J Environ Health Sci Eng. 2014;12(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabanayagam C, Teppala S, Shankar A. Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetol. 2013;50(4):625–631. [DOI] [PubMed] [Google Scholar]
- 22. Beydoun HA, Khanal S, Zonderman AB, Beydoun MA. Sex differences in the association of urinary bisphenol-A concentration with selected indices of glucose homeostasis among U.S. adults. Ann Epidemiol. 2014;24(2):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122(6):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moon MK, Jeong IK, Jung Oh T, Ahn HY, Kim HH, Park YJ, Jang HC, Park KS. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J Endocrinol. 2015;226(1):35–42. [DOI] [PubMed] [Google Scholar]
- 25. Ding S, Fan Y, Zhao N, Yang H, Ye X, He D, Jin X, Liu J, Tian C, Li H, Xu S, Ying C. High-fat diet aggravates glucose homeostasis disorder caused by chronic exposure to bisphenol A. J Endocrinol. 2014;221(1):167–179. [DOI] [PubMed] [Google Scholar]
- 26. Kidani T, Kamei S, Miyawaki J, Aizawa J, Sakayama K, Masuno H. Bisphenol A downregulates Akt signaling and inhibits adiponectin production and secretion in 3T3-L1 adipocytes. J Atheroscler Thromb. 2010;17(8):834–843. [DOI] [PubMed] [Google Scholar]
- 27. Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, Birnbaum LS. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int. 2015;83:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teeguarden JG, Twaddle NC, Churchwell MI, Yang X, Fisher JW, Seryak LM, Doerge DR. 24-hour human urine and serum profiles of bisphenol A following ingestion in soup: Individual pharmacokinetic data and emographics. Data Brief. 2015;4:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15(10):1281–1287. [DOI] [PubMed] [Google Scholar]
- 30. Stahlhut RW, Peterson J, Taylor JA, Nadal A, Dyer JA, vom Saal FS. Experimental BPA exposure and glucose-stimulated insulin response in adult men and women. J Endocr Soc. 2018;2(10):1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schulz KF, Altman DG, Moher D; CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115(5):1063–1070. [DOI] [PubMed] [Google Scholar]
- 32. Morris AP, Olmstead J, Hall K, Phelan S, Hagobian TA. Eight weeks of creatine supplementation, but not creatine plus sodium bicarbonate, increases exercise performance. Gazz Med Ital Arch Sci Med. 2016;175(12):508–515. [Google Scholar]
- 33. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Dis. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 34. European Food Safety Authority Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2-bis(4-hydroxyphenyl)propane. EFSA J. 2007;5(1):1–75. [Google Scholar]
- 35.US Environmental Protection Agency. Chemical Assessment Summary. Bisphenol A; CASRN 80-05-7. Washington, DC: National Center for Environmental Assessment; 1988. [Google Scholar]
- 36.European Food Safety Authority. Scientific opinion on bisphenol A (2015). Available at: www.efsa.europa.eu/sites/default/files/corporate_publications/files/factsheetbpa150121.pdf. Accessed 15 February 2019.
- 37.RRID:AB_2754549.
- 38.RRID:AB_2264887.
- 39.RRID:AB_2750847.
- 40.RRID:AB_2754550.
- 41.RRID:AB_2533915.
- 42. Teeguarden JG, Twaddle NC, Churchwell MI, Doerge DR. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem Toxicol. 2016;92:129–142. [DOI] [PubMed] [Google Scholar]
- 43. Teeguarden JG, Twaddle NC, Churchwell MI, Yang X, Fisher JW, Seryak LM, Doerge DR. 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol. 2015;288(2):131–142. [DOI] [PubMed] [Google Scholar]
- 44. Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect. 2013;121(3):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hagobian T, Smouse A, Streeter M, Wurst C, Schaffner A, Phelan S. Randomized intervention trial to decrease bisphenol A urine concentrations in women: pilot study. J Womens Health (Larchmt). 2017;26(2):128–132. [DOI] [PubMed] [Google Scholar]
- 46. Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113(4):391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77(16):5407–5413. [DOI] [PubMed] [Google Scholar]
- 48. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. [DOI] [PubMed] [Google Scholar]
- 49.Hagobian T, Bird A, Stanelle S, Williams D, Schaffner A, Phelan S. Data from: Effects of varying doses of oral bisphenol A consumption on type 2 diabetes risk markers in healthy adults. Digital Commons at Cal Poly, 2017. Deposited 3 October 2017. https://digitalcommons.calpoly.edu/kine_fac/132/.
- 50. Wendelberger J, Campbell K. Nondetect Data in Environmental Investigations. Los Alamos, NM: Los Alamos National Laboratory: 1994. [Google Scholar]
- 51. Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B. Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009. J Diabetes. 2015;7(2):240–249. [DOI] [PubMed] [Google Scholar]
- 52. Tai X, Chen Y. Urinary bisphenol A concentrations positively associated with glycated hemoglobin and other indicators of diabetes in Canadian men. Environ Res. 2016;147:172–178. [DOI] [PubMed] [Google Scholar]
- 53. Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D Jr. Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30(9):698–702. [DOI] [PubMed] [Google Scholar]
- 54. Nagamatsu S, Grodsky GM. Glucose-regulated proinsulin processing in isolated islets from rat pancreas. Diabetes. 1988;37(10):1426–1431. [DOI] [PubMed] [Google Scholar]
- 55. Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham MK. Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol Sci. 2011;123(1):48–57. [DOI] [PubMed] [Google Scholar]
- 56. Batista TM, Alonso-Magdalena P, Vieira E, Amaral ME, Cederroth CR, Nef S, Quesada I, Carneiro EM, Nadal A. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One. 2012;7(3):e33814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dekant W, Völkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228(1):114–134. [DOI] [PubMed] [Google Scholar]