Abstract
Objectives
Tofacitinib is an oral JAK inhibitor for the treatment of rheumatoid arthritis (RA). We examined response to tofacitinib 5 or 10 mg two times a day in patients with seropositive vs seronegative RA.
Methods
Data were pooled from five Phase III studies of conventional synthetic disease-modifying antirheumatic drug (csDMARD)- or biological DMARD-inadequate responders (ORAL Step [NCT00960440]; ORAL Scan [NCT00847613]; ORAL Solo [NCT00814307]; ORAL Sync [NCT00856544]; ORAL Standard [NCT00853385]). ‘Serotype’ subgroups were: anticyclic citrullinated peptide (CCP) and rheumatoid factor (RF) positive (anti-CCP+/RF+); anti-CCP+/RF negative (-); anti-CCP-/RF+; anti-CCP-/RF-. At month 3, ACR20/50/70 response rates, Disease Activity Score (DAS28-4[ESR])-defined remission (DAS28-4[ESR]<2.6) and low disease activity (LDA; DAS28-4[ESR]≤3.2), changes from baseline (CFB) in Health Assessment Questionnaire-Disability Index (HAQ-DI), Short Form-36 Health Survey (SF-36) physical functioning and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) were evaluated. Safety endpoints were compared.
Results
Baseline demographics/characteristics were similar across subgroups. Tofacitinib significantly improved ACR20/50/70 response rates, DAS28-4(ESR) LDA rates and CFB in HAQ-DI and FACIT-F vs placebo across subgroups. More anti-CCP+/RF+ than anti-CCP-/RF- patients had ACR20/50/70 responses (ACR20/50: both tofacitinib doses; ACR70: 10 mg two times a day). SF-36 physical functioning improved in anti-CCP+/RF+, anti-CCP+/RF- and anti-CCP-/RF+ patients (both tofacitinib doses) and anti-CCP-/RF- patients (10 mg two times a day) vs placebo. More anti-CCP+/RF+ and anti-CCP+/RF- than anti-CCP-/RF- patients achieved DAS28-4(ESR) remission and LDA with tofacitinib 10 mg two times a day. Frequency of adverse events (AEs), serious AEs and discontinuations due to AEs were similar across subgroups.
Conclusion
Generally, tofacitinib efficacy (ACR20/50/70 responses) and safety were similar across subgroups. DAS28-4(ESR) remission rates and SF-36 physical functioning appeared lower in anti-CCP- patients.
Keywords: rheumatoid arthritis, anti-CCP, rheumatoid factor, treatment
Key messages.
What is already known about this subject?
The efficacy and safety of tofacitinib in patients with rheumatoid arthritis (RA) have been demonstrated previously in Phase II and Phase III clinical trials of up to 24 months’ duration and in long-term extension studies with up to 114 months of observation.
Elevated levels of rheumatoid factor (RF) and/or anticyclic citrullinated peptide (CCP) antibodies (seropositivity) are common in patients with RA and may indicate greater disease severity, a higher risk of disease progression and may influence responses to treatments for RA.
What does this study add?
In a posthoc, pooled analysis of five Phase III studies, tofacitinib 5 or 10 mg two times a day significantly improved ACR20/50/70 response rates, DAS28-4(ESR) low disease activity (LDA) rates and change from baseline in Health Assessment Questionnaire-Disability Index and Functional Assessment of Chronic Illness Therapy-Fatigue vs placebo in patients with seropositive or seronegative RA.
Patients who were anti-CCP+/RF+ were more likely to achieve ACR20/50/70 responses with tofacitinib than anti-CCP-/RF- patients (ACR20/50: both tofacitinib doses; ACR70: tofacitinib 10 mg two times a day); anti-CCP+/RF+ or anti-CCP+/RF- patients receiving tofacitinib 10 mg two times a day were more likely to achieve DAS28-4(ESR) remission or LDA than anti-CCP-/RF- patients.
How might this impact on clinical practice?
This study adds to the collective evidence on the relationship between seropositivity and the efficacy of tofacitinib, which may help to inform future therapeutic strategies.
Introduction
Rheumatoid arthritis (RA) is a chronic and debilitating autoimmune disease that has a major effect on health status and quality of life.1 2 RA is characterised by inflammation of the articular synovium leading to deformity, progressive disability and ultimately destruction of joints.
The current guidelines of both the European League against Rheumatism and the American College of Rheumatology (ACR) recommend a ‘treat-to-target’ approach, with the primary goals of treating patients with RA identified as the attainment of remission or low disease activity (LDA) if remission is not achievable.3 4 The use of the conventional synthetic (cs) disease-modifying antirheumatic drug (DMARD) methotrexate (MTX) in conjunction with glucocorticoids (GC), either as monotherapy or in combination with other csDMARDs, is recommended as first-line therapy, with the aim of target attainment by 6 months. If this treatment fails, or if unfavourable prognostic markers such as early erosions, autoantibodies or high disease activity are present, the addition of other csDMARDs, biologic DMARDs or targeted synthetic DMARDs is recommended.3 4 However, clinical outcomes of current treatments remain variable.
Conflicting efficacy results have been observed for tumour necrosis factor inhibitors (TNFi) in different studies. Previously, a randomised double-blind study of etanercept in combination with MTX resulted in 85% of patients achieving a 20% improvement in RA according to ACR criteria (ACR20 response).5 However, an earlier study investigating the same treatment regimen reported an ACR20 response rate of 71%.6 Furthermore, contrasting results have also been observed in different studies of infliximab. While one study from 2000 found ACR20 responses in 42% of patients following treatment with infliximab in combination with MTX,7 other, more recent studies with the same treatments resulted in ACR20 responses in 62.4%,8 60%9 and 58.6%10 of patients. Therefore, gaining a better understanding of the underlying differences in patient characteristics that give rise to variation in response to treatment would be of benefit and would allow the identification of patient subpopulations most likely to respond to specific treatment modalities.
Elevated levels of rheumatoid factor (RF) and/or anticyclic citrullinated peptide (CCP) antibodies (seropositivity) are common in patients with RA and it has been estimated that approximately 80% and 70% are seropositive for RF and CCP, respectively.11 12 Anti-CCP and/or RF seropositivity can occur several years before the onset of RA13 and may indicate greater disease severity and a higher risk of disease progression than seronegativity.14–17 It is possible that anti-CCP and/or RF seropositivity could influence responses to treatments for RA, therefore investigation of the relationship between these markers and treatment efficacy may help to inform future therapeutic strategies. However, assessment of RF or anti-CCP seropositivity as individual diagnostic markers has been questioned due to the moderate sensitivity of serological tests.18 19 An alternative diagnostic approach involving analysis of RF+ and CCP+ in combination has been suggested as a more effective prognostic indicator of structural damage early in the course of the disease than detection of individual markers.20
Tofacitinib is an oral Janus kinase (JAK) inhibitor for the treatment of RA. The efficacy and safety of tofacitinib 5 and 10 mg two times a day administered as monotherapy or in combination with csDMARDs, mainly MTX, in patients with moderately to severely active RA, have been demonstrated in Phase II21–27 and Phase III randomised controlled trials of up to 24 months’ duration28–33 and in long-term extension studies with up to 114 months of observation.34–36
This posthoc subgroup analysis used pooled data from five Phase III studies of tofacitinib to examine treatment response in patients with seropositive vs seronegative RA.
Methods
Clinical trials
Subgroup data were pooled across five Phase III randomised, placebo-controlled, double-blind studies of 6 to 24 months’ duration. Tofacitinib was administered at 5 or 10 mg two times a day, either as monotherapy (ORAL Solo, ClinicalTrials.gov number: NCT00814307)29 or in combination with csDMARDs, mainly MTX (ORAL Sync: NCT00856544;30 ORAL Standard: NCT00853385;33 ORAL Scan: NCT0084761332 and ORAL Step: NCT0096044028) in patients with moderately to severely active RA and an inadequate response to ≥1 csDMARD or biologic DMARD. One Phase III study (ORAL Standard) included an active comparator of adalimumab (40 mg subcutaneously once every 2 weeks). Patients were permitted stable background doses of low-dose oral GCs and non-steroidal anti-inflammatory drugs.
Serotype subgroups
The seropositivity status of each patient was recorded at baseline. For this analysis, patients were categorised as: anti-CCP and RF positive (anti-CCP+/RF+); anti-CCP+/RF negative (-); anti-CCP-/RF+; anti-CCP-/RF-.
Efficacy endpoints and patient-reported outcomes
The following efficacy endpoints were evaluated at month 3 in patients receiving tofacitinib 5 or 10 mg two times a day or placebo for each serotype subgroup: proportions of patients achieving ACR20/50/70 response (defined as 20%, 50% or 70% improvement in ACR criteria, respectively) and Disease Activity Score in 28 joints, erythrocyte sedimentation rate (DAS28-4[ESR])-defined remission (<2.6) and LDA (≤3.2). Patient-reported outcomes (PROs) were also evaluated at month 3 and included changes from baseline in Health Assessment Questionnaire-Disability Index (HAQ-DI), Short Form-36 Health Survey (SF-36) physical functioning domain and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F).
Safety endpoints
Safety was assessed based on reporting of treatment-emergent adverse events (TEAEs), serious AEs (SAEs), discontinuations due to AEs, serious infections, herpes zoster infections, malignancies (excluding non-melanoma skin cancer [NMSC]) and NMSC.
Statistical analyses
Posthoc efficacy and PRO analyses were based on the full analysis set, which included all patients who received at least one dose of study drug and for whom data were available from at least one postbaseline assessment.
Binary endpoints were compared between the tofacitinib 5 mg two times a day, tofacitinib 10 mg two times a day and placebo groups using the normal approximation to the binomial distribution, with non-responder imputation for missing values. In order to compare efficacy between seropositive and seronegative subgroups, Cochran-Mantel-Haenszel adjusted ORs with upper and lower 95% CIs were calculated for ACR20, ACR50 and ACR70 response rates and for DAS28-4(ESR) remission and LDA rates at month 3.
Continuous endpoints were analysed using a linear mixed-effects model for repeated measures. Estimates of mean changes from baseline for each treatment, as well as differences in means vs placebo, were derived from the model as least squares means (LSM), with corresponding 95% CIs. All statistical comparisons were considered exploratory with no adjustment for multiplicity.
The impact of seropositivity status and smoking status on the rate of DAS28-4(ESR) remission and LDA at month 3 was assessed by logistic regression analysis (online supplementary material 1).
rmdopen-2018-000742supp001.docx (20.1KB, docx)
Safety endpoints were reported throughout the duration of each study and were based on all treated patients who received at least one dose of study drug. Incidence rates (IR; unique patients with events per 100 patient-years [PY] of observation) were calculated for discontinuations due to AEs, serious infections and herpes zoster events; 95% CIs for IRs were based on maximum likelihood estimation.
Results
Patient demographics and baseline characteristics
This posthoc analysis pooled data from 3061 patients. Of these, 1194 received tofacitinib 5 mg two times a day, 1197 received tofacitinib 10 mg two times a day and 670 received placebo. Demographics and baseline characteristics were generally similar across treatment groups and across serotype subgroups (anti-CCP+/RF+, n=1961 across all treatment groups; anti-CCP+/RF-, n=337 across all treatment groups; anti-CCP-/RF+, n=182 across all treatment groups; anti-CCP-/RF-, n=581 across all treatment groups; table 1).
Table 1.
Patient demographics and baseline characteristics
| Tofacitinib 5 mg two times a day N=1194 |
Tofacitinib 10 mg two times a day N=1197 |
Placebo N=670 |
||||||||||
| Anti-CCP+/RF+ N=776 |
Anti-CCP+/RF- N=136 |
Anti-CCP-/RF+ N=74 |
Anti-CCP-/RF- N=208 |
Anti-CCP+/RF+ N=775 |
Anti-CCP+/RF- N=113 |
Anti-CCP-/RF+ N=68 |
Anti-CCP-/RF- N=241 |
Anti-CCP+/RF+ N=410 |
Anti-CCP+/RF- N=88 |
Anti-CCP-/RF+ N=40 |
Anti-CCP-/RF- N=132 |
|
| Median age, years (range) | 54.0 (20.0–86.0) |
53.0 (20.0–82.0) |
53.0 (25.0–79.0) |
53.0 (18.0–83.0) |
54.0 (18.0–85.0) |
54.0 (20.0–78.0) |
53.0 (28.0–76.0) |
54.0 (19.0–78.0) |
54.0 (18.0–82.0) |
47.5 (18.0–80.0) |
52.5 (34.0–74.0) |
53.0 (29.0–79.0) |
| Female, n (%)* | 651 (83.9) | 120 (88.2) | 62 (83.8) | 177 (85.1) | 650 (83.9) | 99 (87.6) | 62 (91.2) | 205 (85.1) | 326 (79.5) | 74 (84.1) | 34 (85.0) | 109 (82.6) |
| Smoking status, n (%)* | ||||||||||||
| Never smoked | 492 (63.4) | 107 (78.7) | 52 (70.3) | 144 (69.2) | 485 (62.6) | 87 (77.0) | 48 (70.6) | 175 (72.6) | 252 (61.6) | 59 (67.8) | 26 (65.0) | 81 (61.4) |
| Smoker | 114 (14.7) | 11 (8.1) | 9 (12.2) | 28 (13.5) | 153 (19.7) | 8 (7.1) | 11 (16.2) | 39 (16.2) | 79 (19.3) | 13 (14.9) | 10 (25.0) | 25 (18.9) |
| Ex-smoker | 170 (21.9) | 18 (13.2) | 13 (17.6) | 36 (17.3) | 137 (17.7) | 18 (15.9) | 9 (13.2) | 27 (11.2) | 78 (19.1) | 15 (17.2) | 4 (10.0) | 26 (19.7) |
| BMI mean (range) | 26.6 (14.3–70.3) |
26.0 (17.5–49.7) |
27.7 (16.7–52.1) |
29.2 (16.0–70.8) |
26.8 (14.4–55.6) |
25.8 (15.6–44.4) |
28.1 (16.3–58.1) |
28.4 (17.1–50.2) |
26.9 (16.0–55.1) |
25.1 (15.4–50.4) |
28.3 (17.5–45.9) |
29.5 (14.7–53.8) |
| Duration of RA, years, mean (SD) | 9.4 (8.0) | 9.3 (8.5) | 7.6 (7.4) | 6.7 (7.6) | 9.7 (8.3) | 8.4 (8.6) | 9.6 (8.4) | 7.6 (7.5) | 9.9 (8.6) | 6.6 (6.4) | 11.9 (8.4) | 8.4 (8.9) |
| DAS28-4(ESR), mean (SD) | 6.5 (1.0) | 6.2 (0.9) | 6.3 (0.9) | 6.5 (1.0) | 6.5 (1.0) | 6.1 (0.9) | 6.4 (1.0) | 6.4 (1.0) | 6.5 (0.9) | 6.1 (0.9) | 6.2 (1.1) | 6.4 (1.0) |
| HAQ-DI, mean (SD) | 1.5 (0.7) | 1.4 (0.6) | 1.4 (0.7) | 1.5 (0.6) | 1.5 (0.7) | 1.2 (0.6) | 1.4 (0.7) | 1.5 (0.6) | 1.5 (0.7) | 1.3 (0.7) | 1.4 (0.7) | 1.4 (0.6) |
*Percentages are based on patients with non-missing values.
BMI, body mass index; CCP, cyclic citrullinated peptide; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; HAQ‑DI, Health Assessment Questionnaire-Disability Index; RA, rheumatoid arthritis; RF, rheumatoid factor.
ACR20/50/70 response rates
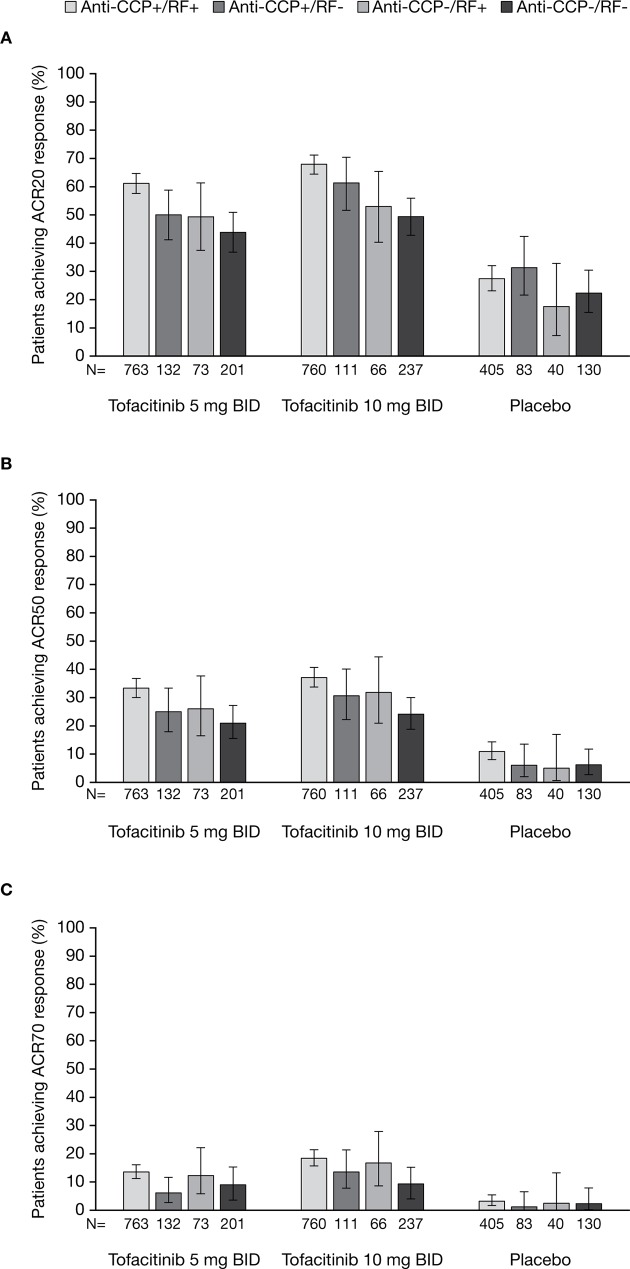
At month 3, ACR20/50/70 response rates were significantly higher among patients receiving tofacitinib 5 and 10 mg two times a day compared with placebo across all serotype subgroups (all p≤0.05 vs placebo; figure 1). Patients who were anti-CCP+/RF+ and receiving tofacitinib 5 or 10 mg two times a day were more likely to achieve ACR20/50 responses than those on the same treatment who were anti-CCP-/RF-. Among patients treated with tofacitinib 10 mg two times a day, those who were anti-CCP+/RF+ were also more likely to achieve an ACR70 response than those who were anti-CCP-/RF-. Anti-CCP+/RF- and anti-CCP-/RF+ patients had similar odds of achieving ACR20/50/70 responses as anti-CCP-/RF- patients (table 2).
Figure 1.
The proportions (and 95% CIs) of anti-CCP+/RF+, anti-CCP+/RF-, anti-CCP-/RF+ and anti-CCP-/RF- patients receiving tofacitinib 5 or 10 mg two times a day or placebo achieving (A) ACR20, (B) ACR50 and (C) ACR70 responses at month 3. For all endpoints, p≤0.05 with tofacitinib 5 and 10 mg two times a day compared with placebo across all serotype subgroups. All data shown are for the full analysis set. Missing data were imputed as non-response to treatment. ACR, American College of Rheumatology; BID, two times a day; CCP, cyclic citrullinated peptide; RF, rheumatoid factor.
Table 2.
ORs (and 95% CIs) for seropositive vs seronegative groups for ACR50 and ACR70 response at month 3
| Anti-CCP+/RF+ vs anti-CCP-/RF- |
Anti-CCP+/RF- vs anti-CCP-/RF- |
Anti-CCP-/RF+ vs anti-CCP-/RF- |
|
| CMH adjusted OR (95% CI) | |||
| ACR20 | |||
| Tofacitinib 5 mg two times a day | 2.0 (1.5 to 2.8) | 1.3 (0.8 to 2.0) | 1.2 (0.7 to 2.1) |
| Tofacitinib 10 mg two times a day | 2.2 (1.6 to 2.9) | 1.6 (1.0 to 2.6) | 1.2 (0.7 to 2.0) |
| Placebo | 1.3 (0.8 to 2.1) | 1.6 (0.9 to 3.0) | 0.7 (0.3 to 1.8) |
| ACR50 | |||
| Tofacitinib 5 mg two times a day | 1.9 (1.3 to 2.7) | 1.3 (0.7 to 2.1) | 1.3 (0.7 to 2.5) |
| Tofacitinib 10 mg two times a day | 1.9 (1.3 to 2.6) | 1.4 (0.8 to 2.3) | 1.5 (0.8 to 2.7) |
| Placebo | 1.9 (0.9 to 4.1) | 1.0 (0.3 to 3.1) | 0.8 (0.2 to 3.9) |
| ACR70 | |||
| Tofacitinib 5 mg two times a day | 1.6 (0.9 to 2.7) | 0.7 (0.3 to 1.6) | 1.4 (0.6 to 3.3) |
| Tofacitinib 10 mg two times a day | 2.2 (1.4 to 3.6) | 1.5 (0.8 to 3.1) | 2.0 (0.9 to 4.3) |
| Placebo | 1.4 (0.4 to 5.0) | 0.5 (0.1 to 5.0) | 1.1 (0.1 to 10.7) |
| DAS28-4(ESR) <2.6 | |||
| Tofacitinib 5 mg two times a day | 0.9 (0.4 to 1.7) | 1.2 (0.5 to 2.9) | 1.1 (0.4 to 3.4) |
| Tofacitinib 10 mg two times a day | 2.8 (1.3 to 5.8) | 5.6 (2.3 to 13.3) | 1.3 (0.3 to 4.9) |
| Placebo | 0.6 (0.2 to 2.6) | 0.6 (0.1 to 5.9) | 1.2 (0.1 to 12.1) |
| DAS28-4(ESR) ≤3.2 | |||
| Tofacitinib 5 mg two times a day | 1.2 (0.7 to 1.9) | 1.4 (0.7 to 2.6) | 1.2 (0.5 to 2.7) |
| Tofacitinib 10 mg two times a day | 1.8 (1.1 to 2.9) | 3.2 (1.7 to 5.8) | 1.7 (0.8 to 3.7) |
| Placebo | 2.2 (0.6 to 7.5) | 0.6 (0.1 to 5.9) | 1.2 (0.1 to 12.1) |
ACR20/50/70, ≥20/50/70% improvement in American College of Rheumatology criteria; CCP, cyclic citrullinated peptide; CMH, Cochran-Mantel-Haenszel; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; RF, rheumatoid factor.
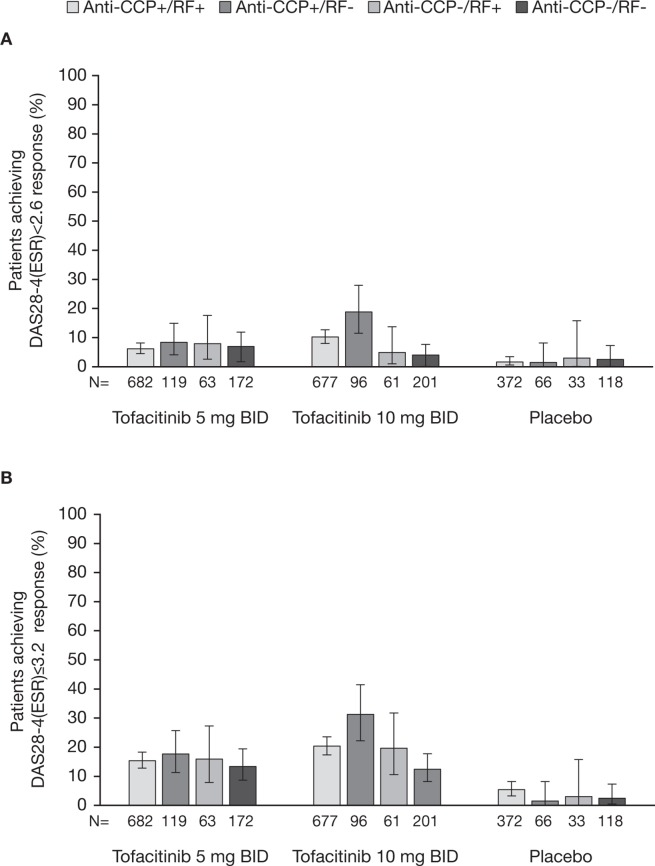
DAS28-4(ESR) response rates
In anti-CCP+/RF+ and anti-CCP+/RF- patients, significantly higher rates of DAS28-4(ESR) remission were achieved by those receiving tofacitinib compared with placebo (all p≤0.05 vs placebo; figure 2A). In anti-CCP-/RF+ and anti-CCP-/RF- patients, DAS28-4(ESR) remission rates were numerically higher in those treated with tofacitinib 5 and 10 mg two times a day than in patients treated with placebo; however, these differences did not reach statistical significance (figure 2A).
Figure 2.
The proportions (and 95% CIs) of anti-CCP+/RF+, anti-CCP+/RF- anti-CCP-/RF+ and anti-CCP-/RF- patients receiving tofacitinib 5 or 10 mg two times a day or placebo achieving DAS28-4(ESR)-defined (A) remission (score <2.6) and (B) LDA (score ≤3.2) at month 3. For DAS28-4(ESR)<2.6, p≤0.05 with tofacitinib 5 and 10 mg two times a day compared with placebo in anti-CCP+/RF+, anti-CCP+/RF- subgroups. For DAS28-4(ESR)≤3.2, p≤0.05 with tofacitinib 5 and 10 mg two times a day compared with placebo across all serotype subgroups. BID, two times a day; CCP, cyclic citrullinated peptide; DAS28-4(ESR), Disease Activity Score in 28 joints, erythrocyte sedimentation rate; LDA, low disease activity; RF, rheumatoid factor.
DAS28-4(ESR) LDA rates were significantly higher among patients receiving tofacitinib 5 or 10 mg two times a day compared with placebo across all serotype subgroups (all p≤0.05 vs placebo; figure 2B).
Comparisons of seropositive and seronegative subgroups showed that patients who were anti-CCP+/RF+ or anti-CCP+/RF- and treated with tofacitinib 10 mg two times were more likely to achieve DAS28-4(ESR) remission or LDA than those on the same treatment who were anti-CCP-/RF- (table 2).
HAQ-DI, FACIT-F and SF-36 physical functioning domain
Patients receiving tofacitinib 5 or 10 mg two times a day reported significantly greater improvements from baseline in HAQ-DI compared with placebo across all serotype subgroups (all p≤0.05 vs placebo; figure 3A).
Figure 3.
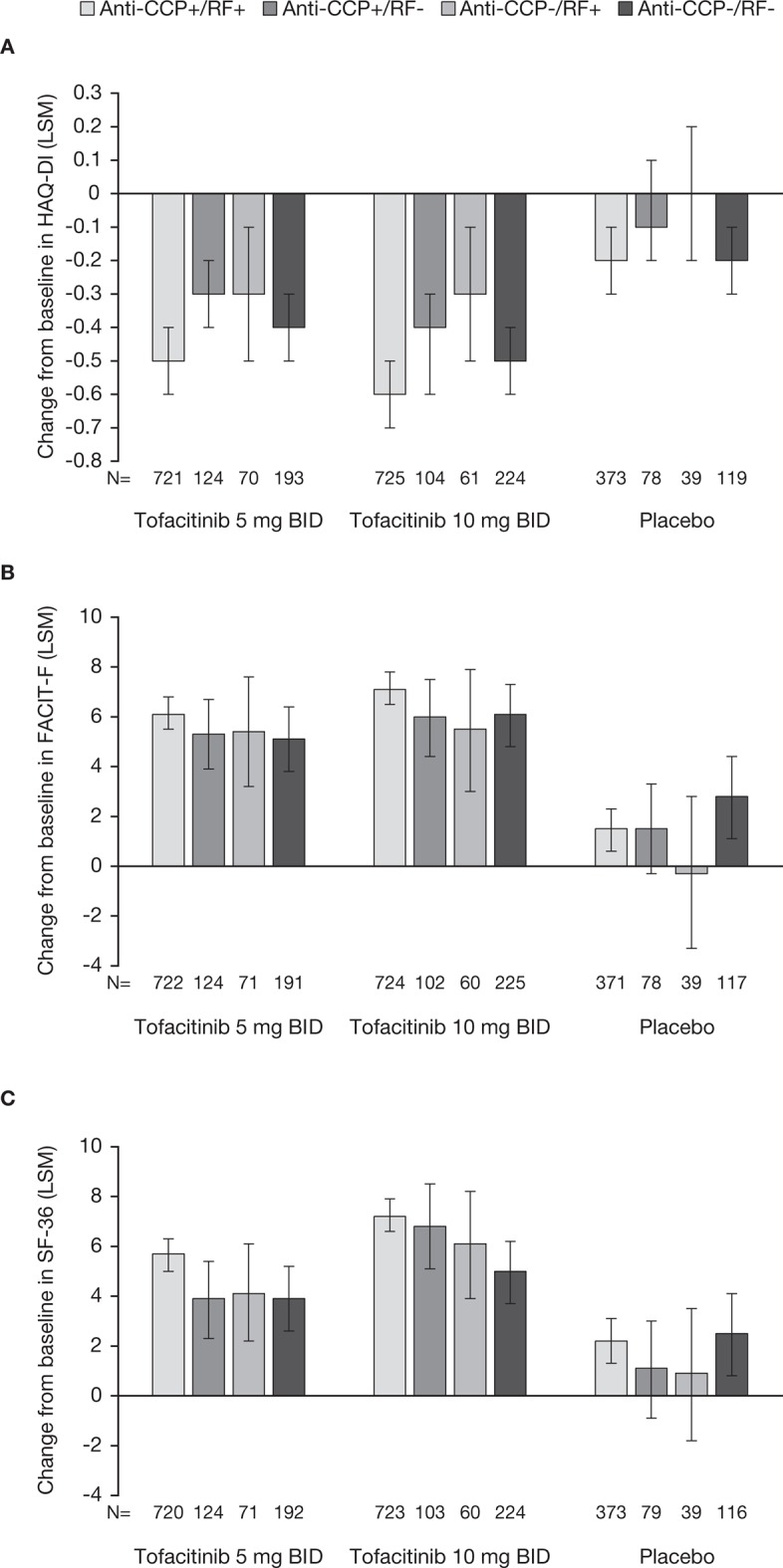
Least squares mean changes from baseline (and 95% CIs) at month three for (A) HAQ-DI, (B) FACIT-F and (C) SF-36 physical functioning domain observed in anti-CCP+/RF+, anti-CCP+/RF-, anti-CCP-/RF+ and anti-CCP-/RF- patients receiving tofacitinib 5 or 10 mg two times a day or placebo. for HAQ-DI and FACIT-F, p≤0.05 with tofacitinib 5 mg two times a day compared with placebo in anti-CCP+/RF+, anti-CCP+/RF- and anti-CCP-/RF+ subgroups and p≤0.05 with tofacitinib 10 mg two times a day compared with placebo across all serotype subgroups. for SF-36 physical functioning domain, p≤0.05 with tofacitinib 5 and 10 mg two times a day compared with placebo across all serotype subgroups. BID, two times a day; CCP, cyclic citrullinated peptide; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability index; LSM, least squares mean; RF, rheumatoid factor; SF-36, Short Form-36 Health Survey.
Similarly, improvements from baseline in FACIT-F were significantly greater in patients receiving tofacitinib 5 or 10 mg two times a day compared with placebo across all serotype subgroups, (all p≤0.05 vs placebo; figure 3B).
SF-36 physical functioning was significantly improved following treatment with tofacitinib 5 or 10 mg two times a day compared with placebo in patients who were anti-CCP+/RF+, anti-CCP+/RF- or anti-CCP-/RF+ (p≤0.05 vs placebo; figure 3C). In the anti-CCP-/RF- subgroup, only patients receiving tofacitinib 10 mg two times a day reported significant improvements in the SF-36 physical functioning domain (p≤0.05); no significant improvement vs placebo was observed in anti-CCP-/RF- patients receiving tofacitinib 5 mg two times a day (figure 3C).
Logistic regression analysis
In separate models, where DAS28-4(ESR) remission or LDA were included as dependent variables and treatment, smoking status, seropositivity status and interaction between smoking status and seropositivity status groups as independent variables, there were no significant differences in remission or LDA, based on smoking status or the interaction between smoking status and seropositivity status. In another model with only treatment and seropositivity status groups as independent variables, significant differences in rates of DAS28-4(ESR) remission or LDA were observed with both doses of tofacitinib (5 and 10 mg two times a day), compared with placebo. In addition, there were significant differences in remission between anti-CCP+/RF- vs anti-CCP-/RF- subgroups, and significant differences in LDA between anti-CCP+/RF+ vs anti-CCP-/RF-, and anti-CCP+/RF- vs anti-CCP-/RF- subgroups (online supplementary table 1).
Safety
Frequencies of AEs were similar across all treatment groups and serotype subgroups (table 3). Across serotype subgroups, TEAEs were observed in: 47.8%–54.3% of patients receiving tofacitinib 5 mg two times a day; 47.8%–57.4% of patients receiving tofacitinib 10 mg two times a day; and 50.2%–58.3% of patients receiving placebo. SAEs were observed in: 2.2%–3.5% of patients receiving tofacitinib 5 mg two times a day; 1.7%–4.4% of patients receiving tofacitinib 10 mg two times a day and 2.5%–5.7% of patients receiving placebo.
Table 3.
Safety endpoints across serotype subgroups for patients receiving tofacitinib or placebo
|
n (%)
IR (95% CI) |
Tofacitinib 5 mg two times a day N=1194 |
Tofacitinib 10 mg two times a day N=1197 |
Placebo N=670 |
|||||||||
| Anti-CCP+/RF+ N=776 |
Anti-CCP+/RF- N=136 |
Anti-CCP-/RF+ N=74 |
Anti-CCP-/RF- N=208 |
Anti-CCP+/RF+ N=775 |
Anti-CCP+/RF- N=113 |
Anti-CCP-/RF+ N=68 |
Anti-CCP-/RF- N=241 |
Anti-CCP+/RF+ N=410 |
Anti-CCP+/RF- N=88 |
Anti-CCP-/RF+ N=40 |
Anti-CCP-/RF- N=132 |
|
| TEAEs | 393 (50.6) | 65 (47.8) | 39 (52.7) | 113 (54.3) | 417 (53.8) | 54 (47.8) | 39 (57.4) | 125 (51.9) | 206 (50.2) | 46 (52.3) | 23 (57.5) | 77 (58.3) |
| SAEs | 27 (3.5) | 3 (2.2) | 2 (2.7) | 7 (3.4) | 21 (2.7) | 5 (4.4) | 2 (2.9) | 4 (1.7) | 13 (3.2) | 5 (5.7) | 1 (2.5) | 4 (3.0) |
| Discontinuations due to AEs* | 77 (9.9) 10.4 [8.3 to 13.0) |
11 (8.1) 7.9 (4.4 to 14.2) |
8 (10.8) 11.2 (5.6 to 22.3) |
16 (7.7) 10.2 (6.3 to 16.7) |
77 (9.9) 10.1 (8.1 to 12.6) |
11 (9.7) 10.1 (5.6 to 18.3) |
8 (11.8) 13.3 (6.6 to 26.5) |
21 (8.7) 10.8 (7.0 to 16.5) |
13 (3.2) 10.3 (6.0 to 17.7) |
5 (5.7) 19.7 (8.2 to 47.4) |
2 (5.0) 16.8 (4.2 to 67.1) |
3 (2.3) 8.6 (2.8 to 26.7) |
| Serious infections* | 27 (3.5) 3.6 (2.5 to 5.3) |
4 (2.9) 2.8 (1.1 to 7.6) |
2 (2.7) 2.8 (0.7 to 11.1) |
3 (1.4) 1.9 (0.6 to 5.9) |
23 (3.0) 3.0 (2.0 to 4.5) |
4 (3.5) 3.7 (1.4 to 9.8) |
1 (1.5) 1.6 (0.2 to 11.6) |
9 (3.7) 4.6 (2.4 to 8.8) |
2 (0.5) 1.6 (0.4 to 6.3) |
1 (1.1) 4.0 (0.6 to 28.1) |
0 (0.0) 0 |
0 (0.0) 0 |
| Herpes zoster*† | 27 (3.5) 3.7 (2.5 to 5.4) |
8 (5.9) 5.9 (3.0 to 11.8) |
6 (8.1) 9.1 (4.1 to 20.3) |
3 (1.4) 1.9 (0.6 to 6.0) |
32 (4.1) 4.3 (3.0 to 6.0) |
9 (8.0) 8.7 (4.5 to 16.8) |
3 (4.4) 5.1 (1.6 to 15.7) |
8 (3.3) 4.2 (2.1 to 8.4) |
3 (0.7) 2.4 (0.8 to 7.4) |
0 (0.0) 0 |
0 (0.0) 0 |
0 (0.0) 0 |
| Malignancies (excluding NMSC) | 6 (0.8) 0.8 (0.4,1.8) |
0 (0.0) 0 |
1 (1.4) 1.4 (0.2 to 9.8) |
1 (0.5) 0.6 (0.1 to 4.5) |
9 (1.2) 1.2 (0.6 to 2.3) |
1 (0.9) 0.9 (0.1,6.5) |
1 (1.5) 1.6 (0.2,11.6) |
0 (0.0) 0 |
0 (0.0) 0 |
0 (0.0) 0 |
0 (0.0) 0 |
0 (0.0) 0 |
| NMSC | 7 (0.9) 0.9 (0.5 to 2.0) |
1 (0.7) 0.7 (0.1 to 5.1) |
0 (0.0) 0 |
1 (0.5) 0.6 (0.1 to 4.5) |
6 (0.8) 0.8 (0.4 to 1.7) |
0 (0.0) 0 |
0 (0.0) 0 |
0 (0.0) 0 |
1 (0.2) 0.8 (0.1 to 5.6) |
1 (1.1) 3.9 (0.6 to 27.9) |
0 (0.0) 0 |
0 (0.0) 0 |
*IRs are representative up to month 24.
†All HZ events include both serious and non-serious events.
AE, adverse event; CCP, cyclic citrullinated peptide; HZ, herpes zoster; IR, incidence rate; NMSC, non-melanoma skin cancer; RF, rheumatoid factor; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
In both tofacitinib- and placebo-treated patients, generally similar IRs for discontinuations due to AEs were observed across all serotype subgroups (tofacitinib 5 mg two times a day: 7.9–11.2; tofacitinib 10 mg two times a day: 10.1–13.3; placebo: 8.6–19.7). Likewise, IRs for serious infections were generally similar across serotype subgroups (tofacitinib 5 mg two times a day: 1.9–3.6; tofacitinib 10 mg two times a day: 1.6–4.6; placebo: 0–4.0).
IRs for herpes zoster were similar between the tofacitinib 5 and 10 mg two times a day groups (1.9–9.1 and 4.2–8.7, respectively) and IRs for both tofacitinib doses were higher than for placebo (0–2.4). With respect to seropositivity, tofacitinib-treated patients who were anti-CCP-/RF+ or anti-CCP+/RF- had numerically greater IRs (5.1–9.1 and 5.9–8.7, respectively) than tofacitinib-treated patients who were anti-CCP-/RF- or anti-CCP+/RF+ (1.9–4.2 and 3.7–4.3, respectively). However 95% CIs were wide and overlapping between groups.
IRs for malignancies (excluding NMSC) were similar between the tofacitinib 5 and 10 mg two times a day groups (0–1.4 and 0–1.6, respectively). No malignancies were observed in patients receiving placebo in any serotype subgroups. IRs for NMSC were similar in both tofacitinib-treated and placebo-treated patients (tofacitinib 5 mg two times a day: 0–0.9; tofacitinib 10 mg two times a day: 0–1.6; placebo: 0–3.9). No cases of NMSC were observed in anti-CCP-/RF+ patients.
Discussion
Identifying factors that predict the likelihood of clinical response may be of benefit in treatment decision making. As elevated levels of RF and/or anti-CCP antibodies are common in patients with RA, it was hypothesised that anti-CCP and/or RF status may influence clinical response and be predictive of treatment efficacy. This posthoc analysis assessed the impact of anti-CCP and RF seropositivity on tofacitinib efficacy and safety in patients with moderately to severely active RA and an inadequate response to DMARDs, based on data pooled from five Phase III studies.
ACR20/50/70 response rates were significantly improved following treatment with tofacitinib 5 and 10 mg two times a day vs placebo across all serotype subgroups. Likewise, significant LSM changes from baseline in HAQ-DI and FACIT-F were observed among all subgroups receiving tofacitinib 5 and 10 mg two times a day vs placebo. However, some differences were observed between serotype subgroups.
ACR20/50/70 ORs demonstrated that a higher proportion of patients who were anti-CCP+/RF+ achieved ACR20/50 responses following treatment with tofacitinib 5 or 10 mg two times a day and ACR70 following treatment with tofacitinib 10 mg two times a day, compared with patients who were anti-CCP-/RF-. Furthermore, DAS28-4(ESR) remission rates following tofacitinib 5 or 10 mg two times a day appeared lower in anti-CCP- patients, regardless of RF status. Tofacitinib 10 mg two times a day was associated with a significant change from baseline (compared with placebo) in SF-36 physical functioning in anti-CCP-/RF- patients; however the effect of tofacitinib 5 mg two times a day treatment was not significant compared with placebo in this subgroup. In addition, among patients who were anti-CCP+ (regardless of RF status), significantly higher DAS28-4(ESR) remission rates were achieved with tofacitinib 5 and 10 mg two times a day compared with placebo, however no significant differences were observed in anti-CCP-/RF+ or anti-CCP-/RF- patients. DAS28-4(ESR) ORs demonstrated that a higher proportion of patients receiving tofacitinib 10 mg two times a day who were anti-CCP+/RF+ or anti-CCP+/RF- achieved DAS28-4(ESR) remission or LDA than patients who were anti-CCP-/RF-, whereas anti-CCP-/RF+patients had the same likelihood of achieving DAS28-4(ESR) remission or LDA as anti-CCP-/RF- patients.
Previous studies have assessed the influence of seropositivity on the efficacy of csDMARDs such as MTX and biologic DMARDs including abatacept, rituximab and TNFi (eg, adalimumab). A study in patients receiving abatacept or TNFi found a positive correlation between seropositivity and greater clinical response to abatacept; patients who were anti-CCP+ and RF+ were more responsive to abatacept than patients who were seropositive for only one marker, who were, in turn, more responsive than seronegative patients.37 However, no correlation was observed between seropositivity for either marker and efficacy with TNFi.37 Another study found that patients who were anti-CCP+ were more responsive to both abatacept and adalimumab than patients who were anti-CCP-.38 Patients with the highest anti-CCP+ concentrations had greater clinical responses with abatacept than those patients with lower anti-CCP+ concentrations; no correlation was found between baseline anti-CCP antibody concentrations and adalimumab efficacy.38 Other studies have also found contrasting relationships between treatment efficacy and seropositivity. In one study of anti-CCP+/RF+, anti-CCP+/RF-, anti-CCP-/RF+ and anti-CCP-/RF- patients receiving rituximab, RF+ was more indicative of response than anti-CCP+.39
With respect to safety endpoints, the incidence of AEs, SAE, serious infections and discontinuations due to AEs were similar in patients receiving tofacitinib 5 mg two times a day, tofacitinib 10 mg two times a day or placebo and across serotype subgroups. The incidence of herpes zoster was higher following tofacitinib treatment compared with placebo, which is consistent with previously reported studies of tofacitinib.40 Some numerical differences in herpes zoster incidence rates were observed between serotype subgroups; however, the 95% CIs were wide and overlapping.
The analysis has a number of limitations. The data used in this study were obtained from pooled posthoc analyses of five Phase III studies. These studies were not designed to show differences based on serological status and this resulted in variations of sample sizes between subgroups. As a result, conclusions may be limited by the small patient numbers in some of the subgroups. Further prospective investigations, involving larger patient numbers, will be required in order to accurately assess potential differences in responses to tofacitinib between CCP and RF seropositive and seronegative patients with RA.
Overall, the results of this analysis of CCP and RF status in tofacitinib-treated patients with RA suggest that endpoints were not markedly influenced by seropositivity versus seronegativity. However, a higher proportion of patients who were anti-CCP+/RF+ achieved ACR20/50 responses following treatment with tofacitinib 5 or 10 mg two times a day and ACR70 following treatment with tofacitinib 10 mg two times a day, compared with patients who were anti-CCP-/RF-. Furthermore, DAS28-4(ESR) remission rates and changes from baseline in SF-36 physical functioning appeared lower in anti-CCP- patients. Further investigation is warranted to fully elucidate the relationship between seropositivity and the efficacy of tofacitinib.
Footnotes
Contributors: All authors meet the ICMJE guidelines for authorship and contributed equally to the design of the project, the discussion of the results and to the preparation of the manuscript. All authors read and approved the final version of the manuscript.
Funding: This study was sponsored by Pfizer Inc.
Competing interests: PB has participated in advisory boards and received honoraria from AbbVie, BMS, Janssen, Pfizer Inc, Roche and UCB. SH has acted as a consultant for AbbVie, Celgene, Eli Lilly, Janssen, Pfizer Inc and Roche. PN has received funding for research and clinical trials, and honoraria for lectures and advice, from Pfizer Inc. CAC and KK are employees of Pfizer Inc and hold stock/stock options in Pfizer Inc. DW and KT are employees of Pfizer Australia Pty. Ltd and hold stock/stock options in Pfizer Inc.
Patient consent for publication: Not required.
Ethics approval: Institutional Review Boards and/or Independent Ethics Committees at each investigational centre.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: On request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programmes that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data-access agreement with Pfizer.
References
- 1. Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs 2010;70:121–45. 10.2165/11531980-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 2. Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients' lives. Clin Exp Rheumatol 2010;28(3 Suppl 59):S32–S40. [PubMed] [Google Scholar]
- 3. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 4. Smolen JS, Landewé R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. 10.1136/annrheumdis-2016-210715 [DOI] [PubMed] [Google Scholar]
- 5. Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. The Lancet 2004;363:675–81. 10.1016/S0140-6736(04)15640-7 [DOI] [PubMed] [Google Scholar]
- 6. Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. 10.1056/NEJM199901283400401 [DOI] [PubMed] [Google Scholar]
- 7. Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594–602. 10.1056/NEJM200011303432202 [DOI] [PubMed] [Google Scholar]
- 8. St Clair EW, van der Heijde DM, Smolen JS, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004;50:3432–43. 10.1002/art.20568 [DOI] [PubMed] [Google Scholar]
- 9. Quinn MA, Conaghan PG, O'Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2005;52:27–35. 10.1002/art.20712 [DOI] [PubMed] [Google Scholar]
- 10. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613–20. 10.1136/annrheumdis-2012-203090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldbach-Mansky R, Lee J, McCoy A, et al. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res 2000;2:236–43. 10.1186/ar93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bas S, Perneger TV, Seitz M, et al. Diagnostic tests for rheumatoid arthritis: comparison of anti-cyclic citrullinated peptide antibodies, anti-keratin antibodies and IgM rheumatoid factors. Rheumatology 2002;41:809–14. 10.1093/rheumatology/41.7.809 [DOI] [PubMed] [Google Scholar]
- 13. Rantapää-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. 10.1002/art.11223 [DOI] [PubMed] [Google Scholar]
- 14. Berglin E, Johansson T, Sundin U, et al. Radiological outcome in rheumatoid arthritis is predicted by presence of antibodies against cyclic citrullinated peptide before and at disease onset, and by IgA-RF at disease onset. Ann Rheum Dis 2006;65:453–8. 10.1136/ard.2005.041376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steiner G, Smolen J. Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res 2002;4(Suppl 2):S1–S5. 10.1186/ar551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szodoray P, Szabó Z, Kapitány A, et al. Anti-citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun Rev 2010;9:140–3. 10.1016/j.autrev.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 17. Rau R, Herborn G, Menninger H, et al. Radiographic outcome after three years of patients with early erosive rheumatoid arthritis treated with intramuscular methotrexate or parenteral gold. Extension of a one-year double-blind study in 174 patients. Rheumatology 2002;41:196–204. 10.1093/rheumatology/41.2.196 [DOI] [PubMed] [Google Scholar]
- 18. Kastbom A, Strandberg G, Lindroos A, et al. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 2004;63:1085–9. 10.1136/ard.2003.016808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niewold TB, Harrison MJ, Paget SA. Anti-CCP antibody testing as a diagnostic and prognostic tool in rheumatoid arthritis. QJM 2007;100:193–201. 10.1093/qjmed/hcm015 [DOI] [PubMed] [Google Scholar]
- 20. Vencovský J, Machácek S, Sedová L, et al. Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 2003;62:427–30. 10.1136/ard.62.5.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coombs JH, Bloom BJ, Breedveld FC, et al. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 2010;69:413–6. 10.1136/ard.2009.108159 [DOI] [PubMed] [Google Scholar]
- 22. Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 2012;64:617–29. 10.1002/art.33383 [DOI] [PubMed] [Google Scholar]
- 23. Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 2009;60:1895–905. 10.1002/art.24567 [DOI] [PubMed] [Google Scholar]
- 24. Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. 10.1002/art.33419 [DOI] [PubMed] [Google Scholar]
- 25. Tanaka Y, Suzuki M, Nakamura H, et al. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res 2011;63:1150–8. 10.1002/acr.20494 [DOI] [PubMed] [Google Scholar]
- 26. Tanaka Y, Takeuchi T, Yamanaka H, et al. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol 2015;25:514–21. 10.3109/14397595.2014.995875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallenstein GV, Kanik KS, Wilkinson B, et al. Effects of the oral Janus kinase inhibitor tofacitinib on patient-reported outcomes in patients with active rheumatoid arthritis: results of two Phase 2 randomised controlled trials. Clin Exp Rheumatol 2016;34:430–42. [PubMed] [Google Scholar]
- 28. Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. The Lancet 2013;381:451–60. 10.1016/S0140-6736(12)61424-X [DOI] [PubMed] [Google Scholar]
- 29. Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012;367:495–507. 10.1056/NEJMoa1109071 [DOI] [PubMed] [Google Scholar]
- 30. Kremer J, Li ZG, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 2013;159:253–61. 10.7326/0003-4819-159-4-201308200-00006 [DOI] [PubMed] [Google Scholar]
- 31. Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370:2377–86. 10.1056/NEJMoa1310476 [DOI] [PubMed] [Google Scholar]
- 32. van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. 10.1002/art.37816 [DOI] [PubMed] [Google Scholar]
- 33. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508–19. 10.1056/NEJMoa1112072 [DOI] [PubMed] [Google Scholar]
- 34. Wollenhaupt J, Silverfield J, Lee EB, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open-label, long-term extension studies over 9 years. Arthritis Reumatol 2017;69(Suppl 10):683–4. 10.1136/annrheumdis-2018-eular.1733 [DOI] [Google Scholar]
- 35. Yamanaka H, Tanaka Y, Takeuchi T, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther 2016;18:34 10.1186/s13075-016-0932-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wollenhaupt J, Silverfield J, Lee EB, et al. Safety and efficacy of tofacitinib, an oral janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 2014;41:837–52. 10.3899/jrheum.130683 [DOI] [PubMed] [Google Scholar]
- 37. Harrold LR, Litman HJ, Connolly SE, et al. OP0178 Impact of anti-cyclic citrullinated peptide and rheumatoid factor status on response to abatacept therapy: findings from a US observational cohort. Ann Rheum Dis 2016;75(Suppl 2):123.2–4. 10.1136/annrheumdis-2016-eular.1277 [DOI] [Google Scholar]
- 38. Sokolove J, Schiff M, Fleischmann R, et al. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann Rheum Dis 2016;75:709–14. 10.1136/annrheumdis-2015-207942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quartuccio L, Fabris M, Salvin S, et al. Rheumatoid factor positivity rather than anti-CCP positivity, a lower disability and a lower number of anti-TNF agents failed are associated with response to rituximab in rheumatoid arthritis. Rheumatology 2009;48:1557–9. 10.1093/rheumatology/kep314 [DOI] [PubMed] [Google Scholar]
- 40. Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2675–84. 10.1002/art.38745 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000742supp001.docx (20.1KB, docx)