Abstract
Background
Existing evidence for the prospective association of vitamin D status with type 2 diabetes (T2D) is focused almost exclusively on circulating total 25-hydroxyvitamin D [25(OH)D] without distinction between its subtypes: nonepimeric and epimeric 25(OH)D3 stereoisomers, and 25(OH)D2, the minor component of 25(OH)D. We aimed to investigate the prospective associations of circulating levels of the sum and each of these three metabolites with incident T2D.
Methods
This analysis in the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct case-cohort study for T2D included 9671 incident T2D cases and 13,562 subcohort members. Plasma vitamin D metabolites were quantified by liquid chromatography–mass spectrometry. We used a multivariable Prentice-weighted Cox regression to estimate hazard ratios (HRs) of T2D for each metabolite. Analyses were performed separately within country, and estimates were combined across countries using random-effects meta-analysis.
Results
The mean concentrations (SD) of total 25(OH)D, nonepimeric 25(OH)D3, epimeric 25(OH)D3, and 25(OH)D2 were 41.1 (17.2), 40.7 (17.3), 2.13 (1.31), and 8.16 (6.52) nmol/L, respectively. Plasma total 25(OH)D and nonepimeric 25(OH)D3 were inversely associated with incident T2D [multivariable-adjusted HR per 1 SD = 0.81 (95% CI, 0.77, 0.86) for both variables], whereas epimeric 25(OH)D3 was positively associated [per 1 SD HR = 1.16 (1.09, 1.25)]. There was no statistically significant association with T2D for 25(OH)D2 [per 1 SD HR = 0.94 (0.76, 1.18)].
Conclusions
Plasma nonepimeric 25(OH)D3 was inversely associated with incident T2D, consistent with it being the major metabolite contributing to total 25(OH)D. The positive association of the epimeric form of 25(OH)D3 with incident T2D provides novel information to assess the biological relevance of vitamin D epimerization and vitamin D subtypes in diabetes etiology.
We examined the prospective association of plasma vitamin D metabolites with type 2 diabetes and found that the epimeric form of plasma 25(OH)D3 was positively associated with type 2 diabetes risk.
The global burden of type 2 diabetes (T2D) continues to expand, making the prevention of T2D a high public health priority (1). Adequacy of vitamin D status, indicated by circulating 25-hydroxyvitamin D [25(OH)D], has been proposed as a modifiable factor for the prevention of T2D (2, 3). However, previous observational studies reporting the association of 25(OH)D with incidence of T2D exclusively refer to either total 25(OH)D, comprised of 25(OH)D3 and 25(OH)D2, or to 25(OH)D3, but little is known about the associations with T2D for individual 25(OH)D metabolites, including 25(OH)D2 and the epimeric form of 25(OH)D3 [3-epi-25(OH)D3] (4), where an epimer refers to one of a pair of stereoisomers including the nonepimeric and epimeric forms.
Circulating 25(OH)D2, which is mainly derived from dietary intake or vitamin supplements, has a low detection rate in general populations (5). Therefore, a large sample size with sufficient incident T2D cases is needed to examine the association of 25(OH)D2 with T2D risk, which has not been reported so far. The epimeric form 3-epi-25(OH)D3 has been recognized only recently as a product of epimerization of the nonepimeric 25(OH)D3 (4, 6, 7), although the enzyme for the epimerization step and also the biochemical roles of 3-epi-25(OH)D3 remain unknown. In a prior study in Switzerland (8), 3-epi-25(OH)D3 as a proportion of total 25(OH)D3 [3-epi-25(OH)D3 plus nonepimeric 25(OH)D3] was positively associated with incident insulin resistance, although its association with incident T2D has not been reported so far. As well as lack of clarity about their associations with incident T2D, the correlates of 3-epi-25(OH)D3 and 25(OH)D2 across European countries are largely unknown, with previous investigations being limited to a single study or country, including several US cohorts (5, 9–11).
Therefore, the aims of this study were to: (i) investigate the correlates of plasma concentrations of nonepimeric 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2 across eight European countries; and (ii) examine the associations with incident T2D for the three 25(OH)D metabolites, as well as total 25(OH)D for comparison with previous literature. We hypothesized that 3-epi-25(OH)D3 would be associated with higher risk of T2D given the prior findings (8) and that nonepimeric 25(OH)D3, 25(OH)D2, and total 25(OH)D would be associated with a lower risk of T2D.
Materials and Methods
Study design
The current analysis used data from the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct case-cohort study (12), conducted in eight European countries. Briefly, a total of 12,403 T2D cases were ascertained and verified from among 340,234 participants of the EPIC cohort study with 3.99 million person-years of follow-up (1991 to 2007). A subcohort was created by randomly selecting 16,835 individuals from those with available stored blood and buffy coat, stratified by center. A total of 16,154 participants remained in the subcohort after exclusion of those with prevalent diabetes (n = 548) and unknown diabetes status (n = 129). There were 778 overlapping incident T2D cases in the subcohort, which is a feature of the case-cohort design. For this analysis, we excluded individuals with no plasma samples available (n = 4969) or with hemolyzed samples (n = 32), or samples that were too low in volume for analysis (n = 49) or failed in biochemical analysis (n = 78). Therefore, our final sample for analysis included 22,651 participants with measurement of at least one 25(OH)D metabolite (9671 T2D cases and 13,562 subcohort individuals, with 582 incident T2D cases in the subcohort) (7). All participants provided written informed consent, and the study was approved by the local ethics committees in the participating centers and by the Internal Review Board of the International Agency for Research on Cancer.
Ascertainment of incident T2D cases was conducted through a review of multiple sources of evidence, including self-report, linkage to primary care registers, secondary care registers, medication use (drug registers), hospital admissions, and mortality data (12). Information from any follow-up visit or external evidence with a date later than the baseline visit was used. T2D cases in Denmark and Sweden were identified through local and national diabetes and pharmaceutical registers, and they were considered to be verified. To increase the specificity of the case definition for other centers, we sought further evidence for all self-reported cases with independent sources, including individual medical record reviews in some centers (12). Follow-up was censored at the date of diagnosis (31 December 2007) or the date of death, whichever occurred first.
Measurement of plasma 25(OH)D metabolites and other blood biomarkers
Plasma 25(OH)D metabolites were measured at Vitas AS (Oslo, Norway; a reference laboratory in Europe with a Vitamin D External Quality Assessment Scheme certificate) (13) using liquid chromatography–tandem mass spectrometry. We measured concentrations of nonepimeric 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2 in citrated plasma samples stored at baseline at –196°C (at −150°C in Denmark). Past evidence indicates stability of 25(OH)D and its metabolites in stored serum or plasma samples for >10 years (14). Briefly, 50 µL of human plasma was diluted with 150 µL of isopropanol with deuterium-labeled nonepimeric 25(OH)D3 as an internal standard. After thorough mixing (10 minutes) and centrifugation (20 minutes, 4000 rpm at 10°C), an aliquot of 30 µL was injected from the supernatant into the HPLC system (Agilent 1260/1290 liquid chromatograph; Agilent Technologies, Palo Alto, CA) interfaced by atmospheric pressure chemical ionization to an Agilent Technologies 6420 Triple Quad liquid chromatography–tandem mass-spectrometry system operated in multiple reaction monitoring mode. 25(OH)D metabolites were separated on an Ascentis® Express F5 150-mm × 4.6-mm column with 2.7 µM particles, maintained at 20°C. A one-point calibration curve was made from analysis of a natural plasma calibrator, where the value was set by the use of reference material from Chromsystems. The coefficient of variation derived from the quality control samples assessed with the study samples was 8.25% for nonepimeric 25(OH)D3, 20.9% for 3-epi-25(OH)D3, and 10.1% for 25(OH)D2. The lower limits of quantification (LLQs) for nonepimeric 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2 were 5 nmol/L, 1 nmol/L, and 3 nmol/L, respectively.
We calculated total 25(OH)D as the sum of nonepimeric 25(OH)D3 and 25(OH)D2 for comparability with most prior literature. As a secondary definition of total 25(OH)D, we also calculated the sum of three metabolites: nonepimeric 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2. The aim of the latter definition was to enable comparability with previous studies that used measurement methods not enabling separation between 3-epi-25(OH)D3 and nonepimeric 25(OH)D3, and hence 25(OH)D included both D3 metabolites (4, 15).
Serum metabolic biomarkers (except for the Umea center in Sweden, where plasma was used) were measured at Stichting Ingenhousz Laboratory (Etten-Leur, Netherlands), including total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, uric acid, creatinine, aspartate transaminase, alanine transaminase, and γ-glutamyltransferase. Low-density lipoprotein cholesterol (LDL-C) was calculated based on the Friedewald formula. Plasma phospholipid fatty acids were measured at Medical Research Council Human Nutrition Research (Cambridge, UK) (16).
Measurement of diet and other covariates
Dietary information was collected using country- or center-specific self- or interviewer-administered dietary questionnaires, which were developed within each country (17, 18). Baseline physical activity was assessed with a questionnaire (19). Other data were collected by questionnaire on a variety of lifestyle and health-related factors, such as education level, occupational status, smoking status, and menstrual and reproductive history (18).
Statistical analysis
Statistical analysis was performed using Stata 14 (StataCorp, College Station, TX). We imputed nonepimeric 25(OH)D3 values below the LLQ (n = 23) by assigning a random value between 0 and the LLQ, but we did not impute 3-epi-25(OH)D3 or 25(OH)D2, as 60.3% and 95.5% of their values, respectively, were below the LLQ. We calculated the ratio of 3-epi-25(OH)D3 to nonepimeric 25(OH)D3, as the ratio reflects the enzyme activity for the epimerization.
We accounted for the seasonality of blood draw using a linear combination of the sine and cosine of the time variable (day of blood draw) (20). All 25(OH)D variables were winsorized using the values representing the first and 99th percentiles of the distribution in the subcohort. We fit a multivariable linear regression model to data from the subcohort to estimate country-specific cross-sectional associations of demographic, lifestyle, and dietary variables and circulating biomarkers with individual 25(OH)D metabolites, except for 3-epi-25(OH)D3 and 25(OH)D2, where Tobit regression was used owing to the large number of left-censored values below the LLQ (21). The analysis of 25(OH)D2 was not stratified by country owing to the small sample size within each country. All metabolites except for nonepimeric 25(OH)D3 had skewed distributions and were log transformed. Estimated associations were combined across countries using random-effects meta-analysis.
We used Prentice-weighted Cox regression to estimate country-specific hazard ratios (HRs) for T2D comparing quintiles and per 1 SD (calculated from the subcohort) of each 25(OH)D variable, and then combined these HRs using random-effects meta-analysis. For 25(OH)D2, we estimated the HRs from models fit to the overall dataset owing to the small sample sizes with available data within each country. We also estimated the HR of T2D for 3-epi-25(OH)D3 or 25(OH)D2 below the LLQ compared with the lowest quintile of the exposure. We fit four statistical models: model 1 included age as the underlying timescale, sex, study center, and seasonality; model 2, as model 1 plus smoking status, physical activity, education, alcohol drinking, total energy intake, Mediterranean diet score as an indicator of diet quality, and circulating lipid biomarkers (HDL-C, LDL-C); model 3, as model 2 plus body mass index (BMI); and model 4, as model 3 plus mutual adjustment for the other 25(OH)D metabolites [nonepimeric 25(OH)D3, 3-epi-25(OH)D3, or 25(OH)D2]. We adjusted for HDL-C and LDL-C, rather than total cholesterol, because circulating 25(OH)D was more specifically associated with HDL-C or LDL-C in previous reports (22–24).
We performed several additional analyses based on the most adjusted model 4 for the 25(OH)D metabolites: model 4a, including dietary factors (dietary intake of fish, egg, red meat, dairy products, cereal, poultry, processed meat, offal, margarine, butter, and mushrooms) and vitamin supplement use as covariates; model 4b, excluding people with HbA1c ≥6.5% (or 48 mmol/mol) at baseline or those confirmed as T2D cases within the first 2 years after baseline to assess the potential influence of reverse causality; model 4c, including baseline HbA1c as a covariate to examine the influence of baseline glycemic status; model 4d, including circulating hepatic (alanine transaminase, aspartate transaminase, and γ-glutamyltransferase) and renal function markers (creatinine, uric acid) as covariates to examine the influence of liver and kidney function; model 4e, including plasma phospholipid saturated fatty acids and polyunsaturated fatty acids as covariates to examine the influence of plasma fatty acids given that vitamin D is a lipid-soluble vitamin; model 4f, including baseline prevalence of stroke, heart disease, or cancer or family history of diabetes as covariates; and model 4g, among women, we additionally adjusted for hormone therapy use and menopausal status. We also performed an interaction analysis between season and nonepimeric 25(OH)D3 on T2D, as well as an analysis stratified by season (in model 4) to investigate whether the association between nonepimeric 25(OH)D3 and incident T2D was consistent across seasons.
We investigated the impact of missing covariate data on the results of both the linear and weighted Cox regression analyses using multiple imputation. Analysis was performed in each of 10 imputed datasets that were created using all the covariates from model 4, with the event variable and Nelson-Aalen estimate of cumulative hazard accounting for the case-cohort design; estimates from each imputation were combined using Rubin’s rules (25).
To explore the shape of the association between 25(OH)D metabolites and incident T2D, we applied a multivariable random-effects meta-analysis to combine the estimated country-specific association of plasma 25(OH)D metabolites with incident T2D (based on model 4 above) using a restricted cubic spline (26, 27); for 25(OH)D2, the model was fit to the overall dataset rather than by country. As the wide range of 3-epi-25(OH)D3 and 25(OH)D2 would potentially distort the shape of the associations, we used log-transformed values for these analyses and plotted the HR of T2D by the log-transformed 3-epi-25(OH)D3 or 25(OH)D2. The P values for nonlinearity were calculated using a Wald test of the relevant parameter from the restricted cubic spline model.
Finally, we investigated potential multiplicative interactions of 25(OH)D metabolites with age, sex, BMI, physical activity, hormone therapy use and menopausal status, modeling age, BMI, and physical activity as continuous variables; others (sex, hormone therapy use and menopausal status) as binary. We estimated associations within the relevant subgroups when a significant interaction (P < 0.05) was observed.
Results
Population characteristics
The mean (SD) plasma concentrations in the subcohort were as follows: plasma total 25(OH)D as a sum of nonepimeric 25(OH)D3 and 25(OH)D2, 41.1 (17.2) nmol/L; nonepimeric 25(OH)D3, 40.7 (17.3) nmol/L; 3-epi-25(OH)D3, 2.13 (1.31) nmol/; and 25(OH)D2, 8.16 (6.52) nmol/L. The baseline population characteristics by cases and noncases are presented in an online repository (7), and baseline population characteristics in the subcohort by quintiles of plasma 25(OH)D metabolites are presented in Table 1 and an online repository (7). In the subcohort, nonepimeric 25(OH)D3 was positively correlated with 3-epi-25(OH)D3 (Spearman r = 0.44, P < 0.001) and negatively correlated with 25(OH)D2 (Spearman r = −0.30, P < 0.001), whereas there was no significant correlation between 3-epi-25(OH)D3 and 25(OH)D2 (P = 0.109) (7).
Table 1.
Population Characteristics by Quintiles of Plasma Nonepimeric 25(OH)D3 and 3-epi-25(OH)D3 in the Subcohort of the EPIC-InterAct Study
| Quintiles of Nonepimeric 25(OH)D3 |
Quintiles of 3-epi-25(OH)D3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n = 2712) | Q2 (n = 2712) | Q3 (n = 2713) | Q4 (n = 2712) | Q5 (n = 2713) | Below the LLQa (n = 8049) | Q1 (n = 1102) | Q2 (n = 1103) | Q3 (n = 1102) | Q4 (n = 1103) | Q5 (n = 1103) | |
| Total 25(OH)Db | 19.6 (5.7) | 30.7 (3.4) | 39.3 (2.9) | 48.8 (3.4) | 66.8 (11.6) | 34.9 (14.5) | 41.4 (14) | 45.3 (14.6) | 47.7 (14.1) | 52.2 (14.7) | 63.7 (17.5) |
| Nonepimeric 25(OH)D3, nmol/L | 18.9 (4.7) | 30.3 (2.7) | 39 (2.5) | 48.6 (3.2) | 66.5 (11.5) | 34.4 (14.5) | 41.1 (14) | 45 (14.6) | 47.4 (14.1) | 52.0 (14.7) | 63.5 (17.5) |
| 3-epi-25(OH)D3, nmol/L | 1.4 (0.4) | 1.6 (0.7) | 1.7 (0.6) | 2.0 (0.9) | 2.8 (1.8) | 1.1 (0.1) | 1.4 (0.1) | 1.8 (0.1) | 2.3 (0.2) | 4.1 (1.7) | |
| Ratio of 3-epi-25(OH)D3 to nonepimeric 25(OH)D3 | 0.07 (0.03) | 0.05 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.03 (0.01) | 0.04 (0.01) | 0.04 (0.01) | 0.05 (0.02) | 0.07 (0.02) | |
| 25(OH)D2, nmol/L | 10.8 (9) | 7.8 (4.7) | 6.7 (4) | 7.1 (4.4) | 6.3 (5.9) | 8.7 (7) | 8.1 (6.9) | 7.7 (4.8) | 6.6 (3.6) | 6.5 (3.5) | 6.7 (7.2) |
| Age, y | 52 (8.8) | 51.9 (8.8) | 51.8 (8.9) | 51.5 (9) | 50.7 (9.8) | 51.7 (9) | 51.5 (9.3) | 51.8 (8.9) | 51.2 (9.3) | 51.4 (9) | 51.1 (9.4) |
| BMI, kg/m2 | 26.7 (4.8) | 26.7 (4.3) | 26.5 (4.2) | 25.8 (4) | 25.0 (3.6) | 26.3 (4.4) | 26.0 (4.3) | 25.9 (4.1) | 25.8 (4.1) | 25.9 (3.9) | 25.7 (3.7) |
| Energy intake, kcal/d | 2150 (656) | 2121 (629) | 2151 (633) | 2129 (635) | 2081 (634) | 2111 (633) | 2131 (649) | 2131 (645) | 2125 (635) | 2165 (633) | 2194 (659) |
| Sex, % | |||||||||||
| Men | 35.8 | 35.8 | 39.0 | 38.6 | 37.5 | 34.3 | 35.8 | 36.5 | 40.0 | 44.3 | 51.8 |
| Women | 64.2 | 64.2 | 61.0 | 61.4 | 62.5 | 65.7 | 64.2 | 63.5 | 60.0 | 55.7 | 48.2 |
| Alcohol consumption, % | |||||||||||
| 0 g/d | 18.7 | 17.4 | 16.8 | 16.3 | 12.1 | 18.1 | 15.7 | 15.4 | 14.9 | 11.8 | 10.2 |
| >0 to <6 g/d | 32.3 | 33.3 | 32.6 | 33.5 | 38.5 | 35.2 | 37.7 | 32.6 | 33.8 | 31.0 | 26.7 |
| 6 to <12 g/d | 12.2 | 14.2 | 15.1 | 14.9 | 15.8 | 14.0 | 14.2 | 16.6 | 14.9 | 14.8 | 14.9 |
| 12 to <24 g/d | 13.6 | 15.8 | 15.6 | 16.8 | 15.5 | 15.1 | 14.8 | 15.0 | 15.8 | 17.0 | 17.5 |
| ≥24 g/d | 22.7 | 18.9 | 19.4 | 18.1 | 17.8 | 17.3 | 17.1 | 20.0 | 19.9 | 25.2 | 30.2 |
| Mediterranean diet score, % | |||||||||||
| Low (score 0–6) | 21.3 | 20.6 | 19.8 | 22.2 | 28.3 | 21.9 | 22.5 | 23.5 | 25.5 | 21.6 | 23.5 |
| Moderate (score 7–10) | 44.1 | 41.8 | 43.4 | 43.4 | 44.3 | 43.2 | 43.6 | 42.0 | 42.9 | 44.7 | 45.1 |
| High (score 11–18) | 32.1 | 35.1 | 34.8 | 32.2 | 25.3 | 32.7 | 31.4 | 32.3 | 29.1 | 32.1 | 28.8 |
| Physical activity, % | |||||||||||
| Inactive | 29.0 | 26.5 | 23.1 | 21.4 | 18.9 | 26.1 | 21.4 | 20.9 | 22.6 | 19.9 | 17.0 |
| Moderately inactive | 34.4 | 34.0 | 34.4 | 30.6 | 28.8 | 33.0 | 32.9 | 32.7 | 30.2 | 32.5 | 29.6 |
| Moderately active | 20.2 | 20.6 | 21.3 | 24.3 | 24.6 | 21.6 | 21.9 | 22.7 | 24.4 | 22.7 | 23.8 |
| Active | 15.6 | 17.8 | 19.6 | 22.1 | 25.3 | 17.6 | 22.1 | 22.1 | 21.7 | 24.1 | 28.4 |
| Smoking status, % | |||||||||||
| Never | 46.6 | 47.5 | 47.7 | 48.3 | 46.2 | 48.3 | 48.3 | 47.3 | 47.5 | 45.9 | 39.8 |
| Former | 21.5 | 25.6 | 26.6 | 28.4 | 28.9 | 24.5 | 27.1 | 25.6 | 26.7 | 29.6 | 34.5 |
| Current | 31.0 | 25.7 | 24.3 | 22.0 | 23.3 | 26.2 | 23.0 | 25.6 | 24.5 | 23.1 | 23.8 |
| Educational level, % | |||||||||||
| None | 10.1 | 10.5 | 9.3 | 7.7 | 5.3 | 9.2 | 7.2 | 7.0 | 8.9 | 7.4 | 8.2 |
| Primary | 34.0 | 32.7 | 33.2 | 30.3 | 28.5 | 32.3 | 29.9 | 33.2 | 30.6 | 31.8 | 29.3 |
| Technical or professional | 20.9 | 21.0 | 21.6 | 22.4 | 24.5 | 21.4 | 23.9 | 23.0 | 22.2 | 23.2 | 23.2 |
| Secondary | 13.9 | 14.9 | 15.2 | 15.5 | 18.3 | 15.4 | 15.7 | 16.0 | 15.8 | 16.3 | 15.5 |
| Higher education | 19.4 | 19.1 | 18.5 | 21.7 | 21.0 | 19.8 | 21.0 | 18.5 | 20.4 | 19.1 | 22.2 |
| Season of blood draw, % | |||||||||||
| Winter (December–February) | 36.6 | 28.8 | 23.1 | 19.2 | 14.1 | 29.1 | 22.8 | 21.2 | 18.9 | 15.2 | 9.2 |
| Spring (March–May) | 40.6 | 34.2 | 28.8 | 21.5 | 14.2 | 31.3 | 29.9 | 27.0 | 24.4 | 19.7 | 13.4 |
| Summer (June–August) | 8.6 | 16.8 | 20.1 | 24.7 | 29.2 | 14.7 | 18.7 | 23.1 | 24.1 | 30.0 | 41.1 |
| Autumn (September–November) | 14.1 | 20.0 | 27.8 | 34.2 | 42.4 | 24.7 | 28.5 | 28.6 | 32.0 | 34.8 | 36.4 |
All values are expressed as mean (SD) or as percentage.
The LLQs for nonepimeric 25(OH)D3 and 3-epi-25(OH)D3 are 5 nmol/L and 1 nmol/L, respectively.
Total 25(OH)D: sum of nonepimeric 25(OH)D3 + 25(OH)D2.
Cross-sectional associations of demographic, lifestyle, and dietary variables and circulating biomarkers with plasma 25(OH)D metabolites
We observed seasonal variation for both nonepimeric 25(OH)D3 and 3-epi-25(OH)D3 across countries with highest levels between July and September, and lowest levels between January and March (P < 0.001 for seasonal variation) (7). No consistent pattern of seasonal variation across countries was seen for 25(OH)D2.
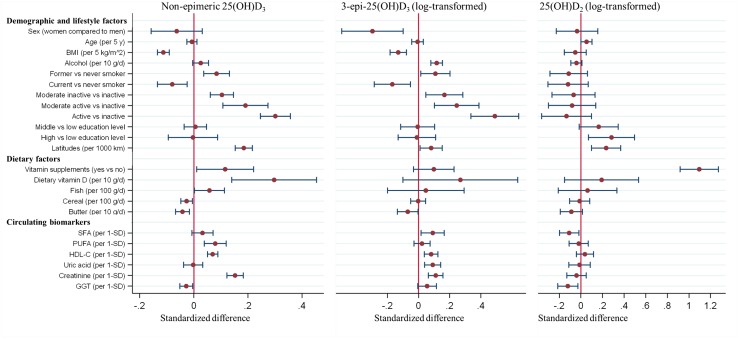
Accounting for the influence of seasonal variation and other potential confounders, several demographic, lifestyle, and dietary factors and circulating biomarkers were associated with nonepimeric 25(OH)D3 (Fig. 1) (7). Dietary vitamin D intake and vitamin supplements as well as physical activity and serum creatinine were positively associated with nonepimeric 25(OH)D3, whereas BMI was inversely associated. Associations with 3-epi-25(OH)D3 were generally weaker in magnitude than, but directionally consistent with, nonepimeric 25(OH)D3. Alcohol intake and physical activity were both positively associated, whereas BMI was inversely associated with 3-epi-25(OH)D3. Vitamin supplements showed the most strongly positive association with 25(OH)D2. Alcohol intake was positively associated with the ratio of 3-epi-25(OH)D3 to nonepimeric 25(OH)D3. Findings from analyses using multiple imputation were similar.
Figure 1.
Association of demographic, lifestyle, dietary variables, or circulating biomarkers with plasma 25(OH)D metabolites in the EPIC-InterAct subcohort. The estimates in the figure represent standardized differences in individual 25(OH)D metabolites (in SD unit) per 1 standardized unit/category difference in each demographic, lifestyle, dietary factor, or circulating biomarker. Linear regression or Tobit regression was used to obtain the country-specific estimates of association (except for latitude due to limited study centers in some countries, or 25(OH)D2 due to limited sample size), which were combined across countries using random-effects meta-analysis. Only associations that were significant (P < 0.05) for at least one metabolite are presented. Estimates for other variables and covariates included in the models are presented in an online repository (7). Results are from complete case analysis, with n = 11,369 for each of the nonepimeric 25(OH)D3, 3-epi-25(OH)D3, and 25(OH)D2. GGT, γ-glutamyltransferase; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids.
Association between plasma 25(OH)D metabolites and incident T2D
Table 2 shows that after adjustment for age, sex, center, and seasonality (model 1), there was an inverse association between nonepimeric 25(OH)D3 and incident T2D (model 1) that was attenuated after adjustment for diabetes risk factors, circulating lipids, and BMI (models 2 and 3) but became stronger after adjustment for the other vitamin D metabolites. Plasma 3-epi-25(OH)D3 was positively associated with T2D in models that adjusted for BMI (model 3) or further adjusted for the other vitamin D metabolites (model 4). Comparing quintiles, a positive association between 3-epi-25(OH)D3 and T2D was present for Q5 vs Q1 (Table 2). There was no evidence of an association with T2D comparing 3-epi-25(OH)D3 below the LLQ with Q1 in any of the models (Table 2). The association of total plasma 25(OH)D [as the sum of nonepimeric 25(OH)D3 and 25(OH)D2] with incident T2D was very similar to that of nonepimeric 25(OH)D3 across the four models (Table 2). The secondary inclusion of 3-epi-25(OH)D3 into the definition of total 25(OH)D produced similar results (7).
Table 2.
Association of Plasma 25(OH)D Metabolites With Incident T2D: EPIC-InterAct Study
| HR (95% CI)a |
|||||||
|---|---|---|---|---|---|---|---|
| Below the LLQb | Q1 | Q2 | Q3 | Q4 | Q5 | Per 1 SD | |
| Total 25(OH)D [nonepimeric 25(OH)D3 + 25(OH)D2], nmol/L | |||||||
| Median (range)c | 20.1 (5.68 to <25.9) | 30.9 (25.9–35.1) | 39.4 (35.1 to <43.7) | 48.7 (43.7 to <54.6) | 63.6 (54.6 to <118.7) | ||
| No. cases per subcohort | 2185/2460 | 1885/2477 | 1612/2488 | 1460/2479 | 1189/2441 | ||
| Model 1 | 1 (ref) | 0.84 (0.71, 0.98) | 0.65 (0.57, 0.73) | 0.55 (0.48, 0.64) | 0.43 (0.39, 0.48) | 0.71 (0.68, 0.73) | |
| Model 2 | 1 (ref) | 0.82 (0.70, 0.96) | 0.67 (0.59, 0.76) | 0.61 (0.53, 0.71) | 0.53 (0.46, 0.60) | 0.76 (0.73, 0.80) | |
| Model 3 | 1 (ref) | 0.91 (0.75, 1.10) | 0.79 (0.67, 0.93) | 0.79 (0.64, 0.98) | 0.72 (0.60, 0.87) | 0.86 (0.81, 0.91) | |
| Model 4 | 1 (ref) | 0.90 (0.74, 1.09) | 0.76 (0.65, 0.90) | 0.74 (0.61, 0.89) | 0.62 (0.54, 0.72) | 0.81 (0.77, 0.86) | |
| Nonepimeric 25(OH)D3, nmol/L | |||||||
| Median (range)c | 19.6 (5.30 to <25.5) | 30.5 (25.5 to <34.8) | 39.0 (34.8 to <43.3) | 48.4 (43.3 to <54.4) | 63.3 (54.4–118.7) | ||
| No. cases per subcohort | 2188/2461 | 1879/2476 | 1595/2491 | 1474/2474 | 1195/2443 | ||
| Model 1 | 1 (ref) | 0.83 (0.72, 0.95) | 0.64 (0.57, 0.72) | 0.57 (0.50, 0.65) | 0.44 (0.39, 0.49) | 0.71 (0.69, 0.74) | |
| Model 2 | 1 (ref) | 0.82 (0.71, 0.94) | 0.67 (0.58, 0.77) | 0.63 (0.55, 0.71) | 0.54 (0.47, 0.61) | 0.77 (0.74, 0.80) | |
| Model 3 | 1 (ref) | 0.89 (0.76, 1.04) | 0.76 (0.63, 0.91) | 0.80 (0.66, 0.97) | 0.72 (0.61, 0.85) | 0.86 (0.82, 0.91) | |
| Model 4 | 1 (ref) | 0.88 (0.75, 1.04) | 0.73 (0.61, 0.87) | 0.74 (0.62, 0.88) | 0.62 (0.54, 0.72) | 0.81 (0.77, 0.86) | |
| 3-epi-25(OH)D3, nmol/L | |||||||
| Median (range)c | <1 | 1.11 (1.00 to <1.25) | 1.40 (1.25 to <1.57) | 1.76 (1.57 to <1.98) | 2.29 (1.98 to <2.74) | 3.49 (2.74–15.4) | |
| No. cases per subcohort | 5143/7312 | 653/1007 | 678/1000 | 599/1006 | 619/1015 | 639/1005 | |
| Model 1 | 1.06 (0.95, 1.19) | 1 (ref) | 1.01 (0.87, 1.17) | 0.89 (0.76, 1.03) | 0.87 (0.75, 1.01) | 0.85 (0.73, 1.00) | 0.99 (0.94, 1.04) |
| Model 2 | 1.04 (0.92, 1.18) | 1 (ref) | 1.06 (0.89, 1.26) | 0.94 (0.79, 1.11) | 0.94 (0.80, 1.11) | 0.99 (0.83, 1.17) | 1.03 (0.97, 1.09) |
| Model 3 | 0.98 (0.83, 1.15) | 1 (ref) | 1.08 (0.86, 1.35) | 0.95 (0.79, 1.14) | 1.00 (0.84, 1.20) | 1.12 (0.91, 1.37) | 1.09 (1.02, 1.17) |
| Model 4 | 0.92 (0.79, 1.08) | 1 (ref) | 1.09 (0.85, 1.38) | 0.99 (0.82, 1.19) | 1.10 (0.92, 1.31) | 1.36 (1.08, 1.71) | 1.16 (1.09, 1.25) |
| 25(OH)D2, nmol/L | |||||||
| Median (range)c | <3 | 3.36 (3.03 to <3.74) | 4.30 (3.74 to <5.06) | 6.12 (5.06 to <7.42) | 8.74 (7.42 to <11.1) | 15.0 (11.1–46.4) | |
| No. cases per subcohort | 7988/11,773 | 50/111 | 72/115 | 70/116 | 88/114 | 63/116 | |
| Model 1 | 1.45 (1.02, 2.05) | 1 (ref) | 1.16 (0.73, 1.83) | 1.07 (0.68, 1.70) | 1.32 (0.85, 2.07) | 1.01 (0.63, 1.61) | 1.00 (0.85, 1.18) |
| Model 2 | 1.36 (0.93, 1.98) | 1 (ref) | 1.04 (0.63, 1.71) | 1.12 (0.68, 1.85) | 1.44 (0.88, 2.38) | 1.02 (0.62, 1.69) | 1.02 (0.84, 1.23) |
| Model 3 | 1.30 (0.86, 1.96) | 1 (ref) | 0.90 (0.50, 1.64) | 1.18 (0.69, 2.02) | 1.18 (0.67, 2.09) | 1.08 (0.62, 1.87) | 1.03 (0.84, 1.25) |
| Model 4 | 1.29 (0.86, 1.95) | 1 (ref) | 0.93 (0.52, 1.66) | 1.15 (0.68, 1.96) | 1.10 (0.62, 1.96) | 1.00 (0.58, 1.73) | 0.94 (0.76, 1.18) |
HRs of T2D comparing quintiles (Q2 to Q5) of 25(OH)D metabolites with Q1 or per 1 SD increase of 25(OH)D metabolites, estimated from country-specific Prentice-weighted Cox regression models; estimates were combined across countries using random-effects meta-analysis. Effect estimates for 25(OH)D2 were derived from analysis of the overall EPIC-InterAct data (i.e., not country-specific) owing to limited sample size. 1 SD (calculated from the subcohort) was 17.3 nmol/L for nonepimeric 25(OH)D3, 1.31 nmol/L for 3-epi-25(OH)D3, and 6.52 nmol/L for 25(OH)D2. The present analyses were based on complete case analyses excluding participants with missing covariates based on model 4. The sample size of total cases per subcohort was 8331/12,345 for nonepimeric 25(OH)D3, 3188/5033 for 3-epi-25(OH)D3 and 343/572 for 25(OH)D2. Models were as follows: model 1, adjusted for age (as underlying timescale), sex, center, and seasonality (continuous: sine and cosine function of the day of blood draw); model 2, model 1 plus smoking status (current, former, never), physical activity (inactive, moderately inactive, moderately active, active), education (none, primary, technical or professional, secondary, higher education), alcohol drinking (never, >0 to <6 g/d, 6 to <12 g/d, 12 to <24 g/d, ≥24 g/d), total energy intake (continuous), Mediterranean diet score (low, moderate, high) and plasma lipid biomarkers (continuous: HDL-cholesterol, LDL-cholesterol); model 3, model 2 plus BMI (continuous); model 4, model 3 plus mutual adjustment for the other 25(OH)D metabolites [nonepimeric 25(OH)D3 (continuous), 3-epi-25(OH)D3 (categorical: below the LLQ, Q1, Q2, Q3, Q4, and Q5), or 25(OH)D2 (categorical: below and above the LLQ)].
The LLQs for 3-epi-25(OH)D3 and 25(OH)D2 are 1 nmol/L and 3 nmol/L, respectively.
Median and range of the 25(OH)D metabolites in each quintile in the InterAct subcohort.
There was no evidence of an association between 25(OH)D2 and T2D in any of the analyses (Table 2). The ratio of 3-epi-25(OH)D3 to nonepimeric 25(OH)D3 was positively associated with T2D in all models when comparing the extreme quintile groups (7). There was little evidence of between-country heterogeneity for vitamin D metabolites (7).
Results were largely unchanged in sensitivity analyses that included adjustment for a range of additional factors or that addressed possible reverse causation or applied multiple imputation (7). The association between nonepimeric 25(OH)D3 and incident T2D was similar across seasons (no significant interaction was found: P interaction = 0.87), with per SD HR 0.85 (0.75 to 0.97), 0.73 (0.62 to 0.86), 0.79 (0.70 to 0.89), and 0.81 (0.73 to 0.89) for winter (December to February), Spring (March to May), Summer (June to August), and Autumn (September to November), respectively.
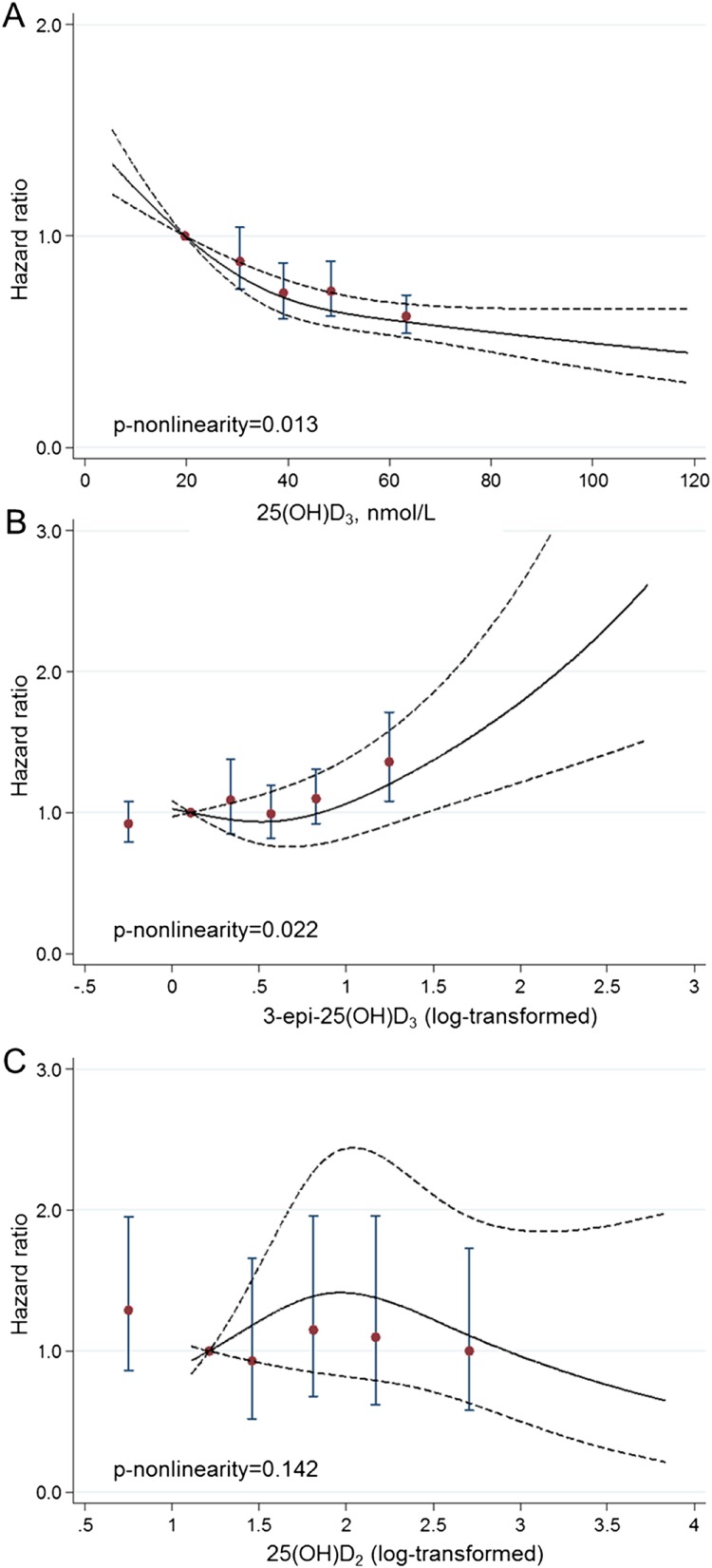
Cubic spline models suggested a potential nonlinear association with T2D for nonepimeric 25(OH)D3 (P nonlinearity = 0.013), 3-epi-25(OH)D3 (P nonlinearity = 0.022), and their ratio (P nonlinearity = 0.014) (Fig. 2) (7). A positive association between 3-epi-25(OH)D3 and T2D was observed within the fifth quintile when the 3-epi-25(OH)D3 was higher than ∼4.48 nmol/L (1.5 in log-transformed value).
Figure 2.
Shape of association between each plasma 25(OH)D metabolite and incident T2D: EPIC-InterAct Study. For 25(OH)D metabolites, restricted cubic spline functions with three knots were used to estimate the association of plasma 25(OH)D metabolites with incident T2D in country-specific [except for 25(OH)D2, where all countries were combined] Prentice-weighted Cox regression models, adjusted for potential confounders (i.e., model 4). We then combined the country-specific estimates using a multivariable random-effects meta-analysis. Reference for the HR estimation was the 10th percentile of the corresponding 25(OH)D metabolite: 19.6 nmol/L, 0.10 (equivalent to 1.11 nmol/L), and 1.21 (equivalent to 3.36 nmol/L) for (A) nonepimeric 25(OH)D3, (B) 3-epi-25(OH)D3 (log transformed), and (C) 25(OH)D2 (log transformed), respectively. To enable the dose-response association to be comparable with the quintile results, we also plotted the HR (95% CI) of T2D across the quintiles 2 through 5 of the 25(OH)D metabolites or at the category of below the lower limit of quantification (quintile 1 as reference, location of each plot corresponds to the median level within each quintile).
There was evidence of interaction (P < 0.001) between both nonepimeric 25(OH)D3 and 3-epi-25(OH)D3 and BMI (7). The inverse association of nonepimeric 25(OH)D3 with T2D and the positive association of 3-epi-25(OH)D3 with T2D were both stronger among those with a higher BMI.
Discussion
The multicenter EPIC-InterAct study identified diverse correlates of plasma vitamin D metabolites in epimeric and nonepimeric forms across eight European countries. Specifically, we found that plasma nonepimeric 25(OH)D3 was inversely associated with T2D, consistent with findings for total 25(OH)D, whereas 3-epi-25(OH)D3 was positively associated with T2D, and 25(OH)D2 was not associated with T2D.
Plasma nonepimeric 25(OH)D3 was correlated with a variety of lifestyle and dietary factors and circulating biomarkers, confirming previous reports (28, 29). Our finding of higher levels of plasma nonepimeric 25(OH)D3 in Northern Europe is consistent with prior reports including European populations (30, 31), but their generalizability was low due to small sample size (<1000 European participants) and limited population representativeness (either in elderly people or in postmenopausal women). Thus, our study with its large sample of European populations provides support for a north-to-south gradient (from high to low) for the distribution of plasma vitamin D3 across Europe. The reason for this apparently counterintuitive distribution is probably because of the high intake of dietary vitamin D or vitamin D supplements in Northern European countries (32).
Prior evidence on the correlates of 3-epi-25(OH)D3 is limited. In the United States–based NHANES study (n = ∼13,000) (5), serum 3-epi-25(OH)D3 was inversely associated with BMI and C-reactive protein, and it was positively associated with vitamin D intake and supplement use. Another United States–based study, ARIC (n = ∼3300) (11), confirmed these associations and additionally found positive associations of serum 3-epi-25(OH)D3 with physical activity levels and HDL-C concentrations. We found directionally consistent association of the 3-epi-25(OH)D3 with BMI, physical activity, and HDL-C, but no association with dietary vitamin D or supplement use in European populations. Of note, in the current study, alcohol intake was correlated with 3-epi-25(OH)D3 or the ratio of 3-epi-25(OH)D3 to nonepimeric 25(OH)D3, which was not reported in prior studies (5, 11).
For 25(OH)D2, owing to the typical low concentration in populations, there have been limited reports of its potential correlates. In a recent Finnish study (33), serum 25(OH)D2 was positively associated with a low sunlight exposure period (compared with a high sunlight period) and with use of oral contraceptives. However, the Finnish study did not have detailed dietary or supplement information. Our current findings indicated that vitamin supplements, but not other dietary sources, were the major positive dietary correlates of plasma 25(OH)D2.
Several meta-analyses based on observational studies consistently suggested that there was a dose-response inverse association between circulating total 25(OH)D and T2D risk (2, 34, 35), although the sample size of the individual studies included in these meta-analyses was typically small, with studies having between 26 and 829 incident T2D cases (34). Our findings of total 25(OH)D and nonepimeric 25(OH)D3, as a major contributor to total 25(OH)D, confirmed results from these previous studies on total 25(OH)D (2, 34, 35) and further confirmed a potential nonlinear association between 25(OH)D and T2D, as suggested previously (36).
No prior study has reported the prospective association between 3-epi-25(OH)D3 and T2D risk. Although the epimerization pathway of vitamin D metabolites has been previously observed within in vitro studies, the existence of the epimer of vitamin D3 in human pediatric and adult populations has only emerged more recently (4), aided by analytic development in resolving the isomeric compounds. To our knowledge, there was only one prior study that assessed 3-epi-25(OH)D3 and examined its association with intermediate markers for T2D incidence (8). The study of a Swiss population assessed incident insulin resistance as an outcome and reported no significant association of 3-epi-25(OH)D3 but a significant positive association of the proportion of the 3-epi-25(OH)D3 in total 25(OH)D3, as a sum of epimeric and nonepimeric D3 (8). Our present findings for the 3-epi-25(OH)D3 and its ratio to nonepimeric 25(OH)D3 are novel in relationship to their positive associations with incident T2D. Our findings that further adjustment for 25(OH)D3 strengthened the positive association of 3-epi-25(OH)D3 supported a potential independent role of the epimer or epimerization process in diabetes etiology.
Although the vitamin D3 metabolic pathway has been well characterized (37–39), a physiologic role of 3-epi-25(OH)D3 or C-3 epimerization is currently unknown. The secosteroid vitamin D3 is derived from the diet and from biosynthesis in the skin (38), undergoing enzymatic hydroxylation to become nonepimeric 25(OH)D3 in the liver (39) and then 1,25(OH)2D3 (the active form of vitamin D3) in the kidney (40). While the enzyme, C-3 epimerase, has not been well characterized (41), C-3 epimerization is thought to convert the major vitamin D3 metabolites, including 1,25(OH)2D3 and nonepimeric 25(OH)D3, to corresponding epimers. These epimers are thought to have lower biological activity, including lower binding affinity to vitamin D receptor and vitamin D–binding protein, compared with their nonepimeric forms (6), but how this might influence their potential roles in the etiology of T2D is currently unclear. Prior genetic Mendelian randomization studies for 25(OH)D indicated no causal effect of total circulating 25(OH)D (34), but these analyses were unable to dissociate nonepimeric and epimeric 25(OH)D3, because a standard assay cannot distinguish between the two (41). The same genetic approach after such decomposition is warranted, because it is possible that the prior null genetic findings reflected both a potentially causal T2D risk–raising effect of epimeric 25(OH)D3 (or C-3 epimerase) and risk-lowering effect of the nonepimeric 25(OH)D3, thereby leading to overall null findings in the genetic studies.
To our knowledge, no previous study has reported on the association between circulating 25(OH)D2 and incident T2D. The lack of research may be due to the technical difficulty in measuring very low concentrations of 25(OH)D2 in a large-scale study; hence, to our knowledge, our current study provides the first investigation of 25(OH)D2 in diabetes etiology. Although circulating vitamin D2 undergoes similar metabolic conversion as vitamin D3, the bioactive form of vitamin D2 [i.e. 1,25(OH)2D2] has a much lower binding affinity to vitamin D–binding protein than to vitamin D3 and its metabolites (42). The lack of association between 25(OH)D2 and incident T2D in our study is therefore consistent with this biological consideration.
The strengths of this study include its large sample size, together with inclusion of measures of multiple 25(OH)D metabolites in epimeric and nonepimeric forms across diverse populations in eight European countries. This enables us to investigate the dietary and lifestyle correlates of these metabolites, as well as their associations with new-onset T2D in a prospective study design. The current study has several limitations. Although 25(OH)D has been shown to be stable in stored samples (14), the stability of the epimeric form has not been confirmed and measurement errors may exist. Nevertheless, observed concentrations of the epimeric 25(OH)D3 metabolites were comparable to those of prior studies (4, 11), and potential measurement errors are unlikely to be differential with respect to case status. Our study included predominantly white European-origin populations, therefore justifying the need for further multiethnic investigations, as genetic differences between race/ethnic groups in vitamin D metabolism are clinically important (43). Our observational findings do not imply causality in the observed associations. Future research, including observational studies to confirm our findings as well as experimental studies and Mendelian randomization studies on different 25(OH)D metabolites, is needed to characterize the physiological roles of 25(OH)D metabolites and vitamin D metabolism in the development of T2D.
The current study suggests that nonepimeric 25(OH)D3, the major component of total 25(OH)D, is inversely associated, whereas its epimeric form is positively associated with incident T2D. These findings indicate that vitamin D metabolism in relationship to the etiology of T2D is more complex than previously understood, and they provide novel information to assess the biological relevance of vitamin D epimerization and vitamin D subtypes in T2D etiology.
Acknowledgments
We thank all EPIC participants and staff for their contribution to the study. We thank Nicola Kerrison (MRC Epidemiology Unit, University of Cambridge) for managing the data for the InterAct project. We thank the technical and functional operational teams of the MRC Epidemiology Unit and laboratory team at Vitas AS (Oslo, Norway) for vitamin D metabolite measurements. We thank staff from the EPIC-CVD and EPIC-InterAct Coordinating Centers for carrying out sample preparation and data-handling work, particularly Sarah Spackman (EPIC-CVD Data Manager), and Cambridge Genomic Services for genotyping.
Financial Support: The InterAct project was funded by the European Union Sixth Framework Program (Grant LSHM_CT_2006_037197). Biomarker measurements for vitamin D metabolites were funded jointly by the MRC Cambridge Initiative (Grants RG71466 and SJAH/004), and by the EPIC-CVD project. EPIC-CVD has been supported by the European Union Seventh Framework Program (Grant HEALTH-F2-2012-279233), the European Research Council (Grant 268834), the UK Medical Research Council (Grants G0800270 and MR/L003120/1), the British Heart Foundation (Grants SP/09/002, RG/08/014, and RG13/13/30194), and by the UK National Institutes of Health Research. Additionally, InterAct investigators acknowledge funding from the following agencies: Medical Research Council Epidemiology Unit MC_UU_12015/1 and MC_UU_12015/5, NIHR Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (Grant IS-BRC-1215-20014), the Regional Government of Asturias, and the Regional Governments of Basque Country. I.S. is supported by Junior Dr. Dekker Grant 2015T019 from the Dutch Heart Foundation. G.F. is supported by Agence Nationale de la Recherche via “Investissement d’Avenir” Grant ANR-10-COHO-0006 and by the IDEX Paris Saclay Nutriperso Project. This project has received funding from the European Union’s Horizon 2020 research and innovation program under Marie Sklodowska-Curie Grant 701708.
Author Contributions: J.-S.Z. and N.G.F. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and they drafted the manuscript; J.-S.Z. performed the statistical analyses; N.J.W., C.L., N.G.F., and S.J.S. coordinated the InterAct project with N.J.W. as the chief investigator. The Working Group (J.-S.Z., F.I., S.J.S., Y.T.v.d.S., I.S., T.E.G., A.S.B., and N.G.F.) and all authors contributed to interpretation of data, revised the article critically for important intellectual content, and approved the final version of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HR
hazard ratio
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- LLQ
lower limit of quantification
- T2D
type 2 diabetes
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D3
25-hydroxyvitamin D3
- 25(OH)D2
25-hydroxyvitamin D2
- 3-epi-25(OH)D3
3-epi-25-hydroxyvitamin D3
References
- 1. Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forouhi NG, Ye Z, Rickard AP, Khaw KT, Luben R, Langenberg C, Wareham NJ. Circulating 25-hydroxyvitamin D concentration and the risk of type 2 diabetes: results from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort and updated meta-analysis of prospective studies. Diabetologia. 2012;55(8):2173–2182. [DOI] [PubMed] [Google Scholar]
- 3. Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65(9):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bailey D, Veljkovic K, Yazdanpanah M, Adeli K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin Biochem. 2013;46(3):190–196. [DOI] [PubMed] [Google Scholar]
- 5. Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, Johnson CL, Pfeiffer CM. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US population during 2007–2010. J Nutr. 2016;146(5):1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1α or C-24 hydroxylation. J Biol Chem. 2004;279(16):15897–15907. [DOI] [PubMed] [Google Scholar]
- 7. Zheng JS. Data from: Association of plasma vitamin D metabolites with incident type 2 diabetes: EPIC-InterAct case-cohort study. figshare 2018. Deposited 18 October 2018. https://doi.org/10.6084/m9.Figshare.6809717.V2. [DOI] [PMC free article] [PubMed]
- 8. Marques-Vidal P, Vollenweider P, Guessous I, Henry H, Boulat O, Waeber G, Jornayvaz FR. Serum vitamin D concentrations are not associated with insulin resistance in Swiss adults. J Nutr. 2015;145(9):2117–2122. [DOI] [PubMed] [Google Scholar]
- 9. Engelman CD, Bo R, Zuelsdorff M, Steltenpohl H, Kirby T, Nieto FJ. Epidemiologic study of the C-3 epimer of 25-hydroxyvitamin D3 in a population-based sample. Clin Nutr. 2014;33(3):421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cashman KD, Kinsella M, Walton J, Flynn A, Hayes A, Lucey AJ, Seamans KM, Kiely M. The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins. J Nutr. 2014;144(7):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lutsey PL, Eckfeldt JH, Ogagarue ER, Folsom AR, Michos ED, Gross M. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC). Clin Chim Acta. 2015;442:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, Kerrison N, Ekelund U, Barroso I, Panico S, Tormo MJ, Spranger J, Griffin S, van der Schouw YT, Amiano P, Ardanaz E, Arriola L, Balkau B, Barricarte A, Beulens JW, Boeing H, Bueno-de-Mesquita HB, Buijsse B, Chirlaque Lopez MD, Clavel-Chapelon F, Crowe FL, de Lauzon-Guillan B, Deloukas P, Dorronsoro M, Drogan D, Froguel P, Gonzalez C, Grioni S, Groop L, Groves C, Hainaut P, Halkjaer J, Hallmans G, Hansen T, Huerta Castaño JM, Kaaks R, Key TJ, Khaw KT, Koulman A, Mattiello A, Navarro C, Nilsson P, Norat T, Overvad K, Palla L, Palli D, Pedersen O, Peeters PH, Quirós JR, Ramachandran A, Rodriguez-Suarez L, Rolandsson O, Romaguera D, Romieu I, Sacerdote C, Sánchez MJ, Sandbaek A, Slimani N, Sluijs I, Spijkerman AM, Teucher B, Tjonneland A, Tumino R, van der A DL, Verschuren WM, Tuomilehto J, Feskens E, McCarthy M, Riboli E, Wareham NJ; InterAct Consortium . Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC Study. Diabetologia. 2011;54(9):2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Snellman G, Melhus H, Gedeborg R, Byberg L, Berglund L, Wernroth L, Michaëlsson K. Determining vitamin D status: a comparison between commercially available assays. PLoS One. 2010;5(7):e11555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hollis BW. Measuring 25-hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutr. 2008;88(2):507S–510S. [DOI] [PubMed] [Google Scholar]
- 15. Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, Eckfeldt JH, Fleet JC, Howard G, Hoofnagle AN, Hui SL, Lensmeyer GL, Massaro J, Peacock M, Rosner B, Wiebe D, Bailey RL, Coates PM, Looker AC, Sempos C, Johnson CL, Picciano MF. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140:2030S–2045S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang LY, Summerhill K, Rodriguez-Canas C, Mather I, Patel P, Eiden M, Young S, Forouhi NG, Koulman A. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013;5(4):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol. 1997;26(Suppl 1):S1–S5. [DOI] [PubMed] [Google Scholar]
- 18. Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondière UR, Hémon B, Casagrande C, Vignat J, Overvad K, Tjønneland A, Clavel-Chapelon F, Thiébaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, González CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B)1113–1124. [DOI] [PubMed] [Google Scholar]
- 19. Peters T, Brage S, Westgate K, Franks PW, Gradmark A, Tormo Diaz MJ, Huerta JM, Bendinelli B, Vigl M, Boeing H, Wendel-Vos W, Spijkerman A, Benjaminsen-Borch K, Valanou E, de Lauzon Guillain B, Clavel-Chapelon F, Sharp S, Kerrison N, Langenberg C, Arriola L, Barricarte A, Gonzales C, Grioni S, Kaaks R, Key T, Khaw KT, May A, Nilsson P, Norat T, Overvad K, Palli D, Panico S, Ramón Quirós J, Ricceri F, Sanchez MJ, Slimani N, Tjonneland A, Tumino R, Feskins E, Riboli E, Ekelund U, Wareham N; InterAct Consortium . Validity of a short questionnaire to assess physical activity in 10 European countries. Eur J Epidemiol. 2012;27(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fanidi A, Muller DC, Midttun Ø, Ueland PM, Vollset SE, Relton C, Vineis P, Weiderpass E, Skeie G, Brustad M, Palli D, Tumino R, Grioni S, Sacerdote C, Bueno-de-Mesquita HB, Peeters PH, Boutron-Ruault MC, Kvaskoff M, Cadeau C, Huerta JM, Sánchez MJ, Agudo A, Lasheras C, Quirós JR, Chamosa S, Riboli E, Travis RC, Ward H, Murphy N, Khaw KT, Trichopoulou A, Lagiou P, Papatesta EM, Boeing H, Kuehn T, Katzke V, Steffen A, Johansson A, Brennan P, Johansson M. Circulating vitamin D in relation to cancer incidence and survival of the head and neck and oesophagus in the EPIC cohort. Sci Rep. 2016;6(1):36017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26(1):24–36. [Google Scholar]
- 22. Lupton JR, Faridi KF, Martin SS, Sharma S, Kulkarni K, Jones SR, Michos ED. Deficient serum 25-hydroxyvitamin D is associated with an atherogenic lipid profile: the Very Large Database of Lipids (VLDL-3) study. J Clin Lipidol. 2016;10(1):72–81.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schnatz PF, Jiang X, Vila-Wright S, Aragaki AK, Nudy M, O’Sullivan DM, Jackson R, LeBlanc E, Robinson JG, Shikany JM, Womack CR, Martin LW, Neuhouser ML, Vitolins MZ, Song Y, Kritchevsky S, Manson JE. Calcium/vitamin D supplementation, serum 25-hydroxyvitamin D concentrations, and cholesterol profiles in the Women’s Health Initiative calcium/vitamin D randomized trial. Menopause. 2014;21(8):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–2006. Am J Clin Nutr. 2011;94(1):225–233. [DOI] [PubMed] [Google Scholar]
- 25. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 26. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29(12):1282–1297. [DOI] [PubMed] [Google Scholar]
- 28. Lips P, van Schoor NM, de Jongh RT. Diet, sun, and lifestyle as determinants of vitamin D status. Ann N Y Acad Sci. 2014;1317(1):92–98. [DOI] [PubMed] [Google Scholar]
- 29. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. [DOI] [PubMed] [Google Scholar]
- 30. Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24(4):693–701. [DOI] [PubMed] [Google Scholar]
- 31. van der Wielen RP, Löwik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346(8969):207–210. [DOI] [PubMed] [Google Scholar]
- 32. Spiro A, Buttriss JL. Vitamin D: an overview of vitamin D status and intake in Europe. Nutr Bull. 2014;39(4):322–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palaniswamy S, Hyppönen E, Williams DM, Jokelainen J, Lowry E, Keinänen-Kiukaanniemi S, Herzig KH, Järvelin MR, Sebert S. Potential determinants of vitamin D in Finnish adults: a cross-sectional study from the Northern Finland birth cohort 1966. BMJ Open. 2017;7(3):e013161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye Z, Sharp SJ, Burgess S, Scott RA, Imamura F, Langenberg C, Wareham NJ, Forouhi NG; InterAct Consortium . Association between circulating 25-hydroxyvitamin D and incident type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2015;3:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song Y, Wang L, Pittas AG, Del Gobbo LC, Zhang C, Manson JE, Hu FB. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buijsse B, Boeing H, Hirche F, Weikert C, Schulze MB, Gottschald M, Kühn T, Katzke VA, Teucher B, Dierkes J, Stangl GI, Kaaks R. Plasma 25-hydroxyvitamin D and its genetic determinants in relation to incident type 2 diabetes: a prospective case-cohort study. Eur J Epidemiol. 2013;28(9):743–752. [DOI] [PubMed] [Google Scholar]
- 37. Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91(6):2552–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT Jr, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210(4466):203–205. [DOI] [PubMed] [Google Scholar]
- 39. Horsting M, DeLuca HF. In vitro production of 25-hydroxycholecalciferol. Biochem Biophys Res Commun. 1969;36(2):251–256. [DOI] [PubMed] [Google Scholar]
- 40. Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1α-hydroxylase and vitamin D synthesis. Science. 1997;277(5333):1827–1830. [DOI] [PubMed] [Google Scholar]
- 41. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84(4):694–697. [DOI] [PubMed] [Google Scholar]
- 43. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]