Abstract
Context
Dual-energy X-ray absorptiometry (DXA) is a cornerstone of pediatric bone health assessment, yet differences in height-for-age confound the interpretation of areal bone mineral density (aBMD) measures. To reduce the confounding of short stature on spine bone density, use of bone mineral apparent density (BMAD) and height-for-age Z-score (HAZ)‒adjusted aBMD (aBMDHAZ) are recommended. However, spine BMAD reference data are sparse, and the degree to which BMAD and aBMDHAZ account for height-related artifacts in bone density remains unclear.
Objective
We developed age-, sex-, and population ancestry‒specific spine BMAD reference ranges; compared height-adjustment methods in accounting for shorter stature; and assessed the stability of these measures over time.
Design
Secondary analysis of data from a previous longitudinal study.
Participants
Children and adolescents aged 5 to 19 years at baseline (n = 2014; 922 males; 22% black) from the Bone Mineral Density in Childhood Study.
Main Outcome Measures
Lumbar spine BMAD and aBMDHAZ from DXA.
Results
Spine BMAD increased nonlinearly with age and was greater in blacks and females (all P < 0.001). Age-specific spine BMAD z-score reference curves were constructed for black and non‒black males and females. Overall, both BMAD and aBMDHAZz scores reduced the confounding influence of shorter stature, but neither was consistently unbiased across all age ranges. Both BMAD and aBMDHAZz scores tracked strongly over 6 years (r = 0.70 to 0.80; all P < 0.001).
Conclusion
This study provided robust spine BMAD reference ranges and demonstrated that BMAD and aBMDHAZ partially reduced the confounding influence of shorter stature on bone density.
Adjusting for effects of short stature on childhood bone density is important. Spine bone mineral apparent density and height-adjusted aBMD z scores reduced stature-related artifacts at most ages.
Dual-energy X-ray absorptiometry (DXA) is a cornerstone of bone health assessment (1). However, there are several challenges in accurately interpreting DXA scans in pediatric patients with chronic conditions that threaten bone health. Because of its two-dimensional nature, which is unable to account for the depth of the bone, DXA is vulnerable to size-related artifacts. Consequently, because childhood bone density is closely linked to height, low (or high) bone density relative to that of same-age peers may be attributed to short (or taller) stature (2). In addition, DXA-acquired measures of bone mineral content (BMC) and areal bone mineral density (aBMD) are dependent on age, sex, and population ancestry and become increasingly variable and nonlinear with age (3–6). Collectively, these points support the need for appropriately constructed age-, sex-, and population ancestry‒specific reference ranges for bone density indices that adequately account for variations in height. For clinicians, such resources are essential for understanding the extent to which low bone density is attributed to short stature (7).
The International Society for Clinical Densitometry has endorsed two height-adjustment approaches for lumbar spine DXA scans in children with short stature or growth delay (7). The first is a two-step procedure wherein height-for-age Z-score (HAZ) is calculated using the Centers for Disease Control and Prevention growth charts (8), and spine aBMD-for-age Z-score is then adjusted for HAZ (aBMDHAZ) (2). The second approach is to calculate bone mineral apparent density (BMAD), which uses a transformation of bone area to estimate the volume of each individual vertebra (L1 to L4) to approximate the effects of bone depth and body size (7, 9). In a study of children with chronic medical conditions, spine BMAD had the numerically greatest diagnostic OR associated with vertebral fracture compared with other lumbar spine and total body measures of bone mass, density, and area. However, the diagnostic accuracy (i.e., CIs) of BMAD did not surpass that of other measures (10). Zemel et al. (2) showed that compared with spine BMAD, aBMDHAZ Z-score provided the least biased measure of bone density relative to height in healthy children. The performance of BMAD and aBMDHAZ in reducing the confounding effect of stature on lumbar spine bone density, notably with respect to height status and age, warrants additional investigation. Further, bone mass accrual in childhood is a strong determinant of peak bone mass in young adulthood; however, it is unknown whether BMAD and aBMDHAZ are stable throughout childhood (4). Quantifying the stability, or tracking, of spine BMAD and aBMDHAZ will help clarify whether these measures are predictive of future risk for osteoporosis and fracture.
The only pediatric reference data for spine BMAD from Hologic fan-beam densitometers are based on 587 white children and adolescents (262 females) aged 4.5 to 20 years from a single clinical center in the United Kingdom (11). To meet criteria for robust reference ranges, a larger, multicenter sample of data including other population ancestry groups is needed. To address this need, we used data from the Bone Mineral Density in Childhood Study (BMDCS), a large, multicenter, multiethnic study of healthy children and adolescents that provides pediatric reference data for aBMD and BMC at several skeletal sites, including the whole body, hip, forearm, and lumbar spine (3, 5). We assessed age-, sex-, and ancestry-dependent changes in spine BMAD during childhood. Second, we developed age-specific spine BMAD reference ranges for black and non‒black males and females. Finally, we compared spine BMAD with aBMD and aBMDHAZ with respect to (i) reducing the effects of shorter stature on bone density and (ii) the stability (i.e., tracking) of these indices within individuals over time.
Methods
Study population
The BMDCS was a multiethnic prospective study of healthy children from five clinical centers across the United States, including Children’s Hospital of Los Angeles (Los Angeles, CA), The Children’s Hospital of Philadelphia (Philadelphia, PA), Cincinnati Children’s Hospital Medical Center (Cincinnati, OH), Columbia University (New York, NY), and Creighton University (Omaha, NE). The study protocols and procedures have been described previously (2, 3, 5). Initially, 1554 healthy children aged 6 to 16 years were enrolled from July 2002 to November 2003 and followed up annually for up to 6 years for seven possible visits. To expand this age range, additional children aged 5 and 19 years were enrolled in the fourth year of the study. These participants were evaluated annually for 2 years, for a maximum of three total visits. Participants were enrolled and evaluated throughout the calendar year. Exclusion criteria included height, weight, or body mass index below the third percentile or above the 97th percentile; delayed or advanced pubertal development; premature birth; scoliosis; use of medications known to influence bone metabolism; or medical conditions that threatened normal bone accretion. In addition, individuals younger than 10 years were excluded for having experienced two or more fractures, and individuals older than 10 years were excluded for having experienced three or more fractures.
For participants younger than 18 years, a parent/guardian provided written informed consent and the participant provided assent. Participants 18 years of age or older provided written informed consent. All study protocols and procedures were approved by the institutional review board for human subjects at each clinical center.
Demographics, anthropometrics, and sexual maturation
Race and ethnicity were queried via questionnaire using National Institutes of Health classification criteria. Ancestry was categorized as black or non‒black, the latter of which included people of European, Hispanic, Asian, and other ancestries. Standing height and body weight were measured while participants were dressed in light clothing, without shoes. Sex-specific HAZ, weight-for-age Z-scores, and body mass index‒for-age Z-scores were calculated using the Centers for Disease Control and Prevention 2000 growth charts (8). An experienced physician or nurse practitioner assessed pubertal stage by physical examination. Stage of maturation was classified using the criteria by Tanner (12).
Bone densitometry
Anterior-posterior lumbar spine (L1 to L4) DXA scans were performed (QDR4500A, QDR4500W, and Delphi A models; Hologic Inc., Bedford, MA) as described previously (2, 3, 5). Measurements following the manufacturer’s guidelines for patient positioning were performed by trained research technicians using a standard protocol. All scans were centrally analyzed at the DXA Core Laboratory at the University of California, San Francisco (San Francisco, CA) using the Hologic software version Discovery 12.3 for baseline measurements and Apex 2.1 for follow-up measurements using the “compare” feature. Spine BMAD (g/cm3) was calculated as shown in Eq. (1) (9, 11):
| (1) |
The volume (V) of each vertebra was calculated as the anterior-posterior bone area (BA) of the respective vertebra, raised to the 1.5 power. For example, L1 V = L1 BA1.5.
Statistical methods
To evaluate whether spine BMAD differed according to age, sex, and population ancestry, we used mixed-effects regression analysis to account for multiple observations per subject. We used polynomial terms (to the fourth power) for age to capture the nonlinear relation of spine BMAD with age. We then tested for sex differences accounting for the nonlinear age effect. In sex-specific models, we tested for differences between population ancestry groups while accounting for age.
Spine BMAD reference curves were generated using the “LMS” approach using LMS Chartmaker version 2.3 (3, 13). Participants older than 20 years were included in the creation of the reference curves to provide stability for curve estimation at the older ages. Separate curves were created for each of the four sex-by-ancestry groups. The LMS analysis generates age-specific values for the median (M), SD (S), and power for the Box-Cox transformation (L), which are used to construct centile curves. For an individual measurement (X), the respective Z-score (Z) can be calculated using the age-specific L, M, and S parameters and Eq (2):
| (2) |
We compared the newly generated distributions for spine BMAD Z-scores with those previously published for children in the United Kingdom (11) and used visual inspection to summarize differences in the distributions.
We calculated spine aBMDHAZ as described previously (5) because this method accounts for the effects of short or taller stature on spine aBMD Z-scores. We compared the association of HAZ with spine BMAD, spine aBMD, and aBMDHAZ Z-scores using mixed-effects regression analysis (xtreg procedure in STATA) to account for multiple observations per subject. The correlations between HAZ and spine Z-scores were determined from the square root of the between-subjects R-square. This analysis was done for the entire group, and by age range (ages 5 to 9.9 years, 10 to 14.9 years, and 15 to 20 years). We divided our study sample into “shorter” (HAZ < −1.0), “average” (HAZ = −1.0 to 1.0), and “taller” (HAZ > 1.0) groups, and used mixed-effects regression to test whether spine Z-scores differed among HAZ groups, accounting for multiple observations per subject.
To assess the stability (or tracking) of spine aBMD, BMAD, and aBMDHAZ Z-scores over time within individuals, we calculated the tracking correlations between each respective measure at baseline and year 6. For these analyses, the sample was restricted to the 896 participants who completed both time points and who were aged ≤20 years. Tracking correlations also were determined stratified by puberty stage at baseline to assess potential effects of pubertal changes on stability of Z-scores. Unless otherwise noted, all statistical analyses were performed using STATA (version 15.1, College Station, TX), and a P value <0.05 was considered statistically significant.
Results
Sample description
This study included 10,722 DXA measurements from 2014 participants (922 males) aged 5 to 19 years at enrollment. Forty-eight percent of the participants were of European ancestry, 24% were black, 17% were Hispanic, and 11% were other ancestries. We excluded 634 follow-up measurements that occurred when study participants were not in their state of usual health (e.g., following extended bed rest, steroid exposure). Unusable spine scans occurred among an additional 60 measurements because of movement or interfering objects, resulting in 10,028 total observations. Sixty-nine percent of participants attended seven study visits, and 12% attended three study visits. Sample characteristics are presented in Table 1.
Table 1.
Description of Sample Used to Create Reference Percentiles
| Non-Black Males | Black Males | Non-Black Females | Black Females | |
|---|---|---|---|---|
| Number of participants | 745 | 246 | 789 | 233 |
| Number of observations | 3813 | 1097 | 3997 | 1121 |
| Puberty Stage | # Obs | n | # Obs | n | # Obs | n | # Obs | n |
|---|---|---|---|---|---|---|---|---|
| Tanner 1 | 1051 | 363 | 277 | 111 | 931 | 364 | 191 | 82 |
| Tanner 2–4 | 944 | 399 | 299 | 125 | 1050 | 467 | 283 | 130 |
| Tanner 5 | 1659 | 444 | 456 | 132 | 1547 | 385 | 559 | 142 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
|---|---|---|---|---|---|---|---|---|
| Age, y | 13.72 | 4.26 | 13.85 | 4.32 | 13.40 | 4.17 | 13.35 | 3.85 |
| Height Z-score | 0.12 | 0.84 | 0.31 | 0.85 | 0.13 | 0.86 | 0.21 | 0.88 |
| Weight Z-score | 0.29 | 0.82 | 0.49 | 0.85 | 0.30 | 0.83 | 0.59 | 0.77 |
| BMI Z-score | 0.24 | 0.90 | 0.38 | 0.89 | 0.29 | 0.83 | 0.58 | 0.82 |
Abbreviations: BMI, body mass index; n, number of participants with observations during the corresponding stage of maturation; # Obs, number of observations.
Age, sex, and ancestry effects on spine BMAD
Longitudinal mixed-effects analyses confirmed nonlinear (significant polynomial age terms) increases in spine BMAD with age (P < 0.003) and significant age × sex (P = 0.0001) and age × ancestry (P = 0.0001) interactions. Specifically, relative to age, females had greater BMAD than males, and blacks had greater BMAD than non‒blacks. Accordingly, separate reference curves were created for males and females of black and non‒black ancestry.
Spine BMAD reference curves
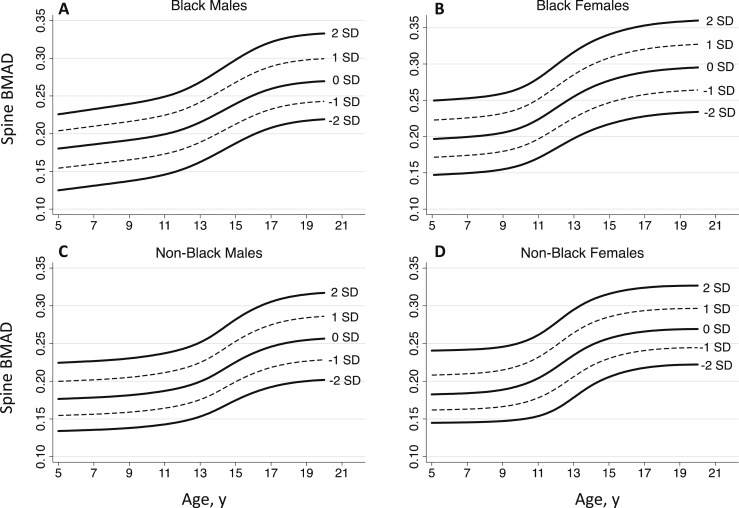
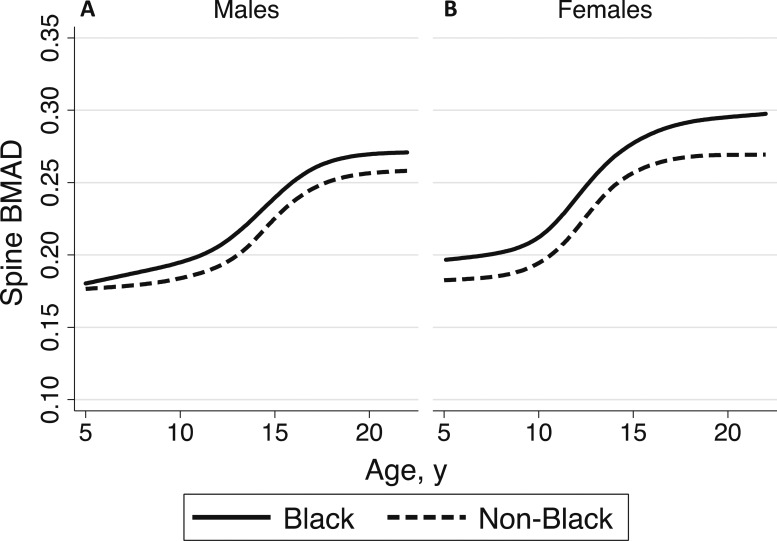
Spine BMAD reference curves are presented in Fig. 1. Age-, sex-, and ancestry-specific spine BMAD reference percentiles, including L, M, and S values, are provided in Table 2. An online repository (14) provides detailed monthly values for calculating BMAD Z-scores for black and non‒black males and females. The 50th centile spine BMAD values for black and non‒black males and females presented in Fig. 2 illustrate the magnitude of the differences between sex and population ancestry groups described previously.
Figure 1.
Spine BMAD reference curves for (A) black males, (B) black females, (C) non‒black males, and (D) non‒black females from the BMDCS.
Table 2.
Spine BMAD LMS Values and Reference Percentiles
| Non‒Black | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Males |
Females |
||||||||||||||||
|
|
|
|
Percentile |
|
|
Percentile |
||||||||||||
| Age (y) | L | S | 2 | 5 | 25 | 50 (M) | 75 | 95 | 98 | L | S | 2 | 5 | 25 | 50 (M) | 75 | 95 | 98 |
| 5.0 | 0.535 | 0.128 | 0.134 | 0.141 | 0.162 | 0.177 | 0.192 | 0.215 | 0.224 | −0.707 | 0.125 | 0.145 | 0.151 | 0.168 | 0.183 | 0.199 | 0.228 | 0.241 |
| 6.0 | 0.535 | 0.128 | 0.135 | 0.142 | 0.163 | 0.177 | 0.193 | 0.217 | 0.226 | −0.651 | 0.126 | 0.145 | 0.151 | 0.169 | 0.183 | 0.200 | 0.229 | 0.241 |
| 7.0 | 0.535 | 0.128 | 0.135 | 0.143 | 0.163 | 0.178 | 0.194 | 0.218 | 0.227 | −0.576 | 0.126 | 0.145 | 0.151 | 0.170 | 0.184 | 0.201 | 0.229 | 0.242 |
| 8.0 | 0.535 | 0.128 | 0.137 | 0.144 | 0.165 | 0.180 | 0.195 | 0.219 | 0.228 | −0.450 | 0.127 | 0.146 | 0.152 | 0.171 | 0.186 | 0.202 | 0.231 | 0.243 |
| 9.0 | 0.535 | 0.127 | 0.138 | 0.145 | 0.166 | 0.181 | 0.197 | 0.221 | 0.230 | −0.225 | 0.128 | 0.147 | 0.154 | 0.173 | 0.189 | 0.206 | 0.234 | 0.246 |
| 10.0 | 0.535 | 0.127 | 0.140 | 0.147 | 0.169 | 0.184 | 0.200 | 0.224 | 0.233 | 0.116 | 0.130 | 0.149 | 0.157 | 0.178 | 0.194 | 0.212 | 0.240 | 0.251 |
| 11.0 | 0.535 | 0.126 | 0.143 | 0.150 | 0.172 | 0.187 | 0.203 | 0.228 | 0.237 | 0.494 | 0.132 | 0.154 | 0.162 | 0.186 | 0.204 | 0.222 | 0.250 | 0.261 |
| 12.0 | 0.535 | 0.125 | 0.147 | 0.155 | 0.176 | 0.192 | 0.208 | 0.233 | 0.243 | 0.732 | 0.131 | 0.163 | 0.173 | 0.199 | 0.218 | 0.237 | 0.266 | 0.277 |
| 13.0 | 0.535 | 0.123 | 0.153 | 0.161 | 0.183 | 0.200 | 0.216 | 0.242 | 0.252 | 0.716 | 0.124 | 0.178 | 0.188 | 0.215 | 0.234 | 0.254 | 0.284 | 0.295 |
| 14.0 | 0.535 | 0.121 | 0.163 | 0.171 | 0.195 | 0.211 | 0.229 | 0.255 | 0.265 | 0.536 | 0.114 | 0.194 | 0.203 | 0.229 | 0.248 | 0.267 | 0.296 | 0.308 |
| 15.0 | 0.535 | 0.119 | 0.175 | 0.183 | 0.208 | 0.225 | 0.243 | 0.271 | 0.282 | 0.328 | 0.107 | 0.206 | 0.214 | 0.239 | 0.257 | 0.276 | 0.305 | 0.316 |
| 16.0 | 0.535 | 0.117 | 0.185 | 0.194 | 0.219 | 0.237 | 0.256 | 0.285 | 0.295 | 0.160 | 0.102 | 0.213 | 0.221 | 0.245 | 0.262 | 0.281 | 0.310 | 0.321 |
| 17.0 | 0.535 | 0.115 | 0.192 | 0.201 | 0.227 | 0.246 | 0.265 | 0.294 | 0.305 | 0.049 | 0.099 | 0.218 | 0.226 | 0.249 | 0.266 | 0.284 | 0.313 | 0.324 |
| 18.0 | 0.535 | 0.114 | 0.197 | 0.206 | 0.233 | 0.251 | 0.271 | 0.300 | 0.312 | −0.015 | 0.098 | 0.220 | 0.228 | 0.251 | 0.268 | 0.286 | 0.314 | 0.326 |
| 19.0 | 0.535 | 0.113 | 0.200 | 0.209 | 0.236 | 0.255 | 0.274 | 0.304 | 0.315 | −0.047 | 0.097 | 0.222 | 0.230 | 0.252 | 0.269 | 0.287 | 0.315 | 0.327 |
| 20.0 | 0.535 | 0.112 | 0.202 | 0.211 | 0.238 | 0.256 | 0.276 | 0.306 | 0.317 | −0.057 | 0.096 | 0.222 | 0.230 | 0.252 | 0.269 | 0.287 | 0.316 | 0.327 |
| Black | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Males |
Females |
||||||||||||||||
|
|
|
|
Percentile |
|
|
Percentile |
||||||||||||
| Age (y) | L | S | 2 | 5 | 25 | 50 (M) | 75 | 95 | 98 | L | S | 2 | 5 | 25 | 50 (M) | 75 | 95 | 98 |
| 5.0 | 1.692 | 0.137 | 0.125 | 0.136 | 0.163 | 0.180 | 0.196 | 0.218 | 0.226 | 0.746 | 0.130 | 0.147 | 0.156 | 0.180 | 0.197 | 0.214 | 0.240 | 0.250 |
| 6.0 | 1.633 | 0.136 | 0.128 | 0.139 | 0.166 | 0.183 | 0.199 | 0.221 | 0.229 | 0.746 | 0.130 | 0.148 | 0.157 | 0.181 | 0.198 | 0.215 | 0.241 | 0.251 |
| 7.0 | 1.574 | 0.135 | 0.131 | 0.142 | 0.169 | 0.186 | 0.202 | 0.225 | 0.233 | 0.746 | 0.129 | 0.150 | 0.158 | 0.182 | 0.200 | 0.217 | 0.243 | 0.253 |
| 8.0 | 1.513 | 0.133 | 0.134 | 0.145 | 0.171 | 0.189 | 0.205 | 0.228 | 0.236 | 0.746 | 0.129 | 0.152 | 0.160 | 0.185 | 0.202 | 0.219 | 0.245 | 0.255 |
| 9.0 | 1.449 | 0.132 | 0.137 | 0.148 | 0.174 | 0.192 | 0.208 | 0.231 | 0.240 | 0.746 | 0.127 | 0.155 | 0.164 | 0.188 | 0.205 | 0.223 | 0.249 | 0.259 |
| 10.0 | 1.372 | 0.131 | 0.141 | 0.151 | 0.178 | 0.195 | 0.212 | 0.235 | 0.244 | 0.746 | 0.126 | 0.161 | 0.170 | 0.195 | 0.212 | 0.230 | 0.257 | 0.267 |
| 11.0 | 1.270 | 0.129 | 0.146 | 0.156 | 0.182 | 0.199 | 0.216 | 0.240 | 0.249 | 0.746 | 0.123 | 0.171 | 0.180 | 0.206 | 0.224 | 0.242 | 0.270 | 0.281 |
| 12.0 | 1.131 | 0.127 | 0.153 | 0.162 | 0.188 | 0.206 | 0.223 | 0.248 | 0.257 | 0.746 | 0.120 | 0.184 | 0.194 | 0.221 | 0.239 | 0.259 | 0.288 | 0.299 |
| 13.0 | 0.952 | 0.123 | 0.162 | 0.172 | 0.198 | 0.215 | 0.233 | 0.259 | 0.269 | 0.746 | 0.117 | 0.197 | 0.208 | 0.235 | 0.255 | 0.275 | 0.305 | 0.316 |
| 14.0 | 0.745 | 0.120 | 0.174 | 0.184 | 0.209 | 0.227 | 0.245 | 0.273 | 0.283 | 0.746 | 0.114 | 0.209 | 0.219 | 0.248 | 0.268 | 0.288 | 0.319 | 0.331 |
| 15.0 | 0.530 | 0.116 | 0.187 | 0.196 | 0.221 | 0.240 | 0.258 | 0.287 | 0.298 | 0.746 | 0.112 | 0.217 | 0.228 | 0.257 | 0.277 | 0.298 | 0.329 | 0.341 |
| 16.0 | 0.330 | 0.112 | 0.199 | 0.208 | 0.233 | 0.251 | 0.270 | 0.300 | 0.311 | 0.746 | 0.110 | 0.223 | 0.234 | 0.263 | 0.284 | 0.305 | 0.336 | 0.348 |
| 17.0 | 0.164 | 0.109 | 0.208 | 0.216 | 0.241 | 0.259 | 0.279 | 0.309 | 0.321 | 0.746 | 0.108 | 0.228 | 0.239 | 0.268 | 0.289 | 0.310 | 0.341 | 0.353 |
| 18.0 | 0.041 | 0.107 | 0.214 | 0.222 | 0.247 | 0.265 | 0.284 | 0.315 | 0.328 | 0.746 | 0.108 | 0.231 | 0.242 | 0.271 | 0.292 | 0.313 | 0.345 | 0.356 |
| 19.0 | −0.037 | 0.105 | 0.217 | 0.226 | 0.250 | 0.268 | 0.287 | 0.319 | 0.331 | 0.746 | 0.107 | 0.233 | 0.243 | 0.273 | 0.294 | 0.315 | 0.347 | 0.358 |
| 20.0 | −0.081 | 0.105 | 0.219 | 0.227 | 0.251 | 0.270 | 0.289 | 0.320 | 0.333 | 0.746 | 0.107 | 0.234 | 0.245 | 0.274 | 0.295 | 0.316 | 0.348 | 0.360 |
Figure 2.
Median spine BMAD values in black (solid lines) and non‒black (dashed lines) (A) males and (B) females from the BMDCS.
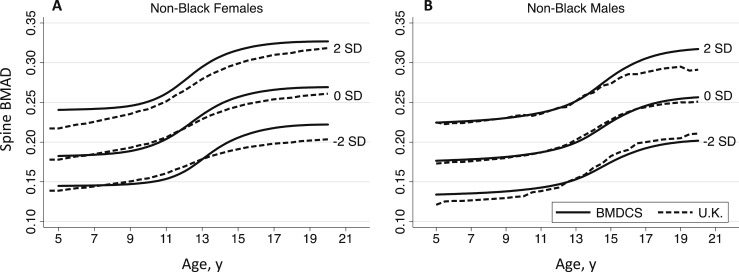
Comparison of the spine BMAD curves for non‒black males (n = 746; 4016 observations) and females (n = 789; 4240 observations) vs published values for males (n = 325) and females (n = 262) aged 4.5 to 20 years from the United Kingdom (11) are graphically displayed in Fig. 3. For males, the curves were approximately similar, with slight differences in the older and younger ages at the −2.0 SD band and in the older ages at the +2.0 SD band. For females, the BMDCS curves showed greater curvature (i.e., inflection) and overall higher BMAD values than those for females from the United Kingdom.
Figure 3.
Comparison of spine BMAD reference curves for non‒black (A) males and (B) females from the BMDCS (solid lines) and United Kingdom (dashed lines) (11).
Comparison of spine aBMD, BMAD, and aBMDHAZ Z-scores
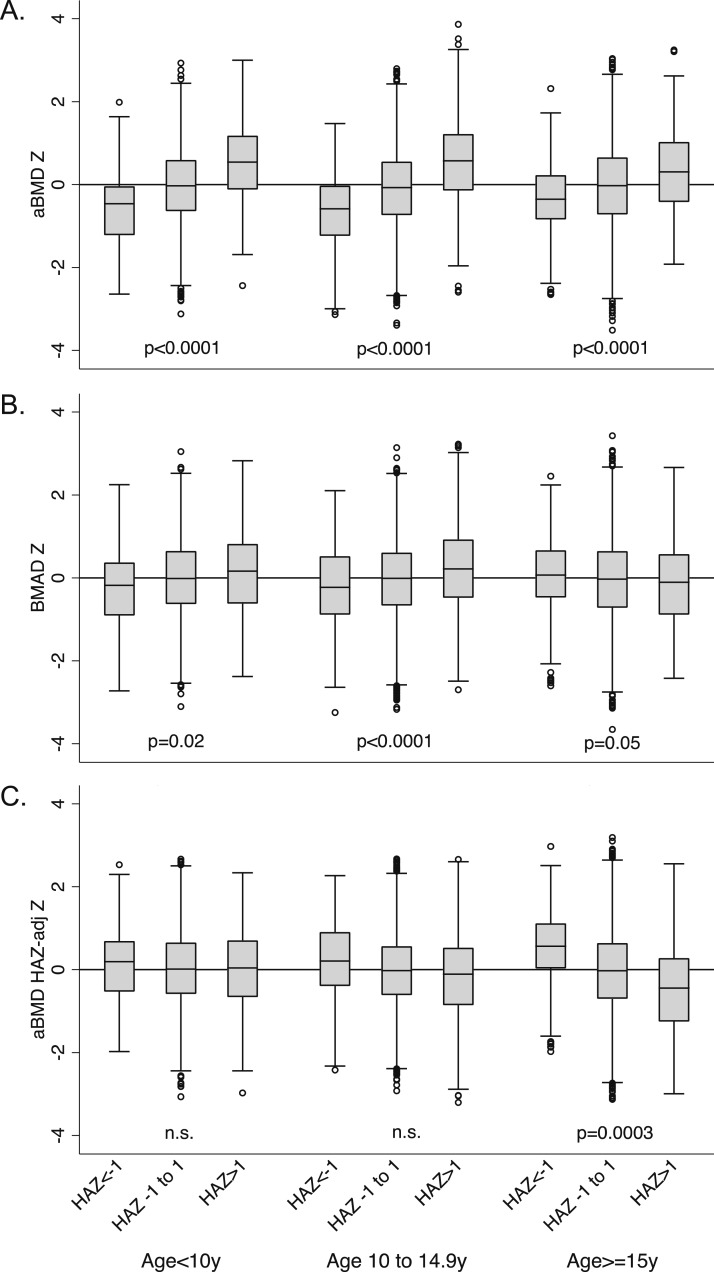
Spine aBMD, BMAD, and aBMDHAZ Z-scores were highly correlated (r = 0.89 to 0.92; all P < 0.0001). For all age groups combined, the correlation with HAZ was greatest for spine aBMD and was modest for BMAD and aBMDHAZ Z-scores. These associations varied by age (Table 3). For children <15 years of age, aBMDHAZ Z-score had the lowest correlation with HAZ, and for ages 15 to 20 years, BMAD Z-score had the lowest correlation with HAZ. The associations with height are further illustrated in Fig. 4, which shows spine Z-scores across shorter (HAZ < −1.0), average (HAZ −1.0 to 1.0), and taller (HAZ > 1.0) groups (15). There were significant differences in spine aBMD Z-score between groups at all ages, with Z-scores increasing from shorter to taller stature. For BMAD, there were significant differences across groups of HAZ for children <15 years of age, with Z-scores increasing from shorter to taller stature. For ages 15 to 20 years, BMAD Z-scores decreased modestly from shorter to taller stature. In children <15 years of age, aBMDHAZ Z-scores did not differ significantly across groups of HAZ. However, for ages 15 to 20 years, aBMDHAZ Z-scores decreased from shorter to taller stature.
Table 3.
Correlations Between HAZ Z-Score and Spine Bone Density Z-Score
|
|
Spine aBMD-for-Age Z-Score |
Spine BMAD Z-Score |
Spine aBMDHAZ Z-Score |
||||||
|---|---|---|---|---|---|---|---|---|---|
| r | # Obs | n | r | # Obs | n | r | # Obs | n | |
| All | 0.28 | 9907 | 2014 | 0.04 | 9912 | 2014 | 0.17 | 9898 | 2014 |
| Age 5 to <10 y | 0.36 | 2193 | 833 | 0.13 | 2198 | 833 | 0.01 | 2198 | 833 |
| Age 10 to <15 y | 0.39 | 4234 | 1321 | 0.16 | 4234 | 1321 | 0.08 | 4234 | 1321 |
| Age 15 to 20 y | 0.18 | 3394 | 1179 | 0.07 | 3394 | 1179 | 0.31 | 3380 | 1179 |
Abbreviations: n, number of participants; # Obs, number of observations.
Figure 4.
Comparison of spine (A) aBMD, (B) BMAD, and (C) aBMDHAZ Z-scores across children with shorter (HAZ < −1.0), average (HAZ = −1.0 to 1.0), and taller (HAZ > 1.0) stature at baseline based on age at baseline. The error bars are the upper and lower adjacent values as defined by Tukey (15). n.s., not statistically significant.
Tracking of spine aBMD indices over time
To estimate the stability, or tracking, of spine bone density Z-scores over time, we assessed the correlation between spine BMAD, aBMD, and aBMDHAZ at baseline compared with the final year of the study. Correlation coefficients for each spine Z-score are presented in Table 4 for the entire group and according to Tanner stage at baseline. Tracking for HAZ was also included for purposes of comparison. Correlations between baseline and final year measurements were strong for each bone density Z-score, ranging from 0.70 to 0.80 (all P < 0.001) and with overlapping CIs within each maturation group.
Table 4.
Correlations in Spine Z-Score Between Baseline and Year 6
| n | HAZ r (95% CI) | Spine aBMD-for-Age Z-Score r (95% CI) | Spine BMAD Z-Score r (95% CI) | Spine aBMDHAZ Z-Score r (95% CI) | |
|---|---|---|---|---|---|
| All | 894 | 0.72 (0.69, 0.75) | 0.72 (0.69, 0.75) | 0.74 (0.71, 0.77) | 0.78 (0.75, 0.80) |
| Tanner 1 at baseline | 496 | 0.80 (0.77, 0.83) | 0.74 (0.69, 0.77) | 0.74 (0.69, 0.77) | 0.76 (0.72, 0.80) |
| Tanner 2–4 at baseline | 342 | 0.67 (0.60, 0.72) | 0.73 (0.68, 0.78) | 0.77 (0.72, 0.81) | 0.80 (0.75, 0.83) |
| Tanner 5 at baseline | 56 | 0.86 (0.77, 0.91) | 0.70 (0.53, 0.81) | 0.73 (0.58, 0.83) | 0.80 (0.69, 0.88) |
Abbreviation: n, number of participants.
Discussion
This study used robust data from the multicenter, multiethnic BMDCS to develop reference ranges for spine BMAD. The reference ranges were based on >10,000 DXA measurements of healthy children and adolescents in the United States that were acquired with contemporary fan-beam densitometers and standardized procedures. These reference ranges for BMAD complement the previously published pediatric reference ranges for other DXA outcomes from the BMDCS (3, 5). Of note, we showed that spine BMAD and aBMDHAZ partially reduced the confounding effect of stature on childhood bone density Z-scores, which tracked strongly during childhood.
These spine BMAD reference ranges represent a major contribution to the field of pediatric bone densitometry. The Pediatric Positions of the International Society for Clinical Densitometry state: “In children with short stature or growth delay, spine and total body less head BMC and areal BMD results should be adjusted. For the spine, adjust using either BMAD or the height Z-score” and “An appropriate reference data set must include a sample of healthy representatives of the general population sufficiently large to capture variability in bone measures that takes into consideration gender, age, and race/ethnicity” (7, 16). To date, the only published spine BMAD reference ranges for Hologic fan-beam densitometers were from Ward et al. (17), which were later expanded by Crabtree et al. (11). These earlier reference data were based on a smaller (n = 587) cohort of children aged 4.5 to 20 years from a single center in the United Kingdom and were restricted to Caucasian children (11, 17). In line with other studies, our spine BMAD values were consistently greater in females (11, 17, 18) and black (18), supporting the need for sex- and ancestry-specific reference data.
To evaluate the comparability of our spine BMAD reference ranges to previously published values, we visually compared the BMDCS reference curves for non‒black children with those for children from the United Kingdom, which included only Caucasians (11). Two distinct dissimilarities are evident when comparing the BMDCS and UK curves. The BMDCS curves show a greater degree of inflection around the ages corresponding to the pubertal growth spurt, particularly for females. In addition, for both males and females, the BMDCS curves had higher values at older ages. The smaller sample size and number of observations of the cross-sectional UK study (262 females and 325 males) may not have been sufficient to adequately capture the skeletal changes that occur during and after pubertal development and provide one possible explanation for differences in values. In addition, differences in inclusion/exclusion criteria and geographic region may have contributed. The UK study was conducted in Manchester, which is located at a considerably higher latitude (53.5° N) than the northernmost BMDCS study site (New York, NY; 40.7° N). The lack of uniformity between reference databases could be problematic (19). For example, a 17-year-old Caucasian female who has a spine BMAD of 0.218 g/cm3 would have a Z-score of −1.29 according to the UK reference data but a Z-score of −2.0 according to the BMDCS reference data. This latter Z-score is considered “low bone mass.” (7, 20) Thus, diagnoses and subsequent decisions to initiate behavioral and/or pharmacologic intervention could differ considerably depending on reference data selection.
In the clinical arena, spine BMAD and aBMDHAZ Z-scores should be used in combination with spine aBMD Z-score to understand the extent to which low (or high) bone mass is attributed to short (or tall) stature. Spine BMAD and aBMDHAZ Z-scores are intended to provide an unbiased assessment of bone density that accounts for the confounding influence of shorter or taller stature relative to same-age peers. Previously, Zemel et al. (2) compared spine BMAD and aBMDHAZ Z-scores and noted differences relative to age and height status. Here, we explored these differences in more detail using a different formula for calculating BMAD and finalized reference ranges for calculating Z-scores. In the current study, aBMD was strongly associated with HAZ, but spine BMAD and aBMDHAZ Z-scores were only modestly associated with HAZ, indicating that both measures help address stature-related artifacts. However, the performance of spine aBMDHAZ and BMAD Z-scores was inconsistent across age ranges. At younger ages (<15 years of age), aBMDHAZ provided a relatively unbiased correction, but at older ages (>15 years of age) it “overcorrected” for shorter stature. In contrast, differences in spine BMAD Z-scores across stature groups were modest in those <15 years of age, but were negligible for children aged 15 to 20 years (P = 0.05). These discrepancies could be problematic because BMAD and aBMDHAZ, which are assumed to adequately account for the confounding of stature across childhood, will likely be used in monitoring patients over an extended period when changes in the structural properties of bone related to growth and sexual maturation have myriad effects. Collectively, these results point to the current limitations of height correction techniques across childhood and highlight the need for additional research in this area.
Beyond providing an unbiased measure of bone density, the clinical utility of BMAD and aBMDHAZ Z-scores depends on whether these measures predict bone health later in life. Two earlier studies from the BMDCS showed that aBMD and BMC Z-scores at several skeletal sites tracked strongly during childhood over 3-year and 6-year periods (21, 22). Our results are consistent with this earlier work, showing that spine BMAD and aBMDHAZ Z-scores also tracked strongly and to a similar degree during childhood. Interestingly, these earlier studies reported smaller tracking coefficients during puberty, but BMAD and aBMDHAZ appeared to track consistently regardless of maturation. This consistency in tracking should benefit clinicians in monitoring patients over time. The confounding of maturation-related changes in aBMD or BMC Z-scores may mask changes in bone health attributed to disease progression and/or clinical intervention, but our results suggest that this may not be a concern for BMAD and aBMDHAZ. In addition, these results suggest that BMAD and aBMDHAZ may be useful indicators of future fracture risk. Fracture is the most important clinical outcome and should be taken into account along with bone density Z-scores when considering osteoporosis diagnoses in pediatric patients (7). Crabtree et al. (10) found that spine BMAD was numerically a stronger predictor of fracture risk than other spine and whole body measures of bone mass, density, and size in children with chronic health conditions. However, the CIs for spine BMAD and aBMD Z-scores overlapped, and the ability of aBMDHAZ Z-scores to predict fracture has not been established. Relationships between height-adjustment bone density measures and fracture will be important to consider in future investigations.
The primary strength of this study was the large, diverse, multicenter sample of black and non‒black males and females in the United States. Our large sample size robustly characterized variability in BMAD in childhood, as reflected in our newly developed reference ranges. This study also had several limitations. Although the creation of reference ranges for BMAD were within the original scope of the BMDCS, the comparison of BMAD and aBMDHAZ represents a retrospective analysis and is limited by the enrollment criteria. The BMDCS inclusion criteria specified that participants had a height between the third and 97th percentiles at enrollment, so “shorter” and “taller” statures were categorized using HAZ cutoffs of −1.0 and +1.0, respectively. Although this approach is consistent with our earlier work (2), we were unable to assess how spine BMAD performed beyond these values. In the clinical setting, height adjustment is most important for individuals with short stature (HAZ < −2.0). Furthermore, it is important to note that the spine BMAD reference data presented in this study are appropriate only for similar DXA scanners and analysis software.
Clinical Perspective
These newly developed age-, sex-, and ancestry-specific lumbar spine BMAD reference data offer a clinical tool for pediatricians and researchers utilizing Hologic fan-beam densitometers. Further studies are needed to determine whether BMAD or aBMDHAZ is preferable in the clinical setting to account for stature-related artifacts in pediatric patients with altered growth and maturation and in predicting fracture in these at-risk patients. Until then, both BMAD and aBMDHAZ Z-scores can be used to compare against lumbar spine aBMD Z-score to understand the extent to which low bone density is attributable to small bone size.
Conclusions
This study fills several key gaps in knowledge regarding the utility of height-adjustment techniques for pediatric DXA scans and addresses a critical need through the development of robust spine BMAD reference data. Both spine BMAD and aBMDHAZ Z-scores reduced the confounding of shorter stature on bone density, but neither did so consistently across age ranges. Spine BMAD and aBMDHAZ tracked strongly and consistently through childhood, but additional studies are needed to determine whether these measures are predictive of fracture in adolescence and adulthood. Our newly developed spine BMAD reference data should play a critical role in accomplishing future research objectives, refining clinical recommendations for pediatric densitometry, and providing an essential clinical resource for identifying and monitoring children with low bone density.
Acknowledgments
We thank the pediatric endocrine divisions of each BMDCS clinical center, the study staff, and the participants and their families. We also acknowledge guidance and advice from Data Safety and Monitoring Board members, including Drs. Clifford Rosen, Ralph D’Agostino, Ingrid Holms, James Reynolds, and Reginald Tsang.
Financial Support: J.M.K. is funded through the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880. This work was funded by National Institute of Child Health and Human Development contracts NO1-HD-1-3228, NO1-HD-1-3329, NO1-HD-1-3330, NO1-HD-1-3331, NO1-HD-1-3332, and NO1-HD-1-3333 and Clinical and Translational Research Center grants 5-MO1-RR-000240 and UL1 RR-026314. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- aBMD
areal bone mineral density
- aBMDHAZ
height-for-age Z-score adjusted areal bone mineral density
- BMAD
bone mineral apparent density
- BMDCS
Bone Mineral Density in Childhood Study
- DXA
dual-energy X-ray absorptiometry
- HAZ
height-for-age Z-score
- L
power for the Box-Cox transformation
- M
median
- S
standard deviation
References
- 1. Bachrach LK, Gordon CM, Section on Endocrinology . Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. [DOI] [PubMed] [Google Scholar]
- 2. Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087–2099. [DOI] [PubMed] [Google Scholar]
- 4. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCormack SE, Cousminer DL, Chesi A, Mitchell JA, Roy SM, Kalkwarf HJ, Lappe JM, Gilsanz V, Oberfield SE, Shepherd JA, Winer KK, Kelly A, Grant SFA, Zemel BS. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017;171(9):e171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, Jaworski M, Gordon CM; International Society for Clinical Densitometry . Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225–242. [DOI] [PubMed] [Google Scholar]
- 8. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 9. Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137–145. [DOI] [PubMed] [Google Scholar]
- 10. Crabtree NJ, Högler W, Cooper MS, Shaw NJ. Diagnostic evaluation of bone densitometric size adjustment techniques in children with and without low trauma fractures. Osteoporos Int. 2013;24(7):2015–2024. [DOI] [PubMed] [Google Scholar]
- 11. Crabtree NJ, Shaw NJ, Bishop NJ, Adams JE, Mughal MZ, Arundel P, Fewtrell MS, Ahmed SF, Treadgold LA, Högler W, Bebbington NA, Ward KA; ALPHABET Study Team . Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults: the ALPHABET Study. J Bone Miner Res. 2017;32(1):172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanner J. Growth at Adolescence. 2nd ed. Oxford, UK: Blackwell Scientific Publications;1962. [Google Scholar]
- 13. Pan H, Cole T. LMSchartmaker, a program to construct growth reference data using the LMS method. Version 2.43. 2010. Available at: http://www.healthforallchildren.com/?product=lmschartmaker-pro. Accessed 21 April 2008.
- 14. Kindler JM, Lappe JM, Gilsanz V, Oberfield S, Shepherd JA, Kelly A, Winer KK, Kalkwarf HJ, Zemel BS. Data from: Lumbar spine bone mineral apparent density in children: results from the Bone Mineral Density in Childhood Study. Figshare. Deposited 10 August 2018. https://doi.org/10.6084/m9.figshare.6954992.v1. [DOI] [PMC free article] [PubMed]
- 15. Tukey JW. Exploratory Data Analysis. Reading, MA: Addison-Wesley Publishing Company; 1977.
- 16. Gordon CM, Leonard MB, Zemel BS; International Society for Clinical Densitometry . 2013 Pediatric Position Development Conference: executive summary and reflections [published correction appears in J Clin Densitom. 2014;17(4):517].J Clin Densitom. 2014;17(2):219–224. [DOI] [PubMed] [Google Scholar]
- 17. Ward KA, Ashby RL, Roberts SA, Adams JE, Zulf Mughal M. UK reference data for the Hologic QDR Discovery dual-energy x ray absorptiometry scanner in healthy children and young adults aged 6-17 years. Arch Dis Child. 2007;92(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bachrach LK, Hastie T, Wang MC, Narasimhan B, Marcus R. Bone mineral acquisition in healthy Asian, Hispanic, black, and Caucasian youth: a longitudinal study. J Clin Endocrinol Metab. 1999;84(12):4702–4712. [DOI] [PubMed] [Google Scholar]
- 19. Kocks J, Ward K, Mughal Z, Moncayo R, Adams J, Högler W. Z-score comparability of bone mineral density reference databases for children. J Clin Endocrinol Metab. 2010;95(10):4652–4659. [DOI] [PubMed] [Google Scholar]
- 20. Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, Makitie O, Munns CF, Shaw N; International Society of Clinical Densitometry . Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom. 2014;17(2):275–280. [DOI] [PubMed] [Google Scholar]
- 21.Wren TA, Kalkwarf HJ, Zemel BS, Lappe JM, Oberfield S, Shepherd JA, Winer KK, Gilsanz V; Bone Mineral Density in Childhood Study Group. Longitudinal tracking of dual-energy X-ray absorptiometry bone measures over 6 years in children and adolescents: persistence of low bone mass to maturity. J Pediatr. 2014;164(6):1280‒1285.e2. [DOI] [PMC free article] [PubMed]
- 22. Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Hangartner TN, Huang X, Frederick MM, Winer KK, Zemel BS. Tracking of bone mass and density during childhood and adolescence. J Clin Endocrinol Metab. 2010;95(4):1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]