Significance
Traditional semiconductor fabrication methods, such as lithography and etching, have been sufficient for the needs of integrated circuits over past decades. Their applicability has also been demonstrated in emerging 2D materials, which offers facile processing over large lateral dimensions, while unique and remarkable properties due to the confinement within atomic thicknesses. Nevertheless, each fabrication step adds cost to the manufacturing and increases the possibility of quality degradation. Here, we developed a method to directly synthesize arbitrary monolayer molybdenum disulfide patterns with high spatial resolution, excellent homogeneity, and electrical performance on insulating SiO2/Si. Significantly, our on-demand method allows for the repeated growth of patterned 2D materials with preserved structural integrity and material qualities, paving the way for simpler and cost-effective fabrication.
Keywords: 2D semiconductor, monolayer MoS2, patterned growth, growth mechanism, recyclable masked growth
Abstract
The 2D van der Waals crystals have shown great promise as potential future electronic materials due to their atomically thin and smooth nature, highly tailorable electronic structure, and mass production compatibility through chemical synthesis. Electronic devices, such as field effect transistors (FETs), from these materials require patterning and fabrication into desired structures. Specifically, the scale up and future development of “2D”-based electronics will inevitably require large numbers of fabrication steps in the patterning of 2D semiconductors, such as transition metal dichalcogenides (TMDs). This is currently carried out via multiple steps of lithography, etching, and transfer. As 2D devices become more complex (e.g., numerous 2D materials, more layers, specific shapes, etc.), the patterning steps can become economically costly and time consuming. Here, we developed a method to directly synthesize a 2D semiconductor, monolayer molybdenum disulfide (MoS2), in arbitrary patterns on insulating SiO2/Si via seed-promoted chemical vapor deposition (CVD) and substrate engineering. This method shows the potential of using the prepatterned substrates as a master template for the repeated growth of monolayer MoS2 patterns. Our technique currently produces arbitrary monolayer MoS2 patterns at a spatial resolution of 2 μm with excellent homogeneity and transistor performance (room temperature electron mobility of 30 cm2 V−1 s−1 and on–off current ratio of 107). Extending this patterning method to other 2D materials can provide a facile method for the repeatable direct synthesis of 2D materials for future electronics and optoelectronics.
In recent years, the family of monolayer transition metal dichalcogenides (TMDs) has become attractive semiconducting crystals for nano- and flexible electronics due to their atomic thinness, high carrier mobility, and excellent flexibility (1, 2). In addition, TMDs have drawn considerable attention in condensed matter due to the unique electronic band structures and intriguing valley–spin coupling, making them promising for valleytronics (3), piezoelectrics (4), bandgap modulation (5), and high-quantum efficiency optical/optoelectronic applications (6, 7).
To fabricate future 2D-based electronics and optoelectronics structures, it is necessary to pattern 2D materials for contacts, gating, optical access, etc. (8, 9). At present, patterning TMDs have relied on either subtractive or additive manufacturing. “Growth and etch” (10–12) is perhaps the most typical method of 2D patterning, where it utilizes subtractive manufacturing (akin to “top down”) to lithographically define and subsequently etch away unwanted areas of 2D material. This method has spatial resolution roughly down to nanometers (when using electron beam lithography), but it has a high manufacturing cost and can become time consuming. Additionally, the inevitable generation of multiple layers (which is necessary when constructing an integrated system) becomes difficult to handle, as selective etching of 2D materials still poses fabrication challenge (also due to their atomic thinness). Furthermore, contaminations between interfaces and rough, amorphous edge terminations may be introduced during fabrication, thus increasing the chance for quality deterioration of as-grown materials. To avoid surface contamination by resist residues, shadow mask (13) and laser cutting (14) can be considered, but the poor spatial resolutions typically are in the range of near millimeters to hundreds of micrometers.
However, for additive manufacturing (“bottom-up”) techniques, inkjet printing (15) has been extensively used for the large-scale preparation of TMD patterns. Nevertheless, it requires solution-based 2D flakes (typically multilayered), and the minimal feature size is usually larger than 10 μm. Various efforts have been devoted to prepattern seed precursors [molybdenum oxide (MoO3), ammonium heptamolybdate (16), or Au catalyst (17)] to generate patterned TMD structures directly, although preventing multilayer islands growing on monolayer regions still turned out to be challenging. Substrate modification by O2 plasma provides a simpler route to patterned TMDs (18, 19), but the reported electrical mobility has been two orders of magnitude lower than as-grown TMDs (1 cm2 V−1 s−1) with poor spatial resolution (∼100 μm).
Herein, through in-depth studies of the growth mechanism and precursor control, we have found that plasma-based substrate modification can be used to directly grow patterned monolayer molybdenum disulfide (MoS2) with high crystal quality, diverse geometries, high spatial resolution (2 μm at present, limited by laser lithography), and high electrical mobility (∼30 cm2 V−1 s−1) on SiO2/Si substrate. The same patterning mask can be recycled for repeated growth with consistent material morphology and quality each time, which demonstrates the potential of this method for a “master–replica” type of repeated manufacturing. Such an additive manufacturing method opens up the possibilities for wide applications of 2D TMD nanostructures in modern electronics and optoelectronics with much simpler fabrication.
Results and Discussions
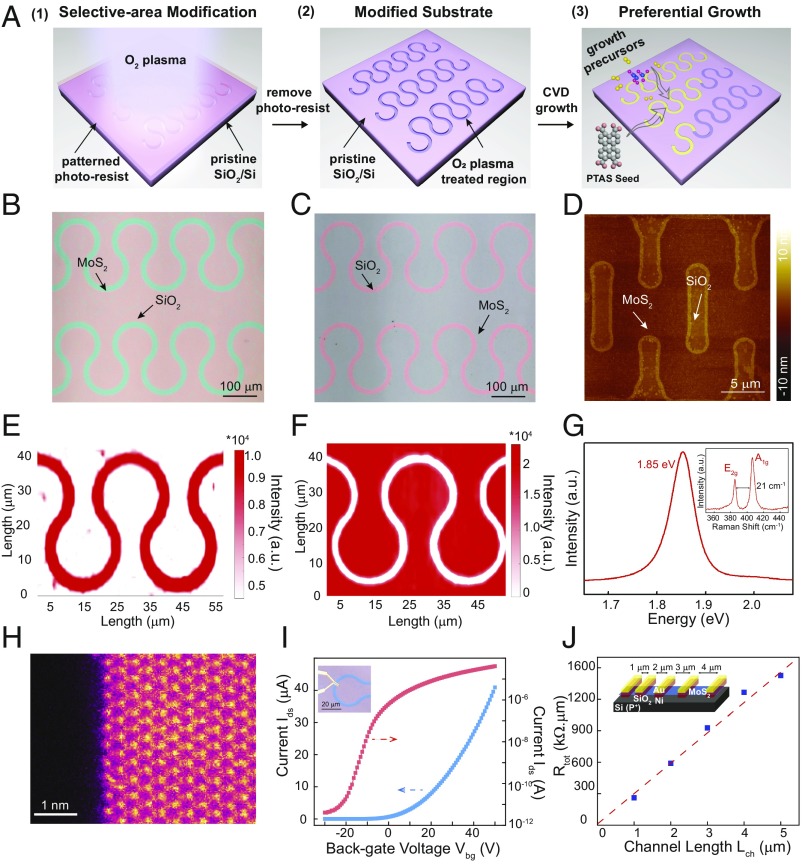
Substrate modification is the first step to achieve the patterned MoS2 structures as shown in Fig. 1A. As a proof of concept, we have chosen to define MoS2 in the shape of kirigami patterns. Traditionally, kirigami is a technique to create 3D structures from 2D sheets and has been applied in 2D materials to build robust macro- or microscale structures with tunable mechanical properties (20–22). Photolithography defines patterns of photoresist on the substrates (SI Appendix, Fig. S1) while undeveloped areas are etched away using O2 plasma, which simultaneously modifies the surface of SiO2 substrate. After removing the developed photoresist, MoS2 can selectively grow on plasma-treated areas, similar to our previous studies (details are in Methods) (23, 24). As the plasma treatment creates a region with higher surface energy and enhanced hydrophilicity (discussion below), the hydrophilic salt perylene-3,4,9,10-tetracarboxylic acid tetrapotassium (PTAS) is used to promote seeded, selective MoS2 growth only at the O2 plasma-treated areas, forming as-grown patterned MoS2.
Fig. 1.
Patterned growth and characterization of MoS2 nanostructures. (A) Schematic representation of the procedures for the direct growth of MoS2 nanostructures via seed-promoted growth and substrate engineering. Step 1: the selective-area modification process by lithography patterning and O2 plasma etching; step 2: the patterned regions are distinguished from pristine SiO2/Si substrates after modification; step 3: the preferential growth of MoS2 on patterned SiO2/Si regions. (B) A typical OM image of a monolayer MoS2 wave array. (C) OM image of a monolayer MoS2 wave film that grows at the opposite locations when a negative photoresist was used for the wave pattern in B. (D) AFM image of a monolayer MoS2 kirigami structure showing the flat surface and ordered edge morphology. (E and F) PL mappings for monolayer MoS2 wave arrays and the inversed wave film pattern. (G) A typical PL spectrum collected on the monolayer MoS2 wave structures. (Inset) A typical Raman spectrum collected on the monolayer MoS2 wave structures. (H) ADF-STEM image of a patterned monolayer MoS2 edge showing the clean and ordered edge at atomic scale. (I) Transfer (Ids − Vbg) characteristic of a typical back-gated transistor fabricated on our CVD-patterned MoS2 film with gate voltages from −30 to 50 V and a bias Vds fixed at 1 V. The room temperature electron mobility of the transistor is 29.3 cm2 V−1 s−1. The red curve shows the transfer characteristic of the same MoS2 transistor in logarithmic scale. The on–off current ratio is ∼107. (Inset) Optical image of a typical MoS2 device based on the wave pattern. (J) Total device resistance RTOT normalized by width vs. channel length Lch measured at a carrier concentration n of ∼ 1.8 × 1012 cm−2, yielding a contact resistance of Rc ∼ 6.7 kΩ.µm from the vertical intercept. (Inset) The schematic of our TLM devices with Ni/Au contacts.
Inspection of the resultant growth using optical microscopy (OM) clearly shows a patterned growth of MoS2 (in the shape of a wave array) on SiO2/Si (Fig. 1B). The reflectance contrast of the green-tinted MoS2, which comprises the wave, is uniform, with a strap width of 20 μm (tunable by changing the patterns on the substrate). Fig. 1C shows that MoS2 grows at opposite locations (in contrast to Fig. 1B) when a negative photoresist was used for the same pattern. Through this technique, monolayer MoS2 patterns with diverse morphologies and different feature sizes can be obtained by only changing the original growth “mask” (SI Appendix, Fig. S2 A1–C2). Currently, the minimal spatial resolution is 2 μm, which is limited by the direct laser writing instrument (SI Appendix, Fig. S2 D1 and D2). To evaluate the surface morphology of the MoS2 patterns, we use atomic force microscopy (AFM). We first observed that the strong O2 plasma etching causes a height difference between the pristine SiO2 regions and the etched regions of about 2 nm (SI Appendix, Fig. S3A). After the growth of MoS2, a flat and pristine monolayer fills in the recessed region (Fig. 1D) and reduces the height difference to ∼1.3 nm (SI Appendix, Fig. S3B), confirming that the thickness of MoS2 patterns is monolayer (∼0.7 nm).
To evaluate the crystallinity of the monolayer MoS2 patterns, characterizations with photoluminescence (PL) and Raman spectroscopy were carried out. A strong PL emission at around 1.85 eV with an FWHM intensity of about 60 meV was observed on all tested samples, consistent with the emission of grown monolayer MoS2 (Fig. 1G) (25). For Raman spectroscopy, the frequency difference between Raman peaks located at 383 cm−1 (E2g) and 404 cm−1 (A1g) is 21 cm−1, further confirming that the patterned MoS2 is monolayers as well (Fig. 1G, Inset). To assess the spatial homogeneity, we performed PL and Raman mappings across the grown structures. PL mappings at 670 nm in Fig. 1 E and F and SI Appendix, Fig. S4 C2 and D2 confirmed that monolayer MoS2 follows the prepared wave and rectangle kirigami patterns reasonably well with crystalline homogeneity. Additionally, Raman mappings of the E2g mode (SI Appendix, Fig. S4 A2, B2, C3, and D3) and the A1g mode (SI Appendix, Fig. S4 A3, B3, C4, and D4) both exhibit a high degree of spatial uniformity. All optical spectroscopy data indicate that this patterned manufacture produces high-quality MoS2.
Through transmission electron microscopy (TEM) examination, it was found that the patterned MoS2 maintains reasonably sharp and clean edges. SI Appendix, Fig. S5A shows a typical bright-field TEM image of an MoS2 edge, while SI Appendix, Fig. S5B shows the corresponding dark-field TEM and diffraction pattern (SI Appendix, Fig. S5B, Inset). Through the dark-field TEM, the patterned MoS2 can be clearly observed as polycrystalline (typical in all of these growth results) with three domains. Excluding a wrinkle likely caused by the TEM transfer process, there is no visible gap or bilayer area over the film. Particularly, the edges formed by domain merging remain flat and sharp. Compared with the growth-and-etch method, one additional advantage for this direct growth is that the as-grown edges are clean, are sharp, and exhibit fewer contaminations or atomic defects as revealed by the atomic-resolution annular dark-field scanning TEM (ADF-TEM) image (shown in Fig. 1H). This is imperative in preserving the intrinsic properties of TMD materials, especially for electrical performance.
In conventional nanofabrication or device manufacturing, photolithography and subsequent plasma etching are necessary to define individual devices from the continuous film, which can degrade the performance of the devices, as photoresist residues may induce scattering centers and high contact resistances at the interface. In comparison, patterned monolayer MoS2 at predefined positions would preserve intrinsic electronic properties and facilitate the system-level fabrication for multifunctional devices (Fig. 1I, Inset). To evaluate the electronic performance, we fabricated back-gated field effect transistors (FETs) on different monolayer MoS2 patterns. Our MoS2 FETs shows high performance based on the average electronic properties (SI Appendix, Fig. S6). Fig. 1I and SI Appendix, Fig. S7A exhibit the typical transfer (Ids − Vbg) and output (Ids − Vds) characteristics, respectively, of an FET fabricated on an MoS2 wave pattern. The MoS2 transistor exhibits n-type conduction with Ion/Ioff ratio up to ∼107. The electron mobility of our MoS2 FET reaches 29.3 cm2 V−1 s−1 at room temperature in air (Fig. 1I), which is comparable with the reported values of metal–organic chemical vapor deposition-grown samples (26). Furthermore, the contact resistance (Rc) of our MoS2 FETs was further extracted based on the transfer length method (TLM). Fig. 1J shows a linear fit to the total resistance normalized by channel width (RTOT) vs. channel length (Lch). We extract Rc ∼ 6.7 kΩ⋅µm at room temperature for a carrier density n of about 1.8 × 1012 cm−2. This contact resistance is consistent with previous reported values of Ni-contacted MoS2 devices on exfoliated samples (27). Significantly, one of the crucial factors to achieve a low contact resistance is the clean interface between MoS2 and metal electrodes, which is attained through the direct growth of MoS2 patterns.
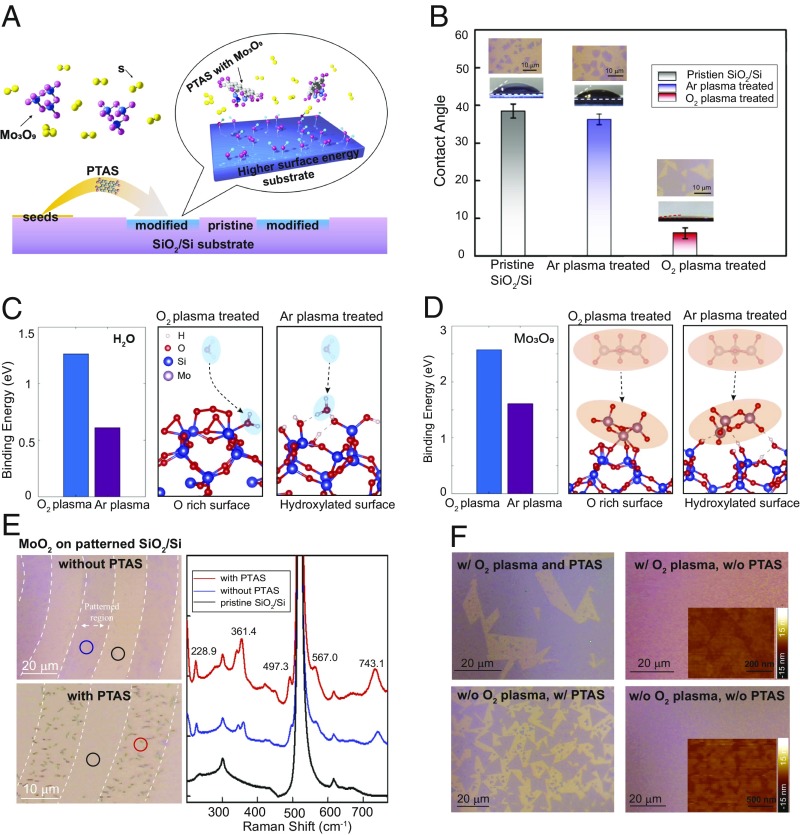
Understanding the growth mechanism is critical to obtaining reliable predefined MoS2 patterns via such direct growth paradigm. To this end, we systematically investigated the substrate modification, seed-promoted nucleation, and the effect of the sulfur vapor on the growth process. A generic mechanism of thin film growth considers chemical potentials and surface energies of epitaxial layers and the substrates (28). Therefore, increasing the surface energy on selective areas of the substrate favors patterned monolayer growth over the growth of multilayer particles. As shown in Fig. 2A, after patterning and O2 plasma treatment, the SiO2/Si substrate was composed of modified regions and pristine regions. We compared the contact angles for each type of surface in Fig. 2B. The contact angle of pristine SiO2/Si substrate is around 38.48°, while it drops rapidly to 6.04° after O2 plasma treating for 10 min, similar to observations in previous reports (19). To compare the change in the physical surface due to O2 plasma treatment, we also measured the contact angle of the surface treated by Ar plasma for the same duration (which gives similar surface roughness) (SI Appendix, Fig. S8). However, the Ar plasma-treated substrate shows a contact angle of about 36.22°, similar to that of the pristine SiO2/Si. Thus, physical roughness is not the main contribution to the surface energy change.
Fig. 2.
Mechanism study for the patterned growth of monolayer MoS2 nanostructures. (A) Schematic illustration of the growth mechanism of the patterned growth of MoS2. (B) Comparison of contact angles for pristine, Ar plasma-treated, and O2 plasma-treated SiO2/Si substrates. (C) DFT calculation for the binding energies between H2O and SiO2/Si substrate treated by O2 plasma and Ar plasma. (D) DFT calculation for the binding energies between Mo3O9 and SiO2/Si substrate treated by O2 plasma and Ar plasma. (E, Upper Left) OM image of MoO2 particles preferentially deposited on patterned SiO2/Si regions without PTAS. (E, Lower Left) OM image of MoO2 particles preferentially deposited on patterned SiO2/Si regions with the presence of PTAS. (E, Right) Raman spectra collected at the regions indicted by the red, blue, and black circles in the OM images in Left; several MoO2 Raman peaks are labeled in the spectra. It is shown that MoO2 particles exhibit a clear preference to deposit on the patterned region and that the Raman peaks of MoO2 are stronger with the presence of PTAS. (F, Upper Left) OM image of monolayer MoS2 flakes grown on O2 plasma-treated SiO2/Si substrate with the assistance of PTAS. The domain size of MoS2 is around 20 μm. (F, Upper Right) OM image of small MoS2 flakes grown on O2 plasma-treated SiO2/Si substrate without PTAS. (Inset) AFM images of MoS2 small flakes with domain size around 300 nm. (F, Lower Left) OM image of monolayer MoS2 flakes grown on pristine SiO2/Si substrate with the assistance of PTAS. The domain size of MoS2 is around 8–10 μm. (F, Lower Right) OM image of small MoS2 flakes grown on pristine SiO2/Si substrate without PTAS seed. (Inset) AFM images of MoS2 small flakes with domain size around 200 nm, indicating the poor quality of MoS2 growth.
To understand the difference in surface energies induced by different treatments, we conducted density functional theory (DFT) calculations. In our model, Ar plasma etching increases the number of dangling bonds on the surface of SiO2/Si substrate, which serve as active sites to dissociate H2O molecules in ambient environment, resulting in a hydroxylated surface (terminated by –OH group) (SI Appendix, Fig. S9). However, in the case of O2 plasma, the oxygen bonds on the unsaturated surface site and yields an oxygen-rich surface. DFT calculations show that the binding energy of water with the oxygen-rich surface is over two times that of the Ar plasma-treated surface (Fig. 2C), consistent with the contact angle measurement in Fig. 2B. When considering the binding energy with Mo3O9 [the intermediate of MoO3−x during the MoS2 growth (29)], the oxygen-rich surface has ∼1-eV higher binding energy than that of the hydroxylated surface (Fig. 2D). Combining the growth result (SI Appendix, Fig. S10) with DFT calculations, it is shown that the O2 plasma-modified substrates have better wettability and higher binding energy to Mo3O9, which is beneficial for the monolayer growth of MoS2. We experimentally verify this via results shown in Fig. 2E. On only supplying MoO3 powder during the growth, Mo-containing species [MoO2 as confirmed by Raman spectroscopy (30)] clearly preferred the O2 plasma-treated regions over the nontreated regions. By adding PTAS, which is a hydrophilic salt and has preference for the patterned regions (23), Mo-based compounds may have higher adsorption within the patterned regions (as given by a stronger Raman signature after PTAS was added). Here, the presence of PTAS may increase the strength of surface adhesion between MoS2 and SiO2/Si, leading to the monolayer growth of MoS2. Based on the different growth results in Fig. 2F, which show obvious growth enhancement under O2 plasma and PTAS, we conclude that the combination of O2 plasma treatment and PTAS exhibits a synergetic effect that promotes the optimized MoS2 growth.
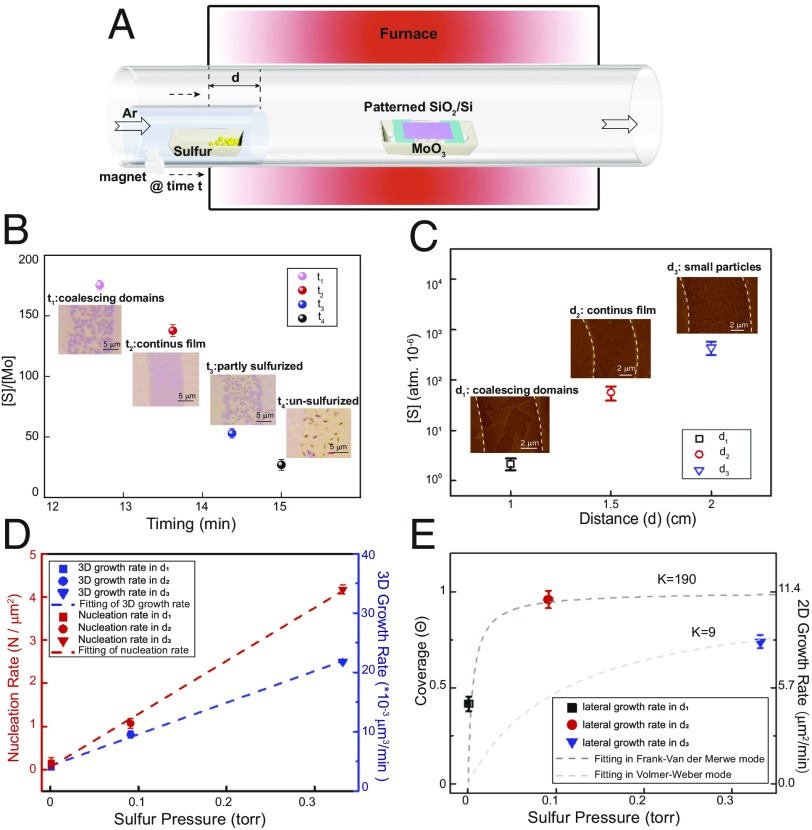
For the MoS2 growth to distinguish the difference between the untreated and treated surfaces, we noted that the entrance timing of sulfur and its amount are critical, both of which will affect the concentration (or partial pressure) of sulfur in the gaseous phase. According to the Mo–O–S phase diagram (31), MoO3 undergoes a two-step reaction in the chemical vapor deposition (CVD) growth of MoS2 (32):
| [1] |
Next, MoO3−x(g) has two possible reaction paths:
| [2.1] |
| [2.2] |
Higher [S]/[Mo] ratio (here, [S] and [Mo] indicate the concentrations or partial pressures of S and Mo species) promotes the reaction in path [2.1], while lower [S]/[Mo] ratio tends to form MoO2 as path [2.2]. To investigate the role of sulfur, we laid several patterned substrates as shown in SI Appendix, Fig. S11A (23). Presumably, the sulfur concentration decreases along the substrate as the distance away from the sulfur source increases, and the quality of the MoS2 patterns in different regions of SiO2/Si reflects the influence of sulfur concentration on the patterned growth. Instead of producing spatially distinguished MoS2 patterns, higher sulfur concentration creates a continuous MoS2 film across the entire surface (SI Appendix, Fig. S11B) (which means that there is no selective patterning by the substrate), while lower sulfur concentration produces discontinuous MoS2 flakes (SI Appendix, Fig. S11E). Only under an appropriate concentration, we can produce patterned monolayer MoS2 (SI Appendix, Fig. S11D). Therefore, we designed an improved CVD setup to control sulfur concentration during the growth. As shown in Fig. 3A, a small quartz tube is used to carry the sulfur boat, and a set of magnets is used to move the position of the sulfur boat. The sulfur powder is initially kept outside of the heating zone, and it is moved inside the heating zone during the ramping period when the MoO3 precursor reaches a suitable temperature (SI Appendix, Fig. S12) (it takes 15 min for the center of the furnace to reach the growth temperature). We first found that the entrance timing of sulfur impacts the ratio of sulfur partial pressure to Mo partial pressure ([S]/[Mo]) due to the temperature difference at different entrance times (SI Appendix, Fig. S13 and Table S1). As shown in Fig. 3B, the [S]/[Mo] ratio has a negative correlation with the entrance timing of sulfur, which essentially dominates the degree of the sulfurization in the process. When sulfur is introduced relatively early (as shown in Fig. 3B) (at t1 or t2; before 14 min), the [S]/[Mo] is high enough to fully sulfurize MoO3 into MoS2. Nevertheless, to make continuous MoS2 patterns, sufficient supply of molybdenum is also required (entering at t2) compared with the lower supply of molybdenum at t1. However, when sulfur enters at t3, the [S]/[Mo] ratio decreases rapidly, resulting in only a part of MoO3 turning into MoS2. If sulfur enters at t4 or later, only black MoO2 particles are obtained on the substrate. Although MoO2 can also be sulfurized into MoS2 (33), the temperature needed (650 °C to 850 °C) is much higher than that in our growth. Additionally, the position where the sulfur boat is introduced (corresponding to the distance d in Fig. 3C and as illustrated in Fig. 3A) is another important factor of sulfur pressure, which therefore, determines the nucleation rate and growth rate of MoS2. According to the temperature variation at different positions (i.e., different d values) (SI Appendix, Fig. S14), we semiquantitatively calculated the nucleation rate and the growth rate of the patterned growth of monolayer MoS2 (SI Appendix, Table S2). In Fig. 3C, the [S] at d1 is insufficient, leading to both low nucleation rate (Fig. 3D) and growth rate (Fig. 3E). While excess [S] at d3 will increase the nucleation rate of MoS2 dramatically, it also accelerates the MoS2 nuclei growth rate in the vertical direction (corresponding to the 3D growth rate in Fig. 3D), which is significantly faster than that in the lateral direction (corresponding to 2D growth rate in Fig. 3E), leading to the formation of multilayer MoS2 particles. If the sulfur partial pressure is kept at an appropriate value (located at d2), we could achieve medium nucleation rate and optimal growth rate in the lateral direction and obtain monolayer MoS2 patterns.
Fig. 3.
The control of sulfur precursor during the patterned growth of monolayer MoS2 nanostructures. (A) Schematic illustration of the modified CVD setup and the controlled process to synthesize patterned MoS2 nanostructures. (B) The relationship between the entrance time of the sulfur precursor and the corresponding [S]/[Mo] ratio during the growth. (Insets) OM images of different growth results according to different entrance times of sulfur. (C) The relationship between the locations of the sulfur precursor (d) and the corresponding vapor pressures of sulfur. (Insets) AFM images of MoS2 patterns grown as a result of different precursor locations. (D) The dependence of nucleation rate as the function of partial pressure of sulfur precursor (left y axis); the dependence of 3D growth rate as the function of partial pressure of sulfur precursor (right y axis). (E) The dependence of surface coverage as the function of partial pressure of sulfur precursor (left y axis); the dependence of 2D growth rate as the function of partial pressure of the sulfur precursor (right y axis).
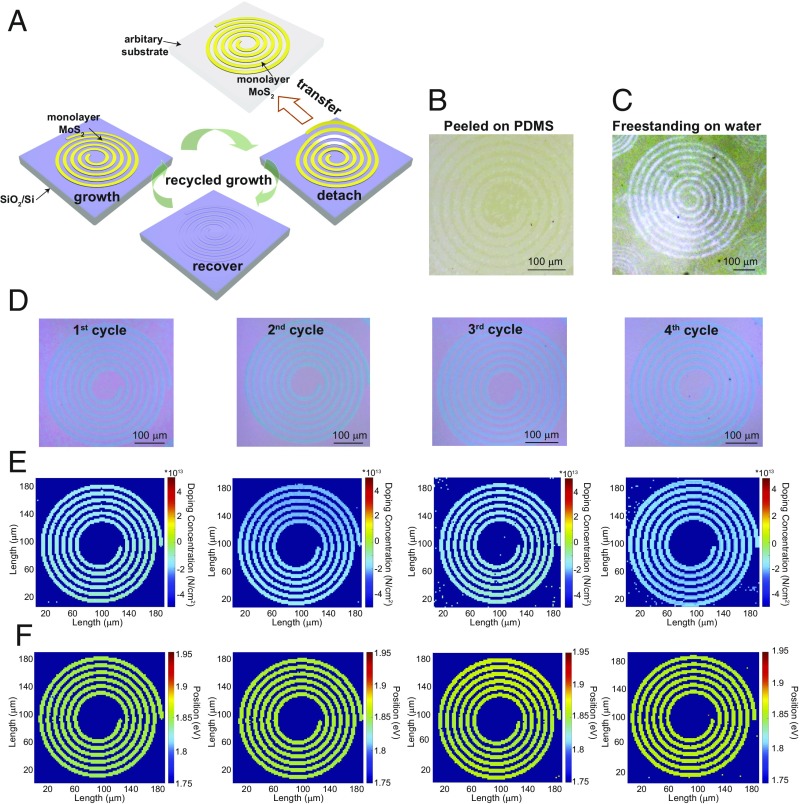
As previously mentioned, electronic applications require patterned materials for device fabrication and integration. Nevertheless, previous methods to produce patterned 2D materials are limited by the single use of patterned substrates or etched materials. Therefore, developing a master–replica type of repeated growth of patterned TMD materials is highly attractive for 2D material device fabrication. Here, we have found that the prepatterned substrate can be repeatedly used through the noninvasive transfer of monolayer MoS2 patterns. As shown in Fig. 4A, due to the weak interaction between the monolayer MoS2 and the SiO2/Si substrate, monolayer MoS2 patterns can be easily detached (with preserved structural integrity) from the substrate by directly peeling with a polydimethylsiloxane (PDMS) stamp via dry transfer (Fig. 4B) or delaminating by water via wet transfer (Fig. 4C and SI Appendix, Figs. S15 and S16). Since the separation process is performed without any intermediate supporting layer or etchant, the released substrate with original patterns is recovered, which makes it possible for the next cycle of patterned growth. In our study, it was found that the patterned substrate can be reused at least four times without seeing signs of wear, and the quality of monolayer MoS2 patterns is well preserved. Fig. 4D shows the OM images of MoS2 spiral patterns grown from four “growth–detach” cycles on the same SiO2/Si substrate after water delamination. It was found that the shape of the MoS2 spiral in each cycle was well reserved and that the reflectance contrast was uniform under the same illumination. The OM images of SiO2/Si substrates before growth and after delamination (SI Appendix, Fig. S17) show that premade patterns on SiO2/Si substrate are retained after this simple and clean transfer without visible damage and contamination. From our calculation, the O2 plasma-treated substrates have lower binding energy with hydrocarbon species (the common contaminate in air) (SI Appendix, Fig. S18), which suggests a “contamination-resilient” feature of O-rich surfaces. This could be verified by our observation that the patterned substrates kept for several weeks are still as effective to patterned growth as freshly made. To evaluate the quality of the MoS2 patterns grown by the recycled growth, we extracted strain (SI Appendix, Fig. S19) and carrier concentration maps (Fig. 4E) from the spatially resolved Raman analysis (34, 35) on the MoS2 spirals grown each time using the same SiO2/Si template. The strain and carrier concentration maps show a relatively small variation, indicating good consistency in the quality of MoS2 patterns for different cycles. Moreover, the PL spectrum on monolayer MoS2 from each cycle has few changes on the emission energy and FWHM (SI Appendix, Fig. S20). The photoluminescence position mappings (Fig. 4F) extracted from the PL spectrum analysis also demonstrate the preserved qualities. After delamination, these MoS2 patterns floating on a water surface can be easily picked up onto arbitrary substrates, such as transparent quartz (SI Appendix, Fig. S21A) and flexible mica (SI Appendix, Fig. S21B), allowing for more efficient integration of complicated 2D device structures.
Fig. 4.
Repeated growth of patterned monolayer MoS2 nanostructures using the same template. (A) Schematic illustration of repeated growth procedures for directly grown MoS2 patterns. (B) OM image of monolayer MoS2 patterns peeled on PDMS. (C) OM image of monolayer MoS2 structures freestanding on water without any additional support. (D) OM images of the monolayer MoS2 spiral pattern after the first, second, third, and fourth growth cycles. (E) The carrier concentration mappings (via Raman) of the monolayer MoS2 spiral after the first, second, third, and fourth growth cycles as given by D. (F) The photoluminescence position mappings (via PL spectrum) of the monolayer MoS2 spiral after the first, second, third, and fourth growth cycles as given by D.
The key for repeated utilization of the same mask relies on the successful delamination (hence, on the quality of the monolayer MoS2 patterns). If MoS2 particles are grown, the patterns are difficult to delaminate by water, which will damage the surface integrity and reduce the lifetime of the master template. With well-controlled growth, successful transfer, and posttreated cleaning, it is anticipated that these prepatterned templates can be used many times. This additive manufacturing method paves the way for broad applications of 2D TMD nanostructures in modern electronics and optoelectronics with much simpler fabrication, lower cost, and higher efficiency.
Methods
To obtain patterned substrates for MoS2 growth, photolithography and O2 plasma treatment steps were first carried out as explained above. After removing the photoresist on the patterned SiO2/Si wafer, the substrate was preannealed at 350 °C for 2 h with 300 standard cubic centimeters per minute (sccm) Ar and 100 sccm H2 gases. Then, a piece of the patterned substrate was suspended between two SiO2/Si substrates with predeposited PTAS (via solution dropping). All of these substrates were placed face down on a crucible containing MoO3 precursor in a 1-inch quartz tube. This crucible was placed in the middle of the heating zone with another smaller tube containing the sulfur crucible upstream close to the cold end of the quartz tube. Before heating, the whole CVD system was purged with 1,000 sccm Ar (99.999% purity) for 3 min. Then, 20 sccm Ar was introduced into the system as a carrier gas. The growth system was heated to 625 °C for 15 min. At around 13 min, the sulfur crucible was pushed into the system by a magnet (as shown in Fig. 3A) at a distance of ∼1.5 cm from the left edge of the furnace. The MoS2 growth was carried out around 620 °C to 630 °C for 3 min under atmospheric pressure. The growth system was finally cooled down to room temperature quickly by opening the furnace and cooling using a fan.
Supplementary Material
Acknowledgments
We acknowledge support from Air Force Office of Scientific Research Multidisciplinary University Research Initiative-Foldable and Adaptive Two-Dimensional Electronics Program Grant FA9550-15-1-0514, the Center for Energy Efficient Electronics Science through NSF Grant 0939514, the US Army Research Office through Massachusetts Institute of Technology Institute for Soldier Nanotechnologies Grant 023574, the Center for Integrated Quantum Materials, Science and Technology Center through NSF Grant DMR-1231319 (to Q.J. and Y.L.), and King Abdullah University of Science and Technology Contract OSR-2015-CRG4-2634. Y.H. and D.A.M. acknowledge the Cornell Center for Materials Research for funding through NSF Materials Research Science and Engineering Centers Program DMR-17198751. X.L. acknowledges the support of Boston University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816197116/-/DCSupplemental.
References
- 1.Radisavljevic B, Kis A. Mobility engineering and a metal-insulator transition in monolayer MoS2. Nat Mater. 2013;12:815–820. doi: 10.1038/nmat3687. [DOI] [PubMed] [Google Scholar]
- 2.Bertolazzi S, Brivio J, Kis A. Stretching and breaking of ultrathin MoS2. ACS Nano. 2011;5:9703–9709. doi: 10.1021/nn203879f. [DOI] [PubMed] [Google Scholar]
- 3.Mak KF, McGill KL, Park J, McEuen PL. Valleytronics. The valley hall effect in MoS2 transistors. Science. 2014;344:1489–1492. doi: 10.1126/science.1250140. [DOI] [PubMed] [Google Scholar]
- 4.Wu W, et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature. 2014;514:470–474. doi: 10.1038/nature13792. [DOI] [PubMed] [Google Scholar]
- 5.Feng Q, et al. Semiconductors: Growth of large-area 2D MoS2(1-x)Se2x semiconductor alloys (Adv. Mater. 17/2014) Adv Mater. 2014;26:2763. doi: 10.1002/adma.201306095. [DOI] [PubMed] [Google Scholar]
- 6.Radisavljevic B, Radenovic A, Brivio J, Giacometti V, Kis A. Single-layer MoS2 transistors. Nat Nanotechnol. 2011;6:147–150. doi: 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- 7.Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat Nanotechnol. 2012;7:699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 8.Zheng W, et al. Patterning two-dimensional chalcogenide crystals of Bi2Se3 and In2Se3 and efficient photodetectors. Nat Commun. 2015;6:6972. doi: 10.1038/ncomms7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Q, et al. Control and characterization of individual grains and grain boundaries in graphene grown by chemical vapour deposition. Nat Mater. 2011;10:443–449. doi: 10.1038/nmat3010. [DOI] [PubMed] [Google Scholar]
- 10.Zhou H, et al. Thickness-dependent patterning of MoS2 sheets with well-oriented triangular pits by heating in air. Nano Res. 2013;6:703–711. [Google Scholar]
- 11.Mahjouri-Samani M, et al. Patterned arrays of lateral heterojunctions within monolayer two-dimensional semiconductors. Nat Commun. 2015;6:7749. doi: 10.1038/ncomms8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue Y, et al. Scalable production of a few-layer MoS2/WS2 vertical heterojunction array and its application for photodetectors. ACS Nano. 2016;10:573–580. doi: 10.1021/acsnano.5b05596. [DOI] [PubMed] [Google Scholar]
- 13.Park W, et al. Photoelectron spectroscopic imaging and device applications of large-area patternable single-layer MoS2 synthesized by chemical vapor deposition. ACS Nano. 2014;8:4961–4968. doi: 10.1021/nn501019g. [DOI] [PubMed] [Google Scholar]
- 14.Cao L, et al. Direct laser-patterned micro-supercapacitors from paintable MoS2 films. Small. 2013;9:2905–2910. doi: 10.1002/smll.201203164. [DOI] [PubMed] [Google Scholar]
- 15.Kelly AG, et al. All-printed thin-film transistors from networks of liquid-exfoliated nanosheets. Science. 2017;356:69–73. doi: 10.1126/science.aal4062. [DOI] [PubMed] [Google Scholar]
- 16.Han GH, et al. Seeded growth of highly crystalline molybdenum disulphide monolayers at controlled locations. Nat Commun. 2015;6:6128. doi: 10.1038/ncomms7128. [DOI] [PubMed] [Google Scholar]
- 17.Song I, et al. Patternable large-scale molybdenium disulfide atomic layers grown by gold-assisted chemical vapor deposition. Angew Chem Int Ed Engl. 2014;53:1266–1269. doi: 10.1002/anie.201309474. [DOI] [PubMed] [Google Scholar]
- 18.Kim H-J, Kim H, Yang S, Kwon J-Y. Grains in selectively grown MoS thin films. Small. 2017;13:1702256. doi: 10.1002/smll.201702256. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, et al. Lithography-free plasma-induced patterned growth of MoS2 and its heterojunction with graphene. Nanoscale. 2016;8:15181–15188. doi: 10.1039/c6nr03318k. [DOI] [PubMed] [Google Scholar]
- 20.Shyu TC, et al. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat Mater. 2015;14:785–789. doi: 10.1038/nmat4327. [DOI] [PubMed] [Google Scholar]
- 21.Lamoureux A, Lee K, Shlian M, Forrest SR, Shtein M. Dynamic kirigami structures for integrated solar tracking. Nat Commun. 2015;6:8092. doi: 10.1038/ncomms9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blees MK, et al. Graphene kirigami. Nature. 2015;524:204–207. doi: 10.1038/nature14588. [DOI] [PubMed] [Google Scholar]
- 23.Ling X, et al. Role of the seeding promoter in MoS2 growth by chemical vapor deposition. Nano Lett. 2014;14:464–472. doi: 10.1021/nl4033704. [DOI] [PubMed] [Google Scholar]
- 24.Ling X, et al. Parallel stitching of 2D materials. Adv Mater. 2016;28:2322–2329. doi: 10.1002/adma.201505070. [DOI] [PubMed] [Google Scholar]
- 25.van der Zande AM, et al. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat Mater. 2013;12:554–561. doi: 10.1038/nmat3633. [DOI] [PubMed] [Google Scholar]
- 26.Kang K, et al. High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity. Nature. 2015;520:656–660. doi: 10.1038/nature14417. [DOI] [PubMed] [Google Scholar]
- 27.English CD, Shine G, Dorgan VE, Saraswat KC, Pop E. Improved contacts to MoS2 transistors by ultra-high vacuum metal deposition. Nano Lett. 2016;16:3824–3830. doi: 10.1021/acs.nanolett.6b01309. [DOI] [PubMed] [Google Scholar]
- 28.ten Wolde PR, Frenkel D. Enhancement of protein crystal nucleation by critical density fluctuations. Science. 1997;277:1975–1978. doi: 10.1126/science.277.5334.1975. [DOI] [PubMed] [Google Scholar]
- 29.Burns RP, DeMaria G, Drowart J, Grimley RT. Mass spectrometric investigation of the sublimation of molybdenum dioxide. J Chem Phys. 1960;32:1363–1366. [Google Scholar]
- 30.Kumari L, et al. X-ray diffraction and Raman scattering studies on large-area array and nanobranched structure of 1D MoO2 nanorods. Nanotechnology. 2007;18:115717. [Google Scholar]
- 31.Li H, Li Y, Aljarb A, Shi Y, Li L-J. Epitaxial growth of two-dimensional layered transition-metal dichalcogenides: Growth mechanism, controllability, and scalability. Chem Rev. 2018;118:6134–6150. doi: 10.1021/acs.chemrev.7b00212. [DOI] [PubMed] [Google Scholar]
- 32.Chhowalla M, et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem. 2013;5:263–275. doi: 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Feng H, Wu Y, Jiao L. Controlled synthesis of highly crystalline MoS2 flakes by chemical vapor deposition. J Am Chem Soc. 2013;135:5304–5307. doi: 10.1021/ja4013485. [DOI] [PubMed] [Google Scholar]
- 34.Michail A, Delikoukos N, Parthenios J, Galiotis C, Papagelis K. Optical detection of strain and doping inhomogeneities in single layer MoS2. Appl Phys Lett. 2016;108:173102. [Google Scholar]
- 35.Chae WH, Cain JD, Hanson ED, Murthy AA, Dravid VP. Substrate-induced strain and charge doping in CVD-grown monolayer MoS2. Appl Phys Lett. 2017;111:143106. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.