Significance
Crystal structures of intermediates in the reaction of oxygen (O2) with cytochrome c oxidase (CcO) have never been determined, limiting our understanding of the coupling between O2 reduction and proton translocation. With time-resolved serial femtosecond crystallography, the structure of a catalytic intermediate was obtained in the reaction of reduced microcrystals of bovine CcO with O2. This intermediate, identified as the PR species formed in the R → A → PR reaction, is composed of a heme a3 ferryl (Fe4+ = O2−) structure and an electronic configuration of the metal centers of [a34+ = O2−, CuB2+-OH−, a3+, CuA1+]. There is a 120° rotation of the heme a farnesyl side chain in PR, suggesting its role in proton gating in mammalian CcOs.
Keywords: bioenergetics, complex IV, X-ray free electron laser, crystallography, catalytic intermediates
Abstract
Cytochrome c oxidase (CcO) reduces dioxygen to water and harnesses the chemical energy to drive proton translocation across the inner mitochondrial membrane by an unresolved mechanism. By using time-resolved serial femtosecond crystallography, we identified a key oxygen intermediate of bovine CcO. It is assigned to the PR-intermediate, which is characterized by specific redox states of the metal centers and a distinct protein conformation. The heme a3 iron atom is in a ferryl (Fe4+ = O2−) configuration, and heme a and CuB are oxidized while CuA is reduced. A Helix-X segment is poised in an open conformational state; the heme a farnesyl sidechain is H-bonded to S382, and loop-I-II adopts a distinct structure. These data offer insights into the mechanism by which the oxygen chemistry is coupled to unidirectional proton translocation.
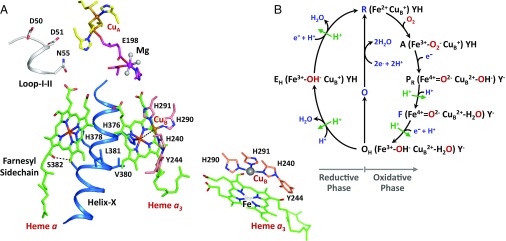
Cytochrome c oxidase (CcO), a member of the oxygen reductase family of enzymes, is the terminal enzyme of the electron transfer chain in the inner mitochondrial membrane. It catalyzes the four-electron reduction of dioxygen to two water molecules and harnesses the redox energy to pump four protons across the membrane against an electrochemical proton concentration gradient (1, 2). Bovine CcO (bCcO) is a member of the family with a molecular weight of ∼400 kDa. It is a homodimer. Each monomer is composed of 13 subunits (SI Appendix, Fig. S1) and four redox centers: a dinuclear copper center (CuA), a heme group (heme a), and a binuclear center consisting of another heme (heme a3) and another copper (CuB) (Fig. 1A). Dioxygen is reduced to two water molecules in the heme a3–CuB binuclear center by using four electrons sequentially transferred to it from cytochrome c, CuA, and heme a, and four protons (the “substrate protons”) taken up from the negative side of the mitochondrial membrane. The energy released from the dioxygen reduction chemistry is used to translocate four additional protons (the “pumped protons”) from the negative side to the positive side of the membrane against an electrochemical gradient.
Fig. 1.
Active site structure of bCcO (A) and the proposed dioxygen reduction reaction cycle (B). The structure shown in A illustrates the relative location of the four redox centers, CuA, heme a and the CuB-heme a3 binuclear center, as well as the Mg site intervening the CuA center and the binuclear center. Helix-X (labeled in blue) contains the axial ligands of heme a3 and heme a, H376 and H378, respectively. (Lower Right Inset) Expanded view of the binuclear center. One of the CuB ligands, H240, is posttranslationally linked to Y244. All these structural elements are in subunit I, except CuA and E198(II) reside in subunit II. (B) Electronic configuration of the heme a3 iron, CuB, and Y244 in each intermediate is depicted. (See Introduction for the full description of the reaction sequence.) Each green arrow indicates the translocation of one pumped proton coupled to the designated reaction step (6).
Time-resolved spectroscopic studies indicate that the dioxygen reduction reaction with the fully reduced enzyme follows a sequential mechanism (Fig. 1B) (3). The reaction is initiated by O2 binding to the heme a3 iron atom in the reduced enzyme (R) to form intermediate A, a ferric-superoxide species. Then, on cleavage of the O–O bond, it converts to a ferryl intermediate, PR, by accepting one electron each from CuB and heme a (2, 4), with the associated formation of a tyrosinate on Y244. Subsequently, a second ferryl intermediate, F, is formed by accepting a proton from the negative side of the membrane. In this state, there is a redox equilibrium between CuA and heme a (4, 5), supported by resonance Raman data, which have shown that in F heme a ∼70% oxidized (3). F converts to a hydroxide bound ferric species, OH, on the addition of another electron from the CuA/heme a pair and another proton from the negative side of the membrane. The oxidation states of the metal centers in each intermediate are summarized in Table 1. Biophysical studies (6) have shown that proton translocation takes place in the PR → F and F → OH steps. In addition, if OH is immediately reduced after the O2 reduction reaction, additional steps of proton translocation may occur during the OH → EH and the EH → R transitions. However, if the reduction is delayed, OH relaxes to the resting fully oxidized species (O), and the subsequent reduction of O to R is not able to drive proton translocation (6).
Table 1.
Proposed redox and coordination states of metal centers for each intermediate in the reaction of fully reduced bCcO with O2
| State | Heme a3 | CuB | Heme a/CuA | Y244 |
| R | Fe2+ | Cu1+ | Fe2+/Cu1+ | YH |
| A | Fe3+-O2− | Cu1+ | Fe2+/Cu1+ | YH |
| PR | Fe4+ = O2- | Cu2+ | Fe3+/Cu1+ | Y− |
| F | Fe4+ = O2- | Cu2+ | Fe3+/Cu1+ | Y− |
| Fe2+/Cu2+ | ||||
| OH | Fe3+-OH− | Cu2+ | Fe3+/Cu2+ | Y− |
In F, as one electron is transferred from either heme a or CuA to the binuclear center, alternate redox states of heme a and CuB are listed.
As no 3D structures of the hypothesized oxygen intermediates have been identified, it remains unclear as to how the dioxygen reduction reaction is coupled to proton translocation. As a first step to characterize the structures of oxygen reaction intermediates of bCcO, we developed a time-resolved serial femtosecond crystallography (TR-SFX) protocol by combining SFX with a “Mix and Inject” technology (7). In SFX experiments, microcrystals suspended in a liquid jet are delivered to the beam of an X-ray free electron laser with a gas dynamic virtual nozzle (8). Diffraction data of randomly oriented microcrystals in the liquid jet are collected by intersecting the liquid jet with a series of ∼40-fs X-ray pulses from the X-ray free electron laser before the microcrystals are destroyed by the high-intensity X-ray pulses. This “diffraction before destruction” method has opened up avenues for structural determination of fragile biomolecules under physiologically relevant conditions (at room temperature and in the absence of cryoprotectants) and without radiation damage (9).
Methods
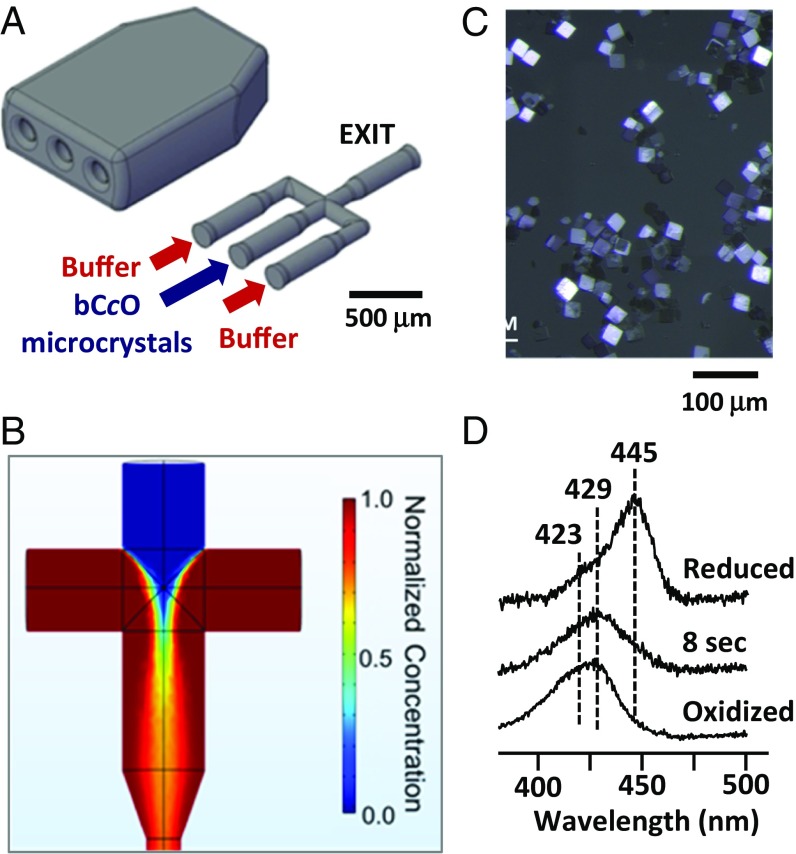
To initiate the in crystallo catalytic reaction, we designed a hydrodynamic focusing microfluidic mixer (Fig. 2A), fabricated using a two-photon 3D printer with submicron resolution. The performance of the mixer was confirmed by computational simulations (Fig. 2B). The bCcO microcrystal suspension enters from the central port of the mixer with a flow rate of 8 μL/min, whereas the O2 saturated buffer is introduced into the two side ports with a faster flow rate, 16 μL/min each. The two oxygenated side solutions focus the microcrystal jet into a narrow stream, such that O2 in the buffer is able to diffuse rapidly (within ∼3 ms) into the microcrystal suspension in the central stream. The bCcO microcrystals have an average dimension of ∼20 × 20 × 4 µm (Fig. 2C). On the basis of Fick’s law (10), we estimated that diffusion of the O2 into the microcrystals occurs within ∼2 ms through the thinnest dimension of the crystal. The mixer is coupled to the gas dynamic virtual nozzle injector via a “timing” loop that is used to tune the reaction time (SI Appendix, Fig. S2). Additional details of the procedures used are described in the SI Appendix, Materials and Methods.
Fig. 2.
Design and evaluation of the hydrodynamic focusing mixer (A and B) and time-resolved spectra associated with in crystallo dioxygen reduction reaction of bCcO (C and D). The schematic drawing in A illustrates the design of the mixer assembly. (B) Mixing behavior of the mixer simulated with COMSOL software, based on a convection-diffusion model. The two side streams, each flowing at a rate of 16 μL/min, squeeze the oxygen-containing central stream, flowing at a rate of 8 μL/min, such that complete mixing takes place before the mixed solution exits the mixer. The color bar indicates the normalized concentration of oxygen. (C) Microscopic image of typical bCcO microcrystals. (D) Optical absorption spectrum of the intermediate populated at 8 s after the mixing of a reduced bCcO microcrystal solution with an O2-containing buffer. The spectra of the reduced and oxidized forms of the enzyme are shown as references.
Results
Identification of Intermediates in the Reaction of O2 with bCcO Microcrystals by Optical Absorption Spectra.
The kinetics of the bCcO reaction illustrated in Fig. 1B have been defined in free solution based on spectroscopic studies (3), but have never been determined in the crystalline state. As a reference to guide the TR-SFX studies, we used optical absorption spectroscopy to monitor the progression of the in crystallo reaction after the mixing of the microcrystal suspension of fully reduced bCcO (R) with an O2 saturated buffer. We identified a unique intermediate at ∼8 s, having a spectral feature with λmax = 429 nm, which is characteristic of a ferryl derivative of CcO (11, 12), such as that in PR or F (Table 1). It is distinct from that of the initial R species (with λmax = 445 nm) or the final relaxed O species (with a characteristic shoulder at ∼423 nm) (Fig. 2D). It also differs from that of the A intermediate (11) with a very broad band centered at ∼437 nm. Intriguingly, in the crystalline state, the intermediate does not convert to the O species until minutes, whereas the entire reaction cycle is completed within a few milliseconds in free solution (3).
Structure and Characterization of a Catalytic Intermediate in bCcO.
On the basis of the time-resolved spectral data, we carried out TR-SFX measurements on the intermediate populated at 8 s after the initiation of the dioxygen reduction reaction. The SFX structure of the intermediate was refined to a resolution of 2.5 Å. Comparable SFX data of the initial reduced enzyme (R) and the fully oxidized enzyme (O) were obtained as references and refined to 2.8 and 2.9 Å, respectively (SI Appendix, Table S1).
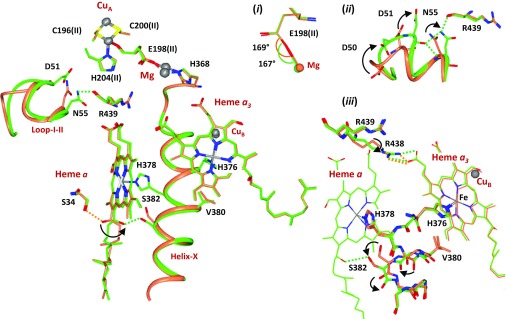
To cleave the O2 bond and generate a ferryl species in a PR or F-intermediate, four electrons are needed in the binuclear center. It is anticipated that one electron comes from CuB, two are derived from heme a3 (hence a transition from the Fe2+ to the Fe4+ state), and one additional electron comes from the CuA/heme a pair (Table 1). It is critical to determine whether the last electron comes from CuA or heme a to fully comprehend protein conformational changes associated with the dioxygen reduction reaction. Fortunately, the redox state changes in the two metal centers are associated with unique structural markers. When heme a is reduced, the OH group on its farnesyl side chain is H-bonded to S34. On oxidation, the OH group rotates by ∼120°, which disrupts the H-bond to S34 and establishes a new H-bond to S382 (13). The fact that the farnesyl side chain of heme a in the intermediate has undergone the ∼120° rotation, and is H-bonded to S382 (Fig. 3 and SI Appendix, Fig. S3), indicates that heme a is in the oxidized form, suggesting that CuA is in the reduced state.
Fig. 3.
Structural changes associated with the R → PR transition. The structure of the PR-intermediate (green) is superimposed on reduced bCcO (R, coral). (Insets) Expanded views of the structural regions of (i) E198(II), highlighting the resemblance of the C-O-Mg angle in PR vs. R, demonstrating that CuA is in its reduced state; (ii) Loop-I-II, highlighting the large-scale movement of D51 and N55; and (iii) the [380-384] segment of Helix-X, showing the partial opening of the helical structure and the rotation in R438. The black curved arrows indicate R → PR structural changes. The coral and green dashed lines represent H-bonds in reduced and intermediate structures, respectively.
CuA is located in a region near a hypothesized site critical for proton translocation (14). It is coordinated by the main chain carbonyl group of E198 in subunit II [referred to as E198(II) hereafter]. The side chain carboxyl group of E198(II) is a ligand to the Mg atom (Fig. 3); it undergoes a rotation upon the oxidation of CuA, such that the C-O-Mg angle is reduced by 35° (SI Appendix, Fig. S4) (14). The observation that the conformation of E198(II), as well as other structural features in its vicinity (SI Appendix, Fig. S5), in the 8-s intermediate, are identical to those in the R species [Fig. 3, Inset (i)] confirms that CuA remains reduced when the intermediate is formed. Taken together the data confirm that the transient species is either the PR or F-intermediate with a [a34+ = O2−, CuB2+-OH−, a3+, CuA1+] or a [a34+ = O2−, CuB2+-OH2, a3+, CuA1+] configuration, respectively, which are distinct from that of the PM intermediate, in which both heme a and CuA are reduced (2), and that of the O species or the OH intermediate formed at the end of the oxidative phase of turnover, where all four redox centers are in their oxidized states (Table 1).
In the intermediate, strong pear-shaped Fo-Fc difference electron density is evident in the binuclear center, which is not present in the R species (Fig. 4A vs. Fig. 4B), confirming an O2 reaction product in the active site. We modeled the binuclear site as either a PR-intermediate with the CuB2+-OH− distance restrained to 1.86 Å or a F-intermediate with the CuB2+-H2O distance restrained to 2.40 Å, based on distances determined by density functional theory calculations (15), whereas the Fe4+ = O2− distances were allowed to vary in the refinements of the PR- and F-intermediates, in which they refined to 1.70 and 1.91 Å, respectively (Table 2). The 1.70-Å distance is characteristic of a ferryl Fe4+ = O2− bond, but 1.91 Å is too long for a ferryl species, making the assignment of the transient species as an F-intermediate unlikely; thus, we tentatively assign the intermediate as PR. The Polder map (16) of the oxygen ligands coordinated to CuB and heme a3 is consistent with the PR assignment (Fig. 4C). (See the SI Appendix for a further discussion of the assignment.)
Fig. 4.
Ligand density in the binuclear center of the PR-intermediate of bCcO. A shows the 2Fo-Fc electron density map of the initial reduced bCcO (R), demonstrating that no ligand density is present in its binuclear center. (B) Fo-Fc difference density map of the PR-intermediate, obtained without any ligand modeled, indicating the presence of exogenous ligand or ligands in the binuclear center. (C and D) Structure of the PR-intermediate modeled with a ferryl oxygen coordinated to the heme a3 and a hydroxide coordinated to CuB. (C) Polder OMIT map for each ligand. (D) 2Fo-Fc map. The CuB to heme a3 iron distance (in Å) is indicated in each structure. The Fo-Fc density map (green) is contoured at σ = 3, the Polder map (black) is contoured at σ = 7, and the 2Fo-Fc density maps (blue) are contoured at σ = 2.
Table 2.
Fe4+ = O2- distance when modeling the intermediate as PR or F with the ligands on CuB, OH− and H2O, respectively, set at their calculated distances (15)
| Intermediate | Fe4+ = O2- (Å) | CuB-L (Å) | Fea3-CuB (Å) |
| PR | 1.70 | 1.86 (OH−) | 4.68 |
| F | 1.91 | 2.40 (H2O) | 4.63 |
The distances are averages of the two subunits in bCcO.
It is important to note that modeling the ligand density in the intermediate with a peroxide leads to extra density that could not be accounted for, excluding the presence of a bridging peroxide (SI Appendix, Fig. S6). In contrast, our fully oxidized O species of bCcO can be modeled with a bridging peroxide (SI Appendix, Fig. S7), as reported previously for bCcO (17) and Paracoccus denitrificans CcO (18). (See SI Appendix, Fig. S7 caption for a discussion of the ligand structure in the oxidized enzyme.) Together, the time-resolved in crystallo optical data and the SFX structural data support the assignment of the 8-s intermediate as the PR-intermediate.
In addition to the aforementioned rotation of the heme a farnesyl side chain, the R → PR transition brings about several distinct protein conformational changes. In particular, the [380-384] segment of Helix-X lying between the two heme groups changes from a closed conformation to an open conformation with disrupted H-bonding interactions [Fig. 3, Inset (iii)], which is presumably induced by the change in ligation state of heme a3 (19). The structural transition in the [380-384] segment moves Helix-X closer to heme a, where its surrounding residues may serve as sites for proton storage and/or gating during translocation (vide infra). The change in the structure of heme a3 also induces a rotation of the central carbon atoms of the R438 residue of ∼120° [Fig. 3, Inset (iii)]. In addition, a very large conformational change in the Loop-I-II region is evident [Fig. 3, Inset (ii)]. In the reduced structure (R), the sidechain of N55 is 5.3 Å away from the main chain carbonyl group of R439. In the PR-intermediate, the distance is reduced to 3.2 Å as a result of a large-scale rotation of the N55 sidechain toward R439, possibly induced by the change in the oxidation state of heme a. These changes are associated with a large-scale motion of the [47-55] loop fragment, as well as the establishment of new main chain and side chain H-bonds between N55 and D51. As D51 has been postulated to be an important proton delivery point (13), the unusual flexibility of this loop (SI Appendix, Fig. S8) is plausibly critical for the proton translocation.
In the catalytic site of the PR-intermediate, a water molecule (labeled as W1 in Fig. 5) was identified in the binuclear center (see Polder map in SI Appendix, Fig. S9). It is within H-bonding distance (2.7 Å) from Y244 and less than 5 Å from the ferryl oxygen atom coordinated to heme a3. A water molecule at this position in bCcO has not been reported in the past, although water molecules near this position were shown to be present in the binuclear center of Rhodobacter sphaeroides CcO (20). The presence of a water molecule near Y244 in the PR-intermediate may play an important role in stabilizing its putative tyrosinate configuration (2). In addition, Y244 is the terminal residue of the K-channel, one of the three postulated proton channels in bCcO (SI Appendix, Fig. S10). In bacterial CcO, the K-channel has been shown to deliver protons to the binuclear center during the reductive phase of the reaction (Fig. 1B), but its functionality has not been demonstrated in bCcO (21). Our structural data suggest that the water molecule may provide a link from Y244 to the binuclear center, and it supports the observation that Y244 is the proton donor for the O–O bond scission during the formation of the PR-intermediate (22, 23).
Fig. 5.
A water molecule detected in the binuclear center of the PR-intermediate. The water molecule (W1) resides at the end of the K-channel, which may stabilize the tyrosinate configuration of Y244 and support proton transfer to the binuclear center. The numbers indicate the distances (in Å). The conserved water molecule, W2, is shown as a reference. The 2Fo-Fc difference map is contoured at σ = 1.
Discussion
The PR-Intermediate.
The PR-intermediate is the first species formed following the cleavage of the O–O bond in the reaction of the fully reduced enzyme with O2 (i.e., R → A → PR). The O–O bond cleavage and the associated formation of the ferryl species results from the transfer of electrons without any associated proton entry into the binuclear center, making the intermediate the only one in which the charge neutrality rule is not upheld (24). The intermediate is poised to uptake a proton to form the F-intermediate, a step in which the first proton translocation event has been shown to occur (6). Despite the fact that the structure of the PR-intermediate is debatable, the oxidation and coordination states of the metal centers inferred from this work are consistent with those defined by electron paramagnetic resonance and optical studies by Morgan et al. (4). The observation that the PR-intermediate may be readily stabilized by triple trapping (4) and is relatively stable in microcrystals suggests that its conversion to the F-intermediate may be limited by protein motion, as has been seen in other enzymes (25), or by proton access to the binuclear center, which has been shown to retard the rate of reduction of the F-intermediate (26). Finally, in the oxygen reaction in solution of the fully reduced Glu-278-GLN (E242 in bovine) mutant enzyme of Paracoccus denitrificans CcO, the reaction stops at the PR state (τ = ∼1.1 s) because of the limited proton availability (22).
Proton Translocation Channels.
Although substantial data on bacterial CcOs, and in particular those derived from mutagenesis studies (27–30), indicate that the four pumped protons are translocated through the d-channel, which connects the negative side of the membrane to the heme a3 site (SI Appendix, Fig. S10), no structural changes are evident in the vicinity of the channel on formation of the PR-intermediate from R in bCcO. In particular, E242 has been postulated to be the diverter valve at the end of the d-channel directing the protons either to the binuclear center for water formation or to a proton loading site for proton translocation (29, 31). Previous Fourier transform-infrared studies showed that redox and CO-photolysis reactions induce conformational changes in E242 (or the residue equivalent to E242) in bCcO, yeast CcO and P. denitrificans CcO, suggesting a change in environment or orientation, supporting its role in proton translocation. However, there is no change in the conformation of E242 in the crystal structure during the R → PR transition (SI Appendix, Fig. S11), or in CO photodissociation studies (32). Although we recognize that the complete side chain rotation cycle required for the diverter valve function of E242 occurs in each elementary step, the E242 residue in the PR-intermediate has the same temperature factors as those of other residues suggesting that it lacks a high degree of flexibility, likely needed for its postulated role in proton gating (31).
In contrast to the absence of changes in residues along the d-channel, large conformational changes were identified in regions associated with the H-channel, and in particular those near the farnesyl side chain of heme a and Loop-I-II. The H-channel, which starts below H413, passes by the farnesyl side chain of heme a and terminates at D51 in Loop-I-II (SI Appendix, Fig. S10), was first proposed to be the major proton translocation pathway by Tsukihara et al. (13), based on structural studies. However, its functional role has been controversial, despite the fact that it was supported by mutagenesis studies of a hybrid bovine/human construct, demonstrating that mutations of residues along the H-channel blocked proton translocation, whereas those along the d-channel did not (33). In addition, several detailed models have been proposed for proton translocation via the H-channel, such as those involving the changes in the redox state of heme a (Redox Bohr effect) (34, 35) and proton accumulation mechanisms (1, 14, 36, 37), although their role in proton translocation has been disputed (38). Recently, a detailed analysis of the H-channel by molecular dynamics simulations was reported by Sharma et al. (39). It was concluded that proton translocation through the upper and central regions of the H-channel is possible, but it is restricted in the region near H413 because of the absence of a sufficient number of water molecules to mediate proton translocation. Instead of translocating protons, it was proposed that the H-channel may serve as a dielectric well in which the dipoles of the polar residues are altered in response to redox state changes in heme a (39). However, Shimada et al. (40) noted that a reanalysis of the simulations is needed, as in a more recent high-resolution structure (PDB ID code: 5B1A; resolution = 1.6 Å) additional waters were identified in the H413 region, which were not present in the lower-resolution structure (PDB ID code: 1V54; resolution = 1.8 Å) originally used by Sharma et al. (39). Interestingly, the conformational changes in the farnesyl side chain of heme a and in Loop-I-II in bCcO were not observed in the bacterial enzyme (R. sphaeroides CcO) when the oxidized and reduced structures were compared (20), suggesting that the mechanism of proton translocation may not be conserved in mammalian and bacterial CcOs.
A Proton Gating Model.
According to the structural data, we postulate that the farnesyl side chain of heme a plays a role in proton gating along the H-channel (Fig. 6). When heme a is in its reduced state, the OH group on its farnesyl side chain, as well as the side chain OH groups on S34 and S382, are protonated. On oxidation of heme a, the farnesyl side chain rotates by 120°, which breaks the H-bond between its OH group and S34 and establishes a new H-bond with S382. The new H-bonding interaction leads to the release of a proton (such that the farnesyl side chain OH group and S382 share a single proton). The partial net charge thereby developed in this structural region is plausibly stabilized by the increased positive charge on the oxidized heme a and the water cluster near the farnesyl OH group (SI Appendix, Fig. S12). The released proton then passes along the heme edge via its formyl group and R38 to a proton loading site, likely in the region near the Mg center, where there is a large water cluster, from which subsequent proton transfer to the positive side of the membrane occurs through the D51 region in Loop-I-II (21) or through other postulated exit pathways (41). Conversely, on reduction of heme a, the OH group on the farnesyl side chain rotates back toward S34 to form an H-bond with it. Concurrently, a new proton is picked up from the negative side of the membrane, leaving all three centers protonated, thereby completing the cycle. In this hypothesized model, one proton is released to a proton loading site each time heme a delivers an electron to the binuclear center (i.e., in each step of the P → F, F → OH, OH → EH and EH → R transitions; Fig. 1B).
Fig. 6.
Postulated heme a gating mechanism. The mechanism is based on conformational changes induced by the change in the oxidation state of heme a and the associated rotation of the farnesyl side chain, as described in the text. PLS, proton loading site.
In summary, the TR-SFX studies allow the structural determination of a key oxygen intermediate of bCcO. It provides insights into the mechanism of proton translocation in the mammalian enzyme compared with that in bacterial CcOs, and paves the way for the determination of the structures of other CcO intermediates, as well as transient species formed in other enzyme reactions.
Supplementary Material
Acknowledgments
The SFX experiments were carried out at the Macromolecular Femtosecond Crystallography experimental station at the Linac Coherent Light Source (LCLS) at the SLAC National Accelerator Laboratory. LCLS is an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University. Use of the LCLS, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science and Office of Basic Energy Sciences under Contract DE-AC02-76SF00515. The helium-rich ambient system for in helium experiments at Macromolecular Femtosecond Crystallography was developed by Bruce Doak and funded by the Max Planck Institute for Medical Research. This work was supported by National Science Foundation Science and Technology Centers Award 1231306, CHE-1404929 (to D.L.R. and S.-R.Y.); Directorate for Biological Sciences Advances in Biological Informatics Grant 1565180 (to N.A.Z. and J.C.H.S.); and National Institutes of Health Awards GM098799 and GM126297 (to D.L.R. and S.-R.Y.) and GM115773 (to S.-R.Y.), and R01GM095583 (to P.F. and A.R.). We also acknowledge support from the Biodesign Center for Applied Structural Discovery at Arizona State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.wwpdb.org (PDB ID codes 6NKN, 6NMF, and 6NMP).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814526116/-/DCSupplemental.
References
- 1.Yoshikawa S, Shimada A. Reaction mechanism of cytochrome c oxidase. Chem Rev. 2015;115:1936–1989. doi: 10.1021/cr500266a. [DOI] [PubMed] [Google Scholar]
- 2.Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxid Redox Signal. 2008;10:1–29. doi: 10.1089/ars.2007.1705. [DOI] [PubMed] [Google Scholar]
- 3.Han S, Takahashi S, Rousseau DL. Time dependence of the catalytic intermediates in cytochrome c oxidase. J Biol Chem. 2000;275:1910–1919. doi: 10.1074/jbc.275.3.1910. [DOI] [PubMed] [Google Scholar]
- 4.Morgan JE, Verkhovsky MI, Palmer G, Wikström M. Role of the PR intermediate in the reaction of cytochrome c oxidase with O2. Biochemistry. 2001;40:6882–6892. doi: 10.1021/bi010246w. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JE, Li PM, Jang DJ, el-Sayed MA, Chan SI. Electron transfer between cytochrome a and copper A in cytochrome c oxidase: A perturbed equilibrium study. Biochemistry. 1989;28:6975–6983. doi: 10.1021/bi00443a030. [DOI] [PubMed] [Google Scholar]
- 6.Verkhovsky MI, Jasaitis A, Verkhovskaya ML, Morgan JE, Wikström M. Proton translocation by cytochrome c oxidase. Nature. 1999;400:480–483. doi: 10.1038/22813. [DOI] [PubMed] [Google Scholar]
- 7.Kupitz C, et al. Structural enzymology using X-ray free electron lasers. Struct Dyn. 2016;4:044003. doi: 10.1063/1.4972069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutet S, et al. High-resolution protein structure determination by serial femtosecond crystallography. Science. 2012;337:362–364. doi: 10.1126/science.1217737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman HN, et al. Femtosecond X-ray protein nanocrystallography. Nature. 2011;470:73–77. doi: 10.1038/nature09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M. Mix and inject: Reaction initiation by diffusion for time-resolved macromolecular crystallography. Adv Condens Matter Phys. 2013;2013:167276. [Google Scholar]
- 11.Wiertz FG, Richter OM, Ludwig B, de Vries S. Kinetic resolution of a tryptophan-radical intermediate in the reaction cycle of Paracoccus denitrificans cytochrome c oxidase. J Biol Chem. 2007;282:31580–31591. doi: 10.1074/jbc.M705520200. [DOI] [PubMed] [Google Scholar]
- 12.Weng LC, Baker GM. Reaction of hydrogen peroxide with the rapid form of resting cytochrome oxidase. Biochemistry. 1991;30:5727–5733. doi: 10.1021/bi00237a014. [DOI] [PubMed] [Google Scholar]
- 13.Tsukihara T, et al. The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process. Proc Natl Acad Sci USA. 2003;100:15304–15309. doi: 10.1073/pnas.2635097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano N, et al. The Mg2+-containing water cluster of mammalian cytochrome c oxidase collects four pumping proton equivalents in each catalytic cycle. J Biol Chem. 2016;291:23882–23894. doi: 10.1074/jbc.M115.711770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaila VR, Johansson MP, Sundholm D, Laakkonen L, Wiström M. The chemistry of the CuB site in cytochrome c oxidase and the importance of its unique His-Tyr bond. Biochim Biophys Acta. 2009;1787:221–233. doi: 10.1016/j.bbabio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Liebschner D, et al. Polder maps: Improving OMIT maps by excluding bulk solvent. Acta Crystallogr D Struct Biol. 2017;73:148–157. doi: 10.1107/S2059798316018210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata K, et al. Determination of damage-free crystal structure of an X-ray-sensitive protein using an XFEL. Nat Methods. 2014;11:734–736. doi: 10.1038/nmeth.2962. [DOI] [PubMed] [Google Scholar]
- 18.Koepke J, et al. High resolution crystal structure of Paracoccus denitrificans cytochrome c oxidase: New insights into the active site and the proton transfer pathways. Biochim Biophys Acta. 2009;1787:635–645. doi: 10.1016/j.bbabio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Ishigami I, et al. Crystal structure of CO-bound cytochrome c oxidase determined by serial femtosecond X-ray crystallography at room temperature. Proc Natl Acad Sci USA. 2017;114:8011–8016. doi: 10.1073/pnas.1705628114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin L, et al. Redox-dependent conformational changes in cytochrome C oxidase suggest a gating mechanism for proton uptake. Biochemistry. 2009;48:5121–5130. doi: 10.1021/bi9001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich PR, Maréchal A. Functions of the hydrophilic channels in protonmotive cytochrome c oxidase. J R Soc Interface. 2013;10:20130183. doi: 10.1098/rsif.2013.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorbikova EA, Belevich I, Wikström M, Verkhovsky MI. The proton donor for O-O bond scission by cytochrome c oxidase. Proc Natl Acad Sci USA. 2008;105:10733–10737. doi: 10.1073/pnas.0802512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorbikova EA, Wikström M, Verkhovsky MI. The protonation state of the cross-linked tyrosine during the catalytic cycle of cytochrome c oxidase. J Biol Chem. 2008;283:34907–34912. doi: 10.1074/jbc.M803511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell R, Rich PR. Proton uptake by cytochrome c oxidase on reduction and on ligand binding. Biochim Biophys Acta. 1994;1186:19–26. doi: 10.1016/0005-2728(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 25.Lewis-Ballester A, et al. Molecular basis for catalysis and substrate-mediated cellular stabilization of human tryptophan 2,3-dioxygenase. Sci Rep. 2016;6:35169. doi: 10.1038/srep35169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadoski RC, Zaslavsky D, Gennis RB, Durham B, Millett F. Exposure of bovine cytochrome c oxidase to high triton X-100 or to alkaline conditions causes a dramatic change in the rate of reduction of compound F. J Biol Chem. 2001;276:33616–33620. doi: 10.1074/jbc.M103640200. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Han H, Pawate A, Gennis RB. Decoupling mutations in the D-channel of the aa(3)-type cytochrome c oxidase from Rhodobacter sphaeroides suggest that a continuous hydrogen-bonded chain of waters is essential for proton pumping. Biochemistry. 2010;49:4476–4482. doi: 10.1021/bi100344x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konstantinov AA, Siletsky S, Mitchell D, Kaulen A, Gennis RB. The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer. Proc Natl Acad Sci USA. 1997;94:9085–9090. doi: 10.1073/pnas.94.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaila VR, Verkhovsky MI, Wikström M. Proton-coupled electron transfer in cytochrome oxidase. Chem Rev. 2010;110:7062–7081. doi: 10.1021/cr1002003. [DOI] [PubMed] [Google Scholar]
- 30.Henry RM, Yu CH, Rodinger T, Pomès R. Functional hydration and conformational gating of proton uptake in cytochrome c oxidase. J Mol Biol. 2009;387:1165–1185. doi: 10.1016/j.jmb.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Kaila VR, Verkhovsky MI, Hummer G, Wikström M. Glutamic acid 242 is a valve in the proton pump of cytochrome c oxidase. Proc Natl Acad Sci USA. 2008;105:6255–6259. doi: 10.1073/pnas.0800770105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada A, et al. A nanosecond time-resolved XFEL analysis of structural changes associated with CO release from cytochrome c oxidase. Sci Adv. 2017;3:e1603042. doi: 10.1126/sciadv.1603042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimokata K, et al. The proton pumping pathway of bovine heart cytochrome c oxidase. Proc Natl Acad Sci USA. 2007;104:4200–4205. doi: 10.1073/pnas.0611627104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egawa T, Yeh SR, Rousseau DL. Redox-controlled proton gating in bovine cytochrome c oxidase. PLoS One. 2013;8:e63669. doi: 10.1371/journal.pone.0063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papa S, Capitanio G, Papa F. The mechanism of coupling between oxido-reduction and proton translocation in respiratory chain enzymes. Biol Rev Camb Philos Soc. 2018;93:322–349. doi: 10.1111/brv.12347. [DOI] [PubMed] [Google Scholar]
- 36.Kubo M, et al. Effective pumping proton collection facilitated by a copper site (CuB) of bovine heart cytochrome c oxidase, revealed by a newly developed time-resolved infrared system. J Biol Chem. 2013;288:30259–30269. doi: 10.1074/jbc.M113.473983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshikawa S, Shimada A, Shinzawa-Itoh K. Respiratory conservation of energy with dioxygen: Cytochrome C oxidase. Met Ions Life Sci. 2015;15:89–130. doi: 10.1007/978-3-319-12415-5_4. [DOI] [PubMed] [Google Scholar]
- 38.Wikström M, Krab K, Sharma V. Oxygen activation and energy conservation by cytochrome c oxidase. Chem Rev. 2018;118:2469–2490. doi: 10.1021/acs.chemrev.7b00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma V, Jambrina PG, Kaukonen M, Rosta E, Rich PR. Insights into functions of the H channel of cytochrome c oxidase from atomistic molecular dynamics simulations. Proc Natl Acad Sci USA. 2017;114:E10339–E10348. doi: 10.1073/pnas.1708628114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimada A, et al. X-ray structural analyses of azide-bound cytochrome c oxidases reveal that the H-pathway is critically important for the proton-pumping activity. J Biol Chem. 2018;293:14868–14879. doi: 10.1074/jbc.RA118.003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugitani R, Stuchebrukhov AA. Molecular dynamics simulation of water in cytochrome c oxidase reveals two water exit pathways and the mechanism of transport. Biochim Biophys Acta. 2009;1787:1140–1150. doi: 10.1016/j.bbabio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.