Significance
In everyday life humans regularly seek participation in highly complex and pleasurable experiences such as music listening, singing, or playing, that do not seem to have any specific survival advantage. The question addressed here is to what extent dopaminergic transmission plays a direct role in the reward experience (both motivational and hedonic) induced by music. We report that pharmacological manipulation of dopamine modulates musical responses in both positive and negative directions, thus showing that dopamine causally mediates musical reward experience.
Keywords: music, dopamine, reward, pleasure, motivation
Abstract
Understanding how the brain translates a structured sequence of sounds, such as music, into a pleasant and rewarding experience is a fascinating question which may be crucial to better understand the processing of abstract rewards in humans. Previous neuroimaging findings point to a challenging role of the dopaminergic system in music-evoked pleasure. However, there is a lack of direct evidence showing that dopamine function is causally related to the pleasure we experience from music. We addressed this problem through a double blind within-subject pharmacological design in which we directly manipulated dopaminergic synaptic availability while healthy participants (n = 27) were engaged in music listening. We orally administrated to each participant a dopamine precursor (levodopa), a dopamine antagonist (risperidone), and a placebo (lactose) in three different sessions. We demonstrate that levodopa and risperidone led to opposite effects in measures of musical pleasure and motivation: while the dopamine precursor levodopa, compared with placebo, increased the hedonic experience and music-related motivational responses, risperidone led to a reduction of both. This study shows a causal role of dopamine in musical pleasure and indicates that dopaminergic transmission might play different or additive roles than the ones postulated in affective processing so far, particularly in abstract cognitive activities.
A fascinating aspect of humans is their capacity to experience feelings of pleasure from highly complex patterns of auditory or visual stimulation such as music and artwork (1–4). Intriguingly, as it is the case for music, these activities do not provide survival values, as primary pleasures (such as food or sex) do, thus raising questions about the ultimate goal of the reward-related signals they can induce in most humans and the neural circuits underlying such particular pleasure.
Previous research has consistently shown that music-evoked pleasure is accompanied by physiological changes in the autonomous nervous system, as well as modulation of the mesolimbic reward pathway, which are similar to those found in response to primary (such as sex or food) and secondary rewards (e.g., money) (refs. 5–17; see also, refs. 18 and 19). Notably, a PET study (11) found that, similar to the processing of biologically relevant rewards, preferred music induces dopamine release in striatal regions, particularly in the nucleus accumbens (NAcc) and the caudate. These findings have led to a model whereby the recruitment of dopaminergic circuits by music—through communication with sensory and cognitive areas involved in the processing of musical information—would result into changes in emotional intensity and arousal, leading to pleasurable and rewarding feelings (20–23). This view challenges previous evidence from primary rewards conducted in rodents, where dopaminergic manipulations show a clear role of dopamine in motivation and learning, but a controversial function in regulating hedonic responses in primary rewards such as food. Indeed, the pleasurable component of reward has been associated with hedonic hotspots in the NAcc regulated by opioids, rather than dopaminergic transmission (23).
However, except for the study of Salimpoor et al. (11), most research on musical pleasure has relied on indirect measures of neuronal activation, with no specificity for neurotransmitter systems that may be involved, and thus their interpretation about the actual neurochemistry supporting musical pleasure has to be taken with caution. In addition, there is no direct evidence showing that dopamine function is causally related to music-evoked pleasure. Indeed, most of the studies conducted rely on correlational methods, such as neuroimaging, or on chemically nonspecific brain stimulation methods (24). Indeed, so far no studies have shown that direct manipulation of synaptic dopaminergic availability can modulate musically induced pleasure. Thus, it remains elusive whether dopamine release and the engagement of dopaminergic circuits observed in prior studies is actually causing/facilitating the pleasure we experience from music or, in contrast, it is a consequence of that pleasure, engaging dopamine-related learning and motivational systems as it has been shown in animal studies using primary rewards. Furthermore, certain authors have distinguished between various kinds of pleasures, ranging from more sensory-based, mastery-competence related, to more aesthetic (refs. 25–27; see ref. 28 for a recent discussion). Although dopamine might not be directly involved in more sensory pleasurable experiences, it could however intertwine differentially or in a more complex way in the processing of diverse types of pleasures, as could be the case in aesthetic experiences (29). Directly manipulating dopaminergic transmission would then critically shed light on the neurobiology and neurochemistry underpinning reward responses to music. More broadly, this approach would also disentangle the causal role of the dopaminergic system, characterized by important differences across species (30, 31), in the pleasurable responses associated with abstract rewards in humans.
We addressed this question through a double blind within-subject pharmacological design in which we directly manipulated dopaminergic synaptic availability while healthy participants listened to self-selected and experimenter-selected musical excerpts (24). Therefore, we orally administrated to each participant a dopamine precursor (levodopa), a dopamine antagonist (risperidone), and a placebo (lactose) in three different sessions (separated by at least 1 wk). The dopamine precursor levodopa does not indiscriminately and massively enhance tonic dopamine levels as other dopamine-enhancing drugs do—like methylphenidate or d-amphetamine. In contrast, levodopa is rapidly taken up by dopaminergic neurons, to be transformed into dopamine and stored in vesicles, enhancing synaptic dopamine levels in association with stimulus-elicited responses. Risperidone is a dopaminergic antagonist that interferes with dopaminergic neurotransmission by binding to a series of dopamine receptors known as D2-like receptors (32). We measured pleasure responses through (i) a physiological measure of arousal, electrodermal activity (EDA), which is a good objective indicator of the hedonic impact of music (19, 33); and (ii) subjective ratings of the experienced pleasure (real-time ratings and general pleasure ratings provided after each song). Motivational responses were measured by asking participants how much of their own money they were willing to spend for each song, using a previously validated auction paradigm (12, 24). To control for other possible dopaminergic-dependent modulations, participants were requested to provide, for each excerpt, subjective ratings of emotional valence, arousal, and familiarity. Crucially, to control for the actual implication of reward processes, we also employed a nonmusic condition, the monetary incentive delay (MID) paradigm, a well-established and extensively used protocol able to activate the dopaminergic system, which was the focus of study here (19, 34, 35). We predicted that if dopamine, beyond its role in learning and motivation, plays a causal role in music-evoked pleasure, levodopa and risperidone administration should lead to opposite effects in measures of both musical pleasure and motivation: while the dopamine precursor levodopa should increase the hedonic experience and the music-related motivational responses, risperidone should lead to a reduction of both. In contrast, if dopamine only plays a role in motivation as previously described in primary rewards, the pharmacological intervention should leave pleasure reactions intact and just modulate music-related motivational responses.
Results
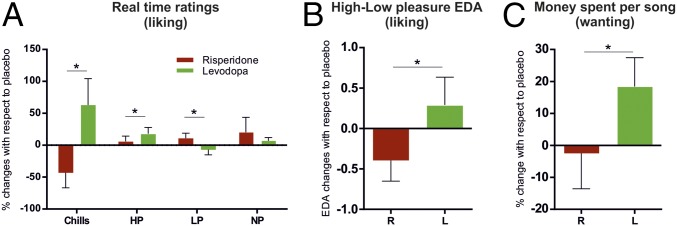
Listening to pleasurable music is often accompanied by measurable bodily reactions such as goose bumps or shivers down the spine, commonly called “chills” or “frissons.” Chills are generally used as indicators of musical pleasure, although not everybody experiences chills in response to music. In the current experiment, 16 (out of 27) participants reported chills during the placebo session with either their own favorite music or our music selection. We examined the time these specific participants reported chills across sessions to assess the effects of the pharmacological interventions on chill responses. To normalize these values across participants (24), we computed changes with respect to the placebo in the amount of time these participants reported chills during music listening (i.e., self-selected and experimenter-selected excerpts), following both levodopa and risperidone administration (but see SI Appendix, Figs. S1–S3 for complete representation of the three pharmacological sessions and SI Appendix, Fig. S4 for distribution of individual responses to drugs). A Wilcoxon signed-rank test indicated that chill responders spent more time reporting chills following levodopa than risperidone (Z = 2.341, P < 0.019). Next, we followed a similar procedure for high-, low-, and no-pleasure ratings (i.e., with the whole sample). The analysis revealed significant differences between pharmacological interventions in the time reporting high- (Z = 1.968, P < 0.049) and low-pleasure (Z = 2.273, P < 0.023) ratings. Under levodopa, participants were more likely to report high-pleasure ratings and less likely to report low-pleasure ratings than following risperidone administration. No differences between levodopa and risperidone were found in the time reporting no pleasure (Z = 0.698, P < 0.485). No significant differences were found when comparing real-time ratings between experimenter-selected (i.e., pop) and self-selected (i.e., favorite) music (all P > 0.173, Wilcoxon signed-rank tests, see also SI Appendix, Fig. S5). Pharmacological interventions (i.e., drugs) bidirectionally affected (i.e., higher pleasure under precursor, lower under antagonist) both types of excerpts in the same way. Importantly, this effect was not due to a general reduction of ratings under risperidone, as no significant differences in total time reporting real-time ratings were found when comparing the two drugs (Z = −0.952, P = 0.341, Wilcoxon signed-rank test). These drug effects did not differ between men and women (all P > 0.706, Kruskal–Wallis H tests).
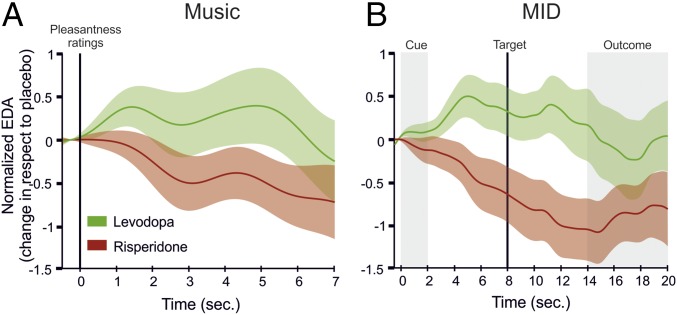
We then evaluated changes across sessions in EDA associated with real-time ratings of pleasure provided by the participants. Previous studies have shown that listening to pleasurable music is generally accompanied by increases of EDA which are modulated by the intensity of the experienced pleasure. Thus, EDA associated with high pleasurable musical excerpts has been generally used as an objective measure of musical pleasure. Therefore, we computed physiological values associated with high-pleasure music listening periods by subtracting changes in EDA following low (real-time ratings 1 and 2) to high pleasantness ratings (i.e., refs. 3 and 4). Placebo-corrected EDA values associated with high-pleasure states during music listening showed an increase under levodopa, significantly different from the decrease observed under risperidone [effect of drug (levodopa/risperidone), t (25) = −2.261, P = 0.033, paired t test] (Figs. 1B and 2A). No EDA modulations were observed during a baseline rest period (before the music listening task). Importantly, these results indicate that pharmacological interventions did not induce changes in EDA itself nor generally in EDA response to music, but rather, affected the signal associated with high hedonic responses to music. In addition, we also analyzed the average EDA while listening to music (thus averaging the 45 s of music listening for all songs listened to) on each session. We did not find differences in EDA amplitude for the entire duration of the songs following levodopa or risperidone [t (25) = 0.873; P = 0.391, paired t test]. This result indicates that the dopaminergic manipulation did not lead to tonic changes in EDA while listening to music, but rather specifically modulated the amplitude of phasic EDA in response to highly pleasant sections of music. For the control monetary reward task (MID), and similarly to music listening, a drug-dependent modulation effect was observed for high incentive condition. EDA activity associated with high monetary gains resulted in increased and decreased conditions under levodopa and risperidone, respectively [t (26) = −2.868, P = 0.008, paired t test] (Fig. 2B), while no significant differences were observed during the neutral (i.e., no rewarding) condition [t (26) = −0.926, P = 0.363), paired t test].
Fig. 1.
Changes with respect to placebo condition under levodopa and risperidone (means ± SEM) for music-related reward responses. (A) Amount of time reporting chills, high pleasure (HP), low pleasure (LP), and no pleasure (NP) real-time ratings (i.e., while listening to each song, including self- and experimenter-selected songs; e.g., risperidone and levodopa, respectively, decreased and increased by 43% and 65% the time reporting chills with respect to the placebo condition). Chills values include chills responders only; HP, LP, and NP values include the entire sample. (B) EDA changes associated with high-pleasure real-time ratings. (C) Motivational ratings, i.e., willingness to spend money for each excerpt. *P < 0.05.
Fig. 2.
EDA changes (means, solid lines ± SEM, lighter colors) over time with respect to placebo condition under levodopa (green) and risperidone (red) during music listening associated with high-rewarding excerpts (i.e., high minus low real-time liking rates) (A) shows very similar responses to EDA changes associated with high-rewarding cues (i.e., high monetary gain minus low monetary gain) in the MID control task (B).
Next, we tested whether dopaminergic manipulation influenced participants’ aesthetic judgments provided after each song (from 1 = no pleasantness to 5 = intense pleasantness). Following the same rationale as with the previous analyses, we computed percent of change following risperidone or levodopa administration with respect to placebo. The analysis revealed a marginal difference between drugs: individuals tend to report higher liking rates following levodopa than risperidone (Z = −1.947, P = 0.052, Wilcoxon signed-rank test). No significant changes were observed between drugs for other subjective ratings provided after each song, i.e., familiarity, arousal, and emotional valence (all P > 0.105 Wilcoxon signed-rank tests).
Finally, and importantly for our hypothesis, the pharmacological intervention did modulate participants’ willingness to pay for the experimenter-selected music: individuals significantly bid more money under levodopa than under risperidone (Z = 2.435, P = 0.015, Wilcoxon signed-rank test). As was the case for pleasure responses, motivational reward responses (monetary bids) increased under dopaminergic stimulation and decreased under dopaminergic inhibition relative to baseline (Fig. 1C). In line with results in real-time ratings, the general drug effect on motivational responses did not differ between men and women [χ2 (1) = 0.462, P = 0.497, Kruskal–Wallis H test].
Discussion
Overall, our results straightforwardly revealed that pharmacological interventions bidirectionally modulated the reward responses elicited by music. In particular, we found that risperidone impaired participants’ ability to experience musical pleasure, whereas levodopa enhanced it. Accordingly, emotional arousal corresponding to high-pleasure real-time ratings, as indexed by changes in EDA, was higher under the dopamine precursor and lower under the dopamine antagonist compared with placebo. These findings parallel those observed in the control MID task, where EDA increased under levodopa and decreased under risperidone in response to high-rewarding, but not to neutral nonrewarding monetary cues. Finally, participants were willing to spend more money under levodopa than under risperidone, indicating that they were more motivated to listen to the music again when promoting the dopaminergic transmission than when blocking it.
Previous studies have consistently shown the implication of the mesolimbic reward system for both hedonic and motivational responses using PET (5, 6, 11, 35), fMRI (7–17), and transcranial magnetic stimulation (TMS) (24). However, except for ref. 11, none of these studies were specific to dopamine transmission, and all were correlational except for ref. 24, which however attempted to indirectly modulate dopamine by means of TMS. Here, we provide causal evidence for the implication of the dopaminergic system in musical reward by directly altering dopaminergic function. Several findings support that our pharmacological modulations specifically affected reward responses, rather than a more general modulation of participants’ arousal or well-being. First, results from the control MID task, previously related to specific brain activity and dopamine release in the NAcc (34, 36), confirm a pharmacological-dependent modulation of the reward system. If the intervention was affecting dopamine-dependent reward responses, changes should be present in a dopamine-dependent task such as the MID. Administration of dopamine precursor was indeed associated with an increase in EDA for high compared with low magnitude of monetary reward-predicting cues, and this response decreased under risperidone. In addition, neutral trials, in which participants did not lose or win money, were used as a negative control to ascertain whether pharmacological modulation was confined to reward-related processes, and thus rule out unspecific drug effects on baseline dopaminergic tone. Crucially, no differences across conditions (i.e., drugs) were found for the neutral condition, indicating an interaction between reward processing specifically and dopaminergic modulation. Second, EDA values were similarly modulated in response to high- compared with low-rewarding musical excerpts, but no differences across sessions were found in the baseline condition. Finally, at the behavioral level, only the reward-related subjective ratings were modulated by pharmacological interventions, while no significant changes in emotional valence, arousal, and familiarity ratings were found across sessions. This is in line with recent findings on word learning [same interventions, same individuals (37); participants completed the word learning task after our music paradigm] showing that the behavioral ratings associated with reward during learning, but not the ones related to a neutral condition or to arousal, were affected by the pharmacological interventions in the same manner as the ones reported here. Altogether, these points offer converging evidence that our findings pointedly reflect a drug-dependent modulation of reward experience and exclude an unspecific and confounding general drug effect affecting participants’ health or mood state.
In this vein, Berridge and Kringelbach (23) describe three interconnected but neurobiologically different psychological components of reward: “liking,” namely the hedonic impact, pleasurable component; “wanting,” namely the motivation for reward; and “learning,” namely the associations, representations, and predictions about future rewards based on past experiences. If on the one hand the role of dopamine in wanting and learning components is widely accepted, on the other hand, its function in regulating hedonic responses appears controversial. For example, near complete destruction of nigrostriatal and mesolimbic dopaminergic neurons led to no changes in pleasure although it was able to completely abolish rats’ interest in food (38). Nevertheless, the studies investigating reward processing usually deal with primary rewards, such as food, and animal models (e.g., refs. 39–41). Here, in contrast, studying responses to abstract rewards in human subjects, we show that manipulation of dopaminergic transmission affects both the pleasure (i.e., amount of time reporting chills and emotional arousal measured by EDA) and the motivational components of musical reward (money willing to spend). These findings suggest that dopaminergic signaling is a sine qua non condition not only for motivational responses, as has been shown with primary and secondary rewards, but also for hedonic reactions to music. This result supports recent findings showing that dopamine also mediates the perceived pleasantness attained by other types of abstract rewards (37) and challenges previous findings in animal models on primary rewards, such as food (42, 43).
Musical pleasure, in contrast to what occurs with primary rewards, may depend on modulations of emotional arousal and intensity (6) triggered by expectations and surprises driven by the presence of structural and temporal regularities in musical patterns (12, 44, 45), associative conditioning (29, 46), or episodic memory (47) among others (48–50). Importantly, most of these elements rely on cognitive computations driven by dopaminergic transmission: from dopamine’s role in learning to its role in memory or attention (see ref. 31). In this regard, levodopa has been shown to enhance cognitive function especially in memory and learning (51–53), but also in other domains, such as semantic activation and priming (54–56), and even feedback-based grammar learning (57). On the contrary, the blockade of D2 receptors may lead to cognitive impairments (58), especially in executive functions and memory (59, 60). Importantly, drug intake only influenced reward-related ratings such as real-time ratings of pleasure, physiological response to pleasure, and participants’ willingness to buy our music selection, but it did not distort other functions important for music perception. Drug intake did not alter their ability to recognize familiar melodies—indicating that the drugs did not interfere with the recall and recognition of previous music experiences—and it did not modulate perceived emotional valence or general arousal, which may be related to concrete acoustic attributes such as pitch, mode, tempo, or loudness (49, 50). Thus, one potential interpretation of our results, together with previous evidence in a task involving implicit reward through successful learning (37), is that dopamine-dependent cognitive processes such as learning and memory—modulated up and down by levodopa and risperidone, respectively—may substantially influence the pleasure generated by abstract activities.
This view does not necessarily imply that dopaminergic transmission generates the hedonic experience per se. It could be generated by other neurotransmitter circuitries that interact with the dopamine system. Previous animal studies with primary rewards have shown the existence of so-called “hedonic hotspots” in the brain that are responsible for the generation of pleasure (61). These hedonic hotspots, found along the reward circuitry in the NAcc, insula, orbitofrontal cortex, and ventral pallidum, are modulated by opioid transmission (62). Both opioid and dopamine systems are colocalized and they interact in complex ways. While opioid modulation of dopamine neurons has been extensively studied due to its relevance in addiction (63–66), a few studies have explored dopamine modulation of endogenous opioid peptide releases (67–69). Interestingly, dopamine-stimulating drugs, acting via D2 dopamine receptors, can cause the release of endogenous opioid peptides within the NAcc (69). Thus, while dopaminergic function may be important and an indispensable step for musical pleasure, the ultimate system responsible might be the opioid circuit, as occurs in primary rewards. However, the exact mechanisms and consequences of this interaction are still unknown and it is unclear to what extent dopaminergic modulation of opioid release may affect hedonic hotspots in particular [representing around 10% of the NAcc neurons (62)]. In this regard, Mallik et al. (70), have recently shown that pharmacological reduction of opioid transmission via naltrexone led to a general reduction of pleasurable responses to both pleasant and nonpleasant music. However, the fact that the manipulation also affected pleasure responses in nonpleasant music—in which no opioid release would have been consequently expected—does not allow the ruling out of unspecific drug effects. Further pharmacological interventions and PET studies targeting opioid and dopamine transmission are warranted to better understand the complex interplay between these two neurotransmitter systems in musical pleasure.
Conversely, an alternative explanation of our results is that the feelings of pleasure evoked by abstract rewards, and music in particular, may differ from those evoked by primary rewards and thus driven by different psychological and neurobiological substrates (refs. 25–27, but see ref. 28). Indeed, the existence of diverse types of pleasurable experiences in humans remains currently an open debate (25–27). As previously mentioned, music-evoked pleasure is driven, among other things, by its intrinsic ability to induce feelings such as anticipation and euphoria. Remarkably, previous studies have shown that dopamine-stimulating drugs such as cocaine or amphetamine elicit similar positive affective states in humans (71). Direct electrical brain stimulation of the NAcc has been shown to elicit smiles and euphoria (72). Similarly, microinjections of psychostimulants into the NAcc elicit 50-kHz ultrasonic vocalizations in rats, proposed to reflect a positive appetitive affective state (73). Although these behaviors have been related to the motivational aspects of reward, one may wonder if these feelings may be interpreted as pleasurable. In this regard, dopamine releases driven by music might increase the attractiveness of the surrounding environment and the motivation to pursue and desire similar experiences leading to positive feelings that are attributed to “pleasurable” (12, 21). Thus, subjective experience of musical pleasure, similar to emotions (74), may arise from motivational signals and cognitive appraisal rather than through the engagement of the hedonic hotspots previously identified with primary rewards.
In conclusion, the present findings show a causal role of dopamine in musical pleasure and shed light on the role of the human dopaminergic system in abstract rewards. More broadly, these results indicate that dopaminergic transmission might play different or additive roles than the ones postulated in affective processing so far, particularly in abstract cognitive activities.
Material and Methods
Participants.
The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization’s Good Clinical Practice Guidelines. All volunteers gave their written informed consent for participation before any procedure. Twenty-nine volunteers (see ref. 37 and SI Appendix for prescreening and selection criteria) were randomized and completed the study (19 females, mean age = 22.83 ± 4.39) in exchange for monetary compensation according to Spanish legislation. The original sample size was chosen to be 30 participants, but 1 participant dropped out early in the study and only 29 finished it. Selected participants were also tested with the extended version of the Barcelona music reward questionnaire (BMRQ) (ref. 19, see also ref. 75). No participants presented signs of amusia. Two participants scored within the ranges considered to indicate musical anhedonia (BMRQ = 61 and 64) and general anhedonia (physical anhedonia scale = 19 and 24), and were therefore excluded from the analysis here reported (total n = 27, 18 females, mean age = 23 ± 4.48, mean BMRQ = 77.07 ± 9.89).
Procedure.
This double-blind, crossover, treatment sequence-randomized study (SI Appendix, Table S3) was performed at the Neuropsychopharmacology Unit and Center for Drug Research of the Santa Creu i Sant Pau Hospital of Barcelona. Experimental testing took place over three sessions. The study was approved by the ethics committee of the Hospital de la Santa Creu i Sant Pau and the Spanish Medicines and Medical Devices Agency (EudraCT 2016–000801-35). For each session, participants arrived at the hospital under fasting conditions and were given a light breakfast. Subsequently, they received in a double-blind masked fashion a capsule containing the treatment (see SI Appendix for more detailed information about drug doses): a dopaminergic precursor with an inhibitor of peripheral dopamine metabolism (levodopa, 100 mg + carbidopa, 25 mg), a dopamine receptor antagonist (risperidone, 2 mg), or a placebo (lactose). One hour after drug administration, the experimental session started. After 3 min of baseline (silence, relaxed, open eyes), the music reward task took place. The session then continued with other tasks (musical memory and language learning, not reported in this article) and ended with the control task, the MID. The total duration of the music reward task was about 15 min. The total duration of the MID task was about 20 min. The total duration of the experimental session was about 2.5 h. Next, participants spent their time in a resting room and were allowed to leave the hospital after 6 h from the treatment administration. At least 1 wk passed between one session and the next.
Music Task.
Participants were requested to listen to the 10 pop songs and to their five favorite musical excerpts following a similar procedure to that in ref. 24. The order of presentation of musical excerpts within each group of songs was randomized across participants. Before starting with each session, a trial pop song (“Sin saber por qué” by Vanessa Martín; neither the song nor the performer was part of the music selected for the experiment) was played to make sure that each participant understood the task and the subjective ratings to provide. Participants were seated in a comfortable chair in front of a screen. Each excerpt (see SI Appendix for details about musical stimuli) was presented via headphones while a musical note (white symbol on black background) appeared on the screen. During each excerpt, participants were asked to indicate in real time the degree of pleasure they were experiencing while listening to the music. Participants were instructed to press one of four available buttons on a keyboard (1 = no pleasure, 2 = low pleasure, 3 = high pleasure, 4 = chills) depending on the pleasure they were experiencing. They were instructed to change the rating by pressing another button as soon as they felt that their experienced pleasure was changing. The same procedure has been extensively used in previous studies on musical reward processing (11, 17, 19, 24, 33). Similarly, after each excerpt, participants provided additional ratings on that song by answering questions appearing on the screen. In particular, they were asked to rate the general pleasantness (from 1 = no general pleasantness to 5 = intense general pleasantness), arousal (from 1 = very relaxing to 5 = very arousing), and emotional valence (from 1 = very sad to 5 = very happy) they felt in response to each excerpt. For pop music, participants were additionally asked to rate the familiarity of each song (from 1 = completely unfamiliar to 5 = I have the song in my PC, mp3, Spotify playlist, etc). Reward ratings (i.e., real-time and pleasantness ratings) may be considered measures of “liking” processes. To have a “wanting” motivational rating, we introduced an auction paradigm in which participants could purchase the pop music with their own money, as an indication that they wanted to hear it again (adapted from refs. 12 and 24). For each experimenter-selected song, participants could indicate whether they were willing to pay €0, €0.29, €0.99, or €1.29 (see refs. 12 and 24 and SI Appendix for more detailed information about the auction paradigm).
MID Task.
The MID task consisted of 35 trials. At the beginning of a trial, participants saw one of five cue shapes (2 s), signaling whether participants were playing to win potential rewards (14 trials; denoted by circles) or to avoid losing potential losses (14 trials; denoted by squares). The magnitude of the possible outcomes was indicated using horizontal lines in the cue, and could be large (1€, three horizontal lines, seven trials for each valence) or small (0.1€, one horizontal line, seven trials for each valence). Six seconds after cue offset, participants had to respond, as fast as possible, with a button press to a white target square that appeared for a variable length of time (target, 160–260 ms). In win trials, if participants responded on time they obtained the corresponding amount of money. In contrast, in loss trials, if participants responded on time they avoided losing the corresponding amount of money. Six seconds after participants’ response, a visual feedback on the screen notified whether they had won or lost money during that trial. Eight seconds later, another cue was presented. Additionally, a neutral condition (seven trials; denoted by a triangle) in which participants were not playing for money was also included. Task difficulty, based on reaction times collected during the practice session, was set such that each participant could succeed on 66% of his/her target responses. Trial types were randomly ordered within each session.
Analysis of Behavioral Ratings and EDA.
The percentage of change under risperidone and levodopa with respect to placebo was computed for each measure. Placebo-corrected values of subjective ratings (i.e., real time and ratings provided after each song) between pharmacological sessions, and gender differences in general drug effects (SI Appendix, Figs. S4 and S5) were compared using nonparametric tests (i.e., Wilcoxon signed-rank and Kruskal–Wallis H tests, as fully described in SI Appendix). EDA was recorded following the procedures described in ref. 17 and analyzed by computing the proportion of amplitude changes corresponding to high- and low-pleasure responses, for both music and MID tasks. Placebo-corrected normalized values were compared between pharmacological sessions using paired-sample t tests (all detailed information reported in SI Appendix).
Supplementary Material
Acknowledgments
We thank the staff of the Centre d’Investigació del Medicament de l’Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau (HSCSP) for their help. The present project has been funded by the Spanish Government (MINECO Grant PSI2011-29219 to A.R.-F. and AP2010-4179 to P.R.). L.F. was partially supported by a Morelli-Rotary postdoctoral fellowship. M.V. was partially supported by Fondo de Investigación en Salud through Grant CP04/00 121 from the Spanish Health Ministry in collaboration with l’Institut de Recerca de HSCSP; she is a member of Centro de Investigación Biomédica en Red Salud Mental (funded by the Spanish Health Ministry, Instituto de Salud Carlos III). H.A. was supported by a grant from the Spanish Government (BES-2013-067440).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 3364.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1811878116/-/DCSupplemental.
References
- 1.Dubé L, Le Bel J. The content and structure of laypeople’s concept of pleasure. Cogn Emotion. 2003;17:263–295. doi: 10.1080/02699930302295. [DOI] [PubMed] [Google Scholar]
- 2.Bigand E, Poulin-Charronnat B. Are we “experienced listeners”? A review of the musical capacities that do not depend on formal musical training. Cognition. 2006;100:100–130. doi: 10.1016/j.cognition.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Patel AD. Science & music: Talk of the tone. Nature. 2008;453:726–727. doi: 10.1038/453726a. [DOI] [PubMed] [Google Scholar]
- 4.Lacey S, et al. Art for reward’s sake: Visual art recruits the ventral striatum. Neuroimage. 2011;55:420–433. doi: 10.1016/j.neuroimage.2010.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 1999;2:382–387. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- 6.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koelsch S. Investigating emotion with music: Neuroscientific approaches. Ann N Y Acad Sci. 2005;1060:412–418. doi: 10.1196/annals.1360.034. [DOI] [PubMed] [Google Scholar]
- 8.Berns GS, Capra CM, Moore S, Noussair C. Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage. 2010;49:2687–2696. doi: 10.1016/j.neuroimage.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montag C, Reuter M, Axmacher N. How one’s favorite song activates the reward circuitry of the brain: Personality matters! Behav Brain Res. 2011;225:511–514. doi: 10.1016/j.bbr.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Pereira CS, et al. Music and emotions in the brain: Familiarity matters. PLoS One. 2011;6:e27241. doi: 10.1371/journal.pone.0027241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14:257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- 12.Salimpoor VN, et al. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340:216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- 13.Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15:170–180. doi: 10.1038/nrn3666. [DOI] [PubMed] [Google Scholar]
- 14.Frühholz S, Trost W, Grandjean D. The role of the medial temporal limbic system in processing emotions in voice and music. Prog Neurobiol. 2014;123:1–17. doi: 10.1016/j.pneurobio.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Mueller K, et al. Investigating the dynamics of the brain response to music: A central role of the ventral striatum/nucleus accumbens. Neuroimage. 2015;116:68–79. doi: 10.1016/j.neuroimage.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Brattico E, et al. It’s sad but I like it: The neural dissociation between musical emotions and liking in experts and laypersons. Front Hum Neurosci. 2016;9:676. doi: 10.3389/fnhum.2015.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Molina N, Mas-Herrero E, Rodríguez-Fornells A, Zatorre RJ, Marco-Pallarés J. Neural correlates of specific musical anhedonia. Proc Natl Acad Sci USA. 2016;113:E7337–E7345. doi: 10.1073/pnas.1611211113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mas-Herrero E, Marco-Pallares J, Lorenzo-Seva U, Zatorre RJ, Rodriguez-Fornells A. Individual differences in music reward experiences. Music Percept. 2013;31:118–138. [Google Scholar]
- 19.Mas-Herrero E, Zatorre RJ, Rodriguez-Fornells A, Marco-Pallarés J. Dissociation between musical and monetary reward responses in specific musical anhedonia. Curr Biol. 2014;24:699–704. doi: 10.1016/j.cub.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 20.Zald DH, Zatorre RJ. Music. In: Gottfried JA, editor. Neurobiology of Sensation and Reward. Chap 19 CRC; Boca Raton, FL: 2011. [Google Scholar]
- 21.Gebauer L, Kringelbach ML, Vuust P. Ever-changing cycles of musical pleasure: The role of dopamine and anticipation. Psychomusicology. 2012;22:152–167. [Google Scholar]
- 22.Zatorre RJ, Salimpoor VN. From perception to pleasure: Music and its neural substrates. Proc Natl Acad Sci USA. 2013;110:10430–10437. doi: 10.1073/pnas.1301228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mas-Herrero E, Dagher A, Zatorre RJ. Modulating musical reward sensitivity up and down with transcranial magnetic stimulation. Nat Hum Behav. 2018;2:27–32. doi: 10.1038/s41562-017-0241-z. [DOI] [PubMed] [Google Scholar]
- 25.Berlyne DE, Madsen KB. In: Pleasure, Reward, Preference: Their Nature, Determinants, and Role in Behaviour. Berlyne DE, Madsen KB, editors. Academic; New York: 1973. [Google Scholar]
- 26.Rozin P. Preadaptation and the puzzles and properties of pleasure. In: Kahneman D, Diener E, Schwarz N, editors. Well-being: The Foundations of Hedonic Psychology. Russell Sage Foundation; New York: 1999. pp. 109–133. [Google Scholar]
- 27.Frijda NH. The nature of pleasure. In: Bargh JA, Apsley DK, editors. Unraveling the Complexities of Social Life: A Festschrift in Honor of Robert B. Zajonc. APA; Washington, DC: 2001. pp. 71–94. [Google Scholar]
- 28.Skov M, Nadal M. Art is not special: An assault on the last lines of defense against the naturalization of the human mind. Rev Neurosci. 2018;29:699–702. doi: 10.1515/revneuro-2017-0085. [DOI] [PubMed] [Google Scholar]
- 29.Panksepp J, Bernatzky G. Emotional sounds and the brain: The neuro-affective foundations of musical appreciation. Behav Processes. 2002;60:133–155. doi: 10.1016/s0376-6357(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 30.Raghanti MA, et al. Human-specific increase of dopaminergic innervation in a striatal region associated with speech and language: A comparative analysis of the primate basal ganglia. J Comp Neurol. 2016;524:2117–2129. doi: 10.1002/cne.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sousa AMM, et al. Molecular and cellular reorganization of neural circuits in the human lineage. Science. 2017;358:1027–1032. doi: 10.1126/science.aan3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burstein ES, et al. Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: Identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J Pharmacol Exp Ther. 2005;315:1278–1287. doi: 10.1124/jpet.105.092155. [DOI] [PubMed] [Google Scholar]
- 33.Salimpoor VN, Benovoy M, Longo G, Cooperstock JR, Zatorre RJ. The rewarding aspects of music listening are related to degree of emotional arousal. PLoS One. 2009;4:e7487. doi: 10.1371/journal.pone.0007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schott BH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown S, Martinez MJ, Parsons LM. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport. 2004;15:2033–2037. doi: 10.1097/00001756-200409150-00008. [DOI] [PubMed] [Google Scholar]
- 36.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ripollés P, et al. Intrinsically regulated learning is modulated by synaptic dopamine signaling. eLife. 2018;7:e38113. doi: 10.7554/eLife.38113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 39.Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: Map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- 40.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 41.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: Enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge KC. Food reward: Brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 43.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer LB. Emotion and Meaning in Music. University of Chicago Press; Chicago: 1956. [Google Scholar]
- 45.Huron DB. Sweet Anticipation: Music and the Psychology of Expectation. MIT Press; Cambridge, MA: 2006. [Google Scholar]
- 46.Sachs ME, Damasio A, Habibi A. The pleasures of sad music: A systematic review. Front Hum Neurosci. 2015;9:404. doi: 10.3389/fnhum.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janata P. The neural architecture of music-evoked autobiographical memories. Cereb Cortex. 2009;19:2579–2594. doi: 10.1093/cercor/bhp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huron D. 2004. Music-engendered laughter: An analysis of humor devices in PDQ Bach. Proceedings of the 8th International Conference on Music Perception and Cognition (Causal Productions, Adelaide, Australia), 700–704.
- 49.Juslin PN, Västfjäll D. Emotional responses to music: The need to consider underlying mechanisms. Behav Brain Sci. 2008;31:559–575, discussion 575–621. doi: 10.1017/S0140525X08005293. [DOI] [PubMed] [Google Scholar]
- 50.Juslin PN. From everyday emotions to aesthetic emotions: Towards a unified theory of musical emotions. Phys Life Rev. 2013;10:235–266. doi: 10.1016/j.plrev.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Knecht S, et al. Levodopa: Faster and better word learning in normal humans. Ann Neurol. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- 52.Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Düzel E. Dopamine modulates episodic memory persistence in old age. J Neurosci. 2012;32:14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shellshear L, et al. Levodopa enhances explicit new-word learning in healthy adults: A preliminary study. Hum Psychopharmacol. 2015;30:341–349. doi: 10.1002/hup.2480. [DOI] [PubMed] [Google Scholar]
- 54.Angwin AJ, et al. Semantic activation in Parkinson’s disease patients on and off levodopa. Cortex. 2009;45:950–959. doi: 10.1016/j.cortex.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Angwin AJ, et al. Dopamine and semantic activation: An investigation of masked direct and indirect priming. J Int Neuropsychol Soc. 2004;10:15–25. doi: 10.1017/S1355617704101033. [DOI] [PubMed] [Google Scholar]
- 56.Copland DA, McMahon KL, Silburn PA, de Zubicaray GI. Dopaminergic neuromodulation of semantic processing: A 4-T FMRI study with levodopa. Cereb Cortex. 2009;19:2651–2658. doi: 10.1093/cercor/bhp017. [DOI] [PubMed] [Google Scholar]
- 57.de Vries MH, Ulte C, Zwitserlood P, Szymanski B, Knecht S. Increasing dopamine levels in the brain improves feedback-based procedural learning in healthy participants: An artificial-grammar-learning experiment. Neuropsychologia. 2010;48:3193–3197. doi: 10.1016/j.neuropsychologia.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Sakurai H, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: Analysis of the CATIE data. Schizophr Bull. 2013;39:564–574. doi: 10.1093/schbul/sbr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hori H, et al. Antipsychotic medication and cognitive function in schizophrenia. Schizophr Res. 2006;86:138–146. doi: 10.1016/j.schres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Uchida H, et al. D2 receptor blockade by risperidone correlates with attention deficits in late-life schizophrenia. J Clin Psychopharmacol. 2009;29:571–575. doi: 10.1097/JCP.0b013e3181bf4ea3. [DOI] [PubMed] [Google Scholar]
- 61.Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 62.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hjelmstad GO, Xia Y, Margolis EB, Fields HL. Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons. J Neurosci. 2013;33:6454–6459. doi: 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Margolis EB, Hjelmstad GO, Fujita W, Fields HL. Direct bidirectional μ-opioid control of midbrain dopamine neurons. J Neurosci. 2014;34:14707–14716. doi: 10.1523/JNEUROSCI.2144-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roth-Deri I, et al. Effect of experimenter-delivered and self-administered cocaine on extracellular β-endorphin levels in the nucleus accumbens. J Neurochem. 2003;84:930–938. doi: 10.1046/j.1471-4159.2003.01584.x. [DOI] [PubMed] [Google Scholar]
- 68.Soderman AR, Unterwald EM. Cocaine-induced mu opioid receptor occupancy within the striatum is mediated by dopamine D2 receptors. Brain Res. 2009;1296:63–71. doi: 10.1016/j.brainres.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colasanti A, et al. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol Psychiatry. 2012;72:371–377. doi: 10.1016/j.biopsych.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 70.Mallik A, Chanda ML, Levitin DJ. Anhedonia to music and mu-opioids: Evidence from the administration of naltrexone. Sci Rep. 2017;7:41952. doi: 10.1038/srep41952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: Implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 72.Okun MS, et al. What’s in a “smile?” Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase. 2004;10:271–279. doi: 10.1080/13554790490507632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- 74.Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annu Rev Psychol. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreri L, Rodriguez-Fornells A. Music-related reward responses predict episodic memory performance. Exp Brain Res. 2017;235:3721–3731. doi: 10.1007/s00221-017-5095-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.