From early embryonic development through adulthood, cilia play crucial roles in unicellular to multicellular organisms. Current taxonomy classifies cilia as two types: motile (conventional) and nonmotile. Motile cilia possess the classic 9 + 2 organization of microtubules, typical of multiciliated epithelia and spermatozoon flagellum, whereas nonmotile cilia characteristically lack the two central microtubules and are common in the primary cilium or monocilium (1). This simplified classification, however, does not accommodate all cilia, leading to calls for reexamining this classification scheme (2). In the embryonic node, some monocilia are motile and others are immotile. Motile nodal cilia have a sloping rotational movement that is essential for establishing left–right asymmetry (1). Patients with Kartagener’s syndrome show reverse orientation of organ development, immotile sperm, and ciliary defects in respiratory epithelium (3), suggesting that both types of cilia are affected. During development, cilia of choroid plexus epithelial cells begin as immotile primary cilia, and then transition through multiple primary cilia before undergoing motile multiciliogenesis after birth (2, 4), which is essential for cerebral spinal fluid (CSF) flow. Although motile nodal monocilia are important for fluid movement during the short period of embryonic development, most cilia responsible for fluid movement are found in differentiated epithelium as motile cilia with the classic 9 + 2 microtubule arrangement (5).
The role of motile cilia extending into the extracellular space is generally assumed to be propulsion of fluid or particles suspended in the fluid through the luminal space of a given organ. In this scenario, cilia merely act as hockey sticks pushing luminal contents from one biological arena to the next. This kind of cilia is typically found in the central nervous system, the trachea and lungs, and the female and male reproductive tracts. Cilia-lined ependymal cells in the brain and spinal cord generate complex flow networks facilitating transport of CSF through the central nervous system (6, 7). In the respiratory tract, motile cilia are essential for propelling mucus with entrapped pathogens and debris out of the lungs (8). Within the oviduct (Fallopian tube), cilia accelerate the rendezvous between oocytes and sperm in the ampullary region. These same cilia then assume the role of transferring preimplantational embryos from the oviduct to the uterus where the conceptus will develop (9). One little-appreciated feature of the male reproductive system is that, when spermatozoa leave the testis, they are incapable of fertilizing an oocyte. Spermatozoa must first complete their journey through the efferent ductules followed by the epididymis (Fig. 1A). Spermatozoa leave the testis through the rete testis channels, bathed in an abundance of seminiferous tubular fluid. They will then enter the efferent ductules, which are lined with motile ciliated and nonciliated cells. It has long been thought that the primary function of such motile cilia is to propel spermatozoa to the epididymis, where fluid reabsorption continues and final processes of spermatozoa maturation occurs (10). It seemed logical to assume that these motile cilia beat in a planar manner and propel spermatozoa and other luminal material down the tract. However, in the male reproductive system, the mechanics of ciliary movement is puzzling. If the sole purpose of cilia in the efferent ductules is to facilitate forward motion of spermatozoa as they exit from the testes to the epididymis, it is unclear why evolution has retained such structures, which represent the sole ciliated region of the male reproductive tract. Why not bypass these structures altogether and allow spermatozoa to transit directly from the testis into the epididymis?
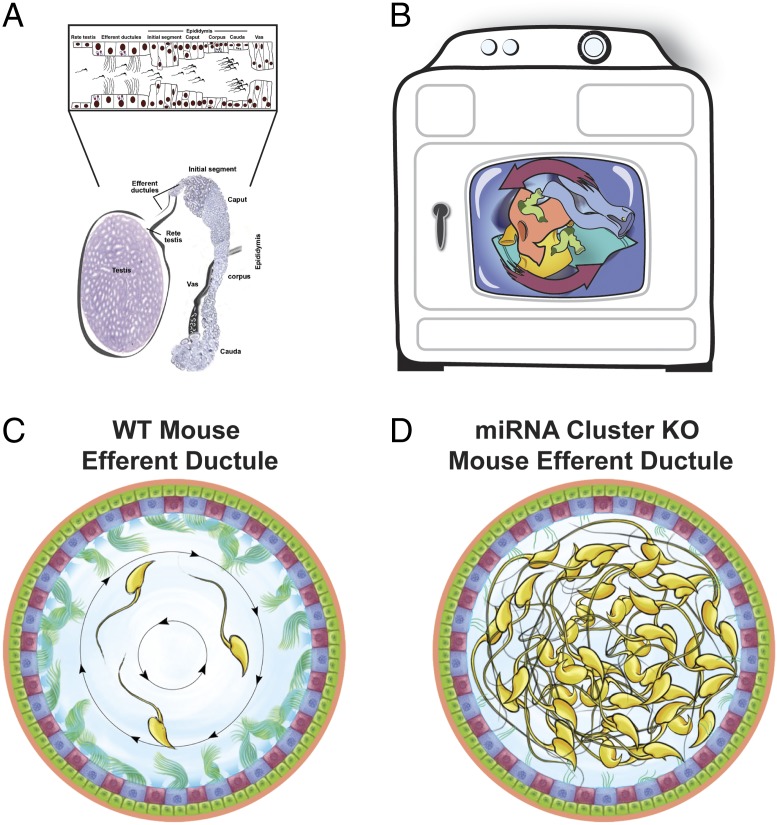
Fig. 1.
Male reproductive system and rotational movement by motile cilia in the efferent ductules. (A, Lower). Male reproductive system showing the testes, rete testis, efferent ductules, and epididymis. Inset (Upper) depicts the epithelial surface lining these structures. Motile cilia are solely present within the efferent ductules. Adapted from ref. 20 by permission of Oxford University Press. (B) Similar to washing machine blades, the motile cilia move in a coordinated and rotational manner to agitate spermatozoa and surrounding fluid. (C) In WT mice, motile cilia swing back and forth to maintain spermatozoa in suspension and prevent agglutination. This rotational ciliary movement might also facilitate reabsorption of luminal fluid. (D) Mice deficient in two miRNA clusters have ductule ciliary dysgenesis (11). Sperm aggregation, luminal obstruction, and sperm granulomas result and lead to a fluid back pressure within the testes and eventual testis degeneration and infertility.
In PNAS, Yuan et al. (11) challenge this conventional wisdom regarding the role of ciliary movement in the male reproductive system and, in the process, demonstrate the importance of a unique pattern of motile ciliary movement in relation to spermatozoa and surrounding fluid within the lumen. By using elegant physiological approaches, the team demonstrates that, rather than moving individually in a wave-like (metachronal) motion, these cilia exert a centripetal force on the spermatozoa collecting in the lumen, and that such ciliary beats can swing back and forth from clockwise to counterclockwise patterns of motion. Just like a washing machine, in which the blades continually stir and separate the clothes (Fig. 1B), this synchronized pattern of ciliary movement keeps spermatozoa in suspension and prevents them from clumping together. In slowing the transit of sperm through the efferent ductules, this rotational ciliary movement might also facilitate reabsorption of luminal fluid.
In addition to demonstrating the unexpected movement of these cilia, Yuan et al. (11) are able to gain some insight into the genetic underpinnings. Genetic ablation of two miRNA clusters (miR-34b/c and miR-449a/b/c) resulted in efferent ductule ciliary dysgenesis (11). As a result of sperm agglutination, luminal obstruction, and sperm granulomas, a fluid back pressure was created within the testes, resulting in testis degeneration and infertility. However, the gene targets of these miRNAs remain unidentified. Fig. 1 C and D compares the fluid hydrodynamics in normal wild-type (WT) mice, in which spermatozoa and surrounding fluid are continuously swirled in both a clockwise and counterclockwise manner, with those of miR-34b/c and miR-449a/b/c KO mice, in which the loss of the turbulent ciliary motion impedes luminal fluid circulation and reabsorption. However, relieving back pressure within the testes of these KO mice restored near-complete spermatogenesis and fertility.
The studies of Yuan et al. (11) uncover unique biophysical properties of cilia lining the efferent ductules of the testes and the importance of such a pattern of ciliary movement in normal male reproduction. The studies also open up an array of questions. What is the drummer these cilia “hear” that differentiates their pace from other motile cilia? Are the movements at least in part driven by extrinsic factors or are they dependent on structural features manifested in ciliated cells? In relation to the first option, serotonin affects ciliary beating in other species (12, 13). Selective serotonin reuptake inhibitor antidepressants bring about reduced sperm concentrations, decreased sperm motility, and greater number of abnormal spermatozoa in men and male mice (14, 15). Effects of serotonin on the motile cilia of efferent ductules remain uncertain, however.
Although various mathematical models have been proposed to explain ciliary movement (8, 16, 17), the phenomenon is most likely structurally based. Orientation of the basal body, formed from a centriole, anchors cilia to the cellular membrane and may influence ciliary movement. Absence of radial spokes (multiunit protein structures present in the axonemes of cilia and flagella) within mouse node cilia governs rotational leftward fluid flow movement, but randomly directed rotation and ultrastructural changes of node cilia result after paclitaxel (Taxol) treatment (18). Intriguingly, motile cilia in the respiratory system of mice deficient in radial spoke head protein, Rsph4a, convert from planar beating to rotational movement. When exposed to Taxol, motile cilia from these transgenic mice demonstrate microtubule rearrangement, which is absent in Taxol-treated motile cilia from WT mice (18). Such findings lend further support in reconsidering ciliary classification based solely on motility and whether more detailed criteria are needed (2). Changes in radial spoke proteins driving efferent ductule ciliary rotational movement is a provocative notion that merits exploration.
Another crucial question raised by the studies of Yuan et al. (11) is, What changes the trajectory of efferent ductule ciliary beat from clockwise to counterclockwise? A single layer of smooth muscle cells encases each efferent ductule. Perhaps contraction of the surrounding smooth muscle layer acts as the metronome driving the ciliary tempo and left–right shifts in direction and is controlled in some manner by serotonin. Ebb and flow of seminiferous tubular fluid within the ductules might also influence ciliary pulse and directionality.
Previous studies (6, 19) raise the question as to whether motile cilia in other systems deviate from planar movement. In human bronchial epithelium, cilia appear to beat with a circular orientation to cause healthy mucus swirling (19). Complexity, as judged by fluid movement, also appears to exist in ciliary beating along the lining of the brain ventricles (6). The churning motion of cilia in the efferent ductules described by Yuan et al. (11) is essential in preventing sperm agglutination and blockage within the efferent ductules and averting spillover damage to the testes as a whole (11). These results provide insight into the genetics and pathophysiology of male infertility arising as a result of disruptions in ciliary formation and/or rhythmic ciliary movements in a crucial region of the male reproductive tract.
Acknowledgments
I thank Dr. Rex A. Hess, Tingting Xie, and Donald L. Connor for the figure drawings. C.S.R. is supported by the NIH National Institute of Environmental Health Sciences Grant 1R01ES025547.
Footnotes
The author declares no conflict of interest.
See companion article on page 3584.
References
- 1.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Takeda S, Narita K. Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation. 2012;83:S4–S11. doi: 10.1016/j.diff.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 4.Narita K, Takeda S. Cilia in the choroid plexus: Their roles in hydrocephalus and beyond. Front Cell Neurosci. 2015;9:39. doi: 10.3389/fncel.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempeneers C, Chilvers MA. To beat, or not to beat, that is question! The spectrum of ciliopathies. Pediatr Pulmonol. 2018;53:1122–1129. doi: 10.1002/ppul.24078. [DOI] [PubMed] [Google Scholar]
- 6.Faubel R, Westendorf C, Bodenschatz E, Eichele G. Cilia-based flow network in the brain ventricles. Science. 2016;353:176–178. doi: 10.1126/science.aae0450. [DOI] [PubMed] [Google Scholar]
- 7.O’Callaghan C, Sikand K, Chilvers MA. Analysis of ependymal ciliary beat pattern and beat frequency using high speed imaging: Comparison with the photomultiplier and photodiode methods. Cilia. 2012;1:8. doi: 10.1186/2046-2530-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niedermayer T, Eckhardt B, Lenz P. Synchronization, phase locking, and metachronal wave formation in ciliary chains. Chaos. 2008;18:037128. doi: 10.1063/1.2956984. [DOI] [PubMed] [Google Scholar]
- 9.Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- 10.Hess RA. Small tubules, surprising discoveries: From efferent ductules in the turkey to the discovery that estrogen receptor alpha is essential for fertility in the male. Anim Reprod. 2015;12:7–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan S, et al. Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence. Proc Natl Acad Sci USA. 2019;116:3584–3593. doi: 10.1073/pnas.1817018116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walentek P, et al. A novel serotonin-secreting cell type regulates ciliary motility in the mucociliary epidermis of Xenopus tadpoles. Development. 2014;141:1526–1533. doi: 10.1242/dev.102343. [DOI] [PubMed] [Google Scholar]
- 13.Doran SA, et al. Effect of serotonin on ciliary beating and intracellular calcium concentration in identified populations of embryonic ciliary cells. J Exp Biol. 2004;207:1415–1429. doi: 10.1242/jeb.00924. [DOI] [PubMed] [Google Scholar]
- 14.Anonymous Semen abnormalities with SSRI antidepressants. Prescrire Int. 2015;24:16–17. [PubMed] [Google Scholar]
- 15.Alzahrani HA. Sister chromatid exchanges and sperm abnormalities produced by antidepressant drug fluoxetine in mouse treated in vivo. Eur Rev Med Pharmacol Sci. 2012;16:2154–2161. [PubMed] [Google Scholar]
- 16.Lukens S, Yang X, Fauci L. Using Lagrangian coherent structures to analyze fluid mixing by cilia. Chaos. 2010;20:017511. doi: 10.1063/1.3271340. [DOI] [PubMed] [Google Scholar]
- 17.Chen D, Zhong Y. A computational model of dynein activation patterns that can explain nodal cilia rotation. Biophys J. 2015;109:35–48. doi: 10.1016/j.bpj.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinohara K, et al. Absence of radial spokes in mouse node cilia is required for rotational movement but confers ultrastructural instability as a trade-off. Dev Cell. 2015;35:236–246. doi: 10.1016/j.devcel.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Khelloufi MK, et al. Spatiotemporal organization of cilia drives multiscale mucus swirls in model human bronchial epithelium. Sci Rep. 2018;8:2447. doi: 10.1038/s41598-018-20882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod. 2011;84:207–217. doi: 10.1095/biolreprod.110.087353. [DOI] [PMC free article] [PubMed] [Google Scholar]