Significance
Particulate matter exposure causes infant respiratory morbidity and mortality, but the role of ultrafine particles (UFPs) with an aerodynamic diameter of less than 0.1 μm in asthma and respiratory tract infections is unclear. Limited mechanistic information is available concerning UFP influence on the etiology of childhood asthma or susceptibility to respiratory infections. Here we exposed two strains of mice (sensitive to oxidative stress or allergen exposure) to UFPs throughout gestation at concentrations relevant to human exposures. Our results reveal a window of pulmonary immunosuppression in offspring following in utero UFP exposure. A dampened host immune response during early development underlies increased childhood susceptibility to respiratory infections, highlighting the necessity to develop strategies to protect the fetus during this vulnerable period.
Keywords: air pollution, ultrafine particulate matter, in utero exposure, prenatal, pulmonary immunosuppression
Abstract
Early life exposure to fine particulate matter (PM) in air is associated with infant respiratory disease and childhood asthma, but limited epidemiological data exist concerning the impacts of ultrafine particles (UFPs) on the etiology of childhood respiratory disease. Specifically, the role of UFPs in amplifying Th2- and/or Th17-driven inflammation (asthma promotion) or suppressing effector T cells (increased susceptibility to respiratory infection) remains unclear. Using a mouse model of in utero UFP exposure, we determined early immunological responses to house dust mite (HDM) allergen in offspring challenged from 0 to 4 wk of age. Two mice strains were exposed throughout gestation: C57BL/6 (sensitive to oxidative stress) and BALB/C (sensitive to allergen exposure). Offspring exposed to UFPs in utero exhibited reduced inflammatory response to HDM. Compared with filtered air (FA)-exposed/HDM-challenged mice, UFP-exposed offspring had lower white blood cell counts in bronchoalveolar lavage fluid and less pronounced peribronchiolar inflammation in both strains, albeit more apparent in C57BL/6 mice. In the C57BL/6 strain, offspring exposed in utero to FA and challenged with HDM exhibited a robust response in inflammatory cytokines IL-13 and Il-17. In contrast, this response was lost in offspring exposed in utero to UFPs. Circulating IL-10 was significantly up-regulated in C57BL/6 offspring exposed to UFPs, suggesting increased regulatory T cell expression and suppressed Th2/Th17 response. Our results reveal that in utero UFP exposure at a level close to the WHO recommended PM guideline suppresses an early immune response to HDM allergen, likely predisposing neonates to respiratory infection and altering long-term pulmonary health.
Early life exposure to fine particulate matter (PM) in air is associated with acute and chronic respiratory morbidities in infants and children (1–3). Fine PM is typically defined as particles with a diameter of smaller than 2.5 μm (or PM2.5), which are directly emitted into the atmosphere (referred to as primary particles) or formed in the atmosphere via the gas-to-particle conversion process (referred to as secondary particles) (4–6). Examples of the fine PM sources include vehicular and industrial emissions as well as combustion of coal and wood for primary particles and new particle formation from biogenic and anthropogenic emissions (7–9). Epidemiologic studies indicate that increased susceptibility to lower respiratory infections correlates with in utero exposure to PM2.5 (3, 10, 11). Furthermore, there is increasing evidence showing that developmental exposure to PM2.5 increases wheeze and asthma risk in childhood (12). Asthma is a heterogeneous immunologically mediated disease with a number of distinct clinical phenotypes. The most common form is allergic asthma, which results from an exacerbated immune response to inhaled allergens in individuals who are termed “atopic asthmatics.” Eosinophilic inflammation, antigen-specific IgE reactivity, airway hyperresponsiveness, and the predominance of T helper (Th) 2 cell-associated cytokine production classically define the phenotypic response. This framework has been extended to include an imbalanced Th17 and regulatory T cell (Treg) response (13). Knowledge on the mechanisms of allergic inflammation has largely been derived from murine models of asthma, which can replicate key features of the human disease (14). Sensitization followed by inhalation challenge with the egg protein ovalbumin (OVA) results in airway hyperreactivity and pulmonary inflammation driven by increased Th2-cytokine production. Th17 cells can exacerbate Th2-cell–mediated eosinophilic inflammation as well as promote airway neutrophilia. The house dust mite (HDM) asthma model primes a strong Th17 response resulting in enhanced pulmonary inflammation and airway hyperresponsiveness (15).
Limited epidemiological studies have evaluated the specific role of early life exposure to ultrafine particles (UFPs) with an aerodynamic diameter of less than 0.1 μm in asthma development, although exposure has been associated with asthma exacerbation in children (16). UFPs represent an important component of ambient PM and traffic-related air pollution (17). However, the current National Ambient Air Quality Standard does not specifically classify UFPs as a criteria pollutant (18). A few mouse models have investigated the offspring allergic inflammatory response to inhaled allergens following in utero exposure to diesel exhaust particulate matter (DEPM), a primary component of traffic-related pollutants. Findings from the previous studies vary considerably, demonstrating either increased airway inflammation and airway hyperreactivity indicative of an asthmatic phenotype (19–21), no effect (22), or even protection from airway inflammation in response to allergen challenge (23). The source and size of particles as well as timing of both PM and allergen exposure may be critical due to the complexity of fetal and infant lung and immune system development during this period. In neonatal mice, which represent an immunologically immature population, coexposure to DEPM (with a mean diameter between 0.1 and 0.3 μm) and HDM from 0 to 3 wk of age markedly enhanced airway inflammation through a mixed Th2 and Th17 response compared with HDM treatment alone (24). Conversely, a model where neonatal mice were exposed to combustion-derived PM (CDPM) with a mean diameter of 0.2 μm before (0–1 wk) and throughout HDM dosing from 1 to 5 wk of age showed a dampened adaptive immune response immediately after exposure in mice coexposed to CDPM and HDM, yet a predisposition to develop asthma upon rechallenge later in life (25). Collectively, these disparate outcomes may be attributable to differences in maternal/neonatal exposure conditions, type and timing of offspring PM or allergen exposure regimens, as well as variance in genetic background of the mouse model. Strain differences in allergic airway inflammation are well established, and reportedly BALB/c mice are more sensitive to allergens than C57BL/6 mice (26). Airway inflammatory response to DEPM also varies significantly among mouse strains. Findings from adult exposure models of prolonged low-dose DEPM exposure with 90% of particles less than 2.5 μm and ∼60% of 0.33-μm particles indicate that genetic differences in host defense response to oxidative stress may underlie variance, suggesting that C57BL/6 mice are more sensitive to DEPM than BALB/c mice (27, 28).
In this study, we investigated the effects of in utero UFP exposure on offspring pulmonary immune response in a mouse model representing a period of immune maturation (Materials and Methods and SI Appendix). We also evaluated the impact of genetic background (i.e., strain difference) on offspring airway inflammatory response. An in utero exposure model was developed to challenge neonates by a subchronic HDM challenge over the juvenile period (0–4 wk). Our exposure system replicated urban UFPs, including atmospherically relevant compositions and concentrations. Since genetic background is an important determinant of airway inflammation, we exposed two common strains employed in murine asthma models, C57BL/6 and BALB/c mice, to a 24-h daily dose of 25 µg/m3 throughout gestation and assessed the pulmonary immune response in offspring.
Particle Generation Replicates Urban UFPs
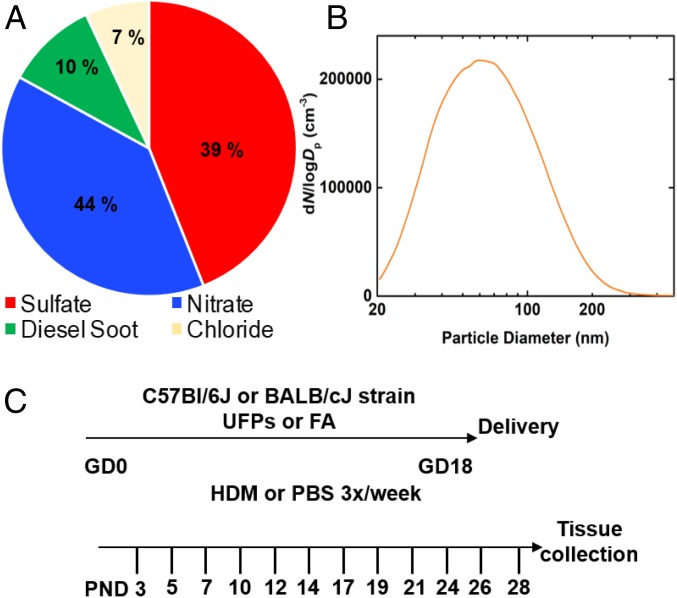
Ambient fine PM is primarily composed of organics, sulfates, nitrates, ammonium, black carbon, and chloride (4–6, 29). The nitrate constituent is attributable to vehicle emissions and industrial source sites, the sulfate is due to the burning of sulfur-containing fuels or coal burning from power plants, and black carbon is produced mainly by diesel vehicles or coal burning from power plants. In our animal model, a multicomponent aerosol mixture representative of PM chemical composition under typically polluted urban environments was produced and introduced into the exposure chamber in a controlled manner (SI Appendix, Figs. S1 and S2). We generated UFPs using an atomizer (SI Appendix, Fig. S1) and a diluted solution consisting of ammonium nitrate, ammonium sulfate, diesel exhaust PM (NIST, SRM 2975), and potassium chloride, with the mass fractions of 44, 39, 10, and 7%, respectively (Fig. 1A). The particle size ranged from 20 to 220 nm, with a peak diameter of about 50 nm (Fig. 1B) and a total surface area of (3.2 ± 0.3) × 103 μm2/cm3. The particle number concentration was measured by a condensational particle counter. In our model, we administered UFPs to time-mated mice 6/h per day from gestation days 0–18 (Fig. 1C). Daily exposure values over the gestational period had an average mass concentration of 101.94 µg/m3 ± 0.0784 (SE), corresponding to a 24-h daily mean dose of 25 µg/m3. While there is no current regulatory standard for UFPs (18), this level is under the US Environmental Protection Agency national ambient air quality standard of 35 μg/m3 for PM2.5 and similar to the WHO recommended guideline of 25 μg/m3 for 24-h average exposure. In utero exposure to UFPs had no effect on litter size. Furthermore, in utero UFP exposure did not affect offspring growth, including weight and length, which were measured beginning on postnatal day 3 until 4 wk of age.
Fig. 1.
In utero UFP exposure model. (A) Particle chemical compositions represent mass compositions measured in typical polluted urban air. The nitrate and sulfate mass fractions, generated from ammonium nitrate and ammonium sulfate, were 44 and 39%, respectively. The chloride mass fraction, from potassium chloride, was 7%. The mass fraction of diesel soot, generated from diesel exhaust PM (NIST, SRM 2975) was 10%. (B) The particle size distribution ranged from 20 to 220 nm with a peak diameter of about 50 nm. (C) Experimental protocol. FA, filtered air; GD, gestational day; HDM, house dust mite; PBS, PBS control; UFPs, ultrafine particles; PND, postnatal day.
In Utero Exposure to UFPs Dampens Offspring Pulmonary Inflammatory Response to HDM
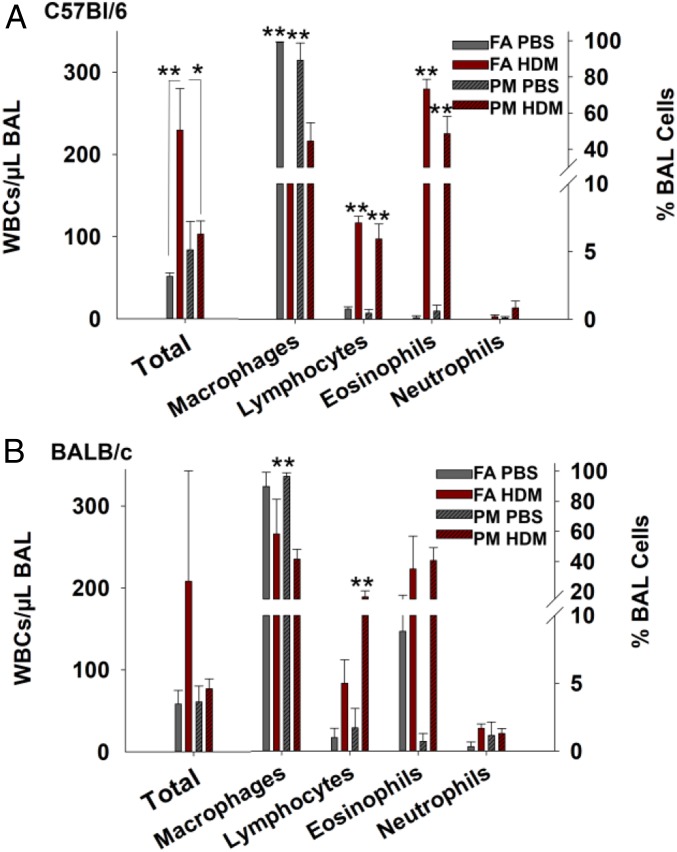
Following birth, offspring exposed to filtered air (FA) or UFPs in utero were placed onto a subchronic allergen challenge using HDM from 0 to 4 wk of age and killed on postnatal day 31 (72 h after the final dose) (Fig. 1C). In both strains of mice, total white blood cell (WBC) counts recovered from bronchoalveolar lavage (BAL) fluid were increased in FA-HDM mice compared with FA-PBS (control) offspring, as expected following HDM exposure. However, in PM-HDM mice the inflammatory response was lower than in FA-HDM mice (Fig. 2A). In C57BL/6 mice, FA-HDM offspring (170 WBC/μL) had significantly higher median BAL WBC counts in comparison with FA-PBS levels (50 WBC/μL) (P = 0.005). Conversely, levels in PM-HDM (79.5 WBC/μL) offspring did not vary significantly from the PM-PBS group (50 WBC/μL) and were significantly lower than the FA-HDM group (P = 0.032), indicating a dampened response. BAL cell differentials showed that macrophages predominated the PBS-treated groups in comparison with HDM-treated offspring (P < 0.001). Levels of lymphocytes and eosinophils were significantly higher in HDM-treated offspring than in PBS controls in both FA and PM groups (P < 0.001). BAL WBC counts in the BALB/c strain (Fig. 2B) did not vary significantly between groups. Significant differences in the number of macrophages and lymphocytes present in the BAL were observed between the PM-PBS and PM-HDM groups (P < 0.001). Sex differences were not apparent in offspring from either strain, likely due to unequal distribution of male and female offspring across the groups and low numbers in some groups. Six weeks after the final allergen challenge, a subset of remaining C57BL/6 mice from the same litters were killed to assess inflammatory response maintenance. BAL WBC counts decreased in all groups, and differential cell counts indicated minimal levels of lymphocytes, eosinophils, and neutrophils (SI Appendix, Fig. S3).
Fig. 2.
Suppression of offspring airway inflammation response. WBCs per μL in BAL expressed as mean ± SE (left axis) and the distribution of macrophages, lymphocytes, eosinophils, and neutrophils in BAL expressed as percentage (right axis). *P < 0.05. **P < 0.01. (A) C57BL/6 offspring pooled from four to five litters per group, FA-PBS (n = 8), FA-HDM (n = 10), PM-PBS (n = 11), PM-HDM (n = 14), P = 0.003 (Kruskal–Wallis test comparing BAL cell counts). Mann–Whitney rank sum values for BAL counts compared between FA-PBS vs. FA-HDM (P = 0.005) and PM-HDM vs. FA-HDM (P = 0.032). FA-PBS vs. FA-HDM (P < 0.001) and PM-PBS vs. PM-HDM (P < 0.001) are shown for percentage of BAL macrophages, lymphocytes, and eosinophils. (B) BALB/c offspring pooled from three litters per group: FA-PBS (n = 6), FA-HDM (n = 3), PM-PBS (n = 7), PM-HDM (n = 7), P = 0.498 (Kruskal–Wallis test comparing BAL cell counts). PM-PBS vs. PM-HDM (P < 0.001) is shown for percentage of BAL macrophages and lymphocytes.
In Utero UFP Exposure Reduces Overall Pulmonary Inflammation in C57BL/6 Mice and Does Not Affect Mucus Production in Either Strain
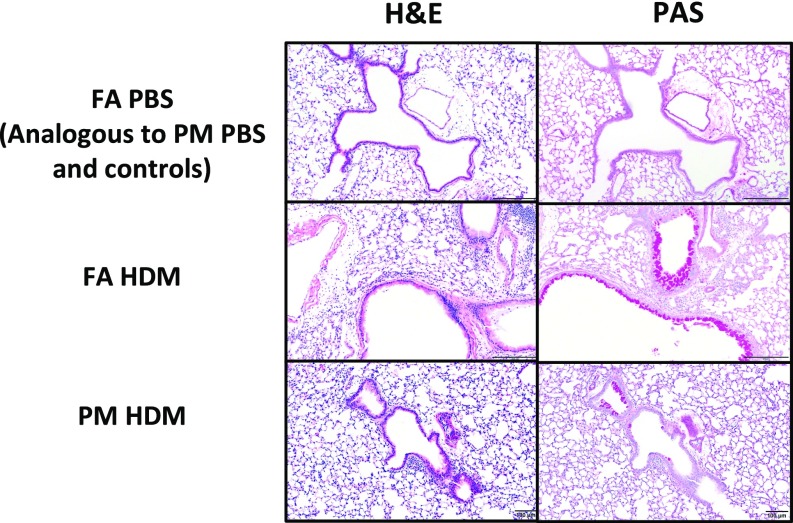
Histological examination of the lungs confirmed the results from the BAL cell counts. Hematoxylin and eosin (H&E) stained lungs isolated from FA-HDM and PM-HDM mice showed similar types of inflammatory responses, with eosinophils as the predominant inflammatory infiltrate and macrophages as the secondary inflammatory cell type. The majority of the inflammation was centered around the bronchioles and vasculature, with alveolar eosinophils becoming apparent only in the most severe cases (Fig. 3). Notably, in C57BL/6 offspring, FA-HDM mice overall demonstrated a more severe inflammatory response, with eight of nine mice (88.9%) classified with marked inflammation and the remaining mouse with moderate inflammation. The average total score for the FA-HDM group was 6.0 (SI Appendix, Table S1). In comparison, the PM-HDM mice had 6/14 mice (42.9%) classified with marked, 5/14 (35.7%) with moderate, and 3/14 (21.4%) with mild inflammation. Their average total score was 4.5, significantly lower than that of FA-HDM mice (P = 0.0483). Average total scores in PM-PBS (0.2) and FA-PBS (0.1) mice did not vary significantly from each other, yet were higher than absolute control mice (0), reflecting a slight response to inhaled saline. Lymphoid hyperplasia was mild to moderate in PM-HDM and FA-HDM lungs, and minimal to not observable in the PBS-dosed mice and absolute controls. To determine the presence of activated T lymphocytes, we used immunohistochemistry targeting FoxP3, a putative marker for Treg cells. Mirroring the BAL WBC differential data showing higher lymphocytes, there were significantly more FoxP3-positive cells in the FA-HDM (39.7 ± 4.2) and PM-HDM (32.5 ± 4.7) groups than in their respective PBS controls, in which no FoxP3+ cells were identified (P < 0.001). Levels between the PM- and FA-HDM groups did not vary significantly.
Fig. 3.
Reduced pulmonary inflammatory response. (Top row) Representative photomicrographs of H&E and PAS-stained sections of lungs in C57BL/6 mice exposed in utero to FA and challenged with PBS show minimal-to-no inflammation and no increased mucus production [no staining of goblet cells in periodic acid–Schiff (PAS)]. Mice exposed in utero to UFPs and challenged with PBS demonstrated analogous histological results. (Middle row) Neonatal mice exposed in utero to FA and challenged with HDM showed the most marked eosinophilic and histiocytic peribronchiolar and perivascular inflammation with bronchus-associated lymphoid tissue (BALT) hyperplasia and increased mucus production with goblet-cell hyperplasia. Note the presence of mucus within the bronchiole in the PAS section. (Bottom row) Neonatal mice exposed in utero to UFPs and challenged with HDM showed an inflammatory response that was similar in character to that in the FA-HDM mice, but on average was less pronounced in severity of inflammation and mucus production. BALT hyperplasia was similar to FA-HDM mice.
In BALB/c offspring, similar to the BAL data, histological examination of the lungs confirmed more variation within the inflammatory response in the FA-HDM group. In this group, two of six mice (33%) were classified as having marked inflammation, 33% mild, and the remaining 33% showed no apparent inflammation. The average total score for the FA-HDM group was 3.0 ± 4.6 (SI Appendix, Table S1). The FA-HDM group averages did not significantly differ from the FA-PBS group across any categories. In comparison, the PM-HDM mice had 7/10 mice (70%) classified as marked, while the remaining 33% showed no apparent inflammation. Their average total score ±SD, 4.3 ± 3.5, was significantly higher than the PM-PBS mean total score (P < 0.001), but was not significantly different from the FA-HDM group.
Mucus production via goblet-cell hyperplasia is common in allergic airway diseases. C57BL/6 offspring from the FA-HDM and PM-HDM groups exhibited marked goblet-cell hyperplasia and mucus production compared with the control groups (Fig. 3). The FA-HDM and PM-HDM mice scored 1.8 ± 0.2 and 1.6 ± 0.4, respectively, which was insignificantly different from each other, while the FA-PBS, PM-PBS, and absolute control mice scored significantly lower: 0.4 ± 0.2, 0.3 ± 0.2, and 0.2 ± 0, respectively (P < 0.001). Similarly, in BALB/c mice, the FA-HDM and PM-HDM groups scored an average of 1.8 ± 0.6 and 2.0 ± 0.3, respectively, which was insignificantly different from each other, while the FA-PBS and PM-PBS groups scored significantly lower: 0.6 ± 0.5 (P = 0.013) and 0.2 ± 0.1 (P < 0.001), respectively.
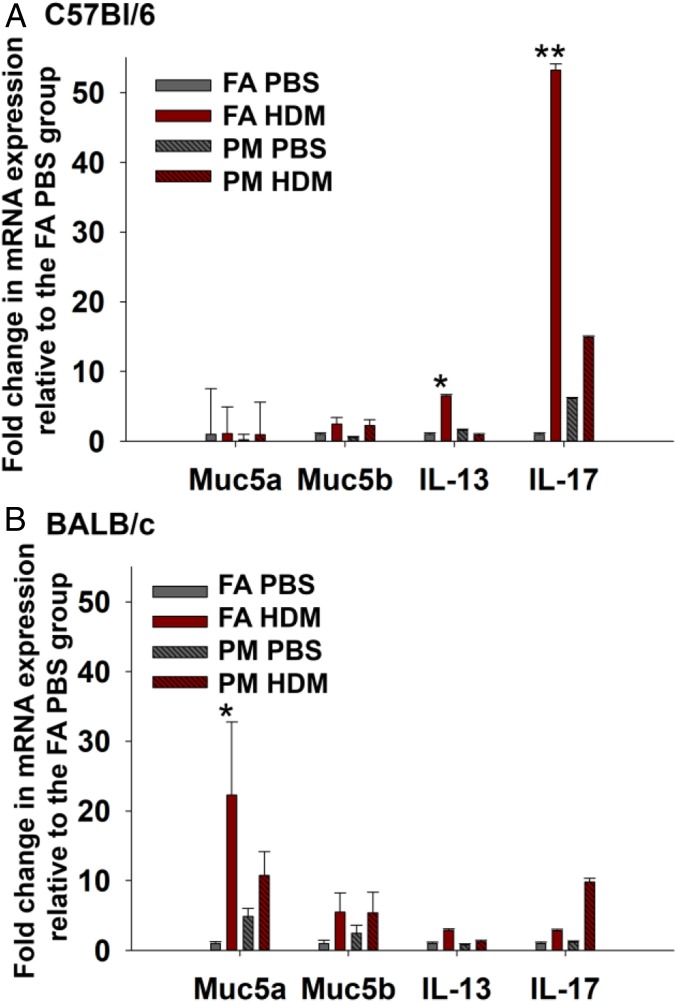
We determined mucus-associated gene expression with quantitative PCR (qPCR) on whole lung tissue (Fig. 4), showing a negligible difference in Muc5a expression among the groups in C57BL/6 mice. In BALB/c mice, Muc5a expression was significantly increased in the FA-HDM group, but not in the PM-HDM group (P < 0.05).
Fig. 4.
Dampened inflammatory response to HDM in the C57BL/6 strain. Lung tissue from offspring was analyzed for relative expression of genes associated with mucus production and selected cytokines. Levels of each transcript were normalized to HPRT and expressed as the fold change in expression relative to the FA-PBS group. *P < 0.05. **P < 0.01. Mean values ± SE are shown for three mice per group (from three separate litters) in C57BL/6 (A). Mann–Whitney rank sum values for IL-13 and IL-17 compared between the FA-PBS vs. FA-HDM groups (P < 0.05) and the FA-HDM and PM-HDM groups (P < 0.05). In the BALB/c strain (B), comparison of Mann–Whitney rank sum values for Muc5a expression between FA-PBS and FA-HDM (P < 0.05) is shown.
In Utero UFP Exposure Diminishes Pulmonary IL-13 and IL-17 Expression in C57BL/6 Mice
We further measured the expression of cytokines associated with a Th1 (IFNγ), Th2 (IL-4, IL-5, Il-13), Th17 (IL-17), or Treg (IL-10, TGF-β) response using qPCR. In C57/Bl6 mice, expression of IL-13 and IL-17 was significantly increased in lung tissue from FA-HDM offspring versus FA-PBS controls (P < 0.05) (Fig. 4A). Notably, IL-13 and IL-17 expression in the PM-HDM group was reduced in comparison with the FA-HDM group and did not significantly differ from PM-PBS controls. In the BALB/c strain, IL-13 and IL-17 expression did not vary significantly between groups. Levels of other cytokines measured in both strains, including IL-4, IL-5, and TGF-β, did not exhibit significant differences between groups. Levels of IL-10 were not detectable.
In Utero UFP Exposure Alters Systemic Inflammatory Response
Systemic inflammatory response was measured by quantifying circulating cytokines in offspring serum. In C57BL/6 mice, reduced systemic IL-9, MCP-1 (i.e., CCL2), and MIP-1α (i.e., CCL3) was observed in PM-HDM offspring compared with PM-PBS controls (Fig. 5A). In comparison with FA-HDM offspring, PM-HDM mice had significantly higher levels of IL-10, an important cytokine involved in Treg cell signaling. In BALB/c mice, the PM-HDM group had significantly higher levels of circulating IL-1β, IL-4, and IL-5 and lower levels of IL-3 than the PM-PBS group (Fig. 5D).
Fig. 5.
Strain differences in offspring systemic inflammatory markers. Circulating cytokines (pg/mL serum) expressed as mean ± SE. Mann–Whitney rank sum values were compared between groups; *P < 0.05. **P < 0.01. (A and C) C57BL/6 strain offspring pooled from four to five litters per group, FA-PBS (n = 5), FA-HDM (n = 4), PM-PBS (n = 4), PM-HDM (n = 4). Differences were observed between PM-PBS and PM-HDM groups for levels of IL-9, MCP-1, and MIP-1a. Differences were observed between the FA-HDM and PM-HDM groups for levels of IL-10. (B and D) BALB/c strain offspring pooled from three litters per group: FA-PBS (n = 4), FA-HDM (n = 3), PM-PBS (n = 4), PM-HDM (n = 4). Differences were observed between PM-PBS and PM-HDM groups for levels of IL-1b, IL-3, IL-4, and IL-5.
Discussion
In this study, we investigated the impact of in utero UFP exposure on offspring pulmonary immune response in a mouse model representing a period of immune maturation. We generated a representative polluted environment by atomizing a mixture of UFPs representative of urban air pollution. The advantages of our systems included the real-time measurement of concentrations at relevant levels and route of exposure. We selected a 24-h dose of 25 μg/m3 (the current WHO recommended guideline for PM2.5). Previously, exposure to PM2.5 in utero at levels of around 25 μg/m3 was attributed to adverse respiratory health outcomes in infants and children (3, 30). PM2.5 exposure during midgestation (during the second trimester) was also identified as a window of susceptibility for childhood respiratory disease (3, 31). Our model captured the relevant window of susceptibly. Notably, there is no current regulatory standard for UFPs. The UFP component of ambient air pollution typically contributes to a negligible portion (less than a few percentage points) of the total PM mass (5). Atmospheric measurements of particle size distributions demonstrated a high-number concentration of UFPs in urban locations because of direct emissions (4, 5) or new particle formation (6–9). For example, under relatively low PM loading, the PM0.1 number concentrations exceeded 200,000 cm−3 because of new particle formation and decreased slightly and remained at about 50,000 cm−3, as the particle size grew and haze events developed (9). Our model employing a peak particle concentration of 20,000 cm−3 represents realistic ambient levels of UFPs (4–8).
Our major conclusion is that in utero UFP exposure results in a reduced pulmonary inflammatory response to allergen challenge during a period of immune maturation. This immune-suppressive phenotype was more pronounced in the C57BL/6 strain, as reflected by significantly decreased white blood cell infiltration in BAL and histopathology; airway eosinophilia elicited by HDM was largely diminished in offspring exposed in utero to UFPs. The observed Th2/Th17-driven inflammatory response (i.e., increased pulmonary expression of IL-13 and IL-17) in the FA-HDM group was lost in the PM-HDM group and was indistinguishable from PBS-treated controls. Conversely, airway mucus production in response to HDM treatment was not attenuated by in utero UFP exposure. While our results differ from the findings of previously described models, i.e., a heightened inflammatory response in PM-exposed C57BL/6 offspring (19–21, 24), they are congruent with the data from a model employing subchronic HDM treatment over a 4-wk period (1–5 wk of age) following early life exposure to combustion-derived particulate matter (CDPM with a mean diameter of 0.2 μm) from 0 to 5 wk of age in C57BL/6 mice (25). Analogous to our results, mice coexposed to CDPM-HDM exhibited reduced Th2-driven pulmonary inflammation in comparison with neonates exposed to FA before and throughout HDM challenge. Airway mucus production did not vary between these groups, consistent with our results. Attenuated allergic airway inflammation has also been shown in cigarette-smoke–exposed mice challenged with OVA (32). On the basis of these findings and our data, an initial suppressive effect may be inferred as beneficial in regard to early asthma symptoms; however, we conclude that an overall adverse effect from UFP exposure as attenuated immune response likely predisposes offspring to respiratory infection and leads to an exacerbated allergic response later in life. In our model, pulmonary inflammation returned to baseline 6 wk after the last allergen challenge when offspring were 12 wk of age. It is possible that, under our conditions, if rechallenged with HDM as adults, offspring may mount an elevated inflammatory response as in prior models (24, 25), following an early window of immunosuppression.
Our study also evaluated the impact of genetic background (i.e., strain difference) on offspring airway inflammatory response. Several previous models demonstrate that mouse strain impacts airway inflammatory response following sensitization and challenge with OVA and HDM (33–35). Furthermore, two commonly employed inbred strains in asthma models, C57BL/6 and BALB/c, have shown differing airway inflammatory responses following prolonged exposure to DEPM (27, 28). In our model, C57BL/6 offspring from both HDM-exposed groups showed greater airway eosinophilia than BALB/c offspring, in agreement with the previous data showing that eosinophil counts in BAL fluid were greater in C57BL/6 (vs. BALB/c) adult mice exposed to diesel exhaust PM from birth to 6 mo (27). Notably, in our model, airway inflammation was significantly attenuated in the C57BL/6 strain, but not in the BALB/c strain; this response likely corresponds to the C57BL/6 strain’s susceptibility to oxidative stress. Numerous previous models highlight that C57BL/6 mice exhibit higher levels of oxidative stress markers in response to inhalation exposure than BALB/c mice. For example, levels of GST mRNA and protein in lung tissue were significantly lower in C57BL/6 than in BALB/c mice, and 8-hydroxy-2′-deoxyguanosine levels in the lung tissues were significantly greater in the C57BL/6 strain following chronic DEPM exposure (27). Similarly, in models of cigarette smoke or water pipe smoke (a form of tobacco smoke) exposure, C57BL/6 mice exhibit decreased levels of glutathione and increased levels of lipid peroxidation products compared with resistant strains, including BALB/c mice (36–38).
The Nrf2-antioxidant response pathway plays an important role in responding to PM-induced oxidative stress (39). Nrf2 is a redox-sensitive basic leucine zipper transcription factor involved in the regulation of many antioxidant genes (40). This signaling pathway represents an adaptive response to environmental stresses, and extensive research has confirmed the importance of Nrf2 in protection against lung pathologies, including asthma (41) and hyperoxia-induced acute lung injury (42). Disruption of Nrf2 has been shown to enhance susceptibility to allergic airway inflammatory responses induced by chronic exposure DEPM (43). Previous findings from a birth cohort in Korea demonstrating increased susceptibility to lower respiratory tract infections in infants exposed in utero to PM2.5 were significantly modified by polymorphisms in maternal genes related to oxidative stress response pathways, especially Nrf2 (44). Likewise, in inbred strains of mice, including C57BL/6 mice, Nrf2 polymorphisms have been shown to influence hyperoxia susceptibility (42), indicating that the differences in Nrf2 response across strains underscore differences in host response to oxidative stress. The mechanisms responsible for the effects of in utero UFP exposure on lung and immune system development are not fully understood, but likely involve maternal systemic inflammation and oxidative stress on the placenta and fetus (2); future work is needed to probe oxidative stress pathways, including Nrf2.
In our model, systemic inflammation data from BALB/c mice indicated increased markers of inflammation, including IFNγ, IL-1β, IL-4, and IL-5, yet decreased IL-3, consistent with a previous study showing that, while neonates dosed with CDPM had a reduced pulmonary immune response to HDM, the total circulating IgE was twofold higher than in air-exposed mice. Conversely, C57BL/6 PM-HDM offspring showed significantly lower IL-9, MCP-1 (i.e., CCL2), and MIP-1α (i.e., CCL3) levels than PM-PBS offspring. Circulating IL-10 levels were significantly higher in the PM-HDM group than in FA-HDM. IL-10 is an important antiinflammatory cytokine secreted by Treg cells. A transient increase in Treg cells likely underlies a decreased inflammatory response. In other models, an increase in pulmonary Treg cells after neonatal CDPM exposure, driven by IL-10 signaling, correlated with increased susceptibility to viral infection (45, 46). We were unable to distinguish Treg cells (i.e., Foxp3+ cells) between FA- and PM-exposed groups using immunohistochemistry in our model; future investigation using flow cytometry may shed light on the role of specific immune subpopulations in the observed response. Another limitation in our study was the small number of BALB/c offspring, particularly in the FA-HDM group (n = 3), leading to high variability in the phenotypic responses. Previous studies have shown that males are more resistant to HDM than females in BALB/c mice (35). Our analysis included offspring of both sexes, and the low numbers rendered the sex-specific analysis difficult in some treatment groups. Future studies evaluating the sex differences in BALB/c offspring are warranted.
In summary, using an in utero exposure model as described in SI Appendix (47, 48), we show that C57BL/6 offspring exposed to UFP in utero and challenged with HDM do not develop a robust airway inflammatory response compared with filtered-air HDM-exposed mice. This indicates an early immunosuppressive environment in the lung and provides a platform to explore the mechanisms of immunosuppression and host defense response to oxidative stress. Our work corroborates the limited earlier evidence that in utero UFP exposure adversely affects offspring pulmonary immune responses and provides insight into the impacts of UFP exposure on infant susceptibility to respiratory infection and overall long-term pulmonary health, highlighting the necessity to reduce UFP exposure and develop strategies to protect the fetus during this vulnerable period.
Materials and Methods
Described here is a summary; additional details are provided in SI Appendix. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee, Texas A&M University.
Mice were housed in a climate-controlled room with a 12/12-h light/dark cycle. Females received a 9% fat breeder diet (Harlan), and males received standard rodent chow (4%) (LabDiet). The exposure chambers consisted of a 12″ × 8″ × 32″ stainless steel box with separated inner compartments and a 1/4″ clear cast acrylic lid (SI Appendix, Figs. S1 and S2). Air was continuously pumped through the chamber by stainless steel aerosol distribution lines attached to the lid and out exhaust lines on the bottom. UFPs were generated utilizing a commercial constant output atomizer, and the number concentration and size distribution of UFPs in the chambers were constantly monitored. To determine offspring response to allergen challenge following in utero UFP exposure, we followed a well-characterized protocol entailing chronic dosing with HDM (15).
Supplementary Material
Acknowledgments
We thank Ms. Valery Roman and Ms. Ana Cardenas for assistance with mouse experiments. This research was supported by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health (R01 ES028866); a Research Enhancement Development Initiative grant from the Texas A&M School of Public Health; and a Tier One Program grant from Texas A&M University. R.Z. acknowledges additional support from Robert A. Welch Foundation Grant A-1417.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1816103116/-/DCSupplemental.
References
- 1.Vieira SE. The health burden of pollution: The impact of prenatal exposure to air pollutants. Int J Chron Obstruct Pulmon Dis. 2015;10:1111–1121. doi: 10.2147/COPD.S40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev. 2017;21:38–46. doi: 10.1016/j.prrv.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Jedrychowski WA, et al. Intrauterine exposure to fine particulate matter as a risk factor for increased susceptibility to acute broncho-pulmonary infections in early childhood. Int J Hyg Environ Health. 2013;216:395–401. doi: 10.1016/j.ijheh.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, et al. Use of a mobile laboratory to evaluate changes in on-road air pollutants during the Beijing 2008 summer Olympics. Atmos Chem Phys. 2009;9:8247–8263. [Google Scholar]
- 5.Levy M, et al. Measurements of submicron aerosols in Houston, Texas during the 2009 SHARP field campaign. J Geophys Res. 2013;118:10,518–10,534. [Google Scholar]
- 6.Guo S, et al. Elucidating severe urban haze formation in China. Proc Natl Acad Sci USA. 2014;111:17373–17378. doi: 10.1073/pnas.1419604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Khalizov A, Wang L, Hu M, Xu W. Nucleation and growth of nanoparticles in the atmosphere. Chem Rev. 2012;112:1957–2011. doi: 10.1021/cr2001756. [DOI] [PubMed] [Google Scholar]
- 8.Yue D, et al. The roles of sulfuric acid in new particle formation and growth in the mega-city of Beijing. Atmos Chem Phys. 2010;10:4953–4960. [Google Scholar]
- 9.Zhang R, et al. Atmospheric new particle formation enhanced by organic acids. Science. 2004;304:1487–1490. doi: 10.1126/science.1095139. [DOI] [PubMed] [Google Scholar]
- 10.Darrow LA, et al. Air pollution and acute respiratory infections among children 0-4 years of age: An 18-year time-series study. Am J Epidemiol. 2014;180:968–977. doi: 10.1093/aje/kwu234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karr CJ, et al. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res. 2009;109:321–327. doi: 10.1016/j.envres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ Res. 2017;159:519–530. doi: 10.1016/j.envres.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Huang F, Yin JN, Wang HB, Liu SY, Li YN. Association of imbalance of effector T cells and regulatory cells with the severity of asthma and allergic rhinitis in children. Allergy Asthma Proc. 2017;38:70–77. doi: 10.2500/aap.2017.38.4076. [DOI] [PubMed] [Google Scholar]
- 14.Debeuf N, Haspeslagh E, van Helden M, Hammad H, Lambrecht BN. Mouse models of asthma. Curr Protoc Mouse Biol. 2016;6:169–184. doi: 10.1002/cpmo.4. [DOI] [PubMed] [Google Scholar]
- 15.Woo LN, et al. A 4-week model of house dust mite (HDM) induced allergic airways inflammation with airway remodeling. Sci Rep. 2018;8:6925. doi: 10.1038/s41598-018-24574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, et al. Influence of ultrafine particle exposure on asthma exacerbation in children: A meta-analysis. Curr Drug Targets. 2018 doi: 10.2174/1389450119666180829114252. [DOI] [PubMed] [Google Scholar]
- 17.HEI Review Panel on Ultrafine Particles . Understanding the Health Effects of Ambient Ultrafine Particles. HEI Perspectives; Boston: 2013. [Google Scholar]
- 18.US Environmental Protection Agency 2013. National Ambient Air Quality Standards for particulate matter: Final rule. 40 CFR parts 50, 51, 52, 53 and 58. Federal Register 78 (No. 10), January 15, pp 3086–3287. [PubMed]
- 19.Fedulov AV, et al. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol. 2008;38:57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiprich M, et al. Inhibition of endotoxin-induced perinatal asthma protection by pollutants in an experimental mouse model. Allergy. 2013;68:481–489. doi: 10.1111/all.12121. [DOI] [PubMed] [Google Scholar]
- 21.Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2014;134:63–72. doi: 10.1016/j.jaci.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharkhuu T, et al. Effects of prenatal diesel exhaust inhalation on pulmonary inflammation and development of specific immune responses. Toxicol Lett. 2010;196:12–20. doi: 10.1016/j.toxlet.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Corson L, et al. Prenatal allergen and diesel exhaust exposure and their effects on allergy in adult offspring mice. Allergy Asthma Clin Immunol. 2010;6:7. doi: 10.1186/1710-1492-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt EB, et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol. 2015;136:295–303.e7. doi: 10.1016/j.jaci.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saravia J, et al. Early-life exposure to combustion-derived particulate matter causes pulmonary immunosuppression. Mucosal Immunol. 2014;7:694–704. doi: 10.1038/mi.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vooght V, et al. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PLoS One. 2010;5:e12581. doi: 10.1371/journal.pone.0012581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YJ, et al. Airway inflammatory responses to oxidative stress induced by prolonged low-dose diesel exhaust particle exposure from birth differ between mouse BALB/c and C57BL/6 strains. Exp Lung Res. 2008;34:125–139. doi: 10.1080/01902140701884406. [DOI] [PubMed] [Google Scholar]
- 28.Li YJ, et al. The effects of oxidative stress induced by prolonged low-dose diesel exhaust particle exposure on the generation of allergic airway inflammation differ between BALB/c and C57BL/6 mice. Immunopharmacol Immunotoxicol. 2009;31:230–237. doi: 10.1080/08923970802383316. [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, et al. Formation of urban fine particulate matter. Chem Rev. 2015;115:3803–3855. doi: 10.1021/acs.chemrev.5b00067. [DOI] [PubMed] [Google Scholar]
- 30.Hsu HH, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. 2015;192:1052–1059. doi: 10.1164/rccm.201504-0658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavigne É, et al. Effect modification of perinatal exposure to air pollution and childhood asthma incidence. Eur Respir J. 2018;51:1701884. doi: 10.1183/13993003.01884-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hizume DC, et al. Cigarette smoke dissociates inflammation and lung remodeling in OVA-sensitized and challenged mice. Respir Physiol Neurobiol. 2012;181:167–176. doi: 10.1016/j.resp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Van Hove CL, et al. Comparison of acute inflammatory and chronic structural asthma-like responses between C57BL/6 and BALB/c mice. Int Arch Allergy Immunol. 2009;149:195–207. doi: 10.1159/000199715. [DOI] [PubMed] [Google Scholar]
- 34.Kelada SN, et al. Strain-dependent genomic factors affect allergen-induced airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 2011;45:817–824. doi: 10.1165/rcmb.2010-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelada SN, et al. Integrative genetic analysis of allergic inflammation in the murine lung. Am J Respir Cell Mol Biol. 2014;51:436–445. doi: 10.1165/rcmb.2013-0501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao H, et al. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1174–L1186. doi: 10.1152/ajplung.00439.2007. [DOI] [PubMed] [Google Scholar]
- 37.Rahman I, De Cunto G, Sundar IK, Lungarella G. Vulnerability and genetic susceptibility to cigarette smoke-induced emphysema in mice. Am J Respir Cell Mol Biol. 2017;57:270–271. doi: 10.1165/rcmb.2017-0175ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan NA, Sundar IK, Rahman I. Strain- and sex-dependent pulmonary toxicity of waterpipe smoke in mouse. Physiol Rep. 2018;6:e13579. doi: 10.14814/phy2.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YJ, Kawada T, Azuma A. Nrf2 is a protective factor against oxidative stresses induced by diesel exhaust particle in allergic asthma. Oxid Med Cell Longev. 2013;2013:323607. doi: 10.1155/2013/323607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 41.Rangasamy T, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho HY, et al. Association of Nrf2 polymorphism haplotypes with acute lung injury phenotypes in inbred strains of mice. Antioxid Redox Signal. 2015;22:325–338. doi: 10.1089/ars.2014.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YJ, et al. Disruption of Nrf2 enhances susceptibility to airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol. 2008;128:366–373. doi: 10.1016/j.clim.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Yang SI, et al. COCOA Study Group Prenatal particulate matter/tobacco smoke increases infants’ respiratory infections: COCOA study. Allergy Asthma Immunol Res. 2015;7:573–582. doi: 10.4168/aair.2015.7.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaligama S, et al. Regulatory T cells and IL10 suppress pulmonary host defense during early-life exposure to radical containing combustion derived ultrafine particulate matter. Respir Res. 2017;18:15. doi: 10.1186/s12931-016-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee GI, et al. Exposure to combustion generated environmentally persistent free radicals enhances severity of influenza virus infection. Part Fibre Toxicol. 2014;11:57. doi: 10.1186/s12989-014-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue H, Khalizov AF, Wang L, Zheng J, Zhang R. Effects of coating of dicarboxylic acids on the mass-mobility relationship of soot particles. Environ Sci Technol. 2009;43:2787–2792. doi: 10.1021/es803287v. [DOI] [PubMed] [Google Scholar]
- 48.Xue H, Khalizov AF, Wang L, Zheng J, Zhang R. Effects of dicarboxylic acid coating on the optical properties of soot. Phys Chem Chem Phys. 2009;11:7869–7875. doi: 10.1039/b904129j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.