Significance
The Western diet (WD) is high in fats and sucrose and low in fiber and is the most prevalent diet in westernized countries. We find that in our model of sepsis, mice fed WD had increased sepsis severity and poorer outcomes. WD-fed mice had higher baseline inflammation, increased sepsis-associated immunoparalysis, and altered neutrophil populations in the blood. The WD-dependent increase in sepsis severity and mortality was independent of the diet-associated microbiome, suggesting that diet may be directly regulating innate immunity. We used our identified disease factors and found WD-fed mice occupy a unique path in sepsis disease progression. Our data provide insight into diet-dependent reprogramming of the immune response and will be important in treating and diagnosing a WD-fed population.
Keywords: Western diet, sepsis, microbiome, metaflammation
Abstract
Sepsis is a deleterious immune response to infection that leads to organ failure and is the 11th most common cause of death worldwide. Despite plaguing humanity for thousands of years, the host factors that regulate this immunological response and subsequent sepsis severity and outcome are not fully understood. Here we describe how the Western diet (WD), a diet high in fat and sucrose and low in fiber, found rampant in industrialized countries, leads to worse disease and poorer outcomes in an LPS-driven sepsis model in WD-fed mice compared with mice fed standard fiber-rich chow (SC). We find that WD-fed mice have higher baseline inflammation (metaflammation) and signs of sepsis-associated immunoparalysis compared with SC-fed mice. WD mice also have an increased frequency of neutrophils, some with an “aged” phenotype, in the blood during sepsis compared with SC mice. Importantly, we found that the WD-dependent increase in sepsis severity and higher mortality is independent of the microbiome, suggesting that the diet may be directly regulating the innate immune system through an unknown mechanism. Strikingly, we could predict LPS-driven sepsis outcome by tracking specific WD-dependent disease factors (e.g., hypothermia and frequency of neutrophils in the blood) during disease progression and recovery. We conclude that the WD is reprogramming the basal immune status and acute response to LPS-driven sepsis and that this correlates with alternative disease paths that lead to more severe disease and poorer outcomes.
Sepsis was recently redefined as a deleterious immune response to infection that leads to life-threatening organ dysfunction (1) and is a leading cause of mortality and critical illness worldwide (2, 3). Currently, sepsis is a major public health concern, and although true incidence is unknown, it is reported that the frequency of sepsis is increasing (4, 5). Despite its clinical relevance, it is still unclear what host factors contribute to the regulation of this deleterious immune response and the outcome of sepsis. By identifying the role of specific host factors that regulate immunological pathways and responses to microbial products, we may begin to define novel relationships between these factors and sepsis severity and mortality.
Recent studies have shed light on the role of diet in regulating the immune system and associated inflammatory diseases; thus, we sought to understand how diet affects sepsis severity and outcome. We are particularly interested in the Western diet (WD), a diet derived from the early agrarian diet introduced nearly 10,000 y ago in the post-Neolithic period that is high in saturated fats and sucrose and low in fiber, because it is one of the most prevalent diets in westernized nations (6, 7) and is associated with obesity. It is known from human studies and animal models that the WD can influence microbial pathogenesis (8–10), chronic inflammation (11–13), and severity of inflammatory diseases (14, 15); however, there is conflicting human data on whether the WD directly alters immunological pathways that contribute to sepsis severity and outcome (16). Thus, our study aims to identify how the WD can influence host immune pathways and immune responses to microbial agents that lead to sepsis and if this alters severity and outcome of sepsis.
In this study, we use a LPS-driven model of sepsis (endotoxemia) to probe the influence of the WD on sepsis. We found that mice fed a Western diet (WD mice) had increased sepsis severity and mortality compared with mice fed a standard fiber-rich chow (SC mice) (see Fig. 1). Our findings are consistent with previous studies showing that WD correlates with increased mortality in mouse models of Staphylococcus aureus-induced sepsis (8) and polymicrobial sepsis (17). Adding to these studies, we provide insight into the regulation of the immune response to microbial products by WD that is independent of bacterial infection. We show that WD-fed untreated mice have increased chronic inflammation, which has been extensively documented in obese patient cohorts (18–22). We also document increased sepsis-associated immunoparalysis during LPS-driven sepsis in WD-fed mice (see Fig. 2) and altered immune cell migration and neutrophil function. We find here that in a germ-free (GF) mouse model, the presence of a diet-associated microbiome is not required for conferring WD-induced sepsis severity and mortality (see Fig. 4). Furthermore, we use plots of our WD-dependent altered disease factors to track disease progression and recovery (see Fig. 5).
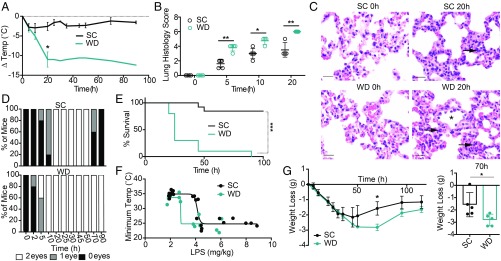
Fig. 1.
Western diet-fed mice have higher susceptibility to LPS-driven sepsis and more severe pathology during disease. (A) Age-matched female BALB/c mice were fed SC or WD for 16 d and injected i.p. with 6 mg/kg of LPS and monitored for temperature loss. (B) Mice were treated with diets as indicated, injected i.p. with 6 mg/kg of LPS, and killed at 0–20 h p.t. for pulmonary histopathologic analysis. (B) Hematoxylin and eosin stained sections of lung (5 μm) were scored for cumulative lung pathology severity, and (C) representative images of neutrophils (arrows) within alveolar septa at 0 and 20 h for SC-fed mice and WD-fed mice are shown (scale bar: 20 μm). Note neutrophil margination within postcapillary venules (asterisk) of WD-fed mice 20 h p.t. Mice treated with diets as indicated and injected i.p. with 6 mg/kg of LPS (D) were monitored for eye exudate formation in zero, one, or two eyes and (E) were monitored for survival up to 100 h p.t. (F) Mice were treated with diets as indicated above and injected i.p. with varying levels of LPS, and temperature was recorded. Temperature loss was plotted against LPS dose to create LPS tolerance curves for this model. (G) Mice were treated with diets as indicated above and injected i.p. with 2 mg/kg of LPS, and weight loss was recorded as a measure of sickness and recovery up to 100 h p.t. n = 4–12 mice/group in each representative experiment. Each experiment was performed two (B and C) or five (A and D–G) times. For A, B, and G a Mann–Whitney U test was used for pairwise comparisons. For all panels, P values less than 0.05 were considered significant (*P < 0.05; **P < 0.01; ***P < 0.001).
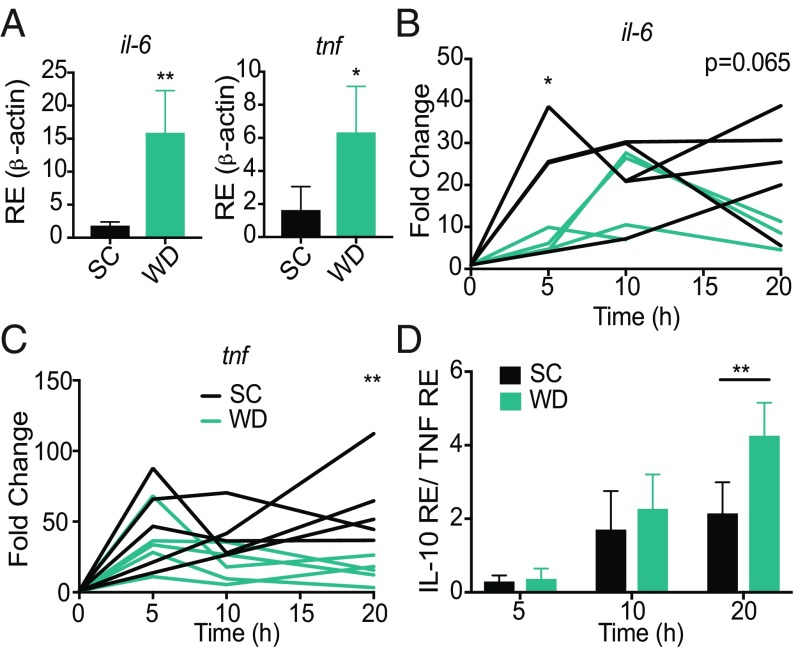
Fig. 2.
Western diet-fed mice have alternative expression of inflammatory cytokines. Age-matched (6–8 wk) female BALB/c mice were fed SC or WD for 16 d before being injected i.p. with 6 mg/kg of LPS, and at indicated times 10 μL of blood were drawn via the tail vein and assessed for expression of il-6 and tnf (A) at 0 h and (B and C) during disease or for (D) il-10 via qRT-PCR. IL-10:TNF ratio was calculated for 5, 10, and 20 h p.t. n = 4–5 mice/group in each representative experiment. Each experiment was performed three times. For A–D, a Mann–Whitney test was used for pairwise comparisons. For all panels, P values less than 0.05 were considered significant (*P < 0.05; **P < 0.01).
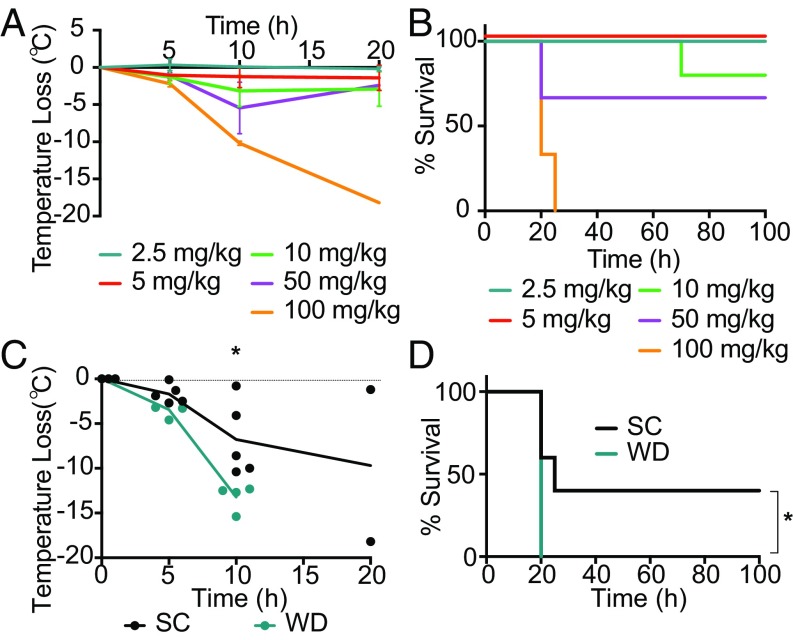
Fig. 4.
Increase in severity of sepsis in Western diet-fed mice is independent of the diet-associated microbiome. Age-matched male germ-free C57B/6 mice were injected i.p. with 2.5, 5, 10, 50, or 100 mg/kg of LPS and monitored for (A) temperature loss as a measure of disease severity and (B) survival. Age-matched male germ-free C57B/6 mice were fed SC or WD for 16 d and then injected i.p. with 50 mg/kg of LPS and monitored for (C) temperature loss and (D) survival. n = 3–5 mice/group in each independent experiment. For C, a Mann–Whitney U test was used for pairwise comparisons. For D, a log-rank test was used. For all panels, P values less than 0.05 were considered significant (*P < 0.05).
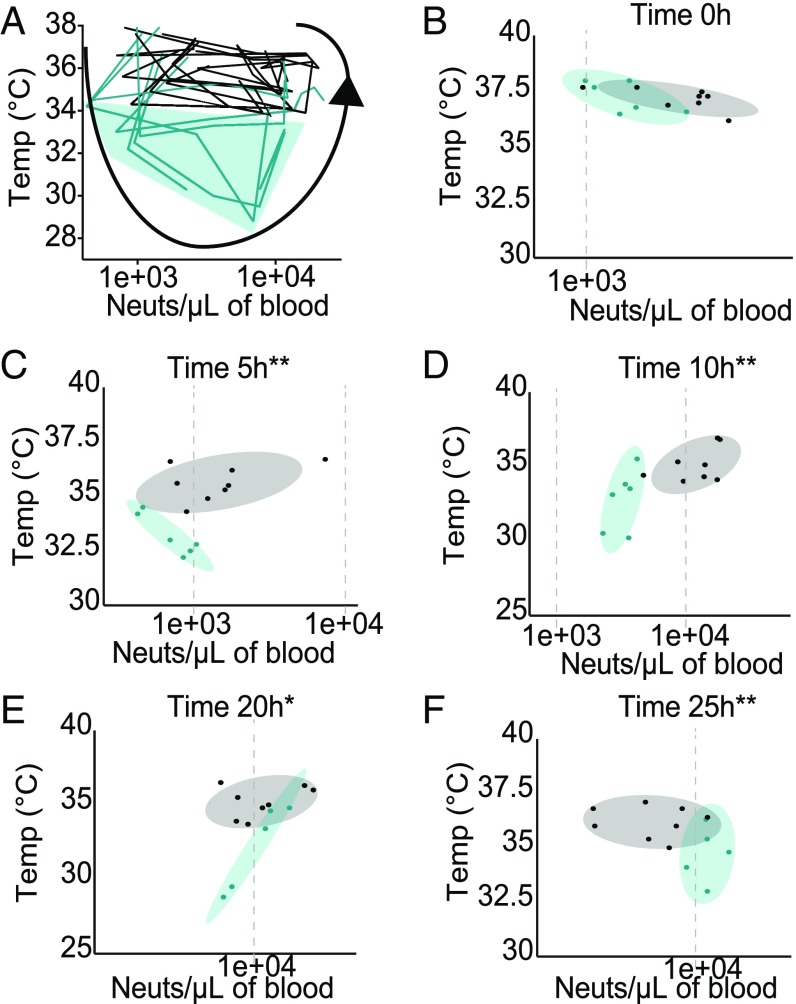
Fig. 5.
Disease space maps of SC- and WD-fed mice during LPS-driven sepsis. (A) Two independent disease markers, temperature (Temp) and neutrophil (Neuts) number in the blood, were plotted against each other over multiple time points (0–20 h p.t.) to create a disease space map for SC-fed (black) and WD-fed (green) mice after a single i.p. injection of 6 mg/kg of LPS. Green shading indicates the portion of the disease map occupied only by WD-fed mice. (B–F) Temperature (y axis) and neutrophil number in the blood (x axis) after i.p. injection of LPS were plotted against each other for each time p.t. Ellipses indicate 70% confidence intervals for the mean of each experimental group, SC fed (gray shading) and WD fed (green shading). A total of four to eight mice per group was used, and each independent experiment was repeated three times. For B–F, multivariate analysis of variance was used to show significant differences in both the temperature and neutrophil number in the blood between the two experimental groups. For panels B–F 0 h, not significant; 5 h, **P < 0.001; 10 h, **P < 0.002; 20 h, *P < 0.047; and 25 h, **P < 0.002.
Our study suggests that WD directly regulates immunity in healthy mice and response to microbial products during LPS-induced sepsis. We have also found multiple immune pathways altered by the WD, and these pathways can be used to predict outcomes of a WD-fed population. Our data suggest patients with chronic exposure to WD may by predisposed to an alternative disease trajectory, and diagnostics and therapeutic interventions should be informed by these data.
Results
Western Diet-Fed Mice Have Increased Sepsis Severity and Poorer Outcomes.
To understand the effect of a chronic high-fat and -sucrose and low-fiber diet on sepsis severity, we fed 6- to 8-wk-old female BALB/c mice the WD for 2 wk. After 2 wk on the WD, mice showed significant weight gain compared with mice fed the SC, as previously published (SI Appendix, Fig. S1A) (8, 23, 24). To assess the impact of chronic WD on sepsis severity and outcome, we induced sepsis in SC- or WD-fed mice by injecting LPS (6 mg/kg) i.p. To measure sepsis severity, we monitored temperature loss (hypothermia), lung histopathology, and eye exudate formation. Sepsis outcome was measured by survival. Mice fed the WD exhibited extreme hypothermia within 20 h of sepsis induction, compared with the SC mice, indicating WD significantly increases LPS-driven sepsis severity (Fig. 1A). Cumulative lung histopathology scores indicated that the septic WD mice showed significantly more lung pathology than septic mice fed the SC from 5 to 20 h posttreatment (p.t.) (Fig. 1 B and C). Additionally, we quantified the development and recovery of eye exudates in septic mice over time as an indication of severe disease. Western diet-fed mice showed signs of eye exudate formation more quickly than SC mice; in the WD mouse that survived there was a severe defect in recovery from eye exudate formation (Fig. 1D). Importantly, WD mice had a significant survival defect compared with the SC mice (Fig. 1E). The dramatic effect of the WD on hypothermia and survival was also evident when mice were administered higher doses of LPS (8 mg/kg; see SI Appendix, Fig. S1B). The increase in disease severity and poorer outcomes in WD mice was not correlated with increased weight gain, suggesting that WD-associated weight gain is not responsible for the WD-dependent increase in sepsis-associated morbidity and mortality (SI Appendix, Fig. S1 C and D). Together, these measures of disease indicate that chronic WD can increase LPS-driven sepsis severity in mice, leading to significantly poorer outcome.
Western Diet Negatively Impacts Tolerance of and Recovery from Sepsis.
Since sepsis severity and outcome were altered by the WD, we determined whether the WD alters general LPS tolerance in mice and recovery from LPS treatment. To understand if LPS tolerance is different among mice fed the WD, we created a “disease tolerance curve” (25–30) by injecting WD mice and SC mice with varying levels of LPS and tracking their minimum temperature as an indicator of health. We found that WD mice showed decreased tolerance to LPS over increasing doses of LPS compared with mice fed the SC (Fig. 1F), indicating that the WD decreases the set dose that is required to induce disease symptoms in our sepsis model.
Another important factor of disease that is often overlooked is recovery. In our model, we measure recovery as kinetics of weight gain after maximum sepsis-induced weight loss. To track recovery, we injected a sublethal dose of LPS (2 mg/kg) i.p. and tracked weight. Both SC- and WD-fed mice treated with 2 mg/kg of LPS lost 10–15% of body weight within a few days after treatment due to sickness-induced anorexia (Fig. 1G). However, at 35 h post-LPS treatment, the SC mice began to regain weight, indicating that they were entering recovery (Fig. 1G). In contrast, the WD mice did not begin to regain weight until 95 h posttreatment (Fig. 1G). The difference in recovery can be seen most dramatically at 70 h post-LPS treatment (Fig. 1G), where the WD mice have lost significantly more weight than the SC mice. Together, these data suggest that WD not only affects sepsis severity and outcome but also affects tolerance of LPS and recovery rate.
Western Diet-Fed Mice Have Higher Baseline Inflammation and Experience Immunoparalysis During Sepsis.
To understand the host pathways that could be driving this WD-dependent increase in disease severity and mortality, we measured differences in the innate immune response in WD mice compared with SC mice before and during sepsis. First, we measured levels of expression of two proinflammatory cytokines, il-6 and tnf, before and after LPS injection. WD mice had increased transcription of il-6 and tnf in the blood before disease compared with SC mice (Fig. 2A), indicating that 2 wk of the WD diet induces increased expression of these proinflammatory cytokines in the blood of mice. This form of chronic inflammation induced by diet is termed metaflammation (31). After LPS treatment, WD mice had lower induction of expression of il-6 and tnf at 5 and 20 h, respectively, compared with the SC mice (Fig. 2B). Together, these data suggest that the WD mice have increased metaflammation and that this is correlated with an inability of WD mice to induce expression of these genes to levels similar to those of SC mice during sepsis.
Considering our previous data indicating that WD mice cannot increase expression of proinflammatory cytokines in response to LPS, we proposed that WD mice may be experiencing higher levels of immunoparalysis during sepsis. The persistence of a suppressed immune state during sepsis has been termed sepsis-associated immunoparalysis and has been correlated with poorer outcomes in patients (32–36). Immunoparalysis can be measured in septic patients by quantifying the ratio of il-10 and tnf, whereby patients with higher IL-10:TNF expression are immunoparalyzed and exhibit poorer sepsis outcomes (37, 38). To elucidate whether the WD induces immunoparalysis in our model, we measured the IL-10:TNF expression ratio in the blood of mice at 0, 5, 10, and 20 h postinduction of sepsis and found that WD mice had a significantly higher ratio of IL-10:TNF expression in the blood at 20 h compared with SC mice (Fig. 2D). Our data agree with previous human studies, suggesting that immunoparalysis is correlated with poorer sepsis outcomes.
There Is a Higher Frequency of Neutrophils in the Blood of Septic Western Diet-Fed Mice.
During sepsis, we found differential expression of inflammatory and antiinflammatory cytokines in the blood; we therefore hypothesized that the migration of immune cells may be altered by the WD. To investigate this hypothesis, we developed a multicolor flow cytometry immune cell panel to determine the relative amounts of monocytes, neutrophils, T cells, B cells, and natural killer (NK) cells within 10 μL of blood in individual mice over time. We found that before disease there were significantly more monocytes and neutrophils circulating in the blood of SC mice compared with WD mice (Fig. 3A). There was no difference in the levels of circulating T cells, B cells, or NK cells (SI Appendix, Fig. S2A) in SC mice compared with WD mice before disease. Together these data suggest that there is altered monocyte and neutrophil recruitment to the blood of WD mice before disease.
Fig. 3.
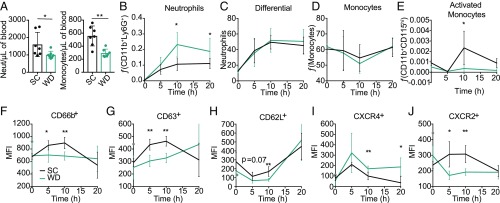
Increased circulating neutrophils with alternative function correlate with Western diet in mice. Age-matched female BALB/c mice were fed SC or WD for 16 d. (A) Mice were killed, and blood was analyzed for the quantity of neutrophils (Neut) (CD11b+/Ly6G+) and monocytes (CD11b+/Ly6G−). Mice were treated with diets as indicated above and injected i.p. with 6 mg/kg of LPS and killed at indicated time points, and neutrophils were analyzed by (B) flow cytometry (CD11b+/Ly6G+) or (C) differential. Additionally, these samples were analyzed by flow cytometry for (D) monocytes (CD11b+/Ly6G−) and (E) activated monocytes (CD11b+/Ly6G−/CD115hi). Neutrophil status was analyzed via mean fluorescence intensity (MFI) of (F) CD66b (secondary granules) and (G) CD63 (primary granules). Neutrophils from the blood were analyzed for recent migration from the bone marrow via MFI of (H) CD62L, (I) CXCR4, and (J) CXCR2. n = 4–7 mice/group in each representative experiment. Each experiment was performed five (A, B, and D) or three (C and E–J) times. For A–J, a Mann–Whitney U test was used for pairwise comparisons. For all panels, P values less than 0.05 were considered significant (*P < 0.05; **P < 0.01).
To identify whether blood immune cell composition was altered in septic mice between WD and SC diets, we quantified the frequency of these cell types in circulation at 5, 10, and 20 h p.t. The frequency of neutrophils circulating in the blood of both SC and WD mice increased after i.p. injection of LPS (Fig. 3B). However, the increase was more dramatic in WD compared with SC mice at 10 and 20 h p.t. (Fig. 3B). Importantly, we analyzed blood from the same animals at the same time points and found that this difference could not be detected in a standard blood differential test (Fig. 3C), suggesting that differentials are not robust enough to detect this significant increase of circulating neutrophils in the WD mice. This finding is important since differentials are standard practice within clinics to assess neutrophil number in the blood and suggests that this method is not sensitive enough to detect significant changes in circulating neutrophils. Furthermore, we did not detect any difference in the relative number of circulating monocytes in the blood between the WD and SC mice (Fig. 3D); however, there are significantly higher numbers of activated monocytes (CD115hi CD11b+) in the blood of SC mice at 10 h p.t. (Fig. 3E). Thus, WD mice have altered neutrophil and activated monocyte frequency in the blood, which may be altering the severity and outcome of sepsis.
Neutrophil populations are heterogeneous and exhibit differential activation states (39). To quantify neutrophil activation and degranulation we used a combination of surface markers. CD63 is a marker for primary granules, and CD66b is a marker for secondary granules (39, 40). We found that neutrophils in the blood of WD mice had lower surface expression of CD63 and CD66b at 5 and 10 h posttreatment compared with the neutrophil populations in the blood of SC mice (Fig. 3 F and G). These data suggest that the neutrophils circulating in the blood of WD mice during sepsis are less activated than the neutrophil populations in the SC mice.
Recently, a specific subset of neutrophils termed “aged neutrophils” were detected in the blood of animals with acute inflammatory diseases (41–43), although their biological role and function are still being defined. During homeostasis, aged neutrophils are removed from circulation in bone marrow, liver, and spleen, and reduced migration of aged neutrophils to the bone marrow has been linked to increased disease severity (41, 42, 44). It is still under debate whether or not aged neutrophils are more or less inflammatory; however, the accepted definition of aged neutrophils is CD62lo and CXCR4hi (41–43). We found that the neutrophils in the blood of WD mice during sepsis had lower surface expression of CD62 (l-selectin) and higher CXCR4 than the neutrophil population within the blood of SC mice (Fig. 3 H and I), indicating the WD mice neutrophils have a more aged phenotype. Furthermore, WD mice neutrophils had lower expression of CXCR2, an inverse indicator of CXCR4 expression (45), compared with SC mice neutrophils in the blood (Fig. 3J). Together, these data support the different immunological status induced by diet. The implications for circulating neutrophils within the WD mice with a different phenotype than the SC mice neutrophils are still unclear.
Considering the significant increase in frequency of neutrophils in the blood of septic mice fed the WD and the alternative phenotype we saw in this cell population, we wanted to assess their role in driving disease severity and recovery. To determine whether neutrophils are driving the phenotypic difference between SC and WD mice in sepsis severity and outcome, we depleted neutrophils before injection with LPS. Mice were fed either the SC or WD for 2 wk, and 24 h before LPS injection mice were treated with a neutralizing antibody against Ly6G (1A8) to decrease neutrophil populations in the blood or treated with the isotype control. We saw that 24 h after injection with anti-Ly6G there was a significant decrease in neutrophil populations in the blood (SI Appendix, Fig. S3A). These mice were then treated i.p. with LPS, and temperature was recorded for severity of disease. Surprisingly, with both diets, mice treated with anti-Ly6G had increased disease severity, as recorded by a significant decrease in temperature at 10 h (SI Appendix, Fig. S3 B and C), suggesting that neutrophils may play a protective role during LPS-driven sepsis in mice. Mice fed SC and treated with anti-Ly6G and LPS had significantly higher mortality than those treated with the isotype control and LPS (SI Appendix, Fig. S3D). Similarly, SC mice treated with anti-Ly6G had outcomes similar to those of WD mice treated with LPS and anti-Ly6G or the isotype control (SI Appendix, Fig. S3D). Additionally, when we measured sepsis recovery, as indicated by the kinetics of weight gain after maximum sepsis-induced weight loss, SC mice showed significantly less weight loss at 70 h compared with WD mice, as seen previously (Fig. 1G and SI Appendix, Fig. S3E). Interestingly, the SC mice treated with anti-Ly6G now showed a lag in weight recovery similar to that of the WD mice at 70 h p.t. (SI Appendix, Fig. S3E), indicating that neutrophils may play a role in the kinetics of recovery from acute sepsis in mice.
Western Diet-Dependent Increase of Sepsis Severity and Poorer Outcome Are Independent of the Microbiome.
It has been shown in mice and humans that chronic WD increases gut permeability, which results in the release of gut-resident microbials into the blood stream (46–51). In agreement with previous published data, we found that healthy WD mice had higher aerobic and anaerobic colony forming units (cfus) isolated from their lungs and aerobic cfus from the blood compared with healthy SC mice (SI Appendix, Fig. S4 A–D). These results led us to hypothesize that increased gut permeability of the WD mice and release of microbial contents may be influencing sepsis severity and outcome in WD mice injected with LPS. Thus, we used a GF mouse model to understand how the microbial contents were affecting sepsis outcome in mice fed either the SC or WD diet.
Since there have been disagreements in the literature over whether GF mice have similar susceptibilities to LPS (52–54), we first identified the tolerance of GF mice to varying doses of LPS. GF mice treated with a single dose of LPS i.p. at 2.5, 5, or 10 mg/kg did not experience quantifiable disease as measured by temperature loss; however, at 50 and 100 mg/kg mice experienced severe hypothermia at 10–20 h p.t. (Fig. 4A). Furthermore, GF mice injected with 2.5, 5, or 10 mg/kg of LPS survived, but at 50 mg/kg ∼50% died, and at 100 mg/kg all of the GF mice died (Fig. 4 A and B). In contrast, the LD100 for sex- and age-matched C57BL/6 conventional mice was 6 mg/kg of LPS (SI Appendix, Fig. S5A). Thus, we found C57BL/6 GF mice are more resistant to i.p. doses of LPS than C57BL/6 conventional mice. These data agree with historical postulations that state GF mice are more resistant to the development of LPS-driven sepsis because (i) sepsis is initiated primarily by the passage of gut-resident microbes into circulation (55, 56) and (ii) GF mice have an immature immune system that correlates with a hyporesponsiveness to microbial products (57–59). Thus, the discrepancies in previously published data may have been due to differences in mouse strains or discordance in germ-free facilities.
Next, we used our GF mouse sepsis model to determine whether the WD-dependent sepsis phenotype is dependent on the microbiota. GF mice were fed either SC or WD for 2 wk and then treated with 50 mg/kg of LPS. At 10 h posttreatment there was a significant decrease in body temperature in mice on the WD (Fig. 4C) compared with GF mice fed the SC. Strikingly, 100% of the WD mice died at 20 h p.t., whereas 50% of the SC mice survived (Fig. 4D). Our results demonstrate that the WD-dependent increase in sepsis severity and poorer outcomes are independent of the microbiota.
Western Diet-Fed Mice Occupy Alternative Space on a Sepsis Disease Map.
Collectively, our results indicated that hosts fed a WD develop a more severe disease. To gain more insights into and predictive power of sepsis outcome, we gathered our various measurements and phenotypes to create disease maps. Disease maps plot the route organisms take through the disease space as they get sick and recover or die (60). By plotting factors that change throughout sickness against each other, you can trace the map of an individual organism through the disease independent of time (60). These maps can be used to identify where the organism is in the disease without knowing exactly when the disease started and can possibly be used to predict the outcome of the disease.
Interestingly, if we plot the frequency of neutrophils in 10 μL of blood against the temperatures of each mouse (in both diets) during sepsis, we find that we can trace mice through sickness and recovery or death (Fig. 5A). Importantly, with this map we can see that the WD animals traverse a much larger disease space (shaded green in Fig. 5A). Much like what has been previously found in malaria disease maps, the organisms with better outcomes trend toward tighter circles in the disease space (black lines), whereas the organisms with poorer outcomes and worse pathologies trend toward larger circles (60). Thus, using the frequency of neutrophils in the blood plotted against temperature, it is obvious that WD mice trend on a path of more severe sepsis and poorer outcomes. Furthermore, if the frequency of neutrophils in blood is plotted against temperature with each time point on a separate graph, we can determine how these two disease factors cluster mice at specific time points during sepsis (Fig. 5 B–F). At 5 and 10 h p.t., the frequency of neutrophils in the blood against temperature clusters SC mice and WD mice separately (Fig. 5D), indicating that these two factors at these time points can report diet and potentially predict disease outcome.
Discussion
In this study we showed that mice fed WD exhibit increased severity and mortality to septic shock induced by LPS (Fig. 1 and SI Appendix, Fig. S1) compared with mice fed a standard high-fiber and low-fat diet. Mice fed WD showed increased metaflammation in healthy mice (before LPS injection) and exacerbated sepsis-associated immunoparalysis during LPS-induced septic shock (Fig. 2). Before LPS treatment, healthy mice fed WD had an increased frequency of monocytes and neutrophils in the blood compared with SC mice. However, WD-fed mice exhibited increased neutrophil populations and decreased activated monocytes in the blood during sepsis compared with SC mice (Fig. 3 and SI Appendix, Fig. S2). Importantly, this increased population of neutrophils in the blood of WD mice had altered surface expression of specific activation and migration markers indicating an aged neutrophil phenotype (Fig. 3) compared with that of SC mice. This neutrophil population will be interesting to further define in the context of systemic inflammation and the role this neutrophil population plays in other inflammatory diseases in mice fed WD.
Our data, along with those of others, have shown that WD increases gut permeability and release of microbial contents into the blood and lungs (SI Appendix, Fig. S4) (46–51). The release of microbial contents is correlated with an increase in sepsis-associated immunoparalysis in WD mice (Fig. 2 B–D) and decreased circulating activated monocytes compared with those of SC mice (Fig. 3E). Thus, WD mice show decreased innate immune responses to LPS in our LPS-driven model of sepsis. Together, these data suggest that microbial contents may be decreasing monocyte sensitivity to a massive LPS challenge, consequently increasing immunoparalysis in WD mice. The association of diet-dependent immunoparalysis with innate immune tolerance has not been investigated but is an interesting and clinically relevant avenue for further investigation. Moreover, analysis of the immunological response of GF mice to LPS will be important in understanding whether diet-dependent sepsis-associated immunoparalysis is driving increased sepsis severity and mortality.
We showed here that the microbiome is not required for the enhanced pathologies and disease severity in hosts fed a WD. Based on previously published results indicating that a WD has many impacts on the gut and microbiome (61, 62), we were surprised that GF mice had increased sepsis severity and mortality when fed WD. Our data suggest that reprogramming of immune profiles and statuses (Figs. 1–3) in WD mice may be driven by dietary constituents. To this point, there have been extensive molecular studies researching the roles dietary fatty acids play in regulating cellular signaling and functions in vitro (63, 64), suggesting that there are specific fatty acids within the WD that may be affecting cellular signaling and functions and thus may be responsible for altered immune cell function and disease outcomes. Moreover, our data suggest that the WD is altering monocyte and neutrophil migration from the bone marrow and tissues and/or myelopoiesis in the bone marrow. Currently, there is a gap in knowledge in understanding the role of dietary metabolites in bone marrow myelopoiesis and immune cell migration. In addition to the fat and sugar present in WD, the loss of fiber, and more specifically microbiota-accessible carbohydrates (MACs) that serve as metabolic input for microbial fermentation, is an important differentiator of the two diets. Diets high in MACs result in more production of fermentation end products like short-chain fatty acids that are known to be absorbed, bind to G protein-coupled receptors, and impact the systemic immune system in a variety of ways (7, 65, 66). Further mechanistic studies are required to uncover the role of diet-induced alterations in metabolites in regulating immune profiles and responses to inflammatory events.
Last, using our identified measurable disease factors (hypothermia and circulating neutrophil numbers), we plotted WD mice vs. SC mice on a disease map and found WD-fed mice have wider loops through space, suggesting more severe disease (Fig. 5A). Together, these data are intriguing because we find that the severity of disease and neutrophil numbers can predict disease pathways depending on diet. In the clinic, the factors we measured may be alternative diagnostics measures. Our findings identify unique diet-induced immune and phenotypic profiles in mice that predict LPS-induced sepsis severity and outcome (Fig. 5). Together, these data suggest two things: (i) WD is manipulating immunity independent of the microbiome, and (ii) circulating neutrophil numbers and temperature loss are two disease factors that, if tracked over time, can predict disease outcome, and at specific time points these factors can predict diet. Considering the inherent variability of human patients in response to sepsis, these results will be interesting to recapitulate in human patients and may be an initial step in increasing the efficacy of sepsis diagnosis and therapeutic treatment.
Materials and Methods
All animal studies were performed in accordance with National Institutes of Health (NIH) guidelines, the Animal Welfare Act, and US federal law. All animal experiments were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC) and were overseen by the Institutional Animal Care and Use Committee (IACUC) under Protocol ID 31047. Animals were housed in a centralized research animal facility certified by the Association of Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. Conventional female BALB/c mice (000651; Jackson Laboratory) or C57BL/6 mice (000664; Jackson Laboratory) between 5 and 7 wk were used for the conventional sepsis model. GF male C57B/6 (Taconic; bred in house) mice between 8 and 10 wk were used for the GF sepsis model. Diet and temperature tracking for mice are detailed in SI Appendix. For sepsis, mice were subject to a single i.p. injection of ultrapure Escherichia coli O111:B4 strain LPS (Invivogen). In GF experiments, a preclinical grade preparation of LPS from the E. coli O111:B4 strain isolated under strict aseptic conditions was used (LPS-EB VacciGrade; Invivogen). In experiments assessing blood cell composition over time, 10 μL of blood were analyzed for the presence of immune cells (SI Appendix). In experiments assessing neutrophil function, plating blood, or lungs for cfus, qRT-PCR analysis, differentials, or lung histology, mice were killed using CO2 and then subject to cardiac puncture for sample collection (details are given in SI Appendix). All samples were acquired on a BD Biosciences LSR II (BD Biosciences) and analyzed using FlowJo (TreeStar, Inc). A drop of blood was collected on a glass slide for differential analysis. Histopathologic evaluation, cfus in lung and blood, and statistical analysis are all described in SI Appendix.
Supplementary Material
Acknowledgments
We thank James Hickman (Portland State University) for formatting and critical reading. This study was supported by National Institute of Allergy and Infectious Diseases (NIAID) Grants 2R01 AI095396-06 (to D.M.M.) and 1F32AI115950-01 (to B.A.N.), Defense Advanced Research Project Agency (DARPA) Grant DARPA-15-21-THoR-FP-006 (to D.M.M.), NIH Grants R01-DK085025 and DP1-AT00989201 (to J.L.S., who is a Chan Zuckerberg Biohub Investigator), and the Stanford School of Medicine Dean’s Postdoctoral Fellowship (to A.J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814273116/-/DCSupplemental.
References
- 1.Singer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL. ICON investigators Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2:380–386. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann R, et al. Adult hemophagocytic lymphohistiocytosis causing multi organ dysfunction in a patient with multiple autoimmune disorders: When the immune system runs amok. Clin Case Rep. 2015;4:165–170. doi: 10.1002/ccr3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 6.Cordain L, et al. Origins and evolution of the Western diet: Health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 7.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779–786. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strandberg L, et al. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One. 2009;4:e7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorthy AN, Tan KB, Wang S, Narasaraju T, Chow VT. Effect of high-fat diet on the formation of pulmonary neutrophil extracellular traps during influenza pneumonia in BALB/c mice. Front Immunol. 2016;7:289. doi: 10.3389/fimmu.2016.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hryckowian AJ, et al. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat Microbiol. 2018;3:662–669. doi: 10.1038/s41564-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruun JM, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:E8–E13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 12.Dalvi PS, et al. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int J Obes. 2017;41:149–158. doi: 10.1038/ijo.2016.183. [DOI] [PubMed] [Google Scholar]
- 13.Christ A, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172:162–175.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzel A, et al. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:404. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H, Bartley GE, Young SA, Davis PA, Yokoyama W. HPMC supplementation reduces abdominal fat content, intestinal permeability, inflammation, and insulin resistance in diet-induced obese mice. Mol Nutr Food Res. 2012;56:1464–1476. doi: 10.1002/mnfr.201200082. [DOI] [PubMed] [Google Scholar]
- 16.Ng PY, Eikermann M. The obesity conundrum in sepsis. BMC Anesthesiol. 2017;17:147. doi: 10.1186/s12871-017-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allman M, Gaskin L, Rivera CA. CCl4-induced hepatic injury in mice fed a Western diet is associated with blunted healing. J Gastroenterol Hepatol. 2010;25:635–643. doi: 10.1111/j.1440-1746.2009.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal R, Faizy AF, Siddiqui SS, Singhai M. Evaluation of TNF-α and IL-6 levels in obese and non-obese diabetics: Pre- and postinsulin effects. N Am J Med Sci. 2012;4:180–184. doi: 10.4103/1947-2714.94944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popko K, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. 2010;15:120–122. doi: 10.1186/2047-783X-15-S2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roytblat L, et al. Raised interleukin-6 levels in obese patients. Obes Res. 2000;8:673–675. doi: 10.1038/oby.2000.86. [DOI] [PubMed] [Google Scholar]
- 21.Ghanim H, et al. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation. 2004;110:1564–1571. doi: 10.1161/01.CIR.0000142055.53122.FA. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmarchelier C, et al. C57Bl/6 N mice on a Western diet display reduced intestinal and hepatic cholesterol levels despite a plasma hypercholesterolemia. BMC Genomics. 2012;13:84. doi: 10.1186/1471-2164-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Råberg L. How to live with the enemy: Understanding tolerance to parasites. PLoS Biol. 2014;12:e1001989. doi: 10.1371/journal.pbio.1001989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 29.Schneider DS, Ayres JS. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louie A, Song KH, Hotson A, Thomas Tate A, Schneider DS. How many parameters does it take to describe disease tolerance? PLoS Biol. 2016;14:e1002435. doi: 10.1371/journal.pbio.1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotamisligil GS. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong HR, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, et al. Interleukin-10/lymphocyte ratio predicts mortality in severe septic patients. PLoS One. 2017;12:e0179050. doi: 10.1371/journal.pone.0179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surbatovic M, et al. Cytokine profile in severe Gram-positive and Gram-negative abdominal sepsis. Sci Rep. 2015;5:11355. doi: 10.1038/srep11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman G, et al. Blood interleukin 10 levels parallel the severity of septic shock. J Crit Care. 1997;12:183–187. doi: 10.1016/s0883-9441(97)90030-7. [DOI] [PubMed] [Google Scholar]
- 36.Chuang TY, et al. High levels of serum macrophage migration inhibitory factor and interleukin 10 are associated with a rapidly fatal outcome in patients with severe sepsis. Int J Infect Dis. 2014;20:13–17. doi: 10.1016/j.ijid.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 37.van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frölich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–953. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 38.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: A marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 39.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 40.Napier RJ, et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 2015;11:e1004770. doi: 10.1371/journal.ppat.1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casanova-Acebes M, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhl B, et al. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood. 2016;128:2327–2337. doi: 10.1182/blood-2016-05-718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang A, et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166:1512–1525.e12. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10:729–734. doi: 10.1097/MCO.0b013e3282efdebb. [DOI] [PubMed] [Google Scholar]
- 47.Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 48.Cani PD, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam YY, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101.e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müller VM, et al. Gut barrier impairment by high-fat diet in mice depends on housing conditions. Mol Nutr Food Res. 2016;60:897–908. doi: 10.1002/mnfr.201500775. [DOI] [PubMed] [Google Scholar]
- 52.Jensen SB, Mergenhagen SE, Fitzgerald RJ. Susceptibility of conventional and garmfree mice to lethal effects of endotoxin. Proc Soc Exp Biol Med. 1963;113:710–714. doi: 10.3181/00379727-113-28469. [DOI] [PubMed] [Google Scholar]
- 53.Landy M, Whitby JL, Michael JG, Woods MW, Newton WL. Effect of bacterial endotoxin in germ-free mice. Proc Soc Exp Biol Med. 1962;109:352–356. doi: 10.3181/00379727-109-27200. [DOI] [PubMed] [Google Scholar]
- 54.Gordon HA, Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971;35:390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schweinburg FB, Frank HA, Fine J. Bacterial factor in experimental hemorrhagic shock; evidence for development of a bacterial factor which accounts for irreversibility to transfusion and for the loss of the normal capacity to destroy bacteria. Am J Physiol. 1954;179:532–540. doi: 10.1152/ajplegacy.1954.179.3.532. [DOI] [PubMed] [Google Scholar]
- 56.Jacob S, et al. Bacterial action in development of irreversibility to transfusion in hemorrhagic shock in the dog. Am J Physiol. 1954;179:523–531. doi: 10.1152/ajplegacy.1954.179.3.523. [DOI] [PubMed] [Google Scholar]
- 57.Fagundes CT, Souza DG, Nicoli JR, Teixeira MM. Control of host inflammatory responsiveness by indigenous microbiota reveals an adaptive component of the innate immune system. Microbes Infect. 2011;13:1121–1132. doi: 10.1016/j.micinf.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Fagundes CT, Amaral FA, Teixeira AL, Souza DG, Teixeira MM. Adapting to environmental stresses: The role of the microbiota in controlling innate immunity and behavioral responses. Immunol Rev. 2012;245:250–264. doi: 10.1111/j.1600-065X.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- 59.Souza DG, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol. 2004;173:4137–4146. doi: 10.4049/jimmunol.173.6.4137. [DOI] [PubMed] [Google Scholar]
- 60.Torres BY, et al. Tracking resilience to infections by mapping disease space. PLoS Biol. 2016;14:e1002436. doi: 10.1371/journal.pbio.1002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagpal R, et al. Gut microbiome composition in non-human primates consuming a Western or Mediterranean diet. Front Nutr. 2018;5:28. doi: 10.3389/fnut.2018.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calder PC. Long-chain fatty acids and inflammation. Proc Nutr Soc. 2012;71:284–289. doi: 10.1017/S0029665112000067. [DOI] [PubMed] [Google Scholar]
- 65.Trompette A, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 66.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.